Abstract
Plakortinic acids A (2) and B (3), two polyketide endoperoxides with a bicyclo[4.2.0]octene unit, were isolated as minor constituents from the sponge–sponge symbiotic association Plakortis halichondrioides–Xestospongia deweerdtae, along with known epiplakinic acid F (1). The structures of the mixture of two inseparable compounds were determined by spectroscopic analysis. Screening for cytotoxic activity of the mixture against two human tumor cell lines revealed that these compounds are very active at sub-micro molar concentration.
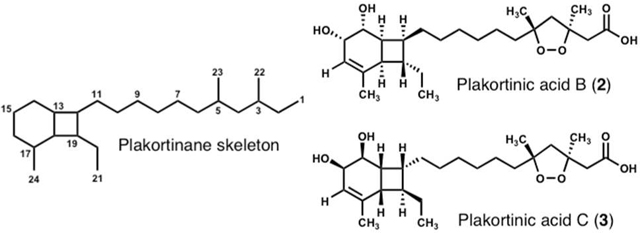
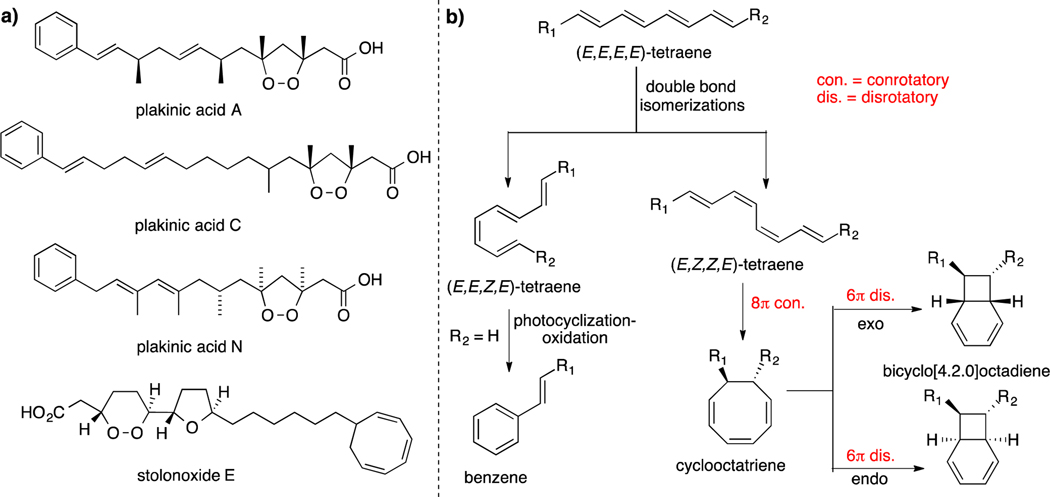
As part of our continuing efforts to identify new bioactive compounds from Caribbean marine sponges, we reinvestigated the symbiotic two-sponge association Plakortis halichondrioides–Xestospongia deweerdtae collected in Mona Island off the west coast of Puerto Rico.1 Marine sponges within the genera Plakortis and Plakinastrella represent an amazing source of cyclic peroxide-containing natural products, which in addition to their interesting biological activity, display a diverse array of molecular architectures.2 Interestingly, many sponges of the Plakinidae family, as well as other marine animals, often contain a plethora of straight- and branched-chain 1,2-dioxolane carboxylic acids of varying length that often incorporate multiple double bonds, terminal phenyl groups and, albeit rarely, cyclooctatriene rings (Figure 1a).3 This observation entails that conjugated linear polyenes are ubiquitous in many marine species.4 Indeed, previous research has demonstrated that from relatively simple (E,E,E,E)-tetraene precursors, structurally diverse scaffolds such as the bicyclo[4.2.0]octadiene core can be obtained through various modes of thermal and photochemical reactions.5 Thus, a suitable polyene could be encouraged by sunlight to undergo selective E/Z double bond isomerizations resulting in the necessary geometry for cascade electrocyclizations to ensue (Figure 1b).6 Although polyene precursors could give rise, among other complex skeletons, to compounds based on a bicyclo[4.2.0]octadiene framework, thus far this type of natural products have yet to be isolated from any Plakortis or Plakinastrella species.7 Herein, we report the re-isolation of known epiplakinic acid F (1)8 and the isolation and structure elucidation of new plakortinic acids A (2) and B (3), the first two members of a new chemical series having an unprecedented bicyclo[4.2.0]octene backbone.9
Figure 1.
a) Previously reported marine natural products arising from a plausible biosynthetic pathway involving a polyene precursor. b) Biogenetic proposals for the formation of the phenyl and bicyclo[4.2.0]octadiene motifs from a linear polyene precursor.
