Abstract
Objective
Proficiency in endoscopic endonasal skull base surgery requires both substantial baseline training and progressive lifelong learning. Endoscopic simulation models continue to evolve in an effort to optimize trainee education and preoperative preparation and improve surgical outcomes. The current scoping review systematically reviews all available literature and synthesizes the current paradigms of simulation models for endoscopic skull base surgery training and skill enhancement.
Methods
In accordance with Preferred Reporting Items for Systemic Review and Meta‐Analyses guidelines, we systematically searched PubMed, Embase, CINAHL, and Cochrane databases. Studies were categorized according to the type of simulation models investigated.
Results
We identified 238 unique references, with 55 studies ultimately meeting inclusion criteria. Of these, 19 studies described cadaveric dissection models, 17 discussed three‐dimensional (3D) printed models, 14 examined virtual surgical planning and augmented reality‐based models, and five 5 articles described task trainers.
Conclusions
There are a wide variety of simulation models for endoscopic skull base surgery, including high‐fidelity cadaveric, virtual reality, and 3D‐printed models. These models are an asset for trainee development and preoperative surgical preparation.
Keywords: resident education, skull base surgery, surgical simulation training, virtual reality, virtual surgical planning
INTRODUCTION
Proficiency in endoscopic skull base surgery requires methodical practice and regular repetition. Currently, most hands‐on training occurs in the operative setting under the guidance of senior surgeons through a traditional apprenticeship model. While this environment provides an ideal venue for observation of actual techniques, the high stakes nature of live surgery lacks adequate opportunity for surgical training and development of an endoscopic skillset. Moreover, trainees are limited by the availability of educators within their training network, and operative cases rarely reflect the breadth of anatomical involvement of various tumors. Providing additional surgical education for trainees outside of the operating room is optimal to lower the risk of patient morbidity and reduce operative times. 1
Surgical simulation models are a widely used modality to develop procedural competency and optimize performance in the operating room. Simulation training improves surgeon confidence and skills, clinical outcomes, and may reduce healthcare costs. 2 , 3 , 4 Simulation is particularly valuable in endoscopic cranial base surgery due to the complex anatomy, challenging ergonomics, and high risk for complications. 5 In addition to surgical training, patient‐specific simulation models are available to guide perioperative planning and to rehearse complicated skull base procedures. 6
Recent technological advancements have facilitated iterative improvement in endoscopic skull base surgery simulation models to accommodate pioneering endoscopic approaches and surgical techniques. Compared to early low‐fidelity synthetic models and task trainers, more recent models have placed a greater emphasis on high‐fidelity, cadaveric, three‐dimensional (3D), and virtual reality (VR)/augmented reality (AR) simulation experiences with supplemented user interaction, such as haptic feedback. 7 These advancements have expanded the field of simulation training and surgical education in general.
In this scoping review, we systematically search and synthesize the literature to provide an up‐to‐date overview of simulation models for endoscopic skull base surgery. We highlight the various applications of simulation models, illustrate the growth of the field throughout the past 20 years, and propose future efforts to continue to optimize endoscopic surgical training
METHODS
Per the Preferred Reporting Items for Systemic Review and Meta‐Analyses guidelines, we performed systematic search queries in PubMed, Embase, CINAHL, and Cochrane Database of Systematic Reviews for skull base simulation models from inception until June 15, 2021. The search queries consisted of a combination of subject headings and search terms grouped by the following three concepts: endoscopy, skull base, and simulation. Appropriate search strings linking these concepts were then combined with the Boolean operator AND to produce the search terms: ([Endoscop*] AND [Simulation]) AND (skull base). PubMed search was also conducted using the following MeSH terms: (“Skull Base” [Mesh]) AND (“Endoscopy” [Mesh]) AND (“Simulation Training” [Mesh]). We then conducted a bibliographic review of included references to ensure completeness of our search, where bibliographies of relevant studies were manually screened to identify additional studies for consideration.
Inclusion criteria consisted of English‐language, peer‐reviewed studies discussing endoscopic skull base surgery simulation models. Studies were excluded if they focused on other anatomic sites in the head and neck or focused primarily on functional endoscopic sinus surgery rather than skull base surgery approaches. Conference abstracts and studies in languages other than English were also excluded. Studies discussing simulation for surgical planning were captured by our search and incorporated in this review.
The following data were extracted: author, date, type of model, the procedure(s) studied, and the primary study outcomes.
RESULTS
Our search yielded a total of 238 studies after the removal of duplicates (Figure 1). After title and abstract review, we identified 68 articles for full‐text review, and ultimately included 49 studies for data extraction. Six additional studies were identified and included after bibliographic review, yielding a total of 55 included studies.
Figure 1.
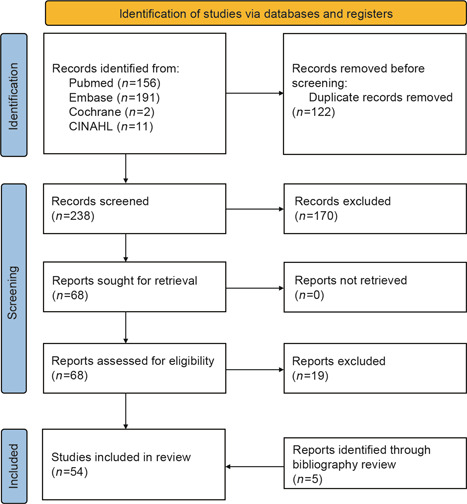
Preferred Reporting Items for Systemic Review and Meta‐Analyses flow chart
Cadaveric models
Nineteen studies of cadaveric models were identified (Table 1). 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 These studies were classified into the following management subcategories: (i) internal carotid artery injury, (ii) vascular clipping, (iii) cerebrospinal fluid (CSF) leak, (iv) tumor resection, and (v) miscellaneous endoscopic endonasal skull base and/or neurosurgical procedures.
