Abstract
Repetitive transcranial magnetic stimulation (rTMS) is used to modulate neural systems and provides the opportunity for experimental tests of hypotheses regarding mechanisms underlying anorexia nervosa (AN). The present pilot study has investigated whether high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) to a region of the right dorsolateral prefrontal cortex (DLPFC) might be associated with change in food selection among adult inpatients with AN. Ten women received one session of sham and one session of HF-rTMS targeting the right DLPFC while completing a computerized Food Choice Task. Compared to sham, HF-rTMS was associated with changes in food ratings and food choice: inpatients reported higher healthiness ratings of low- and high-fat foods and selected a significantly greater proportion of high-fat foods over a neutrally rated reference item while receiving HF-rTMS. Findings suggest that HF-rTMS to the right DLPFC was associated with a reduction of fat avoidance on a food choice task among inpatients with AN and provide additional support for the possibility that this region, and related neural circuits, may underlie restrictive food choice. Research using rTMS to experimentally test neural mechanisms is needed to elucidate the underpinnings of AN and supports the development of novel treatment targets.
Keywords: anorexia nervosa, caloric restriction, choice behavior, feeding and eating disorders, food preferences, magnetic resonance imaging, neuronavigation, TMS, transcranial magnetic stimulation
1 |. INTRODUCTION
Anorexia nervosa (AN) is a debilitating eating disorder with high rates of morbidity and mortality (Arcelus, Mitchell, Wales, & Nielsen, 2011). Restriction of food intake, specifically calories from fat, contributes to the morbidity and high relapse rates (Schebendach et al., 2008; Schebendach, Mayer, Devlin, Attia, & Walsh, 2012). Treatment for adults with AN has limited efficacy and there is a grave need for the development of novel, mechanism-based therapies.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive form of neurostimulation used to modulate neural circits. High frequency rTMS (HF-rTMS) is best-known as a therapeutic intervention for psychiatric disorders, particularly major depressive disorder (MDD; Janicak & Dokucu, 2015), for which it is FDA-approved (Perera et al., 2016). Within research on the clinical therapeutics of AN, ongoing research suggests it yields modest improvements in associated symptoms (McClelland et al., 2013, 2016; Van den Eynde, Guillaume, Broadbent, Campbell, & Schmidt, 2013). However, rTMS can also be used on a more mechanistic level, using as little as a single session to explore the function of neural systems by testing whether modulation of a circuit is associated with a change in behavior (Camprodon, Martínez-Raga, Alonso-Alonso, Shih, & Pascual-Leone, 2007). When used this way, TMS enables researchers to draw inferences about the indirect or direct function of a given brain region (Tik et al., 2017). Targeting a region of the brain that has been implicated specifically in the neurobiology of AN may enable us to test hypothesized mechanisms underlying AN and inform subsequent treatment development.
Behavioral studies of a computerized Food Choice Task have shown that individuals with AN are significantly less likely than healthy controls (HC) to select high-fat foods (Steinglass, Foerde, Kostro, Shohamy, & Walsh, 2015) and that the proportion of high-fat foods chosen during the task significantly correlates with actual intake (Foerde et al., 2020; Foerde, Steinglass, Shohamy, & Walsh, 2015). Neuroimaging research using the same task has repeatedly found that when making food choices, individuals with AN show increased dorsal striatal activity, as compared with HC (Foerde et al., 2015, 2020). Using a psychophysiological interaction (PPI) analysis, our previous study found that patients exhibited peak differential functional connectivity between a specific region of the right DLPFC and dorsal striatum when making decisions about high-fat foods than when making decisions about low-fat foods (Foerde et al., 2015). This finding suggests that frontostriatal circuitry may underlie restrictive food choice in AN, and that the specific anatomical region of the right DLPFC may be a means through which to target the related dorsal striatal circuitry.
The identification of a neural mechanism related to restrictive food choice in AN allows for probing this circuit with rTMS to test whether targeting this specific subregion of the right DLPFC can, in turn, modulate activity in the dorsal striatum to alter decisions about what to eat.
