Abstract
Microbial cell factories for terpenoid synthesis form a less expensive and more environment-friendly approach than chemical synthesis and extraction, and are thus being regarded as mainstream research recently. Organelle compartmentalization for terpenoid synthesis has received much attention from researchers owing to the diverse physiochemical characteristics of organelles. In this review, we first systematically summarized various compartmentalization strategies utilized in terpenoid production, mainly plant terpenoids, which can provide catalytic reactions with sufficient intermediates and a suitable environment, while bypassing competing metabolic pathways. In addition, because of the limited storage capacity of cells, strategies used for the expansion of specific organelle membranes were discussed. Next, transporter engineering strategies to overcome the cytotoxic effects of terpenoid accumulation were analyzed. Finally, we discussed the future perspectives of compartmentalization and transporter engineering strategies, with the hope of providing theoretical guidance for designing and constructing cell factories for the purpose of terpenoid production.
Keywords: Terpenoids, Compartmentalization, Transporter, Metabolic engineering
Introduction
Terpenoids are the largest and most diverse class of natural products, with over 80,000 different structures identified in plants, microorganisms, and marine organisms [1, 2]. All terpenoids are derived from the isoprene (C5) unit isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). Terpenoids are classified as monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes (C > 40) based on the number of isoprene units [3]. For instance, menthol (monoterpene) is one of the most important flavoring additives [4], artemisinic acid (sesquiterpene) is a well-known antimalarial drug [5], paclitaxel (diterpene) can be used as an anticancer drug [6], ginsenoside (triterpene) can inhibit the growth of tumor cells [7], and lycopene (tetraterpene) has a high antioxidant potential [8]. Terpenoids have been extensively applied in pharmaceutical and industrial sectors for decades owing to their diverse biological activities and high bioavailability, thereby resulting in their large market demand.
The two primary methods for obtaining plant terpenoids are plant extraction and chemical synthesis [9]. However, because the terpenoid concentration is low in plants and other problems, such as long cycles and environmental dependence on plant culture, scaling up the production of plant extraction is challenging [10, 11]. While the multiple stereocenters of most terpenoids complicate product synthesis and separation in the traditional chemical synthesis [12], this approach is also accompanied by the use of several organic reagents, which can exert a strain on the environment. To avoid the aforementioned problems, the development of microbial cell factories for efficient terpenoid synthesis has emerged as an important research direction for the sustainable production of terpenoids because microbial cell factories provide benefits of low cost, environmental friendliness, and high production efficiency [13, 14].
Terpenoid production by fermentation of microbial chassis has achieved remarkable results in recent years because of the rapid development of metabolic engineering and synthetic biology [13, 15]. Paddon et al., for instance, developed a strain of Saccharomyces cerevisiae for high-yielding biological production of artemisinic acid, with a fermentation titer of 25 g/L, which met commercialization, by providing the complete biosynthetic pathway and optimizing the fermentation process [16]. Wang et al. successfully constructed a chassis cell with a high yield of ginsenoside aglycone protopanaxadiol through modular engineering of the mevalonic acid (MVA) pathway and optimizing the P450 enzyme expression levels. The production of ginsenoside Rh2 was found to substantially increased, with 179.3 mg/L in shake flakes and 2.25 g/L in 10-L fed-batch fermentation, when combined with the regulation strategy of increasing glycosylation modification [11]. Currently, some researchers have suggested that in addition to enhancing the synthesis pathway [17, 18], rewriting the central carbon metabolism [19], balancing the competition pathway [20, 21], and heterologously expressing cytochrome P450 enzymes may be effective ways for terpenoid synthesis [22, 23]. However, apart from the traditional metabolic strategies, researchers now found that making full use of organelles in microorganisms may provide new insights to further improve the yield of terpenoid, such as increasing the storage capacity [24, 25].
This review focuses on the strategies for improving storage capacity and compartmentalization regulation for plant terpenoid synthesis using microbial cell factories. The physiological properties of various organelles, in addition to their benefits and drawbacks for compartmentalization, were thoroughly analyzed. The efflux pumps or secretion strategies to export terpenoids were also reported. Finally, the future perspectives and challenges associated with compartmentalization and transporter engineering strategies were thoroughly discussed.
Compartmentalization strategies for effective synthesis of terpenoids
Eukaryotes can synthesize and store terpenoids via a complete and orderly production line depending on the various organelles and membrane structures they possess such as the endoplasmic reticulum (ER), Golgi complex, lipid droplets (LDs), peroxisomes, mitochondria, and plasma membrane (PM) [26]. However, terpenoid accumulation has the potential to cause cell toxicity, whereas the compartmentalization strategy can not only improve the catalytic efficiency of enzymes but also avoid the harmful effects of toxic substances on cells [26–28]. As a result, studies are being conducted for immobilizing the enzymes required for terpenoid synthesis in various organelle compartments.
ER engineering
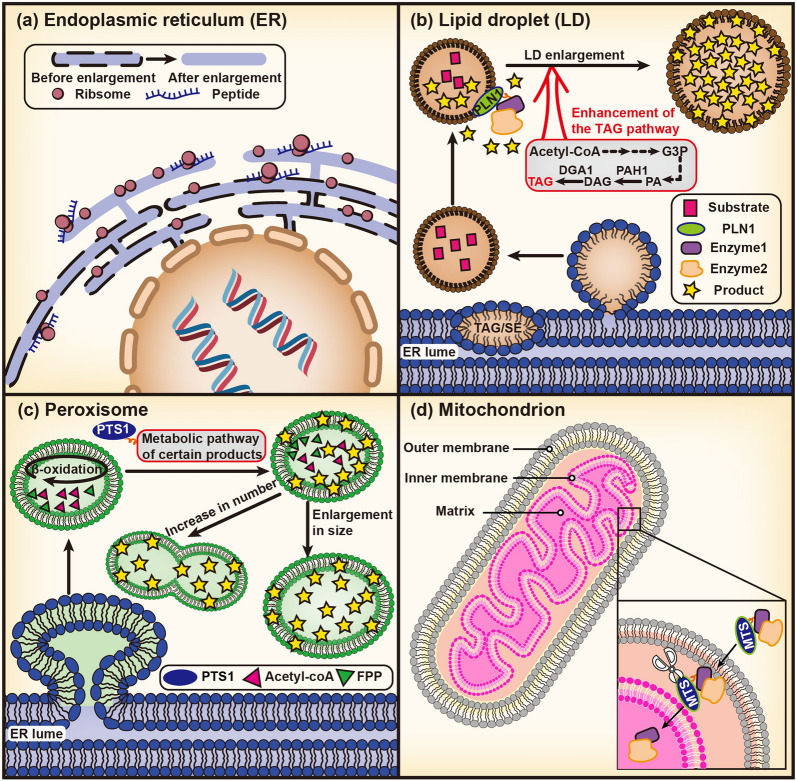
Other than nucleic acids, ER serves as the base for the synthesis of a series of important substances in cells [29]. ER is speculated to have a crucial role in terpenoid synthesis either in the form of enzyme synthesis and processing or specific ER localization of some enzymes such as key enzymes in the MVA pathway and cytochrome P450 [30]. Thus, ER engineering is a focus of research to improve the efficiency of terpenoid synthesis. Recently, ER engineering has primarily focused on increasing the ER size (Fig. 1a) [31].
