Abstract
Purpose
The purpose of this study is to better understand the prevalence of ototoxicity-related hearing loss and its functional impact on communication in a pediatric and young adult cohort with cystic fibrosis (CF) and individuals without CF (controls).
Method
We did an observational, cross-sectional investigation of hearing function in children, teens, and young adults with CF (n = 57, M = 15.0 years) who received intravenous aminoglycoside antibiotics and age- and gender-matched controls (n = 61, M = 14.6 years). Participants completed standard and extended high-frequency audiometry, middle ear measures, speech perception tests, and a hearing and balance questionnaire.
Results
Individuals with CF were 3–4 times more likely to report issues with hearing, balance, and tinnitus and performed significantly poorer on speech perception tasks compared to controls. A higher prevalence of hearing loss was observed in individuals with CF (57%) compared to controls (37%). CF and control groups had similar proportions of slight and mild hearing losses; however, individuals with CF were 7.6 times more likely to have moderate and greater degrees of hearing loss. Older participants displayed higher average extended high-frequency thresholds, with no effect of age on average standard frequency thresholds. Although middle ear dysfunction has not previously been reported to be more prevalent in CF, this study showed that 16% had conductive or mixed hearing loss and higher rates of previous otitis media and pressure equalization tube surgeries compared to controls.
Conclusions
Individuals with CF have a higher prevalence of conductive, mixed, and sensorineural hearing loss; poorer speech-in-noise performance; and higher rates of multiple symptoms associated with otologic disorders (tinnitus, hearing difficulty, dizziness, imbalance, and otitis media) compared to controls. Accordingly, children with CF should be asked about these symptoms and receive baseline hearing assessment(s) prior to treatment with potentially ototoxic medications and at regular intervals thereafter in order to provide otologic and audiologic treatment for hearing- and ear-related problems to improve communication functioning.
Cystic fibrosis (CF) is the most common congenital life-threatening genetic disease in Caucasians occurring in one of 2,500 births and affecting over 70,000 individuals worldwide (Cystic Fibrosis Foundation, 2017; Kreicher et al., 2018). It is characterized by recurrent pulmonary infections that are commonly treated with intravenous aminoglycoside (IV-AG; amikacin, tobramycin, and gentamicin) and glycopeptide (e.g., vancomycin) antibiotics (Barclay et al., 1996; Becker & Cooper, 2013; Jiang et al., 2017; O'Sullivan et al., 2017). These antibiotics are cost-effective and highly efficient against gram-negative organisms, such as Pseudomonas aerugonisa, which are the underlying cause of pulmonary exacerbations in CF (Conway et al., 1985; Szaff et al., 1983). Approximately 46.6% of individuals with CF have Pseudomonas aerugonisa infections, 28.6% are chronic and necessitate repeated courses of IV-AG treatment, and the median age at first occurrence is 5.2 years (Cystic Fibrosis Foundation, 2017). While treatment with AGs alone or in conjunction with glycopeptides is common practice to treat the life-threatening bacterial infections, they have a well-documented ototoxic effect, resulting in irreversible damage to hearing and balance structures within the inner ear (Handelsman et al., 2017; Liu et al., 2013; Pauna et al., 2017; Sone et al., 1998). Ototoxicity can result as a direct effect of AGs, with gentamicin considered to be primarily vestibulotoxic, and amikacin and tobramycin considered to be mainly cochleotoxic. However, in theory, they can all induce damage to both parts of the inner ear (Jiang et al., 2017; Selimoglu, 2007). Glycopeptide antibiotics (e.g., vancomycin) can indirectly cause hearing loss due to their synergistic effect with AGs. For example, vancomycin is highly nephrotoxic, resulting in decreased kidney function/renal clearance of AGs, potentially leading to an increased susceptibility to developing hearing loss (Filippone et al., 2017).
The cochleotoxic effect of AGs, with or without glycopeptides, is characterized by sensorineural hearing loss (SNHL) that originates in the basal turn of the cochlea. High-frequency outer hair cell loss occurs first, followed by inner hair cell loss, after which damage progresses to the lower frequencies (Fausti et al., 1984; Guthrie, 2008; Huizing & de Groot, 1987). Thus, extended high-frequency (EHF; > 8 kHz) audiometry is integral to ototoxicity monitoring due to the progression of hearing loss from high to low frequencies (Fausti et al., 1993, 1992, 1994). Fausti et al. (1999) identified a sensitive (frequency) range for ototoxicity (SRO) that involves the measurement of pure-tone thresholds in one-sixth octaves up to the highest frequency that is audible to the individual. This shortened ototoxicity monitoring test decreases overall test time to approximately 10 min and maintains a 90% detection rate of significant changes in hearing (Fausti et al., 2003, 1999). Even though the use of EHF audiometry was first reported in the 1980s (Ahonen & McDermott, 1984; Fausti et al., 1984; McRorie et al., 1989) and the “sensitive (frequency) range for ototoxicity” method for quickly detecting ototoxicity is available, nearly 40 years later, EHF audiometry is still not routinely used in CF clinics. A survey of Cystic Fibrosis Foundation Accredited Care Centers and Affiliated Programs reported that, while 43% of clinics performed audiologic evaluations, it is unclear if EHFs were included and some centers only measured thresholds if the patient was symptomatic (Van Meter et al., 2009). More recently, Prescott (2011) reported that 39% of pediatric CF programs do not monitor for ototoxicity at all, 32% of clinics only monitor hearing in the standard frequencies (SFs; 0.25–8 kHz), and only 46% include EHF audiometry. Potential explanations for the absence of routine EHF testing include the lack of clear ototoxicity monitoring guidelines and protocols, time constraints, audiology equipment and staffing limitations, absence of EHF audiometry reimbursement codes, and the absence of outcome data that indicate that the preservation of EHF hearing is important and has a positive impact on overall quality of life.
The prevalence of hearing loss in children with CF ranges from 0% to 44% (Al-Malky et al., 2015; Thomsen et al., 1979) and extends up to 59% in adults (Garinis et al., 2017; Scheenstra et al., 2010; Zettner & Gleser, 2018). The substantial variability in prevalence rates across studies is likely caused by differences in hearing loss criteria and audiologic test protocol, population studied, individual susceptibility, and factors related to AG treatment (type of AG and potential co-administration of glycopeptides, mode of administration, total body clearance of the drug, number of doses). In general, studies that only included SF audiometry found lower rates of hearing loss (Cheng et al., 2009; Forman-Franco et al., 1979; Ozcelik et al., 1996; Stavroulaki et al., 2002) compared to studies that included EHF audiometry (Al-Malky et al., 2015, 2011; Garinis et al., 2017; Martins et al., 2010; Pedersen et al., 1987). Furthermore, pediatric patients with minimal AG exposure show a lower prevalence of hearing loss (3%–24%), compared to individuals with higher AG exposure (40%–44%; Al-Malky et al., 2015; Cheng et al., 2009). However, less than half of published pediatric studies of AG-associated ototoxicity include EHF audiometry. This is likely because most pediatric CF studies are retrospective. Since EHF audiometry is not routinely used in clinical practice, the onset and true prevalence of ototoxic hearing loss is likely underestimated.
Early detection of hearing loss is extremely important in pediatric patients due to its documented impact on speech and language development, literacy development (Yoshinaga-Itano, 1999, 2003), and scholastic achievement (Moeller et al., 2007). Children and teens with CF will likely experience EHF hearing loss that accumulates over time into adulthood (Al-Malky et al., 2015, 2011; Garinis et al., 2017). In contrast to the common misconception that EHFs contribute very little to speech understanding, substantial information exists at frequencies above 8 kHz that can be used for localization, speaker identification, and phoneme identification (Best et al., 2005; Brungart & Simpson, 2009; Heffner & Heffner, 2008; Monson et al., 2019). For example, fricatives /ch, f, j, s, sh, th, v, and z/ contain differential spectral information from 3 to 10 kHz that can be used for phoneme identification (Alexander et al., 2014). In adults, poorer EHF thresholds are significantly correlated with poorer speech understanding in noise (Cameron & Dillon, 2007; Yeend et al., 2019) and result in an increased self-report of listening difficulties (Gatehouse & Noble, 2004; Motlagh Zadeh et al., 2019). Despite the aforementioned importance of EHF hearing, there is little, if any, published data on speech-in-noise performance in individuals with CF and AG exposure, a population that typically exhibits EHF loss. Furthermore, children and adults are frequently immersed in noisy environments, such as the classroom, workplace, and social settings. Therefore, the functional impact of hearing loss on speech understanding in noise is highly relevant. Other notable limitations of previous studies include absence of EHF audiometry or bone-conduction (BC) thresholds, which limits their ability to accurately identify the presence and classify type of hearing loss. Most studies did not include an age-matched control group to make a direct and accurate comparison to the general population. Lastly, as previously mentioned, none included a functional measure that examines the effect of hearing loss on daily communication or evaluation of patient and parent perception of perceived hearing difficulties. These patient-reported outcomes are critical for improving patient-centered clinical care approaches and for improving communication between the patient and their clinical care team.
The goal of this study was to better understand the prevalence of ototoxicity-related hearing loss and its functional impact on communication in a pediatric cohort with CF. In order to achieve this goal, we examined SF and EHF audiometry (air conduction [AC] and BC), tympanometry, and middle ear muscle reflexes (MEMRs) to fully describe hearing function in children with CF and age- and gender-matched controls. A standardized pediatric measure of speech in noise as well as a self-report questionnaire for patients and parents on perceived hearing and balance difficulties and the functional impact of hearing loss on communication were also completed.
Materials and Method
Participants
The study was part of a larger longitudinal project to evaluate the prevalence of hearing loss in children, teens, and young adults with CF in relation to the concentration of AG drug exposure and toxicologic response (pharmacodynamics), including ototoxic outcomes. Children and young adults diagnosed with CF (n = 57, M = 15.0 years, range: 6.0–21.0) who received intravenous tobramycin or amikacin antibiotics, alone or in combination with vancomycin, were recruited from the CF inpatient unit at Cincinnati Children's Hospital Medical Center (CCHMC). Age- and gender-matched controls (n = 61, M = 14.6 years, range: 7.5–21.1) were recruited from the same hospital through internal staff e-mails and outpatient study flyers. Additional eligibility criteria for both the CF and control groups included ages between 6 and 21 years and the ability to complete a conventional behavioral hearing test. Controls were not excluded based on hearing status in order to provide a more accurate representation of the hearing levels of the general population. They had no history of AG treatment and no CF diagnoses. The study was approved by the CCHMC Institutional Review Board. Informed parental consent and child assent (for ages 9–17 years) or patient consent (ages 18 years and older) was obtained prior to enrollment. All participants were reimbursed for their participation.
Test Protocol
All participants completed comprehensive diagnostic assessments, including otoscopy, 226-Hz tympanometry, MEMRs, SF and EHF audiometric assessment, and speech understanding in quiet and noise. A hearing and balance questionnaire was administered to determine if the participant or the parent reported any hearing difficulties, tinnitus, balance disturbance, history of otitis media, pressure equalization (PE) tubes, or previous hearing exams. Additional details regarding onset, frequency and type of symptoms, and family history of hearing loss were also queried (see questionnaire in the Appendix). This was a nonvalidated questionnaire, comparable to a case history or intake questionnaire that would be filled out during a clinical audiology appointment. All test procedures were completed in a double-walled sound booth (Industrial Acoustics Company). Otoscopy was completed first to ensure the ear canal was patent, and if necessary, cerumen was removed. The test protocol took approximately 1 hr to complete. Individuals with CF completed testing while they were inpatient at CCHMC, while controls were tested during an outpatient research appointment.
