Abstract
Background
Educational interventions can reduce potentially inappropriate medication (PIM) use in older people. Their effectiveness has been measured mainly as changes in PIM use. In this economic evaluation, we analyse the impact of an educational intervention in terms of costs and quality-adjusted life years (QALYs).
Methods
The educational intervention consisted of activating and interactive training sessions for nursing staff and consulting physicians, and was compared with treatment as usual (TAU). Participants (n = 227) in a cluster randomised trial (cRCT) were residents living permanently in assisted living facilities (n = 20 wards). For economic evaluation, participants’ healthcare service use costs and costs for the intervention were estimated for a 12 month period.
Incremental cost-effectiveness ratios (ICERs) were estimated for QALYs per participant. Cost-effectiveness analysis was conducted from a healthcare perspective. A bootstrapped cost-effectiveness plane and one-way sensitivity analysis were undertaken to analyse the uncertainty surrounding the estimates.
Results
The educational intervention was estimated to be less costly and less effective in terms of QALYs than TAU at the 12 month follow-up [incremental costs –€1,629, confidence interval (CI) –€5,489 to €2,240; incremental effect −0.02, CI –0.06 to 0.02]. The base case ICER was >€80,000/QALY.
Conclusion
The educational intervention was estimated to be less costly and less effective in terms of QALYs compared with TAU, but the results are subject to some uncertainties. Reduction in PIM use or benefits in quality of life did not seem to translate into improvements in QALYs. Our findings emphasise the need for better understanding of the impact of decreasing PIM use on health outcomes.
Keywords: economic evaluation, older people, educational intervention, implementation intervention, potentially inappropriate medication
Key Points
Educational interventions have been studied mainly in terms of potentially inappropriate medication (PIM) use rather than health outcomes or costs.
Educational intervention was estimated to be less costly and less effective in terms of quality-adjusted life years (QALYs), compared with usual treatment.
We found that reduction in PIM use or benefits in terms of quality of life did not seem to translate into improvements in QALYs.
Although QALYs are commonly used in economic evaluations, they might not be suitable in end-of-life care of frail older people.
Introduction
Medication of older people is defined as potentially inappropriate if the associated risks outweigh the potential benefits [1]. Potentially inappropriate medication (PIM) use is associated with adverse drug events, reduced cognitive and physical functioning, decreased quality of life (QoL), hospitalisation and mortality [2–4], and thus with increased healthcare utilisation and costs [5], and higher medication costs [6, 7]. The prevalence of PIM use in Europe is >20% in community-dwelling older people and 49% in older people living in nursing homes [8, 9], and in the USA the prevalence is even higher [10, 11].
The effectiveness of implementation interventions to reduce PIM use has been widely studied. Implementation interventions are usually categorised into medication review services, multidisciplinary interventions, computerised systems, educational interventions and other interventions [12]. Educational interventions, including sessions for health professionals, distribution of materials and training for patients and caregivers, may reduce PIM use and hospitalisation in older people [12]. Educational interventions with fewer educational sessions and poor physician attendance did not show improvement in prescriptions [13, 14]. It appears that interactive approaches with direct feedback are more effective than the dissemination of written material [15]. However, interventions have been studied more in terms of changes in PIM use rather than health outcomes or costs [12, 16].
Although effectiveness studies abound, economic evaluations of implementation interventions to reduce PIMs of older people are rare. There are generally four types of economic evaluations: cost–benefit analysis, cost-minimization analysis, cost-effectiveness analysis and cost-utility analysis. Cost-effectiveness and cost-utility analysis can support optimal patient care and the choice of efficient implementation interventions by comparing the costs of interventions with their health benefits [17]. Recent literature has recognised the need for economic evidence in implementation science, but there is still scope for the use of high-quality cost-effectiveness analyses [18].
