Abstract
mRNA vaccines including Pfizer BioNTech and Moderna have categorically been considered safe when it comes to preventing COVID-19. However, there is still a small associated risk of thromboembolic phenomenon including venous sinus thrombosis with it and our case report highlights one.
We describe a patient who developed severe progressive headache, tinnitus and visual disturbance symptoms post-Pfizer-SARS-CoV-2 vaccination. His medical history included essential tremors, hypertension, type 2 diabetes mellitus, chronic kidney disease stage 3, anxiety, depression and long-term catheterisation. Systemic examination revealed hypotonia, generalised reduced power and central diplopia along with peripheral visual field defect in the left eye. He was extensively investigated, the COVID-19 PCR test was negative and all routine blood tests were in the normal range except a marginally raised D-dimer of 779 ng/mL. CT head was unremarkable. He was also tested for myasthenia gravis; however, acetylcholine receptors antibodies were negative and nerve conduction studies were normal. Subsequent MRI of the brain with venography confirmed venous sinus thrombosis. A 24-hour Holter monitoring test did not reveal any cardiac rate or rhythm abnormality. He was treated with apixaban as per a neurologist’s advice. His clinical condition started to improve and was later discharged from the hospital with an outpatient neurologist clinic follow-up.
Keywords: Venous thromboembolism, COVID-19, Healthcare improvement and patient safety, Vaccination/immunisation, Stroke
Background
In late 2019, a new disease emerged from Wuhan (a city in the Hubei Province of China), this was identified due to SARS-CoV-2 resulting in acute severe pneumonia and respiratory failure. In February 2020, WHO named it COVID-19 and due to its rapid spread declared it a global pandemic.
So far, vaccines have been considered the most promising approach for suppressing the pandemic and are strongly recommended. Different vaccines have been used, for example, Pfizer-BioNTech, Moderna, Johnson & Johnson, AstraZeneca vaccine and so on. In large placebo-controlled trials, these vaccines were highly effective in preventing laboratory-confirmed COVID-19, especially severe/critical disease.1–3
Both local, as well as systemic side effects, have been reported after receiving these vaccines. Local and systemic adverse effects (pain, fever, fatigue, headache) are common but usually non-severe. Johnson & Johnson and AstraZeneca COVID-19 vaccines are, however, associated with an extremely small risk of thrombosis with thrombocytopenia, but their benefits outweigh this rare risk.3 4
We are presenting a rare case of cerebral venous sinus thrombosis (CVST) that appeared after receiving the Pfizer-BioNTech COVID-19 vaccine.
Case presentation
A Caucasian male in his 80s presented to the accident and emergency department with a 2-week history of severe progressive headache more pronounced in the retro-orbital region, associated with tinnitus and visual disturbance. Symptoms started the next day after receiving the first dose of the Pfizer BioNTech vaccine. There was no relief with over the counter analgesia and symptoms worsened causing more psychological distress, impaired concentration and sleeping difficulties. Medical history included essential tremors, hypertension, type 2 diabetes mellitus, chronic kidney disease stage 3, anxiety, depression and long-term catheterisation. He was fully compliant with his regular medications and there was no recent evidence of trauma. He was living alone in his house and mobilising independently.
His initial observations showed raised blood pressure, systemic examination revealed central diplopia and peripheral visual field defect, intentional tremors, positive Romberg’s sign, reduced power in all four limbs, reduced reflexes and down-going planters.
Investigations
He was extensively investigated and all routine blood tests were in the normal range except slightly raised D-dimers (table 1).
Table 1.
