Abstract
Using microwave technique in the presence of citric acid, selenium nanoparticles (SeNPs) were fabricated. The morphological characteristics revealed that the spherical SeNPs with diameters ranging from 10.5 to 20 nm aggregated spherical shapes with sizes ranging from 0.67 to 0.83 mm. Moreover, the antioxidant efficacy was assessed by the DPPH radical scavenging test, which depicted that green-prepared nanoparticle at a 106.3 mg/mL dosage had the maximum scavenging capacity (301.1 ± 11.42 mg/g). Otherwise, with nanoparticle concentrations of 500 mg/ml, in vitro cell viability of SeNPs through human breast cancer MCF-7 cell lines was reduced to 61.2 ± 2.2% after 1 day of exposure. The antibacterial activity was tested against G-negative Pseudomonas aeruginosa (P. aeruginosa) and Escherichia coli (E. coli), G-positive bacteria Bacillus subtilis (B. subtilis), and Staphylococcus aureus (S. aureus), which demonstrated that SeNPs had little activity against S. aureus. Still, it had the highest activity against E. coli, with a zone of inhibition (ZOI) of 25.2 ± 1.5 mm compared to 16.0 ± 0.6 mm for the standard antibiotic. Most notably, biogenic SeNPs have anticoagulant activities using activated partial thromboplastin time (aPTT) assessment. Based on previous findings, SeNPs can be used in medical aid and their cell viability, antioxidant, anticoagulant, and effects on bacteria.
Keywords: Selenium nanoparticles, Green preparation, Microwave technique, Citric acid, Biomedical applications
Introduction
Nanoparticles (NPs) have gained tremendous attention during the last decade’s different applications [1, 2]. Various techniques were implanted to fabricate the nanoparticles, such as physical, chemical, or biological routes categorized under top-down and bottom-up strategies [3–5]. In the case of chemical and physical procedures, dozens of reviews have mentioned several limitations, including high costs and extra time requirements and the usage of numerous harmful compounds. This does not prevent them from being used in biological applications, but green methods are better and safer, especially in medical applications because these hurdles were overcome by using green synthesis and an environmentally friendly approach, which are critical in synthesizing NPs [6–8].
Spherical selenium (Se) nanostructures have been created utilizing a green synthetic procedure with a natural source like citric acid or plant extracts and have demonstrated superior antibacterial efficacy compared to those made chemically [9, 10]. In addition, the physicochemical features of SeNPs may be regulated by the conditions of the chosen production pathway [11, 12]. The creation of SeNPs utilizing a natural substance is legitimate and almost completely free of hazardous forms, compared to a chemically stabilizing agent used for the fabrication of nanoparticles [13]. Additionally, a class of polymers that are water-soluble could be employed as a stabilizing agent to manufacture Se colloids [14].
Selenium is one of the minerals that might be categorized as biocompatible, essential for human health, and a vital antioxidant mineral [15]. Besides, US-FDA approved, it is used as a daily dietary supplement and recommended to protect against cardiovascular disease [16]. Also, it is involved in several biological processes. It is important to play a cofactor for many antioxidative enzymes, including glutathione peroxidase and thioredoxin reductase, which help the body remove free radicals [17, 18]. Selenium is found in the human body in the form of at least 25 selenoproteins that act as anti-inflammatory, antioxidant, anticancer agents, and antiviral [19]. Moreover, it may participate in thiol group oxidation in protein structures like protein kinase C and tyrosine phosphatase [20]. Also, selenium hinders carcinogenic agents from attacking DNA and inhibiting tumor growth and angiogenesis [21, 22].
Recent years have seen an increase in intriguing studies focusing on the synthesis of SeNPs owing to their intriguing biological activity, low toxicity, and high bioavailability [23]. Additionally, some scientists have discovered that SeNPs are more effective and have lower toxicity than silver nanoparticles (Ag-NPs). Besides, it is hypothesized that SeNPs have higher chemical stability than Ag-NPs [24, 25]. It was postulated to prevent coronavirus (COVID-19) penetration through health cells, eliminating their infectious properties [25]. The distinctive antibacterial properties of this nanoparticle are mainly dependent on the preparation circumstances and controlled by cellular redox homeostasis, such as the reactive oxidative species (ROS) elimination and the manipulation of certain enzymes [26]. SeNPs have significant therapeutic potential in cancer chemotherapy due to their ability to prevent cancer cell development and cell membrane toxicity [27, 28].
Bacterial infectious disease is the most serious complication during the wound-healing process [29, 30]. As a part of antibiotic resistance, excessive antibiotic use may result in superbugs. Hence, multidrug resistance may result in the spread of dangerous microorganisms and incurable diseases. Numerous solutions for overcoming these hurdles have been proposed, including metallic nanoparticles such as gold-NPs and silver-NPs. The focus of our study is SeNPs that can be replaced the traditional antibiotics [3, 31–33].
Anticoagulants activity is by preventing the formation of blood clots, while antiplatelet and thrombolytic agents work by reducing blood clots. Some nanoparticles produced from various biological components and used as both reducing and stabilizing agents have been shown to have anticoagulant properties. It was claimed that earthworm extract was used to biosynthesize AuNPs, which improved the anticoagulant properties of heparin [2, 34].
As a result, the present article develops and outlines an environmentally friendly and simple-to-handle method for preparing SeNPs employing citric acid and a biopolymer alginate to reduce and stabilize agents during the formation. The shape, size, optical, and morphological properties of green-produced SeNPs were investigated. Additionally, cell survival when SeNPs are present was determined using the human MCF-7 cell line. To evaluate the antibacterial efficacy of SeNPs, G-positive and G-negative bacteria were used as severe bacterial strains. Moreover, the antioxidant potency was determined by DPPH analysis. Finally, the anticoagulant activity was also evaluated compared to heparin.
