To the editor:
Solid organ transplantation (SOT) recipients are at very high risk of developing severe coronavirus disease 2019 (COVID-19).1 Despite the implementation of a third dose of mRNA vaccine, the efficacy of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination on humoral and cellular immunities is reduced in this population,2 resulting in increased incidence of severe infection and mortality, including in fully vaccinated patients.3 In this context, monoclonal antibodies (mAbs) providing passive immunization have been developed to enhance immunity against SARS-CoV-2 in immunocompromised patients.4 The results of a very recently published randomized trial support the use of a single i.m. dose of Evusheld (tixagevimab/cilgavimab; AstraZeneca) for the prevention of symptomatic and severe COVID-19 (mainly the Alpha, Beta, and Delta strains of SARS-CoV-2), but a relatively small number of immune-suppressed patients and chronic kidney disease patients were included.5 However, although clinical data remain very scarce, several in vitro studies have suggested that mAbs may have reduced efficacy against recent variants, especially Omicron6—the effect of casirivimab-imdevimab was likely to be lost, and that of tixagevimab/cilgavimab was reduced (to an uncertain degree), which led the US Food & Drug Administration to recommend use of a higher dose.
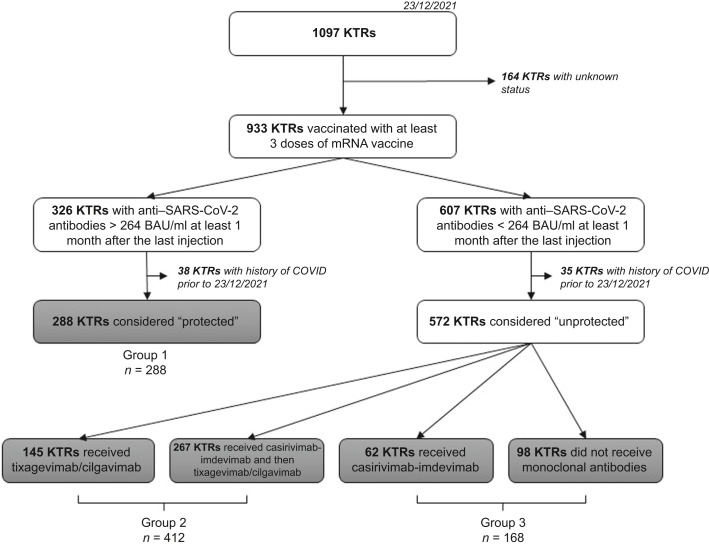
In France, prophylactic use of mAbs (Ronapreve [casirivimab-imdevimab], from September 12, 2021; Evusheld [tixagevimab/cilgavimab], from December 2021) is recommended in solid organ transplantation patients with a complete vaccine scheme and no or weak humoral response (<264 binding antibody units [BAU]/ml)7 1 month after the last injection. Here, we report the occurrence and severity of COVID-19 in 860 fully vaccinated kidney transplant recipients (KTRs) between December 23, 2021 and March 7, 2022, during the Omicron outbreak, from a single kidney transplantation center. During the study period, the BA1 variant was predominant, until February 14, 2022, and then BA2 became predominant.8 Baseline characteristics of the study population are summarized in Supplementary Table S1. All patients were instructed to systematically report potential symptoms of COVID-19 and/or the positivity of a nasopharyngeal swab for SARS-CoV-2. Outcomes were studied according to immunization status, as described in Figure 1 , as follows:
-
•
group 1: vaccine-induced immunization, 288 patients;
-
•
group 2: passive immunization with tixagevimab/cilgavimab, 412 patients. In this group, 267 KTRs received casirivimab-imdevimab as a first step of protection before receiving tixagevimab/cilgavimab. All KTRs of group 2 received 2 i.m. injections of 150 mg tixagevimab + 150 mg cilgavimab between December 23, 2021 and February 7, 2022; and
-
•
group 3: insufficient immunization, 160 patients. In this group, 62 received casirivimab-imdevimab.
Figure 1.