Fig. 1.
a ER, b LD, c peroxisome and d mitochondrion used for compartmentalization strategies. G3P: glycerol-3-phosphate; PA: phosphatidic acid; DAG: diacylglycerol; TAG: triacylglycerol; SE: sterol esters; PLN1: perilipin; PAH1: phosphatidic acid phosphatase; DGA1: diacylglycerol acyltransferase
After deleting the phosphatidic acid phosphatase-encoding PAH1 in S. cerevisiae and Yarrowia lipolytica, a dramatic expansion in ER was observed (Table 1) [32, 33]. The massive proliferation of ER membranes is frequently followed by improvement in their ability to synthesize and fold protein, as well as an increase in protein accumulation levels [34, 35]. For instance, the S. cerevisiae strain with disrupted PAH1 expression could accumulate more Glycyrrhiza glabra β-amyrin synthase (GgbAS), and the accumulation of triterpenoids, including oleanane-type sapogenin β-amyrin, and medicagenic-28-O-glucoside, respectively increased by 8-fold and 16-fold in the Δpah1 strain compared with the control strain [33]. Notably, PAH1 knockout tends to reduce the number of LDs [36], and some key enzymes, such as the oxidosqualene cyclases (OSCs), usually require to be positioned in LDs so that they may function normally. As a result, the trade-off between ER and LDs must be considered [37, 38]. Overexpression of the key ER size regulatory factor INO2 causes ER expansion and a dramatic boost in the yield of squalene and ginsenoside by 128 times and 7 times, respectively [34, 35].
Table 1.
Terpenoid production using different organelle compartmentalization strategies
| Organelle | Yeast species | Products | Titer or yield | Major engineering strategies | References |
|---|---|---|---|---|---|
| Endoplasmic reticulum | S. cerevisiae | β-amyrin | N.A. | Knock out PAH1 | [33] |
| Aedicagenic-28-O-glucoside | 27.1 mg/L | Knock out PAH1 | [33] | ||
| Artemisinic acid | N.A. | Knock out PAH1 | [33] | ||
| Squalene | 634 mg/L | Overexpression of INO2 | [35] | ||
| Ginsenoside | 12.1 mg/L | Overexpression of INO2 | [35] | ||
| Lipid droplet | Y. Lipolytica | Lycopene | 16 mg/g | Strengthen the isoprenoid biosynthesis pathway and block the β-oxidation pathway | [47] |
| Squalene | 731.18 mg/L | Co-overexpression of tHMG1 and DGA1 | [53] | ||
| S. cerevisiae | Squalene | 445.6 mg/L | Co-overexpression of tHMG1 and DGA1 | [54] | |
| Lycopene |
2.37 g/L (73.3 mg/g) |
Strengthen the TAG pathway and modulate TAG fatty acyl composition | [49] | ||
| Ginsenoside | 5 g/L | Target protopanaxadiol synthase to LDs and strengthen the TAG pathway | [44] | ||
| α-amyrin | 1107.9 mg/L | Semi-rational design of MdOSC1, strengthen the MVA pathway and overexpress DGA1 | [48] | ||
| Peroxisome | P. pastoris | Lycopene | 73.9 mg/L | Target heterologous carotenogenic enzymes to peroxisomes | [74] |
| α-humulene | 3.2 g/L | Introduce the α-humulene synthesis pathway to peroxisomes | [77] | ||
| S. cerevisiae | Squalene | 11 g/L | Hybridization of the cytoplasm- and peroxisome-engineered strain | [63] | |
| Geraniol | 2.75 mg/L | Deletion of PEX30 and PEX32 and introduce the geraniol synthesis pathway into peroxisomes | [72] | ||
| 5.5 g/L | Introduce a complete MVA pathway in peroxisomes | [75] | |||
| (R)-(+)-limonene | 2.6 g/L | Introduce a complete MVA pathway in peroxisomes | [75] | ||
| Protopanaxadiol | N.A. | Knock out PEX11, PEX34, and ATG36 | [73] | ||
| α-humulene | 1726.78 mg/L | Introduce the α-humulene biosynthesis pathway into peroxisomes and block the expression of ERG9 | [76] | ||
| β-Amyrin | 2.6 g/L | Introduce the MVA pathway into peroxisomes | [78] | ||
| Mitochondrion | S. cerevisiae | Valencene | 1.5 mg/L | Co-overexpression of tHMG1, mitochondrion-targeted heterologous FDP synthase and amorphadiene synthase | [84] |
| Amorphadiene | 20 mg/L | Co-overexpression of tHMG1, mitochondrion-targeted heterologous FDP synthase and amorphadiene synthase | [84] | ||
| Amorpha-4,11-diene | 427 mg/L | Introduce the amorpha-4,11-diene biosynthesis pathway to mitochondria | [86] | ||
| Linalool | 21 mg/L | Dual mevalonate pathways in mitochondria and cytoplasm | [88] | ||
| Geraniol | 43.3 mg/L | Introduce the geraniol biosynthetic pathway into mitochondria | [89] | ||
| Patchoulol | 19.24 mg/L | Introduce the DMAPP pathway into mitochondria | [90] | ||
| Isoprene | 2527 mg/L | Introduce the complete MVA pathway together with isoprene synthase (ISPS) into mitochondria | [91] | ||
| 11.9 g/L | Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization | [92] | |||
| Plasma membrane | S. cerevisiae | β-Ionone |
184 mg/L (32 mg/g) |
Target the β-carotene cleavage dioxygenase to the membrane | [95] |
| E. coli | Astaxanthin | N.A. | Target CrtW and CrtZ to the membrane via a GlpF protein | [94] | |
| Squalene | 612 mg/L | Overexpression of TSR to expand membrane volume | [93] | ||
| β-carotene | 44.2 mg/g DCW | Overexpression of ALMGS and PLSB/PLSC to increase membrane surface area and enhance membrane synthesis | [24] |
LD engineering
LDs are ER-derived organelles that serve as the primary storage sites for neutral lipids in cells. The LD core is composed of neutral lipids, primarily including triacylglycerols (TAGs) and sterol esters (SEs) [39]. A single-layer phospholipid membrane containing various proteins involved in the biogenesis and function of the organelle surrounds the core [40]. The co-accumulation and co-occurrence of terpenoids and neutral lipids in LDs not only promote further elucidation of the isolation mechanism of the bioactive defense compounds intracellularly, but also provides an opportunity for metabolic engineering and synthetic biology to engineer the high-yield production and storage of terpenoids in the cells with LDs [41–43].
When enzymes and their substrates are compartmentalized, the transformation ability of engineering strains may be extremely low [44]. Anchoring the key enzymes of distinct biosynthetic steps on the surface or inside LDs promotes increased local concentrations of enzymes and hydrophobic substrates, which result in the efficient production of terpenoids. Indeed, the ectopic expression of enzymes in LDs is inextricably linked to the specific localization proteins or corresponding localization peptides, such as PLN1, which is involved in the formation and stability of LDs [45]. The conversion of dammarenediol-II into protopanaxadiol by a normal ER-localized cytochrome P450 enzyme (protopanaxadiol synthase) is a key step in the synthesis pathway of ginsenoside. Given that LDs are the storage organelles of dammarenediol-II, Shi et al. directed protopanaxadiol synthase to LDs using yeast PLN1 protein as the guide protein to obtain a chassis strain. The production of ginsenoside compound K in engineering a chassis strain was 21.8 mg/L/OD, which was nearly 4.4-fold higher than that using the native ER expression strategy [44, 46].
Another key factor in enhancing the ability of cells to synthesize terpenoids is the storage capacity of LDs. Cytosolic LDs are dynamic organelles that vary in size and morphology, and some small LDs can merge to form larger LDs. As the primary factor for TAG synthesis in the oleaginous yeast Y. lipolytica, the expression of diacylglycerol acyltransferases (DGATs) influences the size, quantity, and even distribution of LDs [43]. Overexpression of the DGAT gene YlDGA1 causes Y. lipolytica cells to produce smaller but more numerous LDs, whereas the overexpression of YlDGA2 (also a DGAT gene) results in the production of larger LDs [39]. Furthermore, deletion of GUT2 and POX1–POX6 in Y. lipolytica increases the size of LDs, because the deletion of GUT2 can prevent the reduction of the glycerol-3-phosphate pool, whereas deletion of the latter cuts peroxisomal β-oxidation short (Fig. 1b) [47].
