Abstract
Purpose
Tinnitus and hyperacusis are debilitating conditions often associated with age-, noise-, and drug-induced hearing loss. Because of their subjective nature, the neural mechanisms that give rise to tinnitus and hyperacusis are poorly understood. Over the past few decades, considerable progress has been made in deciphering the biological bases for these disorders using animal models.
Method
Important advances in understanding the biological bases of tinnitus and hyperacusis have come from studies in which tinnitus and hyperacusis are consistently induced with a high dose of salicylate, the active ingredient in aspirin.
Results
Salicylate induced a transient hearing loss characterized by a reduction in otoacoustic emissions, a moderate cochlear threshold shift, and a large reduction in the neural output of the cochlea. As the weak cochlear neural signals were relayed up the auditory pathway, they were progressively amplified so that the suprathreshold neural responses in the auditory cortex were much larger than normal. Excessive central gain (neural amplification), presumably resulting from diminished inhibition, is believed to contribute to hyperacusis and tinnitus. Salicylate also increased corticosterone stress hormone levels. Functional imaging studies indicated that salicylate increased spontaneous activity and enhanced functional connectivity between structures in the central auditory pathway and regions of the brain associated with arousal (reticular formation), emotion (amygdala), memory/spatial navigation (hippocampus), motor planning (cerebellum), and motor control (caudate/putamen).
Conclusion
These results suggest that tinnitus and hyperacusis arise from aberrant neural signaling in a complex neural network that includes both auditory and nonauditory structures.
Clinical disorders in one discipline often spark research in others. A classic example of this was the early use of aspirin, a nonsteroidal anti-inflammatory drug, to treat rheumatoid arthritis (Kersley, 1957; Mainland & Sutcliffe, 1965). The active ingredient in aspirin is salicylate, and when taken in excess, it can lead to death. The optimal serum salicylate dose needed to treat rheumatoid arthritis approaches those that are mildly toxic, which can result in mild temporary hearing loss and tinnitus (Boettcher & Salvi, 1991; Cazals, 2000; Myers & Bernstein, 1965). To optimize dosing, clinicians adopted a simple strategy that involved telling the patient, “Increase the dose of aspirin until your ears start to ring, then lower the dose until the phantom sound of tinnitus disappears” (Mongan et al., 1973). Because the hearing impairments associated with high-dose salicylate are temporary, it has been widely used as a research tool to investigate the perceptual consequences and biological bases of hearing loss, tinnitus, and hyperacusis in humans (McFadden & Plattsmier, 1984; McFadden et al., 1984) and animals (Cazals, 2000). While most early studies of salicylate ototoxicity focused on its effects on the cochlea, more recent studies have revealed its novel effects on structures in the central nervous system, effects that should not come as a surprise given that aspirin is used to treat headache and fever and readily crosses the blood–brain barrier (Boettcher et al., 1990). This review article provides an overview of our research efforts to characterize some of the major salicylate-induced changes that occur in the peripheral and central auditory pathways and the central nervous system, which are relevant to understanding the neural mechanisms likely to contribute to salicylate-induced tinnitus, hyperacusis, and sensorineural hearing loss.
Aspirin-Induced Hearing Loss and Tinnitus
Early insights on salicylate-induced tinnitus and other hearing disorders were originally gleaned from human clinical studies. Unlike other ototoxic drugs that produce a high-frequency hearing loss, aspirin typically induces a relatively flat hearing loss of 10–20 dB; however, losses of up to 40 dB have been reported with high levels of serum salicylate (Cazals, 2000). Aspirin-induced hearing loss is greatest in regions of normal hearing, but in regions of preexisting sensorineural hearing loss, the threshold shifts attributable to salicylate are less pronounced, suggesting that they affect the same underlying mechanisms responsible for most cases of cochlear hearing loss, namely, impairment to the outer hair cell amplification system (Cazals, 2000; Mellado Lagarde et al., 2008; Myers et al., 1965). The hearing loss from oral ingestion of aspirin reportedly begins 24–48 hr later as serum salicylate levels rise. Hearing thresholds typically recover 24–48 hr after the cessation of treatment as serum salicylate levels decline (McFadden, 1982). Aspirin-induced tinnitus is perceived as tonal and high pitched (7–9 kHz), is associated with ear fullness, and is easily suppressed for 30–60 s by low-level tones presented near the perceived tinnitus pitch (McCabe & Dey, 1965; McFadden, 1982; Mongan et al., 1973). Tinnitus onset and offset reportedly occurs before changes in hearing threshold are noted. The masking patterns for the phantom sound of tinnitus are much different than those for a real tone (Feldmann, 1981). Sounds in the contralateral ear can mask tinnitus at relatively low sound levels, and external sounds suppress the tinnitus for many seconds or minutes after the stimulus is turned off, a phenomenon referred to as residual inhibition (Roberts et al., 2008; Sedley et al., 2015; Terry et al., 1983). Collectively, these results suggest that aspirin-induced cochlear hearing loss leads to neuroplastic changes in the central nervous system that give rise to the phantom sound of tinnitus.
Animal Model of Salicylate-Induced Hearing Loss
Operant behavioral techniques have been used to quantify the amount of salicylate-induced hearing loss in animal models. When monkeys were treated with 500–600 mg/kg of salicylate, they developed a flat hearing loss of 17–24 dB several hours later. The hearing loss completely recovered a day or two after treatment cessation, consistent with human clinical reports and data from other species (Crifò, 1975; Myers & Bernstein, 1965; Radziwon et al., 2015; Stebbins et al., 1973).
Animal Model of Salicylate-Induced Tinnitus
A prerequisite for investigating the neural correlates of salicylate-induced tinnitus is the development of an animal behavioral model that can “tell us” that it is experiencing a phantom auditory sensation. Jastreboff et al. (1988) developed the first behavioral model to assess salicylate-induced tinnitus based on a Pavlovian conditioned suppression paradigm. Since then, many different procedures have been developed, as discussed in a recent review (Hayes et al., 2014). We developed a schedule-induced polydipsia avoidance conditioning paradigm to test for tinnitus. This paradigm relies on the fact that food-restricted rats will spontaneously begin to lick for water even though they are not thirsty (i.e., polydipsia) when a food pellet is delivered once per minute (Lobarinas et al., 2004). Rats are trained to avoid a foot shock by not licking for water on trials in which a real sound is presented. However, rats are allowed to lick for water (no shock) when the external sound is off (quiet). Once rats correctly discriminated sound trials (stop licking) from no-sound trials (licking allowed), rats were treated with salicylate. The basic assumption is that rats would stop licking on no-sound trials (quiet) if the dose of salicylate was sufficient to induce the phantom sound of tinnitus, whereas they would continue to lick for water on no-sound trials when the dose of salicylate was extremely low or if the rat was treated with a placebo (saline). When rats were treated with salicylate, they stopped licking for water on no-sound trials when the dose of salicylate was equal to or greater than 150 mg/kg (see Figure 1), a behavior indicating that rats were perceiving a phantom sound. In contrast, rats continued to lick for water on no-sound trials when treated with a placebo (saline) or with a very low dose of salicylate (50–100 mg/kg). Licking was always suppressed on sound trials regardless of the experimental condition, indicating that the rat's behavior was under stimulus control.
Figure 1.
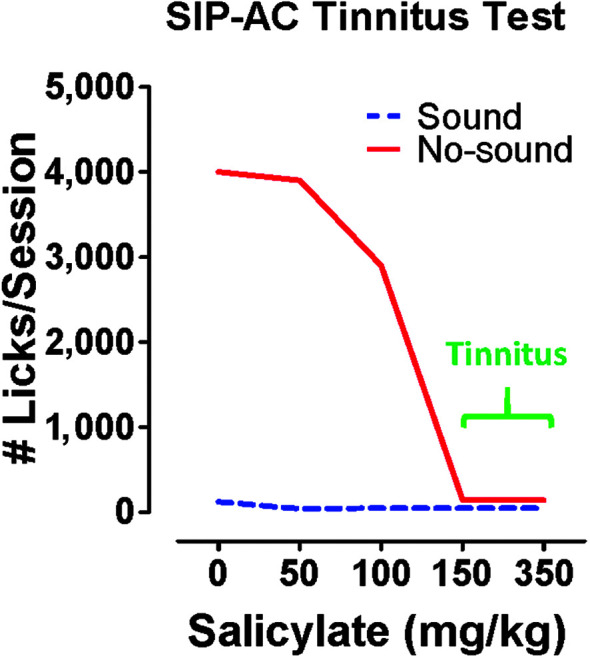
Salicylate dose dependently induces tinnitus-like behavior. Schedule-induced polydipsia avoidance conditioning (SIP-AC) was used to determine the dose of sodium salicylate that could induce tinnitus. Rats were trained to avoid foot shocks on trials in which sound was presented and only lick on trials with no sound (quiet). Here is a schematic illustrating lick suppression data obtained with SIP-AC as a function of salicylate dose. Rats seldom licked on trials when a sound was presented regardless of the salicylate dose or in the absence of salicylate (0 = control). On no-sound trials, rats licked robustly in the absence of salicylate (0 mg/kg, control); however, as the dose of salicylate exceeded 100 mg/kg, licks on no-sound trials largely ceased, which is behavioral evidence that the rats were perceiving the phantom sound of tinnitus in the absence of an external stimulus.
Animal Model of Salicylate-Induced Hyperacusis
Hyperacusis is a loudness intolerance disorder in which moderate-intensity sounds are perceived as intolerably loud. Among patients whose primary complaint is tinnitus, approximately 40% have hyperacusis (Baguley, 2003), although the true percentage is likely higher because most tinnitus patients are unaware they are intolerant of loud sounds (Gu et al., 2010). Conversely, among those with a primary complaint of hyperacusis, 86% have tinnitus (Baguley, 2003). Because hyperacusis is often associated with tinnitus, we predicted that high doses of salicylate would induce hyperacusis.