Flowchart. BAU, binding antibody unit; COVID, coronavirus; KTRs, kidney transplant recipients. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
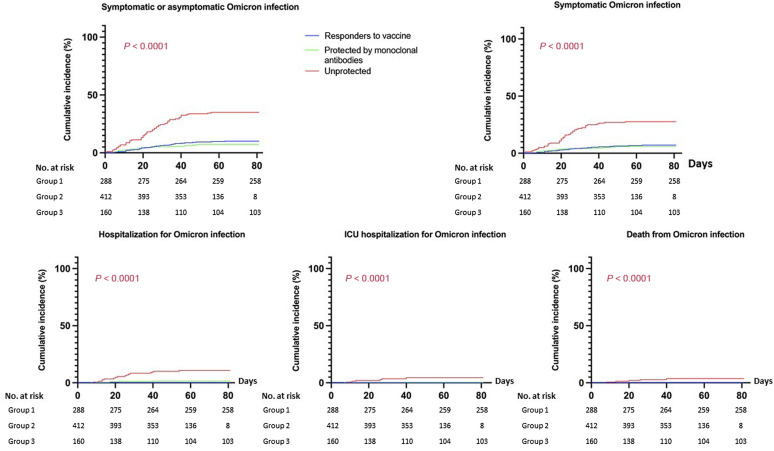
During follow-up, 113 patients (13.1%) presented an Omicron infection, of which 85 were symptomatic (from December 23, 2021 to February 14, 2022—103 cases of infection [91.2%]; from February 14, 2022 to March 7, 2022—10 cases of infection [8.8%]). Twenty-one patients required hospitalization, including 8 in the intensive care unit. Five patients died of COVID-19. The occurrence of infection, symptomatic infection, hospitalization, intensive care unit hospitalization, and COVID-19 death were significantly increased in patients in group 3 (Figure 2 ; Supplementary Table S1). Patients who received passive immunization with tixagevimab/cilgavimab had outcomes similar to those of patients with vaccine-induced immunization, but they had significantly fewer infections (both severe and nonsevere), compared to KTRs considered unprotected.
Figure 2.
Cumulative incidence of Omicron infection. Log rank test was used to compare the 3 groups, andresults were considered significant when P < 0.05. ICU, intensive-care unit.
Despite its potential bias, per the retrospective design, to our knowledge, our study shows for the first time the potential clinical usefulness of mAbs against Omicron in KTRs with weak or no response to vaccine, as a prophylaxis strategy. These results challenge the reduced efficacy of mAbs that has been shown in vitro. No serious adverse event was reported in our cohort who received tixagevimab/cilgavimab. Multicentric prospective or retrospective studies are needed to confirm these encouraging results for the protection of immunosuppressed patients against COVID-19.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the health care professionals of the University hospital of Rouen who were involved in the care of the patients, and particularly the nurse from the kidney transplant unit. We also thank Pr Manuel Etienne and Dr Damien Fuss for their help.
Author Contributions
DB designed the study. DG and DB wrote the paper. All authors provided feedback and critical review.
Footnotes
Supplementary File (PowerPoint)
Table S1. Baseline characteristics of the patients and occurrence of Omicron infection in the 3 groups. The χ2 test (nominal variables) and the t test (continuous variables) were used to compare the 3 groups; results were considered significant when P < 0.05. ∗P: comparison between groups 1 and 2. ∗∗P: comparison between groups 2 and 3. ∗∗∗P: comparison between groups 1 and 3. AZA, azathioprine; eGFR, estimated glomerular filtration rate, estimated by Modification of Diet in Renal Disease (MDRD) formula; F, female; ICU, intensive care unit; M, male; MMF, mycophenolate mofetil.
Supplementary Material
References
- 1.Caillard S., Anglicheau D., Matignon M., et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamar N., Abravanel F., Marion O., et al. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillard S., Chavarot N., Bertrand D., et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:477–479. doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien M.P., Forleo-Neto E., Musser B.J., et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin M.J., Ustianowski A., De Wit S., et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruel T., Hadjadj J., Maes P., et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat Med. 2022;28:1297–1302. doi: 10.1038/s41591-022-01792-5. [DOI] [PubMed] [Google Scholar]
- 7.Feng S., Phillips D.J., White T., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GÉODES. GÉO DONNÉES EN SANTÉ PUBLIQUE. Accessed June 9, 2022. https://geodes.santepubliquefrance.fr
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.