Using lycopene synthesis as an example, Matthäus et al. found that increased LDs formation by Y. lipolytica could improve the storage capacity of cells for lycopene and subsequently improve lycopene synthesis, with an yield of 16 mg/g cell dry weight (CDW) [47]. Similarly, by overexpressing diacylglycerol acyltransferase (DGA1) to increase the intracellular storage capacity of S. cerevisiae, along with increased expression of key genes of the MVA pathway, the fermentation yield of α-amyrin in the engineering strain was found to be 106 times higher than that in the control strain [48]. Furthermore, the lycopene yield in S. cerevisiae strains overexpressing fatty acid desaturase (OLE1) and knocking out seipin (FLD1), which regulates the size of LDs, reached 70.5 mg/g CDW, which was 25% higher than that of the original high-yield strain [49].
The hydrophobic environment within LDs makes them excellent storage organelles for terpenoids. As a result, LD compartmentalization has recently become the focus of research for improving the yield of terpenoids [38]. As more clarity is gained on the mechanism underlying LD formation, mining other ways to manipulate the number and size of LDs other than enhancing the TAG pathway can potentially provide a new basis for further improvement of the terpenoid yield [50–54].
Peroxisome engineering
Peroxisomes are the primary organelles enclosed within a single bilayer membrane, which have an important role in cell detoxification. Peroxisomes can be generated using two pathways: “division” and “regeneration.” In case of division, mature peroxisomes divide to produce offspring peroxisomes [55–57]. Regeneration is a relatively complex process that involves three successive processes: budding from the ER membrane to form precursor membrane vesicles, forming the prototype of the peroxidase body, and finally generating a mature peroxidase body [56]. Peroxisomes have long been associated with fatty acid catabolism, particularly in yeast, because they are the only sites where fatty acid β-oxidation occurs, which implies that this organelle can accommodate hydrophobic chemicals [58–61]. Peroxisomes can also simultaneously produce farnesyl diphosphate (FPP), an important metabolic precursor in isoprenoid biosynthesis, which further suggests that peroxisomes are central to terpenoid synthesis [62].
Peroxisomes can be used as subcellular factories or storage depots for various terpenoids, particularly those in non-oleophilic yeasts, such as S. cerevisiae, which lack lipid storage space [55, 63–65]. Peroxisome size and number, similar to LDs, can be regulated in a dynamic manner to optimize terpenoid production [66–68]. Modulating the expression of peroxin (PEX) and autophagy (ATG) protein family members, which are responsible for peroxisome biogenesis and pexophagy, respectively in S. cerevisiae, may increase the peroxisome proliferation (Fig. 1c) [56, 57, 69–71]. The co-knockout of PEX30 and PEX31, for instance, resulted in a 5.6-fold increase in the peroxisome number [72]. Thus, by improving the tolerance of yeast cells to geraniol and compartmentalizing the geraniol-producing enzymes into peroxisomes, the titer of geraniol was found to increase by 80% [72]. Similar results were also observed in the protopanaxadiol-producing strain [73]. Furthermore, using peroxisome targeting sequence 1, the non-carotenogenic yeast Pichia pastoris was able to produce 73.9 mg/L lycopene by targeting heterologous carotene-producing enzymes to peroxisomes [74]. Similar strategies have been used in the biosynthesis of various monoterpenes [75], sesquiterpenes, including α-humulene [76, 77], and triterpenoids, including squalene [63], β-amyrin [78], and protopanaxadiol [73].
Mitochondrion engineering
Unlike monolayers of LDs and peroxisomes, mitochondria are semi-autonomous organelles surrounded by two layers of membrane, with their inner membrane forming mutliple ridges, which can gradually release energy through respiration to meet the requirements of various cell activities. Because acetyl-CoA is an important precursor for terpenoid synthesis, mitochondria have a much higher acetyl-CoA content (nearly 20–30 times higher) than the cytoplasm, which increases the feasibility of mitochondrial compartmentalization for terpenoid synthesis [79, 80]. However, IPP and DMAPP, which are synthesized from acetyl-CoA via the MVA pathway, are ATP analogs that are strong inhibitors of the mitochondrial respiratory chain. As a result, when the MVA pathway is integrated into the mitochondria, care should be taken to effectively avoid cytotoxicity [81–83].
Rational utilization of FPP pool is a possible strategy for enhancing the production of exogenous terpenoids, and the presence of FPP pools is another advantage of yeast mitochondria [84]. Heterologous FPP synthase and valencene or amorphadiene synthase were targeted into mitochondria using mitochondrial targeting signals (MTSs) and expressed in the S. cerevisiae strain with a truncated and deregulated HMG1, resulting in an 8- and 20-fold increase in the production of valencene and amorphadiene, respectively [84]. The selectivity and applicability of MTSs are frequently associated with the range of mitochondrial compartmentalization applications. At present, the most commonly utilized MTS is the pre-sequence of yeast cytochrome c oxidase subunit IV, which can direct various enzymes into the mitochondrial matrix, both in vitro and in vivo [85]. Of note, once the enzyme has been appropriately transposed into mitochondria, the MTS can be cleaved to avoid the effect of fusion expression on enzyme activity (Fig. 1d) [84, 86].
Mining and screening of more efficient MTSs has been conducted to expand the application of mitochondrial compartmentalization engineering for multigene biosynthetic pathways. Based on the mitochondrial proteome, 6 MTSs were screened for the co-localization of α-santalene synthesis pathway, which consists of 10 expression cassettes capable of converting acetyl-CoA to α-santalene, and the results showed that the production of α-santalene increased by 3.7 times in comparison with the control strain [87]. Indeed, as a promising subcellular organelle for compartmentalization engineering, mitochondria merit further investigation by researchers [88–92].
PM engineering
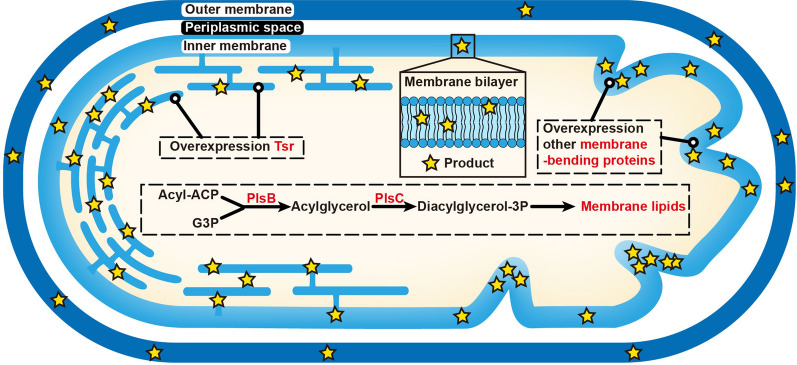
In addition to other organelles with membrane structures, the PM composed of phospholipid bilayers has piqued the interest of those focused on terpenoid research owing to its ability to store lipophilic molecules. PM engineering has received much attention particularly in the case of prokaryotic microorganisms that lack organelles. The combination of engineering membrane morphology and improving the membrane synthesis pathway could result in a 2.9-fold increase in β-carotene production in Escherichia coli [24]. Similarly, overexpression of the serine chemoreceptor Tsr in E. coli to wrinkle the inner membrane inward (also can expand the cell membrane) considerably increases the production of squalene synthesis, which is nearly 2.25 times that of the control strain (Fig. 2) [93]. Furthermore, localizing the enzymes close to their substrates within the PM can further improve catalytic efficiency. For instance, using membrane-anchoring peptides or proteins fused to β-carotene cleavage dioxygenase can increase the production of β-ionone [94, 95].
Fig. 2.