Loudness is a subjective phenomenon that is often assessed in humans using loudness scaling procedures or questionnaires, procedures difficult or impossible to employ in animal models (Hellman, 1984; Khalfa et al., 2002). Fortunately, researchers have been able to employ reaction time to assess loudness, because reaction time decreases in an orderly manner as sound intensity increases. Because of this reliable relationship, reaction time–intensity functions are extremely useful for obtaining reliable estimates of loudness growth in both humans and animals. In this case, reaction time serves as a surrogate metric for loudness, a perceptual measure, whereas intensity is an acoustic property of the stimulus. This procedure is especially powerful when reaction time–intensity functions are obtained from the same subject before and after an experimental manipulation (Marshall & Brandt, 1980; Pfingst et al., 1975; Stebbins, 1966). If reaction time at a given intensity becomes shorter than it was before an experimental treatment (e.g., salicylate or noise exposure), this would indicate that the sound was perceived as louder than normal, evidence of hyperacusis. Conversely, if reaction time became longer than it was before the experimental treatment, it would indicate that the sound was perceived as less loud (i.e., hypoacusis). To determine if salicylate would disrupt loudness growth, we measured reaction time–intensity functions in rats before and after administering salicylate doses between 50 and 300 mg/kg (Radziwon et al., 2017). During baseline testing, reaction times systematically decreased from approximately 250 to 150 ms as broadband noise intensity increased from 30 to 90 dB SPL, as schematized in Figure 2. Treatment with a high dose of salicylate disrupted the reaction time–intensity functions in two important ways. At low intensities, reaction times were longer than pretreatment, indicating that sounds near 30 dB SPL were perceived as less loud (quieter). This change in loudness is most likely caused by the salicylate-induced hearing loss of approximately 20 dB, which would make 30–dB SPL broadband noise just above threshold barely audible. As the intensity of the broadband noise increased, reaction times rapidly declined, such that reaction times were similar to baseline at moderate intensities but much shorter than pretreatment values at higher intensities. These results indicate that moderate- to high-intensity sounds are perceived as louder than normal after high-dose salicylate treatment. Reaction time–intensity functions completely recovered 24–48 hr after treatment was discontinued. Results similar to this were obtained for tone bursts and noise bursts. Importantly, salicylate doses equal to or greater than 150 mg/kg (i.e., the same dose that induced tinnitus) consistently induced hyperacusis-like behaviors (shorter-than-normal reaction time), whereas reaction times were unaffected by lower salicylate doses or a placebo treatment. Because high-intensity sounds are perceived as louder than normal after high-dose salicylate treatment, it is conceivable that salicylate enhances sound-evoked hyperactivity somewhere along the auditory pathway.
Figure 2.
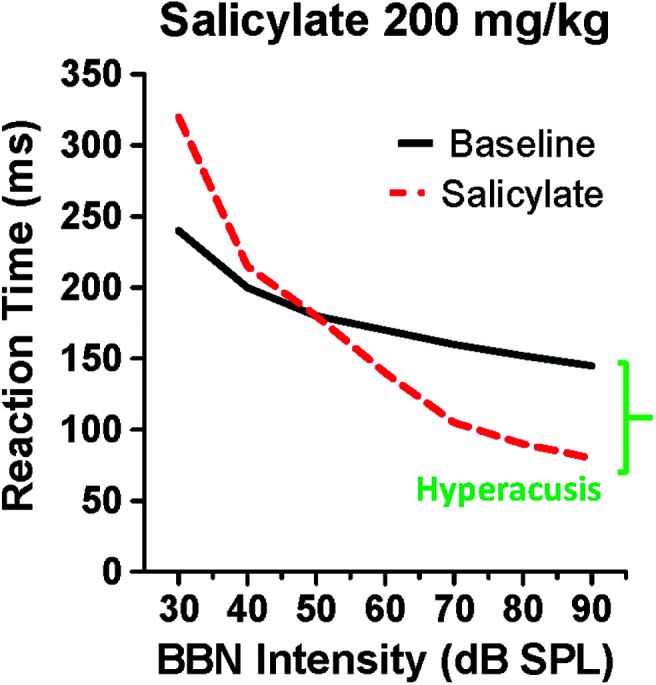
Schematic of reaction time–intensity functions used to assess the growth of loudness during baseline testing and a few hours after salicylate treatment (200 mg/kg). During baseline testing, reaction times gradually decreased as intensity increased. Post-salicylate reaction times increased at low intensities (~30 dB SPL) because salicylate induced a hearing loss of approximately 20 dB, making low-intensity sound less audible. However, as intensity increased, reaction times rapidly increased, catching up to baseline reaction times at moderate intensities (~50 dB SPL) and then becoming shorter than normal at higher intensities. Intensities at which reaction times were shorter than baseline indicate that the sounds were perceived as louder than normal, which is behavioral evidence of hyperacusis. BBN = broadband noise.
Salicylate Upregulates Corticosterone Stress Hormone
Tinnitus and hyperacusis are often associated with hearing loss. However, many patients with hearing loss do not suffer from tinnitus or hyperacusis, suggesting that hearing loss plus other comorbid factors may be needed to induce tinnitus and/or hyperacusis (Nelson & Chen, 2004). In contrast to other forms of hearing loss that may or may not induce tinnitus or hyperacusis, every rat treated with high-dose salicylate invariably develops hearing loss, tinnitus, and hyperacusis. These results suggest that some other factor might be acting synergistically with the cochlear hearing loss to make salicylate a reliable inducer of tinnitus and hyperacusis.
Many patients with tinnitus and hyperacusis indicate that their symptoms are exacerbated by stress (Alpini & Cesarani, 2006; Hasson et al., 2011). Interestingly, cortisol, the human stress hormone, has been linked to tinnitus severity, suggesting that stress hormones might be a mediating factor (Hébert & Lupien, 2009). Taken together, these results suggest that high-dose salicylate might be inducing a stress response that interacts with hearing loss to consistently induce tinnitus and hyperacusis. To test this hypothesis, we measured the corticosterone stress hormone levels in blood samples taken from rats treated with various doses of salicylate (Y.-C. Chen, Chen, et al., 2017). High doses of salicylate that reliably induce tinnitus and hyperacusis (≥ 150 mg/kg) caused a dramatic increase in blood corticosterone levels, whereas low doses of salicylate that failed to induce tinnitus or hyperacusis also failed to increase corticosterone levels. Corticosterone levels declined to baseline levels 24–48 hr posttreatment in concert with the disappearance of tinnitus and hyperacusis (Lobarinas et al., 2004; Radziwon et al., 2017). These results, together with clinical observations, suggest that high levels of stress combined with hearing loss may substantially increase the risk of developing tinnitus and/or hyperacusis.
Salicylate Depresses Distortion Product Otoacoustic Emissions
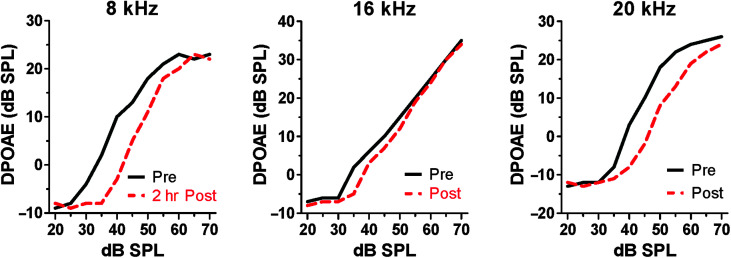
The cochlear amplifier, which imbues the inner ear with its exquisite sensitivity and sharp tuning, is dependent on prestin, an electromotile protein heavily expressed along the lateral wall of outer hair cells (Cheatham et al., 2004; Zheng et al., 2000). Aspirin not only induces tinnitus and hearing loss in humans with salicylate intoxication but also suppresses otoacoustic emissions (Wecker & Laubert, 2004), presumably because it reduces outer hair cell electromotility (Santos-Sacchi et al., 2006; Zheng et al., 2002). It is unclear, however, if salicylate causes hyperacusis in humans. Consistent with these clinical observations, when rats were treated with a high dose of salicylate, it suppressed distortion product otoacoustic emission (DPOAE; Stolzberg et al., 2011). DPOAE input/output functions were shifted rightward to higher intensities (threshold shift), and suprathreshold amplitudes were reduced (see Figure 3). DPOAE amplitudes were moderately reduced at high intensities, and DPOAE threshold shifts were generally 10 dB or less. The DPOAE threshold shifts were smaller than the behavioral threshold shifts reported previously (Myers & Bernstein, 1965), raising the possibility that other pathophysiologies might be occurring elsewhere in the cochlea (e.g., inner hair cells or auditory nerve [AN]).
Figure 3.
Salicylate depresses otoacoustic emissions. Distortion product otoacoustic emission (DPOAE) input/output functions measured pre-exposure and 2 h postexposure. Postexposure input/output functions shifted to the right (threshold shift), and suprathreshold amplitudes reduced. The largest rightward shifts and largest amplitude reductions occurred above and below 16 kHz.
Salicylate Depresses the Cochlear Neural Output
Sound-evoked neural activity transduced by cochlear hair cells is relayed to the central auditory system by Type I AN fibers that innervate the inner hair cells (Spoendlin, 1972). The neural output of the cochlea can be conveniently assessed by presenting tone bursts to elicit the compound action potential (CAP), a local field potential that reflects the synchronous onset response of Type I AN fibers that innervate the inner hair cells (Bourien et al., 2014). CAP input/output functions can be used to quantify changes in the thresholds of the CAP and the amplitude of the CAP at suprathreshold intensities. CAP input/output functions were drastically altered 2 hr after treatment with a high dose of salicylate (see Figure 4). The post-salicylate CAP input/output function was right-shifted (i.e., threshold shift) by approximately 20 dB at low intensities (solid blue line), and the CAP amplitude at high intensities (i.e., the cochlear output) was reduced by more than 60% (dashed green line). The drastic reduction in CAP amplitude observed at intensities greater than 50 dB is difficult to reconcile with the fact that high doses of salicylate induce hyperacusis. How is it possible for moderate- or high-intensity sounds to be perceived as excessively loud when the neural output of the cochlea is significantly reduced?
Figure 4.
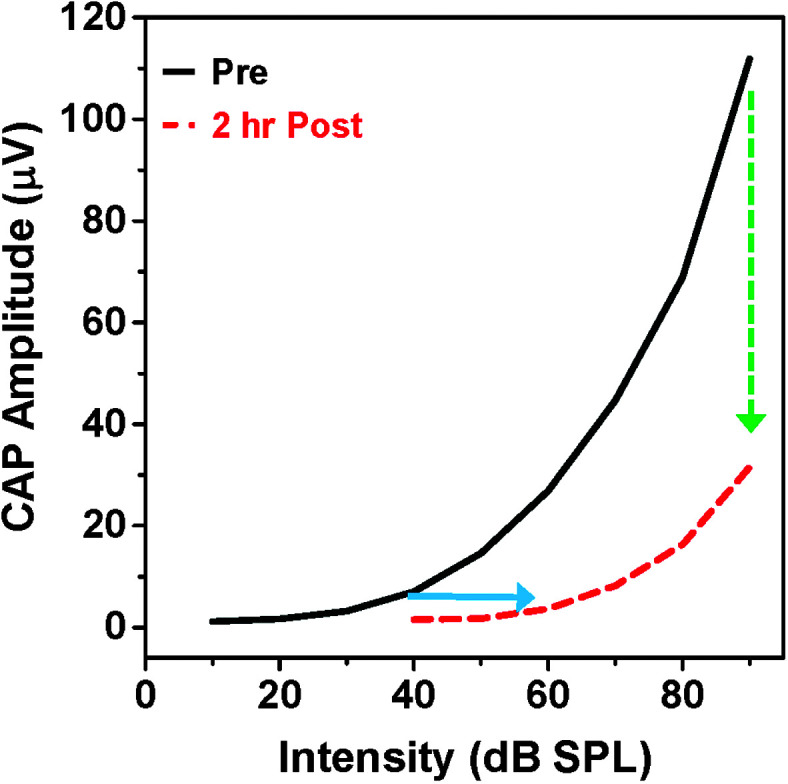
Salicylate suppresses the compound action potential (CAP). Here is a schematic of CAP input/output functions pre-salicylate and 2 hr post-salicylate. Salicylate right-shifted the input/output function by approximately 20 dB (threshold shift, blue line) and reduced the amplitude of the CAP by more than 60% at high intensities (green dashed line), greatly reducing the neural output of the cochlea delivered to the central auditory pathway.
