Abstract
Purpose
Individuals with cystic fibrosis (CF) are often treated with intravenous (IV) aminoglycoside (AG) antibiotics to manage life-threatening bacterial infections. Preclinical animal data suggest that, in addition to damaging cochlear hair cells, this class of antibiotics may cause cochlear synaptopathy and/or damage to higher auditory structures. The acoustic reflex growth function (ARGF) is a noninvasive, objective measure of neural function in the auditory system. A shallow ARGF (small reflex-induced changes in middle ear function with increasing elicitor level) has been associated with synaptopathy due to noise exposure in rodent and human studies. In this study, the ARGF was obtained in CF patients with normal hearing, some of whom have been treated with IV AGs, and a control group without CF. The hypothesis was that patients with IV-AG exposure would have a shallow ARGF due to cochlear synaptopathy caused by ototoxicity.
Method
Wideband ARGFs were examined in four groups of normal-hearing participants: a control group of 29 individuals without CF; and in 57 individuals with CF grouped by lifetime IV-AG exposure: 15 participants with no exposure, 21 with low exposure, and 21 with high exposure. Procedures included pure-tone audiometry, clinical immittance, wideband acoustic immittance battery, including ARGFs, and transient evoked otoacoustic emissions.
Results
CF subjects with normal pure-tone thresholds and either high or low lifetime IV-AG exposure had enhanced ARGFs compared to controls and CF participants without IV-AG exposure. The groups did not differ in transient evoked otoacoustic emission signal-to-noise ratio.
Conclusion
These results diverge from the shallow ARGF pattern observed in studies of noise-induced cochlear synaptopathy and are suggestive of a central mechanism of auditory dysfunction in patients with AG-induced ototoxicity.
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that causes abnormal transport of chloride ions in epithelial cells, resulting in changes to body secretions including sweat and mucus. Over 30,000 individuals in the United States have CF, with about 900 new cases diagnosed each year. Life expectancy for a patient born in 2019 is currently estimated at 48.4 years and respiratory problems are the cause of death for 62.2% of patients (Cystic Fibrosis Foundation, 2020). Patients with CF frequently experience breathing difficulties and potentially life-threatening infections as thickened mucus clogs airways and traps bacteria in the lungs. Severe lung infections are called pulmonary exacerbations and are treated with intravenous (IV) antibiotics, especially aminoglycosides (AGs) such as tobramycin, amikacin, and gentamicin.
AGs are a class of broad-spectrum antibiotics that, when administered intravenously, are trafficked across the stria vascularis into endolymph and absorbed into inner and outer hair cells (H. Li & Steyger, 2011). The hair cells may be particularly susceptible to ototoxic damage due to their unusually high metabolic rate and slow drug clearance, and they have historically been considered the primary target of AG ototoxicity (Cheng et al., 2009; Huth et al., 2011; Mulheran et al., 2001). Other cell types also absorb AGs and display signs of toxicity, including lateral wall fibrocytes, supporting cells, and spiral ganglion neurons (Jiang et al., 2017). In addition, repeated dosing may induce structural changes that further enhance drug uptake into these cells, which has significant implications for the many patients with CF that receive prolonged, repeated antibiotic treatments.
In addition to damaging cochlear hair cells, recent evidence suggests AGs may cause cochlear synaptopathy and/or damage to neural mechanisms in higher auditory brainstem structures. The synapse between the primary afferents and sensory cells can be damaged by ototoxic medications, resulting in a “hidden” hearing loss in the absence of elevated behavioral thresholds (Kujawa & Liberman, 2015). Several rodent studies have identified the inner hair cell ribbon synapse as the primary target of ototoxic damage, with synaptopathy observed prior to hair cell damage (Ishikawa et al., 2019; S. Li et al., 2016, 2013).
However, Xu et al. (2009) suggested that gentamicin damages areas of the brainstem simultaneously or prior to the cochlea after observing a decrease in the cross-sectional area of cochlear nucleus neurons 1 day after administration. AGs can also affect the medial olivocochlear (MOC) efferent system, a descending corticofugal pathway that modulates cochlear outer hair cell function. The MOC reflex is activated by sound in either the ipsilateral or contralateral ear and inhibits the outer hair cells' ability to amplify motion in the organ of Corti (Liberman & Guinan, 1998). In humans, the function of the MOC reflex likely relates to improved hearing in noise (da Costa et al., 1998). Avan et al. (1996) found that gentamicin affected the MOC in guinea pigs, causing a lack of suppression of the outer hair cells that resulted in increased amplitude of otoacoustic emissions (OAEs), a measurement of outer hair cell function.
Prevalence estimates of AG-induced sensorineural hearing loss in persons with CF vary in the literature, likely due to large variability in test protocols and clinical characteristics of the study populations. Additionally, greater lifetime exposure of IV-AGs has been associated with an increased prevalence of hearing loss, typically occurring at higher frequency regions (> 8 kHz; Garinis et al., 2017). Although increases in hearing thresholds may occur after substantial neural damage, the standard audiogram is an insensitive measure for detecting ototoxic damage as significant degeneration of sensory cells has been observed without elevation of hearing thresholds (Lobarinas et al., 2016). Behavioral pure-tone thresholds frequently correlate poorly with reported auditory difficulty or the extent of ototoxic damage observed upon histopathologic analysis (Pauna et al., 2017). Even in patients without behavioral hearing threshold shifts, synaptic or neural damage may still have occurred. Ototoxic damage may manifest as tinnitus, other subjective auditory complaints such as reduced sound tolerance or difficulty understanding speech in background noise, and/or vestibular symptoms (Konrad-Martin et al., 2005). A sensitive physiologic test of neural function in humans is needed to elucidate these functional deficits, particularly in the absence of abnormal audiometric thresholds.
