Abstract
Purpose
Specific classes of antibiotics, such as aminoglycosides, have well-established adverse events producing permanent hearing loss, tinnitus, and balance and/or vestibular problems (i.e., ototoxicity). Although these antibiotics are frequently used to treat pseudomonas and other bacterial infections in patients with cystic fibrosis (CF), there are no formalized recommendations describing approaches to implementation of guideline adherent ototoxicity monitoring as part of CF clinical care.
Method
This consensus statement was developed by the International Ototoxicity Management Working Group (IOMG) Ad Hoc Committee on Aminoglycoside Antibiotics to address the clinical need for ototoxicity management in CF patients treated with known ototoxic medications. These clinical protocol considerations were created using consensus opinion from a community of international experts and available evidence specific to patients with CF, as well as published national and international guidelines on ototoxicity monitoring.
Results
The IOMG advocates four clinical recommendations for implementing routine and guideline adherent ototoxicity management in patients with CF. These are (a) including questions about hearing, tinnitus, and balance/vestibular problems as part of the routine CF case history for all patients; (b) utilizing timely point-of-care measures; (c) establishing a baseline and conducting posttreatment evaluations for each course of intravenous ototoxic drug treatment; and (d) repeating annual hearing and vestibular evaluations for all patients with a history of ototoxic antibiotic exposure.
Conclusion
Increased efforts for implementation of an ototoxicity management program in the CF care team model will improve identification of ototoxicity signs and symptoms, allow for timely therapeutic follow-up, and provide the clinician and patient an opportunity to make an informed decision about potential treatment modifications to minimize adverse events.
Supplemental Material
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that affects over 30,000 persons in the United States and 70,000 worldwide, with an incidence of approximately 1,000 new diagnoses per year (Cystic Fibrosis Foundation [CFF], 2018). CF primarily affects non-Hispanic White persons and is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene; absent or reduced function of the CFTR protein can result in chronic bacterial infections of the lungs, pancreatic exocrine insufficiency, digestive disorders, and elevated sweat chloride concentrations (e.g., CFF, 2018; Knowles & Durie, 2002). Early diagnosis of CF is more common due to newborn screening programs (Scotet et al., 2020). However, the manifestation of CF-related symptoms and severity of disease can dramatically vary across patients. The median predicted survival rate for persons with CF has steadily increased from 28 years in 1986 to 43 years in 2016 (CFF, 2018). Due to the changing landscape of improved CF therapies leading to increased life expectancy, it is critical to consider the negative impacts on quality of life from comorbidities associated with normal aging (e.g., presbycusis) and CF itself, as well as the adverse effects of long-term treatments. Persons with CF most often receive clinical care from a specialized CF care team, which. At minimum, the team includes a pulmonologist, nurse, social worker, respiratory therapist, dietician, and often, a pharmacist. Treatment is highly specialized to the individual and often includes a rigorous daily routine of airway clearance therapies, vitamin supplements, and medications. Despite the advancements in CF clinical care and treatments (including novel CFTR modulator therapies), lung disease remains the primary cause of morbidity and mortality (CFF, 2018; Earnest et al., 2020).
Persons with CF tend to have episodic pulmonary exacerbations that drive lung function decline (Sanders et al., 2010, 2011). The 2018 CF patient registry report showed that almost 25% of pediatric patients (< 18 years) and 43% of adult patients experienced a CF-related exacerbation that year; the group experiencing the highest number of exacerbations was between 15–30 years old (CFF, 2018). During these exacerbations, patients often receive a once-daily treatment course (typically 14 days) including intravenous (IV) aminoglycoside antibiotics (e.g., tobramycin or amikacin), with or without a concomitant glycopeptide (e.g., vancomycin) or beta-lactam (e.g., piperacillin), cephalosporin (e.g., ceftazidime or cefipime) or carbapenem (e.g., meropenem) antibiotic, to manage polymicrobial bacterial infections of the airways. Well-established evidence indicates that aminoglycoside antibiotics, particularly with IV-therapy, may produce ototoxic adverse events including permanent sensorineural hearing loss, tinnitus (i.e., cochleotoxicity; Al-Malky et al., 2015; Elson et al., 2020; Garinis et al., 2017; Jiang et al., 2017; Tan et al., 2003), and/or vestibular problems (i.e., vestibulotoxicity; Ariano et al., 2008; Handelsman, 2018; Handelsman et al., 2017). Synergistic ototoxic effects have also been reported when IV aminoglycoside therapy is paired with vancomycin (Garinis et al., 2017). The reported prevalence of hearing loss, presumably resulting from IV aminoglycoside treatment, in pediatric and adult persons with CF varies, with recent estimates as high as 63% (Al-Malky et al., 2015; Blankenship et al., 2021; Elson et al., 2020; Garinis et al., 2017; reviewed by Zettner & Gleser, 2018). Such rates substantially exceed the ~13% reported prevalence in an age-matched group of individuals (12–49 years old) drawn from the general population (e.g., Lin et al., 2011). A recent study by Elson et al. (2020) showed that 53% of patients with CF treated for ≥ 5 years with inhaled aminoglycosides exhibited hearing abnormalities. The combined use of both inhaled and IV aminoglycosides (or other ototoxic agents) may further increase the risk for developing ototoxicity and needs further investigation. Macrolide antibiotics, especially azithromycin, are also frequently used in CF care for treatment of lung disease, and some case reports describing ototoxicity. This published data suggest that these agents can also produce ototoxicity; however, these effects are still understudied.
These recent prevalence estimates of aminoglycoside-induced hearing loss in the CF population are alarmingly high. Preclinical and human evidence has established that aminoglycosides initially produce high-frequency sensorineural hearing loss (> 8 kHz) by damaging sensory outer hair cells in the basal cochlear region, often progressing to low-frequency hearing loss in the apical cochlear region (Fausti et al., 1984; Guthrie, 2008; Huizing & de Groot, 1987; Jiang et al., 2017; Sha et al., 2001). Thus, it is crucial to include metrics that test this higher frequency region of hearing when implementing an ototoxicity management program into clinical CF care. Ototoxicity management, defined as clinical care that includes identification of hearing- and balance/vestibular-related symptoms, follow-up testing to detect an ototoxic event, and provision of auditory/vestibular rehabilitation is critical to ensure timely detection and management of deficits, particularly in pediatric patients. This is critical to address as even mild high-frequency hearing loss may produce delays in speech and language development in children and compromise speech understanding in noise for both children and adults (e.g., Blankenship et al., 2021; Monson et al., 2019). Other vital auditory functions such as sound localization, phoneme identification, and voice recognition are dependent on high-frequencies and thus preserving the functionality of the basal region of the cochlea is crucially important for communication (Alexander et al., 2014).