2 |. A PRELIMINARY EXPERIMENTAL TEST OF HYPOTHESIZED NEURAL MECHANISMS
Our group conducted a preliminary investigation designed to test whether administering a single dose of HF-rTMS to a targeted region of the right DLPFC implicated in previous neuroimaging studies of AN alters food choice behavior. We tested the hypothesis that compared to sham, one session of HF-rTMS to this region would result in a reduction of restrictive food choice behavior during the Food Choice Task.
2.1 |. Study procedures
Participants were between the ages of 18 and 50 years, met DSM-5 criteria for AN via the Eating Disorders Assessment for DSM-5 clinical interview (Sysko et al., 2015), were receiving treatment at the New York State Psychiatric Institute Eating Disorders Unit, had a body mass index ≥ 14.5 kg/m2, and were medically stable. Participants were excluded if they required treatment with psychotropic medications other than SSRI/SNRI antidepressants, had a comorbid psychiatric disorder requiring separate specialized care, had history of or were at increased risk for seizure, or had contraindications to MRI. Participation occurred on three separate study days, each approximately 1 week apart. On each study day, participants received a standardized breakfast.
On Day 1, participants completed a structural MRI scan, used to localize the target region for TMS. Structural images were collected using a high-resolution T1-weighted MPRAGE pulse sequence (0.83 mm voxel size) for image registration. Identification of the DLPFC target was achieved using the FMRIB Software Library (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). T1-weighted structural images were transformed into MNI-152 standard space and coordinates within the DLPFC were used to identify the region of interest (ROI), defined as the peak voxel within the right DLPFC (MNI xyz = 42 48 22) based on findings from the previous PPI analysis. The inverse of the transformation matrix was then applied to transform the T1-weighted image and ROI coordinates into native space. These images were used in Brainsight software (Rogue Research, Montreal, Canada) for navigation of the TMS coil. participants’ motor threshold (MT), or the minimum amount of stimulation necessary to elicit a motor response at least 50% of the time, was determined prior to TMS administration.
On Days 2 and 3, participants received sham or HF-rTMS (10 pulses/s, 4-s trains, 120% MT, 3000 pulses) using the Neurostar TMS unit. The order of sham versus HF-rTMS was randomly assigned. Blinded coils were used to administer rTMS to the target region of the DLPFC. Sham rTMS was identical in appearance and acoustics and was administered for the same amount of time as HF-rTMS to simulate active treatment.
HF-rTMS or sham was administered while participants completed the Food Choice Task, presented using the Psychophysics toolbox (Brainard, 1997) and Matlab version R2020a (Natick, MA). The task consisted of three blocks: 76 food items were presented in each block, with 38 high-fat and 38 low-fat items. High-fat items were defined as those with greater than 30% total calories from fat. Participants rated food items on Healthiness and Tastiness during the first two blocks, in counterbalanced order. An item that was rated as “neutral” on ratings of both health and taste was selected as a “reference” item. During the third block, participants selected between the reference item and one of the remaining 75 items. To enhance the confidence that participants’ responses reflected true eating preferences, participants were served a snack-size portion of one of their choices, selected at random.
Outcomes from the Food Choice Task include mean ratings of healthiness and tastiness, proportion of trials on which the high-fat food was chosen over the reference item, and choices that reflect self-control. Assessment of self-control involves quantifying the number of opportunities a participant had to exert self-control. That is, trials in which subjective ratings of healthiness and tastiness were incongruent (i.e., a food is rated as tasty and unhealthy, or as not tasty and healthy). Engagement of self-control was quantified as proportion of these trials in which the individual chose the healthy, less tasty item over the reference item, or chose the reference instead of an unhealthy, tasty item. Food ratings and choice data were analyzed using 2 (condition: sham vs. HF-rTMS) × 2 (food type: low-fat vs. high-fat) Repeated Measures Analyses of Variance (RM-ANOVAs). Paired-samples t-tests were used to examine the difference in self-control behavior between conditions.