The sponge specimens were cut into small blocks and blended with MeOH–CHCl3. After filtration, the extract was concentrated to yield a gum that was suspended in H2O and extracted with n-hexane. After concentration, a portion of the oil obtained was chromatographed with n-hexane–acetone. Fractionation and purification of active components guided by our cytotoxicity assay resulted in the isolation of a 1:1 mixture of highly cytotoxic polyketide-derived plakortinic acids A (2) and B (3) (8.0 mg, 0.01% yield), along with known epiplakinic acid F (1, 170 mg, 0.21% yield). The latter metabolite had been isolated earlier in our laboratory while working on a different specimen of the same sponge genus.8 The absolute stereostructure of 1, which was determined by degradation reactions followed by application of Kishi’s method for the assignment of absolute configuration of alcohols, was recently confirmed by total synthesis.10 Exhaustive efforts to separate the mixture of 2 and 3 were unsuccessful in even partially resolving them. Treatment of an aliquot of the mixture of isomers with excess CH2N2 followed by repeated CC, HPLC (normal- and reversed-phase), and PTLC analyses also failed to separate the lesser polar mixture of methyl esters. These facts and the limited quantity made the structure characterization of these isomers very challenging. That notwithstanding, our otherwise analytically pure mixture of diastereomers was well suitable for structure elucidation work.11
The mixture of isomers 2 and 3 was isolated as a colorless oil that was optically active: [α]20D +28.5° (CHCl3). The ESIMS of 2 and 3 showed a single [M+Na]+ ion at m/z 447. Their molecular formulas were subsequently established as C24H40O6 on the basis of HRESIMS data (m/z 447.2726, [M+Na]+, Δ +0.3 mDa) requiring 5 unsaturations.12 The presence of hydroxyl and carboxylic acid groups was implied from the broad stretch at 3391 cm−1 and sharp band at 1714 cm−1, respectively. A cursory examination of the 13C NMR spectra (Table 1) showed 24 resolved signals that, together with the 13C DEPT-135 and HSQC NMR data, were assigned as 4 x CH3, 9 x CH2, 7 x CH, and 4 x C; thus, compounds 2 and 3 had to have 3 OH groups.13 The 1H NMR spectrum (Table 1) showed one olefinic proton (δH 5.56, d, J = 5.8 Hz), two oxymethines (δH 4.11, dd, J = 5.8, 3.3 Hz and 3.93, dd, J = 9.7, 3.3 Hz), three methyl singlets (δH 1.65, 1.46, and 1.29), and one methyl triplet (δH 0.91, t, J = 7.5 Hz). Some of these proton signals and those later ascribed to H2-2 and H2-4 were consistent with a 1,2-dioxolane bearing two methyl groups at C-3 and C-5 and an acetic group at C-3. All of the C–H correlations within 2 and 3 were established from a 1H–13C HSQC experiment. HMBC cross-peaks of H2-2 with C-1, C-3, and C-22 and of H2-4 with C-3, C-5, C-6, C-22, and C-23, confirmed that 2 and 3 contained the same free 1,2-dioxolane carboxylic acid as epiplakinic acid F (1). 1H–1H COSY correlations of H-16−H-15−H-14−H-13−H-12−H-19−H-18−H-13, along with the key HMBC correlations of H-18 (δH 2.34, t, J = 8.4 Hz) with C-13, C-14, C-16, C-17, C-19, C-20, and C-24 and of H-13 (δH 2.41) with C-12, C-14, and C-18 led us to a bicyclo[4.2.0]octene ring system for plakortinic acids A (2) and B (3) (Table S1 and Figure S1a). COSY, HMQC, and HMBC data routinely established the spin systems from H2-11 through H-16, H-18 through H3-21, including H-13 and H-18 and those of H-12/H19. These correlations also helped us establish unequivocally the locus of the 1,2-diol array at C-14/C-15. Furthermore, the HMBC correlation of H3-21 (δH 0.91, t, J = 7.5 Hz) with C-19 and C-20 allowed us to attach an ethyl group to C-19. Based on these observations, we concluded that the remaining NMR signals had to be those of a straight alkyl side chain (C6 through C11) connecting the bicyclo[4.2.0]octene and cycloperoxide ring units.