Acoustic stapedius reflex (ASR) testing is a physiologic measure that assesses the integrity of multiple sites along the auditory pathway, including the middle ear, auditory nerve, and facial nerve. Similar to the MOC reflex, the ASR is elicited by sound in either the ipsilateral or contralateral ear and attenuates the response of the auditory system. While the MOC decreases the cochlear amplification provided by outer hair cells, the ASR stiffens the tympanic membrane to reduce the amount of sound absorbed by the middle ear (Feeney & Schairer, 2015). The acoustic reflex growth function (ARGF) is a function of reflex strength versus elicitor level and has been proposed as a noninvasive, objective measure of neural dysfunction in the auditory system, particularly as a more sensitive measure than behavioral threshold changes. In rodent studies, a shallow ARGF (smaller reflex-induced changes in middle ear absorbance with increasing elicitor level) has been associated with synaptopathy after noise exposure (Valero et al., 2016, 2018). In humans, subjects with normal-hearing thresholds and noise-induced tinnitus had a shallow ARGF (Wojtczak et al., 2017). However, there is a lack of research on the ARGF in persons receiving routine ototoxic treatments. For this study, we analyzed a subset of data from a multiyear investigation of assessments of auditory function of individuals with and without CF, some of whom had been exposed to AGs. As we did not want to compare ARGFs for subjects with sensorineural hearing loss due to ototoxicity with those with normal hearing, all participants included in the study had normal standard pure-tone audiograms. We hypothesized that CF patients treated with IV-AGs would exhibit shallow ARGFs consistent with underlying symptoms of cochlear synaptopathy, compared to groups with low or no IV-AG exposure. This hypothesis suggests that ARGF patterns exhibited in noise-exposed individuals would be similar to those with ototoxic neural damage.
Method
All study materials and test protocols were approved by the Oregon Health & Science University (OHSU) Institutional Review Board. Participants with CF were recruited from the Adult Cystic Fibrosis Care Center at OHSU. Participants without CF and with no history of IV-AG exposure were recruited from OHSU campus and clinics.
Subjects with CF were classified by lifetime IV-AG exposure according to the cumulative weighted dose method described by Garinis et al. (2017). Electronic health records were reviewed for IV-AG exposure from 2007 until the date of study participation, and paper-based medical records were reviewed prior to 2007. All individual IV-AG doses were summed and weighted by the frequency of dosing, with most frequent dosing schedules assigned a higher weight due to the associated increased risk of cochleotoxicity (Tran Ba Huy & Deffrennes, 1988). This method produced a cumulative weighted IV-AG total for each participant. Of the 42 participants with IV-AG exposure, the median number of cumulative doses was 40. Subjects were divided into a Low IV-AG group ranging from two to 40 doses, and a High IV-AG group ranging from 42 to 246 doses. An additional 15 subjects with CF who had no recorded IV-AG exposure formed a third CF group. All participants with CF who received IV-AGs received tobramycin and/or amikacin. Most participants with CF in all dose groups also received regular treatments of nebulized tobramycin, but inhaled administration routes were not included in dose count as this route of administration is considered significantly less ototoxic than IV administration (Moss, 2001). A fourth group consisted of 29 control subjects without CF and with no history of IV-AG exposure who were age- and sex-matched to the CF subgroups (see Table 1).
Table 1.
Participant information for the four subject groups: Control subjects, subjects with cystic fibrosis and no intravenous (IV) aminoglycoside exposure (No IV-AG), subjects with cystic fibrosis and ≤ 40 weighted doses of aminoglycosides (Low IV-AG), and subjects with cystic fibrosis and > 40 weighted doses of aminoglycosides (High IV-AG).
| Variable | Controls | No IV-AG | Low IV-AG | High IV-AG |
|---|---|---|---|---|
| Individuals | 29 | 15 | 21 | 21 |
| Mean age, years (SD) | 28.3 (5.9) | 30.7 (10.0) | 27.5 (11.9) | 25.0 (5.0) |
| Percent male | 58.6% | 60.0% | 57.1% | 57.1% |
| Mean 4-freq PTA, a dB HL (SD) | 3.8 (3.2) | 7.2 (4.3) | 6.1 (4.4) | 7.5 (3.5) |
| Mean HFA, b dB HL (SD) | 5.4 (10.8) | 11.2 (15.9) | 7.3 (11.8) | 11.5 (10.7) |
| Mean 226-Hz static acoustic admittance in millimhos (min/max, SD) | 0.69 (0.3/1.5, 0.27) | 0.80 (0.3/1.6, 0.37) | 0.67 (0.3/1.3, 0.26) | 0.60 (0.2/1.5, 0.30) |
4-frequency pure-tone average (PTA) of thresholds at 0.5, 1.0, 2.0, and 4.0 kHz.
High-frequency average (HFA) of thresholds at 9.0, 10.0, 11.2, 12.5, 14.0, and 16.0 kHz.
All testing was completed by a licensed clinical audiologist in a double-walled sound-attenuated booth approved for clinical audiometric evaluations in either the Otolaryngology Clinic or the Oregon Hearing Research Center at OHSU. The duration of each visit was approximately 2 hr, and the same tests were performed in the same order for all participants.
To be included in data analysis, participants had (a) normal pure-tone air-conduction thresholds ≤ 25 dB HL from 0.25 to 8.0 kHz, (b) audiometric air–bone gaps ≤ 10 dB for 0.25 to 2 kHz and 15 dB at 4.0 kHz, and (c) present ipsilateral wideband ASR at ambient pressure with a maximum test level of 80 dB SPL as described below.
