Abstract
Objective:
To assess the association between serum bicarbonate concentration and cause-specific mortality in the US general population.
Methods:
A total of 31,195 individuals enrolled in the National Health and Nutrition Examination Survey between 1999 and 2010 were followed for a median 6.7 (interquartile range, 3.7–9.8) years. Cause-specific mortality was defined as cardiovascular, malignancy, and noncardiovascular/nonmalignancy causes. Cox proportional hazards adjusted for demographics, comorbidities, medications, and renal function were used to test the association between baseline serum bicarbonate and the outcomes of interest.
Results:
Of the 2798 participants who died, 722 had a cardiovascular- and 620 had a malignancy-related death. Compared with participants with serum bicarbonate 22 to 26 mEq/L, those with a level below 22 mEq/L had an increased hazard of all-cause and malignancy-related mortality (hazard ratio [HR], 1.54; 95% CI, 1.30–1.83; and HR, 1.46; 95% CI 1.00–2.13, respectively). The hazard for cardiovascular mortality was increased by 8% with each 1 mEq/L increase in serum bicarbonate above 26 mEq/L (HR, 1.08; 95% CI, 1.01–1.15). The findings were consistent in participants with or without chronic kidney disease, with no significant interactions observed.
Conclusion:
In a large cohort of US adults, serum bicarbonate concentration level below 22 mEq/L was associated with malignancy-related mortality, whereas a concentration above 26 mEq/L was associated with cardiovascular mortality. Further studies to evaluate potential mechanisms for the differences in cause-specific mortality are warranted.
Chronic low serum bicarbonate concentration, often a manifestation of kidney function decline,1 is associated with a multitude of deleterious effects, including chronic kidney disease (CKD) progression, skeletal muscle breakdown, bone demineralization, and all-cause mortality. 2–8 Whether an imbalance in acid base homeostasis, presenting as low serum bicarbonate concentration, is a cause or a consequence of other pathologic mechanisms linked to adverse clinical outcomes is unclear.
Studies in CKD cohorts have identified a link between serum bicarbonate and all-cause mortality, with an increased risk of death associated with both high and low serum bicarbonate concentration.2,5 However, in non-CKD populations, the findings have been inconsistent.6,9 In a previous National Health and Nutrition Examination Survey (NHANES) analysis, serum bicarbonate less than 22 mEq/L was not observed to be a predictor of mortality in individuals without CKD.9 This was in contrast to an analysis of 2287 elderly participants in the Health, Aging, and Body Composition Study in which low serum bicarbonate concentration was associated with a 24% increase in all-cause mortality.6 Neither study has systematically assessed the underlying cause of death.
Understanding the mortality associated with low serum bicarbonate concentration becomes important in the setting of Westernization of the world’s diet. A heathy adult, consuming a typical Western diet that yields a high daily net acid load,10 sustains a chronic, low degree metabolic acidosis and suffers from its related complications.11 Prior studies have proposed that exposure to chronic acidosis may facilitate cancer cell clonal evolution and metastasis by inducing chromosomal instability and gene mutations.12,13 However there is no firm evidence on the link between serum bicarbonate concentration and cancer, or cancer-related mortality. In the heart, acidosis has been associated with a reduction in Na+−K+−adenosine triphosphatase activity in myocardial cells,14 and reduced contractility15; however, studies on the association between serum bicarbonate and cardiovascular mortality are limited.
The present study assessed the association between serum bicarbonate concentrations and cause-specific mortality in a large cohort of individuals representative of the US adult general population.
METHODS
Study Population
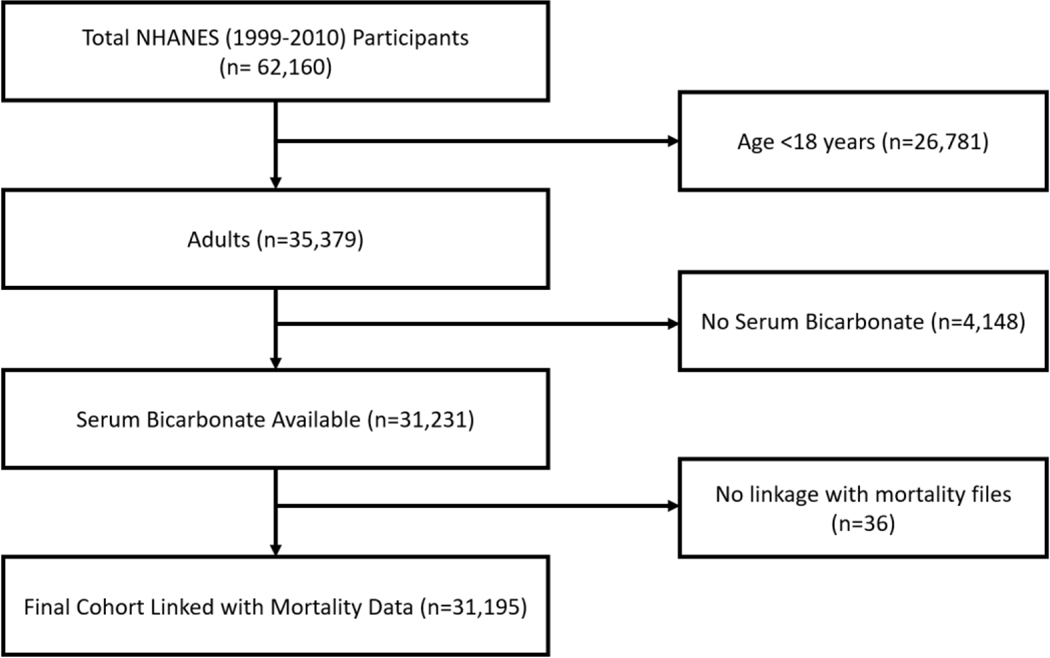
The NHANES survey is a program of studies designed to assess the health and nutritional status in the United States. The current version of the program (NHANES continuous) started in 1999, as a continuation of previous rounds (NHANES I, II, and III). In each cycle that spans 2 years, the program recruits approximately 10,000 participants living in the United States to conduct the studies. Through a multistage probability sample, this program is designed to provide a cohort representative of the civilian noninstitutionalized US population of all ages. NHANES continuous (1999–2010) is linked to the National Death Index through December 31, 2011. The current study enrolled 31,195 adult (≥18 years) participants in NHANES continuous, with available serum bicarbonate concentrations and mortality follow-up data (Figure 1).