Experimental
Materials, human cells, and bacterial strains
Sodium selenite (Na2SeO3, 99%), alginic acid sodium salt (medium viscosity), citric acid monohydrate (reagent grade, ≥ 98%), methylthiazolyl diphenyltetrazolium bromide (MTT,98%) diphenylpicrylhydrazyl (DPPH), DMEM-F12 nutrient mixture, and nutrient agar were supported from Sigma-Aldrich (USA). Any other notified reagents were used as it is.
Green preparation of SeNPs by citric acid
SeNPs were synthesized green to reduce Na2SeO3 with citric acid and use sodium alginate as a stabilizing agent. Sodium selenite 40 mM in aqueous and 0.5% sodium alginate in DDI were combined at room temperature for 1 h with magnetic stirring. Gradually changing the solution color to orange confirms the creation of SeNPs. Then, the solution was transferred to a Teflon-lined (MDS-8, SINEO, China) microwave reactor (900 W, 40% stirring, 75 °C, and 1.2Par). Finally, the solution was centrifuged at 9000 rpm to yield SeNPs, which were then purified by washing thrice with pure water and twice with absolute ethanol, then the sample was left overnight at 50 °C to get dry black powder.
Characterization
UV–vis absorption of the prepared SeNPs was scanned between 200 and 800 nm was evaluated using (Shimadzu 1800, Japan) a double-beam spectrophotometer (UV–vis). TEM and SEM techniques were utilized to assess and analyze the particle size, shape, and surface morphology. The used volt was 120 kV (JEM-2100, Hitachi Limited, Japan). They used an air-dried carbon-coated copper grid to examine the colloidal solution of SeNPs. SEM was used to identify the texture of green-fabricated SeNPs using SEM (Quanta FEG 250, USA). EDX unit was attached to SEM apparatus (ZEISS EVO-MA 10, Germany). FTIR spectra were done using (JASCO, Model No. 4000, Japan) to show the functional groups of prepared NPs after they were formed, and the range was tuned to 4000–500 cm−1.
Antioxidant properties
Based on published elsewhere [35], the antioxidant activity of SeNPs was assessed by the DPPH study. SeNPs concentrations ranged from 106.3 to 6.5 (mg/mL) were prepared [36]. After that, each sample was treated with 2 ml of (0.1 mM of DPPH in methanol), mixed thoroughly, incubated for 40 min in the absence of light. Then, double-beam UV–VIS was used to scan the sample’s absorption at 517 nm. The following equation was used to compute the antioxidant activity:
| 1 |
Cell cytotoxicity assessment
The human breast cancer (MCF-7 cell line) viability with SeNPs was assessed by the MTT method [37], which quantifies the transformation of yellow dye to purple formazan crystals caused by the activity of the enzyme in living cells mitochondria [38]. After cells were cultured in DMEM, Gibpco and incubated in a 5% CO2 incubator at 37 °C environments for 24 h, the cells were seeded at a density of 1 × 104 cells/cm2 in 96-well plates and incubated at 37 °C in a 5% CO2 environment. After separating the media, different diluted doses from the stock solution of 200-μm SeNPs were charged in the medium and incubated to MCF-7 cells. 0.0, 100, 200, 300, 400, and 500 µg/ml were the highest concentrations achieved in the treated wells. Cell compatibility was determined after 1 day of exposure. Each well received a 20 µl of MTT solution in PBS (5 mg/ml), and the cells were incubated for another 4 h at 37 °C in 5% CO2 incubator. Supernatants were released, and the formazan crystals dissolved in 100 µl of DMSO. The optical density at 570 nm was recorded. The studies were validated three times, and the following equation was used to determine the cytotoxicity [39, 40]:
| 2 |
Antibacterial potency
The synthesized SeNP was tested against two distinct G-negative bacteria (E. coli, ATCC-8739, and P. aeruginosa, ATCC-27853) and G-positive bacteria (B. subtilis, ATCC-6633, and S. aureus, ATCC-6538) to evaluate the antibacterial activity. The zone of inhibition (ZOI)-based diameter was used to determine the range of inhibition for each species. The obtained data were compared to those obtained using common antibiotic discs such as ceftriaxone 30 µg, purchased from Bioanalyses. To investigate the antibacterial potency, 10 mg of SeNPs were dissolved in 0.2 ml of purified saline solution to generate a concentration equal to100 mg/ml. Six millimeters of filter paper discs were dipped in the SeNPs solution and allowed to dry. After heating to liquification, the nutrient agar was placed onto sterile Petri dishes and left covered for 15 min. One colony from the aforementioned bacterial strains individually was spread on the Petri dish surface using a sterile loop. In Petri dishes, discs of the examined SeNPs were incubated at 37 °C with the usual antibiotic for 1 day. Three replicates of the experiments were conducted to determine the standard deviation. Both SeNPs and the standard antibiotic inhibitory zone were determined against a conventional antibiotics panel.
Anticoagulation activity
The activated partial thromboplastin time (aPTT) method evaluated anticoagulant action in vitro (Randox kit-APT2749). Plasma deficient in platelets will be taken from the blood of five healthy volunteers in (9:1 v/v) sodium citrate test tube. Then, centrifugation at 3000 rpm for 10 min, and the plasma (50 µl) was mixed with SeNPs (5 to 30 mg/ml) and incubated at 37 °C for 2 min. Then, 50 µl of prewarmed aPTT reagent were thoroughly merged with the mixture and incubated again under the same condition. Following that, induce clotting by adding 50 µl of prewarmed 0.20 M CaCl2 and compare heparin clotting time [41].
