Editor'Severe acute respiratory distress syndrome (ARDS) secondary to H1N1 influenza has a high mortality in children with underlying chronic medical condition, and apart from antiviral agents, no therapy has demonstrated a significant improvement in outcome to date.1 2 Supportive treatment is based on protective mechanical ventilation techniques and extracorporeal membrane oxygenation (ECMO). At present, the pharmacological treatment of ARDS is directed at inhibition of the inflammatory and fibrotic pathways, and is the basis for the use of steroids, although no clear benefits on mortality have been found.3 Activated protein C (aPC) has anticoagulant, anti-inflammatory, and antiapoptotic effects, and its i.v. administration is included in septic shock guidelines for adult,4 but not paediatric patients.5 Nebulized aPC has showed a beneficial effect in different animal models of lung injury.6 Activation of the coagulation pathways can amplify the inflammatory response, and the use of anticoagulants to prevent lung injury or remodelling in ARDS was proposed a decade ago. However, despite the potential benefits of aPC in acute lung injury and the fact that early administration of aPC has been advocated as a potential therapy in ARDS,6 no studies have specifically addressed this issue in humans.
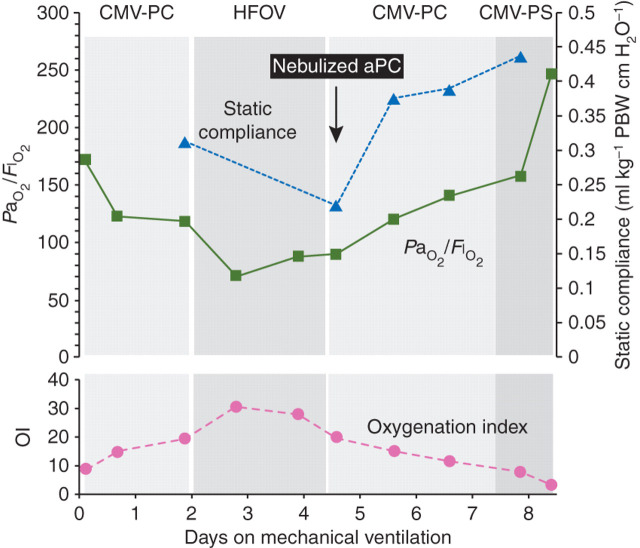
We report a case of paediatric aPC nebulization in a 10-yr-old child with a history of chemotherapy for orbital rhabdomyosarcoma and progressively severe H1N1-related ARDS after pneumonia which did not respond to antibiotics, oseltamivir, steroids, and various modes of ventilatory support [non-invasive, conventional, and high-frequency oscillatory ventilation (HFOV)]. The patient was admitted to the paediatric intensive care unit with respiratory failure and shock. A rapid deterioration in compliance and blood gases was observed after admission, and after 5 days of mechanical ventilation, the patient presented bilateral chest infiltrates, a PaO2/F I O2 ratio of 10.8 kPa, and an oxygenation index of 28 requiring a potentially deleterious high mean airway pressure (25–30 mm Hg) on HFOV to achieve a peripheral oxygen saturation of 86%. The lung injury score was 3.75 (being 4 the maximal severity score), and ECMO was not used due to a low platelet count (35 000 mm−3). The probability of death was estimated to be 70%.7 On the basis of a positive experience in an adult late ARDS,8 and after obtaining informed consent from the relatives, nebulized aPC 1 mg was given over 30 min every 2 h. The use of nebulized aPC in the most severe cases of ARDS (in extremis) is approved by our institution. A progressive improvement in the ventilatory variables was observed (Fig. 1 ), and the trachea was extubated on day 5 after starting aPC nebulization, and the patient transferred to the ward 1 week later. The drug was administered for 4.5 days (total amount of 54 mg in 54 doses), with no evidence of haemorrhagic complications despite the low platelet count. Any beneficial effect of aPC may have been due to local or systemic actions. We consider that systemic effects are unlikely in this case, as no benefits were observed after i.v. aPC in a previous acute lung injury study,9 and a lower dose of aPC than the recommended one by i.v. route was used. On the basis of this case and a previous experience,8 we hypothesize that nebulized aPC may be a useful therapy in severe ARDS, although formal clinical studies are required to establish this.
Fig 1.
Time course of ventilatory variables. CMV-PC, controlled mechanical ventilation-pressure controlled; HFOV, high-frequency oscillatory ventilation; CMV-PS, controlled mechanical ventilation-pressure support; aPC, activated protein C; OI, oxygenation index; PBW, predicted body weight.
Conflict of interest
Dr Pestaña has received lecture fees from Eli Lilly. Dr de la Oliva declares no confict of interest.
References
- 1.Altmann M, Fiebig L, Sokya J, von Kries R, Dehnert M, Haas W. Severe cases of pandemic (H1N1) 2009 in children, Germany. Emerg Infect Dis. 2011;17:186–192. doi: 10.3201/eid1702.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martín-Loeches I, Lisboa T, Rhodes A, et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med. 2011;37:272–283. doi: 10.1007/s00134-010-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meduri GU, Marik PE, Chrousos GP, et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 5.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomized controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu KD, Looney MR, Matthay MA. Inhaled activated protein C: a novel therapy for acute lung injury? Crit Care. 2009;13:150. doi: 10.1186/cc7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold JH, Anas NG, Luckett P, et al. High-frequency oscillatory ventilation in pediatric respiratory failure: a multicenter experience. Crit Care Med. 2000;28:3913–3919. doi: 10.1097/00003246-200012000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Pestaña D, de Blas M, Somoza C, Royo C. Activated protein C nebulization in severe late acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183:280. doi: 10.1164/ajrccm.183.2.280. [DOI] [PubMed] [Google Scholar]
- 9.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]