FIGURE 1.
Consort diagram.
Serum Bicarbonate Measurement
Blood was collected via a venipuncture from consenting NHANES participants. Vials were stored under appropriate frozen (−30°C) conditions and were shipped overnight to the NHANES Central Laboratory for testing. Serum bicarbonate concentrations were analyzed using the phosphoenolpyruvate carboxylase method on Hitachi 917 multichannel analyzer (Roche Diagnostics, Indianapolis, IN) from 1999 to 2001, using a pH-sensitive electrode on Beckman Synchron LX20 (Beckman Coulter Inc., Brea, CA) from 2002 to 2006, using LX20 and Beckman UniCel DxC800 Synchron (Beckman Coulter Inc.) from 2007 to 2008, and using the DxC800 Synchron system from 2009 to 2010. Baseline serum bicarbonate concentration was measured at study baseline and analyzed as a categorical variable by the following clinical relevant strata: low (<22 mEq/L), normal (22–26 mEq/L), and high (>26 mEq/L); as well as a continuous variable estimated using P-splines to allow for nonlinear effects.
Ascertainment of Overall and Cause-Specific Mortality Outcomes
Outcomes were defined as (1) all-cause mortality, (2) cardiovascular mortality, (3) malignancy-related mortality, and (4) other noncardiovascular/noncancer-related mortality. The underlying cause of death was coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD 10). Participants were linked to the National Death Index through December 31, 2011, by the National Center for Health Statistics as previously described.16 Cardiovascular mortality was defined as death due to diseases of the heart, cerebrovascular disease, atherosclerosis, or other diseases of the circulatory system (ICD 10 codes: I00-I09, I11, I13, I20-I78). Cancer-related mortality was defined as death from any cancer (ICD 10 codes: C00-C97). Noncardiovascular/noncancer-related mortality included all other deaths (ie, chronic lower respiratory diseases [ICD 10 codes: J40-J47], Alzheimer’s disease [ICD 10 code: G30], diabetes mellitus [ICD 10 codes: E10-E14], influenza and pneumonia [ICD 10 codes: J09-J18], nephritis, nephrotic syndrome, and nephrosis [ICD 10 codes: N00-N07, N17-N19, N25N27], accidents [ICD 10 codes: V01-X59, Y85-Y86], and all other causes [residual] ).
Covariates
Demographic variables including age, sex, race, along with comorbidities, body mass index, blood pressure, medication use, and laboratory data (total and high-density lipoprotein [HDL] cholesterol, creatinine, proteinuria, hemoglobin, C-reactive protein, and potassium) were obtained at study baseline. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation.17 CKD was defined as eGFR less than 60 mL/min per 1.73 m2. Comorbid conditions including diabetes, heart failure (HF), coronary artery disease (CAD), chronic obstructive pulmonary disease, and malignancy were all self-reported. Diabetes was defined as a self-reported history of diabetes, use of oral hypoglycemic agents or insulin, or fasting plasma glucose greater than or equal to 126 mg/dL. Hypertension was defined as systolic blood pressure greater than140 mm Hg and/or diastolic blood pressure greater than 90 mm Hg, current treatment for hypertension, or if the participant was ever told to take medication for high blood pressure or had hypertension. Cardiovascular disease was defined as a history of myocardial infarction, stroke, or congestive HF. History of smoking included previous and current smokers.
Statistical Analysis
Categorical variables are presented as percentage and continuous variables are presented as mean and 95% CIs. All analyses (unless specified otherwise) accounted for NHANES complex survey design, and followed the analytical guidelines as recommended by the National Center for Health Statistics.18 We compared baseline characteristics across serum bicarbonate strata using χ2 tests for categorical and one-way analysis of variance for continuous variables. We used the Poisson distribution to estimate mortality rates (per 1000 person-years of follow-up) and 95% CI for each leading cause of death within each serum bicarbonate stratum.19,20
Survival analyses were estimated using Cox proportional hazard models in gradually inclusive models defined a priori based on our assessment of the covariates’ likelihood of being a confounder in the relationship between serum bicarbonate and each cause-specific mortality outcome. The relevant confounding variables used for adjustments included demographics (age, sex, and race/ethnicity), traditional cardiovascular risk factors (history of CAD, HF, diabetes, smoking, systolic blood pressure, total and HDL cholesterol, body mass index, malignancy, medications [angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, diuretics]), and kidney function (eGFR and log transformed spot urine albumin/creatinine). Residual plots were used to confirm model assumptions. Serum bicarbonate concentration of 22 to 26 mEq/L was used as the reference group. Demographics (age, sex, and race) were available for all patients. Data missingness was as follows (unweighted data): smoking (N=2601; 8.3%), diabetes (N=480; 1.5%), hypertension (N=213; 0.7%), alcohol (N=8531; 27.3%), history of CAD (N=2716; 8.7%), history of HF (N=2676; 8.6%), history of malignancy (N=2573; 8.2%), history of chronic obstructive pulmonary disease (N=2638; 8.5%), history of liver disease (N=2632; 8.4%), body mass index (N=619; 2%), heart rate (N=30,101; 3.5%), blood pressure (N=1303; 4.2%), glomerular filtration rate (GFR) (N=0; 0%), urine albumin to creatinine ratio (N=506; 1.6%), hemoglobin (N=56; 0.2%), albumin (N=0; 0%), cholesterol (N=1; 0%), potassium (N=2; 0%), and C-reactive protein (N=14; 0%). No assumptions were used for missing data and listwise deletion method was used for missing variables in multivariable models.
