Abstract
Background
Cancer of unknown primary (CUP) is an aggressive rare malignancy with limited treatment options. Data regarding clinical activity of immune checkpoint inhibitors in CUP is lacking. Therefore, we evaluated the efficacy of pembrolizumab, a programmed cell death-1 inhibitor, in patients with CUP.
Methods
The study was designed as a phase 2 basket trial for independent rare tumor cohorts including CUP. Adult patients with CUP who had progressed on previous systemic therapy, performance status 0/1 and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST V.1.1) were eligible. Patients received pembrolizumab (200 mg) intravenously every 21 days. Twenty-nine patients were enrolled and treated between August 2016 and June 2020. The primary endpoint was non-progression rate (NPR) at 27 weeks (NPR-27) per immune-related RECIST. Key prespecified secondary endpoints were confirmed objective response rate (ORR), safety, duration of response (DoR), progression-free survival (PFS) and overall survival (OS). Pretreatment biopsies were examined for biomarkers of response (programmed cell death ligand-1 (PD-L1) expression and tumor infiltrating lymphocytes (TILs)).
Results
Among 25 (of 29 enrolled) eligible and evaluable patients, 14 (56%) had poorly differentiated carcinoma. Patients received a median of two lines of therapy prior to enrollment. Median follow-up was 27.3 months. NPR-27 was observed in seven patients (28.0% (95% CI: 12.1 to 49.4)). ORR was 20.0% (95% CI: 6.8 to 40.7) with five patients achieving immune-related partial response with median DoR of 14.7 months (95% CI: 9.8 to 19.6). Median PFS and OS were 4.1 (95% CI: 3.1 to 5.1) and 11.3 (95% CI: 5.5 to 17.1) months, respectively. Treatment-related adverse events of any and grade ≥3 were seen in 19 (76%) and 4 (16%) patients, respectively. One (4%) patient had grade 3 immune-related acute kidney injury requiring treatment discontinuation. Neither PD-L1 nor TILs were associated with NPR-27. Both positive PD-L1 staining (44.4% vs 6.3%; p=0.040) and intense TIL infiltration (44.4% vs 6.3%; p=0.040) were associated with response.
Conclusion
Pembrolizumab showed encouraging efficacy in patients with CUP with acceptable safety profile.
Trial registration number
Keywords: immunotherapy; therapies, investigational
Key messages.
What is already known on this topic
Empirical treatment of carcinoma of unknown primary (CUP) is mostly guided by histopathologic evaluation to identify the tissue of origin. However, the prospect of meaningful treatment outcome remains unclear. In recent years, programmed cell death-1/programmed cell death ligand-1 pathway inhibitors such as pembrolizumab has demonstrated durable responses in certain tumor types and is well tolerated. Thus, we reasoned that these drugs may be effective and safe in patients with other rare cancers, for which treatment options are lacking.
What this study adds
This is the first phase 2 study of pembrolizumab in patients with carcinoma of unknown primary. Non-progression rate at 27 weeks (primary endpoint) was 28%. Objective response was seen in 20%, disease control in 44% of evaluable patients and 28% were progression free at 27 weeks.
How this study might affect research, practice and/or policy
Pembrolizumab has encouraging clinical activity with an acceptable safety profile in patients with previously treated CUP.
