Abstract
Purpose
The incidence of acute lung injury (ALI) in severe trauma patients is 48% and the mortality rate following acute respiratory distress syndrome evolved from ALI is up to 68.5%. Alveolar epithelial type 1 cells (AEC1s) and type 2 cells (AEC2s) are the key cells in the repair of injured lungs as well as fetal lung development. Therefore, the purification and culture of AEC1s and AEC2s play an important role in the research of repair and regeneration of lung tissue.
Methods
Sprague-Dawley rats (3–4 weeks, 120–150 g) were purchased for experiment. Dispase and DNase I were jointly used to digest lung tissue to obtain a single-cell suspension of whole lung cells, and then magnetic bead cell sorting was performed to isolate T1α positive cells as AEC1s from the single-cell suspension by using polyclonal rabbit anti-T1a (a specific AEC1s membrane protein) antibodies combined with anti-rabbit IgG microbeads. Afterwards, alveolar epithelial cell membrane marker protein EpCAM was designed as a key label to sort AEC2s from the remaining T1α-neg cells by another positive immunomagnetic selection using monoclonal mouse anti-EpCAM antibodies and anti-mouse IgG microbeads. Cell purity was identified by immunofluorescence staining and flow cytometry.
Results
The purity of AEC1s and AEC2s was 88.3% ± 3.8% and 92.6% ± 2.7%, respectively. The cell growth was observed as follows: AEC1s stretched within the 12–16 h, but the cells proliferated slowly; while AEC2s began to stretch after 24 h and proliferated rapidly from the 2nd day and began to differentiate after 3 days.
Conclusion
AEC1s and AEC2s sorted by this method have high purity and good viability. Therefore, our method provides a new approach for the isolation and culture of AEC1s and AEC2s as well as a new strategy for the research of lung repair and regeneration.
Keywords: Alveolar epithelial cells type 1, Alveolar epithelial cells type 2, Three-dimensional culture, Magnetic activated cell sorting
Introduction
With the development of industry as well as the increasingly rampant terrorism, people are more susceptible to injury in daily life or escalating conflicts. WHO estimates that more than 4,000,000 people die from various injuries each year. Lung is not only the most important respiratory organ, but also the most vulnerable target organ in trauma due to its unique anatomical structure and characteristics of gas exchange with the external environment. The incidence of acute lung injury (ALI) in patients with severe trauma (injury severity score ≥16) accounts for 48% and the mortality rate of acute respiratory distress syndrome (ARDS) is up to 68.5%.1 More importantly, respiratory insufficiency is also an important cause of dysfunction of other organs.2 Therefore, prevention and treatment of ALI/ARDS is important for critical diseases such as severe trauma. In recent years, although there are many important progresses in the pathogenesis of ALI/ARDS, the clinical treatment of ALI/ARDS is still very difficult. Respiratory support is the main treatment method, and there is no effective personalized treatment.3 Although adult lung is a relatively static organ in physiological state, it has been reported that the lung has a strong self-repairing ability.4,5 The experiment of lobectomy in mice further confirmed that although no new lobes could grow after resection, many new alveolar could be regenerated rapidly around the unresected lobes to make them enlarged, and the respiratory function of these mice completely repaired within 2–3 weeks.4 Clinical data also showed that pulmonary function of necrotizing pneumonia or ARDS patients can gradually return to normal within 6 months.6, 7, 8
Alveoli are the smallest functional unit of the place of gas exchange in lung. Alveolar walls consist of a single layer of flat epithelium, mainly composed of flat alveolar epithelial type 1 cells (AEC1s) and cuboidal AEC2s. AEC2s are secretory cells which maintain alveolar surface tension by secreting surfactants. AEC2s account for about 5% of the surface area, but the number of AEC2s is almost twice as that of AEC1s. AEC2s are capable of alveolar epithelium repair and regeneration due to its feature of facultative progenitor cells. By genetic lineage tracing in vivo using Cre recombinase driven by surfactant protein C, Desai et al.9 confirmed that AEC2s rarely proliferated and hardly differentiated to AEC1s in stable state. However, after injury caused by bleomycin and hypoxia, AEC2s not only proliferated markedly, but also differentiated into AEC1s immediately after ALI.10,11 It would be of great theoretical significance to explore the mechanism of repair and regeneration of AEC1s and AEC2s after ALI/ARDS. Therefore, isolation, purification and culture of AEC1s and AEC2s are crucial for the study of lung tissue repair and regeneration in vitro.