For survival analyses, the event was defined as the death from any cause (all-cause mortality) or cause-specific deaths (cardiovascular disease [CVD], cancer, and non-CVD/noncancer), censoring for other events (including deaths from other causes). In nonlinear modeling, the association between serum bicarbonate concentrations and all-cause and cause-specific mortality was explored using penalized spline regression models.21 To our knowledge, there is no widely available package to visualize splines under complex survey methods; therefore, splines were performed on unweighted data. Because listwise deletion was used for missing data, effect estimates from incremental models may not be comparable; therefore, sensitivity analyses were performed after excluding participants with missing data from the list of covariates. Additionally, analyses were replicated using Fine-Gray method to consider competing causes of death.22
In secondary analyses, we examined the association between baseline serum bicarbonate and cause-specific mortality rates in subgroups by age, sex, race, CKD, and diabetes status. Subgroup analyses were performed after excluding participants with history of lung disease at baseline.
All analyses were conducted using SPSS, R, SAS, and Stata. All tests were two-sided, and P<.05 was considered statistically significant.
RESULTS
Of the total 31,195 participants, 52% were women, and 11% were African Americans. Mean age SD was 45.3 (95% CI, 45.1–45.5) years. A small proportion (6.2%) had CKD. Serum bicarbonate mean ± SD was 24.5 ± 0.05. Compared with participants with normal (22–26 mEq/L), or high (>26 mEq/L) serum bicarbonate, participants with low (<22 mEq/L) serum bicarbonate concentration at study baseline were more likely to be younger, women, non-black, and with higher eGFR. Participants with high serum bicarbonate level were more likely to have hypertension or history of malignancy at baseline. Baseline characteristics of the study population are presented in Table 1.
TABLE 1.
| Characteristics | Total (N=31,195) | Serum Bicarbonate (mEq/L) |
P valuec | ||
|---|---|---|---|---|---|
| <22 (n=3143) | 22–26 (n=21,801) | > 26 (n=6251) | |||
| Serum bicarbonate, mEq/L | 24.5 (24.4–24.6) | 20.2 (20.1–20.3) | 24.2 (24.1–24.3) | 27.7 (27.6–27.8) | |
| Demographics and Clinical Characteristics | |||||
| Age, years | 45.3 (45.1–45.5) | 40.4 (39.7–41.0) | 44.5 (44.3–44.8) | 50.4 (49.9–50.9) | <.001 |
| Women | 51.6 (50.9–52.3) | 65.5 (63.2–67.8) | 52.5 (51.6–53.3) | 42.0 (40.4–43.5) | <.001 |
| Black | 10.7 (10.4–11.0) | 9.8 (8.9–10.7) | 10.5 (10.2–10.8) | 12.0 (11.3–12.7) | <.001 |
| Ever-smokerd | 48.3 (47.6–49.1) | 51.1 (48.7–53.6) | 48.8 (47.9–49.7) | 45.3 (43.7–46.9) | <.001 |
| Diabetes | 7.3 (7.0–7.7) | 6.9 (5.8–8.0) | 7.1 (6.7–7.5) | 8.3 (7.6–9.1) | .01 |
| Hypertension | 27.6 (27.0–28.2) | 26.4 (24.4–28.5) | 26.0 (25.3–26.7) | 34.1 (32.6–35.5) | <.001 |
| Alcohole | 16.3 (15.7–16.9) | 18.6 (16.5–20.8) | 16.3 (15.6–17.0) | 15.0 (13.8–16.3) | .01 |
| History of coronary disease | 3.4 (3.1–3.6) | 2.7 (2.0–3.4) | 3.1 (2.9–3.4) | 4.4 (3.8–5.0) | <.001 |
| History of heart failure | 2.2 (2.1–2.4) | 2.2 (1.6–2.9) | 2.0 (1.8–2.3) | 2.9 (2.5–3.4) | .001 |
| History of malignancy | 8.3 (7.9–8.7) | 7.1 (5.9–8.3) | 8.0 (7.3–8.2) | 10.9 (9.9–11.8) | <.001 |
| eGFR <60 mL/min per 1.73 m2 | 6.2 (5.9–6.5) | 7.3 (6.3–8.4) | 5.6 (5.3–5.9) | 7.8 (7.1–8.5) | <.001 |
| History of asthma | 13.2 (12.7–13.7) | 15.1 (13.4–16.8) | 13.0 (12.4 –13.6) | 12.8 (11.8–13.9) | .03 |
| BMI, kg/m2 | 28.2 (28.1–28.3) | 29.7 (29.4–30.0) | 28.3 (28.2–28.4) | 27.4 (27.2–27.6) | <.001 |
| Heart rate, beats/min | 72.7 (72.6–72.9) | 76.8 (76.2–77.4) | 72.8 (72.6–73.0) | 70.5 (70.1–70.9) | <.001 |
| Systolic blood pressure, mm Hg | 122.0 (121.7–122.2) | 119.5 (118.7–120.3) | 121.6 (121.3–121.9) | 124.5 (123.9–125.0) | <.001 |
