Abstract
Background
It was urgent and necessary to synthesize the evidence for vaccine effectiveness (VE) against SARS-CoV-2 variants of concern (VOC). We conducted a systematic review and meta-analysis to provide a comprehensive overview of the effectiveness profile of COVID-19 vaccines against VOC.
Methods
Published randomized controlled trials (RCTs), cohort studies, and case-control studies that evaluated the VE against VOC (Alpha, Beta, Gamma, Delta, or Omicron) were searched until 4 March 2022. Pooled estimates and 95% confidence intervals (CIs) were calculated using random-effects meta-analysis. VE was defined as (1-estimate).
Results
Eleven RCTs (161,388 participants), 20 cohort studies (52,782,321 participants), and 26 case-control studies (2,584,732 cases) were included. Eleven COVID-19 vaccines (mRNA-1273, BNT162b2, ChAdOx1, Ad26.COV2.S, NVX-CoV2373, BBV152, CoronaVac, BBIBP-CorV, SCB-2019, CVnCoV, and HB02) were included in this analysis. Full vaccination was effective against Alpha, Beta, Gamma, Delta, and Omicron variants, with VE of 88.0% (95% CI, 83.0–91.5), 73.0% (95% CI, 64.3–79.5), 63.0% (95% CI, 47.9–73.7), 77.8% (95% CI, 72.7–82.0), and 55.9% (95% CI, 40.9–67.0), respectively. Booster vaccination was more effective against Delta and Omicron variants, with VE of 95.5% (95% CI, 94.2–96.5) and 80.8% (95% CI, 58.6–91.1), respectively. mRNA vaccines (mRNA-1273/BNT162b2) seemed to have higher VE against VOC over others; significant interactions (pinteraction < 0.10) were observed between VE and vaccine type (mRNA vaccines vs. not mRNA vaccines).
Conclusions
Full vaccination of COVID-19 vaccines is highly effective against Alpha variant, and moderate effective against Beta, Gamma, and Delta variants. Booster vaccination is more effective against Delta and Omicron variants. mRNA vaccines seem to have higher VE against Alpha, Beta, Gamma, and Delta variants over others.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02397-y.
Keywords: SARS-CoV-2, COVID-19, Variants of concern, Systematic review, Vaccine effectiveness
Background
Since emerging of coronavirus disease 2019 (COVID-19) caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, more than 452 million cases and 6.0 million deaths have been documented worldwide as of 12 March 2022 [1]. COVID-19 vaccines have been rapidly developed and proved to be highly effective in multiple randomized clinical trials (RCTs) [2–5] and observational studies [6–8]. As of 5 March 2022, more than 10 billion vaccine doses have been administered all over the world, but around 150 thousand new cases are diagnosed each day [1]. Most current vaccines used SARS-CoV-2 spike protein as the key antigenic target based on the originally identified Wuhan lineage virus [9]. The B.1.1.7 (Alpha) variant was first identified from genomic sequencing of samples obtained from COVID-19 patients which accounted for an expanding proportion of cases in England in late 2020 [10]. Subsequently, the emergence of the B.1.351 (Beta) variant in South Africa and the P.1 (Gamma) variant in Brazil increased the COVID-19 pandemic. In December 2020, a novel SARS-CoV-2 variant, the B.1.617.2 (Delta) variant was first detected in India, causing a sharp increase in COVID-19 cases and deaths in India and surrounding countries [11]. Recently, the B.1.1.529 (Omicron) variant emerged in December 2021 contains more than 30 mutations in the spike protein, raising concerns for naturally acquired or vaccinated population [12]. The emerging Alpha, Beta, Gamma, Delta, and Omicron variants were classified as variants of concern (VOC), which were associated with the transmission increasing, more severe disease situation (e.g., increased hospitalizations or deaths), significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures [13–18]. The importance of vaccination programs and efficient public health measures will be increased if VOC have increased transmissibility or virulence [19]. It was urgent and necessary to synthesize evidence of the vaccine effectiveness (VE) of COVID-19 vaccines against VOC. To our knowledge, there are some studies evaluating the VE of COVID-19 vaccines against VOC [20–23]. Some relevant systematic review or meta-analysis about COVID-19 vaccines against Delta variant have been published to date [24–26], which did not include many recent studies as the most recent retrieval date was October 2021. Therefore, to gain insight in the VE of COVID-19 vaccines against five kinds of VOC, we conducted a comprehensive systematic review and meta-analysis including both RCTs and observational studies. This review of the VE of COVID-19 vaccines against VOC will support global response on public health measures and vaccination programs timely and evidence based.
Methods
Data sources and searches
We conducted this systematic review according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [27]; the protocol was registered on PROSPERO (CRD42021273986). We searched for literature published on PubMed, Embase, Cochrane Library, and the ClinicalTrials.gov website on or before 4 March 2022. Keywords including “COVID-19,” “SARS-CoV-2,” “vaccine,” and “variant” were used to search; the detailed search strategy was shown in the Additional file 1 (Appendix S1). Additionally, we identified references by searching the reference lists of included studies and relevant reviews.
Selection of studies
We included randomized controlled trials (RCTs), cohort studies, and case-control studies that evaluated the efficacy or effectiveness of COVID-19 vaccines against VOC including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and B.1.1.529 (Omicron). Studies enrolling general population or special populations (e.g., healthcare workers) aged 12 years or older were included. For studies that only reported VE against SARS-CoV-2 infections (without subgroup analysis of VOC), but the specific VOC accounted for 50% or more among positive tests, they were also included in the analysis. We excluded study protocols, editorials, comments, reviews, news, case reports, conference abstracts, animal studies, in vivo experiments, and analysis of antibody neutralization. Searches were limited to English articles. The primary outcome was the VE of full vaccination against VOC; the studies which only reported the VE of partial vaccination were excluded.
Data extraction
Two authors reviewed titles and abstracts independently to identify eligible studies that met pre-specified inclusion criteria and extracted data. When consensus was lacking, a third reviewer was consulted. The journal name, study type, study location, vaccine information, number of participants, characteristics of subjects, and outcomes were extracted from eligible studies. We extracted SARS-CoV-2 infection information if results on both SARS-CoV-2 infection and symptomatic infection were reported. The adjusted VE or estimates of effect size (relative risks, incidence rate ratios, or odds ratios) with corresponding 95% confidence intervals (CIs) were extracted with priority. The risk of bias of RCTs was assessed using the Cochrane Collaboration’s tool [28, 29]. The risk of bias of cohort and case-control studies was assessed using the Newcastle-Ottawa scale (NOS) [30].The NOS contains 8 categories relating to methodological quality, with a maximum of 9 points. A total score of 7–9 points is considered of good quality, while a score of 4–6 points of moderate quality, and a score of 1–3 points of low quality. Two investigators reviewed the studies and judged the risk of bias.