Schematic representation of membrane engineering strategies using E. coli as an example. The overexpression of membrane-bending proteins and the enhancement of membrane lipids will lead to membrane expansion for more terpenoid storage. G3P: glycerol-3-phosphate; Diacylglycerol-3P: diacylglycerol-3-phosphate; Tsr: chemotaxis receptor protein; PlsB: glycerol-3-phosphate acyltransferase; PlsC: 1-acylglycerol- phosphate acyltransferase
However, excessive hydrophobic molecules in the PM would reduce fluidity and stiffen the cell membrane, thereby causing cellular stress [43, 96, 97]. Increasing the proportion of unsaturated fatty acids in the PM could be a breakthrough in resolving this problem [98, 99].
Transport system engineering strategies for effective synthesis of terpenoids
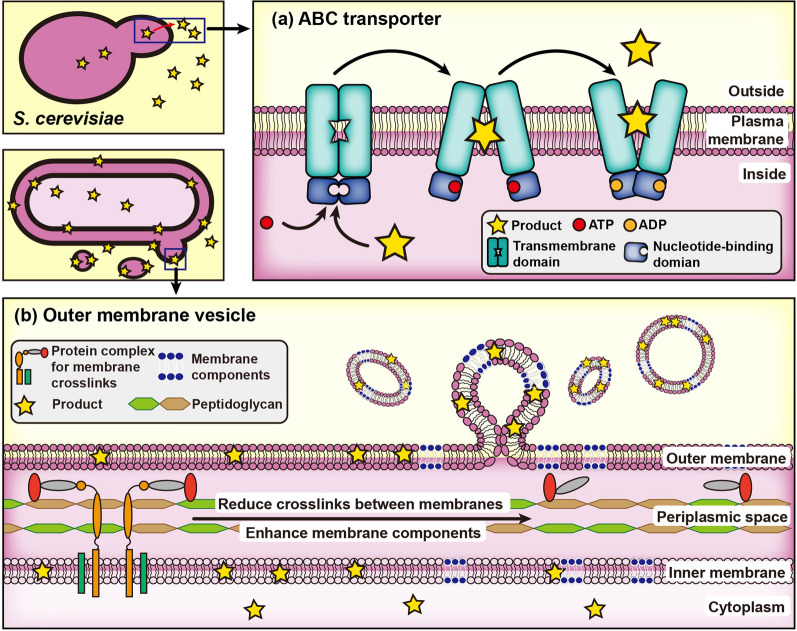
Higher titers may still be difficult to achieve if continuous terpenoid accumulation is noted in cells because of the limited storage capacity and their toxicity to organelles, proteins, DNA, and other biological processes [100, 101]. As a result, engineering efflux pumps to export terpenoids and thus reduce terpenoid cytotoxicity may be a promising solution [102, 103]. This would simultaneously assist in the recovery of the target products [104]. As typical efflux pumps, most ATP-binding cassette (ABC) transporters in the PM can identify and transport hydrophobic compounds [105–107].
Terpenoids and ABC transporters are correlated because they are both hydrophobic compounds. ABC transporter expression levels are higher in the strains that can efficiently synthesize terpenoids [108–110]. When ABC transporters are overexpressed, the synthesis of some terpenoids increases. For instance, overexpression of ABC transporter SNQ2 resulted in a 4.04-fold and a 1.33-fold increase in β‑carotene secretion and intracellular production, respectively [111]. Moreover, increasing ATP supply or improving membrane fluidity can further increase the production of β-carotene, which is likely because ABC transporters are a class of membrane proteins driven by ATP (Fig. 3a) [112].
Fig. 3.
Schematic representation of transport system engineering strategies using S. cerevisiae and E. coli as an example. a Using ABC transporters in S. cerevisiae for terpenoid secretion. b Engineering E. coli cells to produce more outer membrane vesicles by reducing the crosslinks between the inner and outer membrane, and enhancing certain membrane components
Besides a natural protein-based transport system, a novel artificial transport system based on membrane lipids, such as the outer membrane vesicles of E. coli, has initially been beneficial in terms of contributing to carrying and transporting terpenoids [113]. The amount of β-carotene secreted by the recombinant E. coli strain increased 53 times by promoting the formation of outer membrane vesicles and strengthening the synthesis of phosphatidylethanolamine to compensate for the loss of cell membrane components (caused by the formation of outer membrane vesicles), which indicated that this strategy provides a new direction for the extracellular transport of terpenoids (Fig. 3b) [113].
Conclusion and perspectives
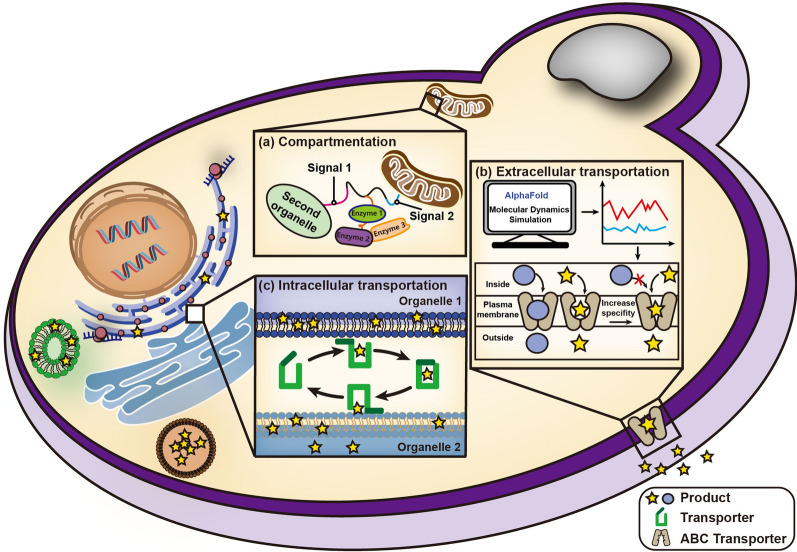
Rationally dividing the terpenoid synthesis pathway by anchoring key enzymes to appropriate organelles can increase the concentration of key enzymes and intermediates, which boosts the efficiency of compartmentalized pathway. Although the endomembrane system of certain eukaryotes has long been studied, new phenomena that provide a better insight into this system have emerged [114]. The interrelationships between organelles also deserve further investigation [115]. Simultaneously, additional research has provided a new theoretical basis for compartmentalization. For instance, the discovery of some dual-localization targeting signals may provide additional ideas and methods for enzyme localization (Fig. 4a) [84, 116–118]. As a result, the application of compartmentalization in terpenoid production remains a viable option. Another bottleneck that needs to be broken to increase terpenoid production is the limited storage capacity. However, it is unclear whether the expansion of organelle membranes will result in disorder between organelles or a stress response [119]. Apart from intracellular compartmentalization, taking advantage of constructing and optimizing the expression system and pathway module in parallel, intercellular compartmentalization can make full use of different biochemical characterizations of hosts and has also been applied in plant terpenoid production recently, such as oxygenated taxanes and strigolactones [120, 121]. Notably, this strategy requires that intermediate metabolites can cross cell membrane. Also, the difference in the doubling time of different hosts makes it particularly important to optimize culture conditions to synthesize more target products. Intercellular compartmentalization provides a promising strategy for complex plant terpenoids, especially when more functional plant-derived proteins are needed to be expressed.
Fig. 4.
Future prospects of strategies that can effectively promote terpenoid synthesis using S. cerevisiae as an example. a Peptides with dual-localization may target enzyme complex to two different organelles. b Using methods like AlphaFold and molecular dynamics simulation for protein engineering to increase the substrate specificity of ABC transporters. c Mining terpenoid transporters to accelerate intracellular transportation
While studies are now focusing more on using ABC transporters to secrete terpenoids to eliminate the toxicity caused by terpenoid accumulation, research that has focused on the mechanism underlying the transportation process is scarce. By analyzing the dynamic process of transportation using methods such as AlphaFold and molecular dynamics simulation, rational protein modification of transporters with poor substrate specificity can most likely be conducted to further improve terpenoid yield (Fig. 4b) [122]. Notably, the synthesis of terpenoids certainly passes through various organelles, such as ER, Golgi LD, and so on. To promote extracellular secretion, further enhancement of intracellular transportation can theoretically improve terpenoid synthesis to a considerable extent [123]. However, research on accelerating intracellular transportation is still in its infancy and more organelle transporters should be mined (Fig. 4c) [52].