Monitoring for ototoxicity in patients with CF has not been a common practice worldwide, likely due to (a) known barriers (e.g., cost, lack of audiology resources) to integrating audiological management into care pathways of clinical specialties (e.g., Konrad-Martin et al., 2018; Maru & Al-Malky, 2018), (b) an already time-intensive clinical burden involving appointments with multiple specialists during routine CF care visits, and (c) lack of clinical guidance for including ototoxicity management in CF care. The lack of audiological care for persons with CF was illustrated through national surveys in the United States reporting that only 26% of adult CF centers (Prescott, 2014) and 39% of pediatric CF centers (Prescott, 2011) include audiometry to monitor adverse effects of aminoglycoside treatments, and that the majority of the audiology protocols in these clinics do not include threshold assessment at the higher frequencies (> 8 kHz) that are known to be sensitive to detection of ototoxicity (Prescott, 2011, 2014). Further evidence is documented by narratives of patients who did not receive ototoxicity monitoring or timely rehabilitation, although they had developed ototoxicity symptoms and suffered personal impacts (videos may be accessed online at: https://www.ncrar.research.va.gov/PatientVoices/Index.asp; National Center for Rehabilitative Auditory Research [NCRAR], 2020a). Thus, improvements in audiological care for CF patients requires alignment of goals among provider groups, institutional leadership, and patient involvement (e.g., Konrad-Martin et al., 2018). Additionally, a thoughtful consideration of the CF care team's interdisciplinary knowledge about the impact of hearing loss, tinnitus, and balance and/or vestibular difficulties on quality of life and its implications for designing CF treatment protocols are important for the success of an ototoxicity management program aligned with CF care (Garinis et al., 2018). Furthermore, the impact on a patient's quality of life from ototoxicity as well as readiness to implement rehabilitation and/or candidacy for modification of their drug regimen to minimize further damage will be highly patient-specific. It is therefore crucial to carefully consider the patient's perspective (Baguley & Prayuenyong, 2020; NCRAR, 2020a).
The most recent update of the U.S. national guidelines on ototoxicity monitoring was published by the American Academy of Audiology (AAA, 2009). These guidelines extend previous recommendations put forth by the American Speech-Language-Hearing Association (ASHA) in 1994 (ASHA, 1994); however, they continue to lack specifics on follow-up care coordination, which is crucial for the management of any auditory and vestibular problems that arise from drug treatment. International guidance ranges from published clinical practice patterns (e.g., Maru & Al-Malky, 2018) to formal guidance for ototoxicity monitoring protocols (e.g., Health Professions Council of South Africa, 2019; World Health Organization, 1994. These did not consider important emerging technologies or audiology practice shifts toward an emphasis on person-centered care that can require substantial cross-disciplinary care coordination (American Academy of Otolaryngology-Head and Neck Surgery, 2015; American Thoracic Society & Infectious Disease Society of America, 2007; Daley et al., 2020). There have also been significant point-of-care (POC) testing and tele-audiology advancements in the assessment of and intervention for hearing loss, tinnitus, and balance/vestibular problems (reviewed in Koleilat et al., 2020; Shaikh et al., 2020). In the domain of ototoxicity, these include the use of validated tablet-based technology with high frequency and patient self-testing capabilities for assessment (e.g., Brungart et al., 2018; Samelli et al., 2020; Vijayasingam et al., 2020) and cochleotoxicity grading within a mobile device (e.g., Hollander et al., 2020). Emerging ototherapeutic clinical trials have promoted the use of optimal grading scales, hearing-related questionnaires, and expanded diagnostic metrics to detect ototoxic adverse events (e.g., Henry et al., 2016; King & Brewer, 2018; Konrad-Martin et al., 2016; Poling et al., 2019). These advances justify the development of clinically feasible expert recommendations for incorporating ototoxicity management into the existing care pathways of patients with CF.
Here, we present expert opinion regarding audiological service provision for patients with CF treated with known ototoxic medications. We also considered published national and international guidelines on ototoxicity monitoring for aminoglycoside antibiotics, along with selected key evidence-based studies, in the development of these recommendations (referenced in Table 1 below and Supplemental Material S1).
Table 1.
Summary of ototoxicity monitoring and management guidance by professional organization.
| Association and document name | Patient population | Key points |
|---|---|---|
| American Academy of Audiology (AAA) | • Adults | • Overview of ototoxic medications |
| Position Statement and Clinical Practice Guidelines: Ototoxicity Monitoring (2009) | • Pediatrics | • Outline of ototoxicity monitoring |
| Website: http://www.audiology.org | ||
|
American Speech-Language-Hearing Association (ASHA)
Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy (1994) Website: https://www.asha.org |
• Adults • Pediatrics • Unresponsive patients |
• Suggested protocols for ototoxicity monitoring |
|
Health Professions Council of South Africa (HPCSA)
Audiological management of patients on treatment that includes ototoxic medications (2019) Website: https://www.hpcsa.co.za |
• General population | • Outline of ototoxicity management • Ototoxic monitoring flow chart • Inclusion of vestibular point-of-care screenings • Interprofessional team collaboration chart |
|
World Health Organization (WHO)
Report of an Informal Consultation on Strategies for Prevention of Hearing Impairment From Ototoxic Drugs (1994) Website: http://www.who.int/pbd/deafness/ototoxic_drugs.pdf |
• General population | • Epidemiology data from different world regions • Emphasis on both professional and public education |
|
American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS)
Position Statement: Ototoxicity (2015) Website: http://www.entnet.org |
• General population | • Role of otolaryngologists in ototoxicity monitoring and management |
|
American Thoracic Society (ATS), European Respiratory Society (ERS), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), and Infectious Diseases Society of America (IDSA)
Daley et al. (2020) Website: https://doi.org/10.1183/13993003.00535-2020 |
• Patients with pulmonary infections | • Diagnostic criteria of Nontuberculous Mycobacterial (NTM) Lung Disease • Clinical diseases caused by NTM • Table (6) of drug-induced adverse events |
Method
Development of a Consensus Statement on Ototoxicity Management in Persons With CF
The International Ototoxicity Management Working Group (IOMG) was formed in response to health care gaps in ototoxicity management worldwide at the 9th Biennial Conference of the NCRAR held in Portland, Oregon in September of 2019 (NCRAR, 2020b). The IOMG executive ototoxic treatment team consists of a chair (Dawn Konrad-Martin, Ph.D.), treatment co-chairs (Angela Garinis, Ph.D.; Gayla Poling, Ph.D.; Carmen Brewer, Ph.D.; and Peter Steyger, Ph.D.), international chair (Lucretia Petersen, M.Sc.), environmental ototoxicants chair (Thais Morata, Ph.D.), and an outreach and dissemination lead (Khaya Clark, Ph.D.). There are over 35 IOMG committee members consisting of expert hearing scientists, audiologists, physicians, patients, and doctoral students. The full IOMG roster is available on the IOMG website at https://www.ncrar.research.va.gov/ClinicianResources/IOMG.asp (NCRAR, 2020b).
