Abstract
Introduction
JTR-161 is a novel allogeneic human cell product consisting of dental pulp stem cells isolated from the extracted teeth of healthy adults. It is currently under development as a cell-based therapy for ischaemic stroke. The aim of this study is to evaluate the safety and efficacy of JTR-161 in patients with acute ischaemic stroke when given as a single intravenous administration within 48 hours of symptom onset.
Methods and analysis
This is a first-in-human, randomised, double-blind, placebo-controlled, multicentre, phase 1/2 clinical trial to be conducted in Japan (from January 2019 to July 2021). Patients with a clinical diagnosis of anterior circulation ischaemic stroke with a National Institutes of Health Stroke Scale (NIHSS)score of 5–20 at baseline were enrolled. Patients previously treated with recombinant tissue-type plasminogen activator and/or endovascular thrombectomy were allowed to be enrolled. The study consists of three cohorts: cohorts 1 and 2 (each eight patients) and cohort 3 (60 patients). Subjects were randomly assigned to receive either JTR-161 or placebo in a 3:1 ratio in cohorts 1 and 2, and in a 1:1 ratio in cohort 3. The number of cells administered was increased sequentially from 1×108 (cohort 1) to 3 x 108 (cohort 2). In cohort 3, the higher tolerated dose among the two cohorts was administered. The primary endpoint is the proportion of patients who achieve an excellent outcome as defined by all of the following criteria at day 91 in cohort 3: modified Rankin Scale ≤1, NIHSS ≤1 and Barthel Index ≥95.
Ethics and dissemination
The protocol and informed consent form were approved by the institutional review board at each participating study site. A manuscript with the results of the primary study will be published in a peer-reviewed journal.
Trial registration number
NCT04608838; JapicCTI-194570 and Clinical Trials. gov.
Keywords: NEUROLOGY, INTERNAL MEDICINE, Neurology, Stroke
Strengths and limitations of this study.
This study is a first-in-human, randomised, double-blind, placebo-controlled phase 1/2 clinical trial of a cell-based therapy for ischaemic stroke using JTR-161, a novel allogeneic human cell product consisting of dental pulp stem cells.
The study consists of three cohorts; patients received 1×108 cells in cohort 1, 3×108 cells in cohort 2 and the higher tolerated dose among the two cohorts (either 1×108 cells or 3×108 cells) in cohort 3.
The results of this study will be used to determine the safe dose of JTR-161 administered as a single intravenous dose within 48 hours of symptom onset.
Primary endpoint is the proportion of patients who achieve an excellent outcome as defined by all of the following criteria at day 91 at the optimised dose: modified Rankin Scale ≤1, National Institutes of Health Stroke Scale 1 and Barthel Index ≥95.
This is a proof-of-concept study; therefore, further study will be required.
Introduction
Stroke is the most prevalent cerebrovascular disease worldwide, and still one of the leading causes of death and severe disability. Ischaemic stroke accounts for about 80% of all stroke events.1 2 The recent advances in reperfusion therapy using endovascular thrombectomy have allowed its benefits to be expanded to a larger population of patients with large-vessel occlusion. However, the rate of favourable clinical outcomes remains low,3 4 underscoring an unmet clinical need for adjunctive neuroprotective treatments. Among them, cell-based therapies using human somatic stem cells have been attracting attention, and there are ongoing clinical studies investigating the use of intravenous or intracerebral human somatic stem cells, mainly using bone marrow-derived mesenchymal stem cells (BM-MSCs), in patients with ischaemic stroke from the acute to the chronic phase.5–8 Administration of human BM-MSCs was safe and well tolerated in patients with acute ischaemic stroke, but no significant clinical improvement was observed.7 8
In 2000, human dental pulp stem cells (DPSCs) were discovered in impacted molar teeth.9 DPSCs are thought to originate from the cranial neural crest derived from the neuroectoderm, thus they express early markers for both mesenchymal and neuroectodermal stem cells.10 11 DPSCs can secrete various neurotrophic factors such as neurotrophin-3, brain-derived neurotrophic factor and vascular endothelial growth factor, which promote neuronal survival, proliferation, differentiation and migration.11 Furthermore, compared with BM-MSCs, DPSCs can be obtained by a less invasive process, are more easily expanded and exert more potent immunosuppressive effects via the inhibition of activated T cell responses,12 which makes them attractive for use in allogeneic transplantation. Several reports have shown the beneficial effects of human DPSC transplantation in animal models of neurological disease.13 14 Sakai et al 14 reported that human DPSC transplantation into the completely transected spinal cord of adult rats resulted in marked recovery of hind limb locomotor functions, whereas transplantation of human BM-MSC or skin-derived fibroblasts led to substantially less recovery of locomotor function. Based on a rat stroke model and an in vitro model of ischaemia,15 human DPSCs are reported to be a better source of cell therapy for ischaemic stroke than human BM-MSCs.