After labeled with AQP5 or T1a, AEC1s with high purity could be isolated by flow cytometry, while it is hard to achieve such an effect for AEC2s due to the lack of specific cell surface markers.12, 13, 14 Percoll density centrifugation has been used to isolate AEC2s and AEC1s.15, 16, 17 However, the upper and lower layers of Percoll could be easily mixed, thus affecting the purity of the isolated cells. Macrophages are easily adhered to IgG, so using IgG-coated plate to remove the macrophages is also too rough for AEC2s isolation.15,18 fluorescence activated cell sorting (FACS) can only isolate AEC2s from proSPC-lineage tracing mice by the auto-fluorescence of AEC2s.19
In this study, we used Dispase and DNase I to digest lung tissue to obtain single cell suspension, from which we separated AEC1s with magnetic beads coated with AEC1s membrane marker protein T1a, and then isolated AEC2s from the remaining cell suspension using alveolar epithelium membrane marker protein EpCAM. Then we evaluate the purity and viability, aiming to prove a method for continuously separation of primary epithelial cells for the research of lung tissue repair and regeneration.
Methods
Single cell isolation
Sprague-Dawley rats, aged 3–4 weeks, weighing 120–150 g, were purchased from the animal center of Daping Hospital, Army Medical University. Rats were anesthetized with 2% pentobarbital at a dose of 60 mg/kg by intraperitoneal injection and then immersed successively in 75% ethanol, iodophor, and 75% ethanol for 5 min. Pharyngeal skin and tissue were dissected to perform tracheal intubation, rinsed the lung via pulmonary artery catheterization after median sternotomy. The lungs were resected en bloc and moved into the clean bench, washed twice with normal saline, and injected 6 mL protease solution (containing 10 U/mL Dispase, 10% fetal bovine serum (FBS), 10 U/mL DNase I solubilized in 1640 Medium) through the trachea. Then transfer the lungs into a 50-mL centrifugal tube containing 10 mL protease solution to digest lung tissues completely at 37°C for 45 min; afterwards cut the lung lobes into small pieces by ophthalmic scissors and filter the digested tissue through a 75 μm filter and then a 40 μm filter; finally centrifugate the samples at a speed of 300 g for 5 min.
Magnetic separation of AEC1s and AEC2s
First, remove the supernatant and resuspend cell precipitation in dispersing agent (containing 10% FBS, 10 U/mL DNase I solubilized in RPMI 1640 medium), blend 2 μL anti-rat T1α (Sigma, #1995, St. Louis, USA) with the suspension, and then incubate at 4 °C for 15 min. Second, wash the suspension twice with buffer and incubate cells in 80 μL buffers supplemented with 20 μL anti-Rabbit IgG Microbeads (Miltenyi Biotec, #130-048-602, Bergisch Gladbach, Germany) for 15 min at 4°C. After two times of wash, cells were resuspended in 500 μL buffers and applied onto the MS column (Miltenyi, #130042-201, Bergisch Gladbach, Germany) and collected flow-through containing unlabeled T1α-neg cells. Third, elute the magnetically labeled T1α-pos cells with 1 mL buffer and centrifugate at a speed of 300 g for 10 min at 4°C. Finally, cells were resuspended and cultured in 8-well plates in a concentration of 5 × 105 cells per 1 mL medium (DMEM/F12, 10% FBS, 200 mM glutamine and 100 × penicillin streptomycin combination). The medium was changed every 48 h.
T1α-neg cells were resuspended in 0.5 mL dispersing buffer and incubated with 2 μL anti-rat EpCAM (Abcam, #ab187276, Cambridgeshire, UK) at 4°C for 45 min. Cell wash was conducted twice with buffer and incubated in 80 μL buffer containing 20 μL anti-mouse IgG Microbeads (Miltenyi Biotec, #130-047-102, Bergisch Gladbach, Germany) for 15 min at 4°C. EpCAM-pos cells labeled with magnetic beads were then isolated using a MS column (Miltenyi, #130042-201, Bergisch Gladbach, Germany) and resuspended in MTEC/plus medium (DMEM/F12, 7.5 M HEPES, 1.8 M sodium bicarbonate, 10% FBS, 200 mM glutamine, 100 × penicillin streptomycin combination and 1 × Insulin Transferrin Selenium). The medium was changed every 48 h.