Neural Amplification in the Central Auditory Pathway
If the auditory system behaved as a simple linear system, the weak neural signals from a damaged cochlea should cause normal everyday sounds to seem muffled and barely audible. However, this is clearly not the case because patients with cochlear hearing loss hear suprathreshold sounds similar to listeners with normal hearing due to loudness recruitment. Growing evidence suggests that cochlear damage leads to neuroplastic changes in the central nervous system that partially compensate for the weak neural output from the cochlea; these neuroplastic changes can occur in a matter of minutes or hours (Auerbach et al., 2014; Salvi et al., 1990, 2016). To determine if rapid central compensation was occurring in the central nervous system following high-dose salicylate treatment, we recorded the local field potentials and multiunit spike discharges from sites along the ascending auditory pathway.
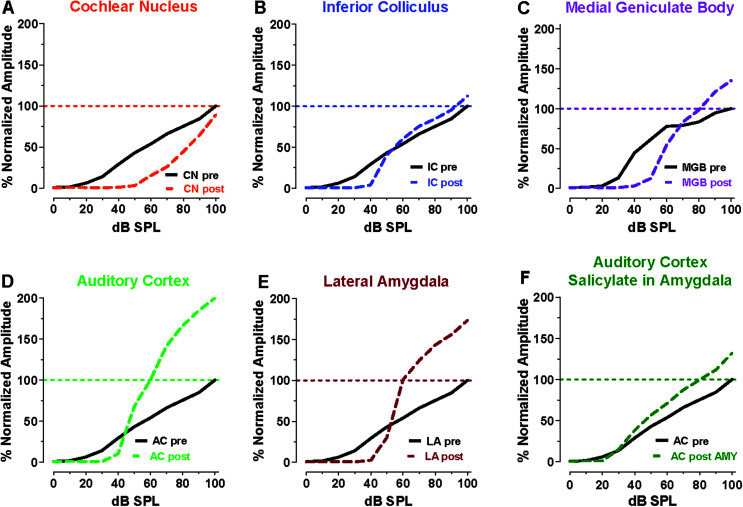
The neural output of the AN is relayed to the ipsilateral cochlear nucleus (CN) complex where it generates a robust local field potential. This is schematized in Figure 5A by plotting the percent normalized local field potential input/output function. In this schematic, 100% represents the maximum amplitude obtained at the highest intensity prior to salicylate treatment; lower intensities evoked proportionately smaller responses relative to the maximum. Pretreatment local field potential gradually increased with intensity, starting around 20 dB SPL. The CN input/output function measured several hours after high-dose salicylate treatment was shifted rightward approximately 20 dB, as previously reported (Jiang et al., 2017). The threshold shift in the CN was similar to that seen in the CAP (see Figure 5, dashed line), indicating that the salicylate-induced hearing loss in the CN was inherited from the AN. Suprathreshold responses from the CN increased rapidly with intensity such that the maximum response at the highest intensity, 100 dB SPL, was approximately 90% of pretreatment values; this is a surprising result given that the maximum response of the CAP (see Figure 4) was reduced by more than 60%. These results suggest that homeostatic changes must have occurred over a relatively short period of time to boost the weak neural signals inherited from the cochlea (Auerbach et al., 2014; Salvi et al., 2000). Nevertheless, the maximum neural response in the CN was still below normal.
Figure 5.
Salicylate depresses sound-evoked neural responses in the auditory periphery, but suprathreshold responses are enhanced in the central auditory pathway. Here are schematics illustrating the input/output functions obtained pre-salicylate (solid line) and post-salicylate (dashed line). Salicylate was administered systemically in Panels A–E but infused into the lateral amygdala (LA) while recordings were obtained from the auditory cortex (AC) in Panel F. Postexposure input/output functions in the cochlear nucleus (CN; Panel A), inferior colliculus (IC; Panel B), medial geniculate body (MGB; Panel C), and AC (Panel D) all shifted to the right of pre-exposure functions at low intensities, reflecting the salicylate-induced cochlear threshold shift. (A) Neural responses in the CN depressed at all intensities post-salicylate. (B) Post-salicylate neural responses in the IC depressed at low intensities but slightly larger than normal at high intensities. (C) Post-salicylate neural responses in the MGB and (D) AC depressed at low intensities but enhanced at high intensities. Salicylate-induced enhancement increases between the MGB and the AC. (E) Post-salicylate input/output function in the LA enhanced at high intensities. (F) Suprathreshold responses in the AC enhanced after salicylate was infused into the LA; however, threshold was unaffected when salicylate was applied to the LA (compare Panel F with Panel D).
To determine if additional changes were occurring at higher levels of the auditory pathway, local field potential input/output functions were recorded from the inferior colliculus (IC), an important binaural processing center in the midbrain (G.-D. Chen et al., 2013; Jiang et al., 2017; Sun et al., 2009). Neural responses from the IC gradually increased in amplitude, beginning around 20 dB SPL (see Figure 5B). The post-salicylate input/output function in the colliculus was right-shifted by approximately 20 dB, consistent with data from the AN and CN. The amplitude of the response from the IC rapidly increased as intensity was increased. At intensities equal to or greater than 60 dB SPL, responses were slightly greater than pretreatment values (see Figure 5B), evidence of enhanced neural gain at suprathreshold intensities.
When local field potentials were recorded more centrally from the medial geniculate body (MGB) (see Figure 5C), an auditory processing area in the thalamus, and from the auditory cortex (AC; see Figure 5D), more dramatic changes were observed at suprathreshold intensities. In both of these regions, the post-salicylate input/output functions were shifted to the right of pretreatment values by approximately 20 dB, similar to that seen at lower levels of the auditory pathway. However, suprathreshold response amplitudes in the MGB and AC were much larger than normal after salicylate treatment, particularly in the AC.
Serial Neural Amplification
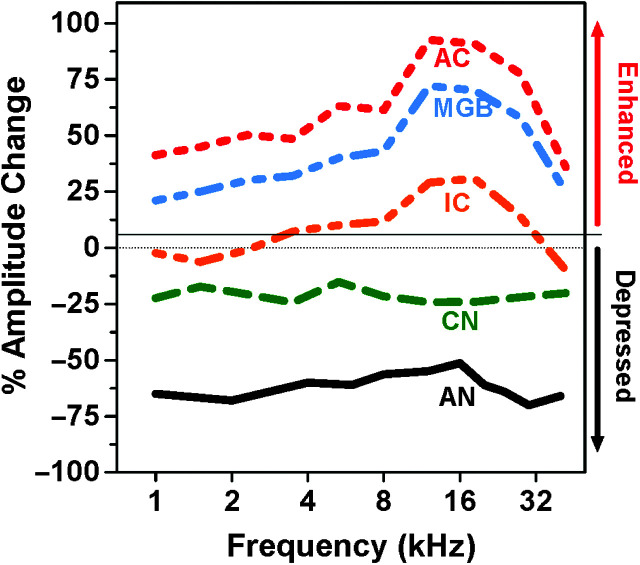
Taken together, these results show that the weak signals from a salicylate-impaired cochlea are progressively amplified as the neural responses are relayed from the periphery up through the central auditory pathway. To visualize these salicylate-induced changes, the schematic in Figure 6 illustrates the frequency-dependent changes in neural activity at different locations along the auditory pathway when suprathreshold (> 60 dB SPL) tone bursts are presented. Neural responses in the AN, reflecting the output of the cochlea, are typically depressed by more than 60% after systemic administration of high-dose salicylate. Neural responses in the CN, which receives its input from the AN, are only reduced by 10%–20%. By the time the neural activity reaches the MGB (IC), the responses are essentially normal or moderately enhanced around 16 kHz. Further neural amplification takes place as the information is progressively relayed to the MGB and then to the AC. Each stage of the auditory pathway appears to cause a small increase in gain such that by the time the signal reaches the AC, it has been amplified multiple times, a process of serial amplification.
Figure 6.
Suprathreshold sound-evoked activity is progressively amplified post-salicylate as neural activity is relayed along the auditory pathway from the auditory nerve (AN), cochlear nucleus (CN), inferior colliculus (IC), medial geniculate body (MGB), and auditory cortex (AC). Here is a schematic depicting percent reduction (depressed, down arrow on the right) or enhancement (enhanced, up arrow on the right) following high-dose salicylate treatment observed in the AN, CN, IC, MGB, or AC as a function of stimulus frequency. Note the massive reduction in neural response from the cochlea. The greatest enhancement of suprathreshold neural activity occurs in the midbrain and cortical areas (IC, MGB, and AC), particularly near 16 kHz.
Frequency-Dependent Neural Amplification
Modern hearing aids include hardware and software to compensate for varying amounts of hearing loss in different frequency regions of the audiogram, a process referred to as frequency-dependent gain or amplification. Salicylate-induced cochlear hearing loss results in a frequency-dependent amplification process at higher levels of the auditory pathway. The amplification that occurred in the IC, MGB, and AC was greatest near 16 kHz, the midfrequency region of the rat's audiogram. What could be occurring in these auditory regions to provide the additional midfrequency amplification? To answer this question, we measured each neuron's threshold (dB SPL) as a function of frequency to delineate the neuron's tuning curve (i.e., threshold vs. frequency function). Tuning curves consist of a low-threshold, narrow V-shaped region of high sensitivity and a high-threshold, broadly tuned low-frequency tail. The frequency with the lowest threshold is referred to as the characteristic frequency (CF). Neurons with high CFs mainly receive information from the high-frequency basal region of the cochlea. Neurons with CFs in the midfrequencies mainly receive information from the midfrequency region in the middle of the cochlea, and low-CF neurons mainly receive information from the low-frequency apical region of the cochlea. Each region in the auditory system has a tonotopic organization such that CFs vary along an anatomical axis (e.g., dorsal to ventral or anterior to posterior) much like the frequency organization of a piano keyboard.
To determine if salicylate disrupted the tonotopic organization of the IC, MGB, and AC, we measured the tuning curve and the CF of neuron multiunit clusters before and after administering a high dose of salicylate (G.-D. Chen et al., 2013; Jiang et al., 2017). Most neural tuning curves in the IC, MGB, and AC had a low-threshold, narrow V-shaped tip with a distinct CF. Following salicylate treatment, the thresholds of most neurons in the IC, MGB, and AC had increased by approximately 20 dB due to the cochlear hearing loss. However, a small percentage (10%–20%) of the tuning curves in each of these regions showed dramatic changes in their CF after salicylate treatment. Some neurons with a low CF showed an expansion of the tuning curve toward the high frequencies, which resulted in an increase in the neuron's CF. That is, the CF of a subpopulation of low-CF neurons shifted toward the 16-kHz region. Conversely, some high-frequency neurons (> 20 kHz) showed an expansion of the tuning curve toward the low frequencies; this resulted in a downshift of the CF toward 16 kHz. One way to conceptualize this is to think of the tonotopic organization of the IC, MGB, and AC as a piano keyboard. Imagine that salicylate caused a small subset of the low-frequency keys on the left side of the keyboard to start to produce midfrequency sounds. Likewise, some high-frequency keys on the right side of the keyboard would begin to produce midfrequency sounds. These salicylate-induced CF upshifts and downshifts would increase the percentage of neurons that responded mainly to mid-frequency sounds (Jiang et al., 2017). Because more neurons have CFs tuned to the midfrequencies after salicylate treatment, neural responses to midfrequency stimuli would be greater than those evoked by low or high sounds, resulting in greater amplification at the midfrequencies.