Following informed written consent, otoscopy was performed to visualize the tympanic membrane and ensure the ear canal was clear of drainage or excessive cerumen. Pure-tone air-conduction thresholds were then measured using a GSI-61 audiometer (Grason-Stadler, Inc.) calibrated to American National Standards Institute standards (2018; S-3.6, 2018). Threshold was defined as the lowest level (in dB HL) at which a response was obtained in at least one half of ascending presentations using the Hughson–Westlake technique, according to standard clinical practice (Carhart & Jerger, 1959). Air-conduction thresholds were measured at conventional frequencies (0.25, 0.50, 1.0, 2.0, 3.0, 4.0, 6.0, and 8.0 kHz) followed by extended high frequencies (9.0, 10.0, 11.2, 12.5, 14.0, and 16.0 kHz). Etymotic Research ER3A insert earphones (Etymotic Research) were used for conventional frequencies and Sennheiser HDA 200 circumaural headphones (Sennheiser Electronic Corp.) for extended high frequencies. A RadioEar B71 bone-oscillator (RadioEar Corp.) was used to measure pure-tone bone-conduction thresholds at 0.25, 0.50, 1.0, 2.0, and 4.0 kHz. Tympanometry and ipsilateral broadband noise (BBN) acoustic reflexes with a 226-Hz probe tone were measured using a Grason Stadler Inc. Tympstar V tympanometer (Grason-Stadler, Inc.) calibrated to American National Standards Institute standards (2020; S3.39-2020). Clinical acoustic reflex thresholds were measured using an ascending and descending bracketing procedure with a 5-dB step size with a reflex criterion of 0.03 mmho.
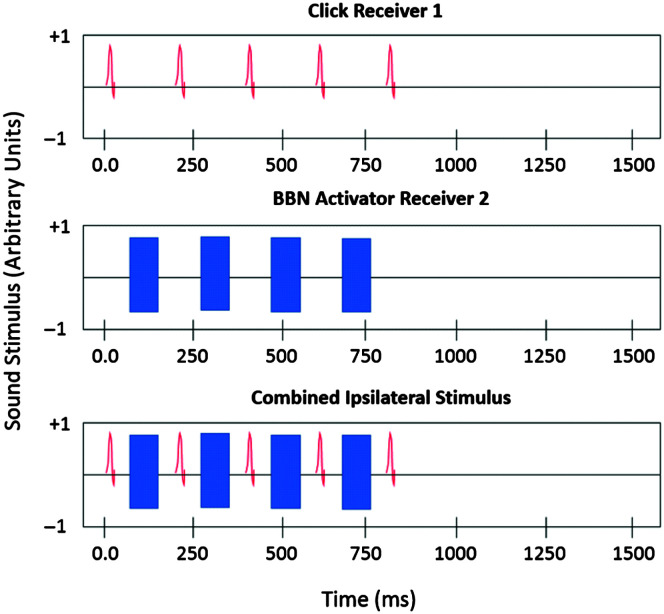
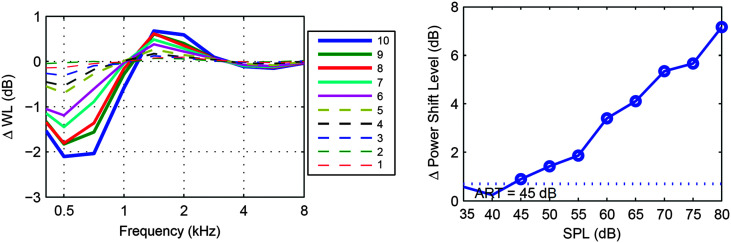
Following pure-tone audiometry and clinical immittance, ipsilateral wideband ASR measurements were obtained using an Interacoustics Wideband Research System (Interacoustics, Inc.) with custom software (Keefe et al., 2010). Like the clinical ASR procedure, the wideband ASR procedure involves measuring a response elicited by a high-level stimulus. The clinical procedure measures a change in admittance with a single-frequency 226-Hz probe, and the wideband procedure measures a change in absorbed sound power across a range of frequencies using a wideband click as the probe. The probe assembly contained a high bandwidth receiver used to generate wideband clicks as a probe stimulus and a second receiver with the same bandwidth that allowed higher levels to generate BBN reflex activator signals (0.2–8.0 kHz). The initial elicitor level was 35 dB SPL and increased in 5-dB steps up to 80 dB SPL. The click-stimulus set (see Figure 1) was presented 2 times at each presentation level. The ipsilateral ASR was measured using the method of Keefe et al. (2017). Figure 1 shows the stimulus waveform in the ear canal. The ASR was classified as present or absent at a given activator presentation level based on multiple factors. Briefly, this was achieved by assessing the difference in absorbed sound power between the initial click and the subsequent four clicks following each broadband activator pulse over a low frequency range of 0.2–2.4 kHz. This absorbed-power shift in dB due to the ASR was measured by adding the absolute value of the shift in half-octave frequency steps starting at 0.2 kHz and ending at 2.4 kHz, which gave the cumulative magnitude of the shift. The shift was considered to be due to the ASR if the cumulative magnitude reached a criterion of 0.7 dB for a given activator level. This is similar in concept to the criterion of a 0.03-mmho shift in admittance to be considered a reflex for the 226-Hz probe tone. The shift in power also had to have the same shape across frequency for two BBN presentations at the same level to be considered a valid reflex response. The ASR threshold (ASRT) was marked as the lowest level of the activator noise at which the 0.7-dB cumulative magnitude criterion was reached while maintaining the same pattern of shift across frequency. Figure 2 shows sample reflex information obtained from one ear. ARGFs were obtained at ambient pressure for each ear.
Figure 1.
The ipsilateral wideband acoustic reflex stimulus waveform: The top panel shows a series of five wideband probe clicks presented every ~200 ms. The broadband noise (BBN) activator pulses (middle panel) were selected from a sampled white-noise signal that was low-pass filtered at 8.0 kHz and had a duration of 116 ms. The overall waveform (bottom panel) had a duration of 1.58 s with the stimuli presented in the initial 0.79 s. Based on Figure 1 from Schairer et al. (2007).
Figure 2.
Sample plots of wideband reflex growth obtained from a single ear. Left: The acoustic stapedius reflex absorbed power (delta WL) level shift across frequency at all activator levels: 1 (35 dB SPL) through 10 (80 dB SPL). The magnitude of the absorbed power shift grows with increasing activator level. Right: acoustic reflex growth function for the reflex response. The dotted line indicates the minimum cumulative absorbed power shift of 0.7 dB that the response must reach in order to be classified as a reflex. The acoustic reflex threshold (ART) for this ear is marked at 45 dB SPL.