A model-based economic evaluation by Sanyal et al. [19] estimated the cost-effectiveness of an educational intervention in discontinuing non-steroidal anti-inflammatory drugs (NSAIDs) in community-dwelling older people. The intervention was dominant, i.e. less costly and more effective in terms of quality-adjusted life years (QALYs) than usual care at 12 month follow-up. To reduce antipsychotic use in persons with dementia living in nursing homes, Ballard et al. [20] focused on an intervention that consisted of an antipsychotic review and staff training in person-centred care and social interaction. They found this educational intervention to be economically dominant at the 12 month follow-up: compared with treatment as usual (TAU), it was more effective in terms of QoL and was also cost-effective.
Economic evaluation studies on other implementation interventions to reduce PIM use exist. They concern multidisciplinary interventions and medication reviews [21–24]. The decision concerning cost-effectiveness in these studies has been dependent on the decision-makers’ valuation of the specific outcome unit [22], but only short-term (≤12 months) cost-effectiveness has been evaluated. The studies used different outcome measures, but the impact on QALYs received less attention.
In this study, we examine the cost-effectiveness of an educational intervention to reduce PIM use and its impact on QALYs in residents in assisted living facilities compared with TAU. The primary outcomes of this trial have been reported earlier [25].
Method
We conducted a cost-effectiveness analysis from a healthcare perspective based on a cluster randomised controlled trial (cRCT) [25]. This economic evaluation adhered to the Consolidated Health Economic Evaluation Reporting Standards Statement (CHEERS) [26].
Study design
In total, 36 assisted living facility wards in Helsinki, Finland were assessed for possible participation in this cRCT. The level of care in assisted living facilities is comparable with that in nursing homes or long-term hospital care.
Of these 36 assisted living facility wards, seven facilities with 20 wards were selected. The minimum data set [27] was used to determine the case mix of each ward. A total of 20 wards were paired into 10 dyads according to their case mix. The wards in each dyad shared similar resident characteristics. These 20 dyads were then randomised to intervention and control groups during the years 2011 and 2012 [28]. The pairs of wards were randomised rather than the participants, in order to prevent contamination. Dyads were randomised using a computerised random number generator.
Intervention
The intervention consisted of two 4 h training sessions organised by a research geriatrician for nursing staff and consulting physicians. Training sessions were based on a constructive learning theory [29, 30]. The aim of the training was to enable nurses to recognise different PIMs and adverse drug events. PIMs were any of the following: Beers criteria medications [1], anticholinergic medications, use of multiple psychotropic medications, NSAIDs and proton pump inhibitors.
The first session was lecture based, and the participants were encouraged to discuss medication-related problems experienced in their residents. The lecture introduced the list of inappropriate medications and suitable alternatives, drug–drug interactions and medication use for residents with renal impairment. The second session was based on participants’ own case studies. The nurses participated in discussions about medication-related problems by presenting and discussing actual cases from their own wards. A list of inappropriate medications was provided for all nurses in the intervention wards. Nurses were invited to identify medication-related problems and inform the consulting physician who was responsible for changes in medications.
The training was especially targeted to those 2–3 registered nurses in the intervention wards who were responsible for residents’ medication. In seven intervention wards, those nurses participated in both sessions. There were two wards in which the nurses did not participate in the first session but participated in the second session. In one ward, the nurses did not participate in either of the sessions and they received tailored individual training. In addition, one geriatrician and one primary care physician were able to participate in one session, and they received tailored individual training.
Participants
Nurses, who were not aware which of the wards were randomised to intervention and control groups, recruited the residents to participate in the study. The residents were included if they were aged >65, living permanently in the assisted living facilities, Finnish speaking, using at least one medication, life expectancy >6 months and able to provide written informed consent (or had a proxy who was able to do so).
Of the 307 eligible residents, 227 participated; 118 residents in the intervention group and 109 in the control group. Those who did not participate either refused or were unavailable. Total loss of residents in the 12 months follow-up was 63 (28%), which included 55 deaths [intervention 33 (28%), control 22 (20%)].