Statistical analysis
Pooled estimates and 95% CIs were calculated using DerSimonian and Laird random-effects meta-analysis [31]. Summary VE was defined as (1-pooled estimate) ×100%. We performed subgroup analysis stratifying by study design, vaccine type, participant, and publication. P for the difference was calculated using random-effects meta-regression, a difference between the estimates of these subgroups was considered significant if pinteraction < 0.10 [32]. Statistical heterogeneity between the studies was assessed with the χ2 test and the I2 statistics. I2 values of 25%, 50%, and 75% have been suggested to be indicators of low, moderate, and high heterogeneity, respectively [33]. All the analyses were performed with STATA 14.
Results
Literature search and study characteristics
This systematic literature search identified 6740 publications; after excluding duplicates and irrelevant papers, 219 published reports were evaluated in full text for eligibility (Additional file 1: Figure S1). Finally, 57 articles were included in the present systematic review [6, 7, 20–23, 34–84]. There were different study designs for included studies, 11 RCTs (161,388 participants) [20, 22, 23, 34–41], 20 cohort studies (52,782,321 participants) [6, 7, 42–59], and 26 case-control studies (2,584,732 cases) [21, 60–84]. In total, 11 COVID-19 vaccines (mRNA-1273, BNT162b2, ChAdOx1, Ad26.COV2.S, NVX-CoV2373, BBV152, CoronaVac, BBIBP-CorV, SCB-2019, CVnCoV, and HB02) and 5 VOC (Alpha, Beta, Gamma, Delta, and Omicron) were included in this study. BNT162b2, mRNA-1273, and CVnCoV are mRNA vaccines; CoronaVac, HB02, BBV152, and BBIBP-CorV are inactivated vaccines; Ad26.COV2.S and ChAdOx1 are non-replicating vector vaccines; and NVX-CoV2373 and SCB-2019 are protein subunit vaccines. Only Ad26.COV2.S is a single-dose vaccine; therefore, a one-dose regimen is regarded as full vaccination. Characteristics of individual studies are summarized in Table 1.
Table 1.
Study characteristics and participants demographics
| First author | Journal | Study design | VOC c | Vaccine | Country | Population characteristics | N |
|---|---|---|---|---|---|---|---|
| Shinde (2021) [20] | N Engl J Med | RCT | Beta (GS) | NVX-CoV2373 | South Africa | GP; age: 18–84 | 4,387 |
| Madhi (2021) [22] | N Engl J Med | RCT | Beta (GS) | ChAdOx1 | South Africa | GP; age: 18–65 | 1,467 |
| Heath (2021) [38] | N Engl J Med | RCT | Alpha (GS) | NVX-CoV2373 | UK | GP; age: 18–84 | 14,039 |
| Sadoff (2022) [40] | N Engl J Med | RCT | Beta (GS) | Ad26.COV2.S | South Africa | GP; age: ≥18 | 4,969 |
| Emary (2021) [23] | Lancet | RCT | Alpha (GS) | ChAdOx1 | UK | GP; age: ≥18 | 8,534 |
| Ella (2021) [37] | Lancet | RCT | Delta (GS) | BBV152 | India | GP; age: 18–98 | 16,973 |
| Thomas (2021) [41] | N Engl J Med | RCT | Beta (GS) | BNT162b2 | South Africa | GP; age: ≥16 | 800 |
| Clemens (2021) [35] | Nat Commun | RCT | Gamma (GS) | ChAdOx1 | Brazil | GP; age: ≥18 | 10,416 |
| Bravo (2022) [34] | Lancet | RCT | Gamma & Delta (GS) | SCB-2019 | Five regions | GP; age: ≥18 | 30,174 |
| Dunkle (2022) [36] | N Engl J Med | RCT | Alpha (GS) | NVX-CoV2373 | USA and Mexico | GP; age: ≥18 | 29,949 |
| Kremsner (2022) [39] | Lancet Infect Dis | RCT | Alpha & Gamma (GS) | CVnCoV | Europe/Latin America | GP; age: ≥18 | 39,680 |
| Lopez Bernal_1 (2021) [21] | N Engl J Med | TNCC | Alpha & Delta (GS) | BNT162b2 or ChAdOx1 | UK | GP; age: ≥16 | 19,109 b |
| Abu-Raddad (2021) [60] | N Engl J Med | TNCC | Alpha & Beta (GS) | BNT162b2 | Qatar | GP; age: 33 (22–40) a | 35,979 b |
| Sheikh (2021) [80] | Lancet | TNCC | Alpha & Delta (GS) | BNT162b2 or ChAdOx1 | UK | GP; age: ≥16 | 19,543 b |
| Chemaitelly_1 (2021) [67] | Nat Med | TNCC | Alpha & Beta (GS) | mRNA-1273 | Qatar | GP; age: 32 (25–39) a | 66,042 b |
| Lopez Bernal_2 (2021) [76] | BMJ | TNCC | Alpha | BNT162b2 | UK | Older adults; age: ≥70 | 3,034 b |
| Chung (2021) [68] | BMJ | TNCC | Alpha & Beta/Gamma (GS) | BNT162b2 and mRNA-1273 | Canada | GP; ≥16 | 324,033 b |
| Ranzani (2021) [79] | BMJ | TNCC | Gamma | CoronaVac | Brazil | Older adults; ≥70 | 43,774 b |
| Carazo (2021) [64] | Clin Infect Dis | TNCC | Alpha | BNT162b2 and mRNA-1273 | Canada | HCWs; age: 18–74 | 901 b |
| Li (2021) [75] | Emerg Microbes Infect | TNCC | Delta (GS) | CoronaVac and BBIBP-CorV | China | GP; age:18–59 | 74 b |
| Charmet (2021) [65] | Lancet Reg Health Eur | Case-control | Alpha & Beta/Gamma | BNT162b2 and mRNA-1273 | France | GP; age: ≥20 | 33,863 b |
| Nasreen (2022) [77] | Nat Microbiol | TNCC | Alpha & Beta & Gamma & Delta (GS) | BNT162b2, mRNA-1273, or ChAdOx1 | Canada | GP; age: ≥16 | 51,440 b |
| Hitchings_1 (2021) [73] | Lancet Reg Health Am | TNCC | Gamma | CoronaVac | Brazil | HCWs; age: ≥18 | 418 b |
| Tang (2021) [82] | Nat Med | TNCC | Alpha & Delta | BNT162b2 or mRNA-1273 | Qatar | GP; age: 27 (12–36) a | 2,934 b |
| Chemaitelly_2 (2021) [66] | N Engl J Med | TNCC | Alpha & Beta & Delta (GS) | BNT162b2 | Qatar | GP; age: 31 (21–39) a | 113,830 b |