After the wideband reflex procedure, cochlear function was assessed using transient evoked OAEs (TEOAEs) measured at ambient pressure using the same probe assembly and a double-evoked wideband chirp stimulus as described in Keefe et al. (2016). This double-evoked procedure allows for assessment of frequencies higher than those measured by standard clinical TEOAE methods. Calibration of this stimulus was completed in a polyethylene tube with an inner diameter equivalent to an average human ear canal and with a long enough length (30.5 cm) to be considered reflectionless (Goodman et al., 2009; Keefe et al., 2016; Putterman et al., 2017). The stimulus was presented at approximately 64 dB SPL and remained relatively constant across the desired measurement bandwidth of 0.7–8.0 kHz, with a chirp sweep across this spectrum of roughly 42 ms (Putterman et al., 2017). A maximum of 288 chirps were averaged across the test duration of 1 min. A signal-to-noise ratio (SNR) of the resulting OAE was calculated at every half octave across the measurement bandwidth.
Results
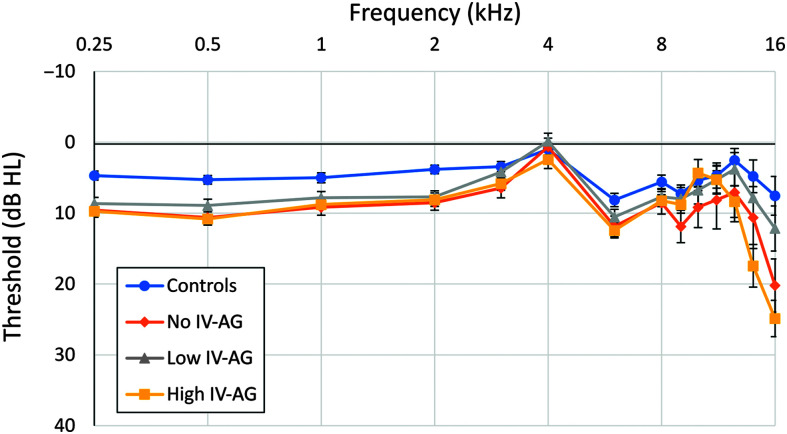
Table 1 contains descriptive statistics for pure-tone thresholds for the four subject groups, and they are plotted in Figure 3. The 29 subjects in the non-CF control group had the lowest mean 4-frequency pure-tone average (PTA; 0.5, 1.0, 2.0, and 4.0 kHz) at 3.8 dB HL, which was significantly lower than that of each of the three CF groups (F = 8.7, p < .001). No other group differences in 4-frequency PTA were significant. A general linear model (GLM) repeated-measures analysis of variance (ANOVA) for the eight standard audiometric frequencies showed a significant effect of frequency, but the Group x Frequency interaction was not significant (F = 1.50, p = .07). The control group also had the lowest mean high-frequency (HF) PTA at 5.4 dB, but there were no significant differences between groups (F = 2.46, p = .07). A GLM repeated-measures ANOVA for the six extended high frequencies showed a significant effect of frequency, the Group x Frequency interaction was significant (F = 5.06, p < .001), and there were significant group mean differences at 14 and 16 kHz. At 14 kHz, the group mean threshold for the High IV-AG group was 12.6 dB higher than for the Control group (F = 3.67, p = .014), and no other group comparisons were significant. At 16 kHz, the group mean threshold for the No IV-AG group was 12.7 dB higher than for the Control group. The group mean threshold for the High IV-AG group was 12.7 dB higher than for the Low IV-AG group and 17.3 dB higher than for the Control group (F = 7.26, p < .001), with no other group mean comparisons being significant. Also shown in Table 1, the mean age and sex composition were similar across the four groups. The mean and range for static acoustic admittance at 226 Hz are shown in the bottom row of Table 1 for each group. An ANOVA revealed that there was no significant difference between groups for the mean static acoustic admittance (F = 2.30, p = .08).
Figure 3.
Mean audiometric thresholds for the four groups at standard (0.25–8.0 kHz) and extended high frequencies (9.0–16.0 kHz). Error bars indicate ± 1 standard error of the mean. Audiometric thresholds at or below 25 dB HL fall within the normal range of hearing. IV = intravenous; AG = aminoglycoside.
The data in Table 2 show both the clinical and wideband ASRTs for the four groups. The first row of Table 2 shows the number of ears for each group that did not meet the inclusion criterion of a reflex threshold for the wideband method of ≤ 80 dB SPL, which was greatest for the No IV-AG group at nine ears and smallest for the High IV-AG group with three ears. Table 2 also shows that the mean ipsilateral reflex thresholds across groups for both the clinical and wideband methods were within about 3 and 2 dB, respectively. There were no statistically significant differences in mean clinical or wideband ASRTs among groups. Similar results were obtained for ASRTs obtained at tympanometric peak pressure (TPP; results not shown).
Table 2.
Clinical and wideband acoustic stapedius reflex threshold (ASRT) information.
| Variable | Controls | No IV-AG | Low IV-AG | High IV-AG |
|---|---|---|---|---|
| Ears excluded: Absent ASRT | 7 | 9 | 8 | 3 |
| Ears included | 51 | 24 | 37 | 37 |
| Mean clinical ASRT, dB SPL (SD) | 74.2 (6.3) | 71.5 (7.9) | 72.0 (9.2) | 70.9 (7.4) |
| Mean WB ASRT, dB SPL (SD) | 59.2 (8.0) | 61.5 (11.9) | 60.7 (8.9) | 60.4 (10.8) |
Note. The number of ears excluded because they did not have a wideband ASRT are shown in the first row of the table. No IV-AG = subjects with cystic fibrosis and no intravenous (IV) aminoglycoside exposure; Low IV-AG = subjects with cystic fibrosis and ≤ 40 weighted doses of aminoglycosides; High IV-AG = subjects with cystic fibrosis and > 40 weighted doses of aminoglycosides. WB = wideband.