Introduction
Cancer of unknown primary site (CUP), a clinically heterogeneous group of metastatic tumors wherein the primary site of origin remains occult after complete clinical evaluation, is a rare cancer with an annual incidence of 4.1/100,000 in the USA.1 2 Despite advances in treatment of metastatic cancers with known primaries, survival with currently available therapies (empirical or site-specific) in CUP is dismal with 1-year survival rate of <50%.3–5 Effectiveness of treatments beyond first-line is limited with response rate of 8%–13% and median survival of 4–5 months; and a critical need to develop novel therapies exists.6–8
While immune checkpoint inhibitors (ICIs) have shown efficacy for diverse cancers, access for patients with CUP is restricted to those with high microsatellite-instability (MSI-H) and tumor-mutation burden (TMB-H), seen in 1.8% and 11.8% cases, respectively.5 9 PD-L1 (programmed cell death ligand-1), a predictive biomarker of ICI response in certain cancers, is expressed in 22% of CUP.9 10 Gene-expression profiling has revealed presence of immune-permissive/sensitive CUP subsets.11 We performed a phase 2 trial to assess efficacy and safety of pembrolizumab, a humanized anti-programmed cell death 1 (PD-1) monoclonal antibody, in patients with previously treated CUP (subcohort of a large multicohort trial in rare tumors).12
Methods
Patients, treatment and assessment
Eligible patients were ≥18 years old and met clinical and histologic criteria for CUP. All patients had comprehensive work-up performed as per standard CUP guidelines (National Comprehensive Cancer Network and European Society of Medical Oncology to confirm a CUP diagnosis, including uniform clinical, radiographic and pathology review performed at MD Anderson Cancer Center (online supplemental methods).5 13 All patients were refractory to at least one line of systemic chemotherapy within past 6 months and had Response Evaluation Criteria in Solid Tumors V.1.1 (RECIST V.1.1) measurable disease, Eastern Cooperative Oncology Group performance status of 0/1 and normal organ functions. Patients with prior immunotherapy and autoimmune disease were excluded.
jitc-2022-004822supp001.pdf (1MB, pdf)
Patients received fixed dose of pembrolizumab (200 mg) intravenously every 3 weeks until disease progression, unacceptable toxicity, completion of 24 months of treatment or withdrawal of consent. No dose modifications were allowed. Imaging studies were performed every 9 weeks or earlier if clinically indicated and response to treatment was assessed using immune-related RECIST (irRECIST).14 Objective response, defined as immune-related complete (irCR) or partial response (irPR), was confirmed by repeat imaging at least 4 weeks after criteria for response were first met. Treatment-related adverse events (TRAEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events V.4.0.03.12.
Biomarker analysis on pretreatment fresh biopsy or archival tumor sample was done at a central laboratory. PD-L1 expression on tumor and mononuclear inflammatory cells in tumor nests were assessed at a central laboratory by immunohistochemistry using Merck 22C3 antibody. PD-L1 positivity was defined as membranous PD-L1 expression of 3+ staining intensity or 2+ in ≥5% cells. H-scores ranging from 0 to 300 were also calculated using the standard formula: (1 × % of cells with 1+ staining intensity) + (2 × % of cells with 2+ staining intensity) + (3 × % of cells with 3+ staining intensity).15 Tumor infiltrating lymphocytes (TILs) were scored from 0 (no TILs), 1 (few TILs), 2 (moderate TILs) to 3 (intense TILs) infiltration. Details are provided in study protocol (online supplemental protocol and methods).12
jitc-2022-004822supp002.pdf (678.5KB, pdf)
Trial design, statistical methods and endpoints
This phase 2 study was an open-label, single-center, multicohort trial (online supplemental figure S1).12 The primary end point was non-progression rate at 27 weeks (NPR-27), defined as proportion of patients alive and progression-free at 27 weeks per irRECIST. Key prespecified secondary endpoints were objective response rate (ORR), clinical benefit rate (CBR) (percentage of patients with irCR, irPR, or immune-related stable disease ≥4 months), duration of response (DoR), progression-free survival (PFS), overall survival (OS) and safety. Exploratory objectives were to examine tissue correlates for clinical activity, specifically association of baseline PD-L1 expression and TIL status and ORR and NPR-27. Details are provided in study protocol (online supplemental protocol and methods).
Results
Patient characteristics
Between August 29, 2016, and June 29, 2020, 29 patients were enrolled and treated with pembrolizumab and 25 were eligible and evaluable (online supplemental figure S1). Baseline characteristics are shown in table 1. Median age was 59 years (range: 33–78). Most patient were women (72%) and had poorly differentiated carcinoma (56%). Patients had received a median of two lines of therapy prior to enrollment.
Table 1.