JTR-161 is an allogeneic cell-based product consisting of human DPSCs isolated from the extracted teeth of healthy adults. In the preclinical study, intravenous administration of DPSCs decreased ischaemic damage and promoted functional improvement in a rodent model of focal cerebral ischaemia by modulating neuroinflammatory reactions.16 17 Preclinical toxicological study of a single intravenous administration of JTR-161 to male and female nude rats showed no notable toxicological findings 2 weeks after administration (in house data). There were no notable findings regarding tumourigenicity 16 weeks after administration. Furthermore, no scaffold-independent proliferation ability was observed. Regarding non-cellular components of the study product and impurities derived from the manufacturing process, because the amount of residual impurities was low, there were negligible concerns regarding safety. Here, we report the protocol of the first-in-human clinical trial of JTR-161 in patients with acute ischaemic stroke.
Methods and analysis
Study design
This is a randomised placebo-controlled multicentre trial to Eevaluate the efficacy and safety of JTR-161, allogeneic human DPSCs, in patients with Acute Ischaemic stRoke (J-REPAIR study). The aims of this phase 1/2 study are to evaluate the efficacy and safety of JTR-161 in Japanese patients with acute ischaemic stroke when given as a single intravenous administration. Patients received 1×108 cells in cohort 1, and 3×108 cells in cohort 2, sequentially. In cohort 3, the higher tolerated dose among the two cohorts (either 1×108 cells or 3×108 cells), determined according to the recommendation by the Data and Safety Monitoring Board (DSMB) (figure 1), was administered. The DSMB consists of three independent external experts. The DSMB does not recommend advancing to the next cohort when two or more product-related death or death for which a causal relationship cannot be ruled out or occur in the same cohort, or any other serious safety concerns are reported. Death due to cerebral infarction itself and concomitant disorders including pneumonia and transtentorial herniation, followed in frequency by cardiac causes and pulmonary embolism, pretreatment with intravenous recombinant tissue-type plasminogen activator (rt-PA) or endovascular treatment, and combination treatment for the primary disease are excluded as causes of death in this study. The study schedule and assessments are shown in table 1.This is A randomised placebo-controlled multicentre trial to evaluate the efficacy and safety of JTR-161, allogeneic human DPSCs, in patients with J-REPAIR study. The aims of this phase 1/2 study are to evaluate the efficacy and safety of JTR-161 in Japanese patients with acute ischaemic stroke when given as a single intravenous administration. Patients received 1×108 cells in cohort 1 and 3×108 cells in cohort 2, sequentially. In cohort 3, the higher tolerated dose among the two cohorts (either 1×108 cells or 3×108 cells) determined according to the recommendation by the Data and Safety Monitoring Board (DSMB) (figure 1), was administered. The DSMB consists of three independent external experts. The DSMB does not recommend advancing to the next cohort when two or more product-related death or death for which a causal relationship cannot be ruled out or occur in the same cohort, or any other serious safety concerns are reported. Death due to cerebral infarction itself and concomitant disorders including pneumonia and transtentorial herniation, followed in frequency by cardiac causes and pulmonary embolism, pretreatment with intravenous recombinant tissue-type plasminogen activator (rt-PA) or endovascular treatment and combination treatment for the primary disease are excluded as causes of death in this study. The study schedule and assessments are shown in table 1.