Table 1.
NMR Spectroscopic Data for the Mixture of Plakortinic Acids A and B (2 and 3) in CDCl3a
| position | δCb | type | δH, mult (J in Hz)c |
|---|---|---|---|
|
| |||
| 1 | 174.5 | C | |
| 2α | 43.9 | CH2 | 2.76, d (14.8) |
| 2β | 2.72, d (14.8) | ||
| 3 | 83.9 | C | |
| 4α | 55.7d | CH2 | 2.25, d (12.5) |
| 4β | 2.44, d (12.5) | ||
| 5 | 86.6a | C | |
| 6α | 39.5 | CH2 | 1.71, dd (14.0, 4.6) |
| 6β | 1.52, dd (14.0, 7.5) | ||
| 7α | 24.3d | CH2 | 1.38, m |
| 7β | 1.30, m | ||
| 8 | 29.8a | CH2 | 1.30, m |
| 9 | 29.7 | CH2 | 1.30, m |
| 10α | 28.2d | CH2 | 1.39, m |
| 10β | 1.28, m | ||
| 11α | 30.7 | CH2 | 1.58, m |
| 11β | 1.38, m | ||
| 12 | 39.8 | CH | 2.03, m |
| 13 | 35.6a | CH | 2.41, ddd (8.4, 9.7 Hz) |
| 14 | 68.9a | CH | 3.93, dd (9.7, 3.3) |
| 15 | 67.8 | CH | 4.11, dd (5.8, 3.3) |
| 16 | 121.3 | CH | 5.56,d (5.8) |
| 17 | 143.1 | C | |
| 18 | 42.1 | CH | 2.34, t (8.4) |
| 19 | 49.2 | CH | 1.66, br m |
| 20α | 28.8 | CH2 | 1.58, br m |
| 20β | 1.49, br m | ||
| 21 | 11.9 | CH3 | 0.91, t (7.5) |
| 22 | 23.8 | CH3 | 1.46, s |
| 23 | 23.2a | CH3 | 1.29, s |
| 24 | 21.3 | CH3 | 1.65, s |
Assignments are based on DEPT, HSQC, HMBC, 1H-1H COSY and NOESY experiments.
Recorded at 125 MHz.
Recorded at 500 MHz.
This resonance consists of two closely-spaced signals with partial overlap. This trait heightens in benzene-d6 solution.
Since these two diastereomers were inseparable under normal conditions, determination of the arrangement of atoms in space was carried out with the mixture. The relative configurations of the stereocenters of the strained ring systems in plakortinic acids A (2) and B (3) were established on the basis of correlations in the NOESY spectrum (see Table S1 and Figure S1b) as well as through interpretation of NMR coupling constant data (Table 1). The H −4 proton (δ 2.44) that is cis to the acetic acid side chain appears downfield from the proton trans to the acetic acid. Thus, H-4β showed strong dipolar coupling to H3-23. H-4α (δH 2.25) showed strong dipolar couplings to H3-22 and H2-6αβ. All of these are consistent with a trans relative stereochemistry for the 1,2-dioxolane carboxylic acid moiety of 2 and 3. Cross-peaks of H-13 with H-12 and H-18, and of H-12 with H3-21 placed these protons on the same face of the bicyclo ring system.14 While those of H-14 with H2-11, H-15, and H-19 were used to place them on the opposite face. The conspicuous absence of NOE’s between H-19, H-12, and H-18 confirmed their trans quasi-diaxial relationship. The coupling constant for H-13 and H-14 (J = 9.7 Hz) is in full agreement with their trans-diaxial orientation, whereas the small coupling constant between H-14 and H-15 (J = 3.3 Hz) supports the cis geometry for the 1,2-diol array in 2 and 3.
