Abstract
Purpose
This review article summarizes our current understanding of the mechanisms underlying acquired hearing loss from hospital-prescribed medications that affects as many as 1 million people each year in Western Europe and North America. Yet, there are currently no federally approved drugs to prevent or treat the debilitating and permanent hearing loss caused by the life-saving platinum-based anticancer drugs or the bactericidal aminoglycoside antibiotics. Hearing loss has long-term impacts on quality-of-life measures, especially in young children and older adults. This review article also highlights some of the current knowledge gaps regarding iatrogenic causes of hearing loss.
Conclusion
Further research is urgently needed to further refine clinical practice and better ameliorate iatrogenic drug-induced hearing loss.
In 2018, the consensus of the Ototoxicity Working Group of Pharmaceutical Interventions for Hearing Loss, 2018 defined ototoxicity as damage to the inner ear, targeting cochlear and vestibular structures and sensory function, due to exposure to certain pharmaceuticals, chemicals, and/or ionizing radiation (i.e., iatrogenic hearing loss). This review article will focus primarily on hospital-based medications: aminoglycoside antibiotics and cisplatin. Aminoglycosides are typically used for serious bacterial and mycobacterial infections, including gentamicin for meningitis and sepsis, tobramycin or amikacin for respiratory infections in individuals with cystic fibrosis, and kanamycin for tuberculosis. Cisplatin- and platinum-based derivatives like carboplatin and oxaliplatin provide efficacious treatment for solid (e.g., testicular and ovarian) tumors, head and neck cancers, and glioblastomas in the brain.
Other ototoxins include solvents and jet fuels (Davis et al., 2002; Fechter et al., 2007; Steyger, 2009), metals such as lead (Counter & Buchanan, 2002; Jamesdaniel et al., 2018), and cyclodextrins, which is a primary component of drug formulations that, at high doses, can sequester membrane cholesterols (Crumling et al., 2017). Ototoxicity is primarily considered a peripheral, inner ear phenomenon; however, ototoxic agents can also affect central neural (or auditory) pathways and are then considered to be neurotoxic (Gopal et al., 2012; Hinduja et al., 2015). Ototoxins can also cause kidney damage and associated renal dysfunction (i.e., nephrotoxicity; Humes, 1999; Jiang et al., 2017; Karasawa & Steyger, 2015).
Adults that lose their exquisite sense of hearing invariably have diminished interactions via spoken language with loved ones, friends, and colleagues, and reduced awareness of environmental sounds with a corresponding loss of safety (Centers for Disease Control and Prevention, 2004; Monson et al., 2019). The clinical consequences of uncorrected or maladaptation to hearing loss can include isolation, depression, and loss of self-esteem that can lead to declining cognitive ability and social withdrawal (Lin et al., 2013). Uncorrected congenital or acquired hearing loss in infants can lead to delayed acquisition of listening and spoken language skills expected of their peers with typical hearing, with concomitant delays meeting academic, linguistic, and psychosocial milestones (Dedhia et al., 2013; Gurney et al., 2007). Loss of vestibular function is also debilitating and can lead to depression and cognitive decline (Smith & Darlington, 2013; Smith & Zheng, 2013). In addition, there is a socioeconomic cost > $1.5 million over the lifetime of each deafened child and > $375,000 for each adult in the United States (extrapolated into 2019 values from Mohr et al., 2000).
Incidence of Iatrogenic Drug-Induced Hearing Loss and Vestibular Disorders
In preclinical models, aminoglycoside-induced hearing loss is typically dose dependent (Wu et al., 2001). The prevalence of clinical aminoglycoside dosing in the United States has decreased from several million in the 1980s due to the use of β-lactams in the 1980s and 1990 to a few hundred thousand during the current era of antibiotic stewardship (Pitiriga et al., 2017; Whelton, 1984). The incidence of hearing loss in humans after aminoglycoside treatment remains unclear due to variable dose stratification regimens and limited pure-tone testing that does not always include frequencies above 8 kHz in audiometric assays; nonetheless, the incidence of hearing loss may occur in 20%–63% of those receiving aminoglycosides for multiple days (Elson et al., 2020; Fausti, Frey, et al., 1992; Fausti, Henry, et al., 1992; Garinis, Cross, et al., 2017; Garinis, Liao, et al., 2017). Patients often report vestibular (and tinnitus) issues before any awareness of aminoglycoside-induced hearing loss (Van Hecke et al., 2017); however, the incidence of aminoglycoside-induced elevations of vestibular thresholds for motion detection (Shayman et al., 2018) is likely underreported due to the widespread absence of clinical apparati (away from major academic medical centers) for determining vestibular function.
Platinum-based drugs, like cisplatin, have long been known to be cochleotoxic in humans and preclinical models (Brock et al., 2012). Cisplatin is clinically very effective in reducing the mass of solid or central nervous system tumors, yet the incidence of hearing loss is cumulatively dose dependent (Callejo et al., 2015; Knight et al., 2005, 2007; Paken et al., 2016). Cisplatin has recently been reported to dose-dependently induce loss of vestibular hair cells and vestibular function in preclinical models (Callejo et al., 2017). Although there are fewer reports of cisplatin-induced vestibular dysfunction, this is more likely due to geographic limitations in determining human vestibular thresholds for motion detection (Prayuenyong et al., 2018), and lack of routine clinical assessments, as for aminoglycosides.
Trafficking of Aminoglycosides and Cisplatin Into the Inner Ear
The trafficking of these ototoxins into the inner ear and hair cells are generally similar, although more details are known for aminoglycosides. The blood–labyrinth barrier (BLB) separates cochlear cells and fluids from the bloodstream, akin to the blood–brain barrier. The endothelial cells of cochlear blood vessels are typically coupled together by tight junctions to form the primary BLB and prevent macromolecules and blood cells from entering the cochlea (Nyberg et al., 2019). Within the cochlea, perilymph has an ionic composition typical of other extracellular fluids (i.e., high in Na+, low in K+, and millimolar Ca2+) and the larger fluid volume (Wangemann, 2006). Although systemically administered aminoglycosides and cisplatin can enter the perilymphatic space surrounding the basolateral membranes of hair cells from the bloodstream (Hellberg et al., 2013; Tran Ba Huy et al., 1986), aminoglycosides do not easily enter hair cells from this domain (Li & Steyger, 2011).
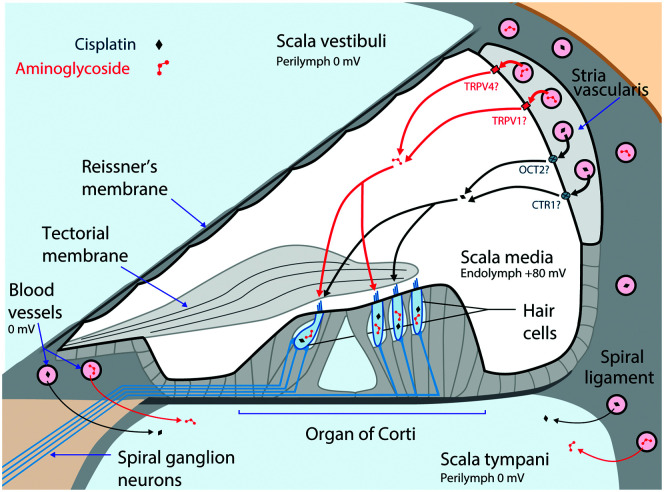
In contrast, endolymph bathes the apical surfaces of hair cells and has a unique extracellular ionic composition in the mammalian body (i.e., high in K+, low in Na+, and ~20-µM Ca2+). Cochlear, but not vestibular, endolymph has a highly positive potential of ~ +80 mV relative to blood or perilymph that is crucial for sensitive hearing. Tight junction–coupled epithelial cells surround the smaller endolymphatic volume (relative to perilymph) to prevent fluid mixing with perilymph (and hearing loss). The endolymphatic potential and its ionic composition are generated by the highly metabolically active cells of the vascularized stria vascularis (see Figure 1) in the lateral wall of the cochlea (Wangemann, 2006). Systemically administered aminoglycosides cross the BLB of the stria vascularis more rapidly than into the adjacent spiral ligament in the perilymphatic domain (Dai & Steyger, 2008). Surprisingly, systemically administered aminoglycosides also enter hair cells more rapidly than when aminoglycosides are directly infused into the perilymphatic compartment that surrounds the basolateral membranes of hair cells (Li & Steyger, 2011). Systemic inflammation and noise, both of which are associated with vasodilation of the blood vessels in the stria vascularis, enhance cochlear uptake of aminoglycosides (Koo et al., 2015; Li et al., 2015). The cellular and molecular mechanisms by which aminoglycosides cross the BLB (i.e., endothelial cells) into the stria vascularis and transverse the tight junction–coupled marginal cells into endolymph remain unknown, and they likely include one or more ion channels, transporters, exchangers, or transcytosis (Koo et al., 2015).
Figure 1.
Main cochlear trafficking routes for systemically administered aminoglycosides and cisplatin. Both blood-borne drugs enter the cochlea primarily from the capillaries in the stria vascularis. Aminoglycosides enter endolymph through as-yet-unidentified ion channels or transporters, although several candidates exist (e.g., transient receptor potential [TRP] vanilloid subtype 1 [TRPV1] and TRP vanilloid subtype 4 [TRPV4]). Cisplatin also enters endolymph potentially trafficking via organic cation transporter-2 (OCT2) and copper-like transport-1 (CTR1) transporters in the marginal cells. From the endolymph, these ototoxins enter hair cells across their apical membranes. Adapted with permission from Figure 1 in the study of Kros and Steyger (2019), copyright © Cold Spring Harbor Laboratory Press.
Less is known about how systemically administered aminoglycoside traffic to vestibular hair cells, although the transitional and dark cells more readily take up aminoglycosides than hair cells and their surrounding supporting cells (Liu et al., 2015). Vestibular endolymph is maintained by dark cells in the vestibule (Wangemann, 2006), akin to the stria vascularis and suggestive of similar mechanisms in trafficking aminoglycosides into endolymph. Intratympanic injection of aminoglycosides also led to more prominent uptake by type I hair cells as well as the crypt cells at the base of vestibular ampullae. It has not been determined in vivo if this was via trafficking into vestibular endolymph, prior to into hair cells and crypt cells (Lyford-Pike et al., 2007; Zhang et al., 2013). Strikingly, intratympanically administered aminoglycosides are taken up by auditory afferent neurons and vestibular efferent neurons from perilymph and transported into the brainstem. Whether this phenomenon has any functional consequences has yet to be established (Zhang et al., 2012).
Several lines of evidence suggest that cisplatin preferentially enters the cochlea via the stria vascularis (Breglio et al., 2017; Chu et al., 2016; van Ruijven et al., 2005) before entering hair cells, presumptively from endolymph (Brock et al., 2012). The putative cisplatin transporters copper-like transport-1 (CTR1) and organic cation transporter-2 (OCT2) are expressed by cells in the stria vascularis but not in the spiral ligament, suggestive of potential trafficking mechanisms for cisplatin transport across the BLB into the endolymph (Ciarimboli et al., 2010; More et al., 2010; Waissbluth & Daniel, 2013). Trafficking of cisplatin into vestibular cells remains largely unexplored, yet in preclinical models, cisplatin can cause loss of vestibular hair cells and sensory function in all five vestibular end organs (Sergi et al., 2003; Takimoto et al., 2016).