Audiometric Assessment
AC thresholds were obtained with an Equinox Audiometer (Interacoustics, Inc.) and Sennheiser HDA-300 circumaural headphones in the SF range at octave test frequencies from 0.25 to 8 kHz and in the EHF range (10, 12.5, 14, and 16 kHz). Calibration was completed according to ISO 389.8 (International Organization for Standardization, 2004) for SF and ISO 389–1 (International Organization for Standardization, 2017) for EHF. BC thresholds were measured using a RadioEar B–71 bone oscillator (RadioEar Corp.) at 0.25, 0.5, 1, 2, and 4 kHz if AC thresholds were ≥ 20 dB HL with appropriate narrowband masking in the contralateral ear. A modified Hughson-Westlake procedure with a 5-dB step size was used to measure threshold. For both the SF and EHF range, audiometric thresholds were used to categorize each ear based on the presence, degree, and type of hearing loss as well as the ear affected (left, right, bilateral). The degree of hearing loss was classified according to the Goodman (1965) and Northern and Downs (1984) criteria for the pure-tone average for 0.5, 1, and 2 kHz: normal (0–15 dB HL), slight (16–25 dB HL), mild (26–40 dB HL), moderate (41–55 dB HL), moderately severe (56–70 dB HL), severe (71–90 dB HL), and profound (91 + dB HL). The type of hearing loss was classified as follows:
Normal hearing (NH): AC and BC thresholds within the normal range (≤ 15 dB HL).
Conductive hearing loss (CHL): BC thresholds of ≤ 15 dB HL, AC thresholds of > 15 dB, and an air–bone gap of 10 dB at two frequencies or 20 dB at one frequency. For some participants, abnormal tympanometry values were used to diagnose conductive loss (see criteria below in 226-Hz tympanometry description).
-
SNHL: AC and BC thresholds of > 15 dB HL without an air–bone gap (defined under CHL) at any test frequency.
Mixed hearing loss (MHL): Presence of a sensorineural and CHL at the same audiometric test frequency or within the same ear.
Middle Ear Measures
Middle ear function was measured using traditional 226-Hz tympanometry using the Titan (Interacoustics, Inc.). Individuals were not excluded due to middle ear dysfunction. If BC thresholds were not measured (due to time restraints or patient fatigue), tympanometry values were used to determine if there was a conductive component to the hearing loss with the following normative ranges for tympanometric peak pressure (−100 to +30 daPa), admittance (0.3–1.5 mmho), and tympanometric width (30–105 daPa; Roup et al., 1998). At least two out of three values needed to be identified as abnormal (i.e., outside normative range) for the individual to be classified as having a conductive component to the hearing loss.
MEMRs
MEMRs were assessed using the Titan (Interacoustics, Inc.) clinical system alone or in combination with a research wideband absorbance technique with custom-designed recording system. MEMR thresholds were measured using a broadband noise stimulus in ipsilateral (same ear) and contralateral (opposite ear) conditions. In both systems, ear canal air pressure was adjusted to the peak tympanometric pressure obtained during wideband tympanometry (results not reported here). For the Interacoustics Titan clinical system, the broadband stimuli (0.5–8 kHz) were presented from 60 to 100 dB SPL in 5–dB steps. An ascending technique was used where the lowest intensity that evoked a reflex using a “very sensitive” criteria of 0.02 ml was marked as threshold. With the research wideband absorbance technique, MEMRs were measured using a pulsed-activator stimulus set that included four broadband noise activator (0.25–8 kHz) pulses that alternated with five wideband (0.25–8 kHz) clicks. Stimulus levels were calibrated in a 2-cm (HA-1) coupler and were presented in 5-dB steps from 60 to 120 dB peSPL. MEMRs measured with the research wideband absorbance technique used response averaging, artifact rejection, and signal processing techniques to measure threshold, onset latency, and amplitude growth. Additional details regarding the measurement and analysis procedures may be found in the study of Keefe et al. (2017). The control group had relatively equal percentages of MEMRs measured using the Titan (52%) compared to the wideband research technique (48%). In comparison, the CF group had a higher percentage of MEMRs measured with the wideband research technique (68%) compared to the Titan (32%). Since the wideband technique results in lower MEMR thresholds, a correction factor was applied in order to combine MEMR measured using the two different recording systems. The correction factor was based on MEMR thresholds measured from a group of children from a separate study using an identical MEMR test protocol (Hunter, Blankenship, et al., 2020). The children all had typical development and NH in the SF region. That normative sample included 48 children with typical development (93 ears) with NH that ranged in age from 6.5 to 14.5 years (M = 10.0 years). The correction factor applied to the research-based thresholds was 15.5 and 12.5 dB SPL for the ipsilateral and contralateral MEMR reflexes, respectively.
Speech Perception Testing
A speech reception threshold (SRT) was measured for each ear, using recorded spondees from the Central Institute for the Deaf W-1 adult or child word list (Auditec, 2015; Hirsch et al., 1952). Speech understanding in noise was evaluated using the Bamford–Kowal–Bench Speech-in-Noise (BKB-SIN) Test (Etymotic Research, 2005) measured at 50 dB HL in the monaural condition with one list pair administered to each ear. This is an adaptive measure with signal-to-noise ratio (SNR) values ranging from +21 to −6 dB and is representative of environments that occur in the classroom and everyday settings. The BKB-SIN sentences were recorded with a 44.1-kHz sampling rate, and a spectral analysis shows that they contain substantial signal energy above the electrical noise floor out to 12 kHz. The participants were instructed to repeat each sentence that they heard and to guess if they were unsure. The number of key words repeated correctly for each sentence was tallied and used to calculate the SNR in dB necessary for the individual to understand 50% of the sentence (SNR-50). The signal-to-noise ratio loss (SNR-Loss) was calculated as the individual SNR-50 minus age-matched normative SNR-50 values from the BKB-SIN manual. The SNR-Loss is used to determine how much greater an SNR is needed for the individual to have equivalent performance to their age-matched peers. Additionally, the SNR-Loss can be categorized as follows to indicate the degree of difficulty understanding speech in noise (Etymotic Research, 2005):
Normal is 0–3 dB.
Mild is greater than 3–7 dB
Moderate is greater than 7–15 dB.
Severe is greater than 15 dB.
Statistical Analysis
Descriptive statistics were used to summarize demographics and outcome measurements to identify any errors and outliers. Interval variables were summarized by central tendency and dispersion, and categorical variables were described by frequencies and percentages. Two-sample t test, chi-square test, or Fisher exact tests were performed as appropriate to compare the demographics between the CF and control groups. Box plots were created to study the distribution of the outcome variables. Mixed models were conducted to study the differences between the CF and control groups, with test ear included as a repeated measure and demographic factors included as covariates (age at test, sex, and race). Where appropriate, test frequency was included as a repeated measure, and hearing status was included as a fixed-effect factor. Interaction terms were also studied and, in some cases, were removed from the final adjusted model for a parsimonious model due to their insignificance (p > .05). Sidak adjustment was applied for pairwise comparisons among the significant factors. Pearson correlations were used to assess the relationship between audiometric thresholds, SNR-Loss, and age at test. All data were collected and managed using REDCap, which is a secure web-based software platform (Harris et al., 2019, 2009) and then exported and formatted for analysis using SAS statistical software, Version 9.3 (SAS Institute). Two-sided significance level was set at .05 for all analyses.
Results
Cohort Characteristics
A total of 74 individuals with CF were approached for the study; however, 10 individuals were not interested in participating (14%), and seven individuals were consented but were unable to complete testing prior to discharge (9%), resulting in 57 individuals who were enrolled and completed test procedures (77%). A control group of children without CF (control; n = 61) were enrolled and completed testing. Therefore, the study included 118 participants, 57 individuals with CF (49%), and 61 controls (51%). For individuals with CF, the mean age at test was 15.0 years (SD = 3.6, range: 6.0–21.0), 42% were males, and 98% were Caucasian. In the control group, the mean age at test was 14.6 years (SD = 2.9, range: 7.5–21.1), 46% were males, and 85% were Caucasian. There were no significant group differences in age at test and sex; however, the control group had a higher number of non-Caucasians than the CF group. See Table 1 for additional group characteristics.
Table 1.
Study sample demographics, questionnaire, and hearing loss characteristics for all participants combined, as well as the cystic fibrosis (CF) and control groups.
| Variable | All | CF | Control | p |
|---|---|---|---|---|
| No. of participants | 118 | 57 | 61 | |
| Age at test | .534 a | |||
| M (SD) | 14.77 (3.28) | 14.96 (3.63) | 14.59 (2.94) | |
| Range | 6.0–21.1 | 6.0–21.0 | 7.5–21.1 | |
| Sex b | .678 c | |||
| Male | 52 (44.07) | 24 (42.11) | 28 (45.90) | |
| Female | 66 (55.93) | 33 (57.89) | 33 (54.10) | |
| Race b | .017 d | |||
| Caucasian | 108 (91.53) | 56 (98.25) | 52 (85.25) | |
| Non-Caucasian | 10 (8.47) | 1 (1.75) | 9 (14.75) | |
| Hearing and balance questionnaire b | ||||
| Hearing problem | 23 (19.49) | 18 (31.57) | 5 (8.19) | .001 c |
| Tinnitus | 39 (33.05) | 30 (52.63) | 9 (16.07) | < .001 c |
| Balance issues | 23 (19.49) | 18 (31.57) | 5 (8.92) | .003 c |
| History of otitis media | 40 (33.89) | 24 (42.10) | 16 (26.22) | .049 c |
| PE tubes | 16 (13.55) | 9 (15.78) | 7 (11.47) | .412 c |
| Previous hearing test | 97 (82.20) | 47 (82.45) | 50 (89.28) | .919 c |
| Hearing status e | ||||
| Normal | 126 (53.39) | 49 (42.98) | 77 (63.11) | — |
| Hearing loss | 110 (46.61) | 65 (57.02) | 45 (36.89) | — |
| Hearing loss frequency range e | ||||
| Standard frequency | 64 (27.59) | 43 (37.72) | 21 (17.21) | — |
| Extended high frequency | 91 (39.22) | 54 (47.37) | 37 (30.33) | — |
| Degree of hearing loss e | ||||
| Slight | 53 (22.46) | 24 (21.05) | 29 (23.77) | — |
| Mild | 27 (11.44) | 15 (13.16) | 12 (9.84) | — |
| Moderate | 21 (8.09) | 18 (15.79) | 3 (2.46) | — |
| Moderately severe | 6 (2.54) | 5 (4.39) | 1 (0.82) | — |
| Severe | 3 (1.27) | 3 (2.63) | 0 (0.00) | — |
| Type of hearing loss e | ||||
| Conductive | 8 (3.39) | 6 (5.26) | 2 (1.64) | — |
| Sensorineural | 82 (34.75) | 47 (41.23) | 35 (28.69) | — |
| Mixed | 18 (7.63) | 12 (10.53) | 6 (4.92) | — |
| Undetermined | 2 (0.85) | 0 (0.00) | 2 (1.64) | — |
Note. Bold italics indicate significant p values. Em dashes indicate data not completed. PE = pressure equalizer.
Two-sample t test.
Number (%) of participants.
Chi-square test.
Fisher's exact test.