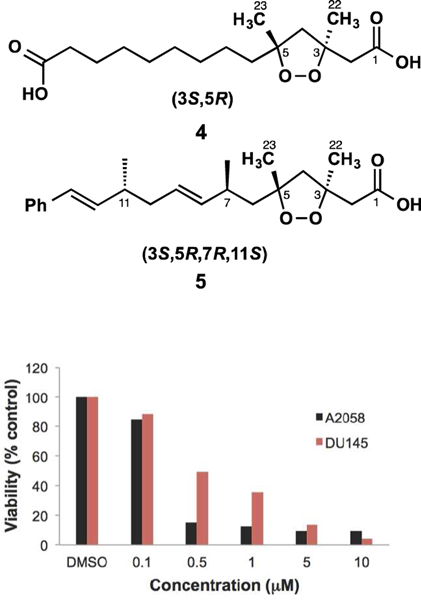
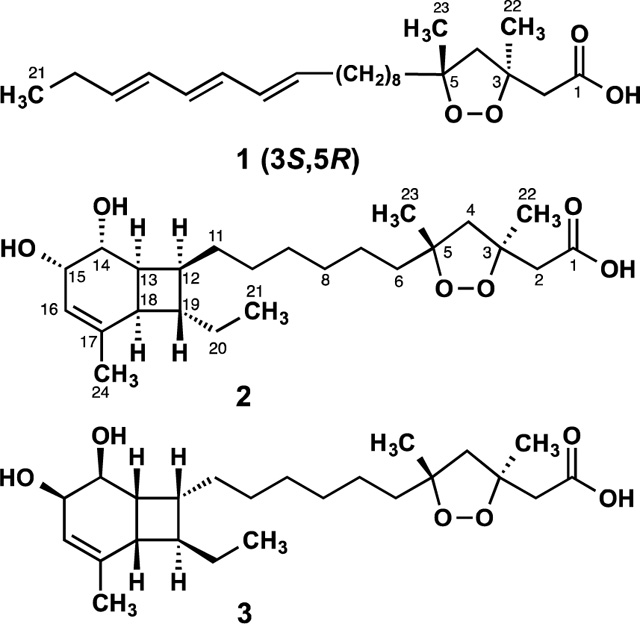
Interestingly, the stereoassignments shown in 2 and 3 of C-3 and C-5 are not arbitrary. Rather, they are based on nearly identical NMR and [α]D data to those reported for co-isolated compound 1 as well as other 1,2-dioxolanes of well-defined configuration (i.e. 4 and 5, see Tables 2 and 3).8,15 In spite of their structural dissimilarities, epiplakinic acid F (1) and the mixture of plakortinic acids A and B have [α]D values of the same positive sign and similar order of magnitude (Table 3).16 In order to account for this observation, we propose that the bicyclic backbone of 2 and 3 is formed spontaneously, i.e., without the assistance of enzymes, from an achiral tetraene precursor through 8π–6π electrocyclization cascades as a 1:1 mixture of diastereomers (see Figure S2). This proposal is consistent with the observation that some natural products whose biosynthesis involves an 8π–6π electrocyclization cascade are typically isolated as racemates.17 We surmise that the specific rotation of the mixture of diastereomers is set exclusively by the dominant influence of a chiral 1,2-dioxolane of well-defined configuration (3S,5R), and that any contributions to [α]D arising from the bicyclo[4.2.0]octene systems cancel out. It is reasonable, therefore, to conclude from the specific rotation and nearly identical 1H and 13C chemical shifts in the vicinity of the 1,2-dioxolane subunit of the mixture of 2 and 3–being comparable to 1, 4 and 5 (and their akin methyl esters)–that the plakortinic acids appear to share the same 3S,5R stereochemistry and that the two structures differ only at the stereocenters about the bicyclo[4.2.0]octene subunit. Therefore, the most likely configurations for plakortinic acid A (2) and plakortinic acid B (2) are 3S, 5R, 12S, 13S, 14R, 15S, 18R, 19S and 3S, 5R, 12R, 13R, 14S, 15R, 18S, 19R, respectively.18
Table 2.