Acknowledgements
Not applicable.
Abbreviations
- ABC
ATP-binding cassette
- CDW
Cell dry weight
- DGAT
Diacylglycerol acyltransferases
- DMAPP
Dimethylallyl diphosphate
- ER
Endoplasmic reticulum
- FPP
Farnesyl diphosphate
- GgbAS
Glycyrrhiza glabra β-amyrin synthase
- IPP
Isopentenyl diphosphate
- LD
Lipid droplet
- MTS
Mitochondrial targeting signals
- MVA
Mevalonic acid
- OSC
Oxidosqualene cyclase
- PM
Plasma membrane
- SE
Sterol ester
- TAG
Triacylglycerol
Author contributions
KJ and HX completed the collection and analysis of relevant literatures and the writing of the first draft. KJ and XL revised the manuscript. KJ, XL and LL designed the manuscript. YL, JL and GD assisted in collecting data and monitor the manuscripts. All authors contributed to the manuscript. All authors read and approved the final manuscript.
Funding
The research has been funded by the National Natural Science Foundation of China (Grant No. 32021005), the National Key R&D Program of China (Grant No. 2018YFA0900300) and the Fundamental Research Funds for the Central Universities (JUSRP52019A, JUSRP121010, JUSRP221013).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueqin Lv, Email: lvxueqin@jiangnan.edu.cn.
Long Liu, Email: longliu@jiangnan.edu.cn.
References
- 1.Pemberton TA, Chen M, Harris GG, Chou WK, Duan L, Köksal M, et al. Exploring the influence of domain architecture on the catalytic function of diterpene synthases. Biochemistry. 2017;56(14):2010–23. doi: 10.1021/acs.biochem.7b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vickers CE, Sabri S. Isoprene. Adv Biochem Eng Biotechnol. 2015;148:289–317. doi: 10.1007/10_2014_303. [DOI] [PubMed] [Google Scholar]
- 3.Chandran SS, Kealey JT, Reeves CD. Microbial production of isoprenoids. Process Biochem. 2011;46(9):1703–10. doi: 10.1016/j.procbio.2011.05.012. [DOI] [Google Scholar]
- 4.Kamatou GP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. doi: 10.1016/j.phytochem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440(7086):940–3. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 6.Skwarczynski M, Hayashi Y, Kiso Y. Paclitaxel prodrugs: toward smarter delivery of anticancer agents. J Med Chem. 2006;49(25):7253–69. doi: 10.1021/jm0602155. [DOI] [PubMed] [Google Scholar]
- 7.Kim TJ, Kim HJ, Kang M, Cho JH, Kim YG, Lee SM, et al. Ginsenoside F2 induces cellular toxicity to glioblastoma through the impairment of mitochondrial function. Phytomedicine. 2021;83:153483. doi: 10.1016/j.phymed.2021.153483. [DOI] [PubMed] [Google Scholar]
- 8.Kim TJ, Kim HJ, Kang M, Cho JH, Kim YG, Lee SM, et al. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018;245:1148–53. doi: 10.1016/j.foodchem.2017.10.116. [DOI] [PubMed] [Google Scholar]
- 9.Quílez del Moral JF, Pérez Á, Barrero AF. Chemical synthesis of terpenoids with participation of cyclizations plus rearrangements of carbocations: a current overview. Phytochem. Rev. 2020;19:559–76. doi: 10.1007/s11101-019-09646-8. [DOI] [Google Scholar]
- 10.Zebec Z, Wilkes J, Jervis AJ, Scrutton NS, Takano E, Breitling R. Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr Opin Chem Biol. 2016;34:37–43. doi: 10.1016/j.cbpa.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Wei W, Ye W, Li X, Zhao W, Yang C, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019;5:5. doi: 10.1038/s41421-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hegazy MF, Elshamy AI, Mohamed TA, Hussien TA, Helaly SE, Abdel-Azim NS, et al. Terpenoid bio-transformations and applications via cell/organ cultures: a systematic review. Crit Rev Biotechnol. 2020;40(1):64–82. doi: 10.1080/07388551.2019.1681932. [DOI] [PubMed] [Google Scholar]
- 13.Gao R, Falkeborg M, Xu X, Guo Z. Production of sophorolipids with enhanced volumetric productivity by means of high cell density fermentation. Appl Microbiol Biotechnol. 2013;97(3):1103–11. doi: 10.1007/s00253-012-4399-z. [DOI] [PubMed] [Google Scholar]
- 14.Mischko W, et al. Modular biomanufacturing for a sustainable production of terpenoid-based insect deterrents. Green Chem. 2018;20:2637–50. doi: 10.1039/C8GC00434J. [DOI] [Google Scholar]
- 15.Xu P, Bhan N, Koffas MA. Engineering plant metabolism into microbes: from systems biology to synthetic biology. Curr Opin Biotechnol. 2013;24(2):291–9. doi: 10.1016/j.copbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–32. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 17.Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci U S A. 2012;109(3):E111-8. doi: 10.1073/pnas.1110740109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schempp FM, Drummond L, Buchhaupt M, Schrader J. Microbial cell factories for the production of terpenoid flavor and fragrance compounds. J Agric Food Chem. 2018;66(10):2247–58. doi: 10.1021/acs.jafc.7b00473. [DOI] [PubMed] [Google Scholar]
- 19.Meadows AL, Hawkins KM, Tsegaye Y, Antipov E, Kim Y, Raetz L, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature. 2016;537(7622):694–7. doi: 10.1038/nature19769. [DOI] [PubMed] [Google Scholar]
- 20.McCarty NS, Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37(2):181–97. doi: 10.1016/j.tibtech.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Lee HL, Heng YC, Chua N, Teo WS, Choi WJ, et al. Synthetic biology toolkits and applications in Saccharomyces cerevisiae. Biotechnol Adv. 2018;36(7):1870–81. doi: 10.1016/j.biotechadv.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Sun W, Xue H, Liu H, Yu Y, Wang Y, Huang M, et al. Controlling chemo- and regioselectivity of a plant p450 in yeast cell toward rare licorice triterpenoid biosynthesis. ACS Catal. 2020;10(7):4253–60. doi: 10.1021/acscatal.0c00128. [DOI] [Google Scholar]
- 23.Dai Z, Liu Y, Sun Z, Wang D, Qu G, Ma X, et al. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories. Metab Eng. 2019;51:70–8. doi: 10.1016/j.ymben.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Ye L, Zhao D, Li S, Li Q, Zhang B, et al. Membrane engineering—a novel strategy to enhance the production and accumulation of β-carotene in Escherichia coli. Metab Eng. 2017;43(Pt A):85–91. doi: 10.1016/j.ymben.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Sun ZJ, Lian JZ, Zhu L, Jiang YQ, Li GS, Xue HL, et al. Combined biosynthetic pathway engineering and storage pool expansion for high-level production of ergosterol in industrial Saccharomyces cerevisiae. Front Bioeng Biotechnol. 2021;9:681666. doi: 10.3389/fbioe.2021.681666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delatte TL, Scaiola G, Molenaar J, de Sousa Farias K, Alves Gomes Albertti L, Busscher J, et al. Engineering storage capacity for volatile sesquiterpenes in Nicotiana benthamiana leaves. Plant Biotechnol. J. 2018;16(12):1997–2006. doi: 10.1111/pbi.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu S, Jiang Z, Kempinski C, Eric Nybo S, Husodo S, Williams R, et al. Engineering triterpene metabolism in tobacco. Planta. 2012;236(3):867–77. doi: 10.1007/s00425-012-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C, Kim Y, Zeng Y, Li M, Wang X, Hu C, et al. Co-compartmentation of terpene biosynthesis and storage via synthetic droplet. ACS Synth Biol. 2018;7(3):774–81. doi: 10.1021/acssynbio.7b00368. [DOI] [PubMed] [Google Scholar]