Statistical analysis
All experiments were carried out in triplicate, and the statistical analysis was carried out using Medcalc software version 15.0. (Medcalc 15.0, Mariakerke and Belgum). The mean and standard deviation of continuous variables were calculated. A sample t test was performed, and a p value was obtained where p values > 0.05 were not statistically significant but those with p < 0.05 were considered significant, and p < 0.01 were considered very significant and p < 0.001 or less were highly significant.
Results and discussion
Optical properties
Surface plasmon resonance (SPR) associated with orange appearances are a distinctive optical feature for the metallic NPs, and the change of color denotes the formation of SeNPs (Fig. 1). Using UV–vis, the main peak at 296 nm is caused by coherent oscillations of the free migration of located electrons in the surface of SeNPs to other bands, and this process is well-known by SPR. Furthermore, the colors stayed steady for a couple of days after the reaction was completed, without noticing color changes. Figure 1 depicts the results that verified citric acid significantly as a bio-reducing agent. SeNPs, in particular, exhibit characteristics that vary according to size and shape. Because of the synthetic nature and quantum confinement effect, SeNPs have been observed to contain a wide range of absorption bands at the UV–vis region. It was reported that Drumstick aqueous extract had been employed to synthesize SeNPs, and the SPR band was strikingly at 390 nm [42]. Kirupagaran et al. also discovered that SeNPs have absorption at 293 nm [43]. Also, at 264 and 310 nm, respectively, strong absorption peaks for PVA-SeNPs and Cs-SeNPs were observed, which is related to the creation of Se-NPs during the reduction process. We found that current absorption values are like prior literature.
Fig. 1.

UV–visible spectra of fabricated SeNPs by citric acid reduction confirmed by color changes with stability for 24 h
FTIR spectra
Figure 2 illustrates the functional moieties existing in the solution phase of SeNPs stabilized by sodium alginate using FTIR spectroscopy. The band at 3419 cm−1 indicates the stretching of the citric acid –OH reducing groups and confirms the surface –OH on SeNPs. The aliphatic C–H groups along the chain were attributed to the band at 2934 cm−1 and the nearby one at the lowest cm−1. The band at 1618 cm−1 conforms to C = O stretching vibration. The C–H bending form in alkanes is responsible for the shifted one at 1412 cm−1. The FTIR spectral analysis confirms the Se reduction. The bands at 823 cm−1 correspond to the combined of SeNPs with the –OH group, indicating coordination links between Se and citric acid. It is worth noting that the particles must be coated to stay away from aggregation. As evidenced by the FTIR data, numerous coating agents were also utilized, such as sodium alginate, the Se particles stabilizing agent in this investigation.
Fig. 2.
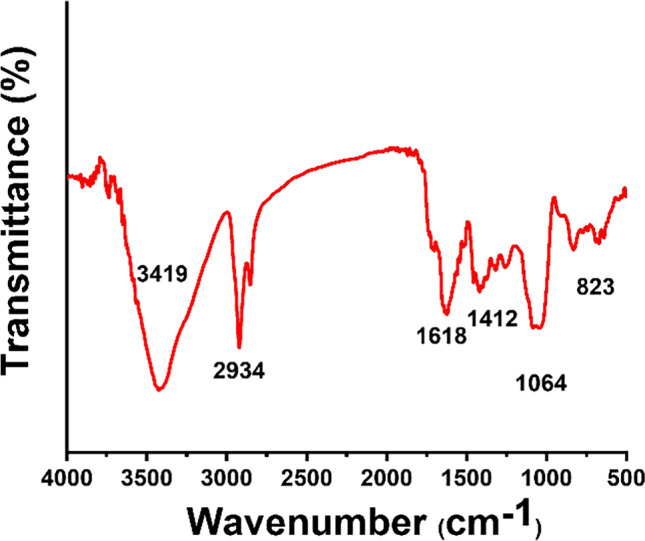
FT-IR spectra of SeNPs/citric acid with sodium alginate
XRD analysis
Powder X-ray crystallography is a useful process for determining the crystalline phase. Figure 3 depicts the positions of the diffraction peaks, and their corresponding planes are listed at 2θ = 23.7 (100), 30.0 (101), 41.8 (110), 44.3 (102), 52.11 (112), 56.2 (202), and 61.7 (210), indicating the existence of crystalline SeNPs and agreeing with JCPDS No.06–0362. At 2θ = 30.0 (101), this sharp peak demonstrated the predominant orientation appeared to assessed facet (101) and also suggested the high purity of SeNPs following the production of the calculated crystallite particle size which is around 37 nm according to the Debye-Scherer equation [44, 45]:
| 3 |
Fig. 3.
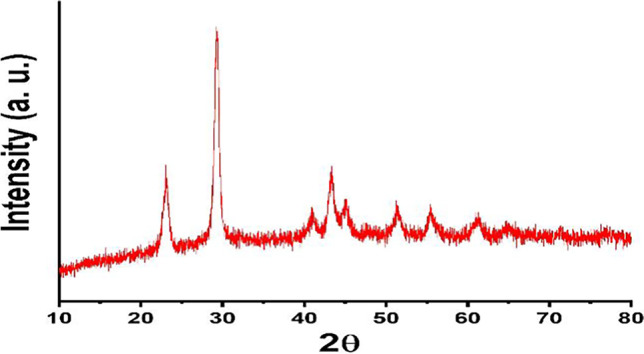
XRD of crystalline SeNPs
K: Scherer constant (0.9).
λ: the X-ray wavelength.
β: the complete width at half point of the XRD peak\
θ: the Bragg angle.
Recently, it was reported that XRD of biosynthesized nanoparticles is crystalline with a spherical structure with the crystalline size of SeNPs ranging from 32 to 86 nm which is near to our findings [46].