Baseline patient characteristics
| Characteristics | Patients (N=25) | % |
| Age at enrollment (years) | ||
| Median (range) | 59 (33–78) | |
| <60 years | 13 | 52 |
| ≥60 years | 12 | 48 |
| Sex | ||
| Female | 18 | 72 |
| Male | 7 | 28 |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 0 | 0 |
| 1 | 25 | 100 |
| Tumor histology | ||
| Adenocarcinoma | 9 | 36 |
| Undifferentiated carcinoma | 14 | 56 |
| Squamous cell carcinoma | 2 | 8 |
| Time to trial since first diagnosis (years) | ||
| Median (range) | 0.9 (0.3–4.5) | |
| <6 months | 6 | 24 |
| 6 months–1 year | 8 | 32 |
| 1 year–2 years | 5 | 20 |
| ≥2 years | 6 | 24 |
| Number of previous anticancer lines of treatment | ||
| Median (range) | 2 (1–5) | |
| 1 | 8 | 32 |
| 2 | 7 | 28 |
| 3 | 4 | 16 |
| 4/5 | 6 | 24 |
| Mismatch-repair (MMR)/microsatellite status (N=11) | ||
| Proficient-MMR/microsatellite-stable | 11 | 100 |
| Deficient-MMR/microsatellite-instability high | 0 | 0 |
| PD-L1 status (H-score) | ||
| 0 | 9 | 36 |
| 1–150 | 12 | 48 |
| 150–300 | 4 | 16 |
| PD-L1 expression status* | ||
| Positive | 9 | 36 |
| Negative | 16 | 64 |
| Tumor infiltrating lymphocytes (TILs) infiltration score | ||
| 0 (no TILs) | 1 | 4 |
| 1 (few TILs) | 11 | 44 |
| 2 (moderate infiltration of TILs) | 4 | 16 |
| 3 (intense infiltration of TILs) | 9 | 36 |
*Programmed cell death ligand-1 (PD-L1) expression was considered positive if immunohistochemistry was 3+ or 2+ in ≥5% cells.
Efficacy analyses
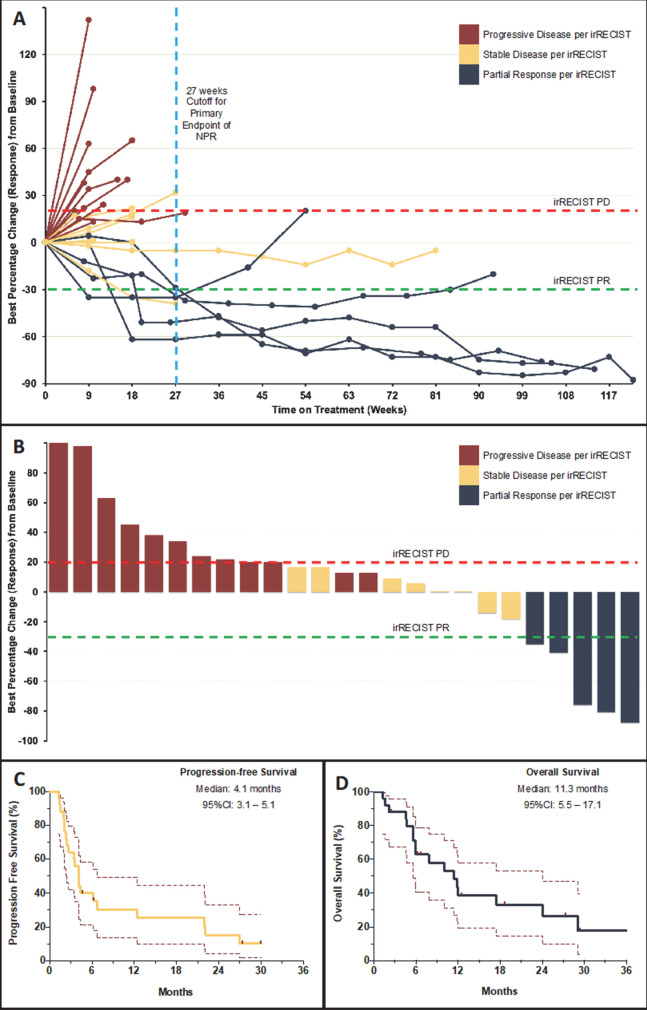
At data cut-off on January 5, 2021, the median follow-up for the evaluable cohort (N=25) was 27.3 months (95% CI: 17.7 to 36.9) and 2 (8%) patients continued treatment. Reasons for discontinuation were disease progression (20 (80%)), toxicity (1 (4%)), and completion of >34 cycles of treatment (2 (8%)). Among 25 evaluable patients, the primary endpoint NPR-27 was 28.0% (95% CI: 12.1 to 49.4) (7 patients) (figure 1A). Five patients had irPR resulting in ORR of 20.0% (95% CI: 6.8 to 40.7) with median DoR of 14.7 months (95% CI: 9.8 to 19.6) in responders and a CBR of 44.0% (95% CI: 24.4 to 65.1) (figure 1B). Median PFS and OS were 4.1 (95% CI: 3.1 to 5.1) and 11.3 (95% CI: 5.5 to 17.1) months, respectively (figure 1C, D). Efficacy outcomes did not differ significantly between key subgroups or for intent-to-treat population (online supplemental tables S1 and S2).