Laboratory investigations of the patient
| Full blood count | Results | Normal values | ||
| Haemoglobin | 121 | g/L | 130–170 | |
| White cell count | 9.7 | 109/L | 4.0–10.0 | |
| Platelet count | 195 | 109/L | 150–400 | |
| Haematocrit | 36 | % | 40–50 | |
| Red cell count | 4.39 | 1012/L | 5.00–6.00 | |
| Mean cell volume | 83 | fL | 83–101 | |
| Mean cell Hb | 28.0 | pg | 27–32 | |
| Neutrophils | 7.49 | 109/L | 1.5–6.5 | |
| Lymphocytes | 1.28 | 109/L | 1.1–3.5 | |
| Monocytes | 0.72 | 109/L | 0.21–0.92 | |
| Eosinophils | 0.15 | 109/L | 0.02–0.67 | |
| Basophils | 0.05 | 109/L | 0.00–0.13 | |
| ESR | 6 | mm/hour | 1–30 | |
| Serum biochemistry | ||||
| CRP | 3 | mg/L | 0–5 | |
| Estimated GFR | 31 | mL/min | ||
| Sodium | 139 | mmol/L | 133–146 | |
| Potassium | 3.9 | mmol/L | 3.5–5.3 | |
| Urea | * | 9.4 | mmol/L | 2.5–7.8 |
| Creatinine | * | 165 | umol/L | 59–104 |
| Total protein | 66 | g/L | 60–80 | |
| Albumin | 43 | g/L | 35–50 | |
| Calcium | * | 2.17 | mmol/L | 2.25–2.55 |
| Corrected calcium | * | 2.15 | mmol/L | 2.20–2.60 |
| Inorganic phosphate | 1.07 | mmol/L | 0.8–1.5 | |
| Total bilirubin | 10 | umol/L | 0–21 | |
| Alkaline phosphatase | 99 | IU/L | 30–130 | |
| Alanine transaminase | 16 | IU/L | 5–41 | |
| Glucose | 5.7 | mmol/L | ||
| Serum vitamin B12 | 501 | ng/L | 197–771 | |
| Vitamin D | 81.1 | nmol/L | 25–50 | |
| Clotting profile and D-dimer | ||||
| INR—clotting profile | 0.9 | Ratio | 0.8–1.2 | |
| APTT—clotting profile | 30 | s | 22–30 | |
| D-dimer | 779 | ng/mL | 500 | |
APTT, activated partial thromboplastin clotting time; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GFR, glomerular filtration rate; INR, international normalized ratio.
COVID-19 PCR test was negative.
CT head showed mild cerebral and cerebellar volume loss but unremarkable otherwise.
He was also investigated for myasthenia gravis, however, acetylcholine receptors antibodies were negative and nerve conduction studies showed no evidence of clinically significant peripheral neuropathy or generalised neuromuscular junction disorder. A 24-hour Holter monitoring test did not reveal any cardiac rate or rhythm abnormality. Brain MRI with venography revealed absent venous flow in the left transverse sinus and the left jugular vein (figure 1A, B) with an impression of cerebral venous sinus thrombosis.
Figure 1.
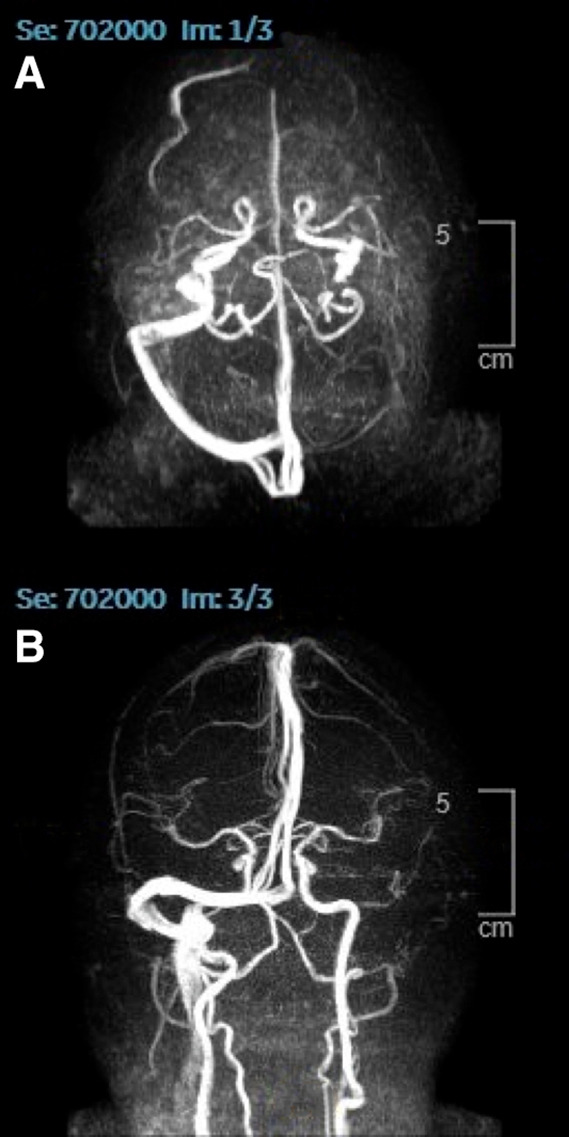
Magnetic resonance venography (MRV) of the cerebral venous sinuses shows absent flow in the left transverse sinus and left jugular.
