Highlights
-
•
Right uncinate fasciculus (UF) integrity predicted socioemotional sensitivity (SS).
-
•
The UF-SS relationship held true in svPPA patient and healthy older adult subgroups.
-
•
Gray matter adjacent to the right UF but not UF integrity predicted SS in bvFTD.
-
•
Other-reported SS may be valuable for clinical trial design for patients with FTLD.
Keywords: Uncinate fasciculus, Socioemotional sensitivity, Diffusion-weighted imaging, Frontotemporal lobar degeneration syndromes
Abstract
The uncinate fasciculus (UF) connects fronto-insular and temporal gray matter regions involved in visceral emotional reactivity and semantic appraisal, but the precise role of this tract in socioemotional functioning is not well-understood. Using the Revised-Self Monitoring (RSMS) informant questionnaire, we examined whether fractional anisotropy (FA) in the right UF corresponded to socioemotional sensitivity during face-to-face interactions in 145 individuals (40 healthy older adults [NC], and 105 patients with frontotemporal lobar degeneration [FTLD] syndromes in whom this tract is selectively vulnerable, including 31 behavioral variant frontotemporal dementia [bvFTD], 39 semantic variant primary progressive aphasia [svPPA], and 35 nonfluent variant primary progressive aphasia [nfvPPA]). Voxelwise and region-of-interest-based DWI analyses revealed that FA in the right but not left UF significantly predicted RSMS score in the full sample, and in NC and svPPA subgroups alone. Right UF integrity did not predict RSMS score in the bvFTD group, but gray matter volume in the right orbitofrontal cortex adjacent to the UF was a significant predictor. Our results suggest that better socioemotional sensitivity is specifically supported by right UF white matter, highlighting a key neuro-affective relationship found in both healthy aging and neurologically affected individuals. The finding that poorer socioemotional sensitivity corresponded to right UF damage in svPPA but was more robustly influenced by gray matter atrophy adjacent to the UF in bvFTD may have important implications for endpoint selection in clinical trial design for patients with FTLD.
1. Introduction
Behavioral variant frontotemporal dementia (bvFTD) (Rascovsky et al., 2011), semantic variant primary progressive aphasia (svPPA) (Gorno-Tempini et al., 2011), and nonfluent variant primary progressive aphasia (nfvPPA) (Gorno-Tempini et al., 2011) belong to a group of early onset neurodegenerative disease syndromes that are caused by frontotemporal lobar degeneration (FTLD) (Mackenzie et al., 2010) in cingulo-insular and anterior temporal lobe networks (Seeley et al., 2009). bvFTD targets the salience (SN) (Seeley et al., 2007) and semantic-appraisal (SAN)/limbic (Seeley et al., 2012, Yeo et al., 2011) networks, and svPPA is associated with early damage to the SAN (Guo et al., 2013, Ranasinghe et al., 2016, Seeley et al., 2008, Seeley et al., 2009). While the SN consists of cortical and subcortical regions that mediate salience-driven attention, the SAN is involved in personal evaluations of social semantic concepts. nfvPPA is characterized by changes in the left fronto-insular speech production network (Mandelli et al., 2014, Seeley et al., 2009) which causes the nonfluent and agrammatic speech symptoms which are the typical features of the syndrome (Gorno-Tempini et al., 2011). In contrast to bvFTD and svPPA, social functions are usually preserved in patients with nfvPPA (Rankin et al., 2006, Sollberger et al., 2009).
Previous studies have shown that the characteristic social dysfunction seen in early bvFTD and in svPPA patients with right temporal lobe damage, including loss of empathy, interpersonal warmth, and emotion reading, correspond to gray matter atrophy and functional connectivity changes in the SN and SAN (Binney et al., 2016, Cerami et al., 2014, Kumfor et al., 2013, Rankin et al., 2006, Rosen et al., 2002, Sollberger et al., 2009, Toller et al., 2018). While these studies examined the relationship of behavior to gray matter and functional connectivity, additional white matter structures likely influence social behavior change as well. The uncinate fasciculus (UF) is a ventral white matter association tract that originates from the anterior temporal lobe and passes through the limen of the insula to the lateral OFC and the frontal pole (Catani et al., 2002, Ebeling et al., 1992). Though there is ongoing controversy around the terminations of the UF (particularly the involvement of the amygdala) (Hau et al., 2016), the tract connects regions of both the SN (insula) and SAN (temporal pole, orbitofrontal cortex [OFC]). Existing evidence shows that both bvFTD (Agosta et al., 2012, Dopper et al., 2013, Mahoney et al., 2014, Whitwell et al., 2010, Zhang et al., 2009) and svPPA (Agosta et al., 2012, Galantucci et al., 2011, Mahoney et al., 2013, Mandelli et al., 2014, Schwindt et al., 2013, Whitwell et al., 2010) are associated with bilateral UF damage. Some studies have also shown that the left UF is affected in patients with nfvPPA (Agosta et al., 2012, Mahoney et al., 2013, Schwindt et al., 2013).
Previous brain imaging studies in healthy participants and human lesion studies have provided some evidence that the UF is involved in memory, semantic, and socioemotional functioning (Von Der Heide et al., 2013), but its precise function particularly for social behavior is still unclear. Moreover, despite the behavioral symptoms at the core of FTLD disorders, few studies have investigated whether social symptoms in FTLD syndromes correspond to white matter changes in specific tracts, particularly those connecting the two key networks underlying social behavior (SN and SAN) (D'Anna et al., 2016, Downey et al., 2015, Multani et al., 2017). Existing investigations did not include the three main FTLD syndromes (bvFTD, svPPA, nfvPPA) that are associated with frontotemporal gray and white matter damage (Galantucci et al., 2011, Mahoney et al., 2014, Schwindt et al., 2013). Precise characterization of syndrome-specific brain-behavior relationships may help to elucidate the natural history of social symptoms seen in different FTLD syndromes, and may provide novel information about clinical and neuroanatomical endpoints for clinical trials that are now being conducted for both tau- and TDP-related FTLD disorders.
For this study, we performed whole-brain voxelwise diffusion-weighted imaging (DWI) analysis to investigate whether individual differences in socioemotional sensitivity corresponded to fractional anisotropy (FA) in the UF in healthy older adults and patients with bvFTD, svPPA, and nfvPPA. We have previously demonstrated that socioemotional sensitivity is associated with both gray matter volume in right anterior temporal and ventral frontal regions (Shdo et al., 2017) and functional connectivity between these areas (Toller et al., 2018). Based on this work, we hypothesized that higher socioemotional sensitivity would correspond to higher FA predominantly in the right UF, both in a whole-brain Tract-Based Spatial Statistics (TBSS) analysis in the full sample (controls + patients), and in region-of-interest (ROI)-based analyses within each diagnostic group.