Number (%) of ears.
Hearing and Balance Questionnaire
Parent and participant hearing and balance questionnaire responses were combined to determine an overall response for each question for each participant. Questions regarding hearing concerns, tinnitus, and balance issues were binary (yes/no), while history of otitis media, PE tubes, and previous hearing test were categorical (yes/no/not sure). Additional details regarding onset, frequency, and type of symptoms were also queried (see questionnaire in the Appendix). Table 1 displays the number of individuals within the CF and control groups and corresponding percentages that indicated “yes” to symptoms included in the questionnaire. Chi-square tests were performed to examine differences in hearing and balance concerns between the CF and control groups. Individuals with CF reported significantly more hearing concerns (CF = 32%, control = 8%), tinnitus (CF = 53%, control = 16%), and balance problems, including dizziness, unsteadiness, or imbalance (CF = 32%, control = 9%) compared to controls. Individuals with CF were also more likely to have a history of middle ear infections than controls (CF = 42%, control = 26%). Although nonsignificant, the CF group reported a slightly higher history of PE tubes (CF = 16%, control = 11%) with controls reporting a similar percentage of having a previous hearing test (CF = 82%, control = 89%).
Audiometric Thresholds
Descriptive statistics for prevalence, degree, and type of hearing loss are displayed in Table 1. Individuals with CF had a significantly higher prevalence of hearing loss in either the SF or EHF ranges (57%) compared to controls (37%). Within the SF range, 38% of the CF group and 17% of the control group had hearing loss. Of the individuals with SF hearing loss in the CF group, most had slight-to-mild hearing loss (88%), while a smaller percentage (12%) had moderate-to-moderately-severe hearing loss. In the control group, the degree of loss only ranged from slight to mild, with no greater degrees of hearing loss present. In the EHF region, both groups displayed even higher rates of hearing loss, with 47% of the CF group and 30% of the control group displaying EHF hearing loss. The CF group had almost equal percentages of slight-to-mild hearing loss (52%) and moderate-to-severe hearing loss (48%). In contrast, most controls had hearing loss in the slight-to-mild range (89%), with few ears in the moderate-to-moderately-severe range (11%). When examining the degree of hearing loss across all audiometric frequencies, the CF and control groups have similar numbers of slight and mild hearing losses; however, individuals with CF had much greater percentages of moderate and greater degrees of hearing loss (CF = 23%, control = 3%). With regard to the type of hearing loss, 5% of the CF group had a CHL, 41% had SNHL, and 11% had MHL. Controls mainly had SNHL (29%), with smaller percentages of CHL (2%) and MHL (5%).
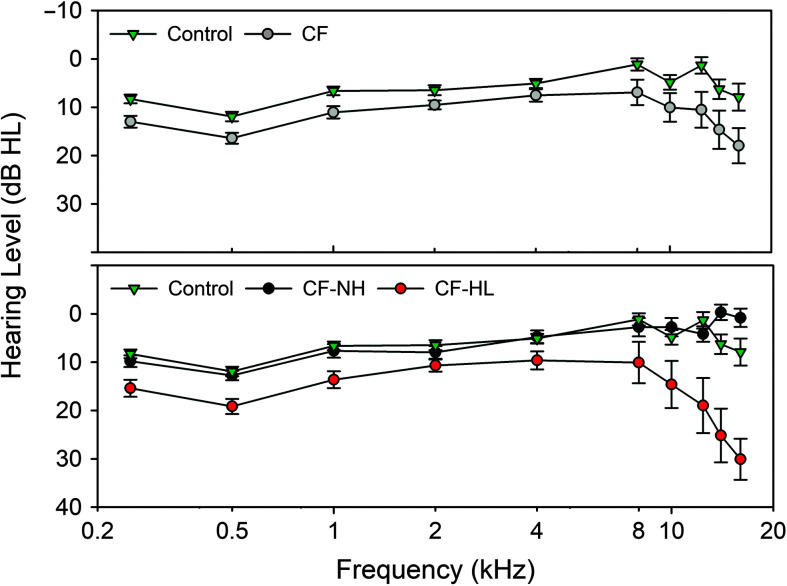
The CF and control groups mean audiometric thresholds and 95% confidence intervals are displayed as a function of test frequency in Figure 1 (top panel). The CF group had significantly poorer audiometric thresholds compared to controls across all test frequencies (p < .001; see Table 2). Audiometric thresholds varied significantly across frequency, with poorer thresholds at 0.25 and 0.5 kHz and in the EHF range (14–16 kHz, p < .001). The interaction between Group × Test Frequency was significant (p = .002), since the mean difference between groups was not constant across all test frequencies. With regard to race, Caucasians had significantly better audiometric thresholds compared to non-Caucasians (p = .004). Minimal mean threshold differences (± 1 dB HL) were observed from 0.25 to 8 kHz. However, at 10 and 12.5 kHz, Caucasians had mean thresholds that were 7–8 dB HL better than non-Caucasians, and at 14–16 kHz mean thresholds, they were 2–3 dB better. Main effects of test ear, sex, and age at test were not significant (p > .05).
Figure 1.
Standard and extended high-frequency audiometric thresholds measured in hearing level (dB HL) and calibrated according to ISO 389–8 and 389–1 (International Organization for Standardization, 2004, 2017). Top panel: Mean and 95% confidence interval (CI) for the cystic fibrosis (CF) and control groups. Bottom panel: Mean and 95% CI for controls with the CF group further classified into normal hearing (CF–NH) and hearing loss (CF–HL) groups.
Table 2.
Mixed model repeated-measures analysis, with p values and F test (degrees of freedom) displayed for factors included in the final model.
| Variable | Group | Test frequency | Group × Frequency | Hearing status | Group × Hearing Status | Ear | Age | Sex | Race |
|---|---|---|---|---|---|---|---|---|---|
| Audiometric threshold | < .001 | < .001 | .002 | — | — | .541 | .094 | .349 | .004 |
| F(df = 262–1524) | 162.18 | 37.12 | 2.94 | — | — | 0.37 | 2.81 | 0.88 | 8.50 |
| Tympanometry | |||||||||
| Peak pressure | .537 | — | — | .001 a | — | .593 | .291 | .002 | .351 |
| F(df = 217) | 0.38 | — | — | 14.67 | — | 0.29 | 1.12 | 10.00 | 0.87 |
| Tympanometric width | .156 | — | — | .614 a | — | .565 | .175 | .144 | .088 |
| F(df = 209–212) | 2.03 | — | — | 0.49 | — | 0.33 | 1.85 | 2.15 | 2.94 |
| Admittance | .002 | — | — | .127 a | .041 a | .612 | .096 | .733 | .299 |
| F(df = 211–215) | 10.35 | — | — | 2.09 | 3.23 | 0.26 | 2.80 | 0.12 | 1.09 |
| MEMR | |||||||||
| Ipsilateral | .042 | — | — | < .001 b | — | .352 | .051 | .972 | .392 |
| F(df = 191) | 4.20 | — | — | 14.19 | — | 0.87 | 3.86 | < 0.00 | 0.74 |
| Contralateral | .004 | — | — | .001 b | — | .800 | .224 | .583 | .462 |
| F(df =183–185) | 8.30 | — | — | 12.42 | — | 0.06 | 1.49 | 0.30 | 0.54 |
| Speech perception | |||||||||
| SNR-Loss | < .001 | — | — | .045 b | — | .328 | .899 | .017 | .302 |
| F(df = 181–182) | 23.95 | — | — | 4.07 | — | 0.96 | 0.02 | 5.83 | 1.07 |
| SRT | < .001 | — | — | — | — | .404 | .796 | .469 | .894 |
| F(df = 190–191) | 30.77 | — | — | — | — | 0.70 | 0.07 | 0.53 | 0.02 |
Note. Bold italics indicate significant p values. Em dashes indicate data not completed. MEMR = middle ear muscle reflex; SNR = signal-to-noise ratio; SRT = speech reception threshold.
Hearing status was categorized into three groups, including normal hearing, conductive or mixed hearing loss, and sensorineural hearing loss.
Hearing status was defined as the presence of hearing loss (> 15 dB HL) at any test frequency (0.25–16 kHz).
In Figure 1 (bottom panel), the CF group was separated into an NH (CF-NH) and hearing loss group (CF-HL; > 15 dB HL at a minimum of one audiometric test frequency), with the control group mean thresholds plotted for comparison. When the CF group was subdivided based on hearing status, the control and CF-NH groups had similar thresholds from 0.25 to 16 kHz. For the CF-HL group, although thresholds are poorer across all test frequencies, the most pronounced difference was from 8 to 16 kHz where maximum thresholds ranged from 55 to 75 dB HL compared to maximum thresholds of 20–40 dB HL in the control group in the same frequency range.
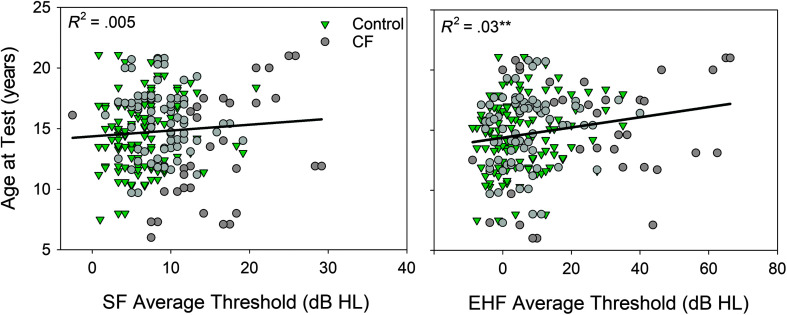
To further evaluate the relationship between hearing status and age at test, age was plotted against mean audiometric thresholds in the SF (0.25–8 kHz) and EHF (10–16 kHz) range for the CF and control groups (see Figure 2). Pearson correlation analysis was conducted to examine the relationship between participant age at test and average thresholds in the SF and EHF range. With all participants combined, a significant positive relationship was found between age at test and average EHF thresholds (r = .183, p = .005), but not for the average SF thresholds (r = .075, p = .249). Results indicate that older participants displayed higher average EHF thresholds, with no difference in SF thresholds. To determine if this relationship was being primarily driven by the CF group, analysis was repeated for each group separately. Correlation analysis results were borderline significant for the CF (r = .180, p = .058) and control group (r = .175, p = .054), indicating that the significant positive relationship between age at test and average EHF thresholds is supported by both groups equally.
Figure 2.
Participant age at test plotted as a function of average hearing thresholds for the control and cystic fibrosis (CF) groups. Standard frequency average thresholds (SF; 0.25–8 kHz) are displayed on the left, and extended high-frequency average thresholds (EHF; 10–16 kHz) are displayed on the right. Regression lines and the coefficient of determination are displayed in each figure. *p < .05, **p < .01, ***p < .001.
Middle Ear Function
Middle ear function was analyzed using repeated-measures analysis to determine if there were differences between CF and control groups (see Table 2). A fixed effect of hearing status was included, where participants were categorized based on the type of hearing loss (NH, SNHL, and CHL/MHL categories). For tympanometric peak pressure, a significant effect of group was not observed (p > .05; CF = −41.2 daPa, control = −46.3 daPa); however, hearing status (p = .001) and sex (p = .002) were both significant in the model. Pairwise comparisons showed similar peak pressure for individuals with NH (M = −19.0 daPa) and SNHL (M = −22.4 daPa). Significantly decreased middle ear pressure was found for participants with a CHL/MHL (M = −89.8 daPa). With regard to sex, females (M = −16.1 daPa) had significantly increased peak pressure compared to males (M = −43.2 daPa).