Three-dimensional (3D) cell culture of AEC2
100 μL matrigel (BD, #354234, Bedford, USA) was pipetted into the 96-well plate and placed in the 5% CO2 incubator for 2 h. 5 × 104 AEC2 cells per 100 μL MTEC/plus medium was pipetted into the well carefully and the well was placed in 5% CO2 incubator. The medium was changed every other day.
Immunofluorescence staining
AEC1s and AEC2s cultured for 3 days were quickly rinsed twice with phosphate buffered saline (PBS) and then fixed for 15 min with 4% paraformaldehyde at 4°C. After permeabilized with 0.1% Triton X-100, cells were blocked for 45 min with 10% donkey serum, and then they were incubated in primary antibodies at 4°C overnight, AQP5 (1:400 dilution) for AEC1s and proSPC (1:400 dilution) for AEC2s. After wash in PBS, cells were incubated with Alexa Fluor-conjugated secondary antibodies donkey-anti-mouse for 1 h at 37 °C and counterstained with nuclear dye DAPI (4′,6-Diamidino-2-Phenylindole, dihydrochloride) after rinsed twice by PBS.
Immunofluorescence staining of 3D cultured AEC2s
Paraffin sections of AEC2 were deparaffinized in xylenes for 30 min and rehydrated in concentration gradients of ethanol. Antigen retrieval was then performed by maintaining sections in citrate buffer using a high-pressure steam boiler. Slides were blocked in 10% donkey serum for 1 h at room temperature after permeabilized with 0.2% Triton X-100, followed by incubation with the primary antibody proSPC (1:400 dilution) at 4 °C (1:400 dilution) overnight. Then, slides were incubated in secondary antibodies for 1 h after three times of wash with PBS. DAPI was used to stain nuclear. Fluorescent images were acquired using Live Cell Station (the United States, Delta Vision).
Evaluation of secretory function of 3D cultured AEC2s
To evaluate the viability and secretion function of 3D cultured AEC2s, we diluted the LysoTracker probes (Life Technologies, L7526, Carlsbad, USA) with medium to finally obtain the working concentration of 50 nM. Then the cells were incubated for 4 h at 37 °C with the working solution. The secretory function was observed both before and after the replacement of loading solution for comparison.