| Diastolic blood pressure, mm Hg | 70.6 (70.5–70.8) | 70.4 (69.8–71.0) | 70.8 (70.6–71.0) | 70.3 (69.9–70.6) | .01 |
| Laboratory data | |||||
| eGFR, mL/min per 1.73 m2 | 97.0 (96.7–97.3) | 102.3 (101.2–103.4) | 97.9 (97.6–98.2) | 91.4 (90.8–92.0) | <.001 |
| Urine albumin/creatinine, median (IQR), mg/g | 0.06 (0.04–0.1) | 0.06 (0.04–0.1) | 0.06 (0.04–0.1) | 0.06 (0.04–0.1) | <.001 |
| Hemoglobin, g/dL | 14.4 (14.3–14.5) | 14.2 (14.1–14.3) | 14.4 (14.3–14.5) | 14.5 (14.50–14.59) | <.001 |
| Albumin, g/dL | 4.3 (4.2–4.4) | 4.19 (4.18–4.21) | 4.3 (4.2–4.4) | 4.33 (4.32–4.34) | <.001 |
| Serum cholesterol, mg/dL | 198.1 (197.5–198.6) | 196.6 (194.5–198.8) | 198.0 (197.3–198.7) | 198.9 (197.7–200.2) | .05 |
| High-density liprotein, mg/dL | 52.6 (52.4–52.9) | 50.7 (49.9–51.4) | 52.4 (52.1–52.7) | 54.4 (53.9–54.9) | <.001 |
| Serum potassium, mEq/L | 4.0 (4.01–4.03) | 4.01 (3.99–4.02) | 4.017 (4.012–4.022) | 4.01 (4.007–4.03) | .57 |
| C-reactive protein, mg/dL, median (IQR) | 0.2 (0.1–0.4) | 0.3 (0.1–0.6) | 0.2 (0.07–0.4) | 0.1 (0.05–0.3) | <.001 |
| Medications | |||||
| Diuretics | 10.3 (9.9–10.7) | 6.6 (5.5–7.6) | 8.7 (8.3–9.2) | 17.8 (16.6–18.9) | <.001 |
| ACEi/ARB | 13.2 (12.8–13.7) | 10.6 (9.3–12.0) | 12.5 (12.0–13.0) | 17.2 (16.1–18.3) | <.001 |
| Beta blockers | 7.9 (7.6–8.3) | 6.2 (5.2–7.2) | 7.2 (6.8–7.6) | 11.5 (10.5–12.4) | <.001 |
| Statins | 11.7 (11.3–12.1) | 7.8 (6.6–9.0) | 10.8 (10.3–11.3) | 16.8 (15.8–17.9) | <.001 |
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Numbers at the top represent the unweighted number of participants. Numbers represent n (%) or mean ± SD unless otherwise specified.
One-way analysis of variance (normal data continuous), Kruskal-Wallis (non-normal continuous), χ2 test (categorical).
Smoking: at least 100 cigarettes/lifetime.
Alcohol: ever had more than 5 drinks per day.
Causes of Death Mortality Rates by Serum Bicarbonate Concentration
Over a median follow-up of 6.7 (interquartile range [IQR], 3.7–9.8) years, 2798 patients died (unweighted). The leading causes of death were CVD (n=722) and malignant neoplasms (n=620) (unweighted). The overall crude unweighted mortality rates per 1000 person-years of follow-up were 14.1 (95% CI, 13.5–14.6) for all-cause deaths, 3.6 (95% CI, 3.4–3.9) for CVD deaths, and 3.1 (95% CI, 2.9–3.3) for malignancy-related deaths. Mortality rates per 1000 person-years across the serum bicarbonate strata overall and by cause-specific mortality are depicted in Table 2.
TABLE 2.
Causes of Death and Mortality Rates per 1000 Person-Years Across Serum Bicarbonate Strata
| Serum Bicarbonate (mEq/L) |
|||
|---|---|---|---|
| <22 | 22–26 | >26 | |
| Cause of death | Mortality rate per 1000 person-years (95% CI) | Mortality rate per 1000 person-years (95% CI) | Mortality rate per 1000 person-years (95% CI) |
| All cause deaths | 9.59 (9.58–9.61) | 8.72 (8.64–8.80) | 13.48 (13.47–13.49) |
| Cardiovascular diseases | 1.65 (1.64–1.66) | 2.02 (2.02–2.02) | 3.6 (3.59–3.61) |
| Malignancy | 2.29 (2.28–2.29) | 2.11 (2.10–2.12) | 3.04 (3.02–3.05) |
| Chronic lower respiratory diseases | 0.41 (0.40–0.42) | 0.47 (0.46–0.48) | 1.13 (1.12–1.14) |
| Alzheimer disease | 0.05 (0.04–0.06) | 0.27 (0.26–0.28) | 0.38 (0.37–0.38) |
| Diabetes mellitus | 0.47 (0.46–0.48) | 0.25 (0.24–0.26) | 0.37 (0.36–0.38) |
| Influenza and pneumonia | 0.16 (0.15–0.17) | 0.21 (0.20–0.21) | 0.31 (0.30–0.33) |
| Kidney disease | 0.42 (0.41–0.43) | 0.17 (0.16–0.18) | 0.14 (0.13–0.15) |
| Accidents (unintentional injuries) | 0.83 (0.82–0.84) | 0.39 (0.38–0.40) | 0.62 (0.61–0.63) |
Serum Bicarbonate Concentration and All-Cause Mortality
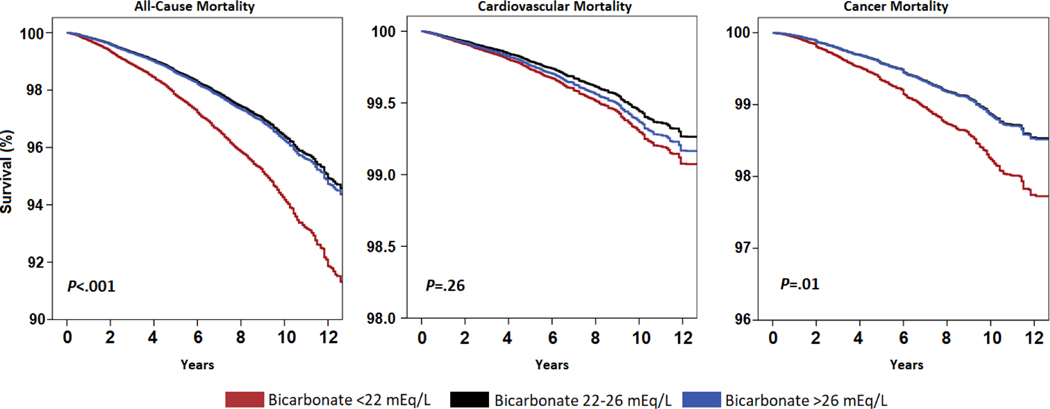
Kaplan-Meier survival curves by baseline serum bicarbonate strata are presented in Figure 2. Participants with serum bicarbonate concentration less than 22 mEq/L had a significantly reduced overall survival compared with participants in the normal or high bicarbonate groups (P<.001) (Figure 2).