What neural mechanisms could account for these CF shifts? Neurons in the central auditory pathway are flanked above and below the CF by strong lateral inhibition that helps sharpen neural tuning and limit excitability at high intensities (Nelken & Young, 1994; Palombi & Caspary, 1996; Wang et al., 2002). Cochlear damage from various forms of trauma reduces the flow of neural activity into the central nervous system, thereby reducing the need for lateral inhibition to limit excitability. The loss of lateral inhibition increases excitability and enhances central gain (Salvi et al., 2016). CF shifts similar to this have been seen in the central auditory pathway after pharmacological treatments that suppress lateral inhibition (Avery et al., 2009; Wang et al., 2002).
Emotional Centers Affect Auditory Gain
The acoustic information transmitted up the auditory pathway is relayed to many other structures in the central auditory pathway where it is used to react to and make informed decisions about the environment, for example, “Wake up, it is the alarm clock!” (arousal), “Does the sound signify danger or reward?” (emotion), and “Where should I go when I hear the sound?” (spatial navigation, motor coordination). The amygdala, a region of the brain that assigns emotional significance to sensory stimulation, can be activated by sounds relayed to it from various parts of the auditory brain (Campeau & Davis, 1995; LeDoux et al., 1991; Quirk et al., 1997; Sander et al., 2007). To determine if salicylate affects this emotional center, local field potential input/output functions were recorded from the lateral amygdala pre- and posttreatment. Pretreatment sound-evoked responses from the lateral amygdala gradually increased as intensity increased (see Figure 5E). A few hours after systemically administering a high dose of salicylate, the input/output function shifted to the right by approximately 20 dB because of the cochlear hearing loss. However, neural responses to suprathreshold sounds were much larger than pretreatment values, similar to what was observed in the MGB and AC. The magnitude of the salicylate-induced changes in the amygdala was similar to that seen in the MGB and AC but greater than that observed in the IC. This suggests that additional sound amplification may be occurring in the amygdala from information received from lower levels of the auditory pathway. However, it is possible that other complex and reciprocal interactions may be occurring between the amygdala and the MGB and AC. Thus, salicylate induces hyperactivity not only in the auditory pathway but also in the amygdala, which adds emotional valence to acoustic stimuli. The changes in the amygdala are relevant to human brain imaging studies in which tinnitus distress and hyperacusis were linked to aberrant neural activity in the amygdala and altered structural interactions between the amygdala and the AC (Gunbey et al., 2017; Hofmeier et al., 2018; Levitin et al., 2003; Vanneste et al., 2010).
Because the amygdala is connected to the AC (Budinger et al., 2008; LeDoux et al., 1991), salicylate-induced changes occurring in the amygdala could affect the AC. To evaluate this possibility, salicylate was infused locally into the amygdala while recording the local field potential from the AC pre- and posttreatment (Y.-C. Chen et al., 2015). Local infusion of salicylate into the amygdala resulted in a large increase of suprathreshold sound-evoked activity in the AC (see Figure 5F). However, unlike systemic salicylate treatment, local administration into the lateral amygdala did not alter threshold or responses to low-intensity sounds. These results illustrate how emotionally driven activity in the amygdala, independent of the cochlea, could alter activity in the AC and other regions in the central auditory pathway.
Functional Brain Imaging
One of the major advances in tinnitus and hyperacusis research during the past two decades is the use of functional brain imaging techniques to identify structures in the central nervous system contributing to these disorders (Y.-C. Chen, Xia, et al., 2017; Husain, 2016; Lockwood et al., 1998; Melcher et al., 2009). Traditionally, auditory electrophysiologists have focused their search for the neural correlates of tinnitus in the auditory pathway (Eggermont, 2008; Kaltenbach, 2000; Ma et al., 2006). However, the auditory system makes extensive direct and indirect connections to other parts of the central nervous system, which likely embellish and add additional features to the raw tinnitus and hyperacusis percepts, for example, “Where is the sound located in space?” “Loud sounds really make me anxious?” “I'm really afraid my tinnitus or hyperacusis will get worse.” and “I need to escape from these loud sounds.”
Functional brain imaging techniques allow researchers to surveil the entire central nervous system to identify novel structures associated with tinnitus and hyperacusis. To identify new structures that could potentially contribute to salicylate-induced tinnitus and hyperacusis, we employed functional magnetic resonance imaging (fMRI) to identify regions of altered brain activity (Y.-C. Chen et al., 2015). Because neurons do not possess an endogenous source of energy, “brain fuel” in the form of sugar and oxygen must be quickly brought in to meet the needs of highly active neurons. The release of oxygen from the blood to support changes in neural activity alters the relative abundance of oxyhemoglobin and deoxyhemoglobin. These activity-dependent changes in brain activity alter the magnetic susceptibility of the blood, which can be detected by fMRI (Ogawa et al., 1990), a technique referred to as blood oxygen level–dependent (BOLD) imaging.
fMRI Spontaneous Activity
Spontaneous brain activity can be evaluated by measuring the amplitude of low-frequency fluctuations (ALFF) in the BOLD response throughout the central nervous system (Hoptman et al., 2010). We used the ALFF to identify regions of the brain in which significant changes in spontaneous neural activity occurred in the ALFF response after treating rats with a high dose of salicylate known to induce tinnitus and hyperacusis. Increases in the ALFF were observed bilaterally in three auditory regions: AC, MGB, and IC (see Table 1). Bilateral increases were observed in two other sensory and/or multisensory regions that communicate with the auditory pathway, the visual cortex, the somatosensory cortex, and the superior colliculus (Foxe et al., 2002; Meredith & Stein, 1990; Skaliora et al., 2004; Stein & Meredith, 1990). Bilateral increases in the ALFF occurred in the amygdala, a region of the brain that assigns emotional significance to auditory and other sensory stimuli (Anders et al., 2008). ALFF activity was strongly upregulated in the reticular formation, a brainstem region that responds robustly to sound, is affected by cortisol levels, and is important for arousal (Born et al., 1988; Kornetsky & Eliasson, 1969; Paus, 2000).
Table 1.
Salicylate-induced changes in spontaneous neural activity reflected in terms of amplitude of low-frequency fluctuations (ALFF) via functional magnetic resonance imaging.
| Brain region | Function | ALFF change |
|---|---|---|
| Auditory cortex | Auditory | ⇧ |
| Medial geniculate body | Auditory | ⇧ |
| Inferior colliculus | Auditory | ⇧ |
| Somatosensory cortex | Somesthesis | ⇧ |
| Visual cortex | Visual | ⇧ |
| Superior colliculus | Visual/multisensory | ⇧ |
| Amygdala | Emotion | ⇧ |
| Reticular formation | Arousal | ⇧ |
| Cerebellum | Motor planning | ⇧ |
| Hippocampus | Memory/spatial navigation | ⇩ |
| Caudate/putamen | Movement regulation | ⇩ |
Note. Up arrow = increase; down arrow = decrease.
Salicylate also increased ALFF activity bilaterally in the cerebellum. The cerebellum plays an important role in motor planning and coordination. Portions of the cerebellum also respond to sound and have been implicated in tinnitus (Azizi et al., 1985; Bauer et al., 2013; Lockwood et al., 1999). In animals with noise-induced tinnitus, imaging studies revealed elevated activity in the parafloccular lobe of the cerebellum (Brozoski et al., 2007). These results suggested that the parafloccular lobe might act as a tinnitus generator. In support of this hypothesis, it was found that ablation of the paraflocculus in animals with preexisting tinnitus abolished tinnitus (Bauer et al., 2013; Brozoski et al., 2017, 2013). Tinnitus was also temporarily suppressed by acute pharmacologic inactivation of the parafloccular lobe.
Salicylate also caused bilateral decreases in the ALFF in two regions, namely, the hippocampus, a structure important for spatial navigation and memory (Bates & Wolbers, 2014; Goodman et al., 2010; Robinson & Bucci, 2012), and the caudate/putamen, a structure important for movement regulation (Herrero et al., 2002; Villablanca, 2010). Both of these structures have been implicated in tinnitus (Larson & Cheung, 2012; Ueyama et al., 2013; Wunderlich et al., 2010). Patients with tinnitus often report that they perceive their tinnitus in the left ear, the right ear, or the head. The aberrant activity in the hippocampus, which is involved in spatial processing, could conceivably be involved in “telling the listener” in which area the phantom sound is located. Activation or inactivation of the locus of the caudate, a region outside the classical auditory pathway, can trigger or suppress the phantom sound of tinnitus, suggesting that this region might serve as a gate to turn tinnitus on or off (Larson & Cheung, 2012, 2013).
fMRI Functional Connectivity
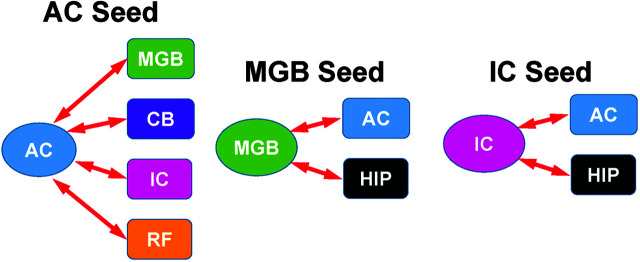
Most regions of the central nervous system form complicated structural and functional networks in order to carry out complex activities such as interpreting speech and movements away from or toward a sound. In order to identify regions of the central nervous system that are working together, we conducted functional connectivity analyses in which the temporal fluctuations in the BOLD response in one region of the auditory system were correlated with BOLD response in all other regions of the central nervous system (Y.-C. Chen et al., 2015). With the “seed” region located in the AC (see Figure 7, left), BOLD responses showed a significant salicylate-induced increase in functional connectivity with two other auditory regions, namely, the MGB and IC. Salicylate also increased functional connectivity between the AC and portions of the cerebellum (lobule IV and parafloccular lobe) and between the AC and the reticular formation. With the seed region placed in the medial geniculate, functional connectivity was significantly increased between the MGB and the AC, consistent with the previous AC analysis, and between the medial geniculate and the hippocampus (see Figure 7, middle). When the seed region was placed in the IC, salicylate treatment significantly increased the functional connectivity between the IC and the AC, consistent with the AC analysis, and between the IC and the hippocampus (see Figure 7, right).