- 29.Read A, Schröder M. The unfolded protein response:an overview. Biology. 2021;10(5):384. doi: 10.3390/biology10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Z, He B, Ma L, Sun Y, Niu Y, Zeng B. Recent advances in ergosterol biosynthesis and regulation mechanisms in Saccharomyces cerevisiae. Indian J Microbiol. 2017;57(3):270–7. doi: 10.1007/s12088-017-0657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Ruijter JC, Koskela EV, Frey AD. Enhancing antibody folding and secretion by tailoring the Saccharomyces cerevisiae endoplasmic reticulum. Microb Cell Fact. 2016;15:87. doi: 10.1186/s12934-016-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerfal M, Claes K, Knittelfelder O, De Rycke R, Kohlwein SD, Callewaert N. Enhanced membrane protein expression by engineering increased intracellular membrane production. Microb Cell Fact. 2013;12:122. doi: 10.1186/1475-2859-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arendt P, Miettinen K, Pollier J, De Rycke R, Callewaert N, Goossens A. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metab Eng. 2017;40:165–75. doi: 10.1016/j.ymben.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187(4):525–36. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JE, Jang IS, Son SH, Ko YJ, Cho BK, Kim SC, et al. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab Eng. 2019;56:50–9. doi: 10.1016/j.ymben.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Milla P, Viola F, Oliaro Bosso S, Rocco F, Cattel L, Joubert BM, et al. Subcellular localization of oxidosqualene cyclases from Arabidopsis thaliana, Trypanosoma cruzi, and Pneumocystis carinii expressed in yeast. Lipids. 2002;37(37):1171–6. doi: 10.1007/s11745-002-1017-9. [DOI] [PubMed] [Google Scholar]
- 37.Adeyo O, Horn PJ, Lee S, Binns DD, Chandrahas A, Chapman KD, et al. The yeast lipin orthologue Pah1p is important for biogenesis of lipid droplets. J Cell Biol. 2011;192(6):1043–55. doi: 10.1083/jcb.201010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo XJ, Yao MD, Xiao WH, Wang Y, Zhao GR, Yuan YJ. Compartmentalized reconstitution of post-squalene pathway for 7-dehydrocholesterol overproduction in Saccharomyces cerevisiae. Front Microbiol. 2021;12:663973. doi: 10.3389/fmicb.2021.663973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajdoš P, Ledesma-Amaro R, Nicaud JM, Čertík M, Rossignol T. Overexpression of diacylglycerol acyltransferase in Yarrowia lipolytica affects lipid body size, number and distribution. FEMS Yeast Res. 2016;16(6):1–8. doi: 10.1093/femsyr/fow062. [DOI] [PubMed] [Google Scholar]
- 40.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2020;7(5):373–8. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 41.Pateraki I, Andersen-Ranberg J, Hamberger B, Heskes AM, Martens HJ, Zerbe P, et al. Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii. Plant Physiol. 2014;164(3):1222–36. doi: 10.1104/pp.113.228429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad A, Feng X, Rasool A, Sun W, Li C. Production of plant natural products through engineered Yarrowia lipolytica. Biotechnol Adv. 2020;43:07555. doi: 10.1016/j.biotechadv.2020.107555. [DOI] [PubMed] [Google Scholar]
- 43.Arhar S, Natter K. Common aspects in the engineering of yeasts for fatty acid- and isoprene-based products. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(12):158513. doi: 10.1016/j.bbalip.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Wang D, Li R, Huang L, Dai Z, Zhang X. Engineering yeast subcellular compartments for increased production of the lipophilic natural products ginsenosides. Metab Eng. 2021;67:104–11. doi: 10.1016/j.ymben.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Gao Q, Binns DD, Kinch LN, Grishin NV, Ortiz N, Chen X, et al. Pet10p is a yeast perilipin that stabilizes lipid droplets and promotes their assembly. J Cell Biol. 2017;216(10):3199–217. doi: 10.1083/jcb.201610013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Wang J, Shi Y, Li R, Fan F, Huang Y, et al. Elucidation of the complete biosynthetic pathway of the main triterpene glycosylation products of Panax notoginseng using a synthetic biology platform. Metab Eng. 2020;61:131–40. doi: 10.1016/j.ymben.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Matthäus F, Ketelhot M, Gatter M, Barth G. Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microbiol. 2014;80(5):1660–9. doi: 10.1128/AEM.03167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Rasool A, Liu H, Lv B, Chang P, Song H, et al. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab Eng. 2020;62:72–83. doi: 10.1016/j.ymben.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Ma T, Shi B, Ye Z, Li X, Liu M, Chen Y, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng. 2019;52:134–42. doi: 10.1016/j.ymben.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137–55. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klug YA, Deme JC, Corey RA, Renne MF, Stansfeld PJ, Lea SM, et al. Mechanism of lipid droplet formation by the yeast Sei1/Ldb16 Seipin complex. Nat Commun. 2021;12(1):1–13. doi: 10.1038/s41467-021-26162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan P, Smolke CD. Engineering cellular metabolite transport for biosynthesis of computationally predicted tropane alkaloid derivatives in yeast. Proc Natl Acad Sci USA. 2021;118(25):e2104460118. doi: 10.1073/pnas.2104460118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang WY, Wang DP, Tian Y, Fan X, Wang C, Lu XY, et al. Metabolic engineering of Yarrowia lipolytica for improving squalene production. Bioresour Technol. 2021;323:124652. doi: 10.1016/j.biortech.2020.124652. [DOI] [PubMed] [Google Scholar]
- 54.Wei LJ, Kwak S, Liu JJ, Lane S, Hua Q, Kweon DH, et al. Improved squalene production through increasing lipid contents in Saccharomyces cerevisiae. Biotechnol Bioeng. 2018;115(7):1793–800. doi: 10.1002/bit.26595. [DOI] [PubMed] [Google Scholar]
- 55.Joshi AS, Cohen S. Lipid droplet and peroxisome biogenesis: Do they go hand-in-hand? Front Cell Dev Biol. 2019;7:92. doi: 10.3389/fcell.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farré JC, Mahalingam SS, Proietto M, Subramani S. Peroxisome biogenesis, membrane contact sites, and quality control. EMBO Rep. 2019;20(1):e46864. doi: 10.15252/embr.201846864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tam YY, Torres-Guzman JC, Vizeacoumar FJ, Smith JJ, Marelli M, Aitchison JD, et al. Pex11-related proteins in peroxisome dynamics: a role for the novel peroxin Pex27p in controlling peroxisome size and number in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14(10):4089–102. doi: 10.1091/mbc.e03-03-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martín JF, Ullán RV, García-Estrada C. Role of peroxisomes in the biosynthesis and secretion of β-lactams and other secondary metabolites. J Ind Microbiol Biotechnol. 2012;39(3):367–82. doi: 10.1007/s10295-011-1063-z. [DOI] [PubMed] [Google Scholar]
- 59.van der Klei IJ, Yurimoto H, Sakai Y, Veenhuis M. The significance of peroxisomes in methanol metabolism in methylotrophic yeast. Biochim Biophys Acta. 2006;1763(12):1453–62. doi: 10.1016/j.bbamcr.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Zhou YJ, Buijs NA, Zhu Z, Gómez DO, Boonsombuti A, Siewers V, Nielsen J. Harnessing yeast peroxisomes for biosynthesis of fatty-acid-derived biofuels and chemicals with relieved side-pathway competition. J Am Chem Soc. 2016;138(47):15368–77. doi: 10.1021/jacs.6b07394. [DOI] [PubMed] [Google Scholar]