Microstructural and shape characteristic
TEM analysis was used to explore the formation of SeNPs (Fig. 4a, b). SeNPs form a spherical particle with uniform distribution and size with few nanometers. DLS was used to precise the diameter, which was found to be 23.68 ± 4.01 nm, as shown in Fig. 4c. The TEM photo indicates a combination of disorderly chains and some nanostructure enrichment. The different magnification of TEM microscopy reveals a spherical shape with a different diameter and smooth margins that are attached. This is consistent with previous studies that synthesized SeNPs using Arabic gum and measured size about 34 nm of spherical and monodispersed particles [47]. SeNPs produced by Vinifera have spherical shapes with sizes ranging from 3 to 18 nm [48]. SEM was used to investigate the morphological behavior of SeNPs, according to research findings [49].
Fig. 4.
TEM of SeNPs at 100, 50, nm (a, b), particles size distribution (c), SEM morphology (d), and EDX profile of fabricated SeNPs (e)
SEM analysis depicts that SeNPs were formed with the prevalent spherical form. The SEM image (Fig. 4d) reveals that the spherical shape of prepared SeNPs is aggregated with each other, returning to the drying process. Furthermore, the grains aggregate during the operation due to the generation of reduced atoms by reduction and nucleation [50]. This brought us back to other functional groups, in which nucleation binds to citric acid and selenious acid ions. Metal aggregation appears to be caused by the more metal ions that are easily accessible are participates in fewer nucleation steps [51]. Earlier studies have shown that spherical agglomerated nanoparticles outperform deformed nanoparticles in terms of biological activity [52].
EDX spectra were also used to confirm the elemental Se. The EDX study supplies the elements that might be used to fabricate nanostructures. Figure 4e shows a profile of a nanoparticle element created with green citric acid. With a ratio of 53.4%, elemental Se has displayed an electrical strong absorption peak at roughly 1.45 keV.
Antioxidant potency
The DPPH radical-scavenging analysis could be utilized to assess the antioxidant potency of SeNPs. As displayed in Fig. 5, the capability of radical-scavenging raises as the quantity of SeNPs increases. It was demonstrated that as the SeNPs quantities rose from 6.5 to 106.3 mg/ml, scavenging capability improved dramatically from 48.45 ± 5.29 to 301.1 ± 11.42 mg/g. The SeNPs’ ability to neutralize these free radicals may be attributed to the dispersibility of the nanoparticles via media due to their tiny particle size, in addition to SeNPs’ high chemical activity [53]. The oxygen-releasing antioxidant might improve the adequacy with which cells multiply, migrate, proliferate, and spread, advancing the healing procedure. As a result, the ability to eliminate free radicals generated by physiological circumstances could prevent cancer cell beginning and, as a result, the advancement of care health operations [28, 54]. Our results were matched with a recent study in which SeNPs exhibited dose-dependent antioxidant activity against ABTS and DDPH radicals [46].
Fig. 5.
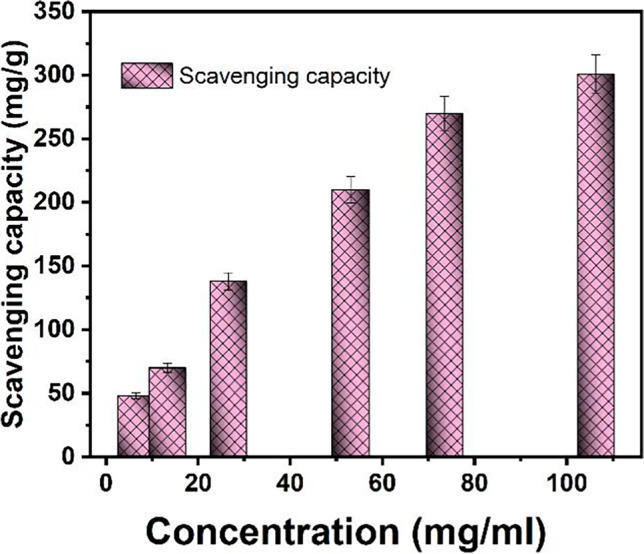
The DPPH radicals scavenging capacity of SeNPs (SD was calculated based on three repetitions) highly significant with (p < 0.0001) (p values > 0.05 non-significant, p < 0.05 significant, p < 0.01 very significant, and p < 0.001 extremely significant)
Cell viability evaluation
In vitro cytotoxicity of SeNPs was tested on the human MCF-7 cell line. Better understanding the cytotoxic potential of various SeNPs quantities was done better, as displayed in Fig. 6. Increasing the concentration of SeNPs drastically reduced cell viability. It began at 96.1 ± 3.2% for the control cell line (untreated) and fell to 61.2 ± 2.2% for the peak level of SeNPs, about 500 mg/ml. This is in agreement with previous studies in which SeNPs-apigenin significantly inhibited MCF-7 cancer cells in a dose-dependent manner. SeNPs-apigenin inhibits MCF-7 cell growth by less than 5% at 1000 M/ml, and the IC50 was determined to be 51.74 M/ml [55]. Furthermore, the cytotoxic capacity of SeNPs at different concentrations on HBL-100 and MDA-MB-231 cells was assessed in vitro. As per the findings, probably half of the undergo apoptosis, the inhibitory concentration (IC50) of rapid synthesized SeNPs against MDA-MB-231 cells was found to be 34 g mL−1 after 48 h. However, at lower doses, cytotoxicity of rapidly synthesized SeNPs against HBL-100 cells was not significant, and cytotoxicity increases when the inhibitory concentration exceeds 50 g mL−1 after 48 h [54].The substantial impact of SeNPs on the cells is attributed to the discharge of NPs colloids through cell culture, which may allow the interaction of ROS to the cell membrane. This situation is thought to degrade living cells and result in a high death rate.
Fig. 6.