FIGURE 2. Demographic adjusted curves for cause-specific mortality by baseline serum bicarbonate strata (< 22 mEq/L, 22 – 26 mEq/L and > 26 mEq/L). (A) All-cause mortality (B) Cardiovascular mortality (C) Cancer mortality.
Analysis was done using Kaplan-Meier method accounting for complex survey design. P-values are of Log-Rank (Mantel-Cox) test.
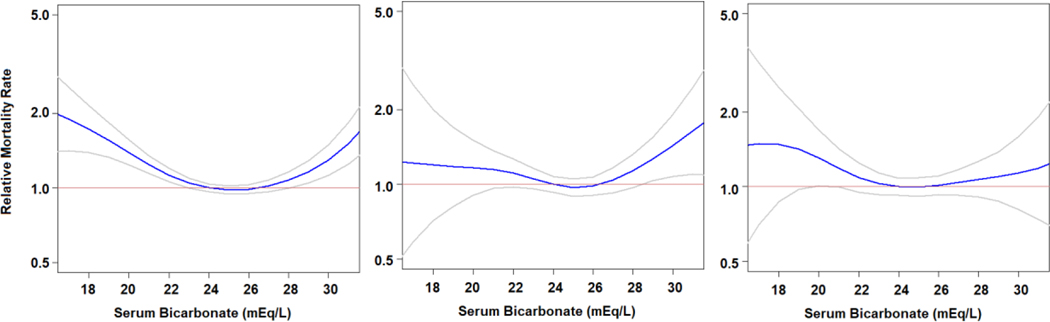
In multivariable adjusted Cox regression models (model 3), the hazard for all-cause mortality was 54% higher (hazard ratio [HR], 1.54; 95% CI, 1.30–1.83; P<.001) for participants with serum bicarbonate concentration less than 22 mEq/L compared with participants with serum bicarbonate level from 22 to 26 mEq/L. Participants with serum bicarbonate concentration greater than 26 mEq/L had similar hazard of all-cause mortality compared with those in the normal bicarbonate group (HR, 1.04; 95% CI, 0.97–1.11; P=.26). Table 3 and Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org) show the incrementally adjusted effect size estimates after excluding participants with missing data. Unadjusted and fully adjusted (model 3) penalized splines models (unweighted) showed a statistically significant U-shaped association between serum bicarbonate concentration and all-cause mortality (Figure 3).
TABLE 3.
Cox Regression Models for the Association Between Serum Bicarbonate Groups and Clinical Outcomes Accounting for Complex Survey Designa,b
| Serum Bicarbonate (mEq/L) |
|||||
|---|---|---|---|---|---|
| <22 | [22–26] | >26 | |||
| All-cause mortality | |||||
| Model 1 | 1.63 (1.42–1.88); <.001 | Ref | 1.02 (0.96–1.07); .51 | ||
| Model 2 | 1.62 (1.37–1.92); <.001 | Ref | 1.03 (0.97–1.10); .31 | ||
| Model 3 | 1.54 (1.30–1.83); <.001 | Ref | 1.04 (0.97–1.11); .26 | ||
| CVD mortality | |||||
| Model 1 | Ref | 1.26 (0.89–1.77); .19 | 1.06 (0.96–1.17); .23 | ||
| Model 2 | 1.26 (0.89–1.79); .20 | Ref | 1.04 (0.93–1.16); .54 | ||
| Model 3 | 1.12 (0.77–1.62); .56 | Ref | 1.06 (0.94–1.19); .33 | ||
| Cancer mortality | |||||
| Model 1 | Ref | 1.57 (1.16–2.11); .003 | 1.01 (0.89–1.14); .88 | ||
| Model 2 | 1.47 (1.01–2.15); .05 | Ref | 1.07 (0.93–1.22); .37 | ||
| Model 3 | 1.46 (1.00–2.13); .05 | Ref | 1.07 (0.93–1.23); .34 | ||
CVD = cardiovascular disease; HR = hazard ratio; Ref = reference.
Values shown are HR (95% CI); P value.
Model 1 is adjusted for age, sex, race (n=31,195).
Model 2 is adjusted for variables in Model 1+ tobacco use, coronary artery disease, heart failure, cholesterol, systolic blood pressure, chronic obstructive pulmonary disease, liver disease, body mass index, high-density lipoprotein, malignancy, diuretics, angiotensin converting enzyme inhibitors, or angiotensin receptor blockers (n=26,652).
Model 3 is adjusted for variables in Model 2 + estimated glomerular filtration rate, and log transformed spot urine albumin/creatinine (n=26,404).
FIGURE 3.
Adjusted (model 3) penalized smoothing splines for all-cause, cardiovascular- and cancer-mortality (unweighted data). Gray lines represent 95% confidence interval. Red line represents HR of 1.
Serum Bicarbonate Concentration and Cardiovascular Mortality
There was no significant association between serum bicarbonate concentration and CVD mortality when serum bicarbonate concentration was analyzed as a categorical predictor using cox-regression estimates (Table 3 and Figure 2) or in Fine-Gray analysis accounting for competing causes of death (Supplemental Table 2 [available online at http://www.mayoclinicproceedings.org]). In a nonlinear approach, using penalized splines we observed an increased risk of CVD mortality for participants with high baseline serum bicarbonate concentration (Figure 3). Multivariable adjusted linear splines revealed an 8% increase in CVD mortality with each 1 mEq/L higher serum bicarbonate concentration above 26 mEq/L (HR, 1.08; 95% CI, 1.01–1.15; P=.04).