2.2 |. Group results
Twelve women with AN were enrolled in the study. One participant discontinued participation due to rTMS-related discomfort and one discharged prior to study participation. The remaining 10 participants (7 with restricting subtype) completed all study procedures. Results are reported for the 10 patients with complete data. Six participants (60%) were randomized to receive HF-rTMS first. Demographics are included in Table 1. Food Choice Task outcomes are depicted in Figure 1; full details on the outcome of statistical analyses comparing health and taste ratings, food choice, and self-control are included in Appendix S1.
TABLE 1.
Participant demographic data
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 30.7 | 7.4 | 18–37 |
| Admission BMI (kg/m2) | 15.6 | 1.9 | 13.9–18.2 |
| Duration of illness (years) | 12.6 | 9.5 | 1.1–30.0 |
| BMI, baseline (kg/m2) | 17.1 | 1.8 | 14.6–20.9 |
| BMI, sham treatment (kg/m2) | 18.3 | 2.3 | 15.5–24.1 |
| BMI, real treatment (kg/m2) | 18.2 | 1.9 | 16.2–22.4 |
| EDE-Q global score | 4.6 | 1.4 | 1.4–5.6 |
Abbreviations: BMI, Body Mass Index; EDE-Q, Eating Disorder Examination-Questionnaire.
FIGURE 1.
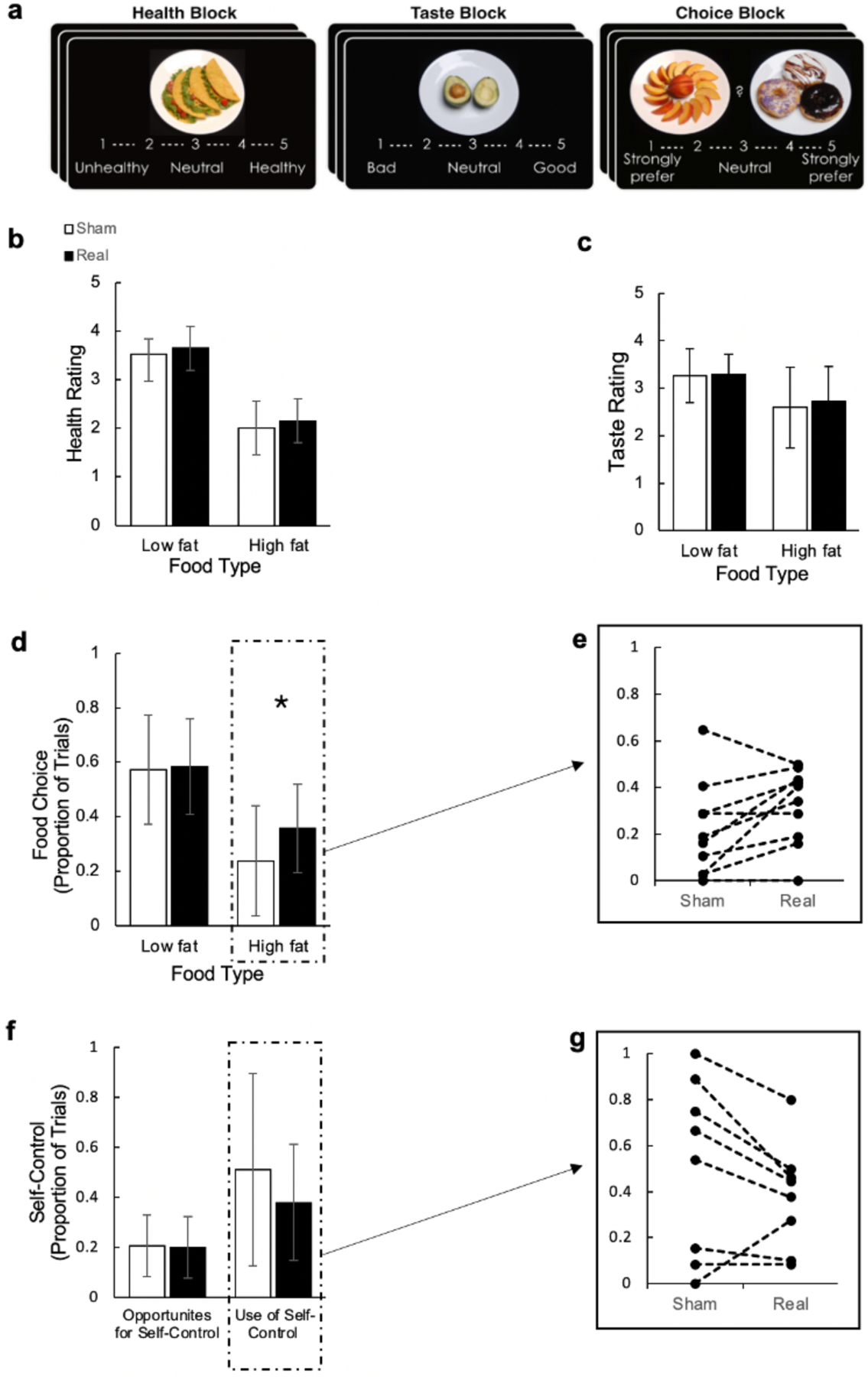
Effects of sham versus real high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) on restrictive eating behavior as measured by the Food Choice Task. (a) Image of the Blocks depicted during the Food Choice Task; (b) Participant healthiness ratings of low and high-fat foods; (c) Participant tastiness ratings of low and high-fat foods; (d) Proportion of trials in which participants selected low and high-fat foods over a neutrally rated reference item; (e) Individual values of change in proportion of high-fat foods selected between sham and real HF-rTMS; (f) Proportion of trials in which there was an opportunity for self-control and participants implemented self-control; (g) Individual values of change in proportion of trials in which participants implemented self-control between sham and real HF-rTMS
3 |. DECIPHERING THE ROLE OF FRONTOSTRIATAL CIRCUITRY IN FOOD CHOICE
The current proof-of-concept study was designed to examine the effects of HF-rTMS to the right DLPFC on food choice behavior among inpatients with AN. Compared to sham, HF-rTMS was associated with increased ratings of healthiness for both low- and high-fat foods. Individuals also selected a significantly greater proportion of high-fat foods relative to a neutrally rated reference item when receiving HF-rTMS, a behavior that has proven very challenging to change and is not reduced following a complete course of inpatient treatment (Foerde et al., 2021). There was no significant difference between conditions in ratings of tastiness for low- or high-fat foods. There was also no significant difference in participants’ opportunities for or use of self-control behavior between conditions. However, the effect size was moderate (.63) for the decreased use of self-control, suggesting the lack of significance may be due to inadequate power in this small sample.