2. Material and methods
2.1. Participants
One hundred and forty-five participants were enrolled in this study, including 40 healthy older adults (NC) and 105 patients with three different FTLD syndromes: 31 were diagnosed with bvFTD (Rascovsky et al., 2011), 39 with svPPA (Gorno-Tempini et al., 2011), and 35 with nfvPPA (Gorno-Tempini et al., 2011). The rationale for including NC and patients with FTLD in this voxelwise analysis was to better reflect the wide range of normal variability in socioemotional sensitivity and white matter in NC, and the wide range of pathological variability occurring in FTLD syndromes (Rankin et al., 2009). This approach maximized our variability at both the brain and behavioral level, thus increasing the likelihood to detect a statistically significant relationship that can be considered generalizable across health and disease (Rankin et al., 2009). We have previously shown that the RSMS scores of healthy older adults and patients with neurodegenerative diseases as well as the functional connectivity in the SN are on a continuum (Toller et al., 2018), demonstrating that the RSMS measures the same construct in both health and disease. Patients were recruited through our memory clinic or external referrals, and diagnoses were determined by a multidisciplinary team of neurologists, neuropsychologists, and nurses, following thorough neurological, neuroimaging, and neuropsychological assessments. Only patients who were in early to moderate disease stage (Clinical Dementia Rating (CDR) score ≤2) were included. NC were required to have unremarkable neurological examination and MRI scan, and no cognitive impairments on formal neuropsychological testing. All participants had an informant who was a first-degree family member or friend, who had known the participant for five or more years and completed the Revised-Self Monitoring Scale (RSMS) informant questionnaire. All participants were required to have RSMS informant ratings and neuropsychological testing obtained within 90 days of DWI. To obtain the final number of 145 participants, four patients (2 svPPA, 2 nfvPPA) and five NC who were otherwise eligible for the study were excluded because of excessive motion during DWI. Demographic and clinical characteristics are shown in Table 1. The study was approved by the Committee on Human Research at the University of California, San Francisco, and all participants and their informants gave their consent to participate.
Table 1.
Demographic and clinical characteristics of study groups (n = 145).
| Median (interquartile range) | NC (n = 40) |
bvFTD (n = 31) |
svPPA (n = 39) |
nfvPPA (n = 35) |
Statistics | p-value | η2 |
|---|---|---|---|---|---|---|---|
| Age | 67 (64–71) | 60 (55–66)* | 66 (61–68) | 68 (61–74) | F(df) = 7.09 (3) | <0.0001 | |
| Sex, M/F | 40%/60% | 61%/39% | 59%/41% | 34%/66% | χ2(df) = 7.73 (3) | =0.05 | |
| Education | 18 (16–18) | 16 (13–17) | 16.5 (14–19) | 17 (14–18) | F(df) = 2.45 (3) | =0.0661 | |
| MMSEa, total (max = 30) | 29 (29–30) | 25 (21–27) | 25 (23–28) | 27 (25–28) | F(df) = 1.56 (2) | =0.2156 | |
| CDRa, total (≤2) | 0 | 1 (0.5–1) | 0.5 (0.5–1) | 0.5 (0–0.5) | F(df) = 14.29 (2) | <0.0001 | |
| CDRa, sum of boxes | 0 | 6 (4–8) | 3.5 (2–5.5) | 1.5 (0–2.5) | F(df) = 31.46 (2) | <0.0001 | |
| RSMS, total (max = 78) | 58 (51–63) | 31 (17–42)** | 33 (24–51)** | 48 (39–61) | F(df) = 17.46 (3) | <0.0001 | 0.28 |
| VOSPb, total (max = 10) | 10 (9–10) | 8 (7–10) | 10 (9–10) | 9 (8–10) | F(df) = 3.67 (3) | <0.05 | 0.10 |
RSMS and VOSP total scores were controlled for age, sex, and MMSE. Dunnett-Hsu post-hoc tests were used to compare mean least-square RSMS and VOSP scores between each patient group and the control group. Group differences in age, sex, MMSE, and CDR were analyzed using Tukey post-hoc tests. NC = healthy older controls, bvFTD = behavioral variant frontotemporal dementia, svPPA = semantic variant primary progressive aphasia, nfvPPA = nonfluent variant primary progressive aphasia. MMSE = Mini-Mental State Examination, CDR = Clinical Dementia Rating, RSMS = Revised Self-Monitoring Scale, VOSP = Visual Object and Space Perception battery.
aPairwise statistical comparisons only across patient groups; b105 out of 145 participants had VOSP scores.
*Group differs from NC at p < 0.05; **Group differs from NC at p < 0.001.
2.2. Behavioral measures
To measure social behavior, rather than asking patients to describe themselves directly, we used an informant-based questionnaire because patients with frontal lobe damage frequently show reduced insight into their social and cognitive deficits (Shany-Ur et al., 2014). The RSMS (Lennox and Wolfe, 1984) is a well-validated 13-item questionnaire that measures people’s ability to read social signals during face-to-face interactions, and the aspects of the scale that measure the ability to modify behavior rely on the core element of social sensitivity to allow individuals to read those signals. Construct validation studies of the questionnaire have shown that it measures one’s sensitivity to the expressive behavior of others, as well as the ability to use those social cues to adjust one’s self-presentation (Anderson, 1991, O'Cass, 2000) and that it reflects neural anatomy specific to affect sharing (Shdo et al., 2017) and detection of socioemotionally salient cues (Toller et al., 2018). The questionnaire was successfully used in previous studies investigating the neuronal correlates of socioemotional sensitivity in both healthy and clinical populations (Hofmann, 2006, Shdo et al., 2017, Toller et al., 2018). A first-degree relative family member or friend, who had known the participant for five or more years, was asked to rate each item on a 6-point Likert scale, ranging from “certainly, always false” to “certainly, always true”. RSMS total score was the primary behavioral outcome measure of the study, and was examined in relation to (1) FA across the whole-brain white matter skeleton, and (2) FA in predefined right and left UF ROIs.
In contrast to social behavior, patient cognitive abilities are best assessed through face-to-face testing rather than indirect informant report alone (Jorm, 1996). The subtest Number Location of the Visual Object and Space Perception (VOSP) battery (Warrington and James, 1991) measures space perception and correlates with parietal regions (Ranasinghe et al., 2014). The Number Location subtest consists of 10 items with two squares that are located one below the other. The upper square contains numbers that are arranged in random order, and the lower square contains one black dot whose position overlaps with the position of one number in the upper square. For each item, participants had to identify which number corresponded to the location of the dot. This test was used as a non-social control task in our within-group analyses to confirm our hypothesis that individual differences in socioemotional but not visuospatial function would be related to FA in the right UF ROI.
2.3. Structural and diffusion-weighted imaging acquisition and processing
Participants underwent structural and DWI using a 3 T Siemens Trio scanner at the University of California, San Francisco. A T1-weighted 3D magnetization prepared rapid gradient echo (MPRAGE) sequence was used to obtain the structural images, with parameters as follows: 160 sagittal slices, 1-mm thick, skip = 0 mm; repetition time (TR) = 2300 ms; echo time (TE) = 2.98 ms; flip angle = 9°; field of view = 240 × 256 mm2; voxel size = 1 mm3; matrix size = 256 × 256. A high angular resolution diffusion-weighted imaging (HARDI) dataset was acquired using a single-shot spin-echo echo-planar imaging (SE-EPI) sequence, including 55 contiguous axial slices acquired in an interleaved order with the following parameters: 2.2-mm thick, TR = 8000 ms, TE = 109 ms, flip angle = 90°, field of view = 220 × 220 mm2, voxel size = 2.2 mm3, matrix size = 100 × 100, 64 non-collinear diffusion sensitization directions at b_2000 s/mm2, 1 at b = 0, and an integrated parallel acquisition technique acceleration factor of 2.