In January 2020, a subgroup of IOMG committee members volunteered to form the Focus Group on Aminoglycoside Antibiotics. The focus group initially met virtually on August 21, 2020, to develop an inventory of barriers and shortcomings of current clinical practices of ototoxicity management in patients receiving aminoglycoside therapies. The outcome of this meeting was to address an immediate need for standardized clinical protocols in patient groups who are routinely treated with aminoglycosides. The group collectively agreed that patients with CF were at a high risk of ototoxicity, and clinical guidance for CF centers, practitioners, and patients was needed promptly. The scope of this CF-specific expert consensus protocol was to provide guidance on the optimal metrics for detecting ototoxicity, as well as the frequency of audiological monitoring during treatments. This foundational framework will lend to the future development of clinical guidelines and detailed best practice recommendations. We did not set out to formally follow a process for the development of these recommendations (e.g., Delphi consensus or Quaker process). Nevertheless, the final document followed an informal consensus-based strategy, using a comprehensive PubMed literature search and consensus from international researchers in ototoxicity and clinicians who manage patients with CF. The members of the group primarily communicated by sharing information through electronic applications and virtual discussions to develop and resolve differences in the recommendations. Recommendations were further vetted through the larger IOMG at teleconferences and there was an opportunity to comment electronically via tracked changes submitted to the first and senior authors. All contributing authors were included in the document preparation, provided edits, and approved the submission for publication.
Scope of the Consensus Document
This document aims to provide a practical and concise set of clinical considerations for implementing audiological care in pediatric and adult CF centers. These recommendations take into account (a) common barriers to implementation of an ototoxicity management program, (b) the necessity of routine audiological surveillance in patients with chronic disease undergoing treatments with potential for causing cochleotoxicity and/or vestibulotoxicity, and (c) the long-term health and social consequences of potentially preventable hearing loss, tinnitus, and balance/vestibular dysfunction that can result from ototoxic medications.
Results and Discussion
Targeted Clinical Considerations for Ototoxicity Management
Utilize POC Measures to Screen for Ototoxicity
POC measures of auditory and vestibular function should be completed in the CF center with coordinated follow-up of abnormal findings or the development of new hearing, tinnitus, and balance or vestibular concerns. Such concerns or symptoms may be patient or caregiver reported or observed by the care team during a clinic visit. Table 2 provides details about POC measures and Figure 1 illustrates the implementation of POC measures into the CF care team referral pathway. CF centers should optimally partner with their institutional or local audiology clinic(s) to implement a POC ototoxicity management program (in-person or through telehealth options) that is feasible for their center. Use of auditory and vestibular measures in CF care will provide a rapid assessment of ototoxicity, which may reduce loss to follow-up and also be convenient for patients who are unable to attend multiple clinic visits due to living proximity, lack of transportation, or illness.
Table 2.
Examples of point-of-care (POC) measures for ototoxicity symptoms.
| Examples of POC measures | Type | Necessary equipment for a CF team member to complete POC measure during clinical care |
|---|---|---|
| Hearing screening | Auditory, bedside | Portable audiometer, tablet-based audiometry, or handheld hearing screener |
| Otoacoustic Emissions (OAE) | Auditory, bedside | Handheld screening device |
| Tinnitus Screener | Auditory, questionnaire | Questionnaire paper and pencil |
| Video Head Impulse Test (vHIT) | Vestibular, bedside | Equipment needed |
| Head Impulse Test (HIT) | Vestibular, bedside | No equipment needed |
| Dizziness Handicap Inventory (DHI) | Vestibular, questionnaire | Questionnaire paper and pencil |
| Dynamic Visual Acuity (DVA) | Vestibular, bedside | Snellen eye chart |
Note. All of the above screening measures can be completed by a trained CF care team member or audiologist who can conduct full diagnostic exams secondary to failed screenings, thus treating the patient timely and effectively. The medical team is encouraged to incorporate an audiologist into the clinical care team. CF = cystic fibrosis.
Figure 1.
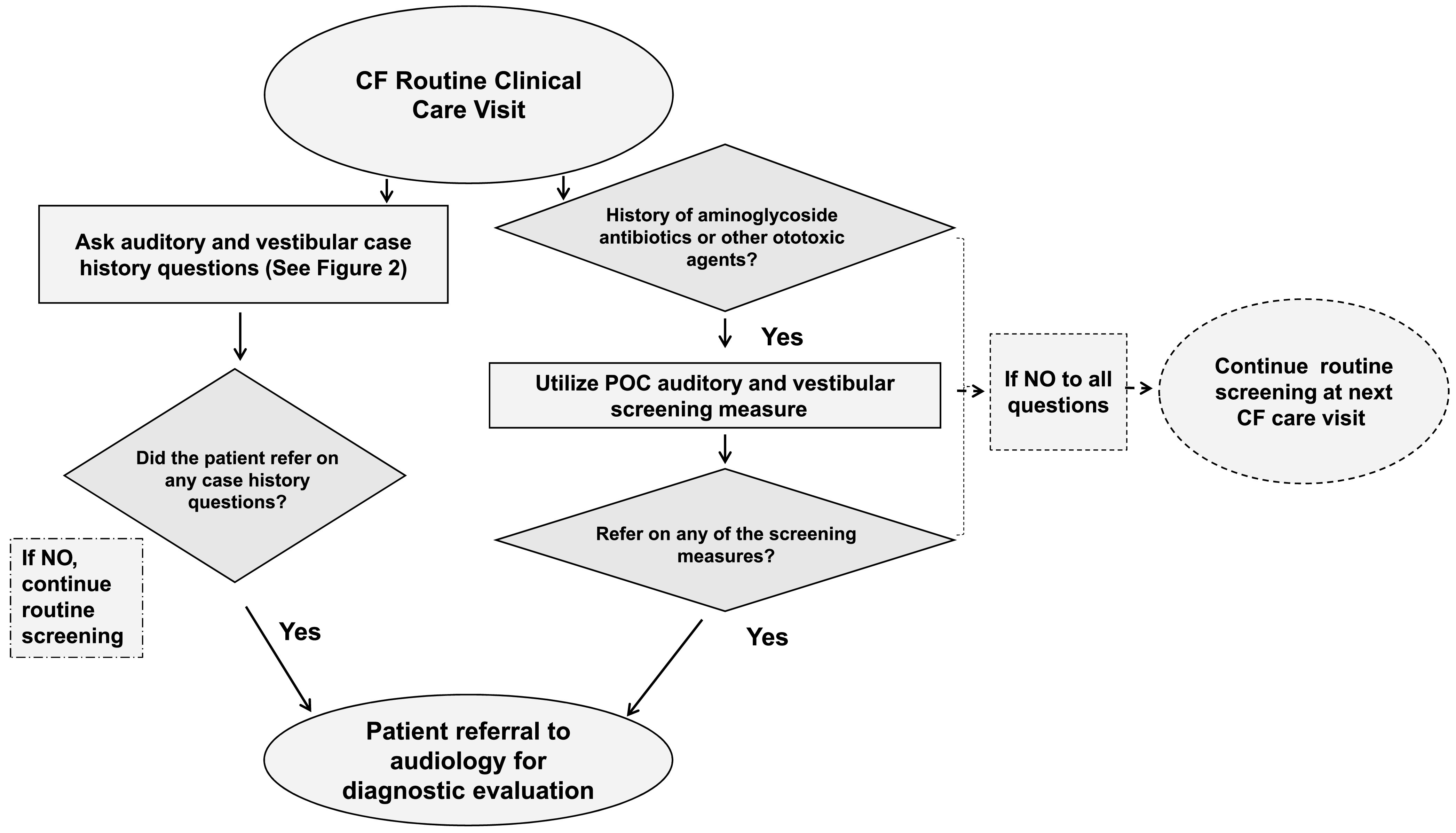
Audiology referral flowchart for adult and pediatric patients with cystic fibrosis (CF). POC = point-of-care.