The Ethics Committee of the Helsinki University Central Hospital approved the study. Written informed consent was obtained from the residents and/or their closest proxy. All study procedures were consistent with good clinical practice and the World Medical Association Declaration of Helsinki.
Outcome measures
Health outcome measures
The primary health outcome indicator for this cost-effectiveness analysis was change in QALYs, as calculated by combining estimates of health-related quality of life (HRQoL) and life years gained. HRQoL was assessed using the 15-dimensional instrument (15D) with one item covering each of the following dimensions: breathing, mental function, speech, vision, mobility, usual activity, vitality, hearing, eating, elimination, sleeping, distress, discomfort and symptoms, depression and sexual activity. Each dimension was divided into five levels from no problems to extreme problems. These dimensions build a weighted 15D index [31]. The assessments were performed by interviewing the residents or the closest proxy at baseline, and at 6 and at 12 months follow-up.
QALYs were derived from the area under a curve (AUC) calculation for the HRQoL values (15D score) from baseline to the last follow-up, and they ranged from 0 to 1, with 1 being equivalent to full health and 0 equivalent to death. The AUC method assumes a linear change between consecutive HRQoL values at 0, 6 and 12 months. There was one participant in the intervention group whose follow-up observations of 15D were missing. When this participant was excluded from the cost-effectiveness analysis, there appeared to be no discernible effect on the results. For those who died between 6 and 12 months follow-up, the life years gained was assumed to be 6 months, and for those who died before the first follow-up, the life years gained was assumed to be 3 months.
Cost measures
Intervention cost included time use of the educating geriatrician, participating nurses, physician and geriatrician. Travel expenses of the educating geriatrician and preparation costs were also calculated (4 h per session).
Seventeen nurses, one physician and one geriatrician participated in the 4 h sessions. We included 1 h of preparation for every session for each participant. Because the education was arranged during working hours, we valued the working hours of the participants according to the national unit costs of social care and healthcare in Finland [32] including social insurance fees, and converted them to 2019 values using the price index of public expenditure [33]. Study materials were offered electronically at zero cost.
The residents’ healthcare services included days spent in assisted living facilities, emergency department visits, outpatient visits, and hospital ward and subacute hospital and rehabilitation days. The data on service utilisation were collected for 12 months and valued according to the national unit costs of social care and healthcare in Finland [32]. The unit costs were converted to 2019 values [33]. Data on primary care physicians’ service use were not collected and therefore not included in the analysis. The difference in the medication costs was not statistically significantly different between the groups at the 12 months follow-up and therefore was not included in this analysis. The unit costs of healthcare services and intervention costs are presented in Table 1.
Table 1.
Intervention cost and unit costs of healthcare services (in 2019 Euros)
| Unit | Unit cost (€) | Total cost (€) | |
|---|---|---|---|
| Intervention cost | |||
| Time use valuation ofa | |||
| Nurses (n = 17) | 86 h | 25 | 2,151 |
| Physician (n = 1) | 5 h | 51 | 255 |
| Participating geriatrician (n = 1) Educating geriatrician (n = 1) |
5 h 18 h |
68 68 |
340 1,223 |
| Travel costb | 4 tickets | 3 | 12 |
| Total intervention cost | 3,981 | ||
| Healthcare services costsc | |||
| Assisted living facilities, daily fee | 134 | ||
| Specialised care | |||
| Emergency department visit | 361 | ||
| Outpatient visit | 301 | ||
| Hospital ward, daily fee | 896 | ||
| Subacute hospital, daily fee | 255 |
Costs were calculated during the follow-up, and baseline costs for both groups were assumed to be zero, and therefore mean costs were divided by person-years. All costs are expressed in Euros (€) in 2019 prices. As the duration of the study was 12 months, we discounted neither costs nor outcomes.