The findings from the present analysis suggest that among a small sample of inpatient women with AN, HF-rTMS targeting a region of the DLPFC previously implicated in food choice results in a reduction of fat avoidance as measured by a food choice task. These findings are consistent with previous studies demonstrating modest improvements in weight and self-reported eating disorder symptoms following HF-rTMS (Bartholdy et al., 2015; Dalton et al., 2018, 2020; McClelland et al., 2013; McClelland, Kekic, Bozhilova, et al., 2016; McClelland, Kekic, Campbell, & Schmidt, 2016). A recent investigation examined the effects of 20 sessions of HF-rTMS versus sham on change in performance during a computerized food choice task among patients with severe and enduring AN. Results indicated that following 20 sessions of rTMS to the left DLPFC, patients receiving HF-rTMS, but not sham, implemented self-control on a significantly fewer proportion of trials than they did at baseline (Dalton et al., 2020). Further examination of choices made during the task revealed that patients who received HF-rTMS made increased selection of foods they considered tasty but unhealthy at posttreatment. These findings of change in self-control behavior suggest rTMS may be able to modify restrictive eating behavior characterizing AN. Other clinical trials have found that a course of HF-rTMS to the left DLPFC yields modest improvements in weight and self-reported eating disorder symptoms, such as urges to restrict food intake. Importantly, however, the effects of TMS in these clinical trials are limited, with symptom improvement observed no longer than 6 months following treatment (McClelland, Kekic, Bozhilova, et al., 2016). Therefore, while rTMS appears to hold some potential, there are limitations to existing protocols. A potential explanation for the modest effects seen in individuals with AN is the region of the brain targeted by TMS. The majority of research in AN has examined the effects of TMS to the same region of the DLPFC as that used in treatment of MDD. The DLPFC is a widespread region within the frontal cortex. Other subregions of the DLPFC, such as the ROI used here, that have been implicated specifically in the neurobiology of AN may confer distinct therapeutic benefit for individuals with AN.