Figure 1.
Tumor response and survival outcomes on pembrolizumab in patients with cancer of unknown primary. (A) (Spider plot) shows the change in sum of target lesion diameters over time in 23 evaluable patients who were treated on the current study and underwent at least one radiological restaging evaluation (two patients had clinical progression prior to first restaging and are reported as default 20% increase). Two patients had unequivocal progression of non-target lesions and were considered as cases with progressive disease (PD). (B) (Waterfall-plot) shows the maximum per cent change from baseline as measured by immune-related Response Evaluation Criteria in Solid Tumors (irRECIST). Partial response (PR) was defined by ≥30% decrease in tumor burden and PD was defined by ≥20% increase in tumor burden, confirmed on a consecutive scan at least 4 weeks apart. (C and D) (KapIan-Meier curves) show progression-free survival and overall survival of patients on study at the time of data cut-off measured from treatment initiation to disease progression/death and death, respectively. Data from patients without an event were censored at date of last follow-up (marks). NPR, non-progression rate.
Safety analyses
TRAEs of any grade were reported by 19 (76%) patients (table 2). Grade 3 TRAEs occurred in 4 (16%) patients. No grade 4/5 events occurred. One (4%) patient had grade 3 immune-related acute kidney injury and required treatment discontinuation.
Table 2.
Treatment-related adverse events
| Adverse event* | All grades† (%) | Grade ≥3 (%) |
| Fatigue | 6 (24) | |
| Hypothyroidism | 5 (20) | |
| Maculo-papular rash | 4 (16) | 1 (4) |
| Aspartate aminotransferase increased | 3 (12) | |
| Alanine aminotransferase increased | 2 (8) | |
| Anorexia | 2 (8) | |
| Diarrhea | 2 (8) | 1 (4) |
| Acute kidney injury‡ | 1 (4) | 1 (4) |
| Arthralgia | 1 (4) | |
| Bullous dermatitis | 1 (4) | |
| Creatinine increased | 1 (4) | |
| Fever | 1 (4) | |
| Hyperthyroidism | 1 (4) | |
| Pneumonitis‡ | 1 (4) | 1 (4) |
| Skin infection | 1 (4) |
*Treatment-related adverse events (TRAEs) listed here include all those that occurred on study patients regardless of grade and all grade ≥3 events. All TRAEs are coded and graded as per the Common Terminology Criteria for Adverse V.4.0.
†No grade 4 or 5 TRAEs occurred on study.
‡Two (8%) patients had treatment-related serious adverse events.
Correlative analyses
PD-L1 and TIL status was determined in all cases and distribution is shown in table 1. PD-L1 H-score was higher in responders (median 55 vs 1; p=0.044) compared with non-responders and a positive PD-L1 staining appeared to be associated with response (44.4% vs 6.3%; p=0.040). TIL score of 3 was associated with response (44.4% vs 6.3%; p=0.040). Patients with either positive PD-L1 or TIL 3 score had higher ORR (38.5% vs 0.0%; p=0.039) compared with those who lacked both these features. Neither PD-L1 nor TILs were associated with NPR-27, PFS or OS (online supplemental figures S2, S3).