Comparison of Selected 13C-NMR Shiftsa
| peak | 1 | 1-ME | 2/3b | 4 | 4-ME | 5 | 5-ME |
|---|---|---|---|---|---|---|---|
|
| |||||||
| C2 | 43.1 | 44.0 | 43.9 | 44.0 | 44.0 | 44.0 | 44.0 |
| C3 | 83.4 | 83.9 | 83.9 | 83.9 | 83.9 | 83.8 | 83.7 |
| C4 | 55.2 | 55.4 | 55.7 | 55.6 | 55.4 | 56.2 | 55.8 |
| C5 | 86.2 | 86.5 | 86.6 | 86.6 | 86.5 | 86.8 | 86.7 |
| C6 | 39.3 | 39.6 | 39.5 | 39.6 | 39.6 | 40.0 | 40.0 |
| C22 | 24.0 | 24.1 | 23.8 | 23.9 | 24.1 | 24.9 | 24.9 |
| C23 | 23.2 | 23.3 | 23.2 | 23.2 | 23.2 | 23.5 | 23.4 |
Table 3.
Comparison of Selected 1H-NMR Spectroscopic and Specific Rotation Dataa
| compd | H-2α | H-2β | H-4α | H-4β | H-6α | H-6β | H3-22 | H3-23 | [α]D |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 2.79, d (14.5) | 2.79 (d, 14.5) | 2.28 (d, 12.5) | 2.46 (d, 12.5) | – | – | 1.50 (s) | 1.29 (s) | +31.2 |
| 1-Me ester | 2.76 (d, 14.5) | 2.65 (d, 14.5) | 2.22 (d, 12.5) | 2.46 (d, 12.5) | 1.69 (br t, 12.2) | 1.53 (br t, 12.2) | 1.43 (s) | 1.28 (s) | +34.4 |
| 2 and 3b | 2.76 (d, 14.8) | 2.72 (d, 14.8) | 2.25 (d, 12.5) | 2.44 (d, 12.5) | 1.71 (dd, 14.0, 4.6) | 1.52 (dd, 14.0, 7.5) | 1.46 (s) | 1.29 (s) | +28.5 |
| 4 | 2.78 (d, 15.0) | 2.71 (d, 15.0) | 2.24 (d, 12.5) | 2.45 (d, 12.5) | – | – | 1.46 (s) | 1.29 (s) | +33.9 |
| 4-Me ester | 2.76 (d, 15.0) | 2.65 (d, 15.0) | 2.22 (d, 15.0) | 2.46 (d, 15.0) | 1.59 (br m) | 1.59 (br m) | 1.43 (s) | 1.28 (s) | +32.3 |
| 5 | 2.72 (AB, 14.8) | 2.72 (AB, 14.8) | – | – | 1.74 (dd, 14.0, 5.5) | 1.59 (dd, 14.0, 7.8) | 1.42 (s) | 1.30 (s) | +8.0 |
| 5-Me ester | 2.75 (d, 14.6) | 2.63 (d, 14.6) | 2.26 (d, 12.3) | 2.45 (d, 12.3) | 1.78 (dd, 14.2, 8.0) | 1.56 (dd, 14.2, 5.7) | 1.42 (s) | 1.30 (s) | +12.0 |
Compounds 1–3 were found to be primarily responsible for the cytotoxicity of the crude extract of the sponge. The cytotoxicity properties for epiplakinic acid (1) have been described elsewhere.8 Figure 2 shows the behavior of the mixture of plakortinic acids A (2) and B (3) in our MTS assay versus A2058 melanoma and DU-145 prostate cancer cells. These cells were strongly sensitive to the composite displaying IC50 values of 0.3 and 0.5 μM, respectively.
Figure 2.
Cytotoxicity of the mixture of isomers 2 and 3.