Entry of Aminoglycosides and Cisplatin Into Sensory Hair Cells
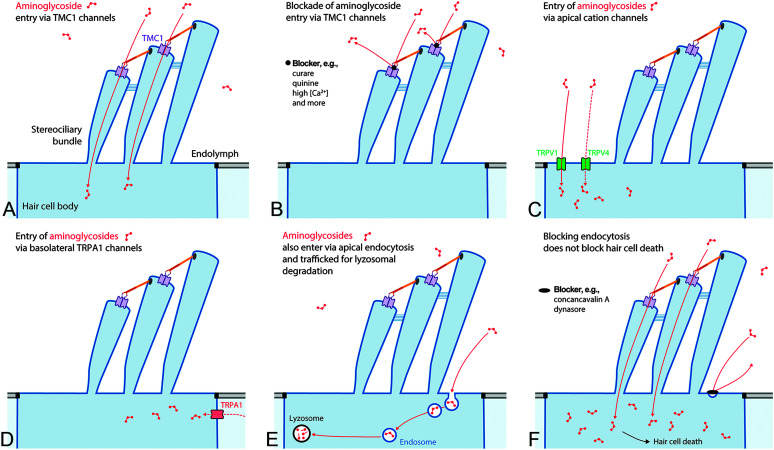
Permanent drug-induced cochleotoxicity typically requires these ototoxins to enter hair cells (Hiel et al., 1993). The mechanically gated transmembrane channel-like protein-1 (TMC1) nonselective cation channels at the tips of most (but not all) hair cell stereocilia (see Figure 2A) that transduce sound or motion into electrical signals are readily permeable to the polycationic aminoglycosides (Alharazneh et al., 2011; Marcotti et al., 2005; Pan et al., 2018). The +80-mV potential of endolymph electrochemically drives cations through the TMC channels into hair cells with their −40- to −70-mV resting potential adding to this electrochemical flux. Aminoglycosides transiently bind to the open-channel pore and are permeant blockers of TMC channels (Marcotti et al., 2005). Aminoglycoside permeation of these large, nonselective cation channels can be blocked by polyvalent cations, including magnesium and calcium (see Figure 2B), as well as organic compounds like curare and quinine (Alharazneh et al., 2011; Coffin et al., 2009). Once in hair cells, aminoglycosides appear unable to readily escape (Hiel et al., 1992; Leitner et al., 2011; Marcotti et al., 2005; Zhai et al., 2013).
Figure 2.
Entry of aminoglycosides into hair cells. Aminoglycosides preferentially enter hair cells across their apical membranes, predominantly via the transmembrane channel-like protein-1 (TMC1) channel complex, consisting of two TMC subunits (purple), each with a permeation pore (A). Entry of aminoglycosides can be blocked by curare, quinine, and high polyvalent cation levels among others (B). Other aminoglycoside-permeant cation channels on the apical membrane of hair cells include transient receptor potential (TRP) vanilloid subtype 1 (TRPV1) and TRP vanilloid subtype 4 (TRPV4) channels (C), and TRP ankyrin 1 (TRPA1) channels on the basolateral membranes of outer hair cells (D). (E) Nonspecific endocytosis also traffics aminoglycosides into hair cells and to lysosomes. (F) Blocking endocytosis does not prevent hair cell death when aminoglycosides can enter hair cells via the TMC1 channel.
Other aminoglycoside-permeant cation channels are also expressed by hair cells, particularly transient receptor potential (TRP) channels, individually activated by various physical or chemical stimuli (Nilius & Szallasi, 2014). TRP vanilloid subtype 1 (TRPV1) and TRP vanilloid subtype 4 (TRPV4) expressed on the apical membranes of hair cells are also aminoglycoside-permeant channels (see Figure 2C), and both are also expressed in the stria vascularis (see Figure 1; Jiang et al., 2019; Karasawa et al., 2008; Myrdal & Steyger, 2005; Zheng et al., 2003). Hair cell expression of TRPV1 is upregulated in the cochlea during inflammation that, in vitro, increases aminoglycoside entry into vestibular hair cells (Jiang et al., 2019; Qian et al., 2020). The TRP ankyrin 1 (TRPA1) channel is another aminoglycoside-permeant channel that is presumptively expressed on the basolateral membranes of outer hair cells (OHCs; see Figure 2D). Activation of TRPA1 by reactive oxygen species enhanced the cellular uptake of fluorescently tagged gentamicin (Stepanyan et al., 2011). This suggests that endogenous signals (e.g., pro-inflammatory cytokines and reactive oxygen species) during inflammation or noise can activate aminoglycoside-permeant TRP channels cochlear stress (e.g., noise and infection) to increase hair cell uptake of aminoglycosides (Goncalves et al., 2020; Kaewpitak et al., 2020).
Another entry route into hair cells is via non–receptor-mediated endocytosis at the apical and synaptic membranes of hair cells and trafficking toward lysosomes (see Figure 2E). However, blocking endocytosis (see Figure 2F) or impeding intracellular trafficking of aminoglycoside-laden endosomes does not prevent hair cell death (Alharazneh et al., 2011; Hailey et al., 2017; Hashino et al., 2000; Qian et al., 2020; Vu et al., 2013).
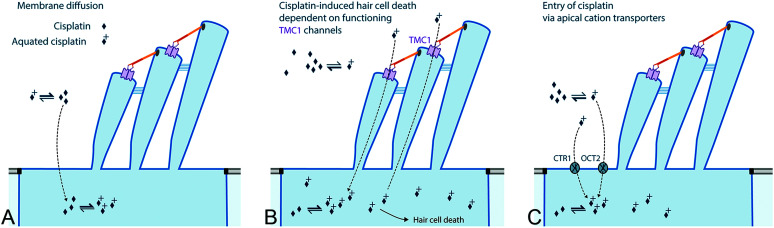
Defining the mechanisms by which cisplatin enters hair cells remains challenging, as cisplatin can enter cultured cells (see Figure 3A) across the cell membrane via passive diffusion (Hall et al., 2008). In zebrafish neuromasts, hair cells with functional TMC1 channels (see Figure 3B) are more susceptible to cisplatin-induced cytotoxicity (Thomas et al., 2013). Cellular uptake of cisplatin can be enhanced by the expression of putative cisplatin transporters, and two (i.e., CTR1 and OCT2) are expressed by cochlear hair cells (see Figure 3C; Ciarimboli et al., 2010; Hall et al., 2008; More et al., 2010; Waissbluth & Daniel, 2013). Systemic administration of cimetidine, an OCT blocker, genomic loss of both OCT1/2, or local administration of copper sulfate all ameliorate cisplatin-induced cochleotoxicity, demonstrating an as-yet-to-be specified (trafficking or hair cell uptake) role for these putative cisplatin transporters in the cochlea (Ciarimboli et al., 2010; More et al., 2010). However, zebrafish hair cells do not express the putative cisplatin transporters CTR1 or OCT2 (Thomas et al., 2013), leaving open the mechanically gated TMC transduction channels as a primary candidate for cisplatin entry into hair cells. Once in the cochlea and hair cells, cisplatin is retained for at least 12–18 months (Breglio et al., 2017).
Figure 3.
Entry of cisplatin into hair cells. Cisplatin has multiple potential entry routes, for which the relative importance has not been established. Neutral cisplatin can enter cells across the plasma membrane via diffusion and, once in the cytoplasm, is readily aquated into the more toxic form of cisplatin to form adducts with proteins and deoxyribonucleic acid. Uptake of (likely) aquated cisplatin is most dependent on a functional, mechanically gated transmembrane channel-like (TMC) channel complexes. Cellular uptake of the aquated form of cisplatin may also occur via copper-like transport-1 (CTR1) and organic cation transporter-2 (OCT2) transport proteins. TMC1 = transmembrane channel-like protein-1.
Patterns of Cochlear Hair Cell Death
Systemically administered aminoglycosides initially induce hair cell death in the outer cells of the basal, high-frequency region of the cochlea. Continued dosing leads to OHC death at lower frequencies in more apical regions of the cochlea, and inner hair cells begin to also die (Forge & Schacht, 2000). Systemically administered cisplatin also initially leads to OHC death in the basal, high-frequency region of the cochlea before affecting hair cells at more apical, lower frequency regions. Increasing cumulative dosing of either ototoxin consistently leads to an increasing risk of permanent hearing loss in humans (Brock et al., 2012; Garinis, Cross, et al., 2017). The apices of dying hair cells are severed from their cell bodies by the expanding phalangeal plates of surrounding supporting cells to form a “scar” and maintain the structural integrity of the reticular lamina, that is, the apical tight junctions between adjacent cells of the organ of Corti facing the scala media. This phenomenon maintains optimal responsiveness in surviving, functional hair cells by preventing the equilibration of endolymphatic and perilymphatic fluids. These “scar” formations are characterized by distinct reticular patterns of junctional and cytoskeletal proteins at the site of the missing hair cell (Leonova & Raphael, 1997; Steyger et al., 1997). The hair cell bodies are typically phagocytosed by adjacent supporting cells and resident macrophages (Monzack et al., 2015). In the vestibule, the preferential uptake of aminoglycosides by a majority of type I hair cells may underlie their pharmacological and physiological sensitivity to aminoglycosides compared with the extra-striolar type II hair cells (Lyford-Pike et al., 2007; Sultemeier & Hoffman, 2017).
Multiple Mechanisms of Ototoxicity
Once inside hair cells, aminoglycosides and cisplatin cause multiple mechanisms of hair cell death that are reviewed in more detail elsewhere (Jiang et al., 2017; Karasawa & Steyger, 2015; Kros & Steyger, 2019). Highlighted here are peripheral mechanisms of ototoxicity that illustrate multiple (yet incompletely) characterized mechanisms of cytotoxicity and pathophysiology induced by aminoglycosides and cisplatin.
Following entry via ion channels into the cytoplasm, aminoglycosides bind to hundreds of proteins (Karasawa et al., 2010, 2011), yet for most protein binding to aminoglycosides, the downstream consequences are not known. Aminoglycosides also bind to the phosphatidylinositol family of lipids, particularly phosphatidylinositol 4,5-bisphosphate (PIP2), that, in mammalian hair cells, blocks voltage-gated, outwardly rectifying potassium channels on the basolateral membranes of OHCs. This blockade prevents the rapid repolarization of hair cells crucial for hair cell survival (Leitner et al., 2011; Schacht et al., 1977). In zebrafish neuromast hair cells, aminoglycosides dysregulate the endoplasmic reticulum that leads to calcium flux into mitochondria and the generation of cytotoxic levels of reactive oxygen species in zebrafish neuromast hair cells (Esterberg et al., 2013, 2014, 2016). Intracellular aminoglycosides have been implicated in the degradation of presynaptic ribbons in hair cells (Liu et al., 2013; Oishi et al., 2015) that may underlie the reported loss of auditory function in cochlear regions despite the presence of many surviving hair cells (Koo et al., 2015; Nicol et al., 1992). Vestibular synaptopathy also occurs after aminoglycoside dosing (Sultemeier & Hoffman, 2017).