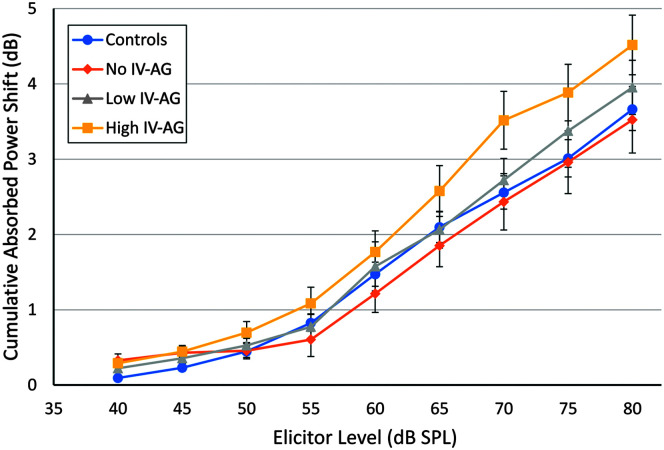
Figure 4 shows the mean ARGFs for the four groups for reflex elicitor levels from 40 to 80 dB SPL in 5-dB steps. For each group, reflex strength increased as a function of elicitor level. A GLM analysis (SPSS V22) was performed to examine the effect of ear on the average reflex growth across level for the four groups. The effect of ear was not significant, and so data were combined across ears and a GLM repeated-measures ANOVA was conducted to evaluate group differences for the cumulative reflex power shift across the nine stimulus levels. The effect of level was significant, but the Group x Level interaction was not significant (F = 1.14, p = .29). Linear best-fit lines were calculated for the ARGFs for each group. The slope of the best fit line (multiplied by 5 for the 5-dB step size) for each group was 0.47, 0.43, 0.49, and 0.57 for the Control, No IV-AG, Low IV-AG, and High IV-AG groups, respectively. Although the High IV-AG group had the steepest ARGF, the differences in slopes among groups were not statistically significant.
Figure 4.
Acoustic reflex strength plotted by broadband noise elicitor level from 40 to 80 dB SPL. Error bars indicate ± 1 standard error of the mean. IV = intravenous; AG = aminoglycoside.
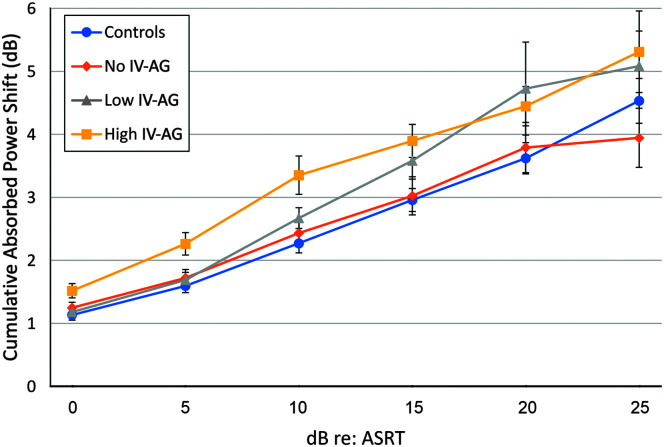
ARGFs were also analyzed relative to each participant's ASRT (see Figure 5). The rationale for aligning data in this way is that any absorbed power shift information occurring for elicitors below an individual subject's ASRT is unlikely to be a reflex response and may contribute noise to the growth functions. Therefore, fewer ears contribute to means at higher levels relative to threshold, and the levels vary across participants depending on the ASRT. The mean ARGFs were plotted relative to the ASRT with the lowest level (0 dB) equivalent to each individual ASRT followed by suprathreshold levels to 25 dB above the ASRT. Higher levels were not used due to the small number of ears with activator presentation > 25-dB re: reflex threshold. A GLM analysis was performed to examine the effect of ear on the reflex growth from ASRT averaged across levels for the four groups. The effect of ear was not significant; therefore, data were combined across ears and a one-way ANOVA was conducted to evaluate group differences for the average cumulative reflex power shift across levels from 0 to 25 dB re: the ASRT. The effect of group was significant (F = 3.41, p < .05). Bonferroni post hoc testing revealed that both the High IV-AG and low IV-AG groups showed significantly greater mean absorbed power shifts, or enhanced ARGFs, compared to the NH controls. No other group differences were significant. The slope of the best fit line for each group was 0.68, 0.58, 0.84, and 0.75 for the Control, No IV-AG, Low IV-AG, and High IV-AG groups, respectively. The Low IV-AG group had the highest slope at 0.84 dB, followed by the slope of the High IV-AG group at 0.75 dB. However, the differences in slopes among groups were not statistically significant.
Figure 5.
Acoustic reflex strength plotted by elicitor level relative to reflex threshold. Error bars indicate ± 1 standard error of the mean. ASRT = acoustic stapedius reflex threshold; IV = intravenous; AG = aminoglycoside.