The CF group had significantly higher admittance (M = 1.1 mmho) using 226-Hz tympanometry compared to controls (M = 0.7 mmho, p = .002). Although hearing status was not significant, there was a significant Group × Hearing Category interaction (p = .041). Controls had similar admittance across hearing status groups (NH = 0.7 mmho, CHL/MHL = 0.6 mmho, SNHL = 0.8 mmho). Within the CF group, individuals with hearing loss had higher admittance (CHL/MHL = 1.5 mmho, SNHL = 1.1 mmho) than those with NH (NH = 0.8 mmho). An effect of age at test, ear, or race did not reach significance in any model for the middle ear function variables (p > .05). Additionally, for tympanometric width, there were no significant effects for any of the factors included in the model (p > .05).
MEMR
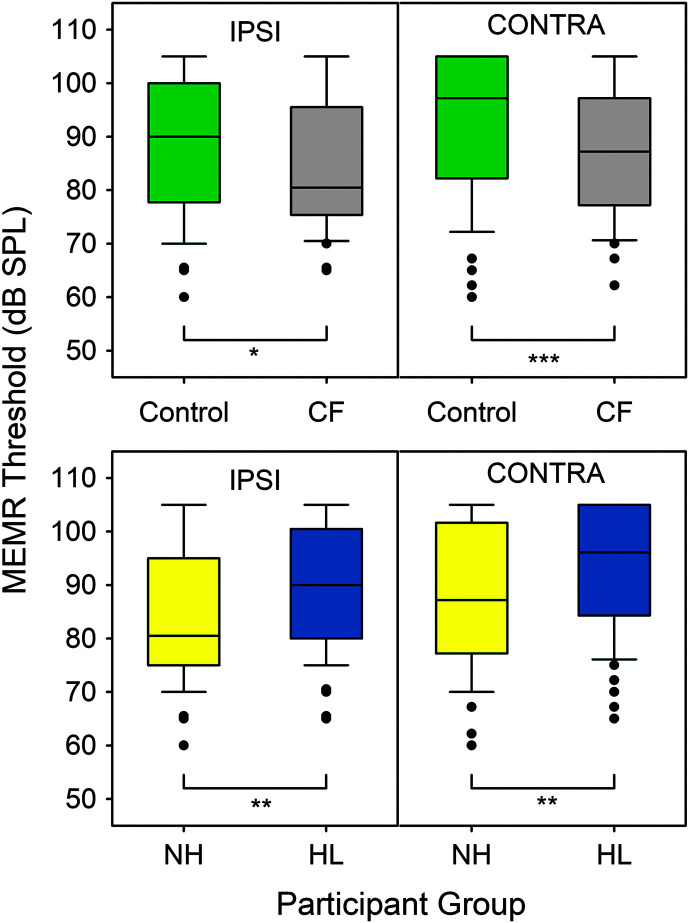
Ipsilateral and contralateral MEMR box plots are displayed in Figure 3 with the repeated-measures analysis results shown in Table 2. A significant effect of Group × Hearing Status (hearing loss at any one frequency > 15 dB HL) was found for both ipsilateral and contralateral MEMR thresholds. For the ipsilateral condition, the CF group had slightly lower MEMR thresholds (M = 84.5 dB SPL) compared to controls (M = 88.3 dB SPL, p = .042). Similarly, for the contralateral condition, individuals with CF had slightly lower MEMR thresholds (M = 88.3 dB SPL) compared to controls (M = 92.8 dB SPL, p = .004). Participants with hearing loss had higher MEMR thresholds than individuals with NH for both the ipsilateral (NH = 83.7 dB SPL, HL = 89.1 dB SPL, p < .001) and contralateral conditions (NH = 87.5 dB SPL, HL = 92.8 dB SPL, p = .001). No significant effects were found for test ear, age at test, sex, or race for either ipsilateral or contralateral MEMR thresholds (p > .05).
Figure 3.
Ipsilateral (IPSI) and contralateral (CONTRA) middle ear muscle reflex (MEMR) thresholds box plots for the cystic fibrosis (CF) and control groups (top panel) and hearing status (bottom panel). Hearing loss was defined as thresholds of > 15 dB HL at any test frequency (0.25–16 kHz) and included normal hearing (NH) and hearing loss (HL) categories. Box plots show median (line), interquartile range (boxes), 95% confidence interval (stems), and outliers (dots). *p < .05, **p < .01, ***p < .001.
Speech Perception
Repeated-measures analysis for SRT and SNR-Loss are shown in Table 2 with SNR-Loss scores displayed in Figure 4. Individuals with CF showed significantly higher SRT (M = 13.8 dB HL) compared to the control (M = 9.5 dB HL, p < .001). In the CF group, SRTs ranged from 0 to 35 dB HL, with 81% of ears having a normal SRT and 19% with an SRT greater than 15 dB HL. In the control group, the SRT ranged from 0 to 20 dB HL, with 94% of ears having a normal SRT value and only 6% with an elevated SRT. Test ear, age at test, sex, and race were not significant predictors (p > .05).
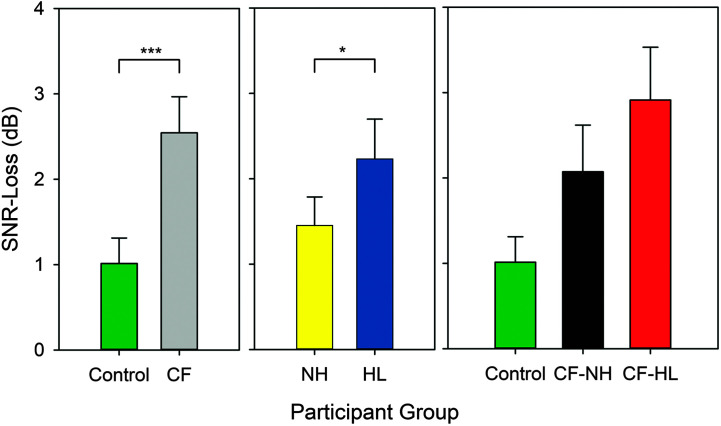
Figure 4.
SNR-Loss displayed based on participant group (left), hearing status (middle), and lastly with the cystic fibrosis (CF) group further classified into normal hearing (CF–NH) and hearing loss (CF–HL) groups (right). The SNR-Loss was calculated as the individual SNR–50 minus age-matched normative SNR-50 values. Error bars indicate 95% confidence intervals. *p < .05, **p < .01, ***p < .001.
For SNR-Loss (age-adjusted scores), individuals with CF had significantly poorer (higher) SNR values (M = 2.5 dB HL) compared to controls (M = 1.1 dB, p < .001; see Figure 4, left). Within the control group, 96% of ears had normal SNR-Loss (range: −2.8 to 3.0 dB), while only 4% had a mild SNR-Loss (range: 3.5–4.4 dB). For the CF group, the SNR-Loss ranged from −1.1 to 10.0 dB HL; 64% of ears had normal SNR-Loss, and 36% of ears had mild or moderate NR-Loss. In addition to a significant group effect, individuals with hearing loss (CF and control groups combined) had slightly higher SNR-Loss (M = 2.2 dB) compared to those with NH (M = 1.5 dB, p = .045; see Figure 3, middle). Lastly, females had significantly higher SNR-Loss values (M = 2.1 dB) compared to males (M = 1.4 dB, p = .017). When the CF group was subdivided based on the presence of hearing loss, 25% of individuals with CF and NH had abnormal SNR-Loss (range: 3.5–6 dB). In contrast, 45% of individuals with CF and hearing loss had abnormal SNR-Loss (range: 3.2–10 dB; see Figure 4, right).
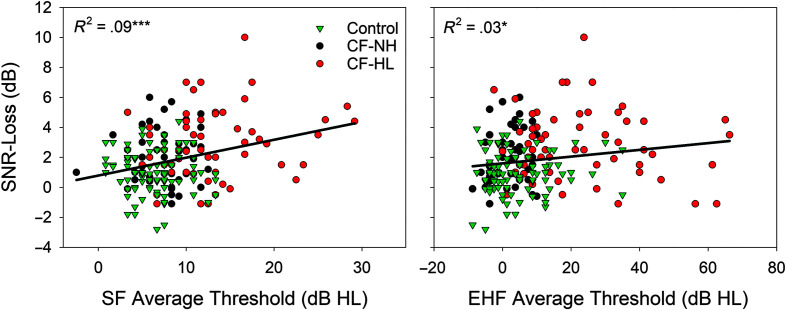
To further evaluate the relationship between hearing status and speech understanding in noise, SNR-Loss was plotted against mean audiometric thresholds in the SF (0.25–8 kHz) and EHF (10–16 kHz) range for the CF-NH, CF-HL, and controls (see Figure 5). Correlation analysis completed with all participants combined showed a significant positive relationship for both the average SF (r = .301, p < .001) and EHF (r = .116, p = .022) thresholds compared to SNR-Loss. The SF range had a stronger relationship with SNR-Loss, explaining a greater amount of variance (9%) compared to the EHF range (3%). Lastly, upon comparison of perceived hearing concerns and speech-in-noise performance, only 53% of the CF group with SNR-Loss reported hearing concerns on the questionnaire while 47% with an abnormal SNR-Loss reported no hearing concerns. Furthermore, only 37% of individuals with CF and hearing loss (any frequency > 15 dB HL) reported hearing concerns on the questionnaire, while 63% with hearing loss reported no hearing concerns.
Figure 5.
SNR-Loss plotted as a function of average hearing thresholds for the control group with the cystic fibrosis (CF) group further classified into normal hearing (CF–NH) and hearing loss (CF–HL) groups. Standard frequency average thresholds (SF; 0.25–8 kHz) are displayed on the left, and extended high-frequency average thresholds (EHF; 10–16 kHz) are displayed on the right. Regression lines and the coefficient of determination are displayed in each figure. *p < .05, **p < .01, ***p < .001.
Discussion
The main purpose of this study was to determine the prevalence of ototoxicity-related hearing loss, tinnitus, and balance and the functional impact on communication in a pediatric cohort with CF compared to age- and gender-matched controls. The CF group had a significantly higher prevalence of hearing loss in either the SF or EHF region (57%) compared to the control group (37%). The majority of controls with hearing loss had slight-to-mild degrees of loss. In contrast, individuals with CF had high percentages of mild-to-moderate hearing loss, with some individuals displaying moderate-to-severe and severe degrees of hearing loss. The CF group also had a higher prevalence of CHL or MHL (16% combined) compared to controls (7%). Individuals with CF were also more likely to have a history of middle ear infections and PE tubes than controls. Lastly, the CF group reported significantly more hearing concerns, tinnitus, balance problems than controls, had higher speech recognition thresholds in quiet, and performed poorer on a clinical speech understanding in noise task.
Prevalence of Hearing Loss
This study showed a very high rate of hearing loss in both the standard (38%) and EHF region (47%) for children, teens, and young adults with CF who have received IV-AG antibiotics to treat lung exacerbations. Estimates of hearing loss vary, in part, due to the criterion for NH; our study included slight hearing loss (> 15 dB HL) in either the SF or EHF range, so the estimates are higher than studies that have used more conservative criteria. Using hearing loss criteria similar to the current study, Al-Malky et al. (2011) reported clinically NH (0–15 dB HL) from 0.25 to 12.5 kHz in children with CF (n = 45) in a low IV-AG exposure group. In a high IV-AG exposure group, 35% of individuals with CF had evidence of ototoxicity in the EHF region, defined as any threshold of ≥ 20 dB HL. Similarly, Al-Malky et al. (2015) reported ototoxicity in 24% of children with CF and previous exposure to IV-AGs, but in the high-exposure group, 44% displayed ototoxicity with thresholds ranging from 25 to 85 dB HL.