Figure 7.
Salicylate enhances functional connectivity. With the seed region in the auditory cortex (AC), functional connectivity was enhanced with the medial geniculate body (MGB), lobule IV and the parafloccular lobe of the cerebellum (CB), inferior colliculus (IC), and portions of the reticular formation (RF). With the seed region in the MGB, functional connectivity was increased with the AC and hippocampus (HIP). With the seed region in the IC, functional connectivity was enhanced with the AC and HIP.
Our ALFF and functional connectivity results identified several regions in the central nervous system that, at first glance, seemed unlikely to be involved in tinnitus or hyperacusis, such as the reticular formation, cerebellum, and hippocampus. To confirm that these were not false positives, subsequent electrophysiological experiments were conducted to test for salicylate-induced aberrant activity in these regions. In each case, we found evidence of sound-evoked hyperactivity in the hippocampus, the reticular formation, and portions of the cerebellum, consistent with the imaging data (Y.-C. Chen, Chen, et al., 2017; Y.-C. Chen et al., 2015).
Sound-Evoked fMRI Hyperactivity
Task-based fMRI paradigms can be used to test for changes in sound-evoked activity, measures relevant to hyperacusis (Gu et al., 2010). To noninvasively assess the neural correlates of salicylate-induced hyperacusis, we used tone bursts to locate regions of aberrant activity in the central nervous system (Wong et al., 2020). fMRI responses in the lateral lemniscus remained at normal levels following salicylate treatment despite the fact that salicylate is known to greatly reduce the neural output of the cochlea (see Figure 4; G.-D. Chen et al., 2013; Stolzberg et al., 2011). In contrast, sound-evoked fMRI responses were significantly enhanced in the MGB at 16 kHz, whereas those in the AC were enhanced to a slightly greater degree at both 8 and 16 kHz (Wong et al., 2020). Interestingly, the temporal profile of sound-evoked fMRI responses varied across regions. Those in the lateral lemniscus and MGB gradually increased over the duration of the 20-s stimulus (buildup), whereas those in the AC were characterized by an onset-like response during the first 8 s of the 20-s stimulus. These results were largely consistent with the electrophysiological results showing evidence of sound-evoked hyperactivity at higher levels of the auditory pathway.
Network Model of Tinnitus and Hyperacusis
Based on the electrophysiological and functional imaging data, a model was proposed in which hubs and neural networks in the central nervous system can account for many of the key features of tinnitus and hyperacusis (Y.-C. Chen et al., 2015). Pitch, loudness, and other sensory features of tinnitus and hyperacusis are likely related to an auditory network that shows enhanced spontaneous and sound-evoked activity as well as increased functional connectivity in the AC, MGB, and IC after salicylate treatment. The increased activity and functional connectivity between the AC and the amygdala provide a pathway through which the aversive and anxiety-provoking aspects of a sound can be linked to negative emotions. Increased activation and functional connectivity between the AC and the reticular formation could further increase arousal, causing an individual to focus on his or her tinnitus or search for environmental cues that might signal an impending loud sound. The hyperactivity and increased functional activity between the hippocampus and the MGB as well as between the MGB and the hippocampus could provide a pathway to consolidate a memory of the phantom sound or to signal the location of the tinnitus or a real sound (“I hear it in my right ear”). The cerebellum would seem to be an unlikely place to be involved in tinnitus or hyperacusis, but the enhanced activity and functional connectivity in selected portions of the cerebellum could aid in coordinating movement of the head and shoulder or aid in directing one's attention to the location of a real or phantom sound.
Limitations of the Model
One of the benefits of using the salicylate model is that it consistently induces hearing loss, tinnitus, and hyperacusis, and the effects are dose dependent and reversible. While the salicylate model has helped propel tinnitus and hyperacusis research forward, the model has a number of limitations. Because the drug effects are completely reversible when salicylate is discontinued, it is unclear if the acute neural and biological changes accurately reflect the biological changes associated with chronic tinnitus and hyperacusis. Less than half of the patients with tinnitus suffer from hyperacusis, whereas many, but not all those with hyperacusis, also have tinnitus (Baguley, 2003). These results suggest that the neural mechanisms that give rise to tinnitus and hyperacusis may not be identical. However, our results show that doses of salicylate that consistently induce tinnitus also induce hyperacusis and hearing loss. Consequently, it is difficult to disentangle the biological mechanisms that give rise to tinnitus from those that cause hyperacusis and/or hearing loss using the salicylate model. Some of these limitations could be overcome by using noise or a drug that causes permanent hearing loss plus tinnitus versus hearing loss alone. Similarly, by comparing the functional changes associated with the combination of hearing loss, tinnitus, and hyperacusis versus just hearing loss plus tinnitus, it might be possible to isolate the neural mechanisms primarily related to hyperacusis.
Other Considerations
It has been suggested that salicylate, the active ingredient in aspirin, might serve as a useful model of sensorineural hearing loss (McFadden et al., 1984). However, it is unclear whether the transient hyperacusis induced by salicylate accurately reflects the persistent tinnitus and hyperacusis associated with intense noise exposure, ototoxic drugs, and aging (Baguley, 2003; Baguley et al., 2013). The central gain enhancement observed with salicylate-induced tinnitus has also been observed in some animal models of noise-induced hearing loss, ototoxicity, and aging (Chambers et al., 2016; Milbrandt et al., 2000; Salvi et al., 1990, 2016, 2007, 2000; Syka et al., 1994).
Some reports have linked enhanced central gain and spontaneous hyperactivity, a putative mechanism for tinnitus, to enhanced excitatory signaling and/or reduced inhibitory neurotransmission in the central auditory pathway (Caspary & Llano, 2017; Chambers et al., 2016; Milbrandt et al., 2000; Salvi et al., 1990, 2016, 2007, 2000; see also Dong et al., 2009, 2010). Decreased inhibition could contribute to some of the temporal processing deficits observed in these models (Chambers et al., 2016; Gleich & Strutz, 2011; Lobarinas, 2006; Salvi et al., 2016) and to disruptions in gap detection that occurs with high-dose salicylate (Radziwon et al., 2015). The temporal processing deficits observed in these models could also arise from dysregulation of ion channels that regulate the temporal firing patterns of neurons in the auditory pathway (Chambers et al., 2017).
It has been suggested that enhanced central gain may be an epiphenomenon unrelated to tinnitus or hyperacusis. One study found that enhanced neural gain was mainly associated with noise-induced hearing loss and was unrelated to tinnitus or hyperacusis (Möhrle et al., 2019). In the context of age-related tinnitus or hyperacusis, it has also been reported that enhanced central gain can compensate for cochlear impairments in young animals, but not in old animals (Möhrle et al., 2016). Moreover, when the cochlear hearing loss is severe, central gain may be insufficient to fully boost the central neural responses back to normal levels (Qiu et al., 2000; Radziwon et al., 2019).
Enhanced central gain has been observed in humans with tinnitus or hyperacusis (Gu et al., 2010; Hébert et al., 2013; Melcher et al., 2009, 2000; Norena, 2011) and subjects with a history of noise exposure and diminished neural output from the cochlea (Bramhall et al., 2018, 2020). In one report, tinnitus subjects with reduced Wave I auditory brainstem response amplitudes had normal Wave V amplitudes, indicative of central gain in the brainstem (Schaette & McAlpine, 2011). Human studies suggesting that enhanced central gain could contribute to tinnitus and hyperacusis are discussed in a recent review (Brotherton et al., 2015). On the other hand, some human studies have found no relationship between enhanced central gain and tinnitus (Shim et al., 2017). Other studies suggest that enhanced neural gain in the brainstem may be necessary, but not sufficient for the induction of tinnitus (Brotherton et al., 2019; Sedley, 2019).
Synopsis
Because high doses of salicylate consistently induce tinnitus, it has been widely used for more than 30 years to investigate the biological bases of tinnitus. More recent studies indicate high doses of salicylate sufficient to cause tinnitus also induce hyperacusis (Radziwon et al., 2017). The reasons why salicylate consistently induces tinnitus and hyperacusis are poorly understood, but several factors likely contribute to this. The first is that salicylate causes a cochlear hearing loss that triggers an increase in central gain, similar to gain increases seen following noise-induced hearing loss. A second factor is that tinnitus readily enters the brain where it can enhance sound-evoked neural activity independent of hearing loss (see Figure 5F). These changes could occur because salicylate disrupts inhibitory circuits in the central nervous system. Third, high doses of salicylate cause a massive upsurge in corticosterone, a stress hormone that has long been implicated in tinnitus and hyperacusis. By combining electrophysiological and functional imaging techniques with validated behavioral models of tinnitus and hyperacusis, it has been possible to identify neuroplastic changes in the central nervous system that are correlated with these subjective perceptual disorders, such as elevated spontaneous activity, increased levels of stress hormones, and enhanced sound-evoked and increased functional connectivity within the central auditory pathway and brain regions associated with emotion, arousal, memory, spatial navigation, and motor planning. Some of the metrics used to assess salicylate-induced tinnitus and hyperacusis in animals could be employed clinically. Auditory evoked potential amplitudes could be measured at multiple levels of the auditory pathway to test for enhanced central gain in patients with tinnitus and/or hyperacusis (Bramhall et al., 2018). These measures could be correlated with tests of anxiety, stress, and depression to identify comorbid factors that may predispose some individuals for developing tinnitus or hyperacusis (Hasson et al., 2013; Hébert & Lupien, 2009).
Acknowledgments
This work was supported in part by National Institute on Deafness and Other Communication Disorders Grants R01DC014452 and R01DC014693 (awarded to R. S.) and F32DC015160 (awarded to B. D. A.) as well as a Hearing Health Foundation grant (awarded to K. R. and S. M.).
Funding Statement
This work was supported in part by National Institute on Deafness and Other Communication Disorders Grants R01DC014452 and R01DC014693 (awarded to R. S.) and F32DC015160 (awarded to B. D. A.) as well as a Hearing Health Foundation grant (awarded to K. R. and S. M.).