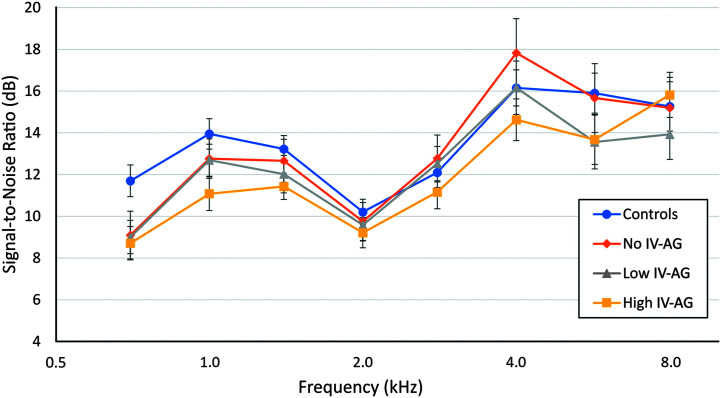
TEOAEs provide an index of cochlear outer hair cell function. TEOAE measurements were taken at both ambient pressure and TPP, but only the ambient condition results are reported here, as results were similar for measurements at TPP (not shown). A criterion of +3 dB SNR or greater was used to determine if an OAE was present at a given frequency. The TEOAE SNRs at half-octave frequencies from 0.7 to 8.0 kHz meeting this criterion are plotted in Figure 6 for the four groups. The percent of cases for which ears in each group failed to meet the + 3-dB criterion at a given frequency was Controls, 4%; No IV-AG, 4%; Low IV-AG, 7%; and High IV-AG, 8%. A GLM repeated- measures ANOVA was conducted for the TEOAE SNR. The effect of frequency was significant, but the Group x Frequency interaction was not significant (F = .54, p = .65). An analysis was also conducted for TEOAE signal level. There was also no significant Group x Frequency interaction (not shown).
Figure 6.
Double-evoked chirp mean signal-to-noise ratios at half octaves from 0.7 to 8.0 kHz at ambient pressure for the four groups. A criterion of +3 dB SNR or greater was used to determine if an otoacoustic emission was present at a given frequency. IV = intravenous; AG = aminoglycoside.
Discussion
The groups differed in extended HF thresholds. At 14 kHz, the significant group mean difference was the High IV-AG group having a higher mean threshold than the Control group. At 16 kHz, groups with IV exposure had higher reflex thresholds than Controls, and the greater the IV exposure, the higher the threshold. Interestingly, at 16 kHz, the No IV-AG group also had a higher mean threshold than Controls. These group mean threshold differences do not occur for frequencies close to the upper frequency cutoff for the 0.2- to 8-kHz BBN; thus, it is not clear how this threshold difference could affect reflex growth, except that it indicates peripheral auditory damage compared to the Control group. Table 1 shows that mean age and middle ear function are similar, as well as PTAs for standard and extended HF thresholds. Moreover, the mean clinical and wideband ASRTs are similar across groups, which rules out a difference between groups in reflex threshold as a source of the disparities in ARGF. Finally, the TEOAE results support the findings with extended HF thresholds in that twice as many ears failed to meet the +3 dB SNR criterion at various frequencies for Low IV-AG and High IV-AG compared to controls and No IV-AG groups (4% vs. ~8% of cases). This and the audiometric data suggest that the CF subjects with IV AG histories likely had outer hair cell damage, but it is not clear how this could account for the enhanced ARGF findings in this study.
Participants with CF who had high and low IV-AG exposure histories had significantly greater shifts in mean cumulative absorbed power for the acoustic reflex compared to the control group and the No IV-AG CF group as measured from the ASRT to ASRT +25 dB in 5-dB steps. This same trend was seen when the ARGFs were measured relative to the activator level rather than relative to the ASRT. These results demonstrate a pattern of an enhanced ARGF in CF patients exposed to IV-AGs compared to CF patients and controls with no IV-AG exposure. This is opposite of the expected finding of a shallow ARGF if ototoxicity would cause synaptopathy similar to that reported in human studies of noise-induced synaptopathy (Wojtczak et al., 2017). Thus, AG-induced ototoxicity in humans in the presence of normal hearing may result in a different mechanism of auditory dysfunction than that induced by high noise exposure.
The increased reflex magnitude could be a sign of central gain, where the auditory system responds to peripheral damage, albeit minimal according to the audiogram, with central hyperactivity. This theory is a proposed mechanism for central tinnitus and hyperacusis generation, as a maladaptive response to reduced sensory input (Noreña, 2011). Animal studies have demonstrated ototoxic damage to the ventral cochlear nucleus (Xu et al., 2009), which is part of the acoustic reflex arc and may control some aspects of central gain (Cao et al., 2019). In humans, temporary acoustic deprivation has been shown to induce changes in the acoustic reflex related to increased central gain (Brotherton et al., 2017). It is possible that, in the IV-AG groups, both central and peripheral (synaptopathy) damage was present, but that central effects predominated.
Although the findings of this study suggest a link between IV-AG exposure and central auditory dysfunction based on a physiological test (ARGF), functional effects in these participants with normal hearing such as reduced speech perception in noise or auditory phenomena such as tinnitus or hyperacusis were not evaluated. Thus, the connection between ototoxicity and some form of “hidden hearing loss” cannot be established based on this study. Another consideration is that some patients may have had incomplete medical records or had IV doses that were not recorded. In addition, inhaled AGs were not included in the lifetime dose counts. This method of administration is considered less ototoxic but may still carry some risk of HF hearing loss or tinnitus. Information about other possible confounding variables such as noise and concomitant drug exposure was not gathered during data collection, and excessive noise or other medications could have a synergistic effect on ototoxic damage from AGs. Finally, another limiting factor in the study was the smaller number of subjects with CF who were in the group with no IV-AG exposure. Future studies should investigate patients at younger ages to identify a greater number of participants without AG histories. Furthermore, future studies should strive to control for noise exposure history when evaluating effects of ototoxicity. Another question of interest is the prevalence of tinnitus and hyperacusis in patients with CF and whether these complaints are related to AG exposure.
Additional exploration of the functional consequences of AG ototoxicity, such as speech perception in noise, and its relationship with enhanced ARGFs is warranted (see Blankenship et al, 2021). Correlations with electrophysiological measures such as the Auditory Brainstem Response Wave I amplitude input–output function may provide an additional method for exploring this phenomenon (Bramhall et al., 2017).
Author Contributions
Martha R. Westman: Data curation (Lead), Methodology (Lead), Writing – original draft (Lead), Writing – review & editing (Lead). Daniel B. Putterman: Data curation (Equal), Methodology (Supporting), Project administration (Supporting), Supervision (Equal), Writing – review & editing (Equal). Angela C. Garinis: Data curation (Equal), Formal analysis (Equal), Methodology (Equal), Supervision (Equal), Writing – review & editing (Equal). Lisa L. Hunter: Funding acquisition (Equal), Methodology (Equal), Resources (Equal), Writing – review & editing (Equal). M. Patrick Feeney: Funding acquisition (Equal), Supervision (Equal), Methodology (Equal), Resources (Equal), Writing – review & editing (Equal).