Figure 1.
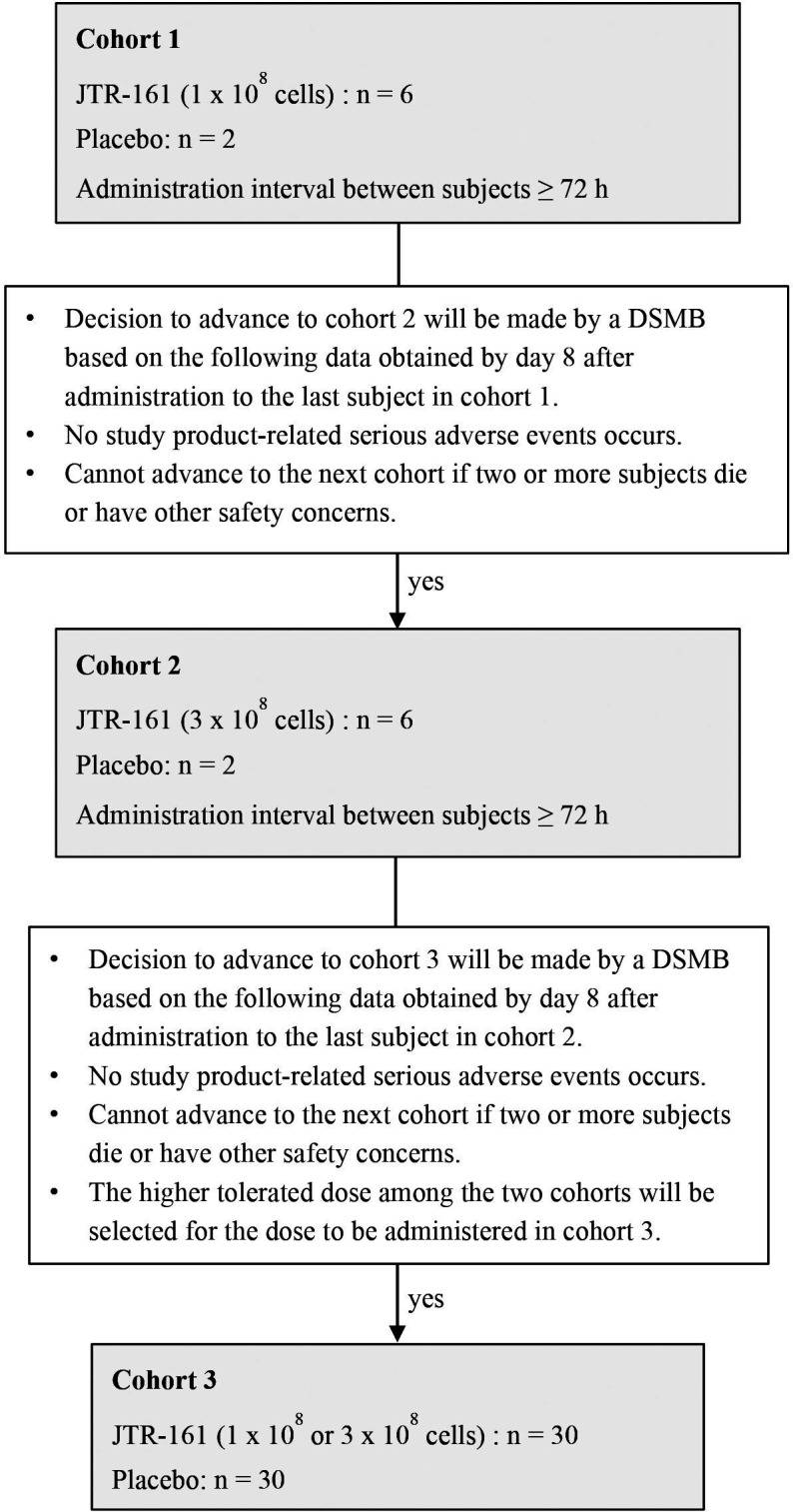
Flowchart of the cohorts. DSMB, Data and Safety Monitoring Board.
Table 1.
Schedule for assessments
| Assessment period | Follow-up period | Dis- charge | Termi- nation | |||||||||||||||||
| Pre-observation period | Observation period | |||||||||||||||||||
| Pre-enrolment | Qualifi-cation | Pre-dosing | Day 1 | Day 2 |
Day 3 |
Day 8 |
Day 31 |
Day 91 |
Day 181 |
Day 366 |
||||||||||
| 0 hour | 1 hour | 2 hour | 4 hour | 6 hour | 12 hours |
|||||||||||||||
| Informed consent | x | |||||||||||||||||||
| Patient characteristics | x¶ | |||||||||||||||||||
| Administration of study product | x | |||||||||||||||||||
| Ability assessment | mRS | x** | x | x | x | x | ||||||||||||||
| Barthel Index | x | x | x | |||||||||||||||||
| Function assessment | NIHSS | x†† | x‡‡ | x | x | x | x | x | ||||||||||||
| QOL assessment | EQ-5D-5L | x | x | x | ||||||||||||||||
| Clinical laboratory tests | Haematology | x†† | x | x | x | x | x | x | x | x | x | |||||||||
| Biochemistry | x†† | x | x | x | x | x | x | x | x | x | ||||||||||
| Blood coagulation test | x†† | x | x | x | x | x | x | x | x | x | ||||||||||
| Biomarker* | x | x | x | |||||||||||||||||
| Urinalysis | x†† | x | x | x | x | x | x | x | x | x | ||||||||||
| Imaging examinations | Safety assessment | x†† | x | x¶¶ | x | |||||||||||||||
| Infarct volume† | x§§ | x¶¶ | x | |||||||||||||||||
| Penumbra region volume† ‡ | x | |||||||||||||||||||
| Body measurements | Height, weight | x†† | ||||||||||||||||||
| Vital signs | Blood pressure, pulse | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Body temperature | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||
| Oxygen saturation | SpO2§ | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Medical examination | Medical examination and interview | x | x | x | x | x | x | x | x | x | x | x | ||||||||
*Assessed in the cohort three only.
†Assessed at the central imaging analysis organisation.
‡Performed at some study sites.
§In addition to the scheduled period in the table, SpO2 is assessed at 15 min, 30 min, 45 min, 1 hour 15 min, 1 hour 30 min, 1 hour 45 min, 2 hour 15 min, 2 hour 30 min, 2 hour 45 min, 3 hour 15 min, 3 hour, 3 hour 15 min, 3 hour 30 min, 3 hour 45 min, 4 hour 30 min, 5 hour, and 5 hour 30 min post-dose.
¶Pregnancy test is performed in premenopausal women or unknown women whether menopause.
**The mRS before ischaemic stroke onset is assessed based on interview from patients or their family.
††Data before obtaining consent are acceptable.
‡‡Assessed at least 4 hours after enrolment.
§§Imaging data after standard treatment are accepted for patients who have undergone standard treatment (rt-PA intravenous or endovascular treatment).
¶¶Assessed once during Day five to Day 8.
mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; QOL, quality of life; SpO2, oxygen salutation of peripheral artery.
Each cohort consists of a 91-day observation period and a 275-day follow-up period (total study period: 366 days). Patients were recruited from 29 stroke centres in Japan between January 2019 and July 2021. The study was registered as JapicCTI-194570, prior to study patient enrolment, and subsequently on Clinical Trials.gov.
