Abstract
Background
Social distancing measures during the Covid-19 pandemic reduced access to health care and concerns were raised over the safety of immunosuppressive disease modifying treatments (DMT) for multiple sclerosis (MS).
Objective
To investigate changes in DMT prescription before and during the pandemic in a large and well-characterized real-world cohort of MS patients.
Methods
From the Vienna MS database (VMSD) we extracted MS patients who were initiated on a new DMT (both treatment-naïve and switching) between January 1st 2017 and December 31st 2021. Two time periods were defined: 1) the preCovid-19 era (January 1st 2017 to March 15th 2020, i.e. the day of the first lockdown in Austria) and the Covid-19 era (March 16th 2020 to December 31st 2021). Average annualized DMT prescription rates were descriptively compared between the two periods.
Results
The average annualized number of prescriptions in the preCovid-19 era was 90.3/year and dropped to 74.8/year (-17.2%) in the Covid-19 era, driven by a marked reduction to 41.7/year (-54%) in the first nine months of the Covid-19 era, partly offset by a rise to 101 in 2021. Use of alemtuzumab (-64%), antiCD20 (-49%), cladribine (-46%), and S1PM (-38%) was reduced, while natalizumab increased by 24%. Lower efficacy treatments remained stable.
Conclusions
The pandemic coincides with a drop in DMT prescription, most markedly for immunosuppressive high-efficacy treatments, strongly suggesting the pandemic as the causal factor. If and how much this affects long-term outcome is yet to be determined.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2, Change, Pandemic, Disease-modifying treatment
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has not only directly affected 282 million patients infected (as by January 03rd, 2022) with 5.4 million dead from CoronaVirus-Disease 2019 (Covid-19), but also disrupted services for patients suffering from chronic disease such as multiple sclerosis (MS).
Social distancing measures caused reduced access to health care facilities and concerns were raised over the safety of immunosuppressive disease modifying treatments (DMT).
The Austrian Society of Neurology (ÖGN) issued guidance in March 2020, advising that the risk from COVID-19 may potentially be increased with alemtuzumab, cladribine, sphingosine-phosphate-1 receptor modulators (S1PM) and antiCD20 monoclonal antibodies (Austrian Society of Neurology (ÖGN), 2020). On the other hand, delay of effective DMT may negatively impact long-term outcome of patients with MS (Hartung et al., 2021).
Here, we aimed to investigate changes in DMT prescription patterns between January 2017 and December 2021 in a large and well-characterized real-world cohort.
2. Methods
To that end, we used the Vienna MS database (VMSD) and extracted MS patients who were initiated on a new DMT (both treatment-naïve and switching) in the period between January 1st 2017 and December 31st 2021 (Bsteh et al., 2021a). Two time periods were defined: (1) the preCovid-19 era (January 1st 2017 to March 15th 2020, i.e. the day of the first lockdown in Austria) and the Covid-19 era (March 16th 2020 to December 31st 2021). Average annualized rates of prescription for licensed DMTs were descriptively compared between the two periods. Subgroup analyses were conducted for treatment-naïve and switching DMT initiations.
The study was approved by the ethics committee of the Medical University of Vienna (EK Nr: EK 1338–2020). As this was a retrospective study of anonymized data obtained in clinical routine, the requirement for informed consent was waived by decision of the ethics committee.
3. Results
Overall, 424 DMT initiations (163 treatment-naïve and 261 switches) were recorded in the VMSD from January 1st 2017 to December 31st 2021. The average annualized number of prescriptions in the preCovid-19 era was 90.3 DMT prescriptions/year and was stable during this era (2017: 88; 2018: 93; 2019: 91). In the Covid-19 era, prescription rate dropped to 74.8/year (−17.2%).
This drop was driven by a marked reduction to 41.7/year (−54% compared to preCovid-19) in the first nine months of the Covid-19 era (from March 16th 2020 to December 31st 2020), which was partly offset by a rise to 101 in 2021.