Initial preprocessing was performed using the FMRIB Software Library (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). First, for each participant, the b0 images were skull stripped, and eddy current distortion and motion correction were performed by registering each diffusion volume to the first b0 volume using affine transformations. Single-subject tensor maps were then generated, and spatially normalized to MNI space with a tensor-based registration algorithm (Keihaninejad et al., 2012, Zhang et al., 2007) using DTI-TK (https://dti-tk.sourceforge.net/pmwiki/pmwiki.php). After tensor diagonalization, FA maps were obtained for each subject in native space. Each FA map was normalized to MNI space with the same affine transformations as used for the tensor realignment (Smith et al., 2006), and was visually checked for registration errors. The FA maps were then used to create a mean FA image of the full sample. The mean FA image was then thinned to derive the mean FA skeleton with a threshold of 0.2, which represented the common white matter tracts across all participants as described in the TBSS approach (Smith et al., 2006). Each subject’s aligned FA image was then projected onto the skeleton and the resulting data was used in a whole-brain voxelwise statistical analysis examining the relationship between socioemotional sensitivity and FA in the full sample (NC + patients). We used FA in our study as a general index of white matter integrity. FA refers to the degree of water diffusion directionality and is high in voxels containing water that moves mainly along a single direction (Le Bihan et al., 2001).
Though our patient subgroup sample sizes were large considering the relatively rare occurrence of the three syndromes (Seelaar et al., 2011), our diagnostic groups were underpowered to detect significant within-group relationships using whole-brain voxelwise brain-behavior analysis. Thus, we performed region-of-interest (ROI)-based analyses to test our specific hypothesis that individual FA differences in the right UF would predict socioemotional sensitivity in any of the diagnostic groups, including healthy older adults. Binarized right and left UF masks were created using the IIT Human Brain Atlas (Zhang and Arfanakis, 2018), and each participant’s mean FA value was extracted from the skeleton for each tract. To examine whether gray matter volume adjacent to the UF predicted the RSMS score in addition to or independent of FA in the UF, right and left gray matter ROIs were defined in the temporal pole (TP), as well as in the medial and posterior OFC using the Neuromorphometrics, INC, brain atlas (https://www.neuromorphometrics.com).
2.4. Statistical analysis
Group differences on potentially confounding covariates (age, sex, education, Mini-Mental State Examination [MMSE]) were analyzed using general linear models in SAS (SAS Proc GLM). GLMs were also used to analyze group differences in RSMS and VOSP score, controlling for age, sex, and MMSE (as a proxy for disease severity). Dunnett-Hsu post-hoc tests were performed to compare each patient group's least square mean RSMS and VOSP score to those of NC.
Voxelwise analysis: A GLM using FSL randomise (Winkler et al., 2014) was conducted to investigate whether individual differences in socioemotional sensitivity predicted FA within the skeleton in the full sample (NC + patients), controlling for age, sex, MMSE, and total intracranial volume (TIV). The threshold for significance was set at p < 0.001, corrected, based on 5000 permutations and threshold-free cluster enhancement (Smith and Nichols, 2009).
ROI analysis: GLMs in SAS were performed to examine whether individual differences in FA (1) in the right UF ROI (=right UF model), and (2) in the left UF ROI (left UF model) predicted RSMS score within any of the diagnostic groups. In a secondary analysis, the right TP, medial OFC, and posterior OFC ROIs were added to the right UF model, and the left TP, medial OFC, and posterior OFC ROIs were added to the left UF model. Age, sex, MMSE, and TIV were included as covariates of no interest in each ROI analysis. Because this was an investigation of brain-behavior relationships in which no human risk would be associated with a false positive result, we chose to accept a p < 0.05 threshold for significance for each of the small number of comparisons (n = 4) in our ROI analyses.
3. Results
3.1. Demographic, clinical, and behavioral scores
Group medians, ranges, and comparisons are shown in Table 1. Mean age in the bvFTD group (M ± SD: 60.7 ± 8.1, p < 0.001) was significantly younger than in NC (68.0 ± 6.2), though no other age differences were found. Though average CDR scores significantly differed between patient groups, all groups had a median CDR between 0.5 and 1, and thus were in early disease stage. Age, sex, and MMSE were included as covariates of no interest in all subsequent analyses. As expected, patients with bvFTD (31.5 ± 2.6; p < 0.001) and svPPA (36.9 ± 2.3; p < 0.001) had significantly lower RSMS scores compared to NC (56.1 ± 2.4). The interaction of sex by diagnostic group did not reach statistical significance for predicting the RSMS score in our sample (p = 0.825). The subgroup comparisons on the visuospatial control task score (VOSP) revealed a significance level of p = 0.141 between patients with bvFTD and NC, p = 0.871 between patients with svPPA and NC, and p = 0.382 between patients with nfvPPA and NC.
3.2. Relationship of behavioral scores to white matter
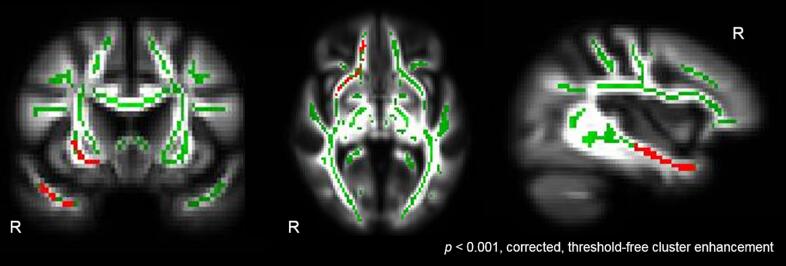
Voxelwise analysis: To investigate whether individual differences in socioemotional sensitivity corresponded to FA in the whole-brain white matter skeleton in the full sample, we performed voxelwise analysis to examine whether FA in any cluster within the skeleton predicted RSMS score. We found that higher RSMS score was significantly associated with higher FA values in several clusters involving the right UF (p < 0.001, cluster enhancement; cluster size: 218 voxels; cluster maximum: x = 82, y = 69, z = 25), controlling for age, sex, MMSE, and TIV (Fig. 1). No other cluster reached statistical significance. The results remained the same when we added group as an additional covariate of no interest to the analysis except that the cluster size was slightly smaller (p < 0.001, cluster enhancement; cluster size: 213 voxels; cluster maximum: x = 82, y = 69, z = 24).
Fig. 1.