Table 1.
Cadaveric dissection skull base simulation models
| Study (first author surname, year of publication) | Procedure studied | Primary outcome |
|---|---|---|
| Internal carotid artery injury | ||
| Donoho, 2019 | ICAI management | Training success and costs |
| Donoho, 2021 | ICAI management | Face and construct validity, trial success rate, time to hemostasis, estimated blood loss, surgeon tachycardia (before and after educational intervention for each outcome) |
| Shen, 2018 | ICAI management | Face and content validity, time to hemostasis, estimated blood loss, surgeon confidence score (before and after educational intervention for each outcome) |
| Pacca, 2017 | ICAI management | Educational value assessed on questionnaire |
| Padhye, 2015 | ICAI management using sheep head and SIMONT | Posttraining operative outcomes |
| Pham, 2014 | ICAI management | Educational value assessed on questionnaire |
| Vascular clipping | ||
| Ciporen, 2016 | Cavernous carotid artery clipping | Feasibility |
| Ciporen, 2017 | Posterior cerebral circulation clipping | Feasibility |
| CSF leak | ||
| AlQatahni, 2021 | CSF leak repair | Face, content, and construct validity |
| Mattavelli, 2020 | CSF leak repair | Feasibility |
| Christian, 2018 | CSF leak repair | Feasibility, pre‐ and posttraining surgeon confidence score |
| AlQatahni, 2018 | CSF leak repair | Feasibility |
| Tumor resection | ||
| Gagliardi, 2018 | Tumor resection (using NICO Myriad System) | Questionnaire assessing the utility of model |
| Gragnaniello, 2014 | Tumor resection | Descriptive report |
| Berhouma, 2013 | Tumor resection | Descriptive report |
| Gragnaniello, 2010 | Tumor resection | Questionnaire assessing similarity of the model to real tumor cases |
| General endoscopic endonasal skull base procedures | ||
| Dias, 2013 | Skull base endoscopic dissection | Cost, portability, image quality |
| Fortes, 2008 | Transpterygoid approach | Feasibility |
| Aboud, 2002 | Various neurosurgical procedures | Descriptive report |
Abbreviations: CSF, cerebrospinal fluid; ICAI, internal carotid artery injury.
Six studies focused on injury to the internal carotid artery, a severe complication of endoscopic skull base procedures. Two studies by Donoho et al. 8 , 9 established the validity, educational utility, and financial costs of using a cadaveric model perfused with artificial blood to practice managing internal carotid artery injury. Using pre‐ and posttraining subjective questionnaires, studies by Pacca et al. 19 and Pham et al. 20 identified that using a perfused cadaveric model added significant educational value for trainees. In a retrospective multicenter case series, Padhye et al. 22 reported improved management techniques among participants who attended a workshop on controlling major arterial bleeding caused by injury to large vessels, including the internal carotid, during endoscopic skull base surgery.
Vascular clipping was the focus of two studies by Ciporen et al. 23 , 24 They demonstrated the feasibility and simplicity of setting up a cost‐effective and replicable cadaveric model to simulate clip placement on the cavernous carotid artery and the posterior cerebral circulation.
Four studies explored simulated repair of CSF leak in cadaveric models. Mattavelli et al. 25 studied the feasibility of simulating a CSF leak in a cadaveric model as well as objectively quantifying the effectiveness of skull base reconstruction by measuring the pressure it can withstand. Christian et al. 26 used a similar model but with intrathecal perfusion of fluorescein‐dyed saline to optimize visual feedback of successful repair. Lastly, AlQatahni et al. 10 , 11 described a similar model using fluorescein‐dyed saline and conducted two studies to establish the feasibility as well as face, content, and construct validity of their model.
Tumor resection was studied in four studies, all of which used an injectable polymer tumor in a cadaveric specimen. 12 , 13 , 14 , 15 The basis for this model was reported by Gragnaniello et al., 13 who first described the utility of a polymer‐based tumor model in 2010. In 2014, the same investigators discussed an optimized polymer model that includes properties such as contrast enhancement, enabling the polymer to be injected under fluoroscopic guidance, as well as the ability to produce intracranial mass effect. 15 Gagliardi et al. 14 illustrated the value of using the NICO Myriad System, a neurosurgical tumor resection tool, in cadaveric simulation models to prepare for similar instrument use during live cases.
Lastly, three studies discussed cadaveric simulation of various endoscopic skull base procedures. Dias et al. 16 described the favorable cost, portability, and image quality of a laptop‐based endoscopic device, as opposed to a costly high‐definition endoscopy system, to aid trainees learning endoscopic dissection techniques. Fortes et al. 17 injected a cadaveric model with colored liquid silicone to aid in the visualization of vascular structures and anatomy of the pterygopalatine fossa. Similarly, Aboud et al. 18 developed a model that enabled dynamic filling of vascular structures and arachnoid cisterns with colored and clear fluid, respectively.