Discussion
CUP is a life-threatening malignancy with limited treatment options. Although role of immunotherapy in CUP is evolving, there is no approved or recommended immunotherapy for this patient population. We report on the first clinical study using single agent pembrolizumab, a PD-L1 inhibitor, in this population. In this study, pembrolizumab demonstrated an NPR-27 and ORR of 28% and 20%, respectively, in treatment refractory CUP with a safety profile consistent with prior reports. Although only five patients had a PR, four of those responses were durable and lasted in excess of 12 months. This activity is similar to that seen with nivolumab in CUP (NivoCUP trial) where the ORR was 24%.16
Clinically, CUP is a very heterogenous disease.2 In our cohort, the seven patients that achieved NPR-27 had undifferentiated carcinoma, adenocarcinoma and squamous cell carcinoma in four, two and one cases, respectively. All but one of these patients had lymph node metastases and none had bone metastases.17 Notably, of these patients that achieved an irPR, two had immunophenotype consistent with putative Mullerian profile (PAX8 +ER+), one had p16+ (squamous cell carcinoma) and one had a urothelial profile (GATA3+, thrombomodulin+). Although, a small subset of patients with CUP who have MSI-H and high TMB now have The United States Food and Drug Administration (FDA)- approved access to pembrolizumab, there is still a critical need to exploit the role of immunotherapy in a large subset.5 9 11 Our study is limited in exploring the clinical subtypes of CUP but algorithms integrating clinical, pathological and molecular profiles may indicate putative profiles that are most likely to benefit from immunotherapy. Moreover, genomic-profiling guided assessment of tissue-of-origin, with further refinement, may help selection of patients for immunopermissive tumor types, adding an additional facet to site-directed therapy in CUP.18
Our study has certain limitations due to its small sample size and single-center design which lends itself to referral and selection biases that can mar generalizability to an unselected patient population with CUP. Larger studies are required to further these findings. However, the single-center enrollment did allow us to ensure the fidelity of CUP diagnosis with rigorous review, which can be a concerning issue with CUP as seen with CUPISCO multicenter trial.19 Although our exploratory biomarker analyses are limited by size and are hypothesis generating, they lend some important insights. Both PD-L1 and TIL was associated with response. No response was seen with PD-L1 H-score <5 and most patients with a response had intense TIL infiltration. All responders were either PD-L1 positive or had a TIL score 3. While TMB status is currently unknown in this study, its predictive ability for ICI response, especially in light of the accelerated FDA approval of pembrolizumab for treatment of patients with metastatic TMB-High (≥10 mutations/megabase) solid tumors that have progressed following prior treatment in June 2020, makes it important to include these biomarkers in future studies of ICI in CUP.20 Ongoing correlative studies with whole-exome sequencing for this study will shed further light on biomarkers of response and resistance, in addition to identifying actionable pathways for tumor characterization and other biomarker-directed targeted therapies.
In conclusion, pembrolizumab was well-tolerated and had encouraging (although somewhat limited) efficacy as a single agent in patients with CUP refractory to prior systemic therapy; though patient selection for PD-L1 expression and TILs may enrich for patients more likely to derive clinical benefit. Further trials using combination of ICI and immunomodulators are needed.
Acknowledgments
We thank the patients, their families and caregivers for participating in the trial and all site personnel, clinical pharmacists and clinical staff for clinical trial support in this trial. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., provided the study drug.
Footnotes
Twitter: @AnaingMD
Contributors: KPR and AN contributed to the conception and design, provision of the study materials or patients, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript and is accountable for all aspects of the work. BSt contributed to the collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript and is accountable for all aspects of the work. DDK, SAP-P, AFW, MO, BSm, RWH, FM-B, GRV, and DSH contributed to the provision of patients, review of manuscript and final approval of manuscript. DJ and DOCO contributed to the collection and assembly of data, review of manuscript and final approval of manuscript. AA contributed to assembly of data, review of manuscript and final approval of manuscript. KPR and AN are responsible for overall content
Funding: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., provided the study drug and funded the study. Support was also provided by the National Institutes of Health/National Cancer Institute under award number P30CA016672 and The University of Texas MD Anderson Cancer Center through the Molecular Evaluation and/or Biopsy Related Support Program (used for performing biopsy).