Supplementary Material
ACKNOWLEDGMENT
Financial support to C.J.-R. was provided by the IFARHUSENACYT Program of Panama. This research was supported by the NIH Grant 1SC1GM086271-01A1 awarded to A. D. R.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Schemes S1–S3, Table S1, Figures S1 and S2, experimental procedures, copies of the NMR spectra of the mixture of 2 and 3 and photograph of the sponge consortium.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Vicente J; Zea S; Powell RJ; Pawlik JR; Hill RT Mar. Biol. 2014, 161, 2803–2818. [Google Scholar]; (b) Vicente J; Zea S; Hill RT Zootaxa 2016, 4178, 209–233. [DOI] [PubMed] [Google Scholar]
- (2).(a) Norris MD; Perkins MV Nat. Prod. Rep. 2016, 33, 861–880. [DOI] [PubMed] [Google Scholar]; (b) Liu D-Z; Liu J-K Nat. Prod. Bioprospect. 2013, 3, 161–206. [Google Scholar]
- (3).(a) Davidson BS J. Org. Chem. 1991, 56, 6722–6724. [Google Scholar]; (b) Jamison MT; Dalisay DS; Molinski TF J. Nat. Prod. 2016, 79, 555–563. [DOI] [PubMed] [Google Scholar]; (c) Reyes F; Rodríguez-Acebes R; Fernández R; Bueno S; Francesch A; Cuevas CJ Nat. Prod. 2010, 73, 83–85. [DOI] [PubMed] [Google Scholar]; (d) Chen Y; McCarthy PJ; Harmody DK; Schimoler-O’Rourke R; Chilson K; Selitrennikoff C,; Pomponi SA; Wright AE J. Nat. Prod. 2002, 65, 1509–1512. [DOI] [PubMed] [Google Scholar]
- (4).Blunt JW; Copp BR; Keyzers RA; Munro MHG; Prinsep MR Nat. Prod. Rep. 2016, 33, 382–431 and previous articles in this series. [DOI] [PubMed] [Google Scholar]
- (5).(a) Bandaranayake WM; Banfield JE; Black D. St. C.; Fallon GD; Gatehouse BM. Aust. J. Chem. 1981, 34, 1655–1667. [Google Scholar]; (b) Bandaranayake WM; Banfield JE; Black D. St. C.; Fallon GD; Gatehouse BM. Aust. J. Chem. 1982, 35, 567–579. [Google Scholar]; (c) Woodward RB; Hoffmann R The Conservation of Orbital Symmetry. VCH, Weinheim, 1970. [Google Scholar]; (d) Marvell EN Thermal Electrocyclic Reactions, Academic Press, New York, 1980. [Google Scholar]; (e) Marvell EN Seubert J; Vogt G; Zimmer G; Moy G; Siegmann JR. Tetrahedron 1978, 34, 1307–1322. [Google Scholar]; (f) Miller AK; Trauner D Angew. Chem. Int. Ed. 2005, 44, 4602–4606. [DOI] [PubMed] [Google Scholar]
- (6).(a) Beaudry CM; Malerich JP; Trauner D Chem. Rev. 2005, 105, 4757–4778. [DOI] [PubMed] [Google Scholar]; (b) Rodriguez R; Adlington RM; Eade SJ; Walter MW; Baldwin JE; Moses JE Tetrahedron 2007, 63, 4500–4509. [Google Scholar]
- (7).Ebada SS; Proksch P In Handbook of Marine Natural Products; Fattorusso E, Gerwick WH, Taglialatela-Scafati O, Eds.; Springer: Netherlands, 2012; Chapter 4, pp 191–293. [Google Scholar]
- (8).Jiménez-Romero C; Ortiz I; Vicente J; Vera B; Rodríguez AD; Nam S; Jove RJ Nat. Prod. 2010, 73, 1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Interestingly, bicyclo[4.2.0]octane-based natural products with different substitution patterns have been reported from various sources, including plants, saccoglossan mollusks, Streptomyces, and marine-derived fungi. For a comprehensive list of structures, references, and biological activities, see Schemes S1–S3 as Supporting Information.