3D‐printed models
Fourteen studies of 3D‐printed models for simulation training of ventral skull base procedures were identified (Table 2). 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Zheng et al. 27 and Shah et al. 35 reported that 3D‐printed models were useful for the purposes of anatomic education of the endoscopic approach to the cranial base. An additional study demonstrated that a 3D model produced using selective laser sintering—fusion of printing materials to form the model—was a feasible and useful simulation of the cranial base for endoscopic approaches. 37 Two studies used laser‐sintered 3D‐printed models to simulate and educate trainees on the management of internal carotid artery injury. Successive training sessions found improved self‐confidence, operating time, blood loss, time to control bleeding, and maintenance of a clear endoscopic view following training on the printed models. 38 , 39 In a study comparing human cadaver bone to four consumer‐grade materials used for 3D printing, polycarbonate was found to be the best substitute for cadaver bone. 32 In addition, one pediatric skull base simulation model for the resection of a pediatric craniopharyngioma was identified. 33 A notable hybrid model by Okuda et al. 30 involved using the commercially produced SurgTrainer 3D‐printed model of the paranasal sinuses and skull base in combination with an egg to simulate endoscopic transsphenoidal resection of pituitary adenomas.
Table 2.
3D‐printed skull base simulation models
| Study (first author surname, year of publication) | Procedure studied | Primary outcome |
|---|---|---|
| London Jr., 2021 | Pediatric skull base/craniopharyngioma resection | Fidelity of skeletonization of the carotid arteries and sella face |
| Maza, 2019 | ICAI | Time to hemostasis, estimated blood loss, trainee self‐confidence |
| Zheng, 2018 | Skull base surgery | Anatomic fidelity and educational value assessed on the questionnaire |
| Zhang, 2018 | Sinus & skull base | Educational value assessed on the questionnaire |
| Hsieh 2018 | Skull base surgery | Anatomic Accuracy, endoscopic anterior craniofacial resection, transpterygoid, and transclival approaches. |
| Muto, 2017 | ICAI | Educational value assessed on the questionnaire |
| Favier, 2017 | Compares four 3D‐printed model to a cadaver for skull base surgery training | 3D printing material best suited for training |
| Wen, 2016 | Skull base surgery | Drilling, curetting, and aspirating performance |
| Tai, 2016 | Endoscopic endonasal drilling techniques | Content validity |
| Shah, 2016 | Skull base surgery | Identification of anatomic structures |
| Oyama, 2015 | Skull base surgery | Exploratory study |
| Narayan, 2015 | Skull base surgery | Ease of learning endoscopic skull base exposure and drilling techniques |
| de Notaris, 2013 | Various endoscopic endonasal approaches | Descriptive report |
| Okuda, 2011 | Transphenoidal resection of pituitary adenoma | Surgical technique using SurgTrainer & egg |
| Preoperative planning | ||
| Huang, 2019 | Pituitary macroadenoma resection | Operative performance in trainees who had pre‐op 3D models versus that of trainees who did not |
| Lin, 2018 | Sellar tumor resection | Fidelity |
| Shinomiya, 2018 | Pituitary adenoma resection | Utility in surgical planning |
Abbreviations: 3D, three‐dimensional; CSF, cerebrospinal fluid; ICAI, internal carotid artery injury.
Three studies discussed the use of 3D‐printed models in preoperative preparation for pituitary and sellar tumor resections (Table 2). 6 , 40 , 41 One retrospective study of endoscopic transsphenoidal surgery reported improved patient outcomes and operative performance when a 3D‐printed model of the patient's skull base was produced and utilized for preoperative planning. 40
Task trainers
Five studies describing surgical task trainers were identified in our search (Table 3). The Sinus Model Otorhino Neuro Trainer (S.I.M.O.N.T) trainer, developed in Brazil, is an anatomical model made of a synthetic thermoretractable rubber called Neoderma. This trainer was found to be a useful tool for endonasal surgical training when used by neurosurgeons. 42 A subsequent prospective study found improved confidence, safety, and performance in trainees and surgeons 6 months after attending a training session. 43 The ENDOtrainer, a more recently developed simulation trainer, mimics endonasal skull base surgery using a training box made from a synthetic polymer structure to mimic constrained working conditions, mandarin oranges and chicken wings to mimic raising of flaps, and a quail egg to mimic tumor resection. 44 In addition, Singh et al. 45 described the Neuro‐Endo‐Trainer, a task trainer that requires trainees to maneuver rings through pegs representing anatomical positions in the ventral skull base. Of note, the distances between the pegs were obtained through analysis of computed tomography (CT) of prior patients to create the most realistic simulation experience. Similarly, a webcam‐based task trainer required trainees to manipulate pegs and rings in a closed box using surgical instruments. Hirayama et al. 46 reported that the use of this trainer was associated with improved speed and efficiency during VR simulation of endoscopic endonasal surgery.
Table 3.