Treatment
There was a discussion with the on-call neurosurgical and neurology team and it was agreed that there was no other contributing factor than the recent Pfizer COVID-19 vaccine. A multidisciplinary team decision agreed on treatment with a course of apixaban (5 mg two times per day) for 3 months. After starting apixaban, patient’s condition started to improve slowly and later he was discharged home with routine outpatient neurology follow-up. Apixaban was advised due to its lower risk of major bleeding and also as characteristics of our case were similar to a normal non-vaccine-related CVST.
Discussion
CVST is a rare condition with a background incidence of about 15 cases per million people each year according to recent studies from Australia and the Netherlands.5 CVST is a rare cause of stroke that generally affects younger adults and women more than men. Although the pathophysiology is not fully understood, prothrombotic conditions are the most common implicated risk factor for CVST. Patients with hereditary thrombophilia have increased chances of developing any form of thrombosis, including CSVT. G20210A prothrombin polymorphism, factor V Leiden and antiphospholipid syndrome are the most usual causes. Protein C, S deficiency and antithrombin III deficiency are less commonly encountered risk factors.6
Studies and clinical data have confirmed that COVID-19 has been associated with hypercoagulability, with a high incidence of the thromboembolic phenomenon.
Since April 2021, CVST (along with low platelets) link has been established with Astra Zeneca vaccine and Johnson & Johnson (Janssen) COVID-19 vaccine, this was named as vaccine-induced immune thrombotic thrombocytopenia (VITT).4 7 This vaccine-associated syndrome provokes immune-mediated thrombotic thrombocytopenia via IgG antibodies that recognise platelet factor (PF) 4 and activate platelets through their Fcγ receptors.4 7–10 Therefore, the pathogenesis of VITT has been considered similar to that of heparin-induced thrombocytopenia.
CVST has also been reported in a very small number of individuals who have received the mRNA vaccines.11 The pathophysiological mechanism leading towards the development of CVST post mRNA-based vaccination remains unclear. One possibility is that before translation, mRNA may induce pro-inflammatory cascades after binding to pattern recognition receptors. A spike protein translated by the mRNA may facilitate platelets aggregation and dense granule secretion12 and activate the alternative pathway.13 Furthermore, the spike protein in epithelial cells promotes IL-6 trans-signalling by activation of the angiotensin II type 1 receptor axis to initiate the collaboration of a hyper-inflammatory response.14 These might be responsible for the trigger of thrombus formation, which is quite dissimilar from the pathogenesis of VITT mediated by PF4-reactive antibodies.4
A USA-based retrospective cohort study (April 2021) was also conducted (537 913 COVID-19 cases) to look at the incidence of CVST and portal vein thrombosis 2 weeks after receiving mRNA-based COVID-19 vaccine. This study suggested that the risk of CVST in the 2 weeks after vaccination with the Moderna and Pfizer vaccines is very low (about 4 per million people), while the incidence of CVST in the 2 weeks after COVID-19 diagnosis is about 8–10 times as high as this (39 per million people). Both of these risks were higher than in the general population over 2 weeks (0.4 per million).15
In a recent US study (June 2021), 3 cases of CVST were observed within the 30 days following Pfizer-BioNTech vaccination (2 females, 1 male) including one individual with a prior history of thrombosis and another individual with recent trauma in the past 30 days. However, there was no statistically significant difference found in the incidence rates of CVST in 30-day time windows before and after vaccination.16
While it is important to consider this reporting in pharmacovigilance decision-making, there is reason to interpret such reports with caution. Spontaneous adverse event reporting is prone to reporting bias and increased event reporting with lay press attention or the introduction of a novel agent.17–19
So far, there is not much data available for post-Pfizer vaccine CVST and it needs further research and studies.