Mammalian sensory hair cells do not undergo cell division, avoiding the primary toxic effect of cisplatin binding to deoxyribonucleic acid (DNA) that initiates apoptotic signaling in proliferating cells (Eastman, 1999). Cisplatin affects many other intracellular pathways and binds to hundreds of proteins that could potentially dysregulate cells and lead to cell death (Karasawa et al., 2013; Karasawa & Steyger, 2015). For cochlear cells, uptake of cisplatin triggers the transcription factor, signal transducer, and activator of transcription 1 (STAT1) to activate the TRPV1 and NADPH oxidase 3 (NOX3) signaling pathways that generate toxic levels of reactive oxygen species that ultimately induce cell death (Mukherjea et al., 2008, 2011).
Ototoxicity can occur in nonsensory cochlear or vestibular cells that have crucial homeostatic functions essential for sensitive hair cell function, that is, cisplatin-induced cytotoxicity in intermediate and marginal cells of the stria vascularis responsible for generating the endolymphatic potential (Laurell et al., 2007; McAlpine & Johnstone, 1990). Aminoglycosides can structurally damage marginal and intermediate cells in the stria vascularis, yet this does not appear to have a major effect on strial generation of the endolymphatic potential (Forge et al., 1987; Ono & Tachibana, 1990; Xiong et al., 2011). More transiently, the medial olivocochlear reflex that protects hair cells from loud sounds is dysregulated by aminoglycosides binding to the efferent synapses at the base of OHCs (Avan et al., 1996; Blanchet et al., 2000).
Factors Enhancing the Risk of Hearing Loss
Exacerbation of Aminoglycoside-Induced Cochleotoxicity
Experimentally induced sepsis (via parenteral injection of lipopolysaccharides [LPS]) potentiates the degree of aminoglycoside-induced hearing loss in mice (Jiang et al., 2019; Koo et al., 2015), and likely vestibulotoxicity (Qian et al., 2020). Pilot data suggest that inflammation (and fever) also increases the risk of aminoglycoside-induced hearing loss (Cross et al., 2015; Henry et al., 1983). Aminoglycosides lyse bacteria and increase blood levels of bacterial immunogens (e.g., LPS) that enhance the severity of inflammatory responses, a phenomenon known as the Jarisch–Herxheimer reaction (Kaplanski et al., 1998; Shenep & Mogan, 1984). Thus, the very patients with infection-induced inflammation and treated with aminoglycosides are likely to be at higher risk of ototoxin-induced hearing loss (Koo et al., 2015), such as individuals with sepsis or cystic fibrosis.
Other clinical risk factors that enhance the risk of aminoglycoside-induced hearing loss include the following: concomitant renal insufficiency that decreases clearance of aminoglycosides from blood (Zager, 1992), increasing age and the decreased glomerular filtration rate associated with aging (Gatell et al., 1987; Manian et al., 1990; McClure, 1992; Triggs & Charles, 1999); depletion of endogenous antioxidants (or poor nutritional status) to counter the aminoglycoside-induced toxic generation of reactive oxygen species (Lautermann, McLaren, et al., 1995); fever (higher-than-normal body temperature); and transient ischemia/hypoxia (Lin et al., 2011). Aminoglycoside dosing in rats during inactive hours (i.e., daytime rest phase for many rodents) has also been associated with greater hearing loss than when administered during active, nighttime hours for these rodents (McKinney et al., 2015; Soulban et al., 1990). How the circadian rhythm modulates ototoxicity is still to be determined.
A variety of mitochondrial mutations in ribosomal ribonucleic acid (RNA; e.g., A1555G and C1494T) have a higher affinity for binding to aminoglycosides. This leads to mistranslation of messenger RNA and inaccurate protein synthesis resulting in greater susceptibility to aminoglycoside-induced hearing loss (Hobbie et al., 2008; Matt et al., 2012; Qian & Guan, 2009). While several genomic polymorphisms are thought to increase the risk of cisplatin-induced hearing loss (see below), none have yet been described to increase (or decrease) susceptibility to aminoglycoside-induced hearing loss, although several studies are underway.
Concomitant dosing with specific therapeutics synergistically enhances aminoglycoside ototoxicity and co-administration should be avoided whenever possible. These cotherapeutics include the loop diuretics in hypertensive individuals (Bates et al., 2002; Mathog & Klein, 1969; Rybak, 1993), and vancomycin, a glycopeptide antibiotic commonly prescribed in the neonatal intensive care unit (Brummett et al., 1990; Rubin et al., 2002). Loop diuretics co-administered with neuromuscular blocking agents (e.g., pancuronium bromide or vecuronium bromide) for patients requiring respiratory assistance (via intubation and ventilation) or surgical procedures that also induce cochleotoxicity (Cheung et al., 1999; Masumoto et al., 2007).
Since the late 1960s and 1970s, loud sound exposures have been known to readily enhance aminoglycoside-induced hearing loss (Brown et al., 1978; Darrouzet, 1963; Gannon & Tso, 1969; Gannon et al., 1979; Vernon et al., 1978). Subototoxic dosing of aminoglycosides becomes ototoxic in the presence of noise that is greater than either insult alone (Collins, 1988). Loud sound exposures up to 2 months prior to aminoglycoside dosing appears to enhance the ototoxicity observed compared with aminoglycoside exposure alone. However, if noise exposure occurs 4 weeks (or more) after aminoglycoside dosing, little or no ototoxic synergy is present. Noise exposure within 3 weeks after aminoglycoside will increase ototoxicity, although with decreasing severity over time compared with simultaneous noise plus aminoglycoside exposure (Ryan & Bone, 1978, 1982). These data will be relevant to those operating in noisy conditions where there is an increased risk of injury and/or infection subsequent to exposure that may require pharmacotherapy with aminoglycosides (e.g., surgery and burns).
Exacerbation of Cisplatin-Induced Cochleotoxicity
Experimentally induced sepsis (via LPS) also potentiates the degree of cisplatin-induced hearing loss in mice (Oh et al., 2011). LPS-induced inflammation in subjects treated with cisplatin occurs when the gastrointestinal tract is irradiated, killing commensal bacteria that then increase blood levels of LPS (Paulos et al., 2007). LPS typically binds to Toll-like receptor 4 (TLR4) that also recognizes endogenous damage-associated molecular patterns including DNA, heat shock proteins, and immunogens from injured cells to induce a sterile inflammatory response (Bhattacharyya et al., 2017; Oblak & Jerala, 2011) that can enhance the ototoxicity of cisplatin or other ototoxic chemotherapeutics. Other risk factors that enhance the ototoxicity of cisplatin include those that potentiate aminoglycoside-induced ototoxicity, including renal insufficiency and dehydration (Gandara et al., 1991), malnutrition that depletes antioxidant levels (Lautermann et al., 1997; Lautermann, Song, et al., 1995), and the use of blood–brain barrier disruption protocols that also disrupt the BLB (Neuwelt et al., 1996).
Specific genomic mutations may predispose individuals to cisplatin-induced hearing loss, including gene variants for DNA adduct repair enzymes like ERCC2 and XPC (Caronia et al., 2009; Turan et al., 2019); antioxidant enzymes; and drug efflux or membrane pumps ACYP2, ABCC3, COMT, and TPMT (Ross et al., 2009; Xu et al., 2015). However, the predictive value in identifying these genomic variants in predisposing individuals for cisplatin-induced ototoxicity is currently poor (Langer et al., 2020; Tserga et al., 2019). Enhanced cisplatin-induced hearing loss also occurs when co-administered with ototoxic therapeutics, including aminoglycosides, loop diuretics, and cranial radiation (Clemens et al., 2016; McAlpine & Johnstone, 1990; Miller et al., 2009; Paulino et al., 2010). Although conflicting data can also be found in the literature, there are many differences in experimental design and treatment that could mitigate against these outcomes. Prior or low dosing with cisplatin (preconditioning) can synergize with subsequent cisplatin dosing to enhance ototoxicity (Harrison et al., 2015). Cisplatin dosing in rats during inactive hours (i.e., daytime) hours is also associated with greater hearing loss (Bielefeld et al., 2018). Noise exposure also increases cisplatin-induced hearing loss (Bokemeyer et al., 1998; Gratton & Kamen, 1990; Gratton et al., 1990; Laurell, 1992).
An additional phenomenon that exacerbates preexisting hearing loss has been identified in patients treated with cisplatin. Those with existing hearing loss at higher frequencies prior to treatment are more likely to experience hearing loss at lower frequencies that extend into the conventional hearing range and important for speech discrimination (Dille et al., 2012), affecting their ability to hear posttreatment compared with pretreatment baselines. The development of predictive ototoxicity modeling can enable pretreatment counseling to prepare for rehabilitating iatrogenic hearing loss (Deutsch et al., 2021; Dille et al., 2012).
Gaps in the Current Ototoxicity Knowledge Base
While the identity of several predisposing factors like aging, renal insufficiency, inflammation, genetic variants, or co-therapeutics are known to enhance the risk of ototoxicity, their interaction with each ototoxin is rarely well characterized, especially in human populations. The prevalence and incidence of these predisposing factors remain to be determined clinically, and this is typically complicated by the variability of each subject's medical history in a clinical population. These individual interactions with each ototoxin can also affect the impact of other predisposing factors and pose an overwhelming challenge to better predict the ototoxic impact of prescribed aminoglycosides and cisplatin, although innovative studies now provide a framework to incorporate additional data points particularly for cisplatin-induced hearing loss (Deutsch et al., 2021; Dille et al., 2012; Hong et al., 2020; Konrad-Martin et al., 2010, 2014; Zhou et al., 2014). To enable better prediction of the risk of ototoxicity, much progress must first be made in better characterizing the key data points for individual medical settings that influence the risk of ototoxicity. Examples of key questions that need answers include the following.
Which molecular mechanisms enable ototoxins to cross the BLB and enter/traverse cochlear cells before entering hair cells?
What are the multiple molecular mechanisms of cytotoxicity for each ototoxin?
Which factors potentiate ototoxin trafficking, cellular entry, and cytotoxicity during inflammation?
What is the threshold for key inflammatory biomarkers to be associated with enhanced drug trafficking across the BLB and inflammation-enhanced drug-induced cochleotoxicity or vestibulotoxicity?
Are these biomarkers the same for inflammatory responses arising from different etiologies (i.e., bacterial, viral, drug-induced, and tumor- or radiation-derived inflammation)?
Are inflammatory responses from different etiologies (e.g., Gram-positive or Gram-negative bacterial species, and viral) equally potent in driving inflammation-enhanced ototoxicity?
These questions can also be rephrased by replacing inflammation with any exacerbating factor. The identification of prognostic, genomic, or diagnostic biomarkers of cochlear damage is an area of intense focus (Landegger et al., 2019; Lassaletta et al., 2019; Schmitt et al., 2018). It will be important for systemic biomarkers (i.e., molecular assays) or noninvasive functional (auditory or vestibular) assessments to efficaciously predict the risk of ototoxicity, especially with the growing list of predisposing factors interacting with the ototoxins. The identification of these biomarkers will require better characterization of these predisposing factors in preclinical models that then must be validated in human studies to be used clinically. Thus, much research lies ahead to provide new insights into iatrogenic ototoxicity. This research will ultimately lead to the identification of druggable targets to preserve or restore hearing during/after iatrogenic ototoxicity. Furthermore, otoprotectants must also efficaciously preserve or restore hearing and/or vestibular function in medical settings that include inflammation, exposure to other ototoxic agents including renal insufficiency, aging, and/or noise.