Include Questions Related to Hearing, Tinnitus, and Balance/Vestibular Problems for All Patients With CF in the Routine Clinical Care Visit
CF routine interval case histories conducted by a trained CF care team member or audiologist should include questions about hearing, tinnitus, and balance or vestibular problems (see Figure 2), including date of last formal hearing and vestibular examinations. The inclusion of the questions should occur at each 3-month CF clinical care visit for any patient treated at a CF center (CFF, 2018). The yes/no questions illustrated in Figure 2 are intended to identify patients who have developed a new auditory issue or ototoxicity symptom since their last CF clinical care visit. Subsequent clinical action based on the patient's response will often lead to a referral from a member of the CF clinical care team to audiology, as described in Figure 1. A detailed understanding of past as well as new symptoms and concerns related to hearing, tinnitus, and balance or vestibular concerns are critical components to early detection, therapeutic intervention, and prevention of continued damage (e.g., AAA, 2009; ASHA, 1994; Handelsman, 2018).
Figure 2.
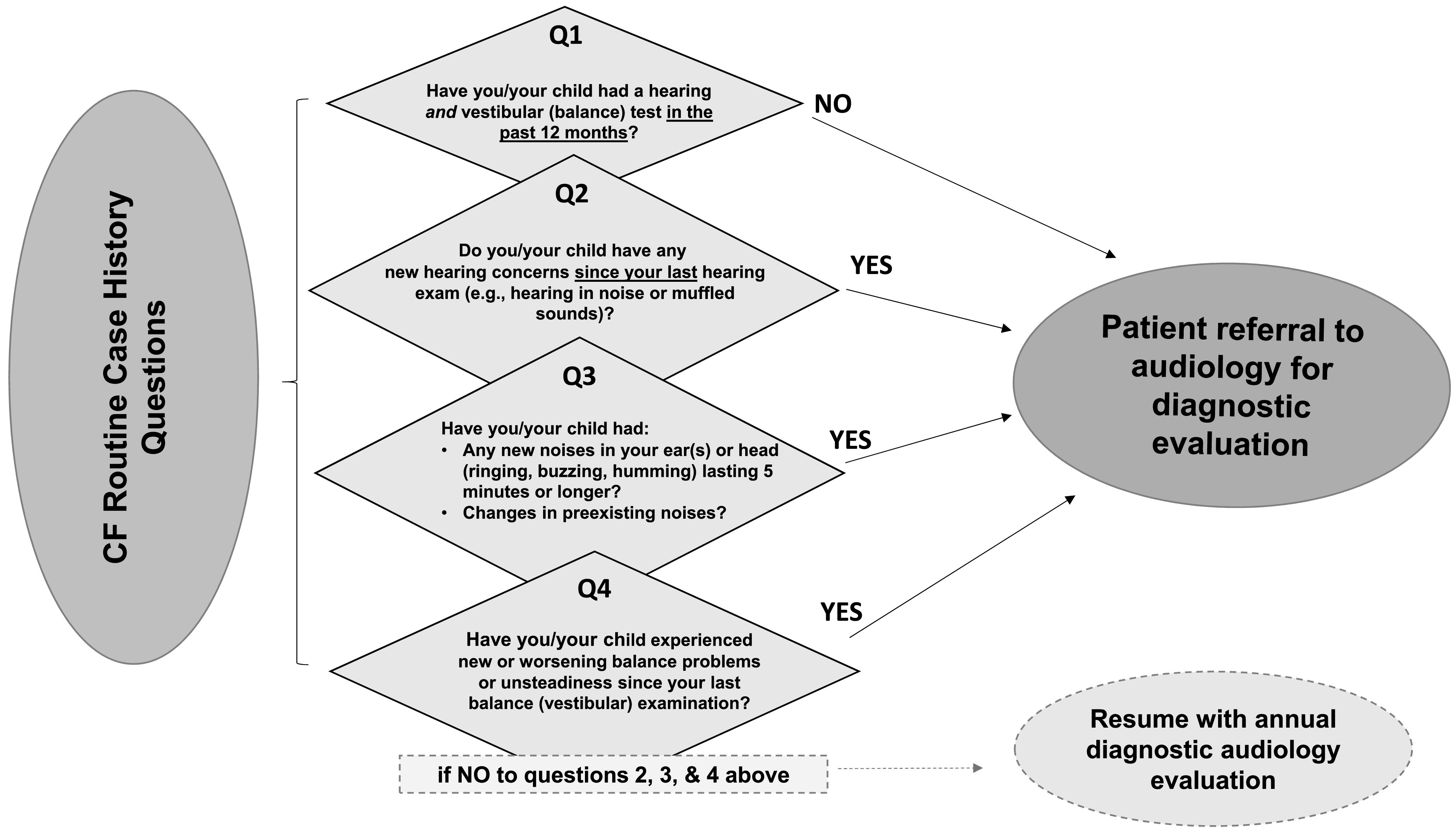
CF-specific case history questions and referral pathway for POC testing and audiological management by a trained CF care team member or audiologist. CF = cystic fibrosis; POC = point-of-care.
Complete Baseline and Posttreatment Hearing Evaluations for IV-Ototoxic Treatments
Patients with CF who are prescribed an IV course of a known cochleotoxic or vestibulotoxic agent should have a baseline ototoxicity monitoring evaluation (before or within 3 days of initial treatment dose) and a posttreatment ototoxicity monitoring evaluation no later than 3 months after completion of treatment (see Figure 3). Testing may be conducted using available POC measures by a trained CF care team member or audiologist, when appropriate. Establishing a baseline measure prior to administration (or as soon as possible after initial dosing) of ototoxic treatment helps serve as a reference for detecting significant changes in subsequent monitoring visits (ASHA, 1994; AAA, 2009). These timeframes were selected to coincide with routine CF clinical care and to detect ototoxicity prior to the next IV course of treatment, which is often unpredictable for a given patient. Interim and immediate referral for assessment is recommended if the patient becomes symptomatic (hearing, tinnitus, or balance/vestibular concerns), or if there are caregiver concerns before the next scheduled follow-up audiology evaluation. More frequent monitoring may be recommended for patients with preexisting ototoxicity symptoms, particularly hearing loss, or those on prolonged courses of IV therapy, such as patients receiving amikacin for nontuberculosis mycobacterial infection. These recommendations are considered the minimal degree of monitoring for patients on IV therapies, and are further supported by new evidence that hearing loss can be evident even after one course of IV-aminoglycoside treatment for some patients with CF (Garinis et al., 2021; Zettner & Gleser, 2018), and that cumulative IV-aminoglycoside exposure increases one's risk for progressive ototoxicity (Garinis et al., 2017). Such audiological data, combined with the patient's response to case history questions (as those shown in Figure 2) are recommended for detecting a patient's ototoxicity symptoms, and facilitating timely referral for audiological follow-up and ongoing ototoxicity management.