| Grannis (2021) [70] | MMWR | TNCC | Delta | BNT162b2, mRNA-1273 or Ad26.COV2.S | USA | GP; age: ≥18 | 3,657 b |
| Sritipsukho (2022) [81] | Emerg Microbes Infect | TNCC | Delta | CoronaVac and/or ChAdOx1 | Thailand | GP; age: ≥18 | 1,118 b |
| Klein (2022) [74] | MMWR | TNCC | Delta & Omicron | BNT162b2 or mRNA-1273 | USA | GP; age: ≥18 | 3,860 b |
| Oliveira (2022) [78] | JAMA Network Open | TNCC | Delta (GS) | BNT162b2 | USA | Adolescents; 12–18 | 186 b |
| Tseng (2022) [84] | Nat Med | TNCC | Delta & Omicron (GS) | mRNA-1273 | USA | GP; age: ≥18 | 23,512 b |
| Ferdinands (2022) [69] | MMWR | TNCC | Delta & Omicron | BNT162b2 or mRNA-1273 | USA | GP; age: ≥18 | 18, 637 b |
| Britton (2022) [63] | JAMA | TNCC | Delta | BNT162b2, mRNA-1273, or Ad26.COV2.S. | USA | GP; age: ≥12 | 329,057 b |
| Grant (2022) [71] | Lancet Reg Health Eur | Case-control | Delta | BNT162b2 or mRNA-1273 | France | GP; age: ≥20 | 8,644 b |
| Andrews_1 (2022) [62] | N Engl J Med | TNCC | Delta | BNT162b2 or ChAdOx1 | UK | GP; age: ≥16 | 1,125,257 b |
| Andrews_2 (2022) [61] | Nat Med | TNCC | Delta (GS) | BNT162b2 | UK | GP; age: ≥18 | 343,955 b |
| Thiruvengadam (2021) [83] | Lancet Infect Dis | TNCC | Delta (GS) | ChAdOx1 | India | GP; age: 35 (28–45) a | 2,766 b |
| Hitchings_2 (2021) [72] | Nat Commun | TNCC | Gamma | ChAdOx1 | Brazil | Older adults; ≥60 | 30,680 b |
| Hall (2021) [6] | Lancet | PRO cohort | Alpha | BNT162b2 | UK | HCWs; age: ≥18 | 23,324 |
| Haas (2021) [46] | Lancet | RETRO cohort | Alpha | BNT162b2 | Israel | GP; age: ≥16 | 6,538,911 |
| Dagan (2021) [7] | N Engl J Med | RETRO cohort | Alpha | BNT162b2 | Israel | GP; age: ≥16 | 1,193,236 |
| Lumley (2021) [49] | Clin Infect Dis | RETRO cohort | Alpha (GS) | BNT162b2 and ChAdOx1 | UK | HCWs; age: 39(30–50) a | 13,109 |
| Williams (2021) [58] | Clin Infect Dis | RETRO cohort | Gamma (GS) | mRNA-1273 | Canada | LTCH | 143 |
| Nanduri (2021) [51] | MMWR | RETRO cohort | Delta | mRNA-1273 or BNT162b2 | US | Nursing home | 5,965,607 |
| Fowlkes (2021) [44] | MMWR | RETRO cohort | Delta | mRNA-1273 and BNT162b2 | US | Frontline workers | 2,840 |
| Pouwels (2021) [53] | Nat Med | RETRO cohort | Alpha & Delta | BNT162b2 or ChAdOx1 or mRNA-1273 | UK | GP; age: 18–64 | 743,526 |
| Flacco (2021) [43] | Vaccines | RETRO cohort | Alpha | BNT162b2 | Italian | GP; age: ≥18 | 204,840 |
| Glatman-Freedman (2021) [45] | Emerg Infect Dis | RETRO cohort | Delta | BNT162b2 | Israel | Adolescents; 12–15 | 601,625 |
| Seppälä (2021) [56] | Euro Surveill | RETRO cohort | Alpha & Delta (GS) | BNT162b2 or mRNA-1273 | Norway | GP; age: ≥18 | 18,431 |
| Fabiani (2022) [42] | BMJ | RETRO cohort | Alpha & Delta | BNT162b2 or mRNA-1273 | Italy | GP; age: ≥16 | 33,250,344 |
| Risk (2022) [55] | Clin Infect Dis | RETRO cohort | Delta | BNT162b2, mRNA-1273, or Ad26.COV2.S. | USA | GP; age: ≥18 | 159,055 |
| Kang (2022) [47] | Ann Intern Med | RETRO cohort | Delta (GS) | CoronaVac or HB02 | China | GP; age: ≥18 | 10,805 |
| Poukka (2022) [52] | Vaccine | RETRO cohort | Delta | BNT162b2 or mRNA-1273 or ChAdOx1 | Finland | 16–69 HCWs | 427,905 |
| Wu (2022) [59] | China CDC Wkly | RETRO cohort | Delta (GS) | BBIBP-CorV or CoronaVac | China | Close contacts; age: ≥18 | 1,462 |
| Katz (2022) [48] | Vaccine | PRO cohort | Alpha (GS) | BNT162b2 | Israel | HCWs; age: 45 (36–55) a | 1,250 |
| Lutrick (2021) [50] | MMWR | PRO cohort | Delta | BNT162b2 | USA | Adolescents; 12–17 | 243 |
| Reis (2021) [54] | N Engl J Med | RETRO cohort | Delta | BNT162b2 | Israel | Adolescents; 12–18 | 188,708 |
| Tartof (2021) [57] | Lancet | RETRO cohort | Delta (GS) | mRNA-1273 | USA | GP; age: ≥12 | 3,436,957 |
Abbreviations: VOC variants of concern, HCWs healthcare workers, TNCC test-negative case-control, LTCH long-term care homes, GP general population, RCT randomized controlled trial, GS genomic sequencing, N number of participants, PRO prospective, RETRO retrospective
aMedian age (interquartile range)
bCases
cVOC were identified by genomic sequencing (GS) or variant circulation dominance
Risk of Bias
All the RCTs were assessed as some concerns for overall risk-of-bias judgment. Fifteen of 20 cohort studies were judged as good quality, and the remaining 5 studies were moderate quality. For 26 case-control studies, 22 were considered as good quality and 4 were moderate quality. The detailed risk of bias assessment is available in Additional file 1 (Tables S1–S3).
Vaccine effectiveness of COVID-19 vaccines against B.1.1.7 (Alpha) variant
Five RCTs [23, 36, 38–40], 9 cohort studies [6, 7, 42, 43, 46, 48, 49, 53, 56], and 10 case-control studies [21, 60, 64–68, 76, 77, 80] had evaluated the VE of COVID-19 vaccines against the Alpha variant. Six COVID-19 vaccines (BNT162b2, mRNA-1273, NVX-CoV2373, ChAdOx1, Ad26.COV2.S and CVnCoV) were included in this analysis. Four studies enrolled healthcare workers [6, 48, 49, 64], one enrolled adults aged 70 or older [76], and the others enrolled the general population. Characteristics of individual studies and VE for Alpha variant are summarized in Fig. 1 and Additional file 1 (Table S4).
Fig. 1.