Statistical methods
Cost-effectiveness
We estimated the incremental cost-effectiveness ratio (ICER), i.e. the ratio of the mean difference in costs to the mean difference in QALYs. The interpretation of ICER is: if the intervention is more costly and more effective, cost-effectiveness is dependent on the decision-makers’ willingness to pay (WTP) for the extra unit of effectiveness. Conversely, if the intervention is less costly and less effective, cost-effectiveness is dependent on the decision-makers’ willingness to accept (WTA) compensation for the lower effectiveness [34].
Statistical comparisons of baseline characteristics between the groups were made using a χ2 test, t-test or bias-corrected bootstrap type t-test. Statistical analyses were performed using Stata statistical software version 15 (StataCorp, College Station, TX, USA).
We recognised the skewed distribution of costs at 12 months, the cluster randomisation and the covariate correlation with costs and effectiveness as recommended [35, 36]. We tested the correlation of the cluster’s size and participants’ baseline characteristics with QALYs and costs. Of the participants’ baseline characteristics, 15D score and age were significantly correlated with QALYs and costs. There was no correlation (intraclass correlation coefficient −0.15 for QALYs and −0.16 for costs) within a cluster, and individuals were independent. Therefore, in the cost-effectiveness analysis, we applied bootstrap analysis adjusted with 15D score and age at baseline. In addition, we generated a bootstrapped cost-effectiveness plane for incremental costs and effects (5,000 subsamples).
We conducted one-way sensitivity analyses by changing costs and effectiveness in the intervention group by 15% in either direction. In addition, we conducted sensitivity analysis including only participants alive at the end of the follow-up.
Results
The mean age of the participants was 83 years, and 93% were diagnosed with dementia (Table 2). The participants’ cognitive impairment was mainly severe in both groups. At baseline, the residents in the intervention group had a higher number of comorbidities [Charlson comorbidity index (CCI) 3.2 versus 2.5, P = 0.004] and lower HRQoL measured by the 15D (0.61 versus 0.66, P = 0.002) than those in the control group. The percentage of females in the intervention group was lower than in the control group. The proportion of participants using PIMs was higher in the intervention group (83.1% versus 71.6%, P = 0.038).
Table 2.
Baseline characteristics
| Intervention group (n = 118) | Control group (n = 109) | P-value | |
|---|---|---|---|
| Females, n (%) | 77 (65.3) | 84 (77.1) | 0.050 |
| Mean age, years (SD) | 82.9 (7.5) | 83.5 (6.9) | 0.41 |
| CCI, mean (SD) | 3.2 (2.0) | 2.5 (1.8) | 0.004 |
| MMSE, mean (SD) | 8.8 (8.2) | 10.0 (8.2) | 0.25 |
| 15D score, mean (SD) | 0.61 (0.12) | 0.66 (0.11) | 0.002 |
| Number of drugs used regularly, mean (SD) | 7.5 (2.8) | 7.8 (3.1) | 0.79 |
| Proportion using PIM, % | 83.1 | 71.6 | 0.038 |
| Mean number of PIM (SD) | 2.9 (1.8) | 2.5 (1.7) | 0.28 |
| Mean number of psychotropics (SD) | 1.13 (.99) | 1.34 (.99) | 0.11 |
Abbreviations: SD, standard deviation; CCI, Charlson comorbidity index; MMSE, Mini-Mental State Examination; 15D, 15-dimensional instrument of health-related quality of life; PIM, potentially inappropriate medication.
Costs of intervention and healthcare service use costs
The total intervention costs were €3,981(Table 3). Unadjusted mean total cost of healthcare services per person-year was lower in the intervention group than in the control group during the follow-up, but the difference was not statistically significant (intervention €40,332 versus control €43,251, P = 0.17). Costs consisted primarily of the costs of assisted living facilities. There was no statistically significant difference between the groups in any of the healthcare services costs.
Table 3.