Task trainer simulation models for general endoscopic endonasal skull base procedures
| Study (first author surname, year of publication) | Name of task trainer | Primary outcome |
|---|---|---|
| Sanroman‐Alvarez, 2017 | ENDO trainer for skull base | Hand‐eye coordination, dexterity and precision utility; for new trainees: time to completion |
| Singh, 2016 | Neuro‐Endo trainer | Utility and validity |
| Fortes, 2016 | SIMONT trainer | Anatomic structure identification and operative performance |
| Hirayama, 2013 | Webcam Box trainer | Performance on VR simulator posttraining |
| Filho, 2011 | SIMONT trainer | Fidelity and development of surgical skills |
Abbreviation: VR, virtual reality.
Virtual surgical planning and VR/AR
Our search identified 14 studies of virtual surgical planning (VSP) and VR/AR‐based endonasal endoscopic skull base surgery models (Table 4). 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 Of these studies, three were based on AR models, including two proof‐of‐concept studies and one validation study. The AR models, which are distinct from VR models, combine physical 3D‐printed models of the paranasal sinuses and skull base with superimposed trackable VR software. The physical model provides additional haptic feedback to the trainee that may be lacking in a strictly virtual experience. 47 , 48 , 53 In one study, the use of an AR model led to less cumulative “error” time, fewer error events, and a marginally significant increase in operative time when compared to the use of an endoscope alone. 48 Three studies examined VR models for educational purposes alone. One such model, the McGill simulator, a previously validated virtual simulator, was found to increase intraoperative skill and performance among trainees who underwent simulation training sessions. 54 Similarly, Rosseau et al. 52 described how the NeuroTouch simulator, originally developed by the National Research Council of Canada, may be applied to endoscopic endonasal transsphenoidal surgical procedures. Three years later, Thawani et al. 49 reported that trainees who used the NeuroTouch haptic simulation platform had improved intraoperative performance on a visual analog scale compared to trainees who used conventional resources alone. Notably, one study described the development of a freely available computer‐based VSP model. For this surgical planning tool, CT scans were converted into a virtual 3D skull model whereby surgeons were able to plan specific surgical steps based on 3D skull base anatomy. 50 , 51 Step‐by‐step interactive presentations explaining the precise surgical method were made available in portable document format to aid in education during cadaveric dissection. 57
Table 4.
Virtual surgical planning and virtual reality/augmented reality skull base simulation models
| Study (first author surname, year of publication) | VSP, VR, or AR | Procedure studied | Primary outcome |
|---|---|---|---|
| Training models | |||
| Kim, 2020 | AR | Skull base surgery | Feasibility |
| Bong, 2017 | AR | Skull base surgery | Proof of concept |
| Thawani, 2016 | VR (NeuroTouch) | Skull base surgery | Whether VR as an adjuvant training tool improved intraoperative performance |
| Mavar‐Haramija, 2015 | VSP (3D computer PDF) | Skull base surgery | Descriptive report |
| Chan, 2015 | AR | Skull base surgery | Validity |
| Varshney, 2014 | VR (McGill Simulator) | Skull base surgery | Pre‐ and posttraining skill performance |
| Rosseau, 2013 | VR (NeuroTouch) | Transsphenoidal approach | Descriptive report |
| de Notaris, 2013 | VSP | Skull base surgery | Technical report |
| de Notaris, 2011 | VSP | Skull base surgery | Descriptive report |
| de Notaris, 2010 | VSP | Skull base surgery | Descriptive report |
| Preoperative planning | |||
| Jean, 2020a | VR | Tuberculum sellae meningioma resection | Descriptive report |
| Jean, 2020b | VR | Pineocytoma resection | Utility in surgical planning |
| Won, 2018 | VR (CardinalSim) | Skull base surgery | Fidelity to prior patient cases |
| de Notaris, 2014 | VR (Dextroscope) | Skull base surgery | Descriptive report |
Abbreviations: AR, augmented reality; PDF, portal document format; VR, virtual reality; VSP, virtual surgical planning.
Four articles discussed VSP simulators for the purpose of preoperative preparation Table 3. 56 , 58 , 59 , 60 For one, de Notaris et al. 56 described the use of the Dextroscope virtual simulator in endoscopic dissections of cadaver specimens. Won et al. 60 demonstrated the utility of the CardinalSim, a virtual simulator with haptic feedback. Two articles discussed the development and use of VR simulators to plan complicated sellar tumor resections. 58 , 59
DISCUSSION
As the volume of endoscopic surgical cases continues to expand over the past decade, simulation models for advanced endoscopic endonasal skull base surgery training have grown in popularity over the past decade. In our review, we identified 55 unique studies on augmenting trainee education with surgical training models. These studies range in scope from cadaveric models to those that incorporate recently developed technologies, such as 3D‐printing, VSP, and VR/AR simulation. Relevant and practical endoscopic surgical simulators are an important addition to the training curriculum for both otolaryngology and neurosurgery trainees, due to the technically challenging nature of endoscopic skull base procedures, actual surgical cases that may not reflect the full anatomical breadth of skull base pathology, and the opportunity to prepare for cases alongside colleagues in this inherently collaborative surgical approach.