Right UF predicting socioemotional sensitivity. Whole-brain voxelwise analysis revealed that higher RSMS score significantly predicted (p < 0.001, corrected, threshold-free cluster enhancement) higher FA in the right UF in the full sample of healthy older adults and FTLD patients. Age, sex, MMSE, and TIV were included as covariates of no interest in the model. The statistical t-map (red), cluster-based corrected for multiple comparisons, was superimposed on both the mean skeleton (green) and mean FA image of the full sample. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Group differences in the UF: We extracted FA from the right and left UF and examined group differences in FA in the UF. Our results showed that patients with bvFTD (p < 0.001) and svPPA (p < 0.001) had significantly lower FA in the right (M ± SD; bvFTD: 0.35 ± 0.01; svPPA: 0.36 ± 0.01; NC: 0.41 ± 0.01; eta squared = 0.27) and left (bvFTD: 0.34 ± 0.01; svPPA: 0.33 ± 0.01; NC: 0.39 ± 0.01; eta squared = 0.34) UF compared to NC. By contrast, the statistical comparison between patients with nfvPPA and NC revealed a significance level of p = 0.808 regarding FA in the right UF, and p = 0.342 regarding FA in the left UF. To examine whether females and males differed with regard to FA in the right and left UF, we examined whether the interaction of sex by diagnostic group predicted the RSMS score. Our results showed that the interaction did not reach statistical significance in both the right (p = 0.175) and left (p = 0.498) UF model.
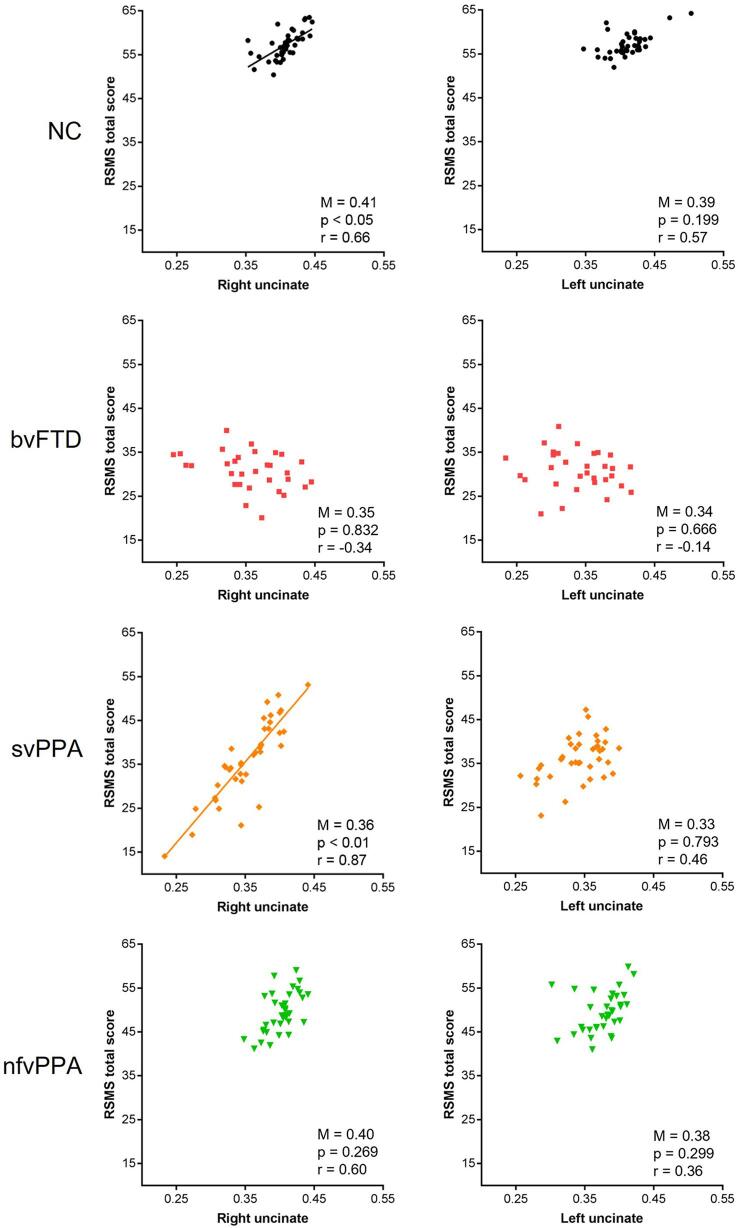
Relationship between UF and RSMS: To test our hypothesis that higher RSMS score would be related to higher FA in the right UF in any of the diagnostic subgroups, we examined (1) whether the right and left UF ROIs separately predicted RSMS score (Main Effects models), and (2) whether the right and left UF ROIs independently predicted RSMS score by entering them into the same regression model (Covariates models).
In NC, higher FA in the right (F1, 35 = 4.65, p = 0.047, r = 0.66, estimate = 135.60, 95%CI = [-0.50, 271.70]) but not left (p = 0.199) UF significantly predicted higher RSMS score in the Main Effects model (Fig. 2), but this relationship lost significance (p = 0.160) after including the two highly correlated tracts (r = 0.78) in the Covariates analysis.
Fig. 2.
Right UF corresponded to socioemotional sensitivity in NC and svPPA patients. Higher FA in the right but not left UF significantly predicted higher Revised Self-Monitoring Scale (RSMS) score in the NC (F1, 35 = 4.65, p = 0.047, r = 0.66, estimate = 135.60, 95%CI = [-0.50, 271.70]) and svPPA group (F1, 33 = 10.74, p = 0.003, r = 0.87, estimate = 191.98, 95%Cl = [72.82, 311.14]) in the Main Effects model. FA in the right and left UF did not significantly predict RSMS score in patients with bvFTD and nfvPPA, though the correlation in the nfvPPA group was high (r = 0.60). RSMS scores were adjusted for age, sex, MMSE, and TIV. Average FA (M) in the right and left UF was significantly lower in patients with bvFTD (p < 0.001) and svPPA (p < 0.001) compared to NC. NC = healthy older adults, bvFTD = behavioral variant frontotemporal dementia, svPPA = semantic variant primary progressive aphasia, nfvPPA = nonfluent variant primary progressive aphasia.
In patients with svPPA, FA in the right UF significantly predicted RSMS score in both the Main Effects (F1, 33 = 10.74, p = 0.003, r = 0.87, estimate = 191.98, 95%Cl = [72.82, 311.14]) and Covariates (F1, 32 = 12.86, p = 0.001, r = 0.82, estimate = 221.59, 95%Cl = [95.72, 347.46]) models (Fig. 2). No significant relationship was found between the left UF and RSMS score in both the Main Effects (p = 0.793) and the Covariates (p = 0.612) model. By contrast, for patients with bvFTD and nfvPPA, FA in the right and left UF did not significantly predict RSMS score in any model (Fig. 2), though the correlation between the right UF and the score was high in the nfvPPA group (r = 0.60). As expected, our control analysis confirmed that individual differences in FA in the right and left UF were not associated with VOSP score in any of the diagnostic groups (Supplementary Fig. 1).