In contrast, several studies have reported much lower rates of hearing loss (0%–12%), possibly due to less stringent hearing loss criteria or the exclusion of EHF audiometry (Martins et al., 2010; Solmaz et al., 2016; Stavroulaki et al., 2002). When the audiometric data (0.25–8 kHz) from Solmaz et al. (2016) were reanalyzed using a more stringent hearing loss criterion (any frequency > 15 dB HL), the percentage of CF ears with hearing loss increased from 12% to 80%, yet all individuals in the control group still had NH. In a large retrospective study of hearing impairment, Kreicher et al. (2018) reported 31.8% of individuals with CF (M = 8.3 years) had hearing loss in at least one ear (> 15 dB HL at any frequency from 0.25 to 8 kHz). Most ears with hearing loss showed a slight-to-mild loss (15%) with a smaller number of moderate-to-profound losses (3%). Lastly, Geyer et al. (2015) reported that both the CF and control groups had NH in the SF region (pure-tone average of 0.25–8 kHz). However, in the CF group, 30.8% had at least one threshold greater than 25 dB HL in the EHF region (9–16 kHz), compared to 2.8% in the control group (Geyer et al., 2015).
The control group also had a high rate of hearing loss in either the SF or EHFs (37%), with only 17% in the SF region and 30% in the EHF region. The high percentage of hearing loss can be attributed to the stringent hearing loss criteria (> 15 dB HL) used in the current study. When the hearing loss criterion was slightly relaxed (> 20 dB HL), only 13% of the control group had hearing loss with 2% and 12% in the SF and EHF range, respectively. These hearing loss percentages are comparable to a cohort of typically developing children (n = 54, age range: 6.5–14.6 years) that all had NH (≤ 20 dB HL) in the SF range but 20% had EHF hearing loss (> 20 dB HL; Hunter, Blankenship, et al., 2020).
Importance of EHFs in Ototoxicity Monitoring
It is well established that AG-induced hearing loss originates in the EHF region and, with increased IV–AG exposure, eventually progresses to the SF region (Fausti et al., 2003, 1999). Therefore, the inclusion of EHF audiometry when monitoring for ototoxicity is essential. Several studies have evaluated the presence of hearing loss based on frequency region. For example, Al-Malky et al. (2015) showed that the use of EHF (9–16 kHz) audiometry identified 15 children with hearing loss, while SF audiometry only detected 13 children. When using an SF pure-tone average (0.5, 1, and 2 kHz), Martins et al. (2010) reported only 4% of CF cases had hearing loss (> 25 dB HL); however, when EHFs (9–12 kHz) were included, 11% had hearing loss. Geyer et al. (2015) reported that, while all individuals with CF had NH in the SFs, 30.8% of the CF group had EHF hearing loss (9–16 kHz). Hearing loss in the aforementioned studies is presumed to be sensorineural since the studies excluded individuals with history of chronic middle ear infections, abnormal middle ear status, middle ear effusion, tympanic membrane perforation, or CHL verified with BC thresholds. In the current study, 38% of the CF group had hearing loss in the SF and 47% had hearing loss in the EHF region. When examining audiometric thresholds across frequency region, 15 individuals with CF (n = 22 ears, 7 bilateral, 8 unilateral) had NH in the SFs but displayed either slight-to-mild (n = 16 ears) or moderate-to-moderately-severe (n = 6 ears) hearing loss in the EHFs. Therefore, the use of only SF audiometry would result in the misdiagnosis of 15 individuals with CF with NH, when they display evidence of significant ototoxicity in the EHFs. Furthermore, older participants (CF and controls grouped together) displayed higher average EHF thresholds, with no effect of age on average SF thresholds. Since individuals in the study are still very young, this may suggest more treatment exposure for individuals with CF when the groups are separated out.
CHL and History of Otitis Media
The mucosal epithelium of the middle ear and Eustachian tube is contiguous with the upper respiratory track. Thus, it was previously assumed that individuals with CF may have a higher than usual incidence of middle ear disease. However, temporal bone studies of individuals with and without CF have shown similar pneumatization and mucosal histology of the middle ear (Berkhout et al., 2014; Seifert et al., 2010; Todd & Martin, 1988; Yildirim et al., 2000). In addition, several studies have suggested that individuals with CF are at no higher risk than age-matched controls, with some studies showing lower rates of inflammatory ear disease compared to controls (Bak-Pedersen & Larsen, 1979; Cepero et al., 1987; Cipolli et al., 1993; Forcucci & Stark, 1972; Jorissen et al., 1998; Martins et al., 2011). However, most previous studies did not include BC audiometry, and if tympanometry was included, individuals with middle ear issues were excluded (otitis media, tympanic membrane perforation). Therefore, most studies were not designed to detect CHL or middle ear disorders, which may help to explain why this issue has gone unrecognized.
In this study, 118 participants (CF = 57, control = 61) were investigated with the use of the traditional approach of audiometric and otologic verification to detect and identify ear disease. In addition, 226-Hz tympanometry and MEMRs were performed. The physiological data were used to analyze the pressure in the middle ear space, integrity and mobility of the tympanic membrane and ossicles, and stapedius muscle function. We found that 42% of participants with CF reported a history of chronic otitis media, 16% have had PE tube surgery, and 16% had a conductive component to their hearing loss, compared to 26%, 11%, and 7% in the control group, respectively. Thus, compared to an age- and gender-matched sample, our CF group had significantly more reports of past otitis media with effusion and PE tube placement. The occurrence of CHL in our sample was mostly mild and was not accompanied by current otitis media with effusion or flat tympanometry. Rather, in most cases, tympanometry showed significantly more negative peak pressure in the ears with CHL, indicating Eustachian tube dysfunction. Most cases of CHL also showed abnormally high admittance for tympanometry, although this was not statistically significant in terms of hearing loss type overall. Histories of PE tubes are frequently associated with high admittance due to tympanic membrane defects related to either spontaneous perforation or myringotomy of the tympanic membrane (Hunter & Blankenship, 2017).
In the CF literature, the prevalence of otitis media ranges from 3% to 45% (Bak-Pedersen & Larsen, 1979; Cepero et al., 1987; Cheng et al., 2009; Forman-Franco et al., 1979; Kreicher et al., 2018). A retrospective analysis of 450 individuals with CF revealed only 3% had a history of otologic disease with only 1% receiving PE tubes (Cepero et al., 1987). Cheng et al. (2009) reported 20% of a pediatric and adult CF cohort had a history of middle ear effusion and or Eustachian tube dysfunction and abnormal tympanometry (Types B and C). More recently, Kreicher et al. (2018) conducted a retrospective study of 217 children with CF and found 45% had acute otitis media, 29% were diagnosed with chronic otitis media (number of infections = 1–66), 8% had PE tubes, and 23% were diagnosed with Eustachian tube dysfunction. Of the 94 ears with tympanometry results, 19% had abnormal tympanograms (Types B and C).
With regard to the prevalence of CHL in individuals with CF, previous studies have reported relatively low percentages ranging from 0% to 10% (Al-Malky et al., 2015, 2011; Geyer et al., 2015; Martins et al., 2010). In a group of children and adults with CF (n = 80) with previous tobramycin and gentamicin exposure, Forman-Franco et al. (1979) reported no individuals displayed CHL. Pedersen et al. (1987) reported a slightly higher rate, with 7% of individuals with CF diagnosed with a CHL. Using BC thresholds measured from 0.25 to 4 kHz, Kreicher et al. (2018) reported that 10% had a conductive component to their hearing loss, with equal percentages of conductive (5.3%) and mixed (5.1%) loss. Lastly, in a cohort of 70 children with CF, Kulczycki et al. (1970) reported a much higher rate with 27% of study participants showing a mild conductive loss.
With regard to MEMR thresholds, the finding of lower (better) thresholds in individuals with CF and AG exposure for both ipsilateral and contralateral stimuli has not been reported previously and does not have a clear explanation (see Westman et al., in press). While MEMR thresholds in this study were overall slightly lower in the CF group, MEMR thresholds were poorer for individuals with hearing loss. This result was expected and is consistent with changes in MEMRs due to outer hair cell loss in kanamycin exposure (Borg & Engström, 1982). Validation in another sample and with non–AG-exposed participants is needed to explore the finding of better MEMR in patients with NH.
Functional Impact
While most previous studies have focused on audiometric assessment, it is important to evaluate the functional impact of the hearing loss on the individual and their communication abilities. In the current study, 32% of individuals with CF reported hearing difficulties and 53% reported tinnitus compared to 8% and 16% in the control group, respectively. In comparison, Al-Malky et al. (2015) reported that most children with ototoxicity did not report any issues with hearing or tinnitus. Of the 15 individuals with CF who displayed hearing loss, only four reported issues with both hearing and tinnitus, two had hearing difficulties, and one child reported issues with tinnitus. The remaining eight children with ototoxicity did not report any issues with hearing or tinnitus. Similarly, Mulheran et al. (2001) reported 17% of the CF group had significant ototoxicity, many with hearing loss that had progressed into the SF region. Yet, most of these individuals were asymptomatic; none of the patients had self-report of hearing difficulties, and only four individuals experienced periodic tinnitus during treatment.
Clinical measures of speech understanding in quiet and noise are important tools to evaluate the impact of hearing impairment on communication abilities. The CF group had significantly poorer speech recognition thresholds and speech-in-noise scores compared to age-matched controls. For speech understanding in quiet, 6% of controls had an abnormal SRT (> 15 dB HL) compared to 19% of the CF group. On the BKB-SIN, 64% of the CF group had an abnormal SNR-Loss that ranged from mild to moderate, yet only 4% of the control group had an abnormal SNR-Loss, all within the mild range. While 45% of individuals with CF with hearing loss also had an abnormal SNR–Loss, 25% of individuals with CF and NH displayed an abnormal SNR-Loss. Furthermore, correlation analysis showed a significant positive relationship between hearing status and SNR-Loss that was stronger for the SF region than for the EHF region. Although the BKB-SIN recordings contain significant spectral information above the electrical noise floor up to 12 kHz, many of the participants had EHF hearing loss that was restricted to higher frequencies. For example, in the CF group, although 47% had EHF hearing loss from 10 to 16 kHz, only 24% had hearing loss from 10 to 12.5 kHz. Similarly, in the control group, 30% had EHF hearing loss, but only 11% had hearing loss in the range of 10–12.5 kHz. The decreased number of participants with hearing loss from 10 to 12.5 kHz may have contributed to the weaker relationship that was found between EHF hearing threshold and SNR-Loss, compared to SF hearing threshold analysis.