Forest plot showing VE of full vaccination against Alpha variant. Abbreviations: VE, vaccine effectiveness; CI, confidence interval; RCT, randomized controlled trial
The summary VE of full vaccination against the Alpha variant was 88.0% (95% CI, 83.0–91.5) (Table 2). The VE against any infection and symptomatic infection with the Alpha variant was 89.4% (95% CI, 82.9–93.5) and 90.9% (95% CI, 84.5–94.7), respectively. Subgroup analysis by study design showed that VE was 77.9% (59.3–88.0) in 5 RCTs and 89.3% (95% CI, 84.4–92.6) in 19 real-world settings (case-control or cohort studies) (pinteraction = 0.067). The VE against Alpha variant of real-world evidence seems to be higher than RCTs. Subgroup analysis of vaccine type showed that VE was 90.1% (95% CI, 85.2–93.4) for mRNA vaccines in 20 study groups, 73.9% (95% CI, 69.9–77.4) for non-replicating vector vaccines in 6 study groups, 89.7% (95% CI, 78.8–95.0) for protein subunit vaccine in 2 study groups, and 82.0% (95% CI, 27.0–95.0) for mixed vaccines (BNT162b2/ChAdOx1) in 1 study group (pinteraction = 0.148). And we detected a significant interaction (pinteraction = 0.026) between VE and vaccine type (mRNA vaccines vs. not mRNA vaccines); the VE of mRNA vaccines seemed to be higher than others. The results of subgroup analysis for participant are shown in Table 2.
Table 2.
Meta-analysis and subgroup analysis for VE of COVID-19 against VOC
| Covariates | Subgroup | Study groups | Pooled estimates | I2 | p (ES=1) | VE% (95% CI) | Pinteraction |
|---|---|---|---|---|---|---|---|
| Alpha | |||||||
| All | 29 | 0.120 (0.085–0.170) | 98% | < 0.001 | 88.0 (83.0–91.5) | ||
| Any infection | 17 | 0.106 (0.065–0.171) | 99% | < 0.001 | 89.4 (82.9–93.5) | ||
| Symptomatic | 18 | 0.091 (0.053–0.155) | 98% | < 0.001 | 90.9 (84.5–94.7) | ||
| Study design | RWE | 24 | 0.107 (0.074–0.156) | 98% | < 0.001 | 89.3 (84.4–92.6) | 0.067 |
| RCT | 5 | 0.221 (0.120–0.407) | 71% | < 0.001 | 77.9 (59.3–88.0) | ||
| Participant | GP | 24 | 0.125 (0.085–0.182) | 98% | < 0.001 | 87.5 (81.8–91.5) | 0.553 |
| HCWs | 4 | 0.087 (0.056–0.133) | 0% | < 0.001 | 91.3 (86.7–94.4) | ||
| Older | 1 | 0.100 (0.061–0.163) | – | < 0.001 | 90.0 (83.7–93.9) | ||
| Vaccine type | mRNA vaccines (BNT162b2/mRNA-1273/CVnCoV) | 20 | 0.099 (0.066–0.148) | 98% | < 0.001 | 90.1 (85.2–93.4) | 0.148 |
| Non-replicating vector vaccine (ChAdOx1/Ad26.COV2.S) | 6 | 0.261 (0.226–0.301) | 0% | < 0.001 | 73.9 (69.9–77.4) | ||
| Protein subunit vaccine (NVX-CoV2373) | 2 | 0.103 (0.050–0.212) | 25% | < 0.001 | 89.7 (78.8–95.0) | ||
| Mixed (BNT162b2/ChAdOx1) | 1 | 0.180 (0.047–0.688) | – | 0.012 | 82.0 (31.2–95.3) | ||
| Not mRNA vaccines | 9 | 0.234 (0.190–0.288) | 28% | < 0.001 | 76.6 (71.2–81.0) | 0.026a | |
| Beta | |||||||
| All | GP | 11 | 0.270 (0.205–0.357) | 82% | < 0.001 | 73.0 (64.3–79.5) | |
| Any infection | 7 | 0.214 (0.169–0.272) | 74% | < 0.001 | 78.6 (72.8–83.1) | 0.044 | |
| Symptomatic | 4 | 0.608 (0.411–0.899) | 20% | 0.013 | 39.2 (10.1–58.9) | ||
| Study design | RWE | 7 | 0.221 (0.178–0.274) | 68% | < 0.001 | 77.9 (72.6–82.2) | 0.032 |
| RCT | 4 | 0.544 (0.282–1.051) | 65% | 0.070 | 45.6 (-5.1–71.8) | ||
| Vaccine type | mRNA vaccines (BNT162b2/mRNA-1273) | 8 | 0.214 (0.170–0.270) | 70% | < 0.001 | 78.6 (73.0–83.0) | 0.057 |
| Non-replicating vector vaccine (ChAdOx1/Ad26.COV2.S) | 2 | 0.690 (0.477–1.000) | 0% | 0.050 | 31.0 (0.0–52.3) | ||
| Protein subunit vaccine (NVX-CoV2373) | 1 | 0.489 (0.238–1.006) | – | 0.052 | 51.1 (-0.6–76.2) | ||
| Gamma | |||||||
| All | 10 | 0.370 (0.263–0.521) | 78% | < 0.001 | 63.0 (47.9–73.7) | ||
| Any infection | 3 | 0.438 (0.295–0.650) | 0% | < 0.001 | 56.2 (35.0–70.5) | 0.960 | |
| Symptomatic | 7 | 0.363 (0.238–0.553) | 85% | < 0.001 | 63.7 (44.7–76.2) | ||
| Study design | RWE | 6 | 0.340 (0.209–0.551) | 86% | < 0.001 | 66.0 (44.9–79.1) | 0.634 |
| RCT | 4 | 0.451 (0.269–0.757) | 40% | 0.003 | 54.9 (24.3–73.1) | ||
| Participant | GP | 5 | 0.287 (0.130–0.633) | 78% | 0.002 | 71.3 (36.7–87.0) | 0.391 |
| Older/LTCH | 4 | 0.378 (0.228–0.628) | 87% | < 0.001 | 62.2 (37.2–77.2) | ||
| HCWs | 1 | 0.632 (0.258–1.549) | – | 0.316 | 36.8 (-54.9–74.2) | ||
| Vaccine type | mRNA vaccines (BNT162b2/mRNA-1273/CVnCoV) | 4 | 0.285 (0.147–0.555) | 68% | < 0.001 | 71.5 (44.5–85.3) | 0.232 |
| Non-replicating vector vaccine (ChAdOx1/Ad26.COV2.S) | 3 | 0.373 (0.163–0.852) | 91% | 0.019 | 62.7 (14.8–83.7) | ||
| Inactivated vaccine (CoronaVac) | 2 | 0.534 (0.465–0.614) | 0% | < 0.001 | 46.6 (38.6–53.5) | ||
| Protein subunit vaccine (SCB-2019) | 1 | 0.082 (0.005–1.361) | – | 0.081 | 91.8 (-36.1–99.5) | ||
| Beta/Gamma | |||||||