With technological advancements offering novel modeling capabilities, one might assume that the field is moving away from cadaveric models and simple VSP in favor of VR/AR simulators or 3D‐printed models. Although these technologically advanced models have gained substantial traction in the literature recently, there is a continued emphasis on the utility of cadaveric models, which are relatively low‐cost and remain a highly realistic representation of a true surgical experience. They also tend to be user‐friendly, given that most trainees have experience with cadaveric dissections throughout their medical education. Studies of these models have focused on a broad range of topics pertaining to endoscopic skull base surgery, including intraoperative complications such as internal carotid artery injury repair and CSF leak while developing alongside the practice of endoscopic skull base approaches. 18 The breadth of research not only provides a strong foundation for practical use in simulation training but also serves as a springboard for future research efforts, enabling these models to become even more reliable and advanced. Ethical and legal factors should always be considered, and preparation and preservation of the specimens may require trained personnel, which may involve additional costs; however, cadaveric models pose few other disadvantages.
3D‐printed models offer different capabilities than cadaveric specimens. For example, in terms of preoperative planning, 3D‐printed models can be produced based on specific patient anatomy. This allows surgeons to better prepare for anatomical challenges and rehearse surgical maneuvers outside of the operative setting, thereby improving surgical performance and outcomes. 40 These models are also often portable or digitally transferrable such that they can be printed in multiple locations. One drawback of 3D models is the high up‐front cost, which includes purchasing 3D printers and model substrates and hiring any personnel who may be required to operate the equipment. However, as this technology becomes more widely available and user‐friendly, costs are expected to decrease, allowing the equipment to become more accessible to surgical departments and institutions. Another important limitation is that 3D models often lack the haptic properties of natural human soft tissue and bone, although efforts are underway to identify the best material to increase the fidelity of these models. 32
In contrast to 3D‐printed and cadaveric models, task trainers typically aim to optimize the performance of specific surgical maneuvers and techniques rather than create a realistic surgical experience. For example, some trainers involve simple tasks, such as placing a ring on pegs while bimanually manipulating surgical instruments in an enclosed box. The box is intended to resemble the constrained working conditions in the endonasal cavity. 45 , 46 Overall, the intention is not to replicate the visual and haptic experience of endoscopic skull base surgery, but to improve psychomotor skills and dexterity while performing tasks that resemble surgical techniques used in these procedures. There is an emphasis on spatial relationships between anatomic structures and simulating successful navigation between them, rather than creating a replica of the structures themselves. As a result, these models are relatively low‐cost and use easily obtainable materials, such as an egg, mandarin orange, and chicken wings. 44 That being said, the SIMONT simulator is slightly different from other trainers in this review, in that it is a real anatomic model based off images and videos of anatomic structures and CT scans. 42 It also uses higher grade synthetic materials and is intended to closely approximate endoscopic dissection. While the current literature supports the utility and validity of many of these task trainers, more comparative research is needed to establish whether a realistic anatomical model, like the SIMONT simulator, or a more basic trainer like the ENDOTrainer, is associated with improved surgical performance, patient outcomes, and educational value.
VR and AR systems are one of the more exciting developments in surgical simulation in recent years. The earliest VR model for endoscopic skull base surgery identified in this review was reported in 2013, which was based off a digital 3D model previously developed in 2010. 51 , 55 The authors expand the capabilities of the digital 3D model to enable virtual dissection using the Dextroscope surgical simulation system (Volume Interactions Pte. Ltd.). While this study was mainly a proof‐of‐concept discussion, subsequent studies of other VR/AR simulators have demonstrated the validity of these models and their utility in improving operative performance. The major advantages of these models include the opportunity to repeat surgical maneuvers multiple times without losing tissue integrity and the ability to integrate instructions and educational feedback into the simulation. 57 The major disadvantage is the high upfront cost to purchase equipment and software for VR simulation. However, there are few repeated consumable material costs in the long term, which differs from 3D‐printed models and cadaveric specimens. Another disadvantage is, similar to 3D models, many VR and AR simulators lack realistic haptic feedback. Particularly for virtual models, which lack the physical component of AR simulators, such feedback helps the user identify anatomical landmarks, adjust their approach, and avoid damaging high‐risk structures. Certain models, such as the NeuroTouch simulator, incorporate haptic feedback to aid with endonasal navigation and drilling. Moving forward, simulators should incorporate haptic guidance wherever possible and provide cues through additional sensory modalities, such as high‐definition visual and auditory output.
Overall, simulation of endoscopic skull base surgery holds great promise and educational value for otolaryngology and neurosurgery trainees as well as more senior surgeons who are interested in preoperative planning or maintenance of surgical skills. There is a growing body of evidence demonstrating the validity and utility of a curriculum that integrates additional hands‐on training in the form of endoscopic task trainers, cadaveric dissections, 3D‐printed models, VSP, and VR/AR models, much of which has been published in the last 5–10 years. Future research efforts should include comparative studies to determine whether certain models or types of models provide superior educational value. In addition, more research is needed to validate the use of simulation models for a broader range of endoscopic skull base procedures. Lastly, cost‐effectiveness would be another topic of interest for potential stakeholders, given that much of the technology for 3D printing and VR simulation can be financially prohibitive.
CONCLUSION
Simulation of endoscopic skull base surgery is a valuable and increasingly popular training method. While cadaveric models were some of the earliest simulation tools developed and remain commonly used today, there is a growing interest in more technologically advanced models, such as 3D‐printed models, high‐fidelity task trainers, and VR/AR systems. Whenever possible, trainees should be exposed to simulations of these procedures outside of the operative setting to enhance surgical skills, improve confidence, and reduce patient morbidity.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
ACKNOWLEDGMENT
None declared.