Acknowledgments
This work was funded by the National Institute on Deafness and Other Communication Disorders Grant DC010202 (awarded to M. P. Feeney, L. L. Hunter, & D. H. Keefe) and DC008764 (awarded to M. P. Feeney). The views expressed above are those of the authors and do not represent the views of the National Institutes of Health or the Department of Veterans Affairs. Portions of this paper were presented at the 2019 9th Annual Biennial NCRAR Conference, Portland, OR, and at the 2019 47th Annual Scientific and Technology Meeting of the American Auditory Society, Scottsdale, AZ.
Funding Statement
This work was funded by the National Institute on Deafness and Other Communication Disorders Grant DC010202 (awarded to M. P. Feeney, L. L. Hunter, & D. H. Keefe) and DC008764 (awarded to M. P. Feeney).
References
- American National Standards Institute. (2018). Specifications for audiometers (ANSI S3.6-2018).
- American National Standards Institute. (2020). Specifications for instruments to measure aural acoustic impedance and admittance (Aural Acoustic Immittance) (ANSI S3.39-1987, R2020).
- Avan, P. , Erre, J. P. , da Costa, D. L. , Aran, J. M. , & Popelár, J. (1996). The efferent-mediated suppression of otoacoustic emissions in awake guinea pigs and its reversible blockage by gentamicin. Experimental Brain Research, 109(1), 9–16. https://doi.org/10.1007/BF00228621 [DOI] [PubMed] [Google Scholar]
- Blankenship, C. M. , Hunter, L. L. , Feeney, M. P. , Cox, M. , Bittinger, L. , Garinis, A. C. , Lin, L. , McPhail, G. , & Clancy, J. P. (2021). Functional impacts of aminoglycoside treatment on speech perception and extended high-frequency hearing loss in a pediatric cystic fibrosis cohort. American Journal of Audiology. Advance online publication. https://doi.org/10.1044/2020_AJA-20-00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall, N. F. , Konrad-Martin, D. , McMillan, G. P. , & Griest, S. E. (2017). Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear and Hearing, 38(1), e1–e12. https://doi.org/10.1097/AUD.0000000000000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton, H. , Plack, C. J. , Schaette, R. , & Munro, K. J. (2017). Using acoustic reflex threshold, auditory brainstem response and loudness judgments to investigate changes in neural gain following acute unilateral deprivation in normal hearing adults. Hearing Research, 345, 88–95. https://doi.org/10.1016/j.heares.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Cao, X. J. , Lin, L. , Sugden, A. U. , Connors, B. W. , & Oertel, D. (2019). Nitric oxide-mediated plasticity of interconnections between T-Stellate cells of the ventral cochlear nucleus generate positive feedback and constitute a central gain control in the auditory system. Journal of Neuroscience, 39(31), 6095–6107. https://doi.org/10.1523/JNEUROSCI.0177-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart, R. , & Jerger, J. F. (1959). Preferred method for clinical determination of pure-tone thresholds. Journal of Speech and Hearing Disorders, 24(4), 330–345. https://doi.org/10.1044/jshd.2404.330 [Google Scholar]
- Cheng, A. G. , Johnston, P. R. , Luz, J. , Uluer, A. , Fligor, B. , Licameli, G. R. , Kenna, M. A. , & Jones, D. T. (2009). Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngology—Head & Neck Surgery, 141(1), 86–90. https://doi.org/10.1016/j.otohns.2009.03.020 [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation. (2020). Cystic Fibrosis Foundation Patient Registry: 2018 annual data report. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2018-Patient-Registry-Annual-Data-Report.pdf
- da Costa, D. L. , Erre, J. P. , & Aran, J. M. (1998). Aminoglycoside ototoxicity and the medial efferent system: I. Comparison of acute and chronic gentamicin treatments. Audiology, 37(3), 151–161. https://doi.org/10.3109/00206099809072969 [DOI] [PubMed] [Google Scholar]
- Feeney, M. P. , & Schairer, K. S. (2015). Acoustic stapedius reflex measurements chapter 10 . In Katz J., Chasin M., English K., Hood L. J., & Tillery K. L. (Eds.), Handbook of clinical audiology (7th ed., pp. 165–186). Lippincott Williams & Wilkins. [Google Scholar]
- Garinis, A. C. , Cross, C. P. , Srikanth, P. , Carroll, K. , Feeney, M. P. , Keefe, D. H. , Hunter, L. L. , Putterman, D. B. , Cohen, D. M. , Gold, J. A. , & Steyger, P. S. (2017). The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. Journal of Cystic Fibrosis, 16(3), 401–409. https://doi.org/10.1016/j.jcf.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, S. S. , Fitzpatrick, D. F. , Ellison, J. C. , Jesteadt, W. , & Keefe, D. H. (2009). High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans. The Journal of the Acoustical Society of America, 125(2), 1014–1032. https://doi.org/10.1121/1.3056566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth, M. E. , Ricci, A. J. , & Cheng, A. G. (2011). Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. International Journal of Otolaryngology, 2011, Article ID 937861. https://doi.org/10.1155/2011/937861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, M. , García-Mateo, N. , Čusak, A. , López-Hernández, I. , Fernández-Martínez, M. , Müller, M. , Rüttiger, L. , Singer, W. , Löwenheim, H. , Kosec, G. , Fujs, Š. , Martínez-Martínez, L. , Schimmang, T. , Petković, H. , Knipper, M. , & Durán-Alonso, M. B. (2019). Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Scientific Reports, 9(1), Article number 2410. https://doi.org/10.1038/s41598-019-38634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M. , Karasawa, T. , & Steyger, P. S. (2017). Aminoglycoside-induced cochleotoxicity: A review. Frontiers in Cellular Neuroscience, 11, 308. https://doi.org/10.3389/fncel.