Finally, additional insights into cochleotoxicity and vestibulotoxicity will come from widespread ototoxicity monitoring, improved objective, data-driven measures of hearing loss (rather than blunt categories of relative hearing deficits), and widespread adoption of extended higher frequency audiometry, potentially via use of tablet-based audiometers.
Brief Resume for Peter Steyger
At 3 years old, Peter Steyger attended The University of Manchester, United Kingdom, to learn to listen and speak following drug-induced hearing loss acquired as a consequence of aminoglycoside treatment for bacterial meningitis. He subsequently obtained a bachelor's degree in Zoology at The University of Manchester in 1984, followed by a PhD degree in Communications and Neuroscience at Keele University, United Kingdom. After postdoctoral training in vestibular neurosciences in San Antonio, Texas, and the Neurological Sciences Institute in Portland, Oregon, Peter joined the Oregon Hearing Research Center at Oregon Health & Science University in 1997 and rose through the professorial ranks. In June 2019, he moved to Creighton University School of Medicine to become the inaugural director of the Translational Hearing Center in the Department of Biomedical Sciences chaired by Dr. Jian Zuo. He has published more than 70 peer-reviewed original research articles and topic reviews. He has two current R01 awards that focus on (a) identifying mechanisms of aminoglycoside-induced ototoxicity and (b) translating that gained knowledge to clinical populations that may lead to refined clinical care guidelines to better ameliorate or prevent the degree of lifelong drug-induced hearing loss (cochleotoxicity).
Acknowledgments
This study was supported by the National Institute on Deafness and Other Communication Disorders through Research Awards R01 DC004555 and R01 DC016880 to author Peter S. Steyger. An early version of this review article was presented at the 9th Annual Biennial National Center for Rehabilitative Auditory Research Conference (September 2019) in Portland, Oregon. The author would like to thank Angela Garinis for specific discussions on this review article, and also current and former staff, trainees, and volunteers in the laboratory for their insights and stimulating discussions.
Funding Statement
This study was supported by the National Institute on Deafness and Other Communication Disorders through Research Awards R01 DC004555 and R01 DC016880 to author Peter S. Steyger.
References
- Alharazneh, A. , Luk, L. , Huth, M. , Monfared, A. , Steyger, P. S. , Cheng, A. G. , & Ricci, A. J. (2011). Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLOS ONE, 6(7), e22347. https://doi.org/10.1371/journal.pone.0022347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avan, P. , Erre, J. P. , da Costa, D. L. , Aran, J. M. , & Popelar, J. (1996). The efferent-mediated suppression of otoacoustic emissions in awake guinea pigs and its reversible blockage by gentamicin. Experimental Brain Research, 109(1), 9–16. https://doi.org/10.1007/BF00228621 [DOI] [PubMed] [Google Scholar]
- Bates, D. E. , Beaumont, S. J. , & Baylis, B. W. (2002). Ototoxicity induced by gentamicin and furosemide [Case reports]. Annals of Pharmacotherapy, 36(3), 446–451. https://doi.org/10.1345/aph.1A216 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, S. , Midwood, K. S. , Yin, H. , & Varga, J. (2017). Toll-like receptor-4 signaling drives persistent fibroblast activation and prevents fibrosis resolution in Scleroderma. Advances in Wound Care (New Rochelle), 6(10), 356–369. https://doi.org/10.1089/wound.2017.0732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld, E. C. , Markle, A. , DeBacker, J. R. , & Harrison, R. T. (2018). Chronotolerance for cisplatin ototoxicity in the rat. Hearing Research, 370, 16–21. https://doi.org/10.1016/j.heares.2018.09.004 [DOI] [PubMed] [Google Scholar]
- Blanchet, C. , Erostegui, C. , Sugasawa, M. , & Dulon, D. (2000). Gentamicin blocks ACh-evoked K+ current in guinea-pig outer hair cells by impairing Ca2+ entry at the cholinergic receptor [Research Support, Non-U.S. Government]. Journal of Physiology, 525(3), 641–654. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00641.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokemeyer, C. , Berger, C. C. , Hartmann, J. T. , Kollmannsberger, C. , Schmoll, H. J. , Kuczyk, M. A. , & Kanz, L. (1998). Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Brain Journal of Cancer, 77(8), 1355–1362. https://doi.org/10.1038/bjc.1998.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breglio, A. M. , Rusheen, A. E. , Shide, E. D. , Fernandez, K. A. , Spielbauer, K. K. , McLachlin, K. M. , Hall, M. D. , Amable, L. , & Cunningham, L. L. (2017). Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nature Communication, 8(1), 1654. https://doi.org/10.1038/s41467-017-01837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, P. R. , Knight, K. R. , Freyer, D. R. , Campbell, K. C. , Steyger, P. S. , Blakley, B. W. , Rassekh, S. R. , Chang, K. W. , Fligor, B. J. , Rajput, K. , Sullivan, M. , & Neuwelt, E. A. (2012). Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. Journal Clinical Oncology, 30(19), 2408–2417. https://doi.org/10.1200/JCO.2011.39.1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. J. , Brummett, R. E. , Meikle, M. B. , & Vernon, J. (1978). Combined effects of noise and neomycin: Cochlear changes in the guinea pig. Acta Oto-laryngologica, 86(5–6), 394–400. https://doi.org/10.3109/00016487809107518 [DOI] [PubMed] [Google Scholar]
- Brummett, R. E. , Fox, K. E. , Jacobs, F. , Kempton, J. B. , Stokes, Z. , & Richmond, A. B. (1990). Augmented gentamicin ototoxicity induced by vancomycin in guinea pigs. Archives of Otolaryngology—Head & Neck Surgery, 116(1), 61–64. https://doi.org/10.1001/archotol.1990.01870010065019 [DOI] [PubMed] [Google Scholar]
- Callejo, A. , Durochat, A. , Bressieux, S. , Saleur, A. , Chabbert, C. , Domenech Juan, I. , Llorens, J. , & Gaboyard-Niay, S. (2017). Dose-dependent cochlear and vestibular toxicity of trans-tympanic cisplatin in the rat. Neurotoxicology, 60, 1–9. https://doi.org/10.1016/j.neuro.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Callejo, A. , Sedo-Cabezon, L. , Juan, I. D. , & Llorens, J. (2015). Cisplatin-induced ototoxicity: Effects, mechanisms and protection strategies. Toxics, 3(3), 268–293. https://doi.org/10.3390/toxics3030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronia, D. , Patino-Garcia, A. , Milne, R. L. , Zalacain-Diez, M. , Pita, G. , Alonso, M. R. , Moreno, L. T. , Sierrasesumaga-Ariznabarreta, L. , Benitez, J. , & Gonzalez-Neira, A. (2009). Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients [Research Support, Non-U.S. Government]. Pharmacogenomics Journal, 9(5), 347–353. https://doi.org/10.1038/tpj.2009.19 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2004). Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment–United States, 2003. Morbidity and Mortality Weekly Report, 53(3), 57–59. https://www.ncbi.nlm.nih.gov/pubmed/14749614 [PubMed] [Google Scholar]
- Cheung, P. Y. , Tyebkhan, J. M. , Peliowski, A. , Ainsworth, W. , & Robertson, C. M. (1999). Prolonged use of pancuronium bromide and sensorineural hearing loss in childhood survivors of congenital diaphragmatic hernia. Journal of Pediatrics, 135(2 Pt. 1), 233–239. https://doi.org/10.1016/S0022-3476(99)70027-2 [DOI] [PubMed] [Google Scholar]
- Chu, Y. H. , Sibrian-Vazquez, M. , Escobedo, J. O. , Phillips, A. R. , Dickey, D. T. , Wang, Q. , Ralle, M. , Steyger, P. S. , & Strongin, R. M. (2016). Systemic delivery and biodistribution of cisplatin in vivo. Molecular Pharmaceutics, 13(8), 2677–2682. https://doi.org/10.1021/acs.molpharmaceut.6b00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli, G. , Deuster, D. , Knief, A. , Sperling, M. , Holtkamp, M. , Edemir, B. , Pavenstadt, H. , Lanvers-Kaminsky, C. , am Zehnhoff-Dinnesen, A. , Schinkel, A. H. , Koepsell, H. , Jurgens, H. , & Schlatter, E. (2010). Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. American Journal of Pathology, 176(3), 1169–1180. https://doi.org/10.2353/ajpath.2010.090610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, E. , de Vries, A. C. , Pluijm, S. F. , Am Zehnhoff-Dinnesen, A. , Tissing, W. J. , Loonen, J. J. , van Dulmen-den Broeder, E. , Bresters, D. , Versluys, B. , Kremer, L. C. , van der Pal, H. J. , van Grotel, M. , van den Heuvel-Eibrink, M. M. , & DCOG-LATER (2016). Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. European Journal of Cancer, 69, 77–85. https://doi.org/10.1016/j.ejca.2016.09.023 [DOI] [PubMed] [Google Scholar]
- Coffin, A. B. , Reinhart, K. E. , Owens, K. N. , Raible, D. W. , & Rubel, E. W. (2009). Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line [Research Support, N.I.H., Extramural Research Support, Non-U.S. Government]. Hearing Research, 253(1–2), 42–51. https://doi.org/10.1016/j.heares.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, P. W. (1988). Synergistic interactions of gentamicin and pure tones causing cochlear hair cell loss in pigmented guinea pigs. Hearing Research, 36(2–3), 249–259. https://doi.org/10.1016/0378-5955(88)90066-4 [DOI] [PubMed] [Google Scholar]
- Counter, S. A. , & Buchanan, L. H. (2002). Neuro-ototoxicity in Andean adults with chronic lead and noise exposure. Journal of Occupational Environmental Medicine, 44(1), 30–38. https://doi.org/10.1097/00043764-200201000-00006 [DOI] [PubMed] [Google Scholar]
- Cross, C. P. , Liao, S. , Urdang, Z. D. , Srikanth, P. , Garinis, A. C. , & Steyger, P. S. (2015). Effect of sepsis and systemic inflammatory response syndrome on neonatal hearing screening outcomes following gentamicin exposure. International Journal of Pediatric Otorhinolaryngology, 79(11), 1915–1919. https://doi.org/10.1016/j.ijporl.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumling, M. A. , King, K. A. , & Duncan, R. K. (2017). Cyclodextrins and iatrogenic hearing loss: New drugs with significant risk. Frontiers in Cellular Neuroscience, 11, 355. https://doi.org/10.3389/fncel.2017.00355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, C. F. , & Steyger, P. S. (2008). A systemic gentamicin pathway across the stria vascularis. Hearing Research, 235(1–2), 114–124. https://doi.org/10.1016/j.heares.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrouzet, J. (1963). Attempts at protection of the organ of Corti against the ototoxicity of antibiotics. Experimental study [in French]. Revue de Laryngologie-Otologie-Rhinologie (Bord), 84, 459–478. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14025153 [PubMed] [Google Scholar]