James J, Irace AL, Gudis DA, Overdevest JB. Simulation training in endoscopic skull base surgery: a scoping review. World J Otorhinolaryngol Head Neck Surg. 2022;8:73‐81. 10.1002/wjo2.11
Joel James and Alexandria L. Irace denotes equal contribution.
REFERENCES
- 1. Fink E, Lord CA, Pinheiro‐Neto CD, et al. Endoscopic endonasal pituitary surgery: impact of surgical education on operation length and patient morbidity. J Neurol Surg B Skull Base. 2012;73:405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter‐related bloodstream infection after simulation‐based education for residents in a medical intensive care unit. Simul Healthc. 2010;5:98‐102. [DOI] [PubMed] [Google Scholar]
- 3. Barsuk JH, Cohen ER, Williams MV, et al. Simulation‐based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93:729‐735. [DOI] [PubMed] [Google Scholar]
- 4. McGaghie WC, Draycott TJ, Dunn WF, Lopez CM, Stefanidis D. Evaluating the impact of simulation on translational patient outcomes. Simul Healthc. 2011;6:S42‐S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner PA, Snyderman CH, Wang EW, Fernandez‐Miranda JC. Complications of endoscopic endonasal skull base surgery. J Neurosurg. 2018;87:207‐212. 10.1016/B978-0-323-50961-9.00036-0 [DOI] [Google Scholar]
- 6. Lin J, Zhou Z, Guan J, et al. Using three‐dimensional printing to create individualized cranial nerve models for skull base tumor surgery. World Neurosurg. 2018;120:e142‐e152. [DOI] [PubMed] [Google Scholar]
- 7. Lavigne P, Yang N. Training and surgical simulation in skull base surgery: a systematic review. Curr Otorhinolaryngol Rep. 2020;8:154‐159. [Google Scholar]
- 8. Donoho DA, Johnson CE, Hur KT, et al. Costs and training results of an objectively validated cadaveric perfusion‐based internal carotid artery injury simulation during endoscopic skull base surgery. Int Forum Allergy Rhinol. 2019;9:787‐794. [DOI] [PubMed] [Google Scholar]
- 9. Donoho DA, Pangal DJ, Kugener G, et al. Improved surgeon performance following cadaveric simulation of internal carotid artery injury during endoscopic endonasal surgery: training outcomes of a nationwide prospective educational intervention. J Neurosurg. 2021:1‐9. [DOI] [PubMed] [Google Scholar]
- 10. AlQahtani AA, Albathi AA, Alhammad OM, Alrabie AS. Innovative real CSF leak simulation model for rhinology training: human cadaveric design. Eur Arch Otorhinolaryngol. 2018;275:937‐941. [DOI] [PubMed] [Google Scholar]
- 11. AlQahtani A, Albathi A, Castelnuovo P, Alfawwaz F. Cerebrospinal fluid leak repair simulation model: face, content, and construct validation. Am J Rhinol Allergy. 2021;35:264‐271. [DOI] [PubMed] [Google Scholar]
- 12. Berhouma M, Baidya NB, Ismaïl AA, Zhang J, Ammirati M. Shortening the learning curve in endoscopic endonasal skull base surgery: a reproducible polymer tumor model for the trans‐sphenoidal trans‐tubercular approach to retro‐infundibular tumors. Clin Neurol Neurosurg. 2013;115:1635‐1641. [DOI] [PubMed] [Google Scholar]
- 13. Gragnaniello C, Nader R, van Doormaal T, et al. Skull base tumor model. J Neurosurg. 2010;113:1106‐1111. [DOI] [PubMed] [Google Scholar]
- 14. Gagliardi F, Chau AM, Mortini P, Caputy AJ, Gragnaniello C. Skull base neuroendoscopic training model using a fibrous injectable tumor polymer and the nico myriad. J Craniofac Surg. 2018;29:e25‐e28. [DOI] [PubMed] [Google Scholar]
- 15. Gragnaniello C, Gagliardi F, Chau A, et al. Intracranial injectable tumor model: technical advancements. J Neurol Surg B Skull Base. 2014;75:301‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dias LA, Gebhard H, Mtui E, Anand VK, Schwartz TH. The use of an ultraportable universal serial bus endoscope for education and training in neuroendoscopy. World Neurosurg. 2013;79:337‐340. [DOI] [PubMed] [Google Scholar]
- 17. Fortes FSG, Sennes LU, Carrau RL, et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118:44‐49. [DOI] [PubMed] [Google Scholar]
- 18. Aboud E, Al‐Mefty O, Yaşargil MG. New laboratory model for neurosurgical training that simulates live surgery. J Neurosurg. 2002;97:1367‐1372. [DOI] [PubMed] [Google Scholar]
- 19. Pacca P, Jhawar SS, Seclen DV, et al. “Live cadaver” model for internal carotid artery injury simulation in endoscopic endonasal skull base surgery. Oper Neurosurg. 2017;13:732‐738. [DOI] [PubMed] [Google Scholar]
- 20. Pham E, Kale A, Marquez Y, et al. A perfusion‐based human cadaveric model for management of carotid artery injury during endoscopic endonasal skull base surgery. J Neurol Surg B Skull Base. 2014;75:309‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen J, Hur K, Zhang Z, et al. Objective validation of perfusion‐based human cadaveric simulation training model for management of internal carotid artery injury in endoscopic endonasal sinus and skull base surgery. Oper Neurosurg. 2018;15:231‐238. [DOI] [PubMed] [Google Scholar]