References
- Alpini, D. , & Cesarani, A. (2006). Tinnitus as an alarm bell: Stress reaction tinnitus model. ORL, 68(1), 31–37. https://doi.org/10.1159/000090488 [DOI] [PubMed] [Google Scholar]
- Anders, S. , Eippert, F. , Weiskopf, N. , & Veit, R. (2008). The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: An fMRI study. Social Cognitive and Affective Neuroscience, 3(3), 233–243. https://doi.org/10.1093/scan/nsn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach, B. D. , Rodrigues, P. V. , & Salvi, R. J. (2014). Central gain control in tinnitus and hyperacusis. Frontiers in Neurology, 5, 206. https://doi.org/10.3389/fneur.2014.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery, M. A. , Sheehan, A. E. , Kerr, K. S. , Wang, J. , & Freeman, M. R. (2009). WldS requires Nmnat1 enzymatic activity and N16–VCP interactions to suppress Wallerian degeneration. Journal of Cell Biology, 184(4), 501–513. https://doi.org/10.1083/jcb.200808042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi, S. A. , Burne, R. A. , & Woodward, D. J. (1985). The auditory corticopontocerebellar projection in the rat: Inputs to the paraflocculus and midvermis. An anatomical and physiological study. Experimental Brain Research, 59(1), 36–49. https://doi.org/10.1007/BF00237663 [DOI] [PubMed] [Google Scholar]
- Baguley, D. M. (2003). Hyperacusis. Journal of the Royal Society of Medicine, 96(12), 582–585. https://doi.org/10.1177/014107680309601203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley, D. M. , McFerran, D. , & Hall, D. (2013). Tinnitus. The Lancet, 382(9904), 1600–1607. https://doi.org/10.1016/S0140-6736(13)60142-7 [DOI] [PubMed] [Google Scholar]
- Bates, S. L. , & Wolbers, T. (2014). How cognitive aging affects multisensory integration of navigational cues. Neurobiology of Aging, 35(12), 2761–2769. https://doi.org/10.1016/j.neurobiolaging.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Bauer, C. A. , Kurt, W. , Sybert, L. T. , & Brozoski, T. J. (2013). The cerebellum as a novel tinnitus generator. Hearing Research, 295, 130–139. https://doi.org/10.1016/j.heares.2012.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher, F. A. , Bancroft, B. R. , & Salvi, R. J. (1990). Concentration of salicylate in serum and perilymph of the chinchilla. Archives of Otolaryngology—Head & Neck Surgery, 116(6), 681–684. https://doi.org/10.1001/archotol.1990.01870060039005 [DOI] [PubMed] [Google Scholar]
- Boettcher, F. A. , & Salvi, R. J. (1991). Salicylate ototoxicity: Review and synthesis. American Journal of Otolaryngology, 12(1), 33–47. https://doi.org/10.1016/0196-0709(91)90071-M [DOI] [PubMed] [Google Scholar]
- Born, J. , Hitzler, V. , Pietrowsky, R. , Pauschinger, P. , & Fehm, H. L. (1988). Influences of cortisol on auditory evoked potentials (AEPs) and mood in humans. Neuropsychobiology, 20(3), 145–151. https://doi.org/10.1159/000118489 [DOI] [PubMed] [Google Scholar]
- Bourien, J. , Tang, Y. , Batrel, C. , Huet, A. , Lenoir, M. , Ladrech, S. , Desmadryl, G. , Nouvian, R. , Puel, J.-L. , & Wang, J. (2014). Contribution of auditory nerve fibers to compound action potential of the auditory nerve. Journal of Neurophysiology, 112(5), 1025–1039. https://doi.org/10.1152/jn.00738.2013 [DOI] [PubMed] [Google Scholar]
- Bramhall, N. F. , Konrad-Martin, D. , & McMillan, G. P. (2018). Tinnitus and auditory perception after a history of noise exposure: Relationship to auditory brainstem response measures. Ear and Hearing, 39(5), 881–894. https://doi.org/10.1097/AUD.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall, N. F. , Niemczak, C. E. , Kampel, S. D. , Billings, C. J. , & McMillan, G. P. (2020). Evoked potentials reveal noise exposure–related central auditory changes despite normal audiograms. American Journal of Audiology, 29(2), 152–164. https://doi.org/10.1044/2019_AJA-19-00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton, H. , Plack, C. J. , Maslin, M. , Schaette, R. , & Munro, K. J. (2015). Pump up the volume: Could excessive neural gain explain tinnitus and hyperacusis. Audiology and Neurotology, 20(4), 273–282. https://doi.org/10.1159/000430459 [DOI] [PubMed] [Google Scholar]
- Brotherton, H. , Turtle, C. , Plack, C. J. , Munro, K. J. , & Schaette, R. (2019). Earplug-induced changes in acoustic reflex thresholds suggest that increased subcortical neural gain may be necessary but not sufficient for the occurrence of tinnitus. Neuroscience, 407, 192–199. https://doi.org/10.1016/j.neuroscience.2019.03.017 [DOI] [PubMed] [Google Scholar]
- Brozoski, T. J. , Brozoski, D. , Wisner, K. , & Bauer, C. (2017). Chronic tinnitus and unipolar brush cell alterations in the cerebellum and dorsal cochlear nucleus. Hearing Research, 350, 139–151. https://doi.org/10.1016/j.heares.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski, T. J. , Ciobanu, L. , & Bauer, C. A. (2007). Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI). Hearing Research, 228(1–2), 168–179. https://doi.org/10.1016/j.heares.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Brozoski, T. J. , Wisner, K. W. , Odintsov, B. , & Bauer, C. A. (2013). Local NMDA receptor blockade attenuates chronic tinnitus and associated brain activity in an animal model. PLOS ONE, 8(10), Article e77674. https://doi.org/10.1371/journal.pone.0077674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinger, E. , Laszcz, A. , Lison, H. , Scheich, H. , & Ohl, F. W. (2008). Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: Bottom-up and top-down processing of neuronal information via field AI. Brain Research, 1220, 2–32. https://doi.org/10.1016/j.brainres.2007.07.084 [DOI] [PubMed] [Google Scholar]
- Campeau, S. , & Davis, M. (1995). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience, 15(3), 2312–2327. https://doi.org/10.1523/JNEUROSCI.15-03-02312.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary, D. M. , & Llano, D. A. (2017). Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology. Hearing Research, 349, 197–207. https://doi.org/10.1016/j.heares.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazals, Y. (2000). Auditory sensori-neural alterations induced by salicylate. Progress in Neurobiology, 62(6), 583–631. https://doi.org/10.1016/S0301-0082(00)00027-7 [DOI] [PubMed] [Google Scholar]
- Chambers, A. R. , Pilati, N. , Balaram, P. , Large, C. H. , Kaczmarek, L. K. , & Polley, D. B. (2017). Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage. Scientific Reports, 7(1) Article 17496. https://doi.org/10.1038/s41598-017-17406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, A. R. , Resnik, J. , Yuan, Y. , Whitton, J. P. , Edge, A. S. , Liberman, M. C. , & Polley, D. B. (2016). Central gain restores auditory processing following near-complete cochlear denervation. Neuron, 89(4), 867–879. https://doi.org/10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham, M. A. , Huynh, K. H. , Gao, J. , Zuo, J. , & Dallos, P. (2004). Cochlear function in Prestin knockout mice. The Journal of Physiology, 560(3), 821–830. https://doi.org/10.1113/jphysiol.2004.069559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G.-D. , Stolzberg, D. , Lobarinas, E. , Sun, W. , Ding, D. , & Salvi, R. (2013). Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hearing Research, 295(1–2), 100–113. https://doi.org/10.1016/j.heares.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.-C. , Chen, G. D. , Auerbach, B. D. , Manohar, S. , Radziwon, K. , & Salvi, R. (2017). Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress. Hearing Research, 349, 208–222. https://doi.org/10.1016/j.heares.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. C. , Li, X. , Liu, L. , Wang, J. , Lu, C. Q. , Yang, M. , Jiao, Y. , Zang, F.-C. , Radziwon, K. , Chen, G.-D. , Sun, W. , Muthaiah, V. P. K. , & Teng, G.-J. (2015). Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife, 4. Article e06576. https://doi.org/10.7554/eLife.06576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.-C. , Xia, W. , Chen, H. , Feng, Y. , Xu, J.-J. , Gu, J.-P. , Salvi, R. , & Yin, X. (2017). Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Human Brain Mapping, 38(5), 2384–2397. https://doi.org/10.1002/hbm.23525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crifò, S. (1975). Aspirin ototoxicity in the guinea pig. ORL, 37(1), 27–34. https://doi.org/10.1159/000275201 [DOI] [PubMed] [Google Scholar]
- Dong, S. , Mulders, W. H. , Rodger, J. , & Robertson, D. (2009). Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neuroscience, 159(3), 1164–1174. https://doi.org/10.1016/j.neuroscience.2009.01.043 [DOI] [PubMed] [Google Scholar]
- Dong, S. , Mulders, W. H. , Rodger, J. , Woo, S. , & Robertson, D. (2010). Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. European Journal of Neuroscience, 31(9), 1616–1628. https://doi.org/10.1111/j.1460-9568.2010.07183.x [DOI] [PubMed] [Google Scholar]
- Eggermont, J. J. (2008). Role of auditory cortex in noise- and drug-induced tinnitus. American Journal of Audiology, 17(2), S162–S169. https://doi.org/10.1044/1059-0889(2008/07-0025) [DOI] [PubMed] [Google Scholar]
- Feldmann, H. (1981). Homolateral and contralateral masking of tinnitus. Journal of Laryngology and Otology. Supplement, (4), 60–70. https://www.ncbi.nlm.nih.gov/pubmed/6946172 [PubMed] [Google Scholar]
- Foxe, J. J. , Wylie, G. R. , Martinez, A. , Schroeder, C. E. , Javitt, D. C. , Guilfoyle, D. , Ritter, W. , & Murray, M. M. (2002). Auditory-somatosensory multisensory processing in auditory association cortex: An fMRI study. Journal of Neurophysiology, 88(1), 540–543. https://doi.org/10.1152/jn.2002.88.1.540 [DOI] [PubMed] [Google Scholar]
- Gleich, O. , & Strutz, J. (2011). The effect of gabapentin on gap detection and forward masking in young and old gerbils. Ear and Hearing, 32(6), 741–749. https://doi.org/10.1097/AUD.0b013e318222289f [DOI] [PubMed] [Google Scholar]
- Goodman, T. , Trouche, S. , Massou, I. , Verret, L. , Zerwas, M. , Roullet, P. , & Rampon, C. (2010). Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience, 171(3), 769–778. https://doi.org/10.1016/j.neuroscience.2010.09.047 [DOI] [PubMed] [Google Scholar]
- Gu, J. W. , Halpin, C. F. , Nam, E.-C. , Levine, R. A. , & Melcher, J. R. (2010). Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. Journal of Neurophysiology, 104(6), 3361–3370. https://doi.org/10.1152/jn.00226.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunbey, H. P. , Gunbey, E. , Aslan, K. , Bulut, T. , Unal, A. , & Incesu, L. (2017). Limbic-auditory interactions of tinnitus: An evaluation using diffusion tensor imaging. Clinical Neuroradiology, 27(2), 221–230. https://doi.org/10.1007/s00062-015-0473-0 [DOI] [PubMed] [Google Scholar]