Statistical analysis
SPSS 17.0 software was used for all mathematical statistical analysis and one-way ANOVA was used to compare the differences between groups. The difference was statistically significant when p < 0.05.
Results
AEC1s and AEC2s purification and identification
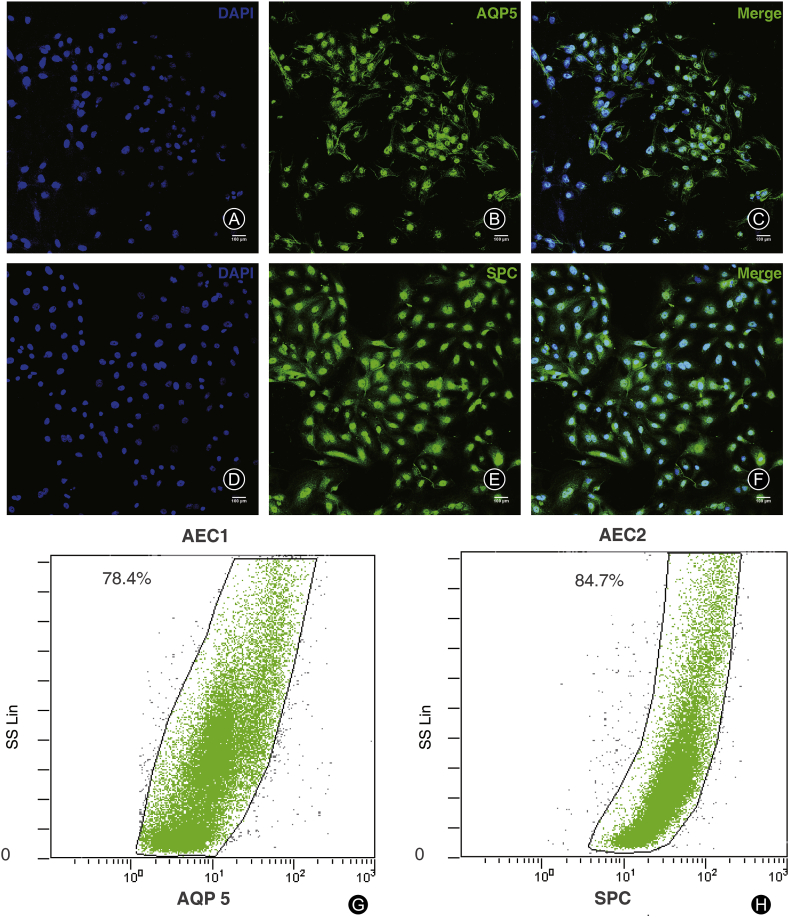
AQP5 is a specific antigen expressed on cell membrane of AEC1s and proSPc is expressed in cytoplasm of AEC2s. By immunofluorescent staining (Fig. 1A-C and 1D-F) and flow cytometry (Fig. 1G and H), AQP5 and proSPC were used to identify AEC1s and AEC2s isolated by magnetic beads. The number of positive cells/total cells × 100% was counted to identify the cell purity. Using flow cytometry analysis, the result showed that the mean purity of AEC1s immediately after magnetic activated cell sorting (MACS) was 88.3% ± 3.8% (Fig. 1G) and that of AEC2s was 92.6% ± 2.7% (Fig. 1H).
Fig. 1.
Identification of the purified alveolar epithelial type 1 cells (AEC1s) and type 2 cells (AEC2s). (A–C) Expression of AQP5 in AEC1s; (D–F) Expression of pro-SPC in AEC2s (scale bars 100 μm); (G–H) Flow cytometry identification of AEC1s′ marker protein AQP5 and AEC2s′ marker protein proSPC right after magnetic activated cell sorting.
Cell growth of AEC1s and AEC2s in vitro
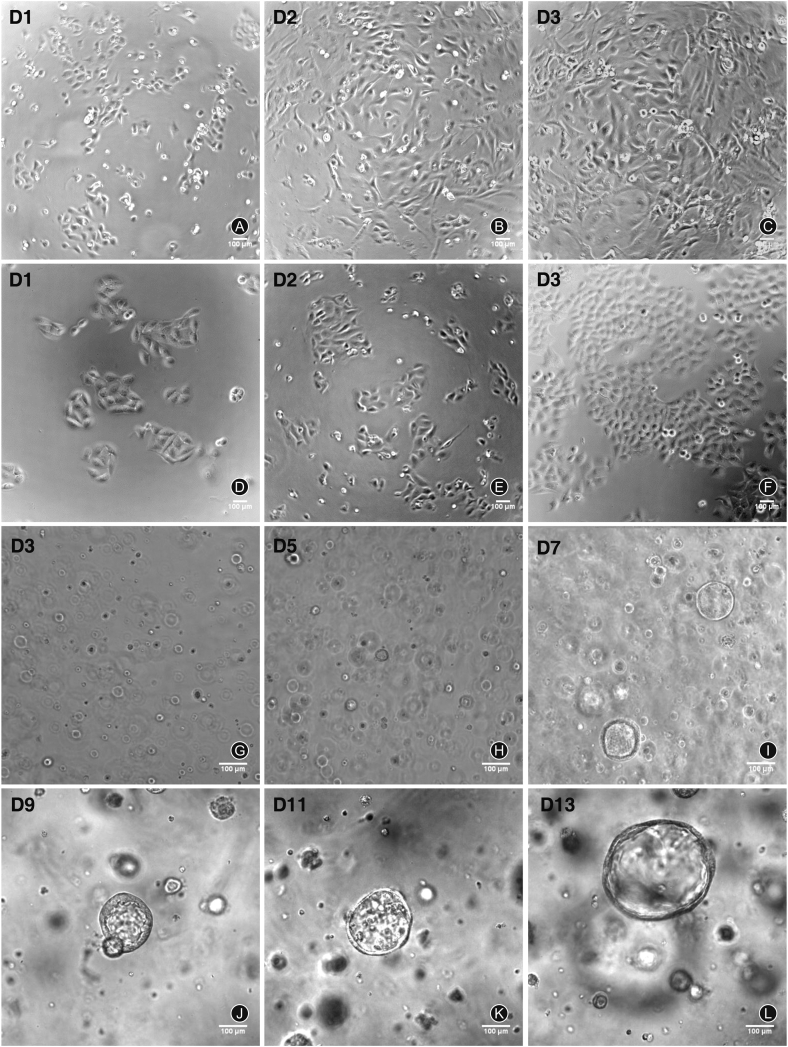
AEC1s and AEC2s isolated by MACS were seeded in 24 well plates at a density of 1.5 × 105 cells/cm2. AEC1s extended and became flattened 24 h after seeding. After 48–72 h, cells extended and became more flattened (Fig. 2A-C). AEC2s extended more slowly than AEC1s. Many cuboidal or round AEC2s formed small clusters 24 h after seeding. The number of clusters increased significantly after two days (Fig. 2D-F). AEC2s proliferated quickly and overspread the bottom of the well after 72 h of seeding (Fig. 2D-F). After three days of seeding, AEC2s began to differentiate to broad- and flat-shaped epithelioid cells.