- 22. Padhye V, Valentine R, Sacks R, et al. Coping with catastrophe: the value of endoscopic vascular injury training. Int Forum Allergy Rhinol. 2015;5:247‐252. [DOI] [PubMed] [Google Scholar]
- 23. Ciporen J, Lucke‐Wold B, Dogan A, Cetas JS, Cameron WE. Dual endoscopic endonasal transsphenoidal and precaruncular transorbital approaches for clipping of the cavernous carotid artery: a cadaveric simulation. J Neurol Surg B Skull Base. 2016;77:485‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciporen JN, Lucke‐Wold B, Dogan A, Cetas J, Cameron W. Endoscopic endonasal transclival approach versus dual transorbital port technique for clip application to the posterior circulation: a cadaveric anatomical and cerebral circulation simulation study. J Neurol Surg B Skull Base. 2017;78:235‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mattavelli D, Ferrari M, Rampinelli V, et al. Development and validation of a preclinical model for training and assessment of cerebrospinal fluid leak repair in endoscopic skull base surgery. Int Forum Allergy Rhinol. 2020;10:89‐96. [DOI] [PubMed] [Google Scholar]
- 26. Christian EA, Bakhsheshian J, Strickland BA, et al. Perfusion‐based human cadaveric specimen as a simulation training model in repairing cerebrospinal fluid leaks during endoscopic endonasal skull base surgery. J Neurosurg. 2018;129:792‐796. [DOI] [PubMed] [Google Scholar]
- 27. Zheng JP, Li CZ, Chen GQ, Song GD, Zhang YZ. Three‐dimensional printed skull base simulation for transnasal endoscopic surgical training. World Neurosurg. 2018;111:e773‐e782. [DOI] [PubMed] [Google Scholar]
- 28. Zhang XD, Li ZH, Wu ZS, et al. A novel three‐dimensional‐printed paranasal sinus‐skull base anatomical model. Eur Arch Otorhinolaryngol. 2018;275:2045‐2049. [DOI] [PubMed] [Google Scholar]
- 29. Wen G, Cong Z, Liu K, et al. A practical 3D printed simulator for endoscopic endonasal transsphenoidal surgery to improve basic operational skills. Childs Nerv Syst. 2016;32:1109‐1116. [DOI] [PubMed] [Google Scholar]
- 30. Okuda T, Yamashita J, Fujita M, Yoshioka H, Tasaki T, Kato A. The chicken egg and skull model of endoscopic endonasal transsphenoidal surgery improves trainee drilling skills. Acta Neurochir. 2014;156:1403‐1407. [DOI] [PubMed] [Google Scholar]
- 31. Hsieh TY, Cervenka B, Dedhia R, Strong EB, Steele T. Assessment of a patient‐specific, 3‐dimensionally printed endoscopic sinus and skull base surgical model. JAMA Otolaryngol Head Neck Surg. 2018;144:574‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Favier V, Zemiti N, Caravaca Mora O, et al. Geometric and mechanical evaluation of 3D‐printing materials for skull base anatomical education and endoscopic surgery simulation—a first step to create reliable customized simulators. PLoS One. 2017;12:e0189486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. London NR, Jr. , Rangel GG, et al. Simulation of pediatric anterior skull base anatomy using a 3D printed model. World Neurosurg. 2021;147:e405‐e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tai BL, Wang AC, Joseph JR, et al. A physical simulator for endoscopic endonasal drilling techniques: technical note. J Neurosurg. 2016;124:811‐816. [DOI] [PubMed] [Google Scholar]
- 35. Shah KJ, Peterson JC, Beahm DD, Camarata PJ, Chamoun RB. Three‐dimensional printed model used to teach skull base anatomy through a transsphenoidal approach for neurosurgery residents. Oper Neurosurg. 2016;12:326‐329. [DOI] [PubMed] [Google Scholar]
- 36. Narayanan V, Narayanan P, Rajagopalan R, et al. Endoscopic skull base training using 3D printed models with pre‐existing pathology. Eur Arch Otorhinolaryngol. 2015;272:753‐757. [DOI] [PubMed] [Google Scholar]
- 37. Oyama K, Filho LFSD, Muto J, et al. Endoscopic endonasal cranial base surgery simulation using an artificial cranial base model created by selective laser sintering. Neurosurg Rev. 2015;38:171‐178. [DOI] [PubMed] [Google Scholar]
- 38. Maza G, VanKoevering KK, Yanez‐Siller JC, et al. Surgical simulation of a catastrophic internal carotid artery injury: a laser‐sintered model. Int Forum Allergy Rhinol. 2019;9:53‐59. [DOI] [PubMed] [Google Scholar]
- 39. Muto J, Carrau RL, Oyama K, Otto BA, Prevedello DM. Training model for control of an internal carotid artery injury during transsphenoidal surgery. Laryngoscope. 2017;127:38‐43. [DOI] [PubMed] [Google Scholar]