Serum Bicarbonate Concentration and Cancer Mortality
Participants with serum bicarbonate concentration less than 22 mEq/L had a significantly increased risk of cancer mortality compared with participants in the normal or high bicarbonate group (P=.01) (Figure 2). The hazard for cancer mortality was 46% higher for participants with serum bicarbonate concentration less than 22 mEq/L (HR, 1.46; 95% CI, 1.00–2.13; P=.05) compared with participants in the normal group (Table 3). The results were replicated using competing risk analyses (Supplemental Table 3 [available online at http://www.mayoclinicproceedings.org]). Findings from penalized spline analyses were generally congruent with the categorical results, indicating a higher risk of cancer mortality for participants with low serum bicarbonate (Figure 3).
Serum Bicarbonate and Noncardiovascular/Noncancer Mortality
The number of events for each other cause of death was small and did not allow individual comparisons (Table 2). Taken as a group, noncardiovascular/noncancer mortality was higher for participants with low serum bicarbonate at baseline compared with participants with normal serum bicarbonate (HR, 1.74; 95% CI, 1.40–2.17; P<.001). There was no association for participants with high serum bicarbonate compared with participants with normal serum bicarbonate (HR, 1.01; 95% CI, 0.92–1.12; P=77).
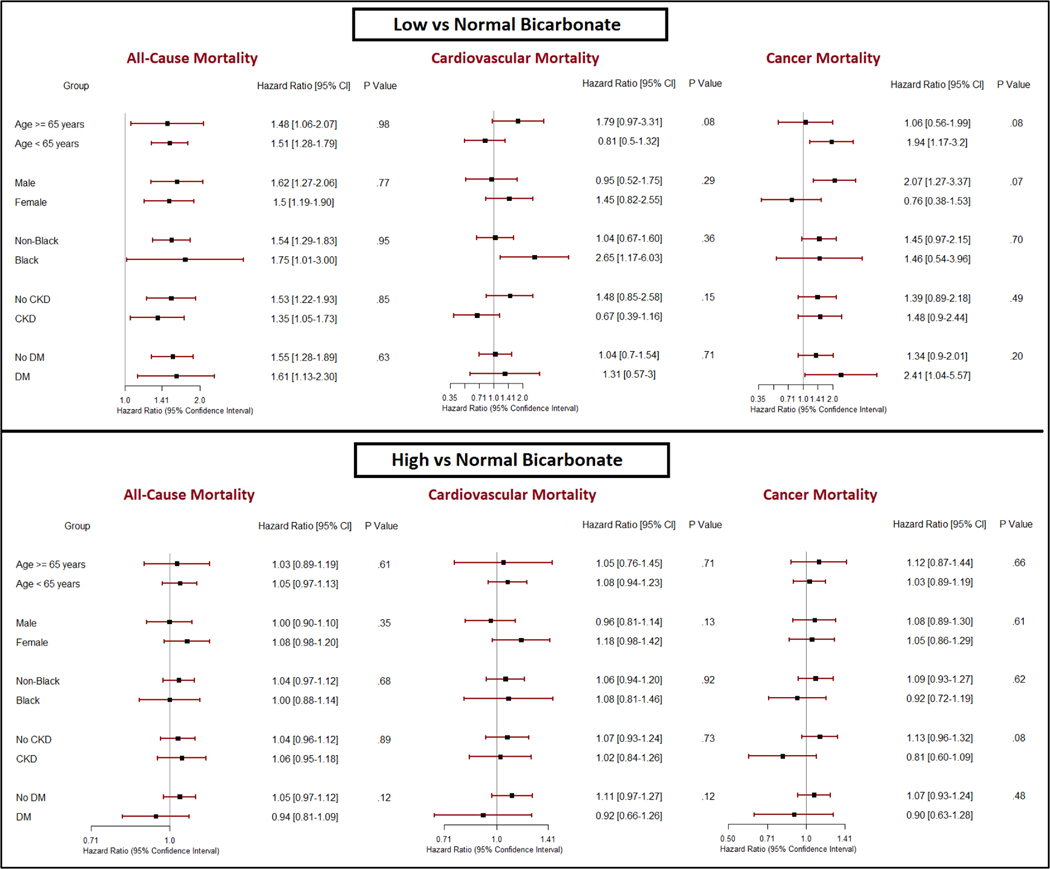
Subgroup Analyses
The association between serum bicarbonate concentrations and cause-specific mortality was consistent in subgroups by age, sex, race, CKD, and diabetes status. There was an increase in the risk of cancer mortality in participants younger than 65 years and in men with low serum bicarbonate concentration, although the differences were not statistically significant (Figure 4). Supplemental Table 3 depicts the association between serum bicarbonate and mortality outcomes after excluding participants with lung disease at baseline.
FIGURE 4. Bicarbonate levels and cause-specific mortality in subgroups.
CKD=chronic kidney disease, DM=Diabetes Mellitus. P values are for interaction between subgroup and bicarbonate levels. Analysis was done using Cox regression accounting for complex survey design
DISCUSSION
In this large cohort representative of the US general population with and without CKD, after accounting for significant confounders, serum bicarbonate concentration less than 22 mEq/L was associated with all-cause and cancer-related mortality, but not with cardiovascular mortality. However, each 1 mEq/L higher serum bicarbonate concentration above 26 mEq/L was associated with an 8% increase in cardiovascular mortality; and a U-shaped association was observed between serum bicarbonate concentration and all-cause mortality. These findings were consistent in individuals with and without CKD. To our knowledge, this is the first largescale study to investigate the cause-specific mortality associated with serum bicarbonate concentration.