Patient population
Inclusion criteria
Patients who met all the following criteria were included:
Japanese male or female patients 20 years of age or older.
Clinical diagnosis of anterior circulation ischaemic stroke based on the results of brain MRI or CT.
National Institutes of Health Stroke Scale (NIHSS) score of ≥5 to ≤20 at screening.
Onset of ischaemic stroke had to have occurred within 48 hours prior to the start of administration of the study product.
A modified Rankin Scale (mRS) of 0 or 1, by either self-report or family report, prior to ischaemic stroke onset.
Exclusion criteria
Patients who met one or more of the following criteria were excluded.
Presence of a new ischaemic lesion in the cerebellum or brainstem at screening.
A marked decline in level of consciousness (NIHSS 1a evaluation of consciousness level is score of 3) at screening.
Patients who had an extensive infarct and for whom maintaining life was expected to be difficult, or who were expected to undergo cranial decompression at screening.
Presence of intracranial haemorrhagic change diagnosed by brain imaging. which was judged to be clinically important by the investigator at screening.
Convulsions after onset of ischaemic stroke.
History of neurological events such as stroke or clinically significant head trauma within 180 days prior to informed consent (IC).
Systolic blood pressure >220 mm Hg or diastolic blood pressure >120 mm Hg, with or without antihypertensive treatment at screening.
Blood glucose level <50 mg/dL or >400 mg/dL at screening.
-
Patients who had any of the serious complication(s) listed below at screening:
End-stage kidney disease for which dialysis was required.
Progressive liver disease such as hepatitis, cirrhosis with Child-Pugh classification class B or C or liver dysfunction with aspartate aminotransferase or alanine aminotransferase over three times the upper limit of the standard value of the study site.
Severe congestive heart failure rated as New York Heart Association class III or IV, active unstable angina or ventricular dysfunction with left ventricular ejection fraction <30%.
Severe pulmonary dysfunction requiring home oxygen therapy.
HIV infection, ongoing systemic infection, severe local infection or immunocompromised condition at screening.
Alzheimer’s disease or other dementias or any other neurological disorder that was judged to affect their ability to give consent to participate in the trial or could confound study assessments performed by the investigator at screening.
Malignant tumour(s) or history of malignant tumour(s) prior to 2 years of ischaemic stroke onset at screening.
Contraindications for MRI such as implanted pacemakers or other metallic prosthesis incompatible with MRI or claustrophobia.
Thrombocytopenia (platelet count <1 00×109/L or heparin-induced thrombocytopenia at screening.
History of allergies to human tissues, bovine or porcine preparations.
History of allergy to streptomycin.
Patients who participated in other clinical trials within 12 weeks prior to IC, or planned to participate in other clinical trials during this trial, or participated in clinical trials of other cell products in the past.
History of splenectomy.
Patients who might have a transient ischaemic attack.
Patients who were scheduled to undergo revascularisation treatment including carotid endarterectomy, stenting, etc by the end of the evaluation (day 91).
Patients who were pregnant or lactating at screening or who wished to become pregnant during the study.
Patients who could not use extremely effective contraception including intrauterine device, intrauterine system, oral contraception (low dose pill), surgical sterilisation, double barrier method (condom with spermicide or combination of condom with pessary) under the guidance of the investigator from the time of IC to 1-year postdose (day 366) or who had a partner who could not take similar contraceptive measures.
Patients who the investigator considered to be inappropriate for inclusion in the study.
Exclusion criteria on eligibility confirmation assessment
After eligibility assessment at screening, the investigator assessed NIHSS again ≥4 hour after the assessment at screening to confirm patient eligibility. Patients who met one or more of the following criteria were excluded:
NIHSS score ≤4 or ≥21.
Change in NIHSS score from screening ≥5.
Administration of the study product could not be started within 48 hours of symptom onset.
Patients who the investigator considered to be inappropriate for inclusion in the study.
Randomisation and blinding
Subjects were randomly assigned to receive either JTR-161 or placebo in a 3:1 ratio in cohorts 1 and 2. In cohort 3, subjects were randomly assigned in a 1:1 ratio to receive either JTR-161 or placebo. Randomisation was performed by the minimisation method, which was adjusted centrally by dynamic assignment with NIHSS at the time of eligibility assessment, with/without standard treatment including intravenous rt-PA or endovascular treatment and age at the time of IC as the allocation factors. The randomisation sequence was generated by an organisation independent of the study sponsors. Allocation of treatment to subjects was randomised via a website. The investigators, patients and the sponsor are masked to the treatment assignment until the observation period is completed. After the final subject in cohort 3 completes the day 91 assessment, the database will be fixed, and the key will be opened. After that, the sponsor, statistical analysts and unblinded personnel will be placed under open blind, and patients and assessors will be blinded until the end of the follow-up period (day 366). JTR-161 and placebo can be identified by the vial appearance; therefore, to ensure masking is maintained, only unblinded persons appointed by the investigator prepared the administration solution, intravenously injected the study product into the patient and cleaned up any spilled administration solution.
Procedure
JTR-161 was manufactured in accordance with good manufacturing practice by JCR Pharmaceuticals. The JTR-161 vial (5.0 mL) contained 1.0×108 cells of DPSC isolated from the extracted teeth of healthy adults and was stored in the gas space of a liquid nitrogen refrigerator.