Competing interests: KPR reports research support from Bayer, AstraZeneca, and Daiichi outside the submitted work; SAP-P reports research support from AbbVie, ABM Therapeutics, Acepodia, Alkermes, Aminex Therapeutics, Amphivena Therapeutics, BioMarin Pharmaceutical, Boehringer Ingelheim, Bristol Myers Squibb, Cerulean Pharma, Chugai Pharmaceutical Co., Curis, Daiichi Sankyo, Eli Lilly, ENB Therapeutics, Five Prime Therapeutics, Gene Quantum, Genmab A/S, GlaxoSmithKline, Helix BioPharma Corp., Incyte Corp., Jacobio Pharmaceuticals Co., Medimmune, LLC., Medivation, Merck Sharp & Dohme Corp., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Pfizer; Principia Biopharma, Puma Biotechnology, Rapt Therapeutics, Seattle Genetics, Silverback Therapeutics, Taiho Oncology, Tesaro, TransThera Bio, NCI/NIH, P30CA016672 – Core Grant (CCSG Shared Resources) outside the submitted work; DSH reports research support from AbbVie, Adaptimmune, Adlai Nortye, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Daichi-Sankyo, Eisai, Eli Lilly, EMD Sereno, Erasca, Fate Therapeutics, Genentech, Genmab, GlaxoSmithKline, Ignyta, Infinity, Kite, Kyowa, LOXO, Merck, MedImmune, Millenium, Mirati, miRNA, Molecular TeMpLaTeS, Mologen, NaVier, nci-cep, Novartis, Numab, Pfizer, Seattle Genetics, Takeda, Turning Point, Vernstam, VM Oncology, and other support from Adaptimmune, Amgen, AstraZeneca, Bayer, Genentech, GlaxoSmithKline, Infinity, Numab, Pfizer, Seattle Genetics, Alpha Insights, Acuta, Axiom, Baxter, Boxer Capital, COG, Ecor1, GLG, Group H, Guidepoint, HCW Precision, Janssen, Merrimack, Medscape, Prime Oncology, STCube, Tavistock, Trieza Therapeutics, Molecular Match, Oncoresponse, Presagia, AACR, ASCO, Celgene, Eli Lilly, SITC, and Phillips, outside of the submitted work; MO reports research support from Merck Sharp & Dohme Corp, AbbVie, Agilvax, Takeda Pharmaceuticals (Japan), Acrotech Biopharma, Janssen Research & Development LLC, Pfizer outside the submitted work; FM-B reports research support from Aileron Therapeutics, AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi Sankyo Co., Debiopharm International, eFFECTOR Therapeutics, Genentech, Guardant Health, Klus Pharma, Millennium Pharmaceuticals, Novartis, Puma Biotechnology, Taiho Pharmaceutical Co.; consulting fees from Aduro BioTech, Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche, Genentech, IBM Watson, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer, Samsung Bioepis, Seattle Genetics, Tyra Biosciences, Xencor, Zymeworks; has served on advisory committees for Immunomedics, Inflection Biosciences, Mersana Therapeutics, Puma Biotechnology, Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, Zentalis; receives honoraria from Chugai Biopharmaceuticals, Mayo Clinic, Rutgers Cancer Institute of New Jersey; and support for travel and accommodation from Beth Israel Deaconess Medical Center outside the submitted work; AN reports research support from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor/Millendo, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera BioSciences, TopAlliance BioSciences, Eli Lilly, Kymab, PsiOxus, Arcus Biosciences, NeoImmuneTech, ImmuneOncia, and Surface Oncology, non-financial support for travel and accommodation from ARMO BioSciences, has served as an advisory board member for Novartis, CytomX Therapeutics, Genome and Company, STCube Pharmaceuticals, OncoSec KEYNOTE-695, and Kymab, reports research funding for his spouse from Immune Deficiency Foundation, Jeffery Modell Foundation and chao physician-scientist, and Baxalta, and his spouse has served as an advisory board member for Takeda, CSL, Behring, Horizon, and Pharming outside the submitted work. BSt, DDK, DJ, DOCO, AA, AFW, BSm, RWH, GRV declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: This paper is dedicated to GRV memory.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the FDA and the Institutional Review Board at The University of Texas MD Anderson Cancer Center. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All the study participants provided written informed consent before enrollment.