- Davis, R. R. , Murphy, W. J. , Snawder, J. E. , Striley, C. A. , Henderson, D. , Khan, A. , & Krieg, E. F. (2002). Susceptibility to the ototoxic properties of toluene is species specific. Hearing Research, 166(1–2), 24–32. https://doi.org/10.1016/s0378-5955(02)00280-0 [DOI] [PubMed] [Google Scholar]
- Dedhia, K. , Kitsko, D. , Sabo, D. , & Chi, D. H. (2013). Children with sensorineural hearing loss after passing the newborn hearing screen. JAMA Otolaryngology—Head & Neck Surgery, 139(2), 119–123. https://doi.org/10.1001/jamaoto.2013.1229 [DOI] [PubMed] [Google Scholar]
- Deutsch, B. C. , Collopy, C. , Kallogjeri, D. , & Piccirillo, J. F. (2021). Validation of hearing loss prediction tool for cisplatin chemotherapy and radiation in head and neck cancer treatment. JAMA Otolaryngology—Head & Neck Surgery, 147(2), 182–189. https://doi.org/10.1001/jamaoto.2020.4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dille, M. F. , Wilmington, D. , McMillan, G. P. , Helt, W. , Fausti, S. A. , & Konrad-Martin, D. (2012). Development and validation of a cisplatin dose-ototoxicity model. Journal of the American Academy of Audiology, 23(7), 510–521. https://doi.org/10.3766/jaaa.23.7.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman, A. (1999). The mechanism of action of cisplatin: From adducts to apoptosis. In Lippert B. (Ed.), Cisplatin: Chemistry and biochemistry of a leading anticancer drug (pp. 111–134). Verlag Helvetica Chimica Acta; Wiley-VCH. https://doi.org/10.1002/9783906390420.ch4 [Google Scholar]
- Elson, E. C. , Meier, E. , & Oermann, C. M. (2020). The implementation of an aminoglycoside induced ototoxicity algorithm for people with cystic fibrosis. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society, 20(2), 284–287. https://doi.org/10.1016/j.jcf.2020.08.002 [DOI] [PubMed] [Google Scholar]
- Esterberg, R. , Hailey, D. W. , Coffin, A. B. , Raible, D. W. , & Rubel, E. W. (2013). Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. Journal of Neuroscience, 33(17), 7513–7525. https://doi.org/10.1523/JNEUROSCI.4559-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg, R. , Hailey, D. W. , Rubel, E. W. , & Raible, D. W. (2014). ER–mitochondrial calcium flow underlies vulnerability of mechanosensory hair cells to damage. Journal of Neuroscience, 34(29), 9703–9719. https://doi.org/10.1523/JNEUROSCI.0281-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg, R. , Linbo, T. , Pickett, S. B. , Wu, P. , Ou, H. C. , Rubel, E. W. , & Raible, D. W. (2016). Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. Journal of Clinical Investigation, 126(9), 3556–3566. https://doi.org/10.1172/JCI84939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti, S. A. , Frey, R. H. , Henry, J. A. , Olson, D. J. , & Schaffer, H. I. (1992). Early detection of ototoxicity using high-frequency, tone-burst-evoked auditory brainstem responses. Journal of the American Academy of Audiology, 3(6), 397–404. https://pubmed.ncbi.nlm.nih.gov/1486202/ [PubMed] [Google Scholar]
- Fausti, S. A. , Henry, J. A. , Schaffer, H. I. , Olson, D. J. , Frey, R. H. , & McDonald, W. J. (1992). High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. Journal of Infectious Diseases, 165(6), 1026–1032. https://doi.org/10.1093/infdis/165.6.1026 [DOI] [PubMed] [Google Scholar]
- Fechter, L. D. , Gearhart, C. , Fulton, S. , Campbell, J. , Fisher, J. , Na, K. , Cocker, D. , Nelson-Miller, A. , Moon, P. , & Pouyatos, B. (2007). JP-8 jet fuel can promote auditory impairment resulting from subsequent noise exposure in rats. Toxicological Sciences, 98(2), 510–525. https://doi.org/10.1093/toxsci/kfm101 [DOI] [PubMed] [Google Scholar]
- Forge, A. , & Schacht, J. (2000). Aminoglycoside antibiotics. Audiology and Neurotology, 5(1), 3–22. https://doi.org/10.1159/000013861 [DOI] [PubMed] [Google Scholar]
- Forge, A. , Wright, A. , & Davies, S. J. (1987). Analysis of structural changes in the stria vascularis following chronic gentamicin treatment. Hearing Research, 31(3), 253–265. https://doi.org/10.1016/0378-5955(87)90195-X [DOI] [PubMed] [Google Scholar]
- Gandara, D. R. , Perez, E. A. , Weibe, V. , & De Gregorio, M. W. (1991). Cisplatin chemoprotection and rescue: Pharmacologic modulation of toxicity. Seminars in Oncology, 18(1 Suppl. 3), 49–55. https://pubmed.ncbi.nlm.nih.gov/1848372/ [PubMed] [Google Scholar]
- Gannon, R. P. , & Tso, S. S. (1969). The occult effect of Kanamycin on the cochlea. Excerpta Medica, 189, 98. [Google Scholar]
- Gannon, R. P. , Tso, S. S. , & Chung, D. Y. (1979). Interaction of kanamycin and noise exposure. Journal of Laryngology and Otology, 93(4), 341–347. https://doi.org/10.1017/S0022215100087119 [DOI] [PubMed] [Google Scholar]
- Garinis, A. C. , Cross, C. P. , Srikanth, P. , Carroll, K. , Feeney, M. P. , Keefe, D. H. , Hunter, L. L. , Putterman, D. B. , Cohen, D. M. , Gold, J. A. , & Steyger, P. S. (2017). The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society, 16(3), 401–409. https://doi.org/10.1016/j.jcf.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis, A. C. , Liao, S. , Cross, C. P. , Galati, J. , Middaugh, J. L. , Mace, J. C. , Wood, A. M. , McEvoy, L. , Moneta, L. , Lubianski, T. , Coopersmith, N. , Vigo, N. , Hart, C. , Riddle, A. , Ettinger, O. , Nold, C. , Durham, H. , MacArthur, C. , McEvoy, C. , & Steyger, P. S. (2017). Effect of gentamicin and levels of ambient sound on hearing screening outcomes in the neonatal intensive care unit: A pilot study. International Journal of Pediatric Otorhinolaryngology, 97, 42–50. https://doi.org/10.1016/j.ijporl.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatell, J. M. , Ferran, F. , Araujo, V. , Bonet, M. , Soriano, E. , Traserra, J. , & SanMiguel, J. G. (1987). Univariate and multivariate analyses of risk factors predisposing to auditory toxicity in patients receiving aminoglycosides. Antimicrobial Agents and Chemotherapy, 31(9), 1383–1387. https://doi.org/10.1128/AAC.31.9.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, E. C. D. , Assis, P. M. , Junqueira, L. A. , Cola, M. , Santos, A. R. S. , Raposo, N. R. B. , & Dutra, R. C. (2020). Citral inhibits the inflammatory response and hyperalgesia in mice: The role of TLR4, TLR2/Dectin-1, and CB2 cannabinoid receptor/ATP-sensitive K(+) channel pathways. Journal of Natural Products, 83(4), 1190–1200. https://doi.org/10.1021/acs.jnatprod.9b01134 [DOI] [PubMed] [Google Scholar]
- Gopal, K. V. , Wu, C. , Shrestha, B. , Campbell, K. C. , Moore, E. J. , & Gross, G. W. (2012). d-Methionine protects against cisplatin-induced neurotoxicity in cortical networks. Neurotoxicology Teratology, 34(5), 495–504. https://doi.org/10.1016/j.ntt.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Gratton, M. A. , & Kamen, B. A. (1990). Potentiation of cisplatin ototoxicity by noise. Journal of Clinical Oncology, 8(12), 2091–2092. https://doi.org/10.1200/JCO.1990.8.12.2091 [DOI] [PubMed] [Google Scholar]
- Gratton, M. A. , Salvi, R. J. , Kamen, B. A. , & Saunders, S. S. (1990). Interaction of cisplatin and noise on the peripheral auditory system. Hearing Research, 50(1–2), 211–223. https://doi.org/10.1016/0378-5955(90)90046-R [DOI] [PubMed] [Google Scholar]
- Gurney, J. G. , Tersak, J. M. , Ness, K. K. , Landier, W. , Matthay, K. K. , Schmidt, M. L. , & Children's Oncology, G. (2007). Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children's Oncology Group. Pediatrics, 120(5), e1229–e1236. https://doi.org/10.1542/peds.2007-0178 [DOI] [PubMed] [Google Scholar]
- Hailey, D. W. , Esterberg, R. , Linbo, T. H. , Rubel, E. W. , & Raible, D. W. (2017). Fluorescent aminoglycosides reveal intracellular trafficking routes in mechanosensory hair cells. Journal of Clinical Investigation, 127(2), 472–486. https://doi.org/10.1172/JCI85052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, M. D. , Okabe, M. , Shen, D. W. , Liang, X. J. , & Gottesman, M. M. (2008). The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annual Review of Pharmacology and Toxicology, 48, 495–535. https://doi.org/10.1146/annurev.pharmtox.48.080907.180426 [DOI] [PubMed] [Google Scholar]
- Harrison, R. T. , DeBacker, J. R. , & Bielefeld, E. C. (2015). A low-dose regimen of cisplatin before high-dose cisplatin potentiates ototoxicity. The Laryngoscope, 125(2), E78. https://doi.org/10.1002/lary.24948 [DOI] [PubMed] [Google Scholar]
- Hashino, E. , Shero, M. , & Salvi, R. J. (2000). Lysosomal augmentation during aminoglycoside uptake in cochlear hair cells. Brain Research, 887(1), 90–97. https://doi.org/10.1016/s0006-8993(00)02971-1 [DOI] [PubMed] [Google Scholar]
- Hellberg, V. , Wallin, I. , Ehrsson, H. , & Laurell, G. (2013). Cochlear pharmacokinetics of cisplatin: An in vivo study in the guinea pig. The Laryngoscope, 123(12), 3172–3177. https://doi.org/10.1002/lary.24235 [DOI] [PubMed] [Google Scholar]
- Henry, K. R. , Guess, M. B. , & Chole, R. A. (1983). Hyperthermia increases aminoglycoside ototoxicity. Acta Oto-laryngologica, 95(3–4), 323–327. https://doi.org/10.3109/00016488309130949 [DOI] [PubMed] [Google Scholar]
- Hiel, H. , Bennani, H. , Erre, J. P. , Aurousseau, C. , & Aran, J. M. (1992). Kinetics of gentamicin in cochlear hair cells after chronic treatment. Acta Oto-laryngologica, 112(2), 272–277. https://doi.org/10.1080/00016489.1992.11665417 [DOI] [PubMed] [Google Scholar]
- Hiel, H. , Erre, J. P. , Aurousseau, C. , Bouali, R. , Dulon, D. , & Aran, J. M. (1993). Gentamicin uptake by cochlear hair cells precedes hearing impairment during chronic treatment. Audiology, 32(1), 78–87. https://doi.org/10.3109/00206099309072930 [DOI] [PubMed] [Google Scholar]