Cell cytotoxicity effect of SeNPs on cultivated MCF-7 cell lines (p < 0.001) (p values > 0.05 non-significant, p < 0.05 significant, p < 0.01 very significant, and p < 0.001 extremely significant)
Furthermore, the cytotoxicity of SeNPs is greatly influenced by their crystallinity, small size, and dispersion [56]. This is related to low crystalline nanoparticles disintegrating rapidly, increasing the effective content of those NPs. As a result, controlling toxic behavior might be accomplished deeply through preparation settings.
Antibacterial properties
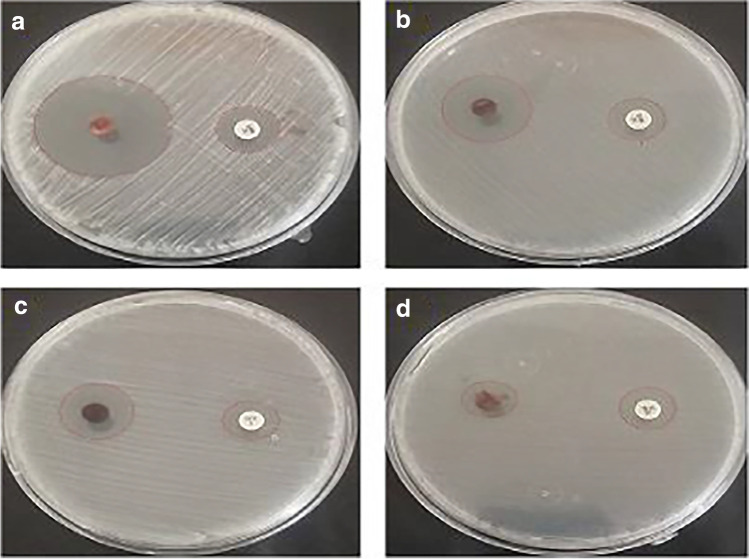
The nanoparticle stability is an important criterion for degrading bacterial cells, which is a critical prerequisite for many biological applications. As a result, the antibacterial properties of SeNPs were tested in vitro against E. coli, P. aeruginosa, S. aureus, and B. subtilis bacterium, using the zone of inhibition method compared to standard antibiotic discs (Fig. 7). The ZOI for these strains is reported in Table 1; it was discovered that SeNPs have little activity against S. aureus compared to the conventional antibiotic. SeNPs had the largest ZOI against E. coli, with a 25.2 ± 1.5 mm ZOI compared to 16.0 ± 0.6 mm for the traditional antibiotic.
Fig. 7.
Antibacterial achievement of SeNPs against E. coli (a), P. aeruginosa (b), B. subtilis (c), and S. aureus (d) compared to the standard antibiotic (p < 0.05) (p values > 0.05 non-significant, p < 0.05 significant, p < 0.01 very significant, and p < 0.001 extremely significant)
Table 1.
The data of ZOI during the interaction between SeNPs and bacterium
| Compound | S. aureus | B. subtilis | E. coli | P. aeruginosa | |
|---|---|---|---|---|---|
| Zone of inhibition (mm) | |||||
| SeNPs | 11.2 ± 1.1 | 15.2 ± 1.4 | 25.2 ± 1.5 | 20.2 ± 1.2 | p < 0.05 |
| Control antibiotic | 12.9 ± 0.4 | 13.5 ± 0.9 | 16.0 ± 0.6 | 15.0 ± 0.8 | |
(p values > 0.05 non-significant, p < 0.05 significant, p < 0.01 very significant, and p < 0.001 extremely significant)
SeNPs may be more active against G-negative than G-positive bacteria. For both types, this behavior is assigned to the cellular composition. The major reasons for bacterial cell death caused by NPs are the disintegration of the bacterial cell wall. Consequently, massive cytoplasmic leakage envelopes like proteins, amino acids, and carbohydrates [57]. The bactericidal potency of SeNPs is connected with ROS activation, which causes severe oxidative stress and, as a result, lipid peroxidation, protein oxidation, and DNA rapidly damaged [58]. Furthermore, the negative charges that have been discovered on the protein content in bacterial walls might cause interaction with ionic species generated by the existence of SeNPs. Previously, the antimicrobial activity of biologically synthesized SeNPs was investigated but using different methodologies and particle sizes and the finding suggests the SeNPs’ antimicrobial activity may be mediated by the generation of reactive oxygen species (ROS), nanoparticle penetration into cells, and disruption of cell survival pathways [46]. SeNPs have the potential for unique antimicrobial properties as well as low mammalian cell toxicity, implying that they should be studied further. Despite their significant potential as an antimicrobial biomaterial, SeNPs’ toxicity is poorly understood. Nonetheless, the findings of this study strongly suggest that, despite their low mammalian cell toxicity, SeNPs have unique anticancer activity and antimicrobial activity [59].
Anticoagulation activity
Anticoagulant effects of substances were assessed using aPTT reagent [60]. The results of the aPTT assay are determined by the clot recognition method and assay kits utilized. Blood clotting normal time was tested in between 25 and 53 s [61]. Blood inside the vasculature remains fluid and clots quickly when it contacts subendothelial surfaces. Thrombosis and bleeding are prevented in normal settings by coagulation/fibrinolysis balance. Any imbalance favors coagulation, which leads to thrombosis, platelet aggregation, fibrin production, and trapped red blood cells in arteries or veins. To treat thrombosis, various antithrombotic medicines are available on the market. Antiplatelet medications prevent platelet activation or aggregation, while anticoagulant medications prevent fibrin production, but fibrinolytic treatments break down fibrin formation [62]. Using biologically produced AgNPs compared with selective active molecules has recently been demonstrated to be successful anticoagulant properties and requires less than active molecules [41, 63]. In another approach, bio-inspired cobalt nanoparticles from red algae have successfully prevented blood clotting formation in vitro [2]. Whereas the mechanisms underlying SeNPs anticoagulant activity have not been theoretically decrypted, previous research on metal oxide nanoparticle anticoagulant activities, particularly on silver and gold nanoparticle anticoagulant activities [64, 65], has aided in clarifying the likely biochemical mechanism. These studies demonstrated that these nanoparticles inhibit the conversion of prothrombin to thrombin, a critical step in the formation of insoluble fibrin strands and the catalysis of other clotting factors [66]. In this study, SeNPs synthesized using citric acid exhibit increased anticoagulant properties with increased SeNPs dose but still less than heparin which is the standard anticoagulant drug (Fig. 8).