The frozen study product was thawed in a constant temperature bath at 37° C±1°C for about 5 min, then the required number of cells (one or three vials) was diluted in 100 mL of saline. The solution was intravenously administered once at a rate of 4 mL/min but ≤6 mL/min within 48 hours of symptom onset. Number of cells administered in each cohort and flowchart of the cohorts are shown in figure 1. The DSMB was primarily involved in deciding whether or not to advance to the next cohort as well as the dose (number of cells) for cohort 3. Surgical revascularisation such as carotid endarterectomy and carotid artery stenting was prohibited during the observation period, and attending any clinical trials other than this study was prohibited until the end of the study. In cohorts 1 and 2, the administration interval between subjects was ≥72 hours.
Baseline assessments were carried out at day 0 prior to administration, including (1) primary disease: initial or recurrent, type of cerebral infarction, infarcted blood vessels, onset time and diffusion-weighted imaging (DWI)—Alberta Stroke Program Early Computed Tomography Score, (2) with/without standard treatment with intravenous rt-PA or endovascular treatment. If yes, treatment start time, degree of recanalisation (modified thrombolysis in cerebral infarction classification), recanalisation time and number of passes. If no, reasons for not implementing standard treatment, (3) NIHSS at time of arrival, preregistration and eligibility tests, (4) mRS before the onset of cerebral infarction reported by patients or her/his family, (5) disease history related to the exclusion criteria and, where relevant, the time of complete cure of any malignant condition, effected at least 2 years before IC and still considered cured at the start of administration of the study product. In addition, a medical history deemed necessary for considering adverse events (AEs) was taken. After administration of the study product, mRS and Barthel Index (BI) were assessed at days 31, 91 and 366. NIHSS was assessed at days 2, 8, 31 and 91, and on the day of discharge. Patients were asked to answer the EuroQOL 5 dimensions 5-level scores (EQ-5D-5L) questionnaire at days 31, 91 and 336. Laboratory tests were performed preregistration, preadministration and on days 2, 3, 8, 31, 91, 181 and 366 after administration. Blood pressures including systolic and diastolic blood pressures and pulse rates were measured preregistration, preadministration, 1, 2, 4, 6, 12 and 24 hours after administration, days 3, 8, 31, 91, 181 and 366 after administration and on the day of discharge. Body temperature was measured preregistration, preadministration, 2, 4, 6 and 24 hours after administration, days 3, 8, 31, 91, 181 and 366 after administration and on the day of discharge. Saturated oxygen was measured preregistration, preadministration, every 15 min between 1 and 4 hours after administration, every 30 min between 4 and 6 hours after administration, 12 and 24 hours after administration and on days 3, 8, 31, 91, 181 and 366 after administration. Imaging tests were performed preregistration, and on days 2, 8 and 31 after administration. Serum cytokines and growth factors including tumour necrosis factor (TNF)-α, IL−1β, IL-6, IL-10, IL-17, IL-23 and angiopoietin-1 (Ang-1) were measured preadministration, and on days 3 and 8 after administration in cohort 3. Infarct volumes were measured on DWI and/or fluid-attenuated inversion recovery using MRI preadministration, and on days 8 and 31 after administration. Ischaemic penumbra was measured using MRI as the mismatch between the hypoperfused area on perfusion-weighted imaging and the abnormal area on DWI preadministration, if available. Assessment of imaging was performed at the central assessment organisation. Discontinuance criteria for individual subjects were (1) AEs, worsening of complications and other safety concerns, (2) no visit to the study site due to inconvenience to patients, (3) termination of the study by the sponsor and (4) termination of the study by the investigator due to safety concerns regarding the study product.
Outcome measures
The primary endpoint is the proportion of patients who achieve an excellent outcome as defined by all of the following criteria at day 91 in cohort 3: mRS ≤1, NIHSS ≤1 and BI ≥95. Secondary endpoints were (1) proportion of patients who achieve mRS ≤1 or mRS ≤2 at days 91 and 366, (2) proportion of patients who achieve BI ≥95 at days 91 and 366, (3) proportion of patients who achieve NIHSS ≤1, who achieve improvement of ≥75% and who achieve improvement of ≥10 points at day 91, (4) changes in EQ-5D-5L scores at day 366, (5) proportion of patients who achieve an excellent outcome (mRS ≤1, NIHSS ≤1 and BI ≥95) at day 91, (6) proportion of patients who achieve overall improvement (mRS ≤2, improvement in NIHSS ≥75% and BI ≥95) at day 91. EQ-5D-5L consists of two parts: the EQ-5D descriptive system and the EQ visual analogue scale (VAS). The descriptive system consists of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has five levels: 1=‘no problems’, 2=‘slight problems’, 3=‘moderate problems’, 4=‘severe problems’ and 5=‘extreme problems’. The EQ VAS was recorded during the patient’s self-rated health assessment on a vertical VAS, where the endpoints were labelled ‘The best health you can imagine’ and ‘The worst health you can imagine’. Safety was assessed based on AEs, laboratory tests, vital signs, transcutaneous oxygen saturation and imaging test including MRI or CT. The investigator assessed the intensity, severity and relatedness of an AE. All serious AEs were reported using a standardised SAE report form. Exploratory assessments were (1) cytokines and growth factors such as TNF-α, IL-1β, IL-6, IL-10, IL-17, IL-23 and Ang-1 as biomarkers in cohort 3, (2) infarct volumes and (3) penumbra area volume if available.