Fig. 2.
Cell culture of alveolar epithelial type 1 cells (AEC1s) and type 2 cells (AEC2s). (A–C) At days 1–3 after cell seeding, the growth of AEC1s cultured on plates; (D–F) At days 1–3 after cell seeding, the growth of AEC2 cultured on plates; (G–L) At days 3, 5, 7, 9, 11, 13 after cell seeding, the growth of AEC2s in matrigel.
The 3D cultured AEC2s were seeded on matrigel at a density of 1.5 × 105 cells/100 μL. In the first two days after culture, AEC2s were scattered single cells. Multiple cell spheres with a diameter of 20–50 μm can be observed on the 3rd day and with a diameter of 30–80 μm at 5–7 days and gradually became larger with a diameter of 100–170 μm at 9–11 days. At 13 days, hollow cysts were observed instead of solid spheres with a diameter of 200–260 μm (Fig. 2G-L).
Identification of 3D cultured AEC2s
The 3D cultured AEC2s after 13 days were embedded in paraffin and sectioned for HE staining. The results showed that 3D cultured AEC2 formed two kinds of spherical cell structure, solid spheres and hollow cyst spheres (Fig. 3A-C). Both AEC2 cell spheres were stained with proSPC, a specific cytoplasm marker of AEC2. The results showed that all cell spheres expressed proSPC and both were 3D spherical structures formed by AEC2 proliferation (Fig. 3D-F and 3G-I).
Fig. 3.
HE and immunofluorescence staining of 3D cell spheres of alveolar epithelial type 2 cells (AEC2s) cultured in matrigel. (A–C) HE staining of solid spheres (arrowheads) and hollow cysts (arrows) formed by AEC2s ( × 400); (D–F) Immunofluorescence staining of DAPI (blue) and proSPC (red) in solid spheres formed by AEC2s; (G–I) Immunofluorescence staining of DAPI (blue) and proSPC (red) in hollow cysts formed by AEC2s.
Secretory function of 3D cultured AEC2s
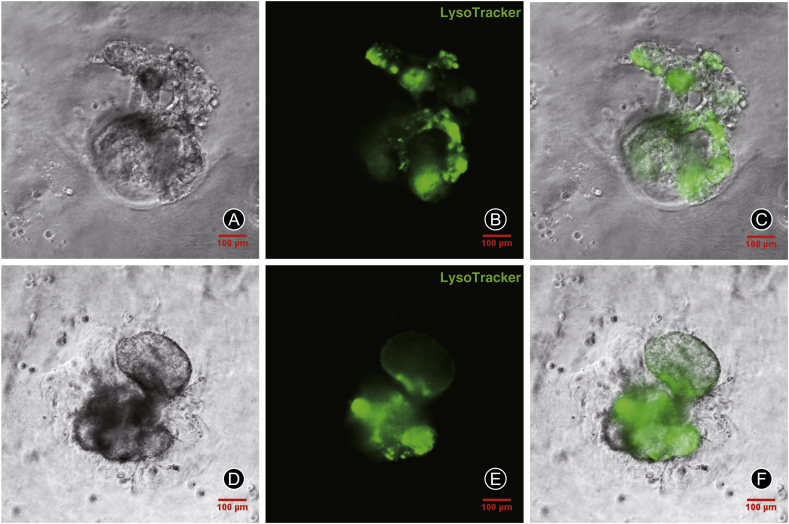
LysoTracker green DND-26 is a fluorescent dye that can stain acid chambers in living cells and has been shown to selectively accumulate in the lamellar bodies of AEC2s. 3D cultured AEC2s were stained with LysoTracker to determine the ability of AEC2s to secrete pulmonary surfactant. The fluorescent of LysoTracker were observed by Live Cell Imaging System (the United States, Delta Vision). The results showed that lamellar bodies in AEC2s cell spheres showed high intensity of LysoTracker staining, indicating a high secretion activity (Fig. 4A-C and 4D-F).