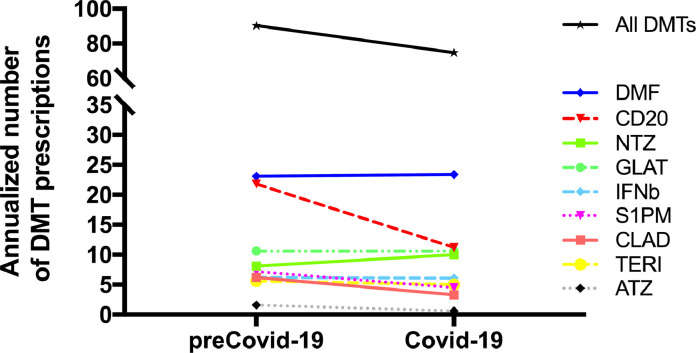
Fig. 1 displays changes in prescriptions/year of individual DMTs between the preCovid-19 and the Covid-19 era. There was a clear drop for alemtuzumab (−64%), antiCD20 (−49%), cladribine (−46%), and S1PM (−38%), while natalizumab prescription relatively increased by 24%. Lower efficacy treatments (interferon beta preparations, glatiramer acetate, dimethyl fumarate and teriflunomide) prescription remained stable. There were no remarkable differences between patterns in treatment-naïve and switching patients.
Fig. 1.
Change of annualized DMT prescription rate from the preCovid-19 to the Covid-19 era. Abbreviations: DMT: disease modifying treatment, DMF: dimethyl fumarate. CD20: B-cell depleting antiCD20 monoclonal antibodies (ocrelizumab, ofatumumab, rituximab). NTZ: natalizumab. GLAT: glatiramer acetate. IFNb: interferon beta preparations. S1PM: sphingosine-phosphate-1 receptor modulator (fingolimod, ozanimod, siponimod). CLAD: cladribine. TERI: teriflunomide. ATZ: alemtuzumab.
Placeholder Fig. 1 . Change of annualized DMT prescription rate from the preCovid-19 to the Covid-19 era.
4. Discussion
In a longitudinal assessment of DMT prescription patterns before and during the Covid-19 pandemic in a population-based cohort of MS patients, we found that the pandemic coincides with a drop in DMT prescription after being stable in earlier years. The most marked reductions were seen for immunosuppressive high-efficacy treatments, for which safety concerns were raised at the start of the pandemic, while DMTs considered safe remained stable or even increased slightly in case of natalizumab, which is the only high-efficacy option not considered systemically immunosuppressive.
The observed pattern strongly suggests the pandemic as the causal factor behind these changes in prescription. After initial concerns over the impact of immunosuppressive DMT on Covid-19 course, reassuring evidence from case series and registry studies was quickly growing that DMT do not increase the risk of severe Covid-19 or mortality with the lone exception of antiCD20 monoclonal antibodies, although data were insufficient to assess the impact of alemtuzumab (Louapre et al., 2020; Bsteh et al., 2021b; Sormani et al., 2021). In addition, the emergence of efficient vaccinations against SARS-CoV2 has changed the landscape providing the possibility of preemptive protection before DMT initiation. Accordingly, various expert consortiums including the ÖGN adjusted guidelines during 2021 recommending vaccination of MS patients before DMT start and choice of DMT with the main focus on parameters of MS course and patient characteristics such as age and comorbidities rather than on the pandemic (Austrian Society of Neurology (ÖGN), 2021). This change of approach is reflected by our data as we found an increase in DMT prescription in 2021 after the stark drop in the first nine months of the Covid era. Our data could also be interpreted as indirect evidence of an impact of the evolving ÖGN guidelines for managing MS patients during the pandemic.
The main strengths of this study are its population-based approach and the high-quality data from a certified specialized MS center. The VMSD includes most of MS patients in its geographical catchment area and is likely representative of a central European MS population (Bsteh et al., 2021a). The main limitation is that we cannot distinguish whether changes in DMT prescription were due to drug safety concerns or other factors affecting patient care such as access to specialized MSologists or diagnostics, tele-medicine or delays in treatment initiation, which may contribute to the reduction in DMT initiations during the pandemic.