Unadjusted mean costs (SD) of healthcare services per person-year during the 12 months of follow-up (in 2019 Euros)
| Intervention group (n = 117) | Control group (n = 109) | P-value | |
|---|---|---|---|
| Mean €/pyr (SE) | Mean €/pyr (SE) | ||
| Assisted living facilities | 39,706 (1,537) | 42,541 (1,367) | 0.18 |
| Specialized care | |||
| Emergency department visit | 83 (22) | 72 (20) | 0.72 |
| Outpatient visit | 82 (23) | 86 (18) | 0.89 |
| Hospital ward | 183 (99) | 238 (130) | 0.74 |
| Subacute hospital | 249 (100) | 314 (100) | 0.65 |
| Intervention cost | 30 | 0 | |
| Total costs including intervention | 40,332 (1,566) | 43,251 (1,376) | 0.17 |
Abbreviations: SE, standard error; pyr, person-year.
Cost-effectiveness
The estimated mean cost per person-year at 12 months follow-up (adjusted with baseline 15D score and age) was €40,954 (95% CI €38,223–€43,686) for the intervention group and €42,584 (95% CI €39,865–€45,302) for the control group (Supplementary Table 1 available in Age and Ageing online). The intervention was associated with an average –€1,629 (95% CI −€5,489 to €2,240) higher but not statistically significant costs per person-year compared with the control (Table 4).
Table 4.
Incremental cost and effectivenessa of the educational intervention compared with the control group during the 12 months of follow-up (in 2019 Euros)
| Incremental cost (€/pyr) |
Incremental effect (QALYs) | ICER (CI) €/QALY | |
|---|---|---|---|
| Base case | –1,629 (−5,489 to 2,240) |
−0.02 (−0.06 to 0.02) |
83,424 (−233,191 to 803,989) |
| Sensitivity analysis | |||
| Participants alive at 12 months (intervention n = 84, control n = 87) |
67 (−551 to 657) |
0.00 (−0.03 to 0.02) |
– |
| Cost (€) +15% | 4,579 (464 to 8,702) |
−0.02 (−0.06 to 0.02) |
Control dominant |
| Cost (€) –15% | −7,838 (−11,487 to 4,287) |
−0.02 (−0.06 to 0.02) |
401,299 |
| QALYs +15% | −1,629 (−5,489 to 2,240) |
0.05 (0.00 to 0.02) |
Intervention dominant |
| QALYs −15% | −1,629 (−5,489 to 2,240) |
−0.09 (−0.13 to 0.05) |
17,641 |
aAdjusted with baseline 15D score and age.
Abbreviations: pyr, person-year; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; CI, confidence interval
Mean QALYs per participant at 12 months follow-up (adjusted with baseline 15D score and age) was estimated to be 0.48 (95% CI 0.45–0.51) in the intervention group and 0.50 (95% CI 0.47–0.53) in the control group (Supplementary Table 1 available in Age and Ageing online). The intervention was associated with an average −0.02 (95% CI −0.06 to 0.02) lower but not statistically significant QALYs per participant compared with the control (Table 4).
ICER estimation in the base case was €83,424/QALY, and the cost saving was €83,424 per QALY lost in the intervention group compared with TAU (Table 4). The educational intervention was estimated to be less costly and less effective than TAU at 12 months follow-up, and therefore the cost-effectiveness of the educational intervention seemed to be dependent on the decision-makers’ WTA.
The bootstrapped cost-effectiveness plane (Figure 1) is positioned mostly in the south–west quadrant, demonstrating a positive ICER value, which shows that the intervention is estimated to be less costly and less effective than TAU. The sensitivity analysis including only participants alive at the end of the 12 months follow-up (Table 4) demonstrates that there was no difference between the groups. The sensitivity analyses also demonstrate that if costs in the intervention group increase by 15% the control group would dominate. On the other hand, if the effectiveness in the intervention group increases by 15% the intervention group would dominate.
Figure 1.