- Hasson, D. , Theorell, T. , Bergquist, J. , & Canlon, B. (2013). Acute stress induces hyperacusis in women with high levels of emotional exhaustion. PLOS ONE, 8(1), Article e52945. https://doi.org/10.1371/journal.pone.0052945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson, D. , Theorell, T. , Wallén, M. B. , Leineweber, C. , & Canlon, B. (2011). Stress and prevalence of hearing problems in the Swedish working population. BMC Public Health, 11(1), Article 130. https://doi.org/10.1186/1471-2458-11-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, S. H. , Radziwon, K. E. , Stolzberg, D. J. , & Salvi, R. J. (2014). Behavioral models of tinnitus and hyperacusis in animals. Frontiers in Neurology, 5, 179. https://doi.org/10.3389/fneur.2014.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert, S. , Fournier, P. , & Noreña, A. (2013). The auditory sensitivity is increased in tinnitus ears. The Journal of Neuroscience, 33(6), 2356–2364. https://doi.org/10.1523/JNEUROSCI.3461-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert, S. , & Lupien, S. J. (2009). Salivary cortisol levels, subjective stress, and tinnitus intensity in tinnitus sufferers during noise exposure in the laboratory. International Journal of Hygiene and Environmental Health, 212(1), 37–44. https://doi.org/10.1016/j.ijheh.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Hellman, R. P. (1984). Growth rate of loudness, annoyance, and noisiness as a function of tone location within the noise spectrum. The Journal of the Acoustical Society of America, 75(1), 209–218. https://doi.org/10.1121/1.390397 [DOI] [PubMed] [Google Scholar]
- Herrero, M.-T. , Barcia, C. , & Navarro, J. M. (2002). Functional anatomy of thalamus and basal ganglia. Child's Nervous System, 18(8), 386–404. https://doi.org/10.1007/s00381-002-0604-1 [DOI] [PubMed] [Google Scholar]
- Hofmeier, B. , Wolpert, S. , Aldamer, E. S. , Walter, M. , Thiericke, J. , Braun, C. , Zelle, D. , Rüttiger, L. , Klose, U. , & Knipper, M. (2018). Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. NeuroImage: Clinical, 20, 637–649. https://doi.org/10.1016/j.nicl.2018.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman, M. J. , Zuo, X.-N. , Butler, P. D. , Javitt, D. C. , D'Angelo, D. , Mauro, C. J. , & Milham, M. P. (2010). Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophrenia Research, 117(1), 13–20. https://doi.org/10.1016/j.schres.2009.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain, F. T. (2016). Neural networks of tinnitus in humans: Elucidating severity and habituation. Hearing Research, 334, 37–48. https://doi.org/10.1016/j.heares.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Jastreboff, P. J. , Sasaki, C. T. , & Brennan, J. F. (1988). An animal model for tinnitus. The Laryngoscope, 98(3), 280–286. https://doi.org/10.1288/00005537-198803000-00008 [DOI] [PubMed] [Google Scholar]
- Jiang, C. , Luo, B. , Manohar, S. , Chen, G.-D. , & Salvi, R. (2017). Plastic changes along auditory pathway during salicylate-induced ototoxicity: Hyperactivity and CF shifts. Hearing Research, 347, 28–40. https://doi.org/10.1016/j.heares.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach, J. A. (2000). Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology, 11(3), 125–137. https://www.ncbi.nlm.nih.gov/pubmed/10755809 [PubMed] [Google Scholar]
- Kersley, G. D. (1957). Aspirin, phenylbutazone or hormones in the treatment of rheumatoid arthritis. Practitioner, 179(1069), 97–99. https://www.ncbi.nlm.nih.gov/pubmed/13453104 [PubMed] [Google Scholar]
- Khalfa, S. , Dubal, S. , Veuillet, E. , Perez-Diaz, F. , Jouvent, R. , & Collet, L. (2002). Psychometric normalization of a hyperacusis questionnaire. ORL, 64(6), 436–442. https://doi.org/10.1159/000067570 [DOI] [PubMed] [Google Scholar]
- Kornetsky, C. , & Eliasson, M. (1969). Reticular stimulation and chlorpromazine: An animal model for schizophrenic overarousal. Science, 165(3899), 1273–1274. https://doi.org/10.1126/science.165.3899.1273 [DOI] [PubMed] [Google Scholar]
- Larson, P. S. , & Cheung, S. W. (2012). Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery, 70(2), 398–405. https://doi.org/10.1227/NEU.0b013e3182320ab5 [DOI] [PubMed] [Google Scholar]
- Larson, P. S. , & Cheung, S. W. (2013). A stroke of silence: Tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. Journal of Neurosurgery, 118(1), 192–194. https://doi.org/10.3171/2012.9.JNS12594 [DOI] [PubMed] [Google Scholar]
- LeDoux, J. E. , Farb, C. R. , & Romanski, L. M. (1991). Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neuroscience Letters, 134(1), 139–144. https://doi.org/10.1016/0304-3940(91)90526-Y [DOI] [PubMed] [Google Scholar]
- Levitin, D. J. , Menon, V. , Schmitt, J. E. , Eliez, S. , White, C. D. , Glover, G. H. , Kadis, J. , Korenberg, J. R. , Bellugi, U. , & Reiss, A. L. (2003). Neural correlates of auditory perception in Williams syndrome: An fMRI study. NeuroImage, 18(1), 74–82. https://doi.org/10.1006/nimg.2002.1297 [DOI] [PubMed] [Google Scholar]
- Lobarinas, E. (2006). Effects of carboplatin-induced inner hair cell loss on auditory perception in chinchilla [Doctoral dissertation, State University of New York at Buffalo] . ProQuest Dissertations & Theses Global. [Google Scholar]
- Lobarinas, E. , Sun, W. , Cushing, R. , & Salvi, R. (2004). A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hearing Research, 190(1–2), 109–114. https://doi.org/10.1016/S0378-5955(04)00019-X [DOI] [PubMed] [Google Scholar]
- Lockwood, A. H. , Salvi, R. J. , Coad, M. L. , Arnold, S. A. , Wack, D. S. , Murphy, B. W. , & Burkard, R. F. (1999). The functional anatomy of the normal human auditory system: Responses to 0.5 and 4.0 kHz tones at varied intensities. Cerebral Cortex, 9(1), 65–76. https://doi.org/10.1093/cercor/9.1.65 [DOI] [PubMed] [Google Scholar]
- Lockwood, A. H. , Salvi, R. J. , Coad, M. L. , Towsley, M. L. , Wack, D. S. , & Murphy, B. W. (1998). The functional neuroanatomy of tinnitus: Evidence for limbic system links and neural plasticity. Neurology, 50(1), 114–120. https://doi.org/10.1212/WNL.50.1.114 [DOI] [PubMed] [Google Scholar]
- Ma, W.-L. , Hidaka, H. , & May, B. J. (2006). Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hearing Research, 212(1–2), 9–21. https://doi.org/10.1016/j.heares.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Mainland, D. , & Sutcliffe, M. I. (1965). Aspirin in rheumatoid arthritis, a seven-day, double-blind trial—Preliminary report. Bulletin on the Rheumatic Diseases, 16(3), 388–391. https://www.ncbi.nlm.nih.gov/pubmed/5318934 [PubMed] [Google Scholar]
- Marshall, L. , & Brandt, J. F. (1980). The relationship between loudness and reaction time in normal hearing listeners. Acta Oto-Laryngologica, 90(1–6), 244–249. https://doi.org/10.3109/00016488009131721 [DOI] [PubMed] [Google Scholar]
- McCabe, P. A. , & Dey, F. L. (1965). The effect of aspirin upon auditory sensitivity. Annals of Otology, Rhinology & Laryngology, 74, 312–325. https://doi.org/10.1177/000348946507400203 [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1982). Tinnitus: Facts, theories, and treatments. The National Academies Press. [PubMed] [Google Scholar]
- McFadden, D. , & Plattsmier, H. S. (1984). Aspirin abolishes spontaneous oto-acoustic emissions. The Journal of the Acoustical Society of America, 76(2), 443–448. https://doi.org/10.1121/1.391585 [DOI] [PubMed] [Google Scholar]
- McFadden, D. , Plattsmier, H. S. , & Pasanen, E. G. (1984). Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hearing Research, 16(3), 251–260. https://doi.org/10.1016/0378-5955(84)90114-X [DOI] [PubMed] [Google Scholar]
- Melcher, J. R. , Levine, R. A. , Bergevin, C. , & Norris, B. (2009). The auditory midbrain of people with tinnitus: Abnormal sound-evoked activity revisited. Hearing Research, 257(1–2), 63–74. https://doi.org/10.1016/j.heares.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher, J. R. , Sigalovsky, I. S. , Guinan , J. J., Jr. , & Levine, R. A. (2000). Lateralized tinnitus studied with functional magnetic resonance imaging: Abnormal inferior colliculus activation. Journal of Neurophysiology, 83(2), 1058–1072. https://doi.org/10.1152/jn.2000.83.2.1058 [DOI] [PubMed] [Google Scholar]
- Mellado Lagarde, M. M. , Drexl, M. , Lukashkina, V. A. , Lukashkin, A. N. , & Russell, I. J. (2008). Outer hair cell somatic, not hair bundle, motility is the basis of the cochlear amplifier. Nature Neuroscience, 11(7), 746–748. https://doi.org/10.1038/nn.2129 [DOI] [PubMed] [Google Scholar]
- Meredith, M. A. , & Stein, B. E. (1990). The visuotopic component of the multisensory map in the deep laminae of the cat superior colliculus. The Journal of Neuroscience, 10(11), 3727–3742. https://doi.org/10.1523/JNEUROSCI.10-11-03727.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt, J. C. , Holder, T. M. , Wilson, M. C. , Salvi, R. J. , & Caspary, D. M. (2000). GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hearing Research, 147(1–2), 251–260. https://doi.org/10.1016/S0378-5955(00)00135-0 [DOI] [PubMed] [Google Scholar]
- Möhrle, D. , Hofmeier, B. , Amend, M. , Wolpert, S. , Ni, K. , Bing, D. , Klose, U. , Pichler, B. , Knipper, M. , & Ruttiger, L. (2019). Enhanced central neural gain compensates acoustic trauma-induced cochlear impairment, but unlikely correlates with tinnitus and hyperacusis. Neuroscience, 407, 146–169. https://doi.org/10.1016/j.neuroscience.2018.12.038 [DOI] [PubMed] [Google Scholar]
- Möhrle, D. , Ni, K. , Varakina, K. , Bing, D. , Lee, S. C. , Zimmermann, U. , Knipper, M. , & Rüttiger, L. (2016). Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiology of Aging, 44, 173–184. https://doi.org/10.1016/j.neurobiolaging.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Mongan, E. , Kelly, P. , Nies, K. , Porter, W. W. , & Paulus, H. E. (1973). Tinnitus as an indication of therapeutic serum salicylate levels. JAMA, 226(2), 142–145. https://doi.org/10.1001/jama.1973.03230020014004 [PubMed] [Google Scholar]
- Myers, E. N. , & Bernstein, J. M. (1965). Salicylate ototoxicity: A clinical and experimental study. Archives of Otolaryngology—Head & Neck Surgery, 82(5), 483–493. https://doi.org/10.1001/archotol.1965.00760010485006 [DOI] [PubMed] [Google Scholar]
- Myers, E. N. , Bernstein, J. M. , & Fostiropolous, G. (1965). Salicylate ototoxicity—A clinical study. The New England Journal of Medicine, 273, 587–590. https://doi.org/10.1056/NEJM196509092731104 [DOI] [PubMed] [Google Scholar]