- 61.Huttanus HM, HM X. Compartmentalized metabolic engineering for biochemical and biofuel production. Biotechnol J. 2017;12(6):1700052. doi: 10.1002/biot.201700052. [DOI] [PubMed] [Google Scholar]
- 62.Kovacs WJ, Olivier LM, Krisans SK. Central role of peroxisomes in isoprenoid biosynthesis. Prog Lipid Res. 2002;41(5):369–91. doi: 10.1016/S0163-7827(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 63.Liu GS, Li T, Zhou W, Jiang M, Tao XY, Liu M, et al. The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction. Metab Eng. 2020;57:151–61. doi: 10.1016/j.ymben.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Zhao Z, Bai F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007;41(3):312–17. doi: 10.1016/j.enzmictec.2007.02.008. [DOI] [Google Scholar]
- 65.Ratledge C, Wynn JP. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol. 2002;51:1–51. doi: 10.1016/S0065-2164(02)51000-5. [DOI] [PubMed] [Google Scholar]
- 66.Sibirny AA. Yeast peroxisomes: structure, functions and biotechnological opportunities. FEMS Yeast Res. 2016;16(4):1–14. doi: 10.1093/femsyr/fow038. [DOI] [PubMed] [Google Scholar]
- 67.van der Klei IJ, Veenhuis M. Yeast peroxisomes: function and biogenesis of a versatile cell organelle. Trends Microbiol. 1997;5(12):502–9. doi: 10.1016/S0966-842X(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 68.Saraya R, Veenhuis M, van der Klei IJ. Peroxisomes as dynamic organelles: peroxisome abundance in yeast. FEBS J. 2010;277(16):3279–88. doi: 10.1111/j.1742-4658.2010.07740.x. [DOI] [PubMed] [Google Scholar]
- 69.Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322(5898):104–10. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tower RJ, Fagarasanu A, Aitchison JD, Rachubinski RA. The peroxin Pex34p functions with the Pex11 family of peroxisomal divisional proteins to regulate the peroxisome population in yeast. Mol Biol Cell. 2011;22(10):1727–38. doi: 10.1091/mbc.e11-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Motley AM, Nuttall JM, Hettema EH. Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae. EMBO J. 2012;31(13):2852–68. doi: 10.1038/emboj.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerke J, Frauendorf H, Schneider D, Wintergoller M, Hofmeister T, Poehlein A, et al. Production of the fragrance geraniol in peroxisomes of a product-tolerant baker’s yeast. Front Bioeng Biotechnol. 2020;8:582052. doi: 10.3389/fbioe.2020.582052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi BH, Kang HJ, Kim SC, Lee PC. Organelle engineering in yeast: enhanced production of protopanaxadiol through manipulation of peroxisome proliferation in Saccharomyces cerevisiae. Microorganisms. 2022;10(3):650. doi: 10.3390/microorganisms10030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhataya A, Schmidt-Dannert C, Lee PC. Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem. 2009;44(10):1095–102. doi: 10.1016/j.procbio.2009.05.012. [DOI] [Google Scholar]
- 75.Dusséaux S, Wajn WT, Liu Y, Ignea C, Kampranis SC. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc Natl Acad Sci U S A. 2020;117(50):31789–99. doi: 10.1073/pnas.2013968117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Li M, Zhao GR, Lu W. Harnessing yeast peroxisomes and cytosol acetyl-Coa for sesquiterpene α-humulene production. J Agric Food Chem. 2020;68(5):1382–9. doi: 10.1021/acs.jafc.9b07290. [DOI] [PubMed] [Google Scholar]
- 77.Guo Q, Shi TQ, Peng QQ, Sun XM, Ji XJ, Huang H. Harnessing Yarrowia lipolytica peroxisomes as a subcellular factory for α-humulene overproduction. J Agric Food Chem. 2021;69(46):13831–7. doi: 10.1021/acs.jafc.1c05897. [DOI] [PubMed] [Google Scholar]
- 78.Du MM, Zhu ZT, Zhang GG, Zhao YQ, Gao B, Tao XY, et al. Engineering Saccharomyces cerevisiae for hyperproduction of β-amyrin by mitigating the inhibition effect of squalene on β-amyrin synthase. J Agric Food Chem. 2022;70(1):229–37. doi: 10.1021/acs.jafc.1c06712. [DOI] [PubMed] [Google Scholar]
- 79.Duran L, López JM, Avalos JL. ¡Viva la mitochondria!: harnessing yeast mitochondria for chemical production. FEMS Yeast Res. 2020;20(6):foaa037. doi: 10.1093/femsyr/foaa037. [DOI] [PubMed] [Google Scholar]
- 80.Weinert BT, Iesmantavicius V, Moustafa T, Schölz C, Wagner SA, Magnes C, et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol Syst Biol. 2015;11(10):833. doi: 10.15252/msb.156513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu ZT, Du MM, Gao B, Tao XY, Zhao M, Ren YH, et al. Metabolic compartmentalization in yeast mitochondria: burden and solution for squalene overproduction. Metab Eng. 2021;68:232–45. doi: 10.1016/j.ymben.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Malwal SR, O’Dowd B, Feng X, Turhanen P, Shin C, Yao J. Bisphosphonate-generated ATP-analogs inhibit cell signaling pathways. J Am Chem Soc. 2018;140(24):7568–78. doi: 10.1021/jacs.8b02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147(4):437–45. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farhi M, Marhevka E, Masci T, Marcos E, Eyal Y, Ovadis M, et al. Harnessing yeast subcellular compartments for the production of plant terpenoids. Metab. Eng. 2011;13(5):474–81. doi: 10.1016/j.ymben.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 85.Maarse AC, Van Loon AP, Riezman H, Gregor I, Schatz G, Grivell LA. Subunit IV of yeast cytochrome c oxidase: cloning and nucleotide sequencing of the gene and partial amino acid sequencing of the mature protein. EMBO J. 1984;3(12):2831. doi: 10.1002/j.1460-2075.1984.tb02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yuan J, Ching CB. Mitochondrial acetyl-CoA utilization pathway for terpenoid productions. Metab. Eng. 2016;38:303–9. doi: 10.1016/j.ymben.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Dong C, Shi Z, Huang L, Zhao H, Xu Z, Lian J. Cloning and characterization of a panel of mitochondrial targeting sequences for compartmentalization engineering in Saccharomyces cerevisiae. Biotechnol Bioeng. 2021;118(11):4269–77. doi: 10.1002/bit.27896. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Wang J, Cao X, Liu W, Yu H, Ye L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzyme Microb Technol. 2020;134:109462. doi: 10.1016/j.enzmictec.2019.109462. [DOI] [PubMed] [Google Scholar]
- 89.Yee DA, DeNicola AB, Billingsley JM, Creso JG, Subrahmanyam V, Tang Y. Engineered mitochondrial production of monoterpenes in Saccharomyces cerevisiae. Metab Eng. 2019;55:76–84. doi: 10.1016/j.ymben.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tao XY, Lin YC, Wang FQ, Liu QH, Ma YS, Liu M, et al. Production of sesquiterpene patchoulol in mitochondrion-engineered Saccharomyces cerevisiae. Biotechnol Lett. 2022;44(4):571–80. doi: 10.1007/s10529-022-03240-3. [DOI] [PubMed] [Google Scholar]
- 91.Lv X, Wang F, Zhou P, Ye L, Xie W, Xu H, et al. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae. Nat Commun. 2016;7:12851. doi: 10.1038/ncomms12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao Z, Zhou P, Su B, Su S, Ye L, Yu H. Enhanced isoprene production by reconstruction of metabolic balance between strengthened precursor supply and improved isoprene synthase in Saccharomyces cerevisiae. ACS Synth Biol. 2018;7(9):2308–16. doi: 10.1021/acssynbio.8b00289. [DOI] [PubMed] [Google Scholar]