Although several studies have reported that speech perception was conducted as part of the audiometric test battery, test results were not provided/reported (Forman-Franco et al., 1979; Martins et al., 2010; Pedersen et al., 1987). Forcucci and Stark (1972) conducted audiometric, ototologic, and speech-language examinations in a group of 31 children with CF, and 22% had deficient speech-language development. Due to the cumulative ototoxic nature of IV-AG, individuals with CF are at a higher risk for developing EHF hearing loss can progress to the SF region and ultimatley impact speech and language development, literacy development, scholastic achievement, and overall quality of life (Moeller et al., 2007; Yoshinaga-Itano, 1999, 2003). Studies have shown EHF information aides in vowel and consonant identification when access to SF energy is restricted or degraded (Lippmann, 1996; Vitela et al., 2015) or when extended bandwidth hearing aids improve audibility of EHFs (Seeto & Searchfield, 2018). EHF information also improves sound localization by providing cues to resolve front/back confusions (Best et al., 2005; Brungart & Simpson, 2009; Heffner & Heffner, 2008) and can help listeners identify the speaker and segregate their voice from background talkers (Monson et al., 2019). Lastly, EHF hearing is significantly correlated with speech perception in noise, which is consistent with studies of adults with hearing loss (Motlagh Zadeh et al., 2019; Yeend et al., 2019). Therefore, ototoxicity monitoring/management programs should include functional impact measures that can be administered quickly, ideally at the patient bedside or at home. Even if the AG treatment schedule cannot be altered to minimize ototoxicity, self-report measures and clinical speech perception assessments are necessary to evaluate the impact of hearing loss and to identify individuals for audiologic treatment. Additionally, there is a substantial lack of patient-reported outcomes in ototoxicity monitoring programs, which are critical for patient-centered clinical care.
Very few studies have reported the effects of AG treatment on vestibular function in individuals with CF (Handelsman et al., 2017; Scheenstra et al., 2009). Furthermore, studies report wide variability in the presence of vestibular impairments, and very little has been reported about vestibular asymmetries. Scheenstra et al. (2009) reported 35% of adults with CF had either a peripheral or central impairment as diagnosed using electronystagmography with caloric irrigation, while only 16% self-reported vestibular issues on a questionnaire. In a group of children and adults with CF, Handelsman et al. (2017) reported that, while most underreported symptoms, 79% had vestibular system dysfunction based on diagnostic test battery (dynamic visual acuity, videonystagmography, sinusoidal and rotational step testing). In the current study, 32% of the CF group reported balance issues compared to 16% in controls. The presence of vestibular impairments is high among individuals with CF, yet most go undiagnosed due to lack of testing and underreport of patient symptoms. Vestibular assessments that can be performed quickly with minimal physical or psychological discomfort, such as the video head impulse test and vestibular evoked myogenic potential test, should be included in ototoxicity monitoring programs.
Limitations and Future Directions
The primary limitation of this study is that individuals with CF had preexisting exposure to AGs, and thus, nearly half had preexisting hearing loss as well. Efforts are underway to continue longitudinal assessment of this sample and to expand the assessment to children not yet exposed to ascertain onset and progression of hearing loss in future studies. In process for this study is analysis of past doses of AGs and pharmacodynamic measures (peak and trough blood levels and deeper tissue estimation of drug exposure over time) to determine relationships to hearing loss and changes over time with repeated hearing tests. While the sample size is relatively modest, it is one of the largest studies published specifically in children with prospective assessment. Diabetes and genetic variants that are related to AG ototoxicity and known mutations (e.g., m1555A>G mutation) that increase risk for developing hearing loss will be included in future studies.
Conclusions
Individuals with CF are at extremely high risk for developing hearing loss and balance disorders due to routine exposure of IV-AGs to treat pulmonary exacerbations. As the predicted median age of survival for individuals with CF continues to increase, many will become exposed to very high cumulative doses of IV-AG, thereby increasing their risk for developing hearing loss. Our results showed high rates of SNHL and CHL in the CF group, higher than most pediatric CF studies due to the inclusion of EHF audiometry, and a more stringent hearing loss criteria (threshold at any frequency > 15 dB HL). Furthermore, older participants display higher average EHF thresholds, with no effect of age on average SF thresholds. Studies that based the presence of hearing loss on the individual thresholds within the SF region, an SF pure-tone average, or higher significant threshold criteria (dB HL) are likely underestimating the incidence of hearing loss in this population. Furthermore, these individuals with CF displayed high rates of middle ear dysfunction and CHL, a problem that has not been identified in studies that did not include BC or excluded individuals with abnormal middle ear status.
Previous studies have not reported speech perception in either quiet or noise. Our study shows that SNHL connected with AG use degrades the listener's ability to perceive speech in noise, which may diminish quality of life and has clear educational implications. The impacts of broadened tuning resulting from EHF hearing loss on speech in noise are just beginning to be appreciated (Besser et al., 2015; Hunter, Monson, et al., 2020) but have received scant attention in patients with ototoxicity. The importance of speech communication for patients with chronic illness also has implications for ongoing care. Medical staff must wear masks due to contact isolation requirements, and they report that communication is challenging with their patients with hearing loss. Armed with evidence of ototoxicity, physicians report that they are willing to consider treatment modifications to minimize permanent hearing loss and the impact on communication (Garinis et al., 2018). The BKB-SIN Test used in this study to evaluate speech-in-noise performance is derived from child language samples, and the sentences are at approximately a first-grade reading level (Nilsson et al., 1994). The test is adaptive, avoiding ceiling and floor effects, has ecological validity, and is reportedly not confounded by language abilities and working memory (Magimairaj et al., 2018). However, other studies have found that sentence in noise performance is associated with language and working memory in children (McCreery et al., 2017). The BKB-SIN Test yields an SNR-Loss score that is age normalized, allowing results to be compared across different populations. Inclusion of an age- and language-appropriate speech-in-noise measure is recommended both to evaluate functional effects of ototoxicity and to assess the need for and benefit of amplification.
Lastly, the CF group reported significantly more issues with hearing (32%), tinnitus (53%), and balance (32%), compared to controls (8%, 16%, 9%, respectively). However, self-report of hearing difficulties was not predictive of hearing loss or speech-in-noise performance; thus, self-report cannot replace formalized testing to monitor for evidence of ototoxicity.
Acknowledgments
This research was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC010202 (multiprincipal investigators: Doug Keefe, Lisa L. Hunter, and Patrick Feeney), Clinical and Translational Science Award Program Grant 5UL1TR001425-04 (Center for Clinical and Translational Science Training at the University of Cincinnati), and the Cincinnati Children's Hospital Medical Center Research Foundation Place Outcomes Research Award (multiprinciple investigators: Lisa L. Hunter and John P. Clancy). The content of this article is solely the responsibility of the authors and does not reflect the official views of the National Institutes of Health, the Department of Veterans Affairs, or Cincinnati Children's Hospital Medical Center. Portions of this study were presented at the World Congress of Audiology 2018, North American Cystic Fibrosis Conference 2019, and the Association for Research in Otolaryngology 2020. We also thank our participants, their families, and the Summer Undergraduate Research Foundation scholars.
Appendix
Participant Questionnaire



Funding Statement
This research was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC010202 (multiprincipal investigators: Doug Keefe, Lisa L. Hunter, and Patrick Feeney), Clinical and Translational Science Award Program Grant 5UL1TR001425-04 (Center for Clinical and Translational Science Training at the University of Cincinnati), and the Cincinnati Children's Hospital Medical Center Research Foundation Place Outcomes Research Award (multiprinciple investigators: Lisa L. Hunter and John P. Clancy).
References
- Ahonen, J. E. , & McDermott, J. C. (1984). Extended high-frequency hearing loss in children with cleft palate. Audiology, 23(5), 467–476. https://doi.org/10.3109/00206098409070086 [DOI] [PubMed] [Google Scholar]
- Al-Malky, G. , Dawson, S. J. , Sirimanna, T. , Bagkeris, E. , & Suri, R. (2015). High-frequency audiometry reveals high prevalence of aminoglycoside ototoxicity in children with cystic fibrosis. Journal of Cystic Fibrosis, 14(2), 248–254. https://doi.org/10.1016/j.jcf.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Al-Malky, G. , Suri, R. , Dawson, S. J. , Sirimanna, T. , & Kemp, D. (2011). Aminoglycoside antibiotics cochleotoxicity in paediatric cystic fibrosis (CF) patients: A study using extended high-frequency audiometry and distortion product otoacoustic emissions. International Journal of Audiology, 50(2), 112–122. https://doi.org/10.3109/14992027.2010.524253 [DOI] [PubMed] [Google Scholar]
- Alexander, J. M. , Kopun, J. G. , & Stelmachowicz, P. G. (2014). Effects of frequency compression and frequency transposition on fricative and affricate perception in listeners with normal hearing and mild to moderate hearing loss. Ear and Hearing, 35(5), 519–532. https://doi.org/10.1097/AUD.0000000000000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auditec. (2015). Spondees and child spondees [Audio CD] .
- Bak-Pedersen, K. , & Larsen, P. K. (1979). Inflammatory middle ear diseases in patients with cystic fibrosis. Acta Oto-Laryngologica, 86 (Suppl. 360), 138–140. https://doi.org/10.3109/00016487809123499 [DOI] [PubMed] [Google Scholar]
- Barclay, M. L. , Begg, E. J. , Chambers, S. T. , Thornley, P. E. , Pattemore, P. K. , & Grimwood, K. (1996). Adaptive resistance to tobramycin in Pseudomonas aeruginosa lung infection in cystic fibrosis. Journal of Antimicrobial Chemotherapy, 37(6), 1155–1164. https://doi.org/10.1093/jac/37.6.1155 [DOI] [PubMed] [Google Scholar]
- Becker, B. , & Cooper, M. A. (2013). Aminoglycoside antibiotics in the 21st century. ACS Chemical Biology, 8(1), 105–115. https://doi.org/10.1021/cb3005116 [DOI] [PubMed] [Google Scholar]
- Berkhout, M. C. , van Rooden, C. J. , Aalbers, R. C. , el Bouazzaoui, L. H. , Fokkens, W. J. , Rijntjes, E. , & Heijerman, H. G. (2014). Temporal bone pneumatization in cystic fibrosis: A correlation with genotype? Laryngoscope, 124(7), 1682–1686. https://doi.org/10.1002/lary.24575 [DOI] [PubMed] [Google Scholar]
- Besser, J. , Festen, J. M. , Goverts, S. T. , Kramer, S. E. , & Pichora-Fuller, M. K. (2015). Speech-in-speech listening on the LiSN-S test by older adults with good audiograms depends on cognition and hearing acuity at high frequencies. Ear and Hearing, 36(1), 24–41. https://doi.org/10.1097/AUD.0000000000000096 [DOI] [PubMed] [Google Scholar]
- Best, V. , Carlile, S. , Jin, C. , & van Schaik, A. (2005). The role of high frequencies in speech localization. The Journal of the Acoustical Society of America, 118(1), 353–363. https://doi.org/10.1121/1.1926107 [DOI] [PubMed] [Google Scholar]
- Borg, E. , & Engström, B. (1982). Acoustic reflex after experimental lesions to inner and outer hair cells. Hearing Research, 6(1), 25–34. https://doi.org/10.1016/0378-5955(82)90005-3 [DOI] [PubMed] [Google Scholar]
- Brungart, D. S. , & Simpson, B. D. (2009). Effects of bandwidth on auditory localization with a noise masker. The Journal of the Acoustical Society of America, 126(6), 3199–3208. https://doi.org/10.1121/1.3243309 [DOI] [PubMed] [Google Scholar]
- Cameron, S. , & Dillon, H. (2007). Development of the Listening in Spatialized Noise–Sentences Test (LISN-S). Ear and Hearing, 28(2), 196–211. https://doi.org/10.1097/AUD.0b013e318031267f [DOI] [PubMed] [Google Scholar]
- Cepero, R. , Smith, R. J. , Catlin, F. I. , Bressler, K. L. , Furuta, G. T. , & Shandera, K. C. (1987). Cystic fibrosis—An otolaryngologic perspective. Otolaryngology—Head & Neck Surgery, 97(4), 356–360. https://doi.org/10.1177/019459988709700403 [DOI] [PubMed] [Google Scholar]
- Cheng, A. G. , Johnston, P. R. , Luz, J. , Uluer, A. , Fligor, B. , Licameli, G. R. , Kenna, M. A. , & Jones, D. T. (2009, July). Sensorineural hearing loss in patients with cystic fibrosis. Otolaryngology—Head & Neck Surgery, 141(1), 86–90. https://doi.org/10.1016/j.otohns.2009.03.020 [DOI] [PubMed] [Google Scholar]
- Cipolli, M. , Canciani, M. , Cavazzani, M. , Uras, P. , Zampieri, P. , & Mastella, G. (1993). Ear disease is not a common complication in cystic fibrosis. European Journal of Pediatrics, 152(3), 265–266. https://doi.org/10.1007/BF01956160 [DOI] [PubMed] [Google Scholar]
- Conway, S. P. , Miller, M. G. , Ramsden, C. , & Littlewood, J. M. (1985). Intensive treatment of pseudomonas chest infection in cystic fibrosis: A comparison of tobramycin and ticarcillin, and netilmicin and ticarcillin. Acta Pædiatrica, 74(1), 107–113. https://doi.org/10.1111/j.1651-2227.1985.tb10929.x [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation. (2017). Patient registry annual data report [Online source] . https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf
- Etymotic Research. (2005). Bamford–Kowal–Bench Speech-in-Noise Test (Version 1.03) [Audio CD] .