Fig. 8.
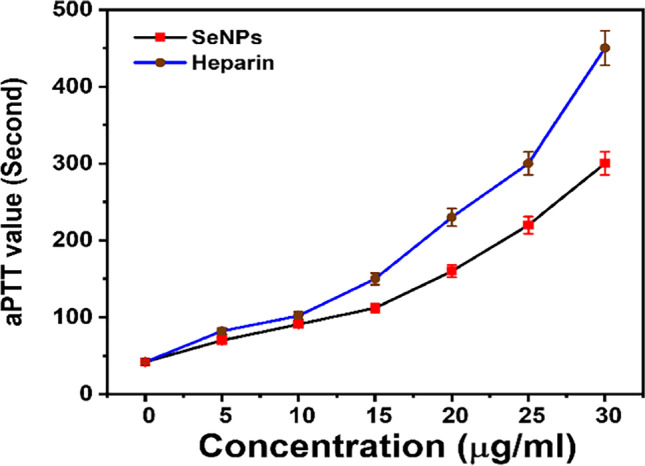
Anticoagulation activity of SeNPs (p < 0.001) (p values > 0.05 non-significant, p < 0.05 significant, p < 0.01 very significant, and p < 0.001 extremely significant)
Conclusion
An optimized synthesis of SeNPs based on citric acid and sodium alginate in the microwave was an easily accessible, simple, and costless technique. The fabricated SeNPs crystallite size was roughly 23.68 nm. The TEM analysis revealed the generation of SeNPs in monodisperse and spherical shapes with diameters ranging from 8.5 to 22 nm. SEM morphological analysis revealed that agglomerated SeNPs were produced in spherical shapes with diameters ranging from 0.67 to 0.83 mm. Furthermore, the antioxidant potency was assessed by DPPH radical scavenging, which revealed that SeNPs with a concentration of 106.3 mg/mL had the maximum scavenging capacity (301.1 ± 11.42 mg/g). Antibacterial activity was particularly strong against E. coli, P. aeruginosa, S. aureus, and B. subtilis. While SeNPs had little effect against S. aureus, they had the largest ZOI against E. coli, with a 25.2 ± 1.5 mm ZOI compared to 16.0 ± 0.6 mm for the conventional antibiotic. SeNPs have clear anticoagulant properties near heparin as standard molecules. Finally, SeNPs’ great potency supports their use in various biological applications.
Acknowledgements
This work is funded by the deanship of Scientific Research at University of Tabuk for through project number S-1442–0092.
Declarations
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shoueir KR, et al. Chitosan based-nanoparticles and nanocapsules: overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int J Biol Macromol. 2021;167:1176–1197. doi: 10.1016/j.ijbiomac.2020.11.072. [DOI] [PubMed] [Google Scholar]
- 2.Ajarem JS et al. Benign synthesis of cobalt oxide nanoparticles containing red algae extract: antioxidant, antimicrobial, anticancer, and anticoagulant activity. Journal of Cluster Science, 2021: p. 1–12.
- 3.Fouda MM, et al. Carboxymethyl cellulose supported green synthetic features of gold nanoparticles: antioxidant, cell viability, and antibacterial effectiveness. Synth Met. 2020;269:116553. doi: 10.1016/j.synthmet.2020.116553. [DOI] [Google Scholar]
- 4.El-Desouky N et al. Synthesis of silver nanoparticles using bio valorization coffee waste extract: photocatalytic flow-rate performance, antibacterial activity, and electrochemical investigation. Biomass Conversion and Biorefinery, 2022: p. 1–15. [DOI] [PMC free article] [PubMed]
- 5.Shoueir K, et al. Fenton-like nanocatalyst for photodegradation of methylene blue under visible light activated by hybrid green DNSA@ Chitosan@ MnFe2O4. Carbohyd Polym. 2018;197:17–28. doi: 10.1016/j.carbpol.2018.05.076. [DOI] [PubMed] [Google Scholar]
- 6.El-Desouky N et al. Bio-inspired green manufacturing of plasmonic silver nanoparticles/Degussa using banana waste peduncles: photocatalytic, antimicrobial, and cytotoxicity evaluation. journal of materials research and technology, 2021. 10: p. 671–686.
- 7.El-Shabasy R, et al. A green synthetic approach using chili plant supported Ag/Ag2O@ P25 heterostructure with enhanced photocatalytic properties under solar irradiation. Optik. 2019;192:162943. doi: 10.1016/j.ijleo.2019.162943. [DOI] [Google Scholar]
- 8.Shoueir K, Mohanty A, Janowska I (2022) Industrial molasses waste in the performant synthesis of few-layer graphene and its Au/Ag nanoparticles nanocomposites. Photocatalytic and supercapacitance applications. J Clean Prod 351:131540
- 9.Alagesan V, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9(1):105–116. doi: 10.1007/s12668-018-0566-8. [DOI] [Google Scholar]
- 10.Rajeshkumar S, Veena P, Santhiyaa R. Exploring the Realms of Nature for Nanosynthesis. Springer; 2018. Synthesis and characterization of selenium nanoparticles using natural resources and its applications; pp. 63–79. [Google Scholar]
- 11.Al Jahdaly BA et al. Selenium nanoparticles synthesized using an eco-friendly method: dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. journal of materials research and technology, 2021. 11: p. 85–97.