Data monitoring body
All data were collected via an electronic case report form prepared using Rave (Medidata Solutions Japan, Tokyo, Japan). Periodic monitoring was performed independently by the sponsor during the trial in order to confirm that the trial was conducted in accordance with the study protocol.
Sample size estimates
In cohorts 1 and 2, eight subjects per cohort (JTR-161, n=6; placebo, n=2) were set as the appropriate number of subjects for the safety evaluation. In cohort 3, 60 subjects (JTR-161, n=30; placebo, n=30) were set as the number sufficient for designing a future clinical trial based on the safety and efficacy data even if a subpopulation analysis is performed.
Statistical analyses
Efficacy analyses will be performed in the full analysis set (FAS); the population of enrolled patients who will have received the study product once and have had a post-dose efficacy assessment, and secondary endpoints will be assessed in the per protocol set; the FAS population excluding those patients with a significant protocol violation. The safety analysis will be performed for patients in the safety analysis set; the population of all enrolled patients who will receive the study product and have a post-dose safety assessment. Categorical variables of patient characteristics and baseline parameters will be aggregated for each treatment group and cohort, and descriptive statistics will be calculated for continuous variables. Comparison analysis will be performed between the JTR-161 and placebo groups in cohort 3, and between the merged JTR-161 groups of cohort 3 and the cohort receiving the same dose as cohort 3 and the merged placebo groups of cohorts 1, 2 and 3. As for the primary endpoint, the proportions and their CIs will be calculated for each administration group. Also, the point estimates of difference in the proportion and its CI will be calculated and compared between the JTR-161 and placebo groups. As for secondary endpoints, the proportions and their confidence intervals for mRS, BI and NIHSS will be calculated for each administration group, and point estimates of the difference in the proportions and its CI will be calculated. The common OR of the mRS will be calculated for each administration group, and the distribution in each category will be shown. Descriptive statistics of mRS, BI, EQ-5D-5L, biomarkers, infarct volumes and penumbra area volume at the time of assessments will be calculated for each treatment group.
For AEs and adverse drug reactions for each administration group, the number of patients, the number of cases and the rate of occurrence will be tabulated according to degree of seriousness, severity and time of onset. AEs will be listed according to MedDRA as lowest level term and are similarly aggregated using the system organ class and preferred term. For laboratory tests, vital signs and oxygen saturation, descriptive statistics will be calculated or tabulated for each administration group and each test time point. The presence or absence of abnormal fluctuations for each test item in individual cases will be summarised. No adjustment for multiplicity will be performed. The two-sided significance level will be set at 5%. Interval estimation will be calculated with a confidence coefficient of 95%.
Study organisation and funding
Teijin Pharma, Tokyo, Japan and JCR Pharmaceuticals, Kobe, Japan were involved in study design, data collection, data analysis, data interpretation, writing of the clinical study report and made the decision to submit the study results for publication. The delegates of the sponsor are Ken-ichi Umino, Teijin Pharma, Clinical Development Department, Research, Development & Technology Unit, 2–1 Kasumigaseki 3-chome, Chiyoda-ku, Tokyo 100–8585, Japan and Kiwamu Imagawa, JCR Pharmaceuticals, Research Division, Drug Discovery Research Institute, 2-2-9 Murotani, Nishi-ku, Kobe, Hyogo, 651–2241 Japan. This study and its publication are funded by Teijin Pharma and JCR Pharmaceuticals.
Patient and public involvement
No patients and/or public were involved in setting the research questions nor they were involved in developing plans for the design (or implementation) of this study protocol.
Ethics and dissemination
The study protocol and IC form were approved by the institutional review board at each participating study site. First approval was obtained from the institutional review board of Nippon Medical School on 20 December 2018. The protocol V.02 issued on 2 November 2018 was reviewed there. All patients gave written IC before initiation of any study-specific procedures. IC from proxies was also allowed due to the pathophysiology of patients with acute cerebral infarction. The study was conducted in accordance with the ethical principles originating in or derived from the Declaration of Helsinki and Good Clinical Practice guidelines. A manuscript with the results of the primary study will be published in a peer-reviewed journal. On completion of the trial, and after publication of the primary manuscript, data requests can be submitted to the corresponding author.