- Fausti, S. A. , Frey, R. H. , Henry, J. A. , Olson, D. J. , & Schaffer, H. I. (1993). High-frequency testing techniques and instrumentation for early detection of ototoxicity. Journal of Rehabilitation Research and Development, 30(3), 333–341. [PubMed] [Google Scholar]
- Fausti, S. A. , Helt, W. J. , Phillips, D. S. , Gordon, J. S. , Bratt, G. W. , Sugiura, K. M. , & Noffsinger, D. (2003). Early detection of ototoxicity using 1/6th-octave steps. Journal of the American Academy of Audiology, 14(8), 444–450. https://doi.org/10.1055/s-0040-1715935 [PubMed] [Google Scholar]
- Fausti, S. A. , Henry, J. A. , Helt, W. J. , Phillips, D. S. , Frey, R. H. , Noffsinger, D. , Larson, V. D. , & Fowler, C. G. (1999). An individualized, sensitive frequency range for early detection of ototoxicity. Ear and Hearing, 20(6), 497–505. https://doi.org/10.1097/00003446-199912000-00005 [DOI] [PubMed] [Google Scholar]
- Fausti, S. A. , Henry, J. A. , Schaffer, H. I. , Olson, D. J. , Frey, R. H. , & McDonald, W. J. (1992). High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. Journal of Infectious Diseases, 165(6), 1026–1032. https://doi.org/10.1093/infdis/165.6.1026 [DOI] [PubMed] [Google Scholar]
- Fausti, S. A. , Larson, V. D. , Noffsinger, D. , Wilson, R. H. , Phillips, D. S. , & Fowler, C. G. (1994). High-frequency audiometric monitoring strategies for early detection of ototoxicity. Ear and Hearing, 15(3), 232–239. https://doi.org/10.1097/00003446-199406000-00004 [DOI] [PubMed] [Google Scholar]
- Fausti, S. A. , Rappaport, B. Z. , Schechter, M. A. , Frey, R. H. , Ward, T. T. , & Brummett, R. E. (1984). Detection of aminoglycoside ototoxicity by high-frequency auditory evaluation: Selected case studies. American Journal of Otolaryngology, 5(3), 177–182. https://doi.org/10.1016/S0196-0709(84)80009-5 [DOI] [PubMed] [Google Scholar]
- Filippone, E. J. , Kraft, W. K. , & Farber, J. L. (2017). The nephrotoxicity of vancomycin. Clinical Pharmacology & Therapeutics, 102(3), 459–469. https://doi.org/10.1002/cpt.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcucci, R. A. , & Stark, E. W. (1972). Hearing loss, speech-language, and cystic fibrosis. Archives of Otolaryngology—Head & Neck Surgery, 96(4), 361–364. https://doi.org/10.1001/archotol.1972.00770090537012 [DOI] [PubMed] [Google Scholar]
- Forman-Franco, B. , Abramson, A. L. , Gorvoy, J. D. , & Stein, T. (1979). Cystic fibrosis and hearing loss. Archives of Otolaryngology—Head & Neck Surgery, 105(6), 338–342. https://doi.org/10.1001/archotol.1979.00790180036007 [DOI] [PubMed] [Google Scholar]
- Garinis, A. C. , Cornell, A. , Allada, G. , Fennelly, K. P. , Maggiore, R. J. , & Konrad-Martin, D. (2018). Ototoxicity monitoring through the eyes of the treating physician: Perspectives from pulmonology and medical oncology. International Journal of Audiology, 57(Suppl. 4), S19–S24. https://doi.org/10.1080/14992027.2017.1381769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis, A. C. , Cross, C. P. , Srikanth, P. , Carroll, K. , Feeney, M. P. , Keefe, D. H. , Hunter, L. L. , Putterman, D. B. , Cohen, D. M. , Gold, J. A. , & Steyger, P. S. (2017). The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. Journal of Cystic Fibrosis, 16(3), 401–409. https://doi.org/10.1016/j.jcf.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse, S. , & Noble, W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85–99. https://doi.org/10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer, L. B. , Menna Barreto, S. S. , Weigert, L. L. , & Teixeira, A. R. (2015). High frequency hearing thresholds and product distortion otoacoustic emissions in cystic fibrosis patients. Brazilian Journal of Otorhinolaryngology, 81(6), 589–597. https://doi.org/10.1016/j.bjorl.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, A. (1965). Reference zero levels for pure-tone audiometer. ASHA, 7(262), 1. [Google Scholar]
- Guthrie, O. W. (2008). Aminoglycoside induced ototoxicity. Toxicology, 249(2–3), 91–96. https://doi.org/10.1016/j.tox.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Handelsman, J. A. , Nasr, S. Z. , Pitts, C. , & King, W. M. (2017). Prevalence of hearing and vestibular loss in cystic fibrosis patients exposed to aminoglycosides. Pediatric Pulmonology, 52(9), 1157–1162. https://doi.org/10.1002/ppul.23763 [DOI] [PubMed] [Google Scholar]
- Harris, P. A. , Taylor, R. , Minor, B. L. , Elliott, V. , Fernandez, M. , O'Neal, L. , McLeod, L. , Delacqua, G. , Delacqua, F. , Kirby, J. , & Duda, S. N. (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. https://doi.org/10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P. A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. , & Conde, J. G. (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. https://doi.org/10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner, H. E. , & Heffner, R. S. (2008). High-frequency hearing. In Dallos P., Oertel D., & Hoy R. (Eds.), Handbook of the senses: Audition (pp. 55–60). Elsevier. https://doi.org/10.1016/B978-012370880-9.00004-9 [Google Scholar]
- Hirsch, I. , Davis, H. , Silverman, S. , Reynolds, E. , Eldert, E. , & Benson, R. (1952). Development of materials for speech audiometry. Journal of Speech and Hearing, 17(3), 321–337. https://doi.org/10.1044/jshd.1703.321 [DOI] [PubMed] [Google Scholar]
- Huizing, E. H. , & de Groot, J. C. (1987). Human cochlear pathology in aminoglycoside ototoxicity—A review. Acta Oto-Laryngologica, 104(436), 117–125. https://doi.org/10.3109/00016488709124984 [DOI] [PubMed] [Google Scholar]
- Hunter, L. , & Blankenship, C. (2017). Middle ear measurement. In Tharpe A. M. & Seewald R. (Eds.), Comprehensive handbook of pediatric audiology (2nd ed.). Plural. [Google Scholar]
- Hunter, L. , Blankenship, C. , Sloat, N. , Perdew, A. , Stewart, H. , & Moore, D. R. (2020). Peripheral auditory involvement in childhood listening difficulty. Ear and Hearing. Advance online publication. https://doi.org/10.1097/AUD.0000000000000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, L. , Monson, B. B. , Moore, D. R. , Sumitrajit, D. , Wright, B. A. , Monro, K. J. , Motlagh Zadeh, L. , Blankenship, C. M. , Stiepan, S. M. , & Siegel, J. H. (2020). Extended high frequency hearing and speech perception implications in adults and children. Hearing Research.. https://doi.org/10.1016/j.heares.2020.107922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization. (2004). ISO 389-8: Acoustics—Reference zero for the calibration of audiometric equipment—Part 8: Reference equivalent threshold sound pressure levels for pure tones and circumaural earphones.
- International Organization for Standardization. (2017). ISO 389-1:Acoustics — Reference zero for the calibration of audiometric equipment—Part 1: Reference equivalent threshold sound pressure levels for pure tones and supra-aural earphones.