| All | 21 | 0.307 (0.238–0.396) | 88% | < 0.001 | 69.3 (60.4–76.2) | ||
| Vaccine type | mRNA vaccines | 12 | 0.228 (0.182–0.287) | 69% | < 0.001 | 77.2 (71.3–81.8) | 0.006a |
| Not mRNA vaccines | 9 | 0.492 (0.360–0.674) | 75% | < 0.001 | 50.8 (32.6–64.0) | ||
| Delta | |||||||
| All | 47 | 0.222 (0.180–0.273) | 99% | < 0.001 | 77.8 (72.7–82.0) | ||
| Any infection | 28 | 0.254 (0.215–0.300) | 95% | < 0.001 | 74.6 (70.0–78.5) | ||
| Symptomatic | 24 | 0.208 (0.154–0.281) | 99% | < 0.001 | 79.2 (71.9–84.6) | ||
| Participant | GP | 35 | 0.245 (0.193–0.312) | 99% | < 0.001 | 75.5 (68.8–80.7) | |
| HCWs | 5 | 0.236 (0.112–0.497) | 93% | < 0.001 | 76.4 (50.3–88.8) | ||
| Older | 5 | 0.403 (0.296–0.550) | 98% | < 0.001 | 59.7 (45.0–70.4) | ||
| Adolescents | 7 | 0.112 (0.076–0.165) | 92% | < 0.001 | 88.8 (83.5–92.4) | ||
| Study design | REW | 44 | 0.214 (0.173–0.266) | 99% | < 0.001 | 78.6 (73.4–82.7) | 0.168 |
| RCT | 3 | 0.407 (0.176–0.940) | 70% | < 0.001 | 59.3 (6.0–82.4) | ||
| Vaccine type | mRNA vaccines (BNT162b2/mRNA-1273) | 28 | 0.166 (0.134–0.204) | 99% | < 0.001 | 83.4 (79.6–86.6) | < 0.001 |
| Non-replicating vector vaccine (ChAdOx1/Ad26.COV2.S) | 12 | 0.350 (0.276–0.445) | 98% | < 0.001 | 65.0 (55.5–72.4) | ||
| Inactivated vaccine (CoronaVac/HB02/CNBG/BBV152/BBIBP-CorV) | 6 | 0.433 (0.358–0.523) | 0% | < 0.001 | 56.7 (47.7–64.2) | ||
| Protein subunit vaccine (SCB-2019) | 1 | 0.213 (0.101–0.449) | – | < 0.001 | 78.7 (55.1–89.9) | ||
| Not mRNA vaccines | 19 | 0.368 (0.303–0.448) | 97% | < 0.001 | 63.2 (55.2–69.7) | < 0.001a | |
| Delta (booster vaccination) | |||||||
| All | 6 | 0.045 (0.035–0.058) | 95% | < 0.001 | 95.5 (94.2–96.5) | ||
| Omicron | |||||||
| All | 3 | 0.441 (0.330–0.591) | 90% | < 0.001 | 55.9 (40.9–67.0) | ||
| Omicron (booster vaccination) | |||||||
| All | 2 | 0.192 (0.089–0.414) | 99% | < 0.001 | 80.8 (58.6–91.1) | ||
Abbreviations: VE vaccine effectiveness, HCWs healthcare workers, LTCH long-term care homes, GP general population, RCT randomized controlled trial, ES effect size
aP for interaction between vaccine effectiveness and vaccine type (mRNA vaccines vs. not mRNA vaccines)
Vaccine effectiveness of COVID-19 vaccines against B.1.351 (Beta) and P.1 (Gamma) variants
Four RCTs [20, 22, 40, 41] and 6 case-control studies [60, 65–67, 77, 82] had evaluated the VE of COVID-19 vaccines against the Beta variant. Four RCTs [34, 35, 39, 40], 1 cohort study [58], and 4 case-control studies [72, 73, 77, 79] had evaluated the VE of COVID-19 vaccines against the Gamma variant. Both Beta and Gamma have N501Y and E484K mutations, and 2 studies used a combined Beta/Gamma group because of insufficient specimens. Eight COVID-19 vaccines (mRNA-1273, BNT162b2, NVX-CoV2373, ChAdOx1, CVnCoV, SCB-2019, CoronaVac, and Ad26.COV2.S) were included in this analysis. For the study population, 1 study enrolled health care workers [73], 2 studies enrolled older adults [72, 79], 1 studies enrolled participants from long-term care homes [58], and the others enrolled the general population. Characteristics of individual studies and VE for Beta and Gamma variants are summarized in Fig. 2 and Additional file 1 (Table S5).
Fig. 2.
Forest plot showing VE of full vaccination against Beta/Gamma variants. Abbreviations: VE, vaccine effectiveness; CI, confidence interval; RCT, randomized controlled trial
The summary VE of full vaccination against Beta variant was 73.0% (95% CI, 64.3–79.5) (Table 2). Subgroup analysis by study design showed that VE for Beta variant was 45.6% (95% CI, −5.1 to 71.8) in 4 RCTs and 77.9% (95% CI, 72.6–82.2) in 7 real-world settings (case-control studies) (pinteraction = 0.067). The VE against Beta variant of real-world evidence seems to be higher than RCTs. Subgroup analysis of vaccine type showed that VE was 78.6% (95% CI, 73.0–83.0) for mRNA vaccines in 8 study groups, 31.0% (95% CI, 0.0–52.3) for non-replicating vector vaccines in 2 study groups, and 51.1% (95% CI, −0.6 to 76.2) for protein subunit vaccine in 1 study group (pinteraction = 0.057). The summary VE of full vaccination against Gamma variant was 63.0% (95% CI, 47.9–73.7) in 10 study groups; the results of subgroup analysis are shown in Table 2.
When Beta/Gamma variant was treated as one group, the summary VE of full vaccination was 69.3% (95% CI, 60.4–76.2) in 21 study groups. Subgroup analysis of vaccine types showed that VE against Beta/Gamma was 77.2% (95% CI, 71.3–81.8) for mRNA vaccines in 12 study groups and 50.8% (95% CI, 32.6–64.0) for not mRNA vaccines in 9 study groups (pinteraction = 0.006); the VE for mRNA vaccines seemed to be higher than others.