Discussion
Bone marrow is a major source of stem cells and systemic delivery of BM-MSCs after cerebral ischaemia has been widely studied.5–8 While collection of BM-MSCs requires invasive bone marrow puncture, DPSCs can be obtained easily and less invasively from the extracted teeth of healthy adults. They exhibit better plasticity and proliferation capability and have more potent immunoregulatory effects.12 18 19 This J-REPAIR study is the first-in-human, randomised, double-blind, placebo-controlled study to evaluate the efficacy and safety of JTR-161 in patients with acute ischaemic stroke. Patients were selected as participants in this first-in-human study from the viewpoint of invasiveness and unknown risk of DPSCs to the subjects, referring to the ‘Guidance on quality, and technical guidance on conducting non-clinical trials and clinical trials of regenerative medicine products (human cell processed products)’.20 The eligible patients were restricted to those with anterior circulation ischaemic stroke because the severity of their symptoms can be assessed using NIHSS,21 one of the key criteria for assessing eligibility and efficacy in our study. It is difficult to confirm the accurate aetiology of stroke on admission; therefore, there is no limitation regarding stroke subtype such as lacuna, atherothrombotic, cardioembolic and others. Our study did not limit the use of standard treatment including intravenous rt-PA and/or endovascular thrombectomy for recruitment. In addition, available treatments for acute ischaemic stroke except revascularisation treatment such as carotid endarterectomy and stenting in routine clinical practice were allowed to be used as a combination therapy. Patients to whom standard treatment could not be given, and patients who received standard treatment but had a NIHSS ≥5 were allowed to be enrolled. However, these pretreatment and combination therapies may make it difficult to evaluate the safety and efficacy of JTR-161 accurately; therefore, a placebo arm was established as a control group. The study is conducted in a double-blinded manner during the observation period. The keys were opened to the sponsor, statistical analysts and unblinded personnel, but patients and assessors continued under blind conditions until the end of the follow-up period, since EQ-5D-5L was assessed at day 366. In order to explore the therapeutic time window, timing of administration was set to be within 48 hours of symptom onset.
The proportion of subjects who achieve an excellent outcome defined as mRS ≤1, NIHSS ≤1 and BI ≥95 was set as the primary endpoint because we considered that this clinical outcome was the most accurate way of detecting any difference in effectiveness between the subjects receiving JTR-161 and the placebo group. As secondary endpoints, the efficacy of JTR-161 was also evaluated using mRS and BI for disability assessments and NIHSS for function assessment, all of which are widely accepted for use as endpoints in clinical trials of acute ischaemic stroke.22 In recent clinical trials of intravenous rt-PA and endovascular treatment, clinical outcomes as per mRS were evaluated 90 days after the start of treatment.23 24 Similarly, period during which the efficacy of JTR-161 was evaluated was set to 90 days after administration of the study product. EQ-5D-5L was used as a patient-reported outcome for evaluating patient health status. It is reported that there was a significant correlation between stroke type and severity and EQ-5D-5L scores; reproducibility and validity have been verified in patients with stroke.25 We measured a variety of serum cytokines and growth factors before and after transplantation of JTR-161 to investigate the mechanism of human DPSCs on acute ischaemic stroke.
In a preclinical study, the distribution of JTR-161 labelled with a radioactive tracer was highest in the lung 2 hours after a single intravenous administration (in-house data), as reported in other types of stem cells.26 The onset of symptoms such as respiratory distress and decreased oxygen saturation should be carefully followed immediately after administration of JTR-161. Oxygen saturation was measured every 15 min for up to 4 hours and every 30 min for up to 6 hours after administration. Imaging tests were performed to assess infarct lesions and the presence or absence of significant haemorrhagic changes. On the other hand, time of disappearance of JTR-161 from the body has not been elucidated. Therefore, we established a follow-up period of up to 1 year after administration (day 366).
In conclusion, JTR-161 will provide a novel therapeutic option for the treatment of patients with ischaemic stroke due to the wider therapeutic time window for human DPSC transplantation.
Supplementary Material
Acknowledgments
The authors thank Ken-ichi Umino, Teijin Pharma Limited and Kiwamu Imagawa, JCR Pharmaceuticals Co., Ltd. for supporting the study design. The authors thank Dr. Tetsuji Asao (SunFlare Co., Ltd.) for writing support. This manuscript was submitted by Kazuo Nakajima (SunFlare Co., Ltd.) on behalf of the authors and all authors have authorised the submission of this manuscript via SunFlare Co., Ltd. This editorial support was funded by Teijin Pharma Ltd.
Footnotes
SS and CN contributed equally.
Collaborators: Seiji Okubo; Masataka Takeuchi; Masaki Takao; Shinichi Takahashi; Masafumi Morimoto; Yasuhisa Akaiwa; Norihiro Ishii; Takao Kanzawa; Mitsuyasu Kanai; Shinichi Yoshimura; Hideo Hara; Akira Tsujino; Eiichiro Kamatsuka; Takeshi Inoue; Takeshi Iwanaga; Yuka Terasawa; Ryuzaburo Kanazawa; Masahiro Yasaka; Kenichi Morita; Norimichi Nakamura; Takeshi Yoshimoto; Yutaka Honma; Taketo Hatano; Tomohiko Izumidani; Shoji Arihiro; Takayuki Mizunari
Contributors: CN, MI, YI, TU, YM, NS and KK were involved in the study design, protocol preparation and acquisition of funding. SS, CN and KK will be responsible for directly accessing and verifying all data. SS and CN were responsible for the first draft. All authors have reviewed and approved the final manuscript. The work is funded by Teijin Pharma Ltd. and JCR Pharmaceuticals Co., Ltd.