4 |. A CALL FOR RTMS MECHANISM-BASED RESEARCH IN AN
Findings from the present study are the first to report a significant reduction in restrictive food choice behavior as measured by the Food Choice Task. There are several limitations to consider when interpreting these results. First, data were collected from a very small sample of individuals who received only one session of HF-rTMS, limiting the generalizability of findings. The small sample size also precludes meaningful comparisons between the subtypes of AN, which could provide further information on the effects of HF-rTMS to the DLPFC among individuals with different symptom presentations. Moreover, despite every effort to maintain blinding, fundamental differences between HF-rTMS and sham rTMS exist, such that changes in food choice behavior may be better explained by a reaction to the physical discomfort experienced during HF-rTMS, or by a placebo effect.
Overall, results suggest this region of the right DLPFC may function as part of a neural system underlying restrictive food choice in AN. To our knowledge, this is the first study to use HF-rTMS to experimentally test hypothesized neural mechanisms underlying a core symptom of AN. The present study demonstrates HF-rTMS’ ability to engender changes in behavior and in turn, enable researchers to draw inferences about the specific function of brain regions and circuits. Researchers are encouraged to harness the strengths of using rTMS in research to better elucidate the neural substrates of a poorly understood disorder. There is a substantial need for more of this research, which can be used to inform the development of novel, mechanism-based treatment targets.
Supplementary Material
ACKNOWLEDGEMENTS
This project was funded by the National Eating Disorders Association (Principal Investigator: Broft); “Repetitive Transcranial Magnetic Stimulation as a New Treatment for Anorexia Nervosa”, 03/01/2018–02/28/2020. This work was also supported by the Neuronetics Investigator-Initiated Trials program.
Funding information
National Eating Disorders Association, Grant/Award Number: The Feeding Hope Fund for Clinical Research; Neuronetics Investigator-Initiated Trials program
Footnotes
CONFLICTS OF INTEREST
Dr. Steinglass receives royalties from UpToDate. Dr. Broft received TMS supplies for the study through a contract with Neuronetics. Dr. Attia serves as a clinical advisor to Equip Health, Inc., and receives royalties from UpToDate. The authors have no other conflicts of interest to report.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arcelus J, Mitchell AJ, Wales J, & Nielsen S (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies. Archives of General Psychiatry, 68(7), 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Bartholdy S, McClelland J, Kekic M, O’Daly OG, Campbell IC, Werthmann J, … Schmidt U (2015). Clinical outcomes and neural correlates of 20 sessions of repetitive transcranial magnetic stimulation in severe and enduring anorexia nervosa (the TIARA study): Study protocol for a randomised controlled feasibility trial. Trials, 16, 548. 10.1186/s13063-015-1069-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih MC, & Pascual-Leone A (2007). One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug and Alcohol Dependence, 86(1), 91–94. 10.1016/j.drugalcdep.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Dalton B, Bartholdy S, McClelland J, Kekic M, Rennalls SJ, Werthmann J, … Schmidt U (2018). Randomised controlled feasibility trial of real versus sham repetitive transcranial magnetic stimulation treatment in adults with severe and enduring anorexia nervosa: The TIARA Study. Mental Health Research, 8(7), e021531. 10.1136/bmjopen-2018-021531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton B, Foerde K, Bartholdy S, McClelland J, Kekic M, Grycuk L, … Steinglass JE (2020). The effect of repetitive transcranial magnetic stimulation on food choice-related self-control in patients with severe. Enduring Anorexia Nervosa, 53(8), 1326–1336. 10.1002/eat.23267 [DOI] [PubMed] [Google Scholar]