Irrespective of the underlying cause, our finding of a drop in DMT prescription during the Covid era suggests an impact of the pandemic on the care for people with MS. If and how much this affects long-term outcome is yet to be determined.
Funding
There was no funding to this research.
Group information
A complete list of the members of the VMSD investigators is given at the end of this article.
VMSD investigators in alphabetical order
Altmann, Patrick; Berger, Thomas; Bsteh, Gabriel; Kornek, Barbara; Krajnc, Nik; Leutmezer, Fritz; Macher, Stefan; Monschein, Tobias; Ponleitner, Markus; Riedl, Katharina; Rinner, Walter; Rommer, Paulus; Schmied, Christiane; Zebenholzer, Karin; Zulehner, Gudrun; Zrzavy, Tobias.
CRediT authorship contribution statement
Gabriel Bsteh: Conceptualization, Visualization, Investigation, Funding acquisition, Formal analysis, Data curation, Writing – original draft, Supervision. Katharina Riedl: Investigation, Funding acquisition, Writing – review & editing. Nik Krajnc: Investigation, Funding acquisition, Writing – review & editing, Data curation. Barbara Kornek: Investigation, Funding acquisition, Writing – review & editing. Fritz Leutmezer: Investigation, Funding acquisition, Writing – review & editing. Stefan Macher: Investigation, Funding acquisition, Writing – review & editing. Paulus Rommer: Investigation, Funding acquisition, Writing – review & editing. Gudrun Zulehner: Investigation, Funding acquisition, Writing – review & editing. Thomas Berger: Conceptualization, Visualization, Investigation, Data curation, Writing – review & editing.
Declaration of Competing Interest
Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis.
Katharina Riedl: declares no conflict of interest relevant to this study.
Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from Merck, Novartis and Roche, and holds a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Barbara Kornek: has received honoraria for speaking and for consulting from Biogen, BMS-Celgene, Johnson&Johnson, Merck, Novartis, Roche, Teva and Sanofi-Genzyme outside of the submitted work. No conflict of interest with respect to the present study.
Fritz Leutmezer: has participated in meetings sponsored by, received speaker honoraria or travel funding from Actelion, Almirall, Biogen, Celgene, MedDay, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Stefan Macher: declares no conflict of interest relevant to this study.
Paulus Rommer: has received honoraria for consultancy/speaking from AbbVie, Allmiral, Alexion, Biogen, Merck, Novartis, Roche, Sandoz, Sanofi Genzyme, has received research grants from Amicus, Biogen, Merck, Roche.
Gudrun Zulehner: has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Thomas Berger: has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, BMS/Celgene, GSK, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi-Genzyme, Teva and UCB. His-institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva.
Acknowledgment
We thank all the VMSD investigators, clinical research staff, and especially the patients for helping to collect these data. The named individuals were not compensated for their help.
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna since data contain potentially sensitive information.
References
- Austrian Society of Neurology (ÖGN). Information multiple sklerose und SARS-COV2/COVID19 #1. 30.03.2020, https://www.oegn.at/covid-19/covid19-und-multiple-sklerose-ms-2/ (2020).
- Austrian Society of Neurology ÖGN. ÖGN information multiple sklerose und SARS-COV2/COVID19 #5, https://www.oegn.at/covid-19/covid19-und-multiple-sklerose-ms-2/ (2021).
- Bsteh G., Hegen H., Riedl K., et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: the VIAADISC score. Eur. J. Neurol. 2021 doi: 10.1111/ene.14705. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G., Assar H., Hegen H., et al. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: insights from a nation-wide Austrian registry. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H.P., Meuth S.G., Thompson A.J. Paradigm shifts: early initiation of high-efficacy disease-modifying treatment in multiple sclerosis. Mult. Scler. J. 2021;27:1473–1476. doi: 10.1177/13524585211033190. [DOI] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. Jama Neurol. 2020;77 doi: 10.1001/jamaneurol.2020.2581. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Rossi N.D., Schiavetti I., et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021 doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request by a qualified researcher and upon approval by the ethics committee of the Medical University Vienna since data contain potentially sensitive information.