References
- 1.Mnatsakanyan E, Tung W-C, Caine B, et al. Cancer of unknown primary: time trends in incidence, United States. Cancer Causes Control 2014;25:747–57. 10.1007/s10552-014-0378-2 [DOI] [PubMed] [Google Scholar]
- 2.Varadhachary GR, Raber MN. Carcinoma of unknown primary site. N Engl J Med 2014;371:2040. 10.1056/NEJMc1411384 [DOI] [PubMed] [Google Scholar]
- 3.Hayashi H, Kurata T, Takiguchi Y, et al. Randomized phase II trial comparing site-specific treatment based on gene expression profiling with carboplatin and paclitaxel for patients with cancer of unknown primary site. J Clin Oncol 2019;37:570–9. 10.1200/JCO.18.00771 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi H, Takiguchi Y, Minami H, et al. Site-specific and targeted therapy based on molecular profiling by next-generation sequencing for cancer of unknown primary site: a nonrandomized phase 2 clinical trial. JAMA Oncol 2020;6:1931. 10.1001/jamaoncol.2020.4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN . National comprehensive cancer network. Occult primary (Version 1.2021 - November 24, 2020), 2020. Available: https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf [Accessed 2 Jan 2021].
- 6.Møller AKH, Pedersen KD, Abildgaard J, et al. Capecitabine and oxaliplatin as second-line treatment in patients with carcinoma of unknown primary site. Acta Oncol 2010;49:431–5. 10.3109/02841861003649240 [DOI] [PubMed] [Google Scholar]
- 7.Hainsworth JD, Spigel DR, Raefsky EL, et al. Combination chemotherapy with gemcitabine and irinotecan in patients with previously treated carcinoma of an unknown primary site: a Minnie pearl cancer research network phase II trial. Cancer 2005;104:1992–7. 10.1002/cncr.21416 [DOI] [PubMed] [Google Scholar]
- 8.Hainsworth JD, Burris HA, Calvert SW, et al. Gemcitabine in the second-line therapy of patients with carcinoma of unknown primary site: a phase II trial of the Minnie pearl cancer research network. Cancer Invest 2001;19:335–9. 10.1081/CNV-100103127 [DOI] [PubMed] [Google Scholar]
- 9.Gatalica Z, Xiu J, Swensen J, et al. Comprehensive analysis of cancers of unknown primary for the biomarkers of response to immune checkpoint blockade therapy. Eur J Cancer 2018;94:179–86. 10.1016/j.ejca.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 10.Raghav K, Overman M, Poage GM, et al. Defining a distinct immunotherapy eligible subset of patients with cancer of unknown primary using gene expression profiling with the 92-gene assay. Oncologist 2020;25:e1807–11. 10.1634/theoncologist.2020-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haratani K, Hayashi H, Takahama T, et al. Clinical and immune profiling for cancer of unknown primary site. J Immunother Cancer 2019;7:251. 10.1186/s40425-019-0720-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naing A, Meric-Bernstam F, Stephen B, et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer 2020;8:e000347. 10.1136/jitc-2019-000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fizazi K, Greco FA, Pavlidis N, et al. Cancers of unknown primary site: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22:vi64–8. 10.1093/annonc/mdr389 [DOI] [PubMed] [Google Scholar]
- 14.Nishino M, Tirumani SH, Ramaiya NH, et al. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 2015;84:1259–68. 10.1016/j.ejrad.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igarashi T, Teramoto K, Ishida M, et al. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open 2016;1:e000083. 10.1136/esmoopen-2016-000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanizaki J, Yonemori K, Akiyoshi K, et al. NivoCUP: an open-label phase II study on the efficacy of nivolumab in cancer of unknown primary. Journal of Clinical Oncology 2020;38:106–06. 10.1200/JCO.2020.38.15_suppl.106 [DOI] [PubMed] [Google Scholar]
- 17.Huey RW, Smaglo BG, Estrella JS, et al. Cancer of unknown primary presenting as bone-predominant or lymph node-only disease: a clinicopathologic portrait. Oncologist 2021;26:e650–7. 10.1002/onco.13700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penson A, Camacho N, Zheng Y, et al. Development of genome-derived tumor type prediction to inform clinical cancer care. JAMA Oncol 2020;6:84–91. 10.1001/jamaoncol.2019.3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauli C, Bochtler T, Mileshkin L, et al. A challenging task: identifying patients with cancer of unknown primary (CUP) according to ESMO guidelines: the CUPISCO trial experience. Oncologist 2021;26:e769–79. 10.1002/onco.13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. 10.1016/S1470-2045(20)30445-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-004822supp001.pdf (1MB, pdf)
jitc-2022-004822supp002.pdf (678.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request and approval from study sponsor according to available guidelines at time of request.