Figure 3.
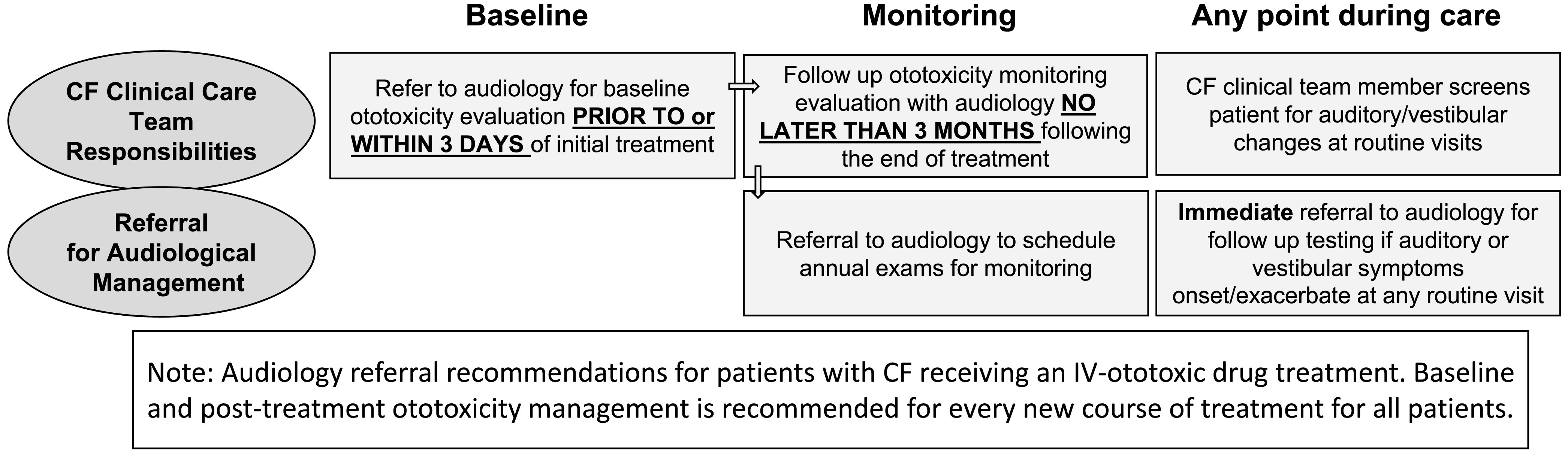
Audiology referral recommendations for patients receiving new IV-ototoxic treatment. IV = intravenous.
Perform Annual Hearing and Vestibular Evaluations for All Patients With CF Exposed to Ototoxic Medications
Annual hearing and vestibular diagnostic evaluations are important to capture subtle changes in hearing and balance/vestibular function for all patients with CF. Changes to auditory function may occur from various sources such as genetic predisposition, noise, ear infections, or the aging process, and may be further exacerbated by exposure to ototoxic. Thus, routine annual hearing and vestibular evaluations should be considered for all pediatric and adult patients with CF, rather than limiting testing to patients receiving IV-ototoxic treatments (see Figures 1 and 3).
As previously described, patients with CF receiving ototoxic treatments are at a high risk for developing ototoxicity and there is no evidence to predict the exact onset of symptoms in a given patient, particularly with inhaled aminoglycosides, thus, routine monitoring is critical for early identification. (e.g., Al-Malky et al., 2015; Blankenship et al., 2021; Dreisbach et al., 2018; Earnest et al., 2020; Garinis et al., 2017, 2021; Handelsman, 2018). Patients who have frequent episodic pulmonary exacerbations should be monitored more closely for ototoxicity symptoms, particularly since they will likely receive a greater cumulative dose of ototoxic treatments in a given year compared to patients without episodic exacerbations. The recommendation for annual audiological care supports the balancing of continued monitoring in a timeframe that aligns with ongoing follow-up with the CF care team.
CF-specific annual hearing evaluations should include both behavioral and physiologic measures of auditory function to capture high frequency changes from ototoxic treatments. Recommended tests include (a) hearing threshold determination for the extended high frequencies (beyond 8 kHz) and (b) cochlear function via otoacoustic emission (OAE) measures to the highest available test frequency, consistent with AAA (2009) and ASHA's (1994) national guidelines. The value of prioritizing extended high-frequency evaluation for the earliest detection of ototoxic changes is well established (e.g., AAA, 2009; Dreisbach et al., 2017; Konrad-Martin et al., 2016; Poling et al., 2019). In patients with CF, higher frequency (> 8 kHz) assessment tools for audiometry (Al-Malky et al., 2015; Garinis et al., 2017; Vijayasingam et al., 2020) as well as objective measures such as OAEs (Dreisbach et al., 2018) are recommended to detect ototoxicity. Pediatric or adult patients with CF who have never received an ototoxic drug would benefit from receiving these ototoxic-specific audiological protocols, given that they will likely receive a future ototoxic treatment for management of bacterial infections. Maintaining a baseline for comparison is crucial for those who develop drug-induced and/or auditory changes.
Individuals with CF, particularly those on aminoglycoside antibiotics, consistently demonstrate a high rate of vestibular impairment warranting annual evaluation (Blankenship et al., 2021; Handelsman et al., 2017; Rogers & Petersen, 2011; Scheenstra et al., 2010). Variable symptoms and degrees of vestibulotoxicity (unilateral or bilateral) may present as oscillopsia, dizziness, motion sickness, or vertigo as well as unsteadiness with standing or walking (Ahmed et al., 2012; Black et al., 2001, 2004; Handelsman, 2018; Ishiyama et al., 2006). Histological studies in animals have suggested the mechanism of damage is likely due to loss of type I vestibular hair cells (e.g., Lyford-Pike et al., 2007). Techniques for detecting vestibulotoxicity may vary depending on the specific vestibular symptoms and may include a thorough case history documenting past and current symptoms or assessment of handicap/disability using specialized instruments and/or bedside techniques (reviewed by Handelsman, 2018). The protocol should minimally include measures such as a Head Impulse Test and/or Video Head Impulse Test examination and dynamic visual acuity assessment to diagnose ear-related balance/vestibular problems, either of which can be accomplished using a time-efficient and non-invasive POC approach. If available, rotational chair testing remains the gold standard to confirm bilateral vestibular weakness.
Recommendations for objective testing suggest that an audiologist should be incorporated into the CF care team to direct ototoxicity monitoring, incorporate timely aural rehabilitation or recommendations for vestibular rehabilitation, and ensure continuity of audiologic care (i.e., ototoxicity management). When an audiologist is not available to conduct evaluations, an appropriately trained health care professional (e.g., nurse or medical assistant trained to administer hearing screenings) should be used to facilitate testing and referrals.