- Hinduja, S. , Kraus, K. S. , Manohar, S. , & Salvi, R. J. (2015). D-methionine protects against cisplatin-induced neurotoxicity in the hippocampus of the adult rat. Neurotoxicity Research, 27(3), 199–204. https://doi.org/10.1007/s12640-014-9503-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie, S. N. , Bruell, C. M. , Akshay, S. , Kalapala, S. K. , Shcherbakov, D. , & Bottger, E. C. (2008). Mitochondrial deafness alleles confer misreading of the genetic code. Proceeding of the National Academy of Sciences of the United States of America, 105(9), 3244–3249. https://doi.org/10.1073/pnas.0707265105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, H. , Dowdy, D. W. , Dooley, K. E. , Francis, H. W. , Budhathoki, C. , Han, H. R. , & Farley, J. E. (2020). Aminoglycoside-induced hearing loss among patients being treated for drug-resistant tuberculosis in South Africa: A prediction model. Clinical Infectious Diseases, 70(5), 917–924. https://doi.org/10.1093/cid/ciz289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, H. D. (1999). Insights into ototoxicity. Analogies to nephrotoxicity [Review]. Annals of the New York Academy of Sciences, 884, 15–18. https://doi.org/10.1111/j.1749-6632.1999.tb00278.x [DOI] [PubMed] [Google Scholar]
- Jamesdaniel, S. , Rosati, R. , Westrick, J. , & Ruden, D. M. (2018). Chronic lead exposure induces cochlear oxidative stress and potentiates noise-induced hearing loss. Toxicology Letters, 292, 175–180. https://doi.org/10.1016/j.toxlet.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M. , Karasawa, T. , & Steyger, P. S. (2017). Aminoglycoside-induced cochleotoxicity: A review. Frontiers in Cellular Neuroscience, 11, 308. https://doi.org/10.3389/fncel.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M. , Li, H. , Johnson, A. , Karasawa, T. , Zhang, Y. , Meier, W. B. , Taghizadeh, F. , Kachelmeier, A. , & Steyger, P. S. (2019). Inflammation up-regulates cochlear expression of TRPV1 to potentiate drug-induced hearing loss. Science Advances, 5(7), eaaw1836. https://doi.org/10.1126/sciadv.aaw1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewpitak, A. , Bauer, C. S. , Seward, E. P. , Boissonade, F. M. , & Douglas, C. W. I. (2020). Porphyromonas gingivalis lipopolysaccharide rapidly activates trigeminal sensory neurons and may contribute to pulpal pain. International Endodontic Journal. https://doi.org/10.1111/iej.13282 [DOI] [PubMed] [Google Scholar]
- Kaplanski, G. , Granel, B. , Vaz, T. , & Durand, J. M. (1998). Jarisch–Herxheimer reaction complicating the treatment of chronic Q fever endocarditis: Elevated TNF-alpha and IL-6 serum levels. Journal of Infection, 37(1), 83–84. https://doi.org/10.1016/S0163-4453(98)91120-3 [DOI] [PubMed] [Google Scholar]
- Karasawa, T. , Sibrian-Vazquez, M. , Strongin, R. M. , & Steyger, P. S. (2013). Identification of cisplatin-binding proteins using agarose conjugates of platinum compounds. PLOS ONE, 8(6), e66220. https://doi.org/10.1371/journal.pone.0066220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa, T. , & Steyger, P. S. (2015). An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicology Letters, 237(3), 219–227. https://doi.org/10.1016/j.toxlet.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa, T. , Wang, Q. , David, L. L. , & Steyger, P. S. (2010). CLIMP-63 is a gentamicin-binding protein that is involved in drug-induced cytotoxicity. Cell Death & Disease, 1, e102. https://doi.org/10.1038/cddis.2010.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa, T. , Wang, Q. , David, L. L. , & Steyger, P. S. (2011). Calreticulin binds to gentamicin and reduces drug-induced ototoxicity. Toxicological Science, 124(2), 378–387. https://doi.org/10.1093/toxsci/kfr196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa, T. , Wang, Q. , Fu, Y. , Cohen, D. M. , & Steyger, P. S. (2008). TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. Journal of Cell Science, 121(Pt. 17), 2871–2879. https://doi.org/10.1242/jcs.023705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, K. R. , Kraemer, D. F. , & Neuwelt, E. A. (2005). Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology, 23(34), 8588–8596. https://doi.org/10.1200/jco.2004.00.5355 [DOI] [PubMed] [Google Scholar]
- Knight, K. R. , Kraemer, D. F. , Winter, C. , & Neuwelt, E. A. (2007). Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. Journal of Clinical Oncology, 25(10), 1190–1195. https://doi.org/10.1200/jco.2006.07.9723 [DOI] [PubMed] [Google Scholar]
- Konrad-Martin, D. , James, K. E. , Gordon, J. S. , Reavis, K. M. , Phillips, D. S. , Bratt, G. W. , & Fausti, S. A. (2010). Evaluation of audiometric threshold shift criteria for ototoxicity monitoring. Journal of the American Academy of Audiology, 21(5), 301–314; quiz 357. https://doi.org/10.3766/jaaa.21.5.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin, D. , Reavis, K. M. , McMillan, G. , Helt, W. J. , & Dille, M. (2014). Proposed comprehensive ototoxicity monitoring program for VA healthcare (COMP-VA). Journal of Rehabilitation Research & Development, 51(1), 81–100. https://doi.org/10.1682/JRRD.2013.04.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, J. W. , Quintanilla-Dieck, L. , Jiang, M. , Liu, J. , Urdang, Z. D. , Allensworth, J. J. , Cross, C. P. , Li, H. , & Steyger, P. S. (2015). Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Science Translational Medicine, 7(298), 298ra118. https://doi.org/10.1126/scitranslmed.aac5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros, C. J. , & Steyger, P. S. (2019). Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harbor Perspectives in Medicine, 9(11). https://doi.org/10.1101/cshperspect.a033548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegger, L. D. , Vasilijic, S. , Fujita, T. , Soares, V. Y. , Seist, R. , Xu, L. , & Stankovic, K. M. (2019). Cytokine levels in inner ear fluid of young and aged mice as molecular biomarkers of noise-induced hearing loss. Frontiers in Neurology, 10, 977. https://doi.org/10.3389/fneur.2019.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, T. , Clemens, E. , Broer, L. , Maier, L. , Uitterlinden, A. G. , de Vries, A. C. H. , van Grotel, M. , Pluijm, S. F. M. , Binder, H. , Mayer, B. , von dem Knesebeck, A. , Byrne, J. , van Dulmen-den Broeder, E. , Crocco, M. , Grabow, D. , Kaatsch, P. , Kaiser, M. , Spix, C. , Kenborg, L. , … . PanCareLIFE consortium. (2020). Usefulness of current candidate genetic markers to identify childhood cancer patients at risk for platinum-induced ototoxicity: Results of the European PanCareLIFE cohort study. European Journal of Cancer, 138, 212–224. https://doi.org/10.1016/j.ejca.2020.07.019 [DOI] [PubMed] [Google Scholar]
- Lassaletta, L. , Calvino, M. , Morales-Puebla, J. M. , Lapunzina, P. , Rodriguez-de la Rosa, L. , Varela-Nieto, I. , & Martinez-Glez, V. (2019). Biomarkers in vestibular schwannoma-associated hearing loss. Frontiers in Neurology, 10, 978. https://doi.org/10.3389/fneur.2019.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell, G. , Ekborn, A. , Viberg, A. , & Canlon, B. (2007). Effects of a single high dose of cisplatin on the melanocytes of the stria vascularis in the guinea pig. Audiology and Neurotology, 12(3), 170–178. https://doi.org/10.1159/000099020 [DOI] [PubMed] [Google Scholar]
- Laurell, G. F. (1992). Combined effects of noise and cisplatin: Short- and long-term follow-up. Annals of Otology, Rhinology & Laryngology, 101(12), 969–976. https://doi.org/10.1177/000348949210101202 [DOI] [PubMed] [Google Scholar]
- Lautermann, J. , Crann, S. A. , McLaren, J. , & Schacht, J. (1997). Glutathione-dependent antioxidant systems in the mammalian inner ear: Effects of aging, ototoxic drugs and noise. Hearing Research, 114(1–2), 75–82. https://doi.org/10.1016/s0378-5955(97)00154-8 [DOI] [PubMed] [Google Scholar]
- Lautermann, J. , McLaren, J. , & Schacht, J. (1995). Glutathione protection against gentamicin ototoxicity depends on nutritional status. Hearing Research, 86(1–2), 15–24. https://doi.org/10.1016/0378-5955(95)00049-A [DOI] [PubMed] [Google Scholar]
- Lautermann, J. , Song, B. , McLaren, J. , & Schacht, J. (1995). Diet is a risk factor in cisplatin ototoxicity. Hearing Research, 88(1–2), 47–53. https://doi.org/10.1016/0378-5955(95)00097-N [DOI] [PubMed] [Google Scholar]
- Leitner, M. G. , Halaszovich, C. R. , & Oliver, D. (2011). Aminoglycosides inhibit KCNQ4 channels in cochlear outer hair cells via depletion of phosphatidylinositol(4,5)bisphosphate [Comparative Study Research Support, Non-U.S. Government]. Molecular Pharmacology: Home, 79(1), 51–60. https://doi.org/10.1124/mol.110.068130 [DOI] [PubMed] [Google Scholar]
- Leonova, E. V. , & Raphael, Y. (1997). Organization of cell junctions and cytoskeleton in the reticular lamina in normal and ototoxically damaged organ of Corti. Hearing Research, 113(1–2), 14–28. https://doi.org/10.1016/S0378-5955(97)00130-5 [DOI] [PubMed] [Google Scholar]
- Li, H. , Kachelmeier, A. , Furness, D. N. , & Steyger, P. S. (2015). Local mechanisms for loud sound-enhanced aminoglycoside entry into outer hair cells. Frontiers in Cellular Neuroscience, 9, 130. https://doi.org/10.3389/fncel.2015.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , & Steyger, P. S. (2011). Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Scientific Reports, 1, 159. https://doi.org/10.1038/srep00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. D. , Kao, M. C. , Tsai, M. H. , Lai, C. H. , Wei, I. H. , Tsai, M. H. , Tang, C. H. , Lin, C. W. , Hsu, C. J. , & Lin, C. Y. (2011). Transient ischemia/hypoxia enhances gentamicin ototoxicity via caspase-dependent cell death pathway. Laboratory Investigation, 91(7), 1092–1106. https://doi.org/10.1038/labinvest.2011.69 [DOI] [PubMed] [Google Scholar]
- Lin, F. R. , Yaffe, K. , Xia, J. , Xue, Q. L. , Harris, T. B. , Purchase-Helzner, E. , Satterfield, S. , Ayonayon, H. N. , Ferrucci, L. , Simonsick, E. M. , & Health ABC Study Group (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173(4), 293–299. https://doi.org/10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Kachelmeier, A. , Dai, C. , Li, H. , & Steyger, P. S. (2015). Uptake of fluorescent gentamicin by peripheral vestibular cells after systemic administration. PLOS ONE, 10(3), e0120612. https://doi.org/10.1371/journal.pone.0120612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Jiang, X. , Shi, C. , Shi, L. , Yang, B. , Shi, L. , Xu, Y. , Yang, W. , & Yang, S. (2013). Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Molecular Neurobiology, 48(3), 647–654. https://doi.org/10.1007/s12035-013-8454-2 [DOI] [PubMed] [Google Scholar]
- Lyford-Pike, S. , Vogelheim, C. , Chu, E. , Della Santina, C. C. , & Carey, J. P. (2007). Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. Journal of the Association for Research, 8(4), 497–508. https://doi.org/10.1007/s10162-007-0093-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manian, F. A. , Stone, W. J. , & Alford, R. H. (1990). Adverse antibiotic effects associated with renal insufficiency. Reviews of Infectious Diseases, 12(2), 236–249. https://doi.org/10.1093/clinids/12.2.236 [DOI] [PubMed] [Google Scholar]