Both low and high bicarbonate concentrations are associated with increased all-cause mortality in CKD.2,5 The association between serum bicarbonate concentration and all-cause mortality in the non-CKD population is not well established. In a study of 15,836 NHANES III participants, serum bicarbonate concentrations less than 22 mEq/L were associated with a 2.6-fold increased hazard of death in individuals with CKD.9 However, serum bicarbonate was not an independent predictor of overall mortality in the non-CKD subgroup.9 Less than 10% in our cohort had CKD; still, in subgroup analysis, we observed no interaction by CKD status. It is unclear whether the analyses are underpowered to detect significant interactions. At the other end of the spectrum, metabolic alkalosis was shown to be associated with all-cause mortality in healthy older individuals.6 The current study validates the U-shaped association between serum bicarbonate concentration and overall mortality and further refines it by adding the causes of death.
Few studies have evaluated the association between serum bicarbonate concentration and CVD with conflicting results. In a cohort of patients with type 2 diabetes, serum bicarbonate concentration was inversely associated with incident coronary disease.23 In contrast, no significant association between bicarbonate and CVD was observed in patients with diabetic kidney disease24 or in healthy older individuals.6
In a study of 3939 participants in the Chronic Renal Insufficiency Cohort study, high serum bicarbonate concentration was associated with increased risk of HF events, but no association with atherosclerotic events, including CAD, was found.3 In a subsequent study, persistently elevated serum bicarbonate concentrations above 26 mEq/L during follow-up remained a robust predictor of incident HF events after adjustments for multiple confounders including diuretic type and doses.4 These studies and others clearly established a link between serum bicarbonate concentration and CVD in CKD. However, this is not well studied in individuals without CKD. The prevalence of CKD in the current study was less than 9% and thus many of the mortality events observed were reflective of a non-CKD population. Whereas low serum bicarbonate concentration was not a predictor of CVD mortality, the current study enhances the earlier findings by showing an association between high serum bicarbonate concentrations and increased cardiovascular mortality in a primarily non-CKD population. Because arterial blood gas was not measured, it is difficult to ascertain if high serum bicarbonate level was a compensatory mechanism for an underlying respiratory acidosis or a reflection of metabolic alkalosis.
Cancer-related mortality was higher in the low bicarbonate group compared with the normal bicarbonate group. The association remained unaltered in increasingly adjusted models, including accounting for eGFR, albuminuria, comorbidities, and medications. The potential mechanisms responsible for this association remain speculative and cannot be proven in this epidemiological cohort, but a few points are important to make. Animal studies suggest that acidosis can induce and propagate carcinogenesis.25 Acid-base disequilibrium modulates molecular activity including adrenal glucocorticoid, insulin growth factor, and adipocyte cytokine signaling, and may result in dysregulated cellular metabolism and osteoclast activation that can induce carcinogenesis.26 Additionally, acidity generated by the tumor microenvironment can drive local invasion of tumor cells by degrading the extracellular matrix.27,28 Also, several families of receptors and ion channels that help cells sense extracellular acidosis have been described in cancer cells.29 pH-sensing G protein-coupled cell receptors including GPR4, TDAG8 (GPR65), OGR1 (GPR68), and G2A (GPR132) are recently discovered pH sensors that are activated by an acidic milieu and may play a role in tumor development, inflammation, and angiogenesis.30–32
Although no statistically significant interaction by sex was observed, men with serum bicarbonate levels below 22 mEq/L had an increased risk of cancer mortality compared to women. The rationale for such potential sex disparity remains unclear, but one can speculate that carcinogenesis in male-specific cancers, such as prostate cancer, finds acidosis a favorable milieu. CKD has been shown to be associated with an increased risk of genitourinary cancers, but not prostate cancer. This raises the possibility that the association with cancer death may be driven by mechanisms other than the chronic metabolic acidosis in the context of declining GFR.
The risk of cancer mortality was also higher for low serum bicarbonate group in participants younger than 65 years, although the difference with the older group was not statistically significant. A potential explanation can reside in the high-energy, high-protein, high-fat (Westernized) diet likely to be consumed in higher amounts by a younger population, therefore increasing the exposure to metabolic acidosis, but also to potentially carcinogenic compounds that are found in meats, including N-nitroso compounds, heterocyclic amines, or polycyclic aromatic hydrocarbons.
Treatment with oral sodium bicarbonate increases tumor pH and reduced lymph node involvement, tumor extravasation, colonization, and distant metastasis formation in animal models.33 The role of sodium bicarbonate use in human cancer treatment is currently under investigation (extended use of sodium bicarbonate in patients with cancer, Clinicaltrials.gov: NCT02531919).
In our study, the association between low serum bicarbonate concentration and cancer deaths were similar in CKD and non-CKD groups, making kidney function a less likely confounder. Diabetes has been shown to increase cancer risk,34 mostly due to chronic inflammation. Higher level of C-reactive protein, a measure of inflammatory state, was found to be prevalent in participants with low serum bicarbonate in our cohort. However, we observed no interaction by diabetes status.
The strengths of this study include a large cohort of individuals representative of the US general population with and without CKD with available serum bicarbonate concentration measurements and cause-specific mortality data. However, few limitations should be acknowledged. The observational study design precludes potential causality between serum bicarbonate concentration and cause-specific mortality. Data on the type of malignancy was not available and the cause of death obtained from the National Death Index may not have been accurately adjudicated. However, these death indexes have been previously used and considered reliable for CVD and cancer mortality.35,36 The lack of available arterial blood gas analysis precluded the assessment of acid-base status. It is possible that participants with low bicarbonate level had an underlying respiratory alkalosis; and high bicarbonate level could be a compensatory mechanism for respiratory acidosis. Additionally, the study is based on a single measurement of serum bicarbonate, which may not be reflective of the temporal variability. Nevertheless, the NHANES dataset was prospectively collected for research purposes, which limits the bias introduced by a retrospective review.
CONCLUSION
In a large cohort of US adults with and without CKD, low serum bicarbonate concentration was an independent predictor of overall and malignancy-related deaths. The risk of cardiovascular mortality was increased for individuals with serum bicarbonate above 26 mEq/L. Further studies to confirm these findings and explain the potential mechanisms for the differences in cause-specific mortality are warranted.