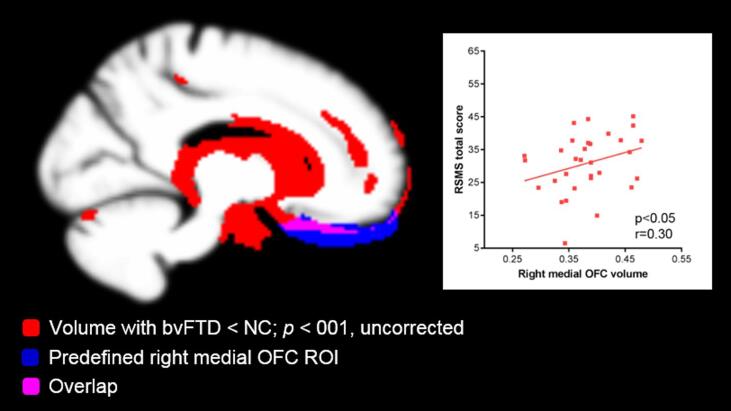
Contribution of gray matter atrophy to the UF/RSMS relationship: Next, to evaluate whether FA in the UF or gray matter volume adjacent to the UF better predicted RSMS score, we performed a secondary analysis of gray matter ROIs, adding (1) the right TP, medial OFC, and posterior OFC ROIs to the right UF Main Effects model, and (2) the left TP, medial OFC, and posterior OFC ROIs to the left UF Main Effects model. Though FA in the right and left UF did not significantly predict RSMS score in the bvFTD group, lower gray matter volume in the right medial OFC ROI did (F1, 23 = 4.33, p = 0.049, r = 0.30, estimate = 161.32, 95%CI = [0.92, 321.72]) (Fig. 3). By contrast, the right posterior OFC volume (p = 0.052) and the right TP (p = 0.807) volume did not significantly predict RSMS score. No significant relationship was found between RSMS score and any of the left ROIs (medial OFC: p = 0.542; posterior OFC: p = 0.518; TP: p = 0.815) in the left UF Main Effects model in bvFTD. None of the GM ROIs significantly predicted RSMS score in any of the other diagnostic subgroups. As an error check of multicollinearity among UF and gray matter ROIs, we calculated pairwise correlations and variance inflator factors (VIFs) for these structures within each diagnostic group. In the NC, nfvPPA, and svPPA groups no correlation was statistically significant and all VIFs were < 5 (Stine, 1995), suggesting that multicollinearity did not have a strong influence in these models. However, in patients with bvFTD, the left UF significantly correlated with each left ROI (left temporal pole: r = 0.39, p = 0.003; left posterior OFC: r = 0.36, p = 0.005; left medial orbitofrontal cortex: r = 0.38, p = 0.003), and the VIFs of the left medial (9.8) and posterior (9.4) OFC were problematically elevated.
Fig. 3.
Gray matter volume predicting socioemotional sensitivity in patients with bvFTD. Though FA in the right UF did not significantly predict RSMS score in the bvFTD group (p = 0.832), lower volume in the right medial OFC ROI (blue) significantly predicted (F1, 23 = 4.33, p = 0.049, r = 0.30, estimate = 161.32, 95%CI = [0.92, 321.72]) lower socioemotional sensitivity. Image confirms that atrophy in patients with bvFTD (red), shown at an uncorrected statistical threshold p < 0.001, overlapped (pink) with the medial OFC gray matter ROI (blue). bvFTD = behavioral variant frontotemporal dementia, NC = healthy older adults, OFC = orbitofrontal cortex. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our results highlight that the right UF underlies socioemotional sensitivity in healthy older adults and patients with neurodegenerative disease through bridging regions of the SN and SAN that together mediate personal evaluations of social semantic concepts based on viscerally experienced valence. Degree of right UF damage was particularly important for predicting socioemotional behavior in svPPA syndrome patients, while in patients with bvFTD gray matter volume in the right medial OFC cortex adjacent to the UF tract predicted their behavior. This more precise model of the neural mechanisms underlying specific social behavior changes in patients with FTLD syndromes further clarifies the natural history of socioemotional symptom development in these patients and can be used to better inform brain-behavior progression models in clinical trials.
4.1. Right UF mediates socioemotional sensitivity in health
We designed our study to maximize the potential variability of FA in the UF by including both healthy individuals and those with degeneration to this tract. In the full sample (patients + NC), we found that higher FA in the right but not left UF predicted higher socioemotional sensitivity. However, we also found this relationship to be significant in the subgroup comprised of healthy older adults alone, showing that there is sufficient variability of FA in the UF in neurologically healthy older individuals to explain individual differences in observer ratings of their socioemotional sensitivity. Thus, this important brain-behavior relationship is a continuum reflected both in normally occurring variability of white matter in the UF across healthy aging individuals, and in the pathological white matter loss resulting from neurodegenerative disease. By contrast, FA in the right UF did not significantly predict the visuospatial control score in any of the diagnostic subgroups, including healthy older adults, which demonstrates the specificity of this brain-behavior relationship to the socioemotional domain, rather than to general cognition.
Our findings also highlight that the laterality of the UF is important as a predictor of behavior. The right UF in particular is better anatomically situated to mediate complex socioemotional behaviors because of its connections to right-sided networks processing visceral emotional reactivity (SN) (Seeley et al., 2007) and the semantic appraisal of person-specific knowledge (SAN) (Olson et al., 2007). Specifically, our findings suggest that degree of white matter in the right UF mediates socioemotional sensitivity through tagging concepts with complex hedonic evaluations and resolving ambiguity about the semantic identity of the concepts based on viscerally experienced valence (this face is happy not sad). This is consistent with previous studies showing that the right UF is involved in emotion recognition and empathy in healthy participants (Coad et al., 2017, Oishi et al., 2015, Parkinson and Wheatley, 2012). By contrast, FA in the left UF did not predict socioemotional sensitivity in our study, which is in line with prior evidence showing that the left UF has a specific role for lexical retrieval of semantic knowledge, and is therefore involved in language more than socioemotional processing (de Zubicaray et al., 2011, Duffau et al., 2009, Papagno et al., 2010).
4.2. Clinical relevance to patients with FTLD syndromes
Early svPPA is characterized by selective loss of semantic knowledge and focal bilateral but typically asymmetric damage to the left anterior temporal lobes (Gorno‐Tempini et al., 2004; Hodges et al., 1992) and UF (Acosta-Cabronero et al., 2011, Galantucci et al., 2011, Mahoney et al., 2013, Schwindt et al., 2013). svPPA patients with predominantly right temporal lobe involvement show many of the same socioemotional symptoms seen in patients with bvFTD, which correspond to gray matter atrophy in the right TP, OFC, and amygdala that are connected through the UF (Gorno-Tempini et al., 2004a, Gorno-Tempini et al., 2004b, Perry et al., 2001, Rosen et al., 2002). Extending these earlier investigations, which primarily examine brain-behavior relationship in gray matter, this study showed that greater damage to the right UF corresponds to lower socioemotional sensitivity in a large group of patients with svPPA. This effect remained significant when gray matter volume in the right TP and OFC was added to the statistical model, suggesting that in patients with early to moderate svPPA, white matter changes in the right UF more strongly predict diminished socioemotional sensitivity than gray matter volume in adjacent regions. Because of the rare occurrence of FTLD syndromes (Seelaar et al., 2011), previous studies have investigated the white matter correlates of social behavior in mixed samples of FTLD patients. In keeping with our findings, one study showed that degree of white matter in the right UF was associated with sarcasm identification in a sample of patients with bvFTD and svPPA (Downey et al., 2015). Furthermore, two other studies revealed that less white matter in right UF corresponded to diminished emotion reading and interpersonal warmth (Multani et al., 2017), as well as to increases in apathy, social inappropriateness, and impulsivity (D'Anna et al., 2016) in patients with primary progressive aphasias. Our findings also support evidence from other clinical populations, including autism (Kumar et al., 2009, Samson et al., 2016), temporal lobe epilepsy (Bell et al., 2011), traumatic brain injury (Johnson et al., 2011), stroke (Oishi et al., 2015), and glioma (Herbet et al., 2015), which may show damage to the right UF and similar symptoms as seen in svPPA such as loss of empathy and impulsive behaviors.