Vaccine effectiveness of COVID-19 vaccines against B.1.617.2 (Delta) variant
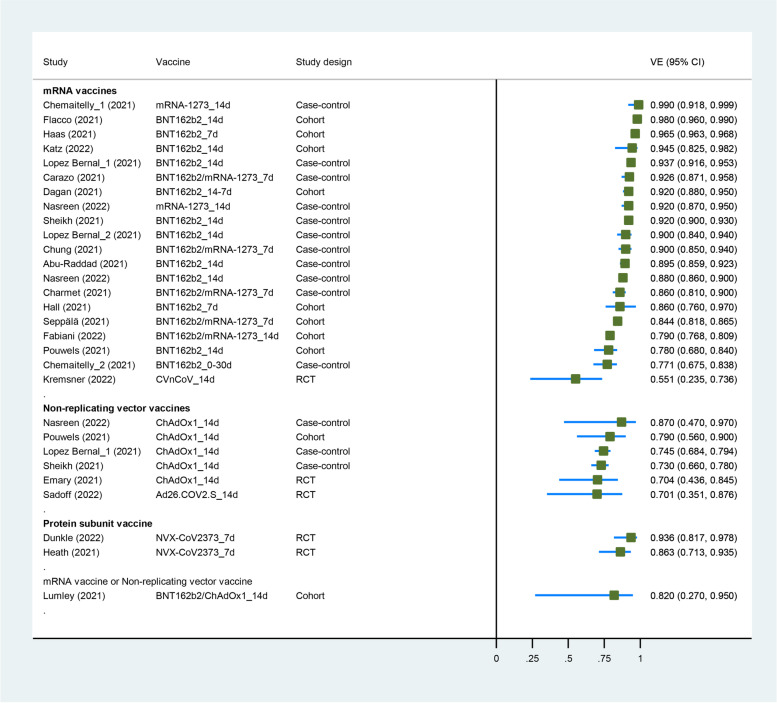
Three RCTs [34, 37, 40], 13 cohort studies [42, 44, 45, 47, 50–57, 59], and 17 case-control studies [21, 61–63, 66, 69–71, 74, 75, 77, 78, 80–84] had evaluated the VE of COVID-19 vaccines against the Delta variant. Ten COVID-19 vaccines (mRNA-1273, BBV152, ChAdOx1, BNT162b2, CoronaVac, Ad26.COV2.S, HB02, CNBG, SCB-2019, and BBIBP-CorV) were included in this analysis. Five studies enrolled adolescents [45, 50, 54, 63, 78], 3 studies enrolled health care workers or frontline workers [42, 44, 52], 1 study enrolled participants in the nursing home [51], and the others enrolled the general population. Three studies reported the VE of booster vaccination against Delta variant [61, 69, 84]. Characteristics of individual studies and VE for Delta variant are summarized in Figs. 3 and 4 and Additional file 1 (Table S6).
Fig. 3.
Forest plot showing VE of full vaccination against Delta variant. Abbreviations: VE, vaccine effectiveness; CI, confidence interval; RCT, randomized controlled trial
Fig. 4.
Forest plot showing VE of full vaccination against Omicron variant, and VE of booster vaccination against Delta or Omicron variant. Abbreviations: VE, vaccine effectiveness; CI, confidence interval; RCT, randomized controlled trial
The summary VE of full vaccination against the Delta variant was 77.8% (95% CI, 72.7–82.0) (Table 2). The VE against any infection and symptomatic infection with the Delta variant were 74.6% (95% CI, 70.0–78.5) and 79.2% (95% CI, 71.9–84.6), respectively. For special populations, the VE was 76.4% (95% CI, 50.3–88.8) in healthcare workers, 59.7% (95% CI, 45.0–70.4) in older adults, and 88.8% (95% CI, 83.5–92.4) in adolescents. Subgroup analysis of study design showed that VE for Delta variant was 59.3% (95% CI, 6.0–82.4) in 3 RCTs and 78.6% (95% CI, 73.4–82.7) in 30 real-world settings (case-control or cohort studies) (pinteraction = 0.168). Subgroup analysis by vaccine type showed that VE was 83.4% (95% CI, 79.6–86.6) for mRNA vaccines in 28 study groups, 65.0% (95% CI, 55.5–72.4) for non-replicating vector vaccines in 12 study groups, 56.7% (95% CI, 47.7–64.2) for inactivated vaccines in 6 study groups, and 78.7% (95% CI, 55.1–89.9) for protein subunit vaccine in 1 study group (pinteraction < 0.001). An interaction (pinteraction < 0.001) between VE and vaccine type (mRNA vaccines vs. not mRNA vaccines) was found; the VE for mRNA vaccines seemed to be higher than others. The summary VE of booster vaccination against the Delta variant was 95.5% (95% CI, 94.2–96.5).
Vaccine effectiveness of COVID-19 vaccines against B.1.1.529 (Omicron) variant
Three case-control studies had evaluated the VE of COVID-19 vaccines against the Omicron variant [69, 74, 84]. Two mRNA vaccines (mRNA-1273 and BNT162b2) were included in this analysis. All three studies enrolled the general population. Characteristics of individual studies and VE for Omicron variant are summarized in Fig. 4 and Additional file 1 (Table S7). The summary VE of full vaccination against the Omicron variant was 55.9% (95% CI, 40.9–67.0), and the VE of booster vaccination against the Omicron variant was 80.8% (95% CI, 58.6–91.1).
Discussion
The VOC have mutations in its spike protein; most breakthrough cases were caused by contemporary variant strains [36]. The VE of current COVID-19 vaccines against VOC is concerning; we conducted this systematic review and meta-analysis to synthesize evidence on this topic during the pandemic. This study has five main findings. First, full vaccination of COVID-19 vaccines was effective against Alpha, Beta, Gamma, Delta, and Omicron variants, with the VE of 88.0%, 73.0%, 63.0%, 77.8%, and 55.9%, respectively. Second, booster vaccination has higher VE against Delta and Omicron variants, with the VE of 95.5% and 80.8%, respectively. Third, mRNA vaccines (BNT162b2 or mRNA-1273) have higher VE against VOC over other vaccines. Fourth, VE against VOC of real-world evidence seemed to be higher than RCTs. Fifth, more evidence was needed to evaluate the VE of COVID-19 against the Omicron variant. To our knowledge, our study is the first comprehensive systematic review and meta-analysis to characterize the VE of COVID-19 vaccines against five kinds of VOC.
The evidence for the Omicron variant was insufficient, which only included three studies evaluating the VE of the mRNA vaccines (BNT162b2 or mRNA-1273). WHO guidelines recommend a lower bound of at least 30% and a vaccine efficacy of at least 50% [85]. The summary VE against Omicron variant was 55.9% of full vaccination and 80.8% of booster vaccination, raising concern for other vaccines. One study showed that Omicron variant extensively but incompletely escaped BNT162b2 neutralization [86]. Owing to multiple spike mutations, over 85% of tested neutralizing antibodies were escaped by Omicron variant, presenting a serious threat to existing therapies and COVID-19 vaccines [12, 87].