Fig. 4.
LysoTracker staining of lamellar bodies in alveolar epithelial type 2 cells (AEC2s) sphere cells cultured in matrigel. The morphology of 3D cultured AEC2s was observed by phase contrast microscope and fluorescence microscope. Lysotracker (green).
Discussion
Adult lung is a highly quiescent organ with low cell renewal rate different from skin, intestine and liver. It takes about 4 months for respiratory epitheliums to renew in physiological state.1 Thus, lung has been regarded as an organ with poor repair ability or even fibrotic repair after damage. However, more and more evidence shows that the lung has remarkable reparative capacity after injury. Our research showed that alveolar epithelial cells can self-repair after acute lung injury. AEC2s are widely accepted as lung stem cells and play a key role in lung repair and regeneration.20 During development, AEC1s and AEC2s arise from a bipotent progenitor cell lineage, whereas after birth, AEC2s can undergo long-term self-renewal and give rise to AEC1s during homeostasis.19,21 Therefore, the separation of AEC1s and AEC2s with highly purification and sufficient viability is important to research of alveolar repair and regeneration.
AEC1s with high purity are usually isolated by flow cytometry. T1α and AQP5 are both specific membrane surface antigens of AEC1s, so that highly purified AEC1s can be obtained by FACS or MACS. However, due to the lack of specific membrane surface antigens, AEC2s are purified by differential adherence on rat IgG-coated plates with low purity or by FACS of proSPC-lineage tracing mice with high purity.12, 13, 14 Chen et al.12 attempted to isolate rat lung cells with anti-T1α. However, they could not get the two kinds of lung cells continuously at the same time. So far, there is still no good way to sort AEC2s from non-lineage tracing mice with high purity. Although the purification of differential adherence on rat IgG-coated plates is easy, the AEC2s purity is only about 60%–70%.22 We digested lung tissue with Dispase and DNase I into single cell suspension which was incubated with anti-rat T1α and then purified by MACS to obtain AEC1s. The remaining cells were incubated with antibody of epithelial cell marker EpCAM and AEC2s were sorted by magnetic beads. EpCAM is a specific membrane surface antigen of epithelium so that highly purified AEC2s can be isolated from remaining cells after removal of AEC1s. By immunofluorescence assay of cell specific antigen, purity of AEC1s and AEC2s sorted by this method was 88.3% ± 3.8% and 92.6% ± 2.7%, respectively, much higher than that of differential adherence on rat IgG-coated plates. Compared to FACS, the activity of AEC2s purified by MACS is better. AEC1s and AEC2s purified by our method were cultured on plates and then 3D cultured in matrigel. In summary, we purified AEC1s and AEC2s by mixed enzymes in combination with magnetic beads sorting for follow-up cell culture. This method provides an important experimental basis for the study of lung stem/progenitor cells, and provides a new purification method for primary epithelial cells for the study of lung tissue repair and regeneration after acute lung injury.
Funding
This study was supported by the National Natural Science Foundation of China (82020108021 and 81530063), the Projects of the State Key Laboratory of Trauma, Burns and Combined Injury (SKLYQ201901), the Chongqing Science and Technology Talents, Chongqing Special Project for Academicians (cstc2020yszx-jcyjX0004), and the Training Plan for Innovation Ability on the Frontiers of Military Medical Research (2019CXJSB014).
Ethical statement
This study was approved by the ethical committee of our institution.