Though bvFTD affects the UF bilaterally (Mahoney et al., 2014, Whitwell et al., 2010, Zhang et al., 2009), degree of white matter in neither right nor left UF predicted socioemotional sensitivity in our sample of patients with early bvFTD. This result was unexpected, and stands in contrast to previous studies demonstrating that less white matter in the UF corresponded to more disinhibition (Hornberger, Brain, 2011) and reduced sarcasm identification (Downey et al., 2015) in patients with bvFTD. However, our secondary analysis showed that lower gray matter volume in the right medial OFC adjacent to the UF tract did significantly predict lower socioemotional sensitivity in the bvFTD group. This suggests that in patients with bvFTD syndrome, reduced socioemotional sensitivity is more robustly influenced by reduction in gray matter volume adjacent to the right UF than by degree of white matter in the UF. bvFTD presents with tremendous clinical, neuroanatomical, and neuropathological heterogeneity, with approximately half of cases showing FTLD-tau and the other half showing FTLD-TDP at autopsy (Mackenzie et al., 2010). By contrast, the pathology underlying svPPA is almost always FTLD-TDP, which is associated with less white matter burden than FTLD-tau (McMillan et al., 2013). Thus, it is unlikely that the reason we found a relationship between right UF and socioemotional sensitivity in svPPA but not in bvFTD would be because patients with bvFTD had less white matter involvement than patients with svPPA. Another consideration is that previous work has shown that patients with bvFTD have divergent patterns of SN and SAN atrophy, and corresponding differences in social behavior (Ranasinghe et al., 2016). It is therefore possible that right UF volume may predict socioemotional sensitivity only in particular bvFTD subtypes, most likely the frontotemporal- and temporal-predominant subtypes (Ranasinghe et al., 2016), which may be associated with more UF damage than the frontal- and subcortical-predominant bvFTD subtypes. Though we included only patients who were in mild to moderate disease stages (CDR ≤ 2) and the average disease severity was mild in all patient groups, the bvFTD group included more patients who were in moderate (CDR = 2) disease stage than the svPPA and nfvPPA groups. Thus, we cannot exclude that the bvFTD group on average had more advanced gray matter atrophy, and that the relationship we found between socioemotional sensitivity and gray matter volume in the right OFC but not white matter in the right UF is a reflection of more pronounced gray matter volume loss. Future studies are warranted to determine whether the degree to which the right UF mediates socioemotional sensitivity varies between distinct bvFTD subtypes and different disease stages.
Typical features of nfvPPA syndrome are motor speech problems and grammatical deficits; these difficulties are caused by gray and white matter damage to the speech production network, which includes left inferior frontal, insular, supplementary motor, and striatal regions (Gorno-Tempini et al., 2006, Grossman et al., 1996, Mandelli et al., 2014). In early nfvPPA, pronounced socioemotional deficits are rare, and patients usually retain intact social functions such as empathy (Rankin et al., 2006) and personality traits like interpersonal warmth (Sollberger et al., 2009). This is consistent with our finding that socioemotional sensitivity did not significantly differ between patients with nfvPPA and the NC group. In addition, and in contrast to some previous studies (Agosta et al., 2012, Mahoney et al., 2013, Schwindt et al., 2013), our results showed that mean FA in the right and left UF in the nfvPPA group was not abnormal. This is probably attributable to the very mild disease stage (median CDR = 0.5) in our nfvPPA group and the focal neurodegeneration in the left fronto-opercular region typically seen in early stages of nfvPPA, with relative sparing of ventral frontotemporal white matter tracts such as the UF.
5. Limitations and conclusions
Study limitations. This study did not include resting-state functional imaging (rs-fMRI), thus it still remains to be investigated to which degree structural integrity in the SN or SAN dictates functional integrity in different disease stages. Our findings in the slightly more advanced bvFTD group suggest that different neuroimaging modalities may predict social symptoms at different disease stages, which may be confirmed in future multimodal neuroimaging studies investigating the neuronal substrates of social function in well-powered samples of bvFTD patients with different disease severities. In addition, our study did not combine micro- and macrostructure approaches, thus the impact of neurodegeneration on white matter remains to be investigated in future studies. Furthermore, we found some evidence for multicollinearity between the left UF and left OFC in the bvFTD group, which may have reduced our power to detect statistically significant brain-behavior relationships. However, this does not weaken our main finding that the right UF mediates socioemotional sensitivity through tagging social semantic entities with hedonic evaluations. Finally, we have used a fully-automated TBSS approach in which each subject’s FA data is projected onto the mean FA skeleton in such a way that each skeleton voxel takes the FA value from the local center of the nearest relevant tract, thus alleviating issues of alignment and correspondence compared to other voxelwise approaches. The limitations of TBSS include within-scan motion, interpretation of data in regions of crossing tracts or tract junctions, and the possibility that pathology could reduce FA so strongly that potential areas of interest may be wrongly excluded from the analysis. Although FA is the most widely used among DTI-derived metrics, FA is sensitive to several confounds, including image and motion artifacts, partial volume effects, and regions of crossing white matter tracts (Alexander et al., 2007). To minimize these errors, we have performed motion correction during preprocessing and we have excluded participants with excessive motion and atrophy as well as patients with severe disease stage (CDR = 3). Finally, we did not include other DTI-derived metrics such as axial (AD) and radial (RD) diffusivity because it still remains controversial how to interpret these metrics in the context of pathology, particularly when inflammation, axonal loss, axonal injury, and demyelination co-occur (Wheeler-Kingshott and Cercignani, 2009, Winklewski et al., 2018).
Summary. Overall, we conclude that individual differences in other-rated socioemotional sensitivity are reflected in degree of white matter specifically in the right UF, and that this brain-behavior relationship exists not only in patients with degeneration to this tract, but also in neurologically healthy older adults. Our findings extend our neuroscientific understanding about the role of the UF in normal socioemotional behavior, and may also have important clinical implications for patients with neurological and neuropsychiatric diseases in whom this tract is selectively vulnerable, including FTLD, temporal lobe epilepsy, traumatic brain injury, stroke, and autism.