Conclusions
The IOMG clinical care considerations for ototoxicity management in patients with CF were developed to address a critical need for identifying and advocating for incorporating audiological care into the CF care model. Effective management of ototoxicity is crucial for patient awareness and clinical decision making related to the long-term hearing health impacts of their treatment regimen, as well as guidance for rehabilitative follow-up (e.g., hearing amplification, vestibular rehabilitation, tinnitus management). To date, it is unknown to what extent novel CFTR modulator therapy will increase life expectancy of patients with CF, or what the long-term effects of preexisting disease, including hearing loss, will have on a given individual's quantity and quality of life. In addition, due to the changing landscape and challenges of health care, many outpatient appointments have shifted from in person to virtual and avoidance of clinical settings may be preferred for clinical care even when feasible in the future. Thus, novel solutions for efficient, remote ototoxicity monitoring metrics are also needed to facilitate routine hearing health care in this patient population, particularly in patients receiving home IV therapies. Thus, it is important to highlight the crucial need to improve hearing health care in this population given the likely advancement of age with its attendant risk for presbycusis in combination with long-term ototoxicity effects on the quality and quantity of life in persons with CF.
Supplementary Material
Acknowledgments
Funding for this work was supported in part by the National Institute on Deafness and Other Communication Disorders (Grant ZIA-DC000064, awarded to C. C. B.; Grant 1R21DC016128-01A1, awarded to A. C. G.; Grants DC004555 and DC016680, awarded to P. S. S.; Grant 1R01DC017867, awarded to L. L. H.; Grant 1R01DC017425, awarded to T. E. H.), as well as the Cystic Fibrosis Foundation (Grant GARINI1A90, awarded to A. C. G, P. S. S., and R. C. R.). D. M. B is supported by the UK National Institute for Health Research (NIHR): his views herein are his own and do not represent those of NIHR nor the UK Department of Health and Social Care. We would like to acknowledge the National Center for Rehabilitative Auditory Research at the Portland VA Health Care System for providing their support and resources to hold our first in-person IOMG meeting (VA Rehabilitation Research and Development Services Center Award-C2361C). A special thanks to Patrick Feeney and Thais Morata for their efforts to review and comment on this document. We would also like to thank all committee members of the IOMG for contributing to the development of this working group aimed to improve the ototoxicity monitoring and management process across clinical specialties worldwide.
Funding Statement
Funding for this work was supported in part by the National Institute on Deafness and Other Communication Disorders (Grant ZIA-DC000064, awarded to C. C. B.; Grant 1R21DC016128-01A1, awarded to A. C. G.; Grants DC004555 and DC016680, awarded to P. S. S.; Grant 1R01DC017867, awarded to L. L. H.; Grant 1R01DC017425, awarded to T. E. H.), as well as the Cystic Fibrosis Foundation (Grant GARINI1A90, awarded to A. C. G, P. S. S., and R. C. R.). D. M. B is supported by the UK National Institute for Health Research (NIHR): his views herein are his own and do not represent those of NIHR nor the UK Department of Health and Social Care.
References
- Ahmed, R. M. , Hannigan, I. P. , MacDougall, H. G. , Chan, R. C. , & Halmagyi, G. M. (2012). Gentamicin ototoxicity: A 23-year selected case series of 103 patients. Medical Journal of Australia, 196(11), 701–704. https://doi.org/10.5694/mja11.10850 [DOI] [PubMed] [Google Scholar]
- Alexander, J. M. , Kopun, J. G. , & Stelmachowicz, P. G. (2014). Effects of frequency compression and frequency transposition on fricative and affricate perception in listeners with normal hearing and mild to moderate hearing loss. Ear and Hearing, 35(5), 519–532. https://doi.org/10.1097/AUD.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Malky, G. , Dawson, S. J. , Sirimanna, T. , Bagkeris, E. , & Suri, R. (2015). High-frequency audiometry reveals high prevalence of aminoglycoside ototoxicity in children with cystic fibrosis. Journal of Cystic Fibrosis, 14(2), 248–254. https://doi.org/10.1016/j.jcf.2014.07.009 [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology. (2009). Position statement and clinical practice guidelines: Ototoxicity monitoring. Accessed January 27, 2017, from http://www.audiology.org [Google Scholar]
- American Academy of Otolaryngology-Head and Neck Surgery. (2015). Position statement: Ototoxicity. Accessed October 26, 2018, from http://www.entnet.org [Google Scholar]
- American Speech-Language-Hearing Association. (1994). Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA, 36(Suppl. 12), 11–19. [Google Scholar]
- American Thoracic Society, & Infectious Disease Society of America. (2007). An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial disease. American Journal of Respiratory and Critical Care Medicine, 175, 367–416. https://doi.org/10.1164/ajrccm.175.7.744b [DOI] [PubMed] [Google Scholar]
- Ariano, R. E. , Zelenitsky, S. A. , & Kassum, D. A. (2008). Aminoglycoside-induced vestibular injury: Maintaining a sense of balance. The Annals of Pharmacotherapy, 42(9), 1282–1289. https://doi.org/10.1345/aph.1L001 [DOI] [PubMed] [Google Scholar]
- Baguley, D. M. , & Prayuenyong, P. (2020). Looking beyond the audiogram in ototoxicity associated with platinum-based chemotherapy. Cancer Chemotherapy and Pharmacology, 85(2), 245–250. https://doi.org/10.1007/s00280-019-04012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, F. O. , Gianna-Poulin, C. , & Pesznecker, S. C. (2001). Recovery from vestibular ototoxicity. Otology & Neurotology, 22(5), 662–671. https://doi.org/10.1097/00129492-200109000-00018 [DOI] [PubMed] [Google Scholar]
- Black, F. O. , Pesznecker, S. C. , & Stallings, V. (2004). Permanent gentamicin vestibulotoxicity. Ototology & Neurotology, 25(4), 559–569. https://doi.org/10.1097/00129492-200407000-00025 [DOI] [PubMed] [Google Scholar]
- Blankenship, C. M. , Hunter, L. L. , Feeney, M. P. , Cox, M. , Bittinger, L. , Garinis, A. C. , Lin, L. , McPhail, G. , & Clancy, J. P. (2021). Functional impacts of aminoglycoside treatment on speech perception and extended high-frequency hearing loss in a pediatric cystic fibrosis cohort. American Journal of Audiology, 19, 1–20. https://doi.org/10.1101/2020.04.29.20084848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brungart, D. , Schurman, J. , Konrad-Martin, D. , Watts, K. , Buckey, J. , Clavier, O. , Jacobs, P. G. , Gordon, S. , & Dille, M. F. (2018). Using tablet-based technology to deliver time-efficient ototoxicity monitoring. International Journal of Audiology, 57(Suppl. 4), S25−S33. https://doi.org/10.1080/14992027.2017.1370138 [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation. (2018). CF Patient Registry Report [Online source] . Accessed December 23, 2020, https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf [Google Scholar]