- 93.Meng Y, Shao X, Wang Y, Li Y, Zheng X, Wei G, et al. Extension of cell membrane boosting squalene production in the engineered Escherichia coli. Biotechnol Bioeng. 2020;117:3499–507. doi: 10.1002/bit.27511. [DOI] [PubMed] [Google Scholar]
- 94.Ye L, Zhu X, Wu T, Wang W, Zhao D, Bi C, et al. Optimizing the localization of astaxanthin enzymes for improved productivity. Biotechnol Biofuels. 2018;11:278. doi: 10.1186/s13068-018-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Werner N, Ramirez-Sarmiento CA, Agosin E. Protein engineering of carotenoid cleavage dioxygenases to optimize β-ionone biosynthesis in yeast cell factories. Food Chem. 2019;299:125089. doi: 10.1016/j.foodchem.2019.125089. [DOI] [PubMed] [Google Scholar]
- 96.Csáky Z, Garaiová M, Kodedová M, Valachovič M, Sychrová H, Hapala I. Squalene lipotoxicity in a lipid droplet-less yeast mutant is linked to plasma membrane dysfunction. Yeast. 2020;37(1):45–62. doi: 10.1002/yea.3454. [DOI] [PubMed] [Google Scholar]
- 97.Liu P, Sun L, Sun Y, Shang F, Yan G. Decreased fluidity of cell membranes causes a metal ion deficiency in recombinant Saccharomyces cerevisiae producing carotenoids. J Ind Microbiol Biotechnol. 2016;43:525–35. doi: 10.1007/s10295-015-1728-0. [DOI] [PubMed] [Google Scholar]
- 98.Hong J, Park SH, Kim S, Kim SW, Hahn JS. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl Microbiol Biotechnol. 2019;103(1):211–23. doi: 10.1007/s00253-018-9449-8. [DOI] [PubMed] [Google Scholar]
- 99.Zhang JL, Bai QY, Peng YZ, Fan J, Jin CC, Cao YX, et al. High production of triterpenoids in Yarrowia lipolytica through manipulation of lipid components. Biotechnol Biofuels. 2020;13:133. doi: 10.1186/s13068-020-01773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nicolaou SA, Gaida SM, Papoutsakis ET. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng. 2010;12:307–31. doi: 10.1016/j.ymben.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 101.Fordjour E, Mensah EO, Hao Y, Yang Y, Liu X, Li Y, et al. Toward improved terpenoids biosynthesis: strategies to enhance the capabilities of cell factories. Bioresour Bioprocess. 2022;91(9):1–33. doi: 10.1186/s40643-022-00493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406(6797):775–81. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 103.Martinez JL, Sánchez MB, Martínez-Solano L, Hernandez A, Garmendia L, Fajardo A, et al. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev. 2009;33(2):430–49. doi: 10.1111/j.1574-6976.2008.00157.x. [DOI] [PubMed] [Google Scholar]
- 104.Hara KY, Kobayashi J, Yamada R, Sasaki D, Kuriya Y, Hirono-Hara Y, et al. Transporter engineering in biomass utilization by yeast. FEMS Yeast Res. 2017 doi: 10.1093/femsyr/fox061. [DOI] [PubMed] [Google Scholar]
- 105.Jungwirth H, Kuchler K. Yeast ABC transporters—a tale of sex, stress, drugs and aging. FEBS Lett. 2006;580(4):1131–8. doi: 10.1016/j.febslet.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 106.Godinho CP, Costa R, Sá-Correia I. The ABC transporter Pdr18 is required for yeast thermotolerance due to its role in ergosterol transport and plasma membrane properties. Environ Microbiol. 2021;23(1):69–80. doi: 10.1111/1462-2920.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Claus S, Jezierska S, Van Bogaert INA. Protein-facilitated transport of hydrophobic molecules across the yeast plasma membrane. FEBS Lett. 2019;593(13):1508–27. doi: 10.1002/1873-3468.13469. [DOI] [PubMed] [Google Scholar]
- 108.Ro DK, Ouellet M, Paradise EM, Burd H, Eng D, Paddon CJ, et al. Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol. 2008;8:83. doi: 10.1186/1472-6750-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bi X, Guo N, Jin J, Liu J, Feng H, Shi J, et al. The global gene expression profile of the model fungus Saccharomyces cerevisiae induced by thymol. J Appl Microbiol. 2010;108(2):712–22. doi: 10.1111/j.1365-2672.2009.04470.x. [DOI] [PubMed] [Google Scholar]
- 110.Hu F, Liu J, Du G, Hua Z, Zhou J, Chen J. Key cytomembrane ABC transporters of Saccharomyces cerevisiae fail to improve the tolerance to d-limonene. Biotechnol Lett. 2012;348(34):1505–9. doi: 10.1007/s10529-012-0931-6. [DOI] [PubMed] [Google Scholar]
- 111.Lee JJ, Chen L, Cao B, Chen WN. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl Microbiol Biotechnol. 2016;100(2):869–77. doi: 10.1007/s00253-015-7102-3. [DOI] [PubMed] [Google Scholar]
- 112.Bu X, Lin JY, Cheng J, Yang D, Duan CQ, Koffas M, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels. 2020;13:168. doi: 10.1186/s13068-020-01809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu T, Li S, Ye L, Zhao D, Fan F, Li Q, et al. Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of β-carotene in Escherichia coli. ACS Synth Biol. 2019;8(5):1037–46. doi: 10.1021/acssynbio.8b00472. [DOI] [PubMed] [Google Scholar]
- 114.Vasdekis AE, Silverman AM, Stephanopoulos G. Exploiting bioprocessing fluctuations to elicit the mechanistics of de novo lipogenesis in Yarrowia lipolytica. PLoS ONE. 2017;12(1):e0168889. doi: 10.1371/journal.pone.0168889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joshi AS, Nebenfuehr B, Choudhary V, Satpute-Krishnan P, Levine TP, Golden A, et al. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat Commun. 2018;9(1):2940. doi: 10.1038/s41467-018-05277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ast J, Stiebler AC, Freitag J, Bölker M. Dual targeting of peroxisomal proteins. Front Physiol. 2013;4:297. doi: 10.3389/fphys.2013.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben-Menachem R, Pines O. Detection of dual targeting and dual function of mitochondrial proteins in yeast. Methods Mol Biol. 2017;1567:179–95. doi: 10.1007/978-1-4939-6824-4_11. [DOI] [PubMed] [Google Scholar]
- 118.Yogev O, Pines O. Dual targeting of mitochondrial proteins: mechanism, regulation and function. Biochim Biophys Acta. 2011;1808(3):1012–20. doi: 10.1016/j.bbamem.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 119.Kumar V, Maity S. ER stress-sensor proteins and er-mitochondrial crosstalk-signaling beyond (ER) stress response. Biomolecules. 2021;11(2):173. doi: 10.3390/biom11020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou K, Qiao K, Edgar S, Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33(4):377–83. doi: 10.1038/nbt.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu S, Ma X, Zhou A, Valenzuela A, Zhou K, Li Y. Establishment of strigolactone-producing bacterium-yeast consortium. Sci Adv. 2021;7(38):eabh4048. doi: 10.1126/sciadv.abh4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hofmann S, Januliene D, Mehdipour AR, Thomas C, Stefan E, Brüchert S, et al. Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature. 2019;571(7766):580–3. doi: 10.1038/s41586-019-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alkhadrawi AM, Xue H, Ahmad N, Akram M, Wang Y, Li C. Molecular study on the role of vacuolar transporters in glycyrrhetinic acid production in engineered Saccharomyces cerevisiae. Biochim Biophys Acta Biomembr. 2022;1864(6):183890. doi: 10.1016/j.bbamem.2022.183890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.