CRediT authorship contribution statement
Gianina Toller: Conceptualization, Formal analysis, Visualization, Writing – original draft, Funding acquisition. Maria Luisa Mandelli: Formal analysis, Visualization, Writing – original draft. Yann Cobigo: Formal analysis, Writing – review & editing. Howard J. Rosen: Writing – review & editing. Joel H. Kramer: Resources, Writing – review & editing, Funding acquisition. Bruce L. Miller: Resources, Writing – review & editing, Funding acquisition. Maria Luisa Gorno-Tempini: Writing – review & editing, Supervision, Funding acquisition. Katherine P. Rankin: Conceptualization, Writing – original draft, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the patients and their caregivers for participating in this research. This study was supported by grants R01AG029577, K23-AG021606, P01AG019724, P50AG0235015R01AG032289-08, and R01 NS050915 from the National Institutes of Health, grants 2002/2J and 2014-A-004-NET from the Larry L. Hillblom Foundation, and grant P2ZHP1_165073 from the Swiss National Science Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102994.
Contributor Information
Gianina Toller, Email: Gianina.Toller@ucsf.edu.
Maria Luisa Mandelli, Email: MariaLuisa.Mandelli@ucsf.edu.
Yann Cobigo, Email: Yann.Cobigo@ucsf.edu.
Howard J. Rosen, Email: Howie.Rosen@ucsf.edu.
Joel H. Kramer, Email: Joel.Kramer@ucsf.edu.
Bruce L. Miller, Email: Bruce.Miller@ucsf.edu.
Maria Luisa Gorno-Tempini, Email: MariaLuisa.GornoTempini@ucsf.edu.
Katherine P. Rankin, Email: Kate.Rankin@ucsf.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Acosta-Cabronero J., Patterson K., Fryer T.D., Hodges J.R., Pengas G., Williams G.B., Nestor P.J. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134(7):2025–2035. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- Agosta F., Scola E., Canu E., Marcone A., Magnani G., Sarro L., Comi G. White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex. 2012;22(12):2705–2714. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L.R. Test-retest reliability of the revised self-monitoring scale over a two-year period. Psychol. Rep. 1991;68(3):1057–1058. doi: 10.2466/pr0.1991.68.3.1057. [DOI] [PubMed] [Google Scholar]
- Bell B., Lin J.J., Seidenberg M., Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat. Rev. Neurol. 2011;7(3):154. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney R.J., Henry M.L., Babiak M., Pressman P.S., Santos-Santos M.A., Narvid J., Rankin K.P. Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex. 2016;82:147–163. doi: 10.1016/j.cortex.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Canessa N., Crespi C., Marcone A., Cortese F., Cappa S.F. Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimer's & Dementia. 2014;10(6):827–834. doi: 10.1016/j.jalz.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Coad B.M., Postans M., Hodgetts C.J., Muhlert N., Graham K.S., Lawrence A.D. Structural connections support emotional connections: Uncinate fasciculus microstructure is related to the ability to decode facial emotion expressions. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna L., Mesulam M.M., Thiebaut de Schotten M., Dell'Acqua F., Murphy D., Wieneke C., Catani M. Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology. 2016;86(15):1393–1399. doi: 10.1212/WNL.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G.I., Rose S.E., McMahon K.L. The structure and connectivity of semantic memory in the healthy older adult brain. NeuroImage. 2011;54(2):1488–1494. doi: 10.1016/j.neuroimage.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Dopper E.G., Rombouts S.A., Jiskoot L.C., Heijer T., de Graaf J.R., Koning I., van Swieten J.C. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2013;80(9):814–823. doi: 10.1212/WNL.0b013e31828407bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L.E., Mahoney C.J., Buckley A.H., Golden H.L., Henley S.M., Schmitz N., Fox N.C. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. NeuroImage: Clinical. 2015;8:640–651. doi: 10.1016/j.nicl.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Moritz-Gasser S., Mandonnet E. Is the left uncinate fasciculus essential for language? J. Neurol. 2009;256(3):382. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Ebeling U., Cramon D.V. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir. 1992;115(3):143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Galantucci S., Tartaglia M.C., Wilson S.M., Henry M.L., Filippi M., Agosta F., Miller B.L. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134(10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J., Miller B.L. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Rankin K.P., Woolley J.D., Rosen H.J., Phengrasamy L., Miller B.L. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex. 2004;40(4):631–644. doi: 10.1016/s0010-9452(08)70159-x. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L., Ogar, J. M., Brambati, S. M., Wang, P., Jeong, J. H., Rankin, K. P., Miller, B. L. (2006). Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology, 67(10), 1849-1851. doi:01.wnl.0000237038.55627.5b [pii]. [DOI] [PubMed]
- Grossman M., Mickanin J., Onishi K., Hughes E., D'Esposito M., Ding X., Reivich M. Progressive nonfluent aphasia: Language, cognitive, and PET measures contrasted with probable alzheimer's disease. J. Cognit. Neurosci. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Guo C.C., Gorno-Tempini M.L., Gesierich B., Henry M., Trujillo A., Shany-Ur T., Seeley W.W. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain: A J. Neurol. 2013;136(Pt 10):2979–2991. doi: 10.1093/brain/awt222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau J., Sarubbo S., Perchey G., Crivello F., Zago L., Mellet E., Tzourio-Mazoyer N. Cortical terminations of the inferior fronto-occipital and uncinate fasciculi: Anatomical stem-based virtual dissection. Front. Neuroanat. 2016;10:58. doi: 10.3389/fnana.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G., Lafargue G., Moritz-Gasser S., de Champfleur N.M., Costi E., Bonnetblanc F., Duffau H. A disconnection account of subjective empathy impairments in diffuse low-grade glioma patients. Neuropsychologia. 2015;70:165–176. doi: 10.1016/j.neuropsychologia.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Patterson K., Oxbury S., Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G. The emotional consequences of social pragmatism: The psychophysiological correlates of self-monitoring. Biol. Psychol. 2006;73(2):169–174. doi: 10.1016/j.biopsycho.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Johnson C.P., Juranek J., Kramer L.A., Prasad M.R., Swank P.R., Ewing-Cobbs L. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J. Int. Neuropsychol. Soc. 2011;17(4):663–673. doi: 10.1017/S1355617711000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm A.F. Assessment of cognitive impairment and dementia using informant reports. Clin. Psychol. Rev. 1996;16(1):51–73. [Google Scholar]
- Keihaninejad S., Ryan N.S., Malone I.B., Modat M., Cash D., Ridgway G.R., Ourselin S. The importance of group-wise registration in tract based spatial statistics study of neurodegeneration: A simulation study in alzheimer's disease. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0045996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Sundaram S.K., Sivaswamy L., Behen M.E., Makki M.I., Ager J., Chugani D.C. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb. Cortex. 2009;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0067457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N., Chabriat H. Diffusion tensor imaging: concepts and applications. J. Magnetic Resonance Imag. 2001;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lennox R.D., Wolfe R.N. Revision of the self-monitoring scale. J. Pers. Soc. Psychol. 1984;46(6):1349–1364. doi: 10.1037//0022-3514.46.6.1349. [DOI] [PubMed] [Google Scholar]