- Marcotti, W. , van Netten, S. M. , & Kros, C. J. (2005). The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. Journal of Physiology, 567(Pt. 2), 505–521. https://doi.org/10.1113/jphysiol.2005.085951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto, K. , Nagata, K. , Uesugi, T. , Yamada, T. , & Taguchi, T. (2007). Risk factors for sensorineural hearing loss in survivors with severe congenital diaphragmatic hernia. European Journal of Pediatrics, 166(6), 607–612. https://doi.org/10.1007/s00431-006-0300-3 [DOI] [PubMed] [Google Scholar]
- Mathog, R. H. , & Klein, W. J., Jr. (1969). Ototoxicity of ethacrynic acid and aminoglycoside antibiotics in uremia. New England Journal of Medicine, 280(22), 1223–1224. https://doi.org/10.1056/NEJM196905292802208 [DOI] [PubMed] [Google Scholar]
- Matt, T. , Ng, C. L. , Lang, K. , Sha, S. H. , Akbergenov, R. , Shcherbakov, D. , Meyer, M. , Duscha, S. , Xie, J. , Dubbaka, S. R. , Perez-Fernandez, D. , Vasella, A. , Ramakrishnan, V. , Schacht, J. , & Bottger, E. C. (2012). Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proceeding of the National Academy of Sciences, 109(27), 10984–10989. https://doi.org/10.1073/pnas.1204073109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine, D. , & Johnstone, B. M. (1990). The ototoxic mechanism of cisplatin. Hearing Research, 47(3), 191–203. https://doi.org/10.1016/0378-5955(90)90151-E [DOI] [PubMed] [Google Scholar]
- McClure, C. L. (1992). Common infections in the elderly. American Academy of Family Physicians, 45(6), 2691–2698. [PubMed] [Google Scholar]
- McKinney, W. , Yonovitz, A. , & Smolensky, M. H. (2015). Circadian variation of gentamicin toxicity in rats. The Laryngoscope, 125(7), E252–E256. https://doi.org/10.1002/lary.25116 [DOI] [PubMed] [Google Scholar]
- Miller, M. W. , Riedel, G. , Hoistad, D. , Sutherland, C. , Juhn, S. K. , Adams, G. L. , Griffin, R. , & Ondrey, F. G. (2009). Ototoxicity after combined platinum and fractionated radiation in a novel guinea pig model. American Journal of Otolaryngology, 30(1), 1–7. https://doi.org/10.1016/j.amjoto.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Mohr, P. E. , Feldman, J. J. , Dunbar, J. L. , McConkey-Robbins, A. , Niparko, J. K. , Rittenhouse, R. K. , & Skinner, M. W. (2000). The societal costs of severe to profound hearing loss in the United States. International Journal of Technology Assessment in Health Care, 16(4), 1120–1135. https://doi.org/10.1017/S0266462300103162 [DOI] [PubMed] [Google Scholar]
- Monson, B. B. , Rock, J. , Schulz, A. , Hoffman, E. , & Buss, E. (2019). Ecological cocktail party listening reveals the utility of extended high-frequency hearing. Hearing Research, 381, 107773. https://doi.org/10.1016/j.heares.2019.107773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzack, E. L. , May, L. A. , Roy, S. , Gale, J. E. , & Cunningham, L. L. (2015). Live imaging the phagocytic activity of inner ear supporting cells in response to hair cell death. Cell Death & Differentiation, 22(12), 1995–2005. https://doi.org/10.1038/cdd.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, S. S. , Akil, O. , Ianculescu, A. G. , Geier, E. G. , Lustig, L. R. , & Giacomini, K. M. (2010). Role of the copper transporter, CTR1, in platinum-induced ototoxicity. Journal of Neuroscience, 30(28), 9500–9509. https://doi.org/10.1523/JNEUROSCI.1544-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea, D. , Jajoo, S. , Sheehan, K. , Kaur, T. , Sheth, S. , Bunch, J. , Perro, C. , Rybak, L. P. , & Ramkumar, V. (2011). NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss [Research Support, N.I.H., Extramural Research Support, Non-U.S. Government]. Antioxidants & Redox Signaling, 14(6), 999–1010. https://doi.org/10.1089/ars.2010.3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea, D. , Jajoo, S. , Whitworth, C. , Bunch, J. R. , Turner, J. G. , Rybak, L. P. , & Ramkumar, V. (2008). Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat [Research Support, N.I.H., Extramural Research Support, Non-U.S. Government]. Journal of Neuroscience, 28(49), 13056–13065. https://doi.org/10.1523/JNEUROSCI.1307-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrdal, S. E. , & Steyger, P. S. (2005). TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hearing Research, 204(1–2), 170–182. https://doi.org/10.1016/j.heares.2005.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt, E. A. , Brummett, R. E. , Remsen, L. G. , Kroll, R. A. , Pagel, M. A. , McCormick, C. I. , Guitjens, S. , & Muldoon, L. L. (1996). In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Research, 56(4), 706–709. http://cancerres.aacrjournals.org/content/56/4/706.full.pdf [PubMed] [Google Scholar]
- Nicol, K. M. , Hackney, C. M. , Evans, E. F. , & Pratt, S. R. (1992). Behavioural evidence for recovery of auditory function in guinea pigs following kanamycin administration. Hearing Research, 61(1–2), 117–131. https://doi.org/10.1016/0378-5955(92)90042-L [DOI] [PubMed] [Google Scholar]
- Nilius, B. , & Szallasi, A. (2014). Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacological Reviews, 66(3), 676–814. https://doi.org/10.1124/pr.113.008268 [DOI] [PubMed] [Google Scholar]
- Nyberg, S. , Abbott, N. J. , Shi, X. , Steyger, P. S. , & Dabdoub, A. (2019). Delivery of therapeutics to the inner ear: The challenge of the blood–labyrinth barrier. Science Translational Medicine, 11(482). https://doi.org/10.1126/scitranslmed.aao0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak, A. , & Jerala, R. (2011). Toll-like receptor 4 activation in cancer progression and therapy. Clinical and Developmental Immunology, 2011, Article ID 609579. https://doi.org/10.1155/2011/609579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, G. S. , Kim, H. J. , Choi, J. H. , Shen, A. , Kim, C. H. , Kim, S. J. , Shin, S. R. , Hong, S. H. , Kim, Y. , Park, C. , Lee, S. J. , Akira, S. , Park, R. , & So, H. S. (2011). Activation of lipopolysaccharide-TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. Journal of Immunology, 186(2), 1140–1150. https://doi.org/10.4049/jimmunol.1002183 [DOI] [PubMed] [Google Scholar]
- Oishi, N. , Duscha, S. , Boukari, H. , Meyer, M. , Xie, J. , Wei, G. , Schrepfer, T. , Roschitzki, B. , Boettger, E. C. , & Schacht, J. (2015). XBP1 mitigates aminoglycoside-induced endoplasmic reticulum stress and neuronal cell death. Cell Death & Disease, 6(5), e1763. https://doi.org/10.1038/cddis.2015.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, T. , & Tachibana, M. (1990). Origin of the endolymphatic DC potential in the cochlea and ampulla of the guinea pig. European Archives of Oto-Rhino-Laryngology, 248(2), 99–101. https://doi.org/10.1007/BF00240229 [DOI] [PubMed] [Google Scholar]
- Paken, J. , Govender, C. D. , Pillay, M. , & Sewram, V. (2016). Cisplatin-associated ototoxicity: A review for the health professional. Journal of Toxicology, 2016, 1809394. https://doi.org/10.1155/2016/1809394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B. , Akyuz, N. , Liu, X. P. , Asai, Y. , Nist-Lund, C. , Kurima, K. , Derfler, B. H. , Gyorgy, B. , Limapichat, W. , Walujkar, S. , Wimalasena, L. N. , Sotomayor, M. , Corey, D. P. , & Holt, J. R. (2018). TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron, 99(4), 736–753. e736. https://doi.org/10.1016/j.neuron.2018.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino, A. C. , Lobo, M. , Teh, B. S. , Okcu, M. F. , South, M. , Butler, E. B. , Su, J. , & Chintagumpala, M. (2010). Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. International Journal of Radiation Oncology, 78(5), 1445–1450. https://doi.org/10.1016/j.ijrobp.2009.09.031 [DOI] [PubMed] [Google Scholar]
- Paulos, C. M. , Wrzesinski, C. , Kaiser, A. , Hinrichs, C. S. , Chieppa, M. , Cassard, L. , Palmer, D. C. , Boni, A. , Muranski, P. , Yu, Z. , Gattinoni, L. , Antony, P. A. , Rosenberg, S. A. , & Restifo, N. P. (2007). Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling [Research Support, N.I.H., Intramural]. Journal of Clinical Investigation, 117(8), 2197–2204. https://doi.org/10.1172/JCI32205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitiriga, V. , Dimitroulia, E. , Saroglou, G. , & Tsakris, A. (2017). The challenge of curbing aminoglycoside resistance: can antimicrobial stewardship programs play a critical role? Expert Review of Anti-infective Therapy, 15(10), 947–954. https://doi.org/10.1080/14787210.2017.1382355 [DOI] [PubMed] [Google Scholar]
- Prayuenyong, P. , Taylor, J. A. , Pearson, S. E. , Gomez, R. , Patel, P. M. , Hall, D. A. , Kasbekar, A. V. , & Baguley, D. M. (2018). Vestibulotoxicity associated with platinum-based chemotherapy in survivors of cancer: A scoping review. Frontiers in Oncology, 8, 363. https://www.frontiersin.org/article/10.3389/fonc.2018.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X. , He, Z. , Wang, Y. , Chen, B. , Hetrick, A. , Dai, C. , Chi, F. , Li, H. , & Ren, D. (2020). Hair cell uptake of gentamicin in the developing mouse utricle. Journal of Cellular Physiology. https://doi.org/10.1002/jcp.30228 [DOI] [PubMed] [Google Scholar]
- Qian, Y. , & Guan, M. X. (2009). Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrobial Agents and Chemotherapy, 53(11), 4612–4618. https://doi.org/10.1128/AAC.00965-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C. J. , Katzov-Eckert, H. , Dube, M. P. , Brooks, B. , Rassekh, S. R. , Barhdadi, A. , Feroz-Zada, Y. , Visscher, H. , Brown, A. M. , Rieder, M. J. , Rogers, P. C. , Phillips, M. S. , Carleton, B. C. , & Hayden, M. R. (2009). Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature Genetics, 41(12), 1345–1349. https://doi.org/10.1038/ng.478 [DOI] [PubMed] [Google Scholar]
- Rubin, L. G. , Sanchez, P. J. , Siegel, J. , Levine, G. , Saiman, L. , Jarvis, W. R. , & Pediatric Prevention, N. (2002). Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists' practices. Pediatrics, 110(4), e42. https://doi.org/10.1542/peds.110.4.e42 [DOI] [PubMed] [Google Scholar]
- Ryan, A. F. , & Bone, R. C. (1978). Potentiation of kanamycin ototoxicity by a history of noise exposure. Otolaryngology, 86(1), ORL-125–ORL-128. https://doi.org/10.1177/019459987808600130 [DOI] [PubMed] [Google Scholar]