Learning points.
The SARS-CoV-2 infection has been associated with hypercoagulability, with a high incidence of venous thromboembolism including pulmonary embolism and deep vein thrombosis with a glaring-associated thrombocytopenia.
Physicians should also be alert for signs and symptoms related to cerebral venous sinus thrombosis (CVST), for example, headache or other neurological symptoms when they occur in patients who have recently been vaccinated with the COVID-19 vaccines, for example, AstraZeneca vaccine, Pfizer and so on.
Features of CVST after mRNA-based COVID-19 vaccines are similar to those of non-vaccine-related CVST including a normal platelet count. Therefore, anticoagulation may be appropriate and should not be avoided in contrast to patients with vaccine-induced immune thrombotic thrombocytopenia after virus vector COVID-19 vaccination, in which heparinisation or platelet transfusion is contraindicated.
Acknowledgments
Dr Imran contributed to the writing of this case report.
Footnotes
Contributors: MIAQ was involved in the conception and design, interpretation of data, and drafting and revising it critically before final approval. BA helped in data gathering and compiling the evidence. MAW and AI assisted in drafting and revising up critically before final submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HAN Archive - 00442 [Internet]. Cdc.gov, 2021. Available: https://emergency.cdc.gov/han/2021/han00442.asp [Accessed 16 Oct 2021].
- 4.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med Overseas Ed 2021;384:2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicherla CB, Pauley RA, de Havenon A, et al. Cerebral venous sinus thrombosis in the COVID-19 pandemic. J Neuroophthalmol 2020;40:457–62. 10.1097/WNO.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 6.Idiculla PS, Gurala D, Palanisamy M, et al. Cerebral venous thrombosis: a comprehensive review. Eur Neurol 2020;83:369–79. 10.1159/000509802 [DOI] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–30. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–11. 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir K-L, Kallam A, Koepsell SA, et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021;384:1964–5. 10.1056/NEJMc2105869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus Thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021;325:2448–56. 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thrombosis with thrombocytopenia syndrome - hematology.Org [Internet]. Hematology.org:443. Available: https://www.hematology.org:443/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia [Accessed 16 Oct 2021].
- 12.Yu J, Yuan X, Chen H, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood 2020;136:2080–9. 10.1182/blood.2020008248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to "potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases". Clin Immunol 2021;224:108665. 10.1016/j.clim.2021.108665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patra T, Meyer K, Geerling L, et al. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog 2020;16:e1009128. 10.1371/journal.ppat.1009128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taquet M. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases [Internet]. Open Science Framework, 2021. Available: https://osf.io/h2mt7/ [Accessed 16 Oct 2021]. [DOI] [PMC free article] [PubMed]
- 16.Pawlowski C, Rincón-Hekking J, Awasthi S. Cerebral venous sinus thrombosis (CVST) is not significantly linked to COVID-19 vaccines or non-COVID vaccines in a large multi-state US health system. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McConeghy KW, Bress A, Qato DM, et al. Evaluation of dabigatran bleeding adverse reaction reports in the FDA adverse event reporting system during the first year of approval. Pharmacotherapy 2014;34:561–9. 10.1002/phar.1415 [DOI] [PubMed] [Google Scholar]
- 18.McAdams MA, Governale LA, Swartz L, et al. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: revisiting the Weber effect. Pharmacoepidemiol Drug Saf 2008;17 :882–9. vol.. 10.1002/pds.1633 [DOI] [PubMed] [Google Scholar]
- 19.Bohn J, Kortepeter C, Muñoz M, et al. Patterns in spontaneous adverse event reporting among branded and generic antiepileptic drugs. Clin Pharmacol Ther 2015;97:508–17. 10.1002/cpt.81 [DOI] [PubMed] [Google Scholar]