- Daley, C. L. , Laccarino, J. M. , Lange, C. , Cambau, E. , Wallace, R. J., Jr. , Andrejak, C. , Böttger, E. C. , Brozek, J. , Griffith, D. E. , Guglielmetti, L. , Huitt, G. A. , Knight, S. L. , Leitman, P. , Marras, T. K. , Olivier, K. N. , Santin, M. , Stout, J. E. , Tortoli, E. , van Ingen, J. , Wagner, D. , & Winthrop, K. L. (2020). Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clinical Infectious Diseases, 71(4), e1–e36. https://doi.org/10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach, L. , Ho, M. , Reid, E. , & Siegel, J. (2017). Effects of oxaliplatin, carboplatin, and cisplatin across treatment on high-frequency objective and subjective auditory measures in adults. Perspectives of the ASHA Special Interest Groups, 2(6), 17–36. https://doi.org/10.1044/persp2.SIG6.17 [Google Scholar]
- Dreisbach, L. , Zettner, E. , Chang, M. , Meuel, C. , MacPhee, I. , & Boothroyd, A. (2018). High-frequency distortion-product otoacoustic emission repeatability in a patient population. Ear and Hearing, 39(1), 85–100. https://doi.org/10.1097/AUD.0000000000000465 [DOI] [PubMed] [Google Scholar]
- Earnest, A. , Salimi, F. , Wainwright, C. E. , Bell, S. C. , Ruseckaite, R. , Ranger, T. , Kotsimbos, T. , & Ahern, S. (2020). Lung function over the life course of paediatric and adult patients with cystic fibrosis from a large multi-centre registry. Scientific Reports, 10, 17421. https://doi.org/10.1038/s41598-020-74502-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson, E. C. , Meier, E. , & Oermann, C. M. (2020). The implementation of an aminoglycoside induced ototoxicity algorithm for people with cystic fibrosis. Journal of Cystic Fibrosis, 20(2), 284–287. https://doi.org/10.1016/j.jcf.2020.08.002 [DOI] [PubMed] [Google Scholar]
- Fausti, S. A. , Rappaport, B. Z. , Schechter, M. A. , Fray, R. H. , Ward, T. T. , & Brummett, R. E. (1984). Detection of aminoglycoside ototoxicity by high-frequency auditory evaluation: Selected case studies. American Journal of Otolaryngology, 5(3), 177–182. https://doi.org/10.1016/S0196-0709(84)80009-5 [DOI] [PubMed] [Google Scholar]
- Garinis, A. C. , Cornell, A. , Allada, G. , Fennelly, K.P. , Maggiore, R. , & Konrad-Martin, D. (2018). Ototoxicity monitoring through the eyes of the treating physician: Perspectives from pulmonology and medical oncology. International Journal of Audiology, 57(Suppl. 4), S19−S24. https://doi.org/10.1080/14992027.2017.1381769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis, A. C. , Cross, C. P. , Srikanth, P. , Carroll, K. , Feeney, M. P. , Keefe, D. H. , Hunter, L. L. , Putterman, D. B. , Cohen, D. M. , Gold, J. A. , & Steyger, P. S. (2017). The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. Journal of Cystic Fibrosis, 16(3), 401–409. https://doi.org/10.1016/j.jcf.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis, A. C. , Gleser, M. , Johns, A. , Larsen, E. , & Vachhani, J. (2021). Prospective cohort study of ototoxicity in persons with cystic fibrosis following a single course of intravenous tobramyci. Journal of Cystic Fibrosis, 20(2), 278–283. https://doi.org/10.1016/j.jcf.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, O. W. (2008). Aminoglycoside induced ototoxicity. Toxicology, 249(2–3), 91–96. https://doi.org/10.1016/j.tox.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Handelsman, J. A. (2018). Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. International Journal of Audiology, 57(Suppl. 4), S69−S77. https://doi.org/10.1080/14992027.2018.1468092 [DOI] [PubMed] [Google Scholar]
- Handelsman, J. A. , Nasr, S. Z. , Pitts, C. , & King, W. M. (2017). Prevalence of hearing and vestibular loss in cystic fibrosis patients exposed to aminoglycosides. Pediatric Pulmonology, 52(9), 1157–1162. https://doi.org/10.1002/ppul.23763 [DOI] [PubMed] [Google Scholar]
- Health Professions Council of South Africa. (2019). Audiological management of patients on treatment that includes ototoxic medications. Year 2018. Acessed October 15, 2020, from https://www.hpcsa.co.za/Uploads/SLH/Guidelines%20for%20Audiological%20Management%20of%20Patients%20on%20Treatment%20that%20includes%20Ototoxic%20Medications.pdf [Google Scholar]
- Henry, J. A. , Griest, S. , Austin, D. , Helt, W. , Gordon, J. , Theodoroff, S. M. , Lewis, M. S. , Blankenship, C. , Zaugg, T. L. , & Carlson, K. (2016). Tinnitus screener: Results from the first 100 participants in an epidemiology study. American Journal of Audiology, 25(2), 153–160. https://doi.org/10.1044/2016_AJA-15-0076 [DOI] [PubMed] [Google Scholar]
- Hollander, C. , Joubert, K. , & Schellack, N. (2020). An ototoxicity grading system within a mobile app (OtoCalc) for a resource-limited setting to guide grading and management of drug-induced hearing loss in patients with drug-resistant tuberculosis: Prospective, cross-sectional case series. JMIR mHealth and uHealth, 8(1), Article e14036. https://doi.org/10.2196/14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing, E. H. , & de Groot, J. C. (1987). Human cochlear pathology in aminoglycoside ototoxicity—A review. Acta Oto-Laryngologica. Supplementum, 436, 117–125. https://doi.org/10.3109/00016488709124984 [DOI] [PubMed] [Google Scholar]
- Ishiyama, G. , Ishiyama, A. , Kerber, K. , & Baloh, R. W. (2006). Gentamicin ototoxicity: Clinical features and the effect on the human vestibulo-ocular reflex. Acta Oto-Laryngologica, 126(10), 1057–1061. https://doi.org/10.1080/00016480600606673 [DOI] [PubMed] [Google Scholar]
- Jiang, M. , Karasawa, T. , & Steyger, P. S. (2017). Aminoglycoside-induced cochleotoxicity: A review. Frontiers in Cellular Neuroscience, 11, 308. https://doi.org/10.3389/fncel.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, K. A. , & Brewer, C. (2018). Clinical trials, ototoxicity grading scales and the audiologist's role in therapeutic decision making. International Journal of Audiology, 57(Suppl. 4), S19−S28. https://doi.org/10.1080/14992027.2017.1417644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, M. R. , & Durie, P. R. (2002). What is cystic fibrosis? The New England Journal of Medicine, 347(6), 439–442. https://doi.org/10.1056/NEJMe020070 [DOI] [PubMed] [Google Scholar]