Funding: This study was funded by Teijin Pharma Ltd., JCR Pharmaceuticals Co., Ltd.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Expert Witness from Teijin Pharma Ltd. (SS, CN, KK). Research funding from Teijin Pharma Ltd. (KK). Lecture fee from Teijin Pharma Ltd. (YI). The other authors report no conflicts.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
on behalf of the J- REPAIR trial group:
Seiji Okubo, Masataka Takeuchi, Masaki Takao, Shinichi Takahashi, Masafumi Morimoto, Yasuhisa Akaiwa, Norihiro Ishii, Takao Kanzawa, Mitsuyasu Kanai, Shinichi Yoshimura, Hideo Hara, Akira Tsujino, Eiichiro Kamatsuka, Takeshi Inoue, Takeshi Iwanaga, Yuka Terasawa, Ryuzaburo Kanazawa, Masahiro Yasaka, Kenichi Morita, Norimichi Nakamura, Takeshi Yoshimoto, Yutaka Honma, Taketo Hatano, Tomohiko Izumidani, Shoji Arihiro, and Takayuki Mizunari
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Toyoda K. Epidemiology and registry studies of stroke in Japan. J Stroke 2013;15:21–6. 10.5853/jos.2013.15.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindsay MP, Norrving B, Sacco RL, et al. World stroke organization (WSO): global stroke fact sheet 2019. Int J Stroke 2019;14:806–17. 10.1177/1747493019881353 [DOI] [PubMed] [Google Scholar]
- 3. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5. Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011;134:1790–807. 10.1093/brain/awr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shichinohe H, Kawabori M, Iijima H, et al. Research on advanced intervention using novel bone marrOW stem cell (rainbow): a study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrOW stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol 2017;17:179. 10.1186/s12883-017-0955-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (masters): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2017;16:360–8. 10.1016/S1474-4422(17)30046-7 [DOI] [PubMed] [Google Scholar]
- 8. Savitz SI, Yavagal D, Rappard G, et al. A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-Stroke). Circulation 2019;139:192–205. 10.1161/CIRCULATIONAHA.117.030659 [DOI] [PubMed] [Google Scholar]
- 9. Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000;97:13625–30. 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerkis I, Kerkis A, Dozortsev D, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing Oct-4 and other embryonic stem cell markers. Cells Tissues Organs 2006;184:105–16. 10.1159/000099617 [DOI] [PubMed] [Google Scholar]
- 11. Nosrat IV, Smith CA, Mullally P, et al. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci 2004;19:2388–98. 10.1111/j.0953-816X.2004.03314.x [DOI] [PubMed] [Google Scholar]
- 12. Pierdomenico L, Bonsi L, Calvitti M, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005;80:836–42. 10.1097/01.tp.0000173794.72151.88 [DOI] [PubMed] [Google Scholar]
- 13. Sugiyama M, Iohara K, Wakita H, et al. Dental pulp-derived CD31⁻/CD146⁻ side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A 2011;17:1303–11. 10.1089/ten.tea.2010.0306 [DOI] [PubMed] [Google Scholar]
- 14. Sakai K, Yamamoto A, Matsubara K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 2012;122:80–90. 10.1172/JCI59251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song M, Lee J-H, Bae J, et al. Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transplant 2017;26:1001–16. 10.3727/096368916X694391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nito C, Sowa K, Nakajima M, et al. Transplantation of human dental pulp stem cells ameliorates brain damage following acute cerebral ischemia. Biomed Pharmacother 2018;108:1005–14. 10.1016/j.biopha.2018.09.084 [DOI] [PubMed] [Google Scholar]
- 17. Sowa K, Nito C, Nakajima M, et al. Impact of dental pulp stem cells overexpressing hepatocyte growth factor after cerebral ischemia/reperfusion in rats. Mol Ther Methods Clin Dev 2018;10:281–90. 10.1016/j.omtm.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponnaiyan D, Jegadeesan V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur J Dent 2014;8:307–13. 10.4103/1305-7456.137631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawashima N, Noda S, Yamamoto M, et al. Properties of dental Pulp-derived mesenchymal stem cells and the effects of culture conditions. J Endod 2017;43:S31–4. 10.1016/j.joen.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 20. Ministry of health, labor and welfare, office memorandum June 27, 2016, the guidance of quality, and technical guidance on conducting non-clinical trials and clinical trials of regenerative medicine products (human cell processed products) (Japanese). Available: https://www.pmda.go.jp/files/000212850.pdf [Accessed 10 Jan 2021].
- 21. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603–12. 10.1016/S1474-4422(06)70495-1 [DOI] [PubMed] [Google Scholar]
- 22. Stroke Therapy Academic Industry Roundtable II (STAIR-II) . Recommendations for clinical trial evaluation of acute stroke therapies. Stroke 2001;32:1598–606. 10.1161/01.STR.32.7.1598 [DOI] [PubMed] [Google Scholar]
- 23. Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015;314:1832–43. 10.1001/jama.2015.13767 [DOI] [PubMed] [Google Scholar]
- 24. Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364–72. 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Graaf JA, Kuijpers M, Visser-Meily J, et al. Validity of an enhanced EQ-5D-5L measure with an added cognitive dimension in patients with stroke. Clin Rehabil 2020;34:545–50. 10.1177/0269215520907990 [DOI] [PubMed] [Google Scholar]
- 26. Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009;18:683–92. 10.1089/scd.2008.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