The main results in this study were in consistent with a recent meta-analysis for neutralizing antibodies against SARS-CoV-2 variants, which showed that Alpha, Beta, Gamma, and Delta variants significantly escaped natural-infection-mediated neutralization, with an average of 1.4-fold, 4.1-fold, 1.8-fold, and 3.2-fold reduction in live virus neutralization assay [88]. Despite the reduction in neutralization titers against Alpha variant, they remain robust, and there is no evidence of vaccine escape in one study [89]. Escape of Beta variant from neutralization by convalescent plasma and vaccine-induced sera was observed in some studies [13, 90, 91]. Although neutralization titers against Gamma variant are reduced, it is hoped that immunization with vaccines designed against parent strains will protect Gamma variant infection [92]. The Delta variant escapes neutralization by some antibodies that target the receptor-binding domain or N-terminal domain; the neutralization titers against Delta were three to fivefolds than Alpha variant when two-dose of the vaccine administrated [15]. This study also supports the two-dose vaccine regimen recommended by the FDA and EMA, which is consistent with an in vitro study for SARS-CoV-2 variants of concern [93]. Also, booster vaccination demonstrates high VE against Delta infection in our study.
We did not evaluate VE against asymptomatic infection due to poor reporting in included studies. The summary VE against asymptomatic infection was slightly higher than any infection for Alpha, Gamma, and Delta variants, which was consistent with primary studies. The summary VE was higher against any infection with the Beta variant, which was probably confounded by study design and vaccine type. Three of 4 RCTs used symptomatic infection as an outcome, but 5 of 6 case-control studies used any infection. Most studies enrolled general population; only a few studies analyzed the VE in older adults or adolescents. We have performed subgroup analyses for VE against Delta variant stratifying by participants; the VE was 59.7% in older adults which was lower than general population (75.5%), healthcare workers (76.4%), and adolescents (88.8%). Vaccine type may be a confounder for this analysis, because one study showed that the VE of Ad26.COV2.S was much lower than BNT162b2 and mRNA-1273 in adolescents [63]. More evidence is needed for evaluating the VE against VOC in special population.
This review included 3 study designs evaluating 11 COVID-19 vaccines against 5 VOC in different populations. There is high heterogeneity between studies, and high statistical heterogeneity is also observed in most analysis. Other factors like the definition of outcomes (all SARS-CoV-2 or symptomatic infection), days after vaccination, and participant’s characteristics (e.g., age and race) may also contribute to the heterogeneity. Therefore, we mainly performed narrative descriptive synthesis.
This study has some limitations. First, 19% of studies (11 of 57) are nonrandomized. The imbalance between groups in observational studies is a concern, so potential selection bias may be existent. Second, we did not evaluate VE against asymptomatic infection due to poor reporting in included studies. Third, although we performed qualitative analysis by different stratifications, heterogeneity was still high in most quantitative analysis. Fourth, VE against hospitalization or death related to VOC is not included in our analysis. Finally, the evidence of COVID-19 vaccines against Omicron variant is not enough, more research is needed in the future.
Conclusions
Full vaccination of COVID-19 vaccines is highly effective against Alpha variant and moderate effective against Beta, Gamma, and Delta variant. Booster vaccination has more effectiveness against Delta and Omicron variants. mRNA vaccines (BNT162b2 or mRNA-1273) seem to have higher VE against Alpha, Beta, Gamma, or Delta over other vaccines. SARS-CoV-2 Omicron is raising concern for vaccinated individuals, and more evidence is needed to evaluate the VE of COVID-19 vaccines against the Omicron variant.
Supplementary Information
Additional file 1: Supplementary Materials. Search strategy (Appendix S1). Flow chart of literature search and study selection (Figure S1). Risk of bias for included randomized controlled trials (Table S1). Risk of bias for included cohort studies (Table S2). Risk of bias for included case-control studies (Table S3). VE of COVID-19 vaccines against Alpha variant (Table S4). VE of COVID-19 vaccines against Beta and Gamma variant (Table S5). VE of COVID-19 vaccines against Delta variant (Table S6). VE of COVID-19 vaccines against Omicron variant (Table S7).
Acknowledgements
Not applicable.
Abbreviations
- CI
Confidence interval
- NOS
Newcastle-Ottawa scale
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RCT
Randomized controlled trial
- VE
Vaccine effectiveness
- VOC
Variants of concern
Authors’ contributions
KY and FS conceived the study. BZ and KY designed the study. BZ, LG, and QZ undertook the literature review and extracted the data. BZ and QZ coded the statistical analysis, figures, and appendix. BZ and LG interpreted the data and wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by National Key R&D Program of China (2021YFC2301601).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
No human subjects, human material, or human data were involved in this research, which is based on literature review.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kai Yu, Email: wzxkjk@126.com.
Feng Sun, Email: sunfeng@bjmu.edu.cn.
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://www.who.int. Accessed 10 March 2022.
- 2.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet (London, England) 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le TT, Cramer JP, Chen R, Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 10.Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. December, 2020. Available at: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563.
- 11.European Centre for Disease Prevention and Control. Threat assessment brief: emergence of SARS-CoV-2 B.1.617 variants in India and situation in the EU/EEA. May 11, 2021 Available at: https://www.ecdc.europa.eu/en/publications-data/threat-assessment-emergence-sars-cov-2-b1617-variants.
- 12.Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384–93.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–83.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 16.Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walensky RP, Walke HT, Fauci AS. SARS-CoV-2 variants of concern in the United States-challenges and opportunities. JAMA. 2021;325:1037–1038. doi: 10.1001/jama.2021.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. SARS-CoV-2 variant classifications and definitions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html. Accessed 10 March 2022.