- Foerde K, Schebendach JE, Davis L, Daw N, Walsh BT, Shohamy D, & Steinglass JE (2020). Restrictive eating across a spectrum from healthy to unhealthy: Behavioral and neural mechanisms. Psychological Medicine, 1–10. 10.1017/S0033291720003542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Steinglass JE, Shohamy D, & Walsh BT (2015). Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nature Neuroscience, 18(11), 1571–1573. 10.1038/nn.4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Walsh BT, Dalack M, Daw N, Shohamy D, & Steinglass JE (2021). Changes in brain and behavior during foodbased decision-making following treatment of anorexia nervosa. Journal of Eating Disorders, 9(1), 48. 10.1186/s40337-021-00402-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicak PG, & Dokucu ME (2015). Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatric Disease and Treatment, 11, 1549–1560. 10.2147/NDT.S67477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- McClelland J, Bozhilova N, Nestler S, Campbell IC, Jacob S, Johnson-Sabine E, & Schmidt U (2013). Improvements in symptoms following neuronavigated repetitive transcranial magnetic stimulation (rTMS) in severe and enduring anorexia nervosa: Findings from two case studies. European Eating Disorders Review, 21(6), 500–506. 10.1002/erv.2266 [DOI] [PubMed] [Google Scholar]
- McClelland J, Kekic M, Bozhilova N, Nestler S, Dew T, Van den Eynde F, … Schmidt U (2016). A randomised controlled trial of Neuronavigated repetitive transcranial magnetic stimulation (rTMS) in anorexia nervosa. PLoS One, 11(3), e0148606–e0148606. 10.1371/journal.pone.0148606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J, Kekic M, Campbell IC, & Schmidt U (2016). Repetitive transcranial magnetic stimulation (rTMS) treatment in enduring anorexia nervosa: A case series. European Eating Disorders Review, 24(2), 157–163. 10.1002/erv.2414 [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, & Wirecki TS (2016). The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebendach JE, Mayer LE, Devlin MJ, Attia E, Contento IR, Wolf RL, & Walsh BT (2008). Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. The American Journal of Clinical Nutrition, 87(4), 810–816. 10.1093/ajcn/87.4.810 [DOI] [PubMed] [Google Scholar]
- Schebendach JE, Mayer LES, Devlin MJ, Attia E, & Walsh BT (2012). Dietary energy density and diet variety as risk factors for relapse in anorexia nervosa: A replication. The International Journal of Eating Disorders, 45(1), 79–84. 10.1002/eat.20922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinglass J, Foerde K, Kostro K, Shohamy D, & Walsh BT (2015). Restrictive food intake as a choice—A paradigm for study. The International Journal of Eating Disorders, 48(1), 59–66. 10.1002/eat.22345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysko R, Glasofer DR, Hildebrandt T, Klimek P, Mitchell JE, Berg KC, … Walsh BT (2015). The eating disorder assessment for DSM-5 (EDA-5): Development and validation of a structured interview for feeding and eating disorders. The International Journal of Eating Disorders, 48(5), 452–463. 10.1002/eat.22388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tik M, Hoffmann A, Sladky R, Tomova L, Hummer A, Navarro de Lara L, … Windischberger C (2017). Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes networkspecific increase in functional connectivity. NeuroImage, 162, 289–296. 10.1016/j.neuroimage.2017.09.022 [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Guillaume S, Broadbent H, Campbell IC, & Schmidt U (2013). Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. European Psychiatry, 28(2), 98–101. 10.1016/j.eurpsy.2011.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