- 12.Ferro C, Florindo HF, Santos HA. Selenium nanoparticles for biomedical applications: from development and characterization to therapeutics. Adv Healthcare Mater. 2021;10(16):2100598. doi: 10.1002/adhm.202100598. [DOI] [PubMed] [Google Scholar]
- 13.Ikram M, et al. Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int J Nanomed. 2021;16:249. doi: 10.2147/IJN.S295053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javed R, et al. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. Journal of Nanobiotechnology. 2020;18(1):1–15. doi: 10.1186/s12951-020-00704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakr TM, Korany M, Katti KV. Selenium nanomaterials in biomedicine—an overview of new opportunities in nanomedicine of selenium. Journal of Drug Delivery Science and Technology. 2018;46:223–233. doi: 10.1016/j.jddst.2018.05.023. [DOI] [Google Scholar]
- 16.Chen N, Zhao C, Zhang T. Selenium transformation and selenium-rich foods. Food Biosci. 2021;40:100875. doi: 10.1016/j.fbio.2020.100875. [DOI] [Google Scholar]
- 17.Xu C, et al. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohyd Polym. 2018;195:576–585. doi: 10.1016/j.carbpol.2018.04.110. [DOI] [PubMed] [Google Scholar]
- 18.Sherlock LG, et al. Neonatal selenoenzyme expression is variably susceptible to duration of maternal selenium deficiency. Antioxidants. 2021;10(2):288. doi: 10.3390/antiox10020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minich WB. Selenium metabolism and biosynthesis of selenoproteins in the human body. Biochem Mosc. 2022;87(1):S168–S177. doi: 10.1134/S0006297922140139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng F, et al. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020;34:101475. doi: 10.1016/j.redox.2020.101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon S, et al. Selenium nanoparticles: a potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf, B. 2018;170:280–292. doi: 10.1016/j.colsurfb.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Collery P. Strategies for the development of selenium-based anticancer drugs. J Trace Elem Med Biol. 2018;50:498–507. doi: 10.1016/j.jtemb.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Shi X-D, et al. Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit Rev Food Sci Nutr. 2021;61(13):2225–2236. doi: 10.1080/10408398.2020.1774497. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim ATA. Toxicological impact of green synthesized silver nanoparticles and protective role of different selenium type on Oreochromis niloticus: hematological and biochemical response. J Trace Elem Med Biol. 2020;61:126507. doi: 10.1016/j.jtemb.2020.126507. [DOI] [PubMed] [Google Scholar]
- 25.Mittal AK, et al. Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. J Mater Nanosci. 2016;3(1):22–27. [Google Scholar]
- 26.Zhang C, et al. Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment. Advanced Science. 2021;8(3):2002797. doi: 10.1002/advs.202002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokarz P, Woźniak K. SENP proteases as potential targets for cancer therapy. Cancers. 2021;13(9):2059. doi: 10.3390/cancers13092059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An Y and J. Zhao, Functionalized selenium nanotherapeutics synergizes with zoledronic acid to suppress prostate cancer cell growth through induction of mitochondria-mediated apoptosis and cell cycle S phase arrest. Frontiers in Oncology, 2021. 11. [DOI] [PMC free article] [PubMed]
- 29.Zhang P, et al. Wound healing acceleration by antibacterial biodegradable black phosphorus nanosheets loaded with cationic carbon dots. J Mater Sci. 2021;56(10):6411–6426. doi: 10.1007/s10853-020-05766-1. [DOI] [Google Scholar]
- 30.Zeng Q, et al. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioactive materials. 2021;6(9):2647–2657. doi: 10.1016/j.bioactmat.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, et al. In situ growth gold nanoparticles in three-dimensional sugarcane membrane for flow catalytical and antibacterial application. J Hazard Mater. 2021;402:123445. doi: 10.1016/j.jhazmat.2020.123445. [DOI] [PubMed] [Google Scholar]
- 32.Hassan AA, et al. Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: Morphology, cell adhesion, and antibacterial activity. Int J Pharm. 2021;593:120143. doi: 10.1016/j.ijpharm.2020.120143. [DOI] [PubMed] [Google Scholar]
- 33.Salayová A, et al. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: morphology and antibacterial efficacy. Nanomaterials. 2021;11(4):1005. doi: 10.3390/nano11041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lateef A, et al. Nanomedical applications of nanoparticles for blood coagulation disorders. Environmental nanotechnology, 2018: p. 243–277.
- 35.Lakshme PT, et al. Evaluation of antioxidant and cytotoxic effect of selenium nanoparticles synthesised using Capparis decidua. Evaluation, 2020. 32(19).
- 36.Boroumand, S., et al., Selenium nanoparticles: synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Materials Research Express, 2019. 6(8): p. 0850d8.
- 37.Alkhudhayri AA, et al. Selenium nanoparticles induce cytotoxicity and apoptosis in human breast cancer (MCF-7) and liver (HEPG2) cell lines. Nanosci Nanotechnol Lett. 2020;12(3):324–330. doi: 10.1166/nnl.2020.3115. [DOI] [Google Scholar]
- 38.Chen J, et al. CuO NPs@ Starch as a novel chemotherapeutic drug for the treatment of several types of gastrointestinal system cancers including gastric, pancreatic, and colon cancers. Arabian Journal of Chemistry, 2022: p. 103681.