- 19.Krause PR, Fleming TR, Longini IM, Peto R, Briand S, Heymann DL, et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385:179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder T, Külper-Schiek W, Reda S, Treskova-Schwarzbach M, Koch J, Vygen-Bonnet S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021;26:2100920. doi: 10.2807/1560-7917.ES.2021.26.41.2100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahumud RA, Ali MA, Kundu S, Rahman MA, Kamara JK, Renzaho AMN. Effectiveness of COVID-19 vaccines against Delta variant (B.1.617.2): a meta-analysis. Vaccines. 2022;10:277. doi: 10.3390/vaccines10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Aghayari Sheikh Neshin S, Khatami A, et al. Effectiveness of COVID-19 vaccines against Delta (B.1.617.2) variant: a systematic review and meta-analysis of clinical studies. Vaccines. 2021;10:23. doi: 10.3390/vaccines10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 30.Wells G, Shea B, O'Connell D, Peterson J, Welch, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2008. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Global Health. 2019;7:192–198. doi: 10.1016/j.cegh.2018.05.005. [DOI] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399:461–472. doi: 10.1016/S0140-6736(22)00055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemens SAC, Folegatti PM, Emary KRW, Weckx LY, Ratcliff J, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nat Commun. 2021;12:5861. doi: 10.1038/s41467-021-25982-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ella R, Reddy S, Blackwelder W, Potdar V, Yadav P, Sarangi V, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kremsner PG, Ahuad Guerrero RA, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22:329–340. doi: 10.1016/S1473-3099(21)00677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386:847–860. doi: 10.1056/NEJMoa2117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas SJ, Moreira ED, Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabiani M, Puopolo M, Morciano C, Spuri M, Spila Alegiani S, Filia A, et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ. 2022;376:e069052. doi: 10.1136/bmj-2021-069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flacco ME, Soldato G, Acuti Martellucci C, Carota R, Di Luzio R, Caponetti A, et al. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian province. Vaccines (Basel) 2021;9:628. doi: 10.3390/vaccines9060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fowlkes A, Gaglani M, Groover K, Thiese MS, Tyner H, Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance - eight U.S. locations, December 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis. 2021;27:2919–2922. doi: 10.3201/eid2711.211886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, et al. Effectiveness of inactivated COVID-19 vaccines against illness caused by the B.1.617.2 (Delta) variant during an outbreak in Guangdong, China: a cohort study. Ann Intern Med. 2022;175:533–540. doi: 10.7326/M21-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katz MA, Harlev EB, Chazan B, Chowers M, Greenberg D, Peretz A, et al. Early effectiveness of BNT162b2 Covid-19 vaccine in preventing SARS-CoV-2 infection in healthcare personnel in six Israeli hospitals (CoVEHPI) Vaccine. 2022;40:512–520. doi: 10.1016/j.vaccine.2021.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumley SF, Rodger G, Constantinides B, Sanderson N, Chau KK, Street TL, et al. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis. 2022;74:1208–1219. doi: 10.1093/cid/ciab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutrick K, Rivers P, Yoo YM, Grant L, Hollister J, Jovel K, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12-17 years - Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1761–1765. doi: 10.15585/mmwr.mm705152a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poukka E, Baum U, Palmu AA, Lehtonen TO, Salo H, Nohynek H, et al. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020 - October 2021. Vaccine. 2022;40:701–705. doi: 10.1016/j.vaccine.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis BY, Barda N, Leshchinsky M, Kepten E, Hernán MA, Lipsitch M, et al. Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med. 2021;385:2101–2103. doi: 10.1056/NEJMc2114290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Risk M, Shen C, Hayek SS, Holevinski L, Schiopu E, Freed G, et al. Comparative effectiveness of COVID-19 vaccines against the Delta variant. Clin Infect Dis. 2022:ciac106. 10.1093/cid/ciac106. [DOI] [PMC free article] [PubMed]
- 56.Seppälä E, Veneti L, Starrfelt J, Danielsen AS, Bragstad K, Hungnes O, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26:2100793. doi: 10.2807/1560-7917.ES.2021.26.35.2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams C, Al-Bargash D, Macalintal C, Stuart R, Seth A, Latham J, et al. COVID-19 outbreak associated with a SARS-CoV-2 P.1 lineage in a long-term care home after implementation of a vaccination program - Ontario, April-May 2021. Clin Infect Dis. 2022;74:1085–1088. doi: 10.1093/cid/ciab617. [DOI] [PubMed] [Google Scholar]
- 59.Wu D, Zhang Y, Tang L, Wang F, Ye Y, Ma C, et al. Effectiveness of inactivated COVID-19 vaccines against symptomatic, pneumonia, and severe disease caused by the Delta variant: real world study and evidence - China, 2021. China CDC Wkly. 2022;4:57–65. doi: 10.46234/ccdcw2022.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, et al. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat Med. 2022;28:831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Britton A, Fleming-Dutra KE, Shang N, Smith ZR, Dorji T, Derado G, et al. Association of COVID-19 vaccination with symptomatic SARS-CoV-2 infection by time since vaccination and Delta variant predominance. JAMA. 2022;327(11):1032–1041. doi: 10.1001/jama.2022.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carazo S, Talbot D, Boulianne N, Brisson M, Gilca R, Deceuninck G, et al. Single-dose mRNA vaccine effectiveness against SARS-CoV-2 in healthcare workers extending 16 weeks post-vaccination: a test-negative design from Quebec, Canada. Clin Infect Dis. 2021:ciab739. 10.1093/cid/ciab739. [DOI] [PMC free article] [PubMed]
- 65.Charmet T, Schaeffer L, Grant R, Galmiche S, Chény O, Von Platen C, et al. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France. Lancet Reg Health Eur. 2021;8:100171. doi: 10.1016/j.lanepe.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 68.Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance - nine states, June-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1291–1293. doi: 10.15585/mmwr.mm7037e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grant R, Charmet T, Schaeffer L, Galmiche S, Madec Y, Von Platen C, et al. Impact of SARS-CoV-2 Delta variant on incubation, transmission settings and vaccine effectiveness: results from a nationwide case-control study in France. Lancet Reg Health Eur. 2022;13:100278. doi: 10.1016/j.lanepe.2021.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hitchings MDT, Ranzani OT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of ChAdOx1 vaccine in older adults during SARS-CoV-2 Gamma variant circulation in São Paulo. Nat Commun. 2021;12:6220. doi: 10.1038/s41467-021-26459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. 2021;1:100025. doi: 10.1016/j.lana.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5-17 years - VISION Network, 10 States, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. doi: 10.15585/mmwr.mm7109e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, et al. Efficacy of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021;10:1751–1759. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7:379–385. doi: 10.1038/s41564-021-01053-0. [DOI] [PubMed] [Google Scholar]
- 78.Oliveira CR, Niccolai LM, Sheikha H, Elmansy L, Kalinich CC, Grubaugh ND, et al. Assessment of clinical effectiveness of BNT162b2 COVID-19 vaccine in US adolescents. JAMA network open. 2022;5:e220935. doi: 10.1001/jamanetworkopen.2022.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect. 2022;11:585–592. doi: 10.1080/22221751.2022.2037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 83.Thiruvengadam R, Awasthi A, Medigeshi G, Bhattacharya S, Mani S, Sivasubbu S, et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect Dis. 2022;22:473–482. doi: 10.1016/S1473-3099(21)00680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022. 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed]
- 85.WHO. Considerations for evaluation of COVID19 vaccines. November, 2020. Available at: https://cdn.who.int/media/docs/default-source/in-vitro-diagnostics/covid19/considerations-who-evaluation-of-covid-vaccine_v25_11_2020.pdf.
- 86.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 88.Chen X, Chen Z, Azman AS, Sun R, Lu W, Zheng N, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734–742. doi: 10.1093/cid/ciab646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–11.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184:2348–61.e6. doi: 10.1016/j.cell.2021.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H, et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell. 2021;184:2362–71.e9. doi: 10.1016/j.cell.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dejnirattisai W, Zhou D, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939–54.e9. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skelly DT, Harding AC, Gilbert-Jaramillo J, Knight ML, Longet S, Brown A, et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nature Commun. 2021;12:5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Materials. Search strategy (Appendix S1). Flow chart of literature search and study selection (Figure S1). Risk of bias for included randomized controlled trials (Table S1). Risk of bias for included cohort studies (Table S2). Risk of bias for included case-control studies (Table S3). VE of COVID-19 vaccines against Alpha variant (Table S4). VE of COVID-19 vaccines against Beta and Gamma variant (Table S5). VE of COVID-19 vaccines against Delta variant (Table S6). VE of COVID-19 vaccines against Omicron variant (Table S7).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.