- Nelken, I. , & Young, E. D. (1994). Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus type IV units to narrowband and wideband stimuli. Journal of Neurophysiology, 71(6), 2446–2462. https://doi.org/10.1152/jn.1994.71.6.2446 [DOI] [PubMed] [Google Scholar]
- Nelson, J. J. , & Chen, K. (2004). The relationship of tinnitus, hyperacusis, and hearing loss. Ear, Nose & Throat Journal, 83(7), 472–476. https://doi.org/10.1177/014556130408300713 [PubMed] [Google Scholar]
- Norena, A. J. (2011). An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neuroscience & Biobehavioral Reviews, 35(5), 1089–1109. https://doi.org/10.1016/j.neubiorev.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Ogawa, S. , Lee, T. M. , Kay, A. R. , & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87(24), 9868–9872. https://doi.org/10.1073/pnas.87.24.9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi, P. S. , & Caspary, D. M. (1996). GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. Journal of Neurophysiology, 75(6), 2211–2219. https://doi.org/10.1152/jn.1996.75.6.2211 [DOI] [PubMed] [Google Scholar]
- Paus, T. (2000). Functional anatomy of arousal and attention systems in the human brain. Progress in Brain Research, 126, 65–77. https://doi.org/10.1016/S0079-6123(00)26007-X [DOI] [PubMed] [Google Scholar]
- Pfingst, B. E. , Hienze, R. , Kimm, J. , & Miller, J. (1975). Reaction time procedure for measurement of hearing. I. Suprathreshold functions. The Journal of the Acoustical Society of America, 57, 421–430. https://doi.org/10.1121/1.380465 [DOI] [PubMed] [Google Scholar]
- Qiu, C. , Salvi, R. , Ding, D. , & Burkard, R. (2000). Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: Evidence for increased system gain. Hearing Research, 139(1–2), 153–171. https://doi.org/10.1016/S0378-5955(99)00171-9 [DOI] [PubMed] [Google Scholar]
- Quirk, G. J. , Armony, J. L. , & LeDoux, J. E. (1997). Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron, 19(3), 613–624. https://doi.org/10.1016/S0896-6273(00)80375-X [DOI] [PubMed] [Google Scholar]
- Radziwon, K. , Auerbach, B. D. , Ding, D. , Liu, X. , Chen, G.-D. , & Salvi, R. (2019). Noise-induced loudness recruitment and hyperacusis: Insufficient central gain in auditory cortex and amygdala. Neuroscience, 422, 212–227. https://doi.org/10.1016/j.neuroscience.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon, K. , Holfoth, D. , Lindner, J. , Kaier-Green, Z. , Bowler, R. , Urban, M. , & Salvi, R. (2017). Salicylate-induced hyperacusis in rats: Dose- and frequency-dependent effects. Hearing Research, 350, 133–138. https://doi.org/10.1016/j.heares.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon, K. , Stolzberg, D. J. , Urban, M. E. , Bowler, R. A. , & Salvi, R. J. (2015). Salicylate-induced hearing loss and gap detection deficits in rats. Frontiers in Neurology, 6, 31. https://doi.org/10.3389/fneur.2015.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, L. E. , Moffat, G. , Baumann, M. , Ward, L. M. , & Bosnyak, D. J. (2008). Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. JARO, 9(4), 417–435. https://doi.org/10.1007/s10162-008-0136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. , & Bucci, D. J. (2012). Fear conditioning is disrupted by damage to the postsubiculum. Hippocampus, 22(6), 1481–1491. https://doi.org/10.1002/hipo.20987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi, R. J. , Saunders, S. S. , Gratton, M. A. , Arehole, S. , & Powers, N. (1990). Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hearing Research, 50(1–2), 245–257. https://doi.org/10.1016/0378-5955(90)90049-U [DOI] [PubMed] [Google Scholar]
- Salvi, R. J. , Sun, W. , Ding, D. , Chen, G.-D. , Lobarinas, E. , Wang, J. , Radziwon, K. , & Auerbach, B. D. (2016). Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Frontiers in Neuroscience, 10, 621. https://doi.org/10.3389/fnins.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi, R. J. , Wang, J. , & Caspary, D. (2007). Functional changes in the central auditory system after noise-induced cochlear damage. In Luxon L. & Prasher D. (Eds.), Noise and its effects (pp. 110–126). Wiley. [Google Scholar]
- Salvi, R. J. , Wang, J. , & Ding, D. (2000). Auditory plasticity and hyperactivity following cochlear damage. Hearing Research, 147(1–2), 261–274. https://doi.org/10.1016/S0378-5955(00)00136-2 [DOI] [PubMed] [Google Scholar]
- Sander, K. , Frome, Y. , & Scheich, H. (2007). FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Human Brain Mapping, 28(10), 1007–1022. https://doi.org/10.1002/hbm.20333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi, J. , Song, L. , Zheng, J. , & Nuttall, A. L. (2006). Control of mammalian cochlear amplification by chloride anions. The Journal of Neuroscience, 26(15), 3992–3998. https://doi.org/10.1523/JNEUROSCI.4548-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette, R. , & McAlpine, D. (2011). Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. The Journal of Neuroscience, 31(38), 13452–13457. https://doi.org/10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley, W. (2019). Tinnitus: Does gain explain. Neuroscience, 407, 213–228. https://doi.org/10.1016/j.neuroscience.2019.01.027 [DOI] [PubMed] [Google Scholar]
- Sedley, W. , Gander, P. E. , Kumar, S. , Oya, H. , Kovach, C. K. , Nourski, K. V. , Kawasaki, H. , Howard , M. A., III , & Griffiths, T. D. (2015). Intracranial mapping of a cortical tinnitus system using residual inhibition. Current Biology, 25(9), 1208–1214. https://doi.org/10.1016/j.cub.2015.02.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, H. J. , An, Y. H. , Kim, D. H. , Yoon, J. E. , & Yoon, J. H. (2017). Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLOS ONE, 12(12), Article e0189157. https://doi.org/10.1371/journal.pone.0189157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaliora, I. , Doubell, T. P. , Holmes, N. P. , Nodal, F. R. , & King, A. J. (2004). Functional topography of converging visual and auditory inputs to neurons in the rat superior colliculus. Journal of Neurophysiology, 92(5), 2933–2946. https://doi.org/10.1152/jn.00450.2004 [DOI] [PubMed] [Google Scholar]
- Spoendlin, H. (1972). Innervation densities of the cochlea. Acta Oto-Laryngologica, 73(2–6), 235–248. https://doi.org/10.3109/00016487209138937 [DOI] [PubMed] [Google Scholar]
- Stebbins, W. C. (1966). Auditory reaction time and the derivation of equal loudness contours for the monkey. Journal of the Experimental Analysis of Behavior, 9(2), 135–142. https://doi.org/10.1901/jeab.1966.9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins, W. C. , Clark, W. W. , Pearson, R. D. , & Weiland, N. G. (1973). Noise- and drug-induced hearing loss in monkeys. Advances in Oto-Rhino-Laryngology, 20, 42–63. https://doi.org/10.1159/000393088 [DOI] [PubMed] [Google Scholar]
- Stein, B. E. , & Meredith, M. A. (1990). Multisensory integration: Neural and behavioral solutions for dealing with stimuli from different sensory modalities. Annals of the New York Academy of Sciences, 608(1), 51–65. https://doi.org/10.1111/j.1749-6632.1990.tb48891.x [DOI] [PubMed] [Google Scholar]
- Stolzberg, D. , Chen, G.-D. , Allman, B. L. , & Salvi, R. J. (2011). Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience, 180, 157–164. https://doi.org/10.1016/j.neuroscience.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, W. , Lu, J. , Stolzberg, D. , Gray, L. , Deng, A. , Lobarinas, E. , & Salvi, R. J. (2009). Salicylate increases the gain of the central auditory system. Neuroscience, 159(1), 325–334. https://doi.org/10.1016/j.neuroscience.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka, J. , Rybalko, N. , & Popelář, J. (1994). Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hearing Research, 78(2), 158–168. https://doi.org/10.1016/0378-5955(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Terry, A. M. , Jones, D. M. , Davis, B. R. , & Slater, R. (1983). Parametric studies of tinnitus masking and residual inhibition. British Journal of Audiology, 17(4), 245–256. https://doi.org/10.3109/03005368309081485 [DOI] [PubMed] [Google Scholar]
- Ueyama, T. , Donishi, T. , Ukai, S. , Ikeda, Y. , Hotomi, M. , Yamanaka, N. , Shinosaki, K. , Terada, M. , & Kaneoke, Y. (2013). Brain regions responsible for tinnitus distress and loudness: A resting-state fMRI study. PLOS ONE, 8(6), Article e67778. https://doi.org/10.1371/journal.pone.0067778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste, S. , Plazier, M. , van der Loo, E. , Van de Heyning, P. , Congedo, M. , & De Ridder, D. (2010). The neural correlates of tinnitus-related distress. NeuroImage, 52(2), 470–480. https://doi.org/10.1016/j.neuroimage.2010.04.029 [DOI] [PubMed] [Google Scholar]
- Villablanca, J. R. (2010). Why do we have a caudate nucleus. Acta Neurobiologiae Experimentalis, 70(1), 95–105. https://doi.org/7011[pii] [DOI] [PubMed] [Google Scholar]
- Wang, J. , McFadden, S. L. , Caspary, D. , & Salvi, R. (2002). Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Research, 944(1–2), 219–231. https://doi.org/10.1016/S0006-8993(02)02926-8 [DOI] [PubMed] [Google Scholar]
- Wecker, H. , & Laubert, A. (2004). Reversible hörminderung bei akuter salizylatintoxikation [Reversible reduction in hearing with acute salicylate intoxication]. HNO, 52(4), 347–351. https://doi.org/10.1007/s00106-004-1065-5 [DOI] [PubMed] [Google Scholar]
- Wong, E. , Radziwon, K. , Chen, G.-D. , Liu, X. , Manno, F. A. , Manno, S. H. , Auerbach, B. , Wu, E. X. , Salvi, R. , & Lau, C. (2020). Functional magnetic resonance imaging of enhanced central auditory gain and electrophysiological correlates in a behavioral model of hyperacusis. Hearing Research, 389, 107908. https://doi.org/10.1016/j.heares.2020.107908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich, A. P. , Schonfeldt-Lecuona, C. , Wolf, R. C. , Dorn, K. , Bachor, E. , & Freund, W. (2010). Cortical activation during a pitch discrimination task in tinnitus patients and controls—An fMRI study. Audiology and Neurotology, 15(3), 137–148. https://doi.org/10.1159/000241094 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Madison, L. D. , Oliver, D. , Fakler, B. , & Dallos, P. (2002). Prestin, the motor protein of outer hair cells. Audiology and Neurotology, 7(1), 9–12. https://doi.org/10.1159/000046855 [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Shen, W. , He, D. Z. , Long, K. B. , Madison, L. D. , & Dallos, P. (2000). Prestin is the motor protein of cochlear outer hair cells. Nature, 405(6783), 149–155. https://doi.org/10.1038/35012009 [DOI] [PubMed] [Google Scholar]