Declaration of competing interest
All authors declare that they have no competing financial interests.
Author contributions
Ling Zeng and Jian-Xin Jiang designed this study. Di Liu and Jian-Hui Sun performed experiments, collected and analyzed data, and wrote the initial draft. Hua-Cai Zhang provided reagents, materials, animals, and instrumentation. Ling Zeng and Di Liu reviewed and revised the manuscript.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Jiang J.X. Nosocomial infection in trauma patients: risk factors and counter measures. J Third Milit Med Univ. 2009;31:13–16. 1000-5404(2009)01-0013-04. [Google Scholar]
- 2.Whitmore L.C., Goss K.L., Newell E.A., et al. NOX2 protects against progressive lung injury and multiple organ dysfunction syndrome. Am J Physiol Lung Cell Mol Physiol. 2014;307:71–82. doi: 10.1152/ajplung.00054.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monsel A., Zhu Y.G., Gennai S., et al. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014;121:1099–1121. doi: 10.1097/ALN.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravikumar P., Yilmaz C., Bellotto D.J., et al. Separating in vivo mechanical stimuli for postpneumonectomy compensation: imaging and ultrastructural assessment. J Appl Physiol. 2013;114:961–970. doi: 10.1152/japplphysiol.01394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dane D.M., Yilmaz C., Estrera A.S., et al. Separating in vivo mechanical stimuli for postpneumonectomy compensation: physiological assessment. J Appl Physiol. 2013;114:99–106. doi: 10.1152/japplphysiol.01213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawicki G.S., Lu F.L., Valim C., et al. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur Respir J. 2008;31:1285–1291. doi: 10.1183/09031936.00099807. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox M.E., Patsios D., Murphy G., et al. Radiologic outcomes at 5 years after severe ARDS. Chest. 2013;143:920–926. doi: 10.1378/chest.12-0685. [DOI] [PubMed] [Google Scholar]
- 8.Herridge M.S., Cheung A.M., Tansey C.M., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Desai T.J., Brownfield D.G., Krasnow M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkauskas C.E., Cronce M.J., Rackley C.R., et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock J.R., Barkauskas C.E., Cronce M.J., et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial-to-mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:1475–1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Chen Z., Narasaraju T., et al. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest. 2004;84:727–735. doi: 10.1038/labinvest.3700095. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez R.F., Dobbs L.G. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. Methods Mol Biol. 2013;945:145–159. doi: 10.1007/978-1-62703-125-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabata A., Hampl A., Koledova Z. In: Koledova Z., editor. vol. 1612. Humana Press; New York, NY: 2017. Lungosphere assay: 3D culture of lung epithelial stem/progenitor cells. (3D Cell Culture. Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 15.Dobbs L.G., Gonzalez R., Williams M.C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 16.Richards R.J., Davies N., Atkins J., et al. Isolation, biochemical characterization, and culture of lung type II cells of the rat. Lung. 1987;165:143–158. doi: 10.1007/BF02714430. [DOI] [PubMed] [Google Scholar]
- 17.Skillrud D.M., Martin W.J., 2nd The isolation of rat alveolar type II cells: a simplified approach using Percoll density centrifugation. Lung. 1984;162:245–252. doi: 10.1007/BF02715652. [DOI] [PubMed] [Google Scholar]
- 18.Ambesi-Impiombato F.S., Parks L.A., Coon H.G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci USA. 1980;77:3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C., Sun J., Du J., et al. The Hippo-YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury. Cell Biol Int. 2019;43:1174–1183. doi: 10.1002/cbin.11098. [DOI] [PubMed] [Google Scholar]
- 20.Zeng L., Yang X., Li H., et al. The cellular kinetics of lung alveolar epithelial cells and its relationship with lung tissue repair after acute lung injury. Respir Res. 2016;17:164. doi: 10.1186/s12931-016-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Tang Z., Huang H., et al. Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc Natl Acad Sci USA. 2018;115:2407–2412. doi: 10.1073/pnas.1719474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leland G.D., Robert F.G. In: Culture of Epithelial Cells. second ed. Freshney R.I., Freshney M.G., editors. Wiley-Liss; New York, NY: 2002. Isolation and culture of pulmonary alveolar epithelial type II cells; pp. 277–303. [Google Scholar]