- Jiang, M. , Karasawa, T. , & Steyger, P. S. (2017). Aminoglycoside-induced cochleotoxicity: A review. Frontiers in Cellular Neuroscience, 11, 308. https://doi.org/10.3389/fncel.2017.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen, M. , De Boeck, K. , & Feenstra, L. (1998). Middle ear disease in cystic fibrosis. International Journal of Pediatric Otorhinolaryngology, 43(2), 123–128. https://doi.org/10.1016/S0165-5876(97)00172-9 [DOI] [PubMed] [Google Scholar]
- Keefe, D. H. , Feeney, M. P. , Hunter, L. L. , & Fitzpatrick, D. F. (2017). Aural acoustic stapedius-muscle reflex threshold procedures to test human infants and adults. Journal of the Association for Research in Otolaryngology, 18(1), 65–88. https://doi.org/10.1007/s10162-016-0599-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreicher, K. L. , Bauschard, M. J. , Clemmens, C. S. , Riva, C. M. , & Meyer, T. A. (2018). Audiometric assessment of pediatric patients with cystic fibrosis. Journal of Cystic Fibrosis, 17(3), 383–390. https://doi.org/10.1016/j.jcf.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Kulczycki, L. L. , Butler, J. S. , McCord-Dickman, D. , & Herer, G. R. (1970). The hearing of patients with cystic fibrosis. Archives of Otolaryngology—Head & Neck Surgery, 92(1), 54. https://doi.org/10.1001/archotol.1970.04310010080009 [DOI] [PubMed] [Google Scholar]
- Lippmann, R. P. (1996). Accurate consonant perception without mid-frequency speech energy. IEEE Transactions on Speech and Audio Processing, 4(1), 66. https://doi.org/10.1109/TSA.1996.481454 [Google Scholar]
- Liu, K. , Jiang, X. , Shi, C. , Shi, L. , Yang, B. , Shi, L. , Xu, Y. , Yang, W. , & Yang, S. (2013). Cochlear inner hair cell ribbon synapse is the primary target of ototoxic aminoglycoside stimuli. Molecular Neurobiology, 48(3), 647–654. https://doi.org/10.1007/s12035-013-8454-2 [DOI] [PubMed] [Google Scholar]
- Magimairaj, B. M. , Nagaraj, N. K. , & Benafield, N. J. (2018). Children's speech perception in noise: Evidence for dissociation from language and working memory. Journal of Speech, Language, and Hearing Research, 61(5), 1294–1305. https://doi.org/10.1044/2018_JSLHR-H-17-0312 [DOI] [PubMed] [Google Scholar]
- Martins, L. , Camargos, P. A. , Becker, H. M. , Becker, C. G. , & Guimaraes, R. E. (2010). Hearing loss in cystic fibrosis. International Journal of Pediatric Otorhinolaryngology, 74(5), 469–473. https://doi.org/10.1016/j.ijporl.2010.01.021 [DOI] [PubMed] [Google Scholar]
- Martins, L. , Guimarães, R. E. , Becker, H. M. , Bedran, M. B. , Medeiros, M. , & Camargos, P. (2011). Low prevalence of middle ear disease in cystic fibrosis patients. Jornal de Pediatria, 87(1), 80–83. https://doi.org/10.2223/JPED.2061 [DOI] [PubMed] [Google Scholar]
- McCreery, R. W. , Spratford, M. , Kirby, B. , & Brennan, M. (2017). Individual differences in language and working memory affect children's speech recognition in noise. International Journal of Audiology, 56(5), 306–315. https://doi.org/10.1080/14992027.2016.1266703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRorie, T. I. , Bosso, J. , & Randolph, L. (1989). Aminoglycoside ototoxicity in cystic fibrosis. Evaluation by high-frequency audiometry. The American Journal of Diseases of Children, 143(11), 1328–1332. https://doi.org/10.1001/archpedi.1989.02150230086028 [DOI] [PubMed] [Google Scholar]
- Moeller, M. P. , Tomblin, J. B. , Yoshinaga-Itano, C. , Connor, C. M. , & Jerger, S. (2007). Current state of knowledge: Language and literacy of children with hearing impairment. Ear and Hearing, 28(6), 740–753. https://doi.org/10.1097/AUD.0b013e318157f07f [DOI] [PubMed] [Google Scholar]
- Monson, B. B. , Rock, J. , Schulz, A. , Hoffman, E. , & Buss, E. (2019). Ecological cocktail party listening reveals the utility of extended high-frequency hearing. Hearing Research, 381, 107773. https://doi.org/10.1016/j.heares.2019.107773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh Zadeh, L. , Silbert, N. H. , Sternasty, K. , Swanepoel, W. , Hunter, L. L. , & Moore, D. R. (2019). Extended high-frequency hearing enhances speech perception in noise. Proceedings of the National Academy of Sciences of the United States of America, 116(47), 23753–23759. https://doi.org/10.1073/pnas.1903315116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulheran, M. , Degg, C. , Burr, S. , Morgan, D. W. , & Stableforth, D. E. (2001). Occurrence and risk of cochleotoxicity in cystic fibrosis patients receiving repeated high-dose aminoglycoside therapy. Antimicrobial Agents and Chemotherapy, 45(9), 2502–2509. https://doi.org/10.1128/AAC.45.9.2502-2509.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, M. , Soli, S. D. , & Sullivan, J. A. (1994). Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. The Journal of the Acoustical Society of America, 95(2), 1085–1099. https://doi.org/10.1121/1.408469 [DOI] [PubMed] [Google Scholar]
- Northern, J. L. , & Downs, M. P. (1984). Hearing in children (3rd ed.). Lippincott Williams & Wilkins. [Google Scholar]
- O'Sullivan, M. E. , Perez, A. , Lin, R. , Sajjadi, A. , Ricci, A. J. , & Cheng, A. G. (2017). Towards the prevention of aminoglycoside-related hearing loss. Frontiers in Cellular Neuroscience, 11, 325. https://doi.org/10.3389/fncel.2017.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelik, T. , Ozgirgin, N. , Ozcelik, U. , Gocmen, A. , Gurcan, B. , & Kiper, N. (1996). Auditory nerve-brainstem responses in cystic fibrosis patients. International Journal of Pediatric Otorhinolaryngology, 35(2), 165–169. https://doi.org/10.1016/0165-5876(95)01321-0 [DOI] [PubMed] [Google Scholar]
- Pauna, H. F. , Monsanto, R. C. , Kurata, N. , Paparella, M. M. , & Cureoglu, S. (2017). Changes in the inner ear structures in cystic fibrosis patients. International Journal of Pediatric Otorhinolaryngology, 92, 108–114. https://doi.org/10.1016/j.ijporl.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, S. S. , Jensen, T. , Osterhammel, D. , & Osterhammel, P. (1987). Cumulative and acute toxicity of repeated high-dose tobramycin treatment in cystic fibrosis. Antimicrobial Agents and Chemotherapy, 31(4), 594–599. https://doi.org/10.1128/AAC.31.4.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott, W. A., Jr. (2011). National survey of extended-interval aminoglycoside dosing in pediatric cystic fibrosis pulmonary exacerbations. The Journal of Pediatric Pharmacology and Therapeutics, 16(4), 262–269. https://doi.org/10.5863/1551-6776-16.4.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roup, C. M. , Wiley, T. L. , Safady, S. H. , & Stoppenbach, D. T. (1998). Tympanometric screening norms for adults. American Journal of Audiology, 7(2), 55–60. https://doi.org/10.1044/1059-0889(1998/014) [DOI] [PubMed] [Google Scholar]
- Scheenstra, R. J. , Heijerman, H. G. , Zuur, C. L. , Touw, D. J. , & Rijntjes, E. (2010). No hearing loss after repeated courses of tobramycin in cystic fibrosis patients. Acta Oto-Laryngologica, 130(2), 253–258. https://doi.org/10.3109/00016480903015150 [DOI] [PubMed] [Google Scholar]
- Scheenstra, R. J. , Rijntjes, E. , Tavy, D. L. J. , Kingma, H. , & Heijerman, H. G. M. (2009). Vestibulotoxicity as a consequence of systemically administered tobramycin in cystic fibrosis patients [Article]. Acta Oto-Laryngologica, 129(1), 4–7. https://doi.org/10.1080/00016480801968534 [DOI] [PubMed] [Google Scholar]
- Seeto, A. , & Searchfield, G. D. (2018). Investigation of extended bandwidth hearing aid amplification on speech intelligibility and sound quality in adults with mild-to-moderate hearing loss. Journal of the American Academy of Audiology, 29(3), 243–254. https://doi.org/10.3766/jaaa.16180 [DOI] [PubMed] [Google Scholar]
- Seifert, C. M. , Harvey, R. J. , Mathews, J. W. , Meyer, T. A. , Ahn, C. , Woodworth, B. A. , & Schlosser, R. J. (2010). Temporal bone pneumatization and its relationship to paranasal sinus development in cystic fibrosis. Rhinology, 48(2), 233–238. https://doi.org/10.4193/Rhin09.145 [DOI] [PubMed] [Google Scholar]
- Selimoglu, E. (2007). Aminoglycoside-induced ototoxicity. Current Pharmaceutical Design, 13(1), 119–126. https://doi.org/10.2174/138161207779313731 [DOI] [PubMed] [Google Scholar]
- Solmaz, F. , Gündoğdu, E. , Akduman, D. , Haksever, M. , Dikici, O. , & Ünal, F. (2016). Does amikacin treatment cause subclinical hearing loss in patients with cystic fibrosis? Toxicology Reports, 3, 401–404. https://doi.org/10.1016/j.toxrep.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone, M. , Schachern, P. A. , & Paparella, M. M. (1998). Loss of spiral ganglion cells as primary manifestation of aminoglycoside ototoxicity. Hearing Research, 115(1–2), 217–223. https://doi.org/10.1016/S0378-5955(97)00191-3 [DOI] [PubMed] [Google Scholar]
- Stavroulaki, P. , Vossinakis, I. C. , Dinopoulou, D. , Doudounakis, S. , Adamopoulos, G. , & Apostolopoulos, N. (2002). Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Archive of Otolaryngology—Head & Neck Surgery, 128(2), 150–155. https://doi.org/10.1001/archotol.128.2.150 [DOI] [PubMed] [Google Scholar]
- Szaff, M. , Høiby, N. , & Flensborg, E. W. (1983). Frequent antibiotic therapy improves survival of cystic fibrosis patients with chronic Pseudomonas aeruginosa infection. Acta Pædiatrica, 72(5), 651–657. https://doi.org/10.1111/j.1651-2227.1983.tb09789.x [DOI] [PubMed] [Google Scholar]
- Thomsen, J. , Friis, B. , Jensen, K. , Bak-Pedersen, K. , & Larsen, P. K. (1979). Tobramycin ototoxicity. Repeated courses of high dosage treatment in children with cystic fibrosis. Journal of Antimicrobial Chemotherapy, 5(3), 257–260. https://doi.org/10.1093/jac/5.3.257 [DOI] [PubMed] [Google Scholar]
- Todd, N. W. , & Martin, W. S. (1988). Temporal bone pneumatization in cystic fibrosis patients. Laryngoscope, 98(10), 1046–1049. https://doi.org/10.1288/00005537-198810000-00004 [DOI] [PubMed] [Google Scholar]
- Van Meter, D. J. , Corriveau, M. , Ahern, J. W. , & Lahiri, T. (2009). A survey of once-daily dosage tobramycin therapy in patients with cystic fibrosis. Pediatric Pulmonology, 44(4), 325–329. https://doi.org/10.1002/ppul.20985 [DOI] [PubMed] [Google Scholar]
- Vitela, A. D. , Monson, B. B. , & Lotto, A. J. (2015). Phoneme categorization relying solely on high-frequency energy. The Journal of the Acoustical Society of America, 137(1), El65–E170. https://doi.org/10.1121/1.4903917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman, M. R. , Putterman, D. B. , Garinis, A. C. , Hunter, L. L. , & Feeney, M. P. (in press). Wideband acoustic reflex growth in adults with cystic fibrosis. American Journal of Audiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend, I. , Beach, E. F. , & Sharma, M. (2019). Working memory and extended high-frequency hearing in adults: diagnostic predictors of speech-in-noise perception. Ear and Hearing, 40(3), 458–467. https://doi.org/10.1097/AUD.0000000000000640 [DOI] [PubMed] [Google Scholar]
- Yildirim, N. , Sone, M. , Mutlu, C. , Schachern, P. A. , Paparella, M. M. , & Le, C. T. (2000). Histopathologic features of the temporal bone in patients with cystic fibrosis. Archive of Otolaryngology—Head & Neck Surgery, 126(1), 75–78. https://doi.org/10.1001/archotol.126.1.75 [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano, C. (1999). Benefits of early intervention for children with hearing loss. Otolaryngologic Clinics of North America, 32(6), 1089–1102. https://doi.org/10.1016/S0030-6665(05)70196-1 [DOI] [PubMed] [Google Scholar]
- Yoshinaga-Itano, C. (2003). From screening to early identification and intervention: Discovering predictors to successful outcomes for children with significant hearing loss. The Journal of Deaf Studies and Deaf Education, 8(1), 11–30. https://doi.org/10.1093/deafed/8.1.11 [DOI] [PubMed] [Google Scholar]
- Zettner, E. M. , & Gleser, M. A. (2018). Progressive hearing loss among patients with cystic fibrosis and parenteral aminoglycoside treatment. Otolaryngology—Head & Neck Surgery, 159(5), 887–894. https://doi.org/10.1177/0194599818782444 [DOI] [PubMed] [Google Scholar]