- Koleilat, A. , Argue, D. P. , Schimmenti, L. A. , Ekker, S. C. , & Poling, G. L. (2020). The GoAudio Quantitative Mobile Audiology Test enhances access to clinical hearing assessments. American Journal of Audiology, 29(4), 887–897. https://doi.org/10.1044/2020_AJA-20-00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin, D. , Poling, G. L. , Dreisbach, L. , Reavis, K. , McMillan, G. , Lapsley Miller, J. , & Marshall, L. (2016). Serial monitoring of otoacoustic emissions in clinical trials. Otology & Neurotology, 37(8), e286–e294. https://doi.org/10.1097/MAO.0000000000001134 [DOI] [PubMed] [Google Scholar]
- Konrad-Martin, D. , Poling, G. L. , Garinis, A. C. , Ortiz, C. E. , Hopper, J. , O'Connell Bennett, K. , & Dille, M. F. (2018). Applying U.S. national guidelines for ototoxicity monitoring in adult patients: Perspectives on patient populations, service gaps, barriers and solutions. International Journal of Audiology, 57(Suppl. 4), S3−S18. https://doi.org/10.1080/14992027.2017.1398421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. R. , Niparko, J. K. , & Ferrucci, L. (2011). Hearing loss prevalence in the United States. Archives of Internal Medicine, 17(20), 1851–1852. https://doi.org/10.1001/archinternmed.2011.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford-Pike, S. , Vodelheim, C. , Chu, E. , Santina, C. D. , & Carey, J. P. (2007). Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. Journal of the Association for Research in Otolaryngology, 8(4), 497–508. https://doi.org/10.1007/s10162-007-0093-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru, D. , & Al-Malky, G. (2018). Current practice of ototoxicity management across the United Kingdom (UK). International Journal of Audiology, 57(Suppl. 4), S29–S41. https://doi.org/10.1080/14992027.2018.1460495 [DOI] [PubMed] [Google Scholar]
- Monson, B. B. , Rock, J. , Schulz, A. , Hoffman, E. , & Buss, E. (2019). Ecological cocktail party listening reveals the utility of extended high-frequency hearing. Hearing Research, 381, 107773. https://doi.org/10.1016/j.heares.2019.107773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Auditory Rehabilitative Research. (2020a). Patient Voices. Accessed December 31, 2020, from https://www.ncrar.research.va.gov/PatientVoices/Index.asp [Google Scholar]
- National Center for Auditory Rehabilitative Research. (2020b). International Ototoxicity Management Group (IOMG) charter and complete roster. Accessed December 31, 2020, from https://www.ncrar.research.va.gov/ClinicianResources/IOMG.asp [Google Scholar]
- Poling, G. L. , Vlosich, B. , & Dreisbach, L. (2019). Emerging distortion product otoacoustic emission techniques to identify preclinical warning signs of basal cochlear dysfunction due to ototoxicity. Applied Sciences, 9(15), 3132. https://doi.org/10.3390/app9153132 [Google Scholar]
- Prescott, W. A., Jr. (2011). National survey of extended-interval aminoglycoside dosing in pediatric cystic fibrosis pulmonary exacerbations. Journal of Pediatric Pharmacology and Therapeutics, 16(4), 262–269.https://doi.org/10.5863/1551-6776-16.4.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott, W. A., Jr. (2014). A survey of extended-interval aminoglycoside dosing practices in United States adult cystic fibrosis programs. Respiratory Care, 59(1359), 1353–1359. https://doi.org/10.4187/respcare.02980 [DOI] [PubMed] [Google Scholar]
- Rogers, C. , & Petersen, L. (2011). Aminoglycoside-induced balance deficits: A review of vestibulotoxicity. South African Family Practice, 53(5), 419–424. https://doi.org/10.1080/20786204.2011.10874126 [Google Scholar]
- Samelli, A. G. , Rabelo, C. M. , Sanches, S. G. G. , Martinho, A. C. , & Matas, C. G. (2020). Tablet-based tele-audiometry: Automated hearing screening for schoolchildren. Journal of Telemedicine and Telecare, 26, 140–149. https://doi.org/10.1177/1357633X18800856 [DOI] [PubMed] [Google Scholar]
- Sanders, D. B. , Bittner, R. , Rosenfeld, M. , Hoffman, L. R. , Redding, G. J. , & Goss, C. H. (2010). Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. American Journal of Respiratory and Critical Care Medicine, 182(5), 627–632. https://doi.org/10.1164/rccm.200909-1421OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, D. B. , Bittner, R. , Rosenfeld, L. R. , Redding, G. J. , & Goss, C. H. (2011). Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatric Pulmonology, 46(4), 393–400. https://doi.org/10.1002/ppul.21374 [DOI] [PubMed] [Google Scholar]
- Scheenstra, R. J. , Heijerman, H. G. , Zuur, C. L. , Touw, D. J. , & Rijntjes, E. (2010). No hearing loss after repeated courses of tobramycin in cystic fibrosis patients. Acta Oto-Laryngologica, 130(2), 253–258. https://doi.org/10.3109/00016480903015150 [DOI] [PubMed] [Google Scholar]
- Scotet, V. , Gutierrez, H. , & Farrell, P. M. (2020). Newborn screening for CF across the globe—Where is it worthwhile? International Journal of Neonatal Screening, 6(1), 18. https://doi.org/10.3390/ijns6010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, S. H. , Taylor, R. , Forge, A. , & Schacht, J. (2001). Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hearing Research, 155(1–2), 1–8. https://doi.org/10.1016/S0378-5955(01)00224-6 [DOI] [PubMed] [Google Scholar]
- Shaikh, A. G. , Bronstein, A. , Carmona, S. , Cha, Y.-H. , Cho, C. , Ghasia, F. F. , Gold, D. , Green, K. E. , Helmchen, C. , Ibitoye, R. T. , Kattah, J. , Kim, J.-S. , Kothari, S. , Manto, M. , Seemungal, B. M. , Straumann, D. , Strupp, M. , Szmulewicz, D. , Tarnutzer, A. , … Kheradmand, A. (2020). Consensus on virtual management of vestibular disorders: Urgent versus expedited care. The Cerebellum, 20, 4–8. https://doi.org/10.1007/s12311-020-01178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, K. H. , Mulheran, M. , Knox, A. J. , & Smyth, A. R. (2003). Aminoglycoside prescribing and surveillance in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine, 167(6), 819–823. https://doi.org/10.1164/rccm.200109-012CC [DOI] [PubMed] [Google Scholar]
- Vijayasingam, A. , Frost, E. , Wilkins, J. , Gillen, L. , Premachandra, P. , Mclaren, K. , Gilmartin, D. , Picinali, L. , Vidal-Diez, A. , Borsci, S. , Ni, M. Z. , Tang, W. Y. , Morris-Rosendahl, D. , Harcourt, J. , Elston, C. , Simmonds, N. J. , & Shah, A. (2020). Tablet and web-based audiometry to screen for hearing loss in adults with cystic fibrosis. Thorax, 75(8), 632–639. https://doi.org/10.1136/thoraxjnl-2019-214177 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1994). Report of an informal consultation on strategies for prevention of hearing impairment from ototoxic drugs [Online] . Accessed August 7, 2017, from http://www.who.int/pbd/deafness/ototoxic_drugs.pdf [Google Scholar]
- Zettner, E. M. , & Gleser, M. A. (2018). Progressive hearing loss among patients with cystic fibrosis and parenteral aminoglycoside treatment. Otolaryngology—Head and Neck Surgery, 159(5), 887–894. https://doi.org/10.1177/0194599818782444 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