- (10).Tian X-Y; Han J-W; Zhao Q; Wong HNC. Org. Biomol. Chem. 2014, 12, 3686–3700. [DOI] [PubMed] [Google Scholar]
- (11).For illustrative reports of natural products isolated as two or more inseparable compounds, see: [Google Scholar]; (a) Rudi A; Yosief T; Schleyer M; Kashman Y. Org. Lett. 1999, 1, 471–472. [DOI] [PubMed] [Google Scholar]; (b) Liu L; Liu S; Niu S; Guo L; Chen X; Che YJ Nat. Prod. 2009, 72, 1482–1486. [DOI] [PubMed] [Google Scholar]; (c) Cheng J-F; Lee J-S; Sakai R; Jares-Erijman EA; Silva MV; Rinehart KL J. Nat. Prod. 2007, 70, 332–336. [DOI] [PubMed] [Google Scholar]; (d) Liu Y; Mansoor TA; Hong J; Lee C-O; Sim C-J; Im KS; Kim ND; Jung JH J. Nat. Prod. 2003, 66, 1451–1456. [DOI] [PubMed] [Google Scholar]; (e) Choi K; Hong J; Lee C-O; Kim D-K; Sim CJ; Im KS; Jung JH J. Nat. Prod. 2004, 67, 1186–1189. [DOI] [PubMed] [Google Scholar]; (f) Reynolds WF; McLean S; D’Armas HT; Mootoo BS Magn. Reson. Chem. 2001, 39, 94–97. [Google Scholar]
- (12).Plakortinic acids A (2) and B (3): colorless oil; [α]D20 +28.5° (c 1.3, CHCl3); IR (film) υmax 3391, 2930, 2855, 1714, 1455, 1376, 1220, 1061 cm−1; HRESIMS m/z [M+Na]+ 447.2726 (calcd for C24H40O6Na, 447.2723).
- (13).The blend of isomers was so uniform (both chromatographically and spectroscopically) that at the outset we were not able to distinguish between them from the NMR spectra. However, when the 13C NMR spectrum was expanded considerably, we noticed that some lines appeared as narrowly split pairs, and the intensity indicated that the isomers were present in a ratio close to 1:1. In addition, the carbon resonances around the bicyclic subunit were observed in pairs, suggesting that they might be epimers at C12–C15, C18, and C19.
- (14).Due to the near coincidence in chemical shifts between H-13 and H-18 in CDCl3, no consideration about their relative orientation could be established on the basis of NOESY experiments. However, in benzene-d6 greater dispersion in the chemical shifts made it possible to detect an NOE cross-peak between these nuclei.
- (15).Dai P; Trullinger TK; Liu X; Dussault PH J. Org. Chem. 2005, 71, 2283–2292. [DOI] [PubMed] [Google Scholar]
- (16).Likewise, chiral 1,2-dioxolanes 1, 4, and 5 (and their methyl esters) have [α]D values of the same positive sign and similar magnitudes (Table 3), indicating they share the same absolute configuration of the substituted 1,2-dioxolane ring. Even in 5 and its methyl ester the specific rotations are dominated by the C-3 and C-5 configurations, and the signs appear to be insensitive to stereocenters remote from the dioxolane ring. For the full spectroscopic data of compounds 1, 1-Me ester, 4, and 4-Me ester, see refs 8 and 10. For the data of 5, 5-Me ester and other stereodefined “plakinates”, see ref 15.
- (17).(a) Asai T; Luo D; Yamashita K; Oshima Y Org. Lett. 2012, 15, 1020–1023. [DOI] [PubMed] [Google Scholar]; (b) Leverrier A; Elise M; Dau TH; Retailleau P; Awang K; Guéritte F; Litaudon M Org. Lett. 2010, 12, 3638–3641. [DOI] [PubMed] [Google Scholar]; (c) Bandaranayake WM.; Banfield JE.; Black D. St. C.; Fallon GD; Gatehouse BM. Chem. Commun. 1980, 162–163. [Google Scholar]
- (18).While the most likely configurations (3S,5R)-2 and (3S,5R)-3 are uniform, the assignments for the centers of asymmetry about the bicyclo[4.2.0]octene system of 2 and 3 are interchangeable.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.