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H. , Feeney, M. P. , Hunter, L. L. , & Fitzpatrick, D. F. (2016). Comparisons of transient evoked otoacoustic emissions using chirp and click stimuli. The Journal of the Acoustical Society of America, 140(3), 1949–1973. https://doi.org/10.1121/1.4962532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H. , Feeney, M. P. , Hunter, L. L. , & Fitzpatrick, D. F. (2017). Aural acoustic stapedius-muscle reflex threshold procedures to test human infants and adults. Journal of the Association for Research in Otolaryngology, 18(1), 65–88. https://doi.org/10.1007/s10162-016-0599-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H. , Fitzpatrick, D. , Liu, Y. W. , Sanford, C. A. , & Gorga, M. P. (2010). Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction. Hearing Research, 263(1–2), 52–65. https://doi.org/10.1016/j.heares.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin, D. , Helt, W. J. , Reavis, K. M. , Gordon, J. S. , Coleman, L. L. , Bratt, G. W. , & Fausti, S. A. (2005). Ototoxicity: Early detection and monitoring. The ASHA Leader, 10(7). https://doi.org/10.1044/leader.FTR1.10072005.1 [Google Scholar]
- Kujawa, S. G. , & Liberman, M. C. (2015). Synaptopathy in the noise exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research, 330(Part B), 191–199. https://doi.org/10.1016/j.heares.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Hang, L. , & Ma, Y. (2016). FGF22 protects hearing function from gentamycin ototoxicity by maintaining ribbon synapse number. Hearing Research, 332, 39–45. https://doi.org/10.1016/j.heares.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Li, H. , & Steyger, P. S. (2011). Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Scientific Reports, 1, Article number 159. https://doi.org/10.1038/srep00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, M. C. , & Guinan, J. J., Jr. (1998). Feedback control of the auditory periphery: Anti-masking effects of middle ear muscles vs. olivocochlear efferents. Journal of Communication Disorders, 31(6), 471–483. https://doi.org/10.1016/S0021-9924(98)00019-7 [DOI] [PubMed] [Google Scholar]
- Liu, K. , Jiang, X. , Shi, C. , Shi, L. , Yang, B. , Shi, L. , Xu, Y. , Yang, W. , & Yang, S. (2013). Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Molecular Neurobiology, 48(3), 647–654. https://doi.org/10.1007/s12035-013-8454-2 [DOI] [PubMed] [Google Scholar]
- Lobarinas, E. , Salvi, R. , & Ding, D. (2016). Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. Journal of the Association for Research in Otolaryngology, 17(2), 89–101. https://doi.org/10.1007/s10162-015-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, R. B. (2001). Administration of aerosolized antibiotics in cystic fibrosis patients. Chest, 120(3), 107S–113S. https://doi.org/10.1378/chest.120.3_suppl.107S [DOI] [PubMed] [Google Scholar]
- Mulheran, M. , Degg, C. , Burr, S. , Morgan, D. W. , & Stableforth, D. E. (2001). Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrobial Agents and Chemotherapy, 45(9), 2502–2509. https://doi.org/10.1128/AAC.45.9.2502-2509.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña, A. J. (2011). An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neuroscience & Biobehavioral Reviews, 35(5), 1089–1109. https://doi.org/10.1016/j.neubiorev.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Pauna, H. F. , Monsanto, R. C. , Kurata, N. , Paparella, M. M. , & Cureoglu, S. (2017). Changes in the inner ear structures in cystic fibrosis patients. International Journal of Pediatric Otorhinolaryngology, 92, 108–114. https://doi.org/10.1016/j.ijporl.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterman, D. B. , Keefe, D. H. , Hunter, L. L. , Garinis, A. C. , Fitzpatrick, D. F. , McMillan, G. P. , & Feeney, M. P. (2017). Assessing sensorineural hearing loss using various transient-evoked otoacoustic emission stimulus conditions. Ear and Hearing, 38(4), 507–520. https://doi.org/10.1097/AUD.0000000000000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer, K. S. , Ellison, J. C. , Fitzpatrick, D. , & Keefe, D. H. (2007). Wideband ipsilateral measurements of middle-ear muscle reflex thresholds in children and adults. The Journal of the Acoustical Society of America, 121(6), 3607–3616. https://doi.org/10.1121/1.2722213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy, P. , & Deffrennes, D. (1988). Aminoglycoside ototoxicity: Influence of dosage regimen on drug uptake and correlation between membrane binding and some clinical features. Acta Oto-Laryngologica, 105(5–6), 511–515. https://doi.org/10.3109/00016488809119511 [DOI] [PubMed] [Google Scholar]
- Valero, M. D. , Hancock, K. E. , & Liberman, M. C. (2016). The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hearing Research, 332, 29–38. https://doi.org/10.1016/j.heares.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero, M. D. , Hancock, K. E. , Maison, S. F. , & Liberman, M. C. (2018). Effects of cochlear synaptopathy on middle-ear muscle reflexes in unanesthetized mice. Hearing Research, 363, 109–118. https://doi.org/10.1016/j.heares.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak, M. , Beim, J. A. , & Oxenham, A. J. (2017). Weak middle-ear-muscle reflex in humans with noise-induced tinnitus and normal hearing may reflect cochlear synaptopathy. eNeuro, 4(6), ENEURO.0363-17.2017. https://doi.org/10.1523/ENEURO.0363-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Hu, H. T. , Jin, Z. , Chen, G. , Wang, W. X. , Fan, Y. L. , Anniko, M. , & Duan, M. (2009). Ototoxicity on cochlear nucleus neurons following systemic application of gentamicin. Acta Oto-Laryngologica, 129(7), 745–748. https://doi.org/10.1080/00016480802454716 [DOI] [PubMed] [Google Scholar]