- Mackenzie I.R., Neumann M., Bigio E.H., Cairns N.J., Alafuzoff I., Kril J., Holm I.E. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010;119(1):1. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Malone I.B., Ridgway G.R., Buckley A.H., Downey L.E., Golden H.L., Rossor M.N. White matter tract signatures of the progressive aphasias. Neurobiol. Aging. 2013;34(6):1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Ridgway G.R., Malone I.B., Downey L.E., Beck J., Kinnunen K.M., Schott J.M. Profiles of white matter tract pathology in frontotemporal dementia. Hum. Brain Mapp. 2014;35(8):4163–4179. doi: 10.1002/hbm.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli M.L., Caverzasi E., Binney R.J., Henry M.L., Lobach I., Block N., Gorno-Tempini M.L. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan C.T., Irwin D.J., Avants B.B., Powers J., Cook P.A., Toledo J.B., Grossman M. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry. 2013;84(9):949–955. doi: 10.1136/jnnp-2012-304418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani N., Galantucci S., Wilson S.M., Shany-Ur T., Poorzand P., Growdon M.E., Rankin K.P. Emotion detection deficits and changes in personality traits linked to loss of white matter integrity in primary progressive aphasia. NeuroImage: Clinical. 2017;16:447–454. doi: 10.1016/j.nicl.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cass A. A psychometric evaluation of a revised version of the lennox and wolfe revised self-monitoring scale. Psychol. Market. 2000;17(5):397–419. [Google Scholar]
- Oishi K., Faria A.V., Hsu J., Tippett D., Mori S., Hillis A.E. Critical role of the right uncinate fasciculus in emotional empathy. Ann. Neurol. 2015;77(1):68–74. doi: 10.1002/ana.24300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Papagno C., Miracapillo C., Casarotti A., Romero Lauro L.J., Castellano A., Falini A., Bello L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2010;134(2):405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Parkinson C., Wheatley T. Relating anatomical and social connectivity: white matter microstructure predicts emotional empathy. Cereb. Cortex. 2012;24(3):614–625. doi: 10.1093/cercor/bhs347. [DOI] [PubMed] [Google Scholar]
- Perry R., Rosen H., Kramer J., Beer J., Levenson R., Miller B. Hemispheric dominance for emotions, empathy and social behaviour: Evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7(2):145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Ranasinghe K.G., Hinkley L.B., Beagle A.J., Mizuiri D., Dowling A.F., Honma S.M., Nagarajan S.S. Regional functional connectivity predicts distinct cognitive impairments in alzheimer’s disease spectrum. NeuroImage: Clinical. 2014;5:385–395. doi: 10.1016/j.nicl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe K.G., Rankin K.P., Pressman P.S., Perry D.C., Lobach I.V., Seeley W.W., Shany-Ur T. Distinct subtypes of behavioral variant frontotemporal dementia based on patterns of network degeneration. JAMA Neurology. 2016;73(9):1078–1088. doi: 10.1001/jamaneurol.2016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin K.P., Salazar A., Gorno-Tempini M.L., Sollberger M., Wilson S.M., Pavlic D., Miller B.L. Detecting sarcasm from paralinguistic cues: Anatomic and cognitive correlates in neurodegenerative disease. NeuroImage. 2009;47(4):2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin, K. P., Gorno-Tempini, M. L., Allison, S. C., Stanley, C. M., Glenn, S., Weiner, M. W., Miller, B. L. (2006). Structural anatomy of empathy in neurodegenerative disease. Brain: J. Neurol.gy, 129(Pt 11), 2945-2956. doi:awl254 [pii]. [DOI] [PMC free article] [PubMed]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: A J. Neurol. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Perry R.J., Murphy J., Kramer J.H., Mychack P., Schuff N., Miller B.L. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125(10):2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Samson A.C., Dougherty R.F., Lee I.A., Phillips J.M., Gross J.J., Hardan A.Y. White matter structure in the uncinate fasciculus: implications for socio-affective deficits in autism spectrum disorder. Psychiatry Res.: Neuroimaging. 2016;255:66–74. doi: 10.1016/j.pscychresns.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Schwindt G.C., Graham N.L., Rochon E., Tang-Wai D.F., Lobaugh N.J., Chow T.W., Black S.E. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum. Brain Mapp. 2013;34(4):973–984. doi: 10.1002/hbm.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H., Rohrer J.D., Pijnenburg Y.A., Fox N.C., van Swieten J.C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: A review. J. Neurol. Neurosurg. Psychiatry. 2011;82(5):476–486. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R., Rascovsky K., Kramer J.H., Weiner M., Miller B.L., Gorno-Tempini M.L. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 2008;65(2):249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Zhou J., Kim E. Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? The Neuroscientist. 2012;18(4):373–385. doi: 10.1177/1073858411410354. [DOI] [PubMed] [Google Scholar]
- Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci., 27(9), 2349–2356. doi:27/9/2349 [pii]. [DOI] [PMC free article] [PubMed]
- Shany-Ur T., Lin N., Rosen H.J., Sollberger M., Miller B.L., Rankin K.P. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain: A J. Neurol. 2014;137(Pt 8):2368–2381. doi: 10.1093/brain/awu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shdo S.M., Ranasinghe K.G., Gola K.A., Mielke C.J., Sukhanov P.V., Miller B.L., Rankin K.P. Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia. 2017;116:126–135. doi: 10.1016/j.neuropsychologia.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Matthews P.M. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sollberger M., Stanley C.M., Wilson S.M., Gyurak A., Beckman V., Growdon M., Rankin K.P. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47(13):2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine R.A. Graphical interpretation of variance inflation factors. Am. Statistician. 1995;49(1):53–56. [Google Scholar]
- Toller G., Brown J., Sollberger M., Shdo S.M., Bouvet L., Sukhanov P., Rankin K.P. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex. 2018;103:211–223. doi: 10.1016/j.cortex.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide V., Rebecca J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E.K., James M. Thames Valley Test Company; Bury St Edmunds: 1991. The Visual Object and Space Perception Battery. [Google Scholar]
- Wheeler-Kingshott C.A., Cercignani M. About “axial” and “radial” diffusivities. Magn. Resonance Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Avula R., Senjem M.L., Kantarci K., Weigand S.D., Samikoglu A., Jack C.R., Jr. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74(16):1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski P.J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes—what do we know? Front. Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Avants B.B., Yushkevich P.A., Woo J.H., Wang S., McCluskey L.F., Gee J.C. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Trans. Med. Imaging. 2007;26(11):1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- Zhang S., Arfanakis K. Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. NeuroImage. 2018;172:40–50. doi: 10.1016/j.neuroimage.2018.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Schuff N., Du A., Rosen H.J., Kramer J.H., Gorno-Tempini M.L., Weiner M.W. White matter damage in frontotemporal dementia and alzheimer's disease measured by diffusion MRI. Brain. 2009;132(9):2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.