Supplementary Material
Abbreviations and Acronyms:
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- IQR
interquartile range
- NHANES
National Health and Nutrition Examination Survey
Footnotes
SUPPLEMENTAL ONLINE MATERIAL Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Data Previously Presented: The abstract of this manuscript was accepted as an oral presentation and published in the conference proceedings at the 54th European Renal Association d European Dialysis and Transplant Association (ERA-EDTA) Congress that took place in Madrid, Spain, on June 3–6, 2017.
Potential Conflicts of Interest: The authors report no conflict of interest.
Contributor Information
Sadeer G. Al-Kindi, Division of Cardiovascular Medicine, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center and Case Western Reserve University, Cleveland, OH.
Anuja Sarode, College of Public Health, Kent State University, Kent, OH.
Melissa Zullo, College of Public Health, Kent State University, Kent, OH.
Sanjay Rajagopalan, Division of Cardiovascular Medicine, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center and Case Western Reserve University, Cleveland, OH.
Mahboob Rahman, Division of Nephrology and Hypertension, University Hospitals Cleveland Medical Center and Case Western Reserve University, Cleveland, OH.
Thomas Hostetter, Division of Nephrology and Hypertension, University Hospitals Cleveland Medical Center and Case Western Reserve University, Cleveland, OH.
Mirela Dobre, Division of Nephrology and Hypertension, University Hospitals Cleveland Medical Center and Case Western Reserve University, Cleveland, OH.
REFERENCES
- 1.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45(6): 978–993. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24(4): 1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobre M, Yang W, Chen J, et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2013;62(4):670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobre M, Yang W, Pan Q, et al. Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): a report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc. 2015; 4(4):e001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navaneethan SD, Schold JD, Arrigain S, et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(10):2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raphael KL, Murphy RA, Shlipak MG, et al. Bicarbonate Concentration, Acid-Base Status, and Mortality in the Health, Aging, and Body Composition Study. Clin J Am Soc Nephrol. 2016;11(2):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011;79(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobre M, Rahman M, Hostetter TH. Current status of bicarbonate in CKD. J Am Soc Nephrol. 2015;26(3):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raphael KL, Zhang Y, Wei G, Greene T, Cheung AK, Beddhu S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant. 2013;28(5):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemann J Jr. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron. 1999;81(suppl 1):18–25. [DOI] [PubMed] [Google Scholar]
- 11.Frassetto LA, Todd KM, Morris RC Jr, Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. 1998;68(3): 576–583. [DOI] [PubMed] [Google Scholar]
- 12.Morita T, Nagaki T, Fukuda I, Okumura K. Clastogenicity of low pH to various cultured mammalian cells. Mutat Res. 1992; 268(2):297–305. [DOI] [PubMed] [Google Scholar]
- 13.Xiao H, Li TK, Yang JM, Liu LF. Acidic pH induces topoisomerase II-mediated DNA damage. Proc Natl Acad Sci U S A. 2003; 100(9):5205–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown RH Jr, Cohen I, Noble D. The interactions of protons, calcium and potassium ions on cardiac Purkinje fibres. J Physiol. 1978;282:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JH, Wildenthal K, Johnson RL Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1(5):375–389. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. The Linkage of National Center for Health Statistics Survey Data to the National Death Index e 2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. Centers for Disease Control and Prevention website, https://www.cdc.gov/nchs/data/datalinkage/LMF2015_Methodology_Analytic_Considerations.pdf. Accessed April 4, 2019.
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 19992010. Vital Health Stat 2. 2013;161:1–24. [PubMed] [Google Scholar]
- 19.Patil V, Kulkarni H. Comparison of confidence intervals for the Poisson mean: some new aspects. REVSTAT-Stat J. 2012;10(2):211–227. [Google Scholar]
- 20.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990; 131(2):373–375. [DOI] [PubMed] [Google Scholar]
- 21.Eilers PH, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11(2):89–121. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446):496–509. [Google Scholar]
- 23.Paul Chubb SA, Davis WA, Peters KE, Davis TM. Serum bicarbonate concentration and the risk of cardiovascular disease and death in type 2 diabetes: the Fremantle Diabetes Study. Cardiovasc Diabetol. 2016;15(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schutte E, Lambers Heerspink HJ, Lutgers HL, et al. Serum bicarbonate and kidney disease progression and cardiovascular outcome in patients with diabetic nephropathy: a post hoc analysis of the RENAAL (Reduction of End Points in NonInsulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) study and IDNT (Irbesartan Diabetic Nephropathy Trial). Am J Kidney Dis. 2015;66(3):450–458. [DOI] [PubMed] [Google Scholar]
- 25.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev. 2014;33(4):1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond). 2012;9(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estrella V, Chen T, Lloyd M, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013; 73(5):1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brisson L, Reshkin SJ, Gore J, Roger S. pH regulators in invadosomal functioning: proton delivery for matrix tasting. Eur J Cell Biol. 2012;91(11–12):847–860. [DOI] [PubMed] [Google Scholar]
- 29.Holzer P. Acid-sensitive ion channels and receptors. Handb Exp Pharmacol. 2009;194:283–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyder L, Suply T, Ricoux B, et al. Reduced pathological angiogenesis and tumor growth in mice lacking GPR4, a proton sensing receptor. Angiogenesis. 2011;14(4):533–544. [DOI] [PubMed] [Google Scholar]
- 31.Jing Z, Xu H, Chen X, et al. The proton-sensing G-protein coupled receptor GPR4 promotes angiogenesis in head and neck cancer. PLoS One. 2016;11(4):e0152789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol. 2013;4:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robey IF, Baggett BK, Kirkpatrick ND, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CJ, Weir HK, Fink AK, et al. The impact of National Death Index linkages on population-based cancer survival rates in the United States. Cancer Epidemiol. 2013;37(1): 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olubowale OT, Safford MM, Brown TM, et al. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2017;6(5):e004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.