- Ryan, A. F. , & Bone, R. C. (1982). Non-simultaneous interaction of exposure to noise and kanamycin intoxication in the chinchilla. American Journal of Otolaryngology, 3(4), 264–272. https://doi.org/10.1016/S0196-0709(82)80065-3 [DOI] [PubMed] [Google Scholar]
- Rybak, L. P. (1993). Ototoxicity of loop diuretics. Otolaryngologic Clinics of North America, 26(5), 829–844. https://doi.org/10.1016/S0030-6665(20)30770-2 [PubMed] [Google Scholar]
- Schacht, J. , Lodhi, S. , & Weiner, N. D. (1977). Effects of neomycin on polyphosphoinositides in inner ear tissues and monomolecular films. Advance in Experimental Medicine and Biology, 84, 191–208. https://doi.org/10.1007/978-1-4684-3279-4_9 [DOI] [PubMed] [Google Scholar]
- Schmitt, H. , Roemer, A. , Zeilinger, C. , Salcher, R. , Durisin, M. , Staecker, H. , Lenarz, T. , & Warnecke, A. (2018). Heat shock proteins in human perilymph: Implications for cochlear implantation. Otology & Neurotology, 39(1), 37–44. https://doi.org/10.1097/MAO.0000000000001625 [DOI] [PubMed] [Google Scholar]
- Sergi, B. , Ferraresi, A. , Troiani, D. , Paludetti, G. , & Fetoni, A. R. (2003). Cisplatin ototoxicity in the guinea pig: Vestibular and cochlear damage [Research Support, Non-U.S. Government]. Hearing Research, 182(1–2), 56–64. https://doi.org/10.1016/S0378-5955(03)00142-4 [DOI] [PubMed] [Google Scholar]
- Shayman, C. S. , Seo, J. H. , Oh, Y. , Lewis, R. F. , Peterka, R. J. , & Hullar, T. E. (2018). Relationship between vestibular sensitivity and multisensory temporal integration. Journal of Neurophysiology, 120(4), 1572–1577. https://doi.org/10.1152/jn.00379.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep, J. L. , & Mogan, K. A. (1984). Kinetics of endotoxin release during antibiotic therapy for experimental Gram-negative bacterial sepsis. Journal of Infectious Diseases, 150(3), 380–388. https://doi.org/10.1093/infdis/150.3.380 [DOI] [PubMed] [Google Scholar]
- Smith, P. F. , & Darlington, C. L. (2013). Personality changes in patients with vestibular dysfunction. Frontiers in Human Neuroscience, 7, 678. https://doi.org/10.3389/fnhum.2013.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P. F. , & Zheng, Y. (2013). From ear to uncertainty: Vestibular contributions to cognitive function. Frontiers in Integrative Neuroscience, 7, 84. https://doi.org/10.3389/fnint.2013.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulban, G. , Smolensky, M. H. , & Yonovitz, A. (1990). Gentamicin-induced chronotoxicity: Use of body temperature as a circadian marker rhythm. Chronobiology International, 7(5-6), 393–402. https://doi.org/10.3109/07420529009059150 [DOI] [PubMed] [Google Scholar]
- Stepanyan, R. S. , Indzhykulian, A. A. , Velez-Ortega, A. C. , Boger, E. T. , Steyger, P. S. , Friedman, T. B. , & Frolenkov, G. I. (2011). TRPA1-mediated accumulation of aminoglycosides in mouse cochlear outer hair cells. Journal of the Association for Research in Otolaryngology, 12(6), 729–740. https://doi.org/10.1007/s10162-011-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyger, P. S. (2009). Potentiation of chemical ototoxicity by noise. Seminar Hearing, 30(1), 38–46. https://doi.org/10.1055/s-0028-1111105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyger, P. S. , Burton, M. , Hawkins, J. R. , Schuff, N. R. , & Baird, R. A. (1997). Calbindin and parvalbumin are early markers of non-mitotically regenerating hair cells in the bullfrog vestibular otolith organs. International Journal of Developmental Neuroscience, 15(4–5), 417–432. https://doi.org/10.1016/s0736-5748(96)00101-3 [DOI] [PubMed] [Google Scholar]
- Sultemeier, D. R. , & Hoffman, L. F. (2017). Partial aminoglycoside lesions in vestibular epithelia reveal broad sensory dysfunction associated with modest hair cell loss and afferent calyx retraction. Frontiers in Cellular Neuroscience, 11, 331. https://doi.org/10.3389/fncel.2017.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto, Y. , Imai, T. , Kondo, M. , Hanada, Y. , Uno, A. , Ishida, Y. , Kamakura, T. , Kitahara, T. , Inohara, H. , & Shimada, S. (2016). Cisplatin-induced toxicity decreases the mouse vestibulo-ocular reflex. Toxicology Letters, 262, 49–54. https://doi.org/10.1016/j.toxlet.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Thomas, A. J. , Hailey, D. W. , Stawicki, T. M. , Wu, P. , Coffin, A. B. , Rubel, E. W. , Raible, D. W. , Simon, J. A. , & Ou, H. C. (2013). Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. Journal Neuroscience, 33(10), 4405–4414. https://doi.org/10.1523/JNEUROSCI.3940-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy, P. , Bernard, P. , & Schacht, J. (1986). Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. Journal of Clinical Investigation, 77(5), 1492–1500. https://doi.org/10.1172/JCI112463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs, E. , & Charles, B. (1999). Pharmacokinetics and therapeutic drug monitoring of gentamicin in the elderly [Review]. Clinical Pharmacokinetics, 37(4), 331–341. https://doi.org/10.2165/00003088-199937040-00004 [DOI] [PubMed] [Google Scholar]
- Tserga, E. , Nandwani, T. , Edvall, N. K. , Bulla, J. , Patel, P. , Canlon, B. , Cederroth, C. R. , & Baguley, D. M. (2019). The genetic vulnerability to cisplatin ototoxicity: A systematic review. Scientific Report, 9(1), 3455. https://doi.org/10.1038/s41598-019-40138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan, C. , Kantar, M. , Aktan, C. , Kosova, B. , Orman, M. , Bilgen, C. , & Kirazli, T. (2019). Cisplatin ototoxicity in children: Risk factors and its relationship with polymorphisms of DNA repair genes ERCC1, ERCC2, and XRCC1. Cancer Chemotherapy and Pharmacology, 84(6), 1333–1338. https://doi.org/10.1007/s00280-019-03968-2 [DOI] [PubMed] [Google Scholar]
- Van Hecke, R. , Van Rompaey, V. , Wuyts, F. L. , Leyssens, L. , & Maes, L. (2017). Systemic aminoglycosides-induced vestibulotoxicity in humans. Ear and Hearing, 38(6), 653–662. https://doi.org/10.1097/AUD.0000000000000458 [DOI] [PubMed] [Google Scholar]
- van Ruijven, M. W. , de Groot, J. C. , Hendriksen, F. , & Smoorenburg, G. F. (2005). Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hearing Research, 203(1–2), 112–121. https://doi.org/10.1016/j.heares.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Vernon, J. , Brown, J. , Meikle, M. , & Brummett, R. E. (1978). The potentiation of noise-induced hearing loss by neomycin. Otolaryngology, 86(1), ORL-123–ORL-124. https://doi.org/10.1177/019459987808600129 [DOI] [PubMed] [Google Scholar]
- Vu, A. A. , Nadaraja, G. S. , Huth, M. E. , Luk, L. , Kim, J. , Chai, R. , Ricci, A. J. , & Cheng, A. G. (2013). Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PLOS ONE, 8(1), e54794. https://doi.org/10.1371/journal.pone.0054794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waissbluth, S. , & Daniel, S. J. (2013). Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hearing Research, 299, 37–45. https://doi.org/10.1016/j.heares.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Wangemann, P. (2006). Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. Journal of Physiology, 576(Pt. 1), 11–21. https://doi.org/10.1113/jphysiol.2006.112888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton, A. (1984). The aminoglycosides. Clinical Orthopaedics and Related Research, 190, 66–74. https://doi.org/10.1097/00003086-198411000-00010 [PubMed] [Google Scholar]
- Wu, W. J. , Sha, S. H. , McLaren, J. D. , Kawamoto, K. , Raphael, Y. , & Schacht, J. (2001). Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hearing Research, 158(1–2), 165–178. https://doi.org/10.1016/S0378-5955(01)00303-3 [DOI] [PubMed] [Google Scholar]
- Xiong, H. , Chu, H. , Zhou, X. , Huang, X. , Cui, Y. , Zhou, L. , Chen, J. , Li, J. , Wang, Y. , Chen, Q. , & Li, Z. (2011). Simultaneously reduced NKCC1 and Na,K-ATPase expression in murine cochlear lateral wall contribute to conservation of endocochlear potential following a sensorineural hearing loss [Research Support, Non-U.S. Gov't]. Neuroscience Letters, 488(2), 204–209. https://doi.org/10.1016/j.neulet.2010.11.030 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Robinson, G. W. , Huang, J. , Lim, J. Y. , Zhang, H. , Bass, J. K. , Broniscer, A. , Chintagumpala, M. , Bartels, U. , Gururangan, S. , Hassall, T. , Fisher, M. , Cohn, R. , Yamashita, T. , Teitz, T. , Zuo, J. , Onar-Thomas, A. , Gajjar, A. , Stewart, C. F. , & Yang, J. J. (2015). Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nature Genetics, 47(3), 263–266. https://doi.org/10.1038/ng.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager, R. A. (1992). Endotoxemia, renal hypoperfusion, and fever: interactive risk factors for aminoglycoside and sepsis-associated acute renal failure. American Journal of Kidney Diseases, 20(3), 223–230. https://doi.org/10.1016/S0272-6386(12)80694-9 [DOI] [PubMed] [Google Scholar]
- Zhai, F. , Zhang, R. , Zhang, T. , Steyger, P. S. , & Dai, C. F. (2013). Preclinical and clinical studies of unrelieved aural fullness following intratympanic gentamicin injection in patients with intractable Meniere's disease. Audiology and Neurotology, 18(5), 297–306. https://doi.org/10.1159/000351805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Zhang, Y. B. , Dai, C. F. , & Steyger, P. S. (2013). Temporal and spatial distribution of gentamicin in the peripheral vestibular system after transtympanic administration in guinea pigs. Hearing Research, 298, 49–59. https://doi.org/10.1016/j.heares.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. B. , Zhang, R. , Zhang, W. F. , Steyger, P. S. , & Dai, C. F. (2012). Uptake of gentamicin by vestibular efferent neurons and superior olivary complex after transtympanic administration in guinea pigs. Hearing Research, 283(1–2), 169–179. https://doi.org/10.1016/j.heares.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Dai, C. , Steyger, P. S. , Kim, Y. , Vass, Z. , Ren, T. , & Nuttall, A. L. (2003). Vanilloid receptors in hearing: Altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of Corti. Journal of Neurophysiology, 90(1), 444–455. https://doi.org/10.1152/jn.00919.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Li, G. B. , Huang, L. Y. , Xie, H. Z. , Zhao, Y. L. , Chen, Y. Z. , Li, L. L. , & Yang, S. Y. (2014). A prediction model of drug-induced ototoxicity developed by an optimal support vector machine (SVM) method. Computers in Biology and Medicine, 51, 122–127. https://doi.org/10.1016/j.compbiomed.2014.05.005 [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a