- 39.Al-Wafi R, Mansour S, Ahmed M. Mechanical, microstructural properties and cell adhesion of Sr/Se-hydroxyapatite/graphene/polycaprolactone nanofibers. J Thermoplast Compos Mater. 2021;34(4):536–556. doi: 10.1177/0892705720912781. [DOI] [Google Scholar]
- 40.Ahmed M, Menazea A, Abdelghany A. Blend biopolymeric nanofibrous scaffolds of cellulose acetate/ε-polycaprolactone containing metallic nanoparticles prepared by laser ablation for wound disinfection applications. Int J Biol Macromol. 2020;155:636–644. doi: 10.1016/j.ijbiomac.2020.03.257. [DOI] [PubMed] [Google Scholar]
- 41.Shanthi N, et al. Extraction of fucoidan from Turbinaria decurrens and the synthesis of fucoidan-coated AgNPs for anticoagulant application. ACS Omega. 2021;6(46):30998–31008. doi: 10.1021/acsomega.1c03776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassanien R, Abed-Elmageed AA, Husein DZ. Eco-friendly approach to synthesize selenium nanoparticles: photocatalytic degradation of sunset yellow azo dye and anticancer activity. ChemistrySelect. 2019;4(31):9018–9026. doi: 10.1002/slct.201901267. [DOI] [Google Scholar]
- 43.Kirupagaran R, Vedhi C. Journal of Nanoscience and Technology. Journal of Nanoscience and Technology. 2016;2(5):224–226. [Google Scholar]
- 44.Shoueir K, et al. Thallium and selenite doped carbonated hydroxyapatite: microstructural features and anticancer activity assessment against human lung carcinoma. Ceram Int. 2020;46(4):5201–5212. doi: 10.1016/j.ceramint.2019.10.268. [DOI] [Google Scholar]
- 45.Ahmed M, et al. Structural, mechanical and thermal features of Bi and Sr co-substituted hydroxyapatite. J Mater Sci. 2019;54(3):1977–1991. doi: 10.1007/s10853-018-2999-4. [DOI] [Google Scholar]
- 46.Abdel-Moneim A-ME, et al. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi Journal of Biological Sciences. 2022;29(2):1197–1209. doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu W-Y, et al. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf, B. 2018;170:692–700. doi: 10.1016/j.colsurfb.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Moneim A-ME, et al. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol Trace Elem Res. 2022;200(2):768–779. doi: 10.1007/s12011-021-02662-w. [DOI] [PubMed] [Google Scholar]
- 49.Menon S, et al. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid and Interface Science Communications. 2019;29:1–8. doi: 10.1016/j.colcom.2018.12.004. [DOI] [Google Scholar]
- 50.Yang H, et al. Targeting bone microenvironments for treatment and early detection of cancer bone metastatic niches. J Control Release. 2022;341:443–456. doi: 10.1016/j.jconrel.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, et al. Aerobic and anaerobic biosynthesis of nano-selenium for remediation of mercury contaminated soil. Chemosphere. 2017;170:266–273. doi: 10.1016/j.chemosphere.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Jamkhande PG, et al. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. Journal of drug delivery science and technology. 2019;53:101174. doi: 10.1016/j.jddst.2019.101174. [DOI] [Google Scholar]
- 53.Liu J, et al. Recent progress of surface modified nanomaterials for scavenging reactive oxygen species in organism. Bioconjug Chem. 2021;32(11):2269–2289. doi: 10.1021/acs.bioconjchem.1c00402. [DOI] [PubMed] [Google Scholar]
- 54.Cittrarasu V, et al. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci Rep. 2021;11(1):1–15. doi: 10.1038/s41598-020-80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Otaibi AM et al. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environmental Science and Pollution Research, 2022: p. 1–10. [DOI] [PubMed]
- 56.Varlamova EG, et al. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int J Mol Sci. 2021;22(15):7798. doi: 10.3390/ijms22157798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makvandi P, et al. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv Func Mater. 2020;30(22):1910021. doi: 10.1002/adfm.201910021. [DOI] [Google Scholar]
- 58.Nastulyavichus A, et al. Antibacterial coatings of Se and Si nanoparticles. Appl Surf Sci. 2019;469:220–225. doi: 10.1016/j.apsusc.2018.11.011. [DOI] [Google Scholar]
- 59.Tran PA et al. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology, 2015. 27(4): p. 045101. [DOI] [PubMed]
- 60.Miranda VMAM, et al. Evaluation of partial thromboplastin time, thrombin time and prothrombin time over treated plasma using a fibrinolytic protease. Research, Society and Development. 2022;11(2):e15311225439–e15311225439. doi: 10.33448/rsd-v11i2.25439. [DOI] [Google Scholar]
- 61.Henderson RA, et al. Hematologic evaluation of intraoperative autologous blood collection and allogeneic transfusion in cardiac surgery. Transfusion. 2021;61(3):788–798. doi: 10.1111/trf.16259. [DOI] [PubMed] [Google Scholar]
- 62.Altaf F, Wu S, Kasim V. Role of fibrinolytic enzymes in anti-thrombosis therapy. Front Mol Biosci. 2021;8:476. doi: 10.3389/fmolb.2021.680397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lateef A, et al. Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Preparative Biochemistry and Biotechnology, 2018. 48(7): p. 646–652. [DOI] [PubMed]
- 64.Li T, D. Yuan, and J. Yuan Antithrombotic drugs—pharmacology and perspectives. Coronary Artery Disease: Therapeutics and Drug Discovery, 2020: p. 101–131. [DOI] [PubMed]
- 65.Talank N, et al. Bioengineering of green-synthesized silver nanoparticles: In vitro physicochemical, antibacterial, biofilm inhibitory, anticoagulant, and antioxidant performance. Talanta. 2022;243:123374. doi: 10.1016/j.talanta.2022.123374. [DOI] [PubMed] [Google Scholar]
- 66.Jin NZ, Gopinath SC. Potential blood clotting factors and anticoagulants. Biomed Pharmacother. 2016;84:356–365. doi: 10.1016/j.biopha.2016.09.057. [DOI] [PubMed] [Google Scholar]