- 40. Huang X, Liu Z, Wang X, et al. A small 3D‐printing model of macroadenomas for endoscopic endonasal surgery. Pituitary. 2019;22:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shinomiya A, Shindo A, Kawanishi M, et al. Usefulness of the 3D virtual visualization surgical planning simulation and 3D model for endoscopic endonasal transsphenoidal surgery of pituitary adenoma: technical report and review of literature. Interdiscip Neurosurg Adv Tech Case Manag. 2018;13:13‐19. [Google Scholar]
- 42. Filho FV, Coelho G, Cavalheiro S, Lyra M, Zymberg ST. Quality assessment of a new surgical simulator for neuroendoscopic training. Neurosurg Focus. 2011;30:E17. [DOI] [PubMed] [Google Scholar]
- 43. Fortes B, Balsalobre L, Weber R, et al. Endoscopic sinus surgery dissection courses using a real simulator: the benefits of this training. Braz J Otorhinolaryngol. 2016;82:26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanromán‐Álvarez P, Simal‐Julián JA, García‐Piñero A, Miranda‐Lloret P. Multitask box trainer for endoscopic endonasal skull base surgery: ENDOtrainer. World Neurosurg. 2017;101:304‐307. [DOI] [PubMed] [Google Scholar]
- 45. Singh R, Baby B, Damodaran N, et al. Design and validation of an open‐source, partial task trainer for endonasal neuro‐endoscopic skills development: Indian experience. World Neurosurg. 2016;86:259‐269. [DOI] [PubMed] [Google Scholar]
- 46. Hirayama R, Fujimoto Y, Umegaki M, et al. Training to acquire psychomotor skills for endoscopic endonasal surgery using a personal webcam trainer. J Neurosurg. 2013;118:1120‐1126. [DOI] [PubMed] [Google Scholar]
- 47. Kim DH, Kim HM, Park JS, Kim SW. Virtual reality haptic simulator for endoscopic sinus and skull base surgeries. J Craniofac Surg. 2020;31:1811‐1814. [DOI] [PubMed] [Google Scholar]
- 48. Bong JH, Song HJ, Oh Y, Park N, Kim H, Park S. Endoscopic navigation system with extended field of view using augmented reality technology. Int J Med Robot. 2018;14. [DOI] [PubMed] [Google Scholar]
- 49. Thawani JP, Ramayya AG, Abdullah KG, et al. Resident simulation training in endoscopic endonasal surgery utilizing haptic feedback technology. J Clin Neurosci. 2016;34:112‐116. [DOI] [PubMed] [Google Scholar]
- 50. de Notaris M, Solari D, Cavallo LM, et al. The use of a three‐dimensional novel computer‐based model for analysis of the endonasal endoscopic approach to the midline skull base. World Neurosurg. 2011;75:106‐113. [DOI] [PubMed] [Google Scholar]
- 51. de Notaris M, Prats‐Galino A, Cavallo LM, et al. Preliminary experience with a new three‐dimensional computer‐based model for the study and the analysis of skull base approaches. Childs Nerv Syst. 2010;26:621‐626. [DOI] [PubMed] [Google Scholar]
- 52. Rosseau G, Bailes J, del Maestro R, et al. The development of a virtual simulator for training neurosurgeons to perform and perfect endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2013;73(suppl 1):85‐93. [DOI] [PubMed] [Google Scholar]
- 53. Chan HH, Siewerdsen JH, Vescan A, Daly MJ, Prisman E, Irish JC. 3D rapid prototyping for otolaryngology‐head and neck surgery: applications in image‐guidance, surgical simulation and patient‐specific modeling. PLoS One. 2015;10:e0136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Varshney R, Frenkiel S, Nguyen LHP, et al. Development of the McGill simulator for endoscopic sinus surgery: a new high‐fidelity virtual reality simulator for endoscopic sinus surgery. Am J Rhinol Allergy. 2014;28:330‐334. [DOI] [PubMed] [Google Scholar]
- 55. de Notaris M, Topczewski T, de Angelis M, et al. Anatomic skull base education using advanced neuroimaging techniques. World Neurosurg. 2013;79(2):S16.e9‐13. [DOI] [PubMed] [Google Scholar]
- 56. de Notaris M, Palma K, Serra L, et al. A three‐dimensional computer‐based perspective of the skull base. World Neurosurg. 2014;82:S41‐S48. [DOI] [PubMed] [Google Scholar]
- 57. Mavar‐Haramija M, Prats‐Galino A, Méndez JA, Puigdelívoll‐Sánchez A, de Notaris M. Interactive 3D‐PDF presentations for the simulation and quantification of extended endoscopic endonasal surgical approaches. J Med Syst. 2015;39:127. [DOI] [PubMed] [Google Scholar]
- 58. Jean WC. Virtual reality surgical rehearsal and 2‐dimensional operative video of a paramedian supracerebellar infratentorial approach endoscopic resection of pineocytoma: 2‐dimensional operative video. Oper Neurosurg. 2020;20:E51‐E52. [DOI] [PubMed] [Google Scholar]
- 59. Jean WC, Huang MC, Felbaum DR. Optimization of skull base exposure using navigation‐integrated, virtual reality templates. J Clin Neurosci. 2020;80:125‐130. [DOI] [PubMed] [Google Scholar]
- 60. Won TB, Hwang P, Lim JH. Early experience with a patient‐specific virtual surgical simulation for rehearsal of endoscopic skull‐base surgery. Int Forum Allergy Rhinol. 2018;8:54‐63. [DOI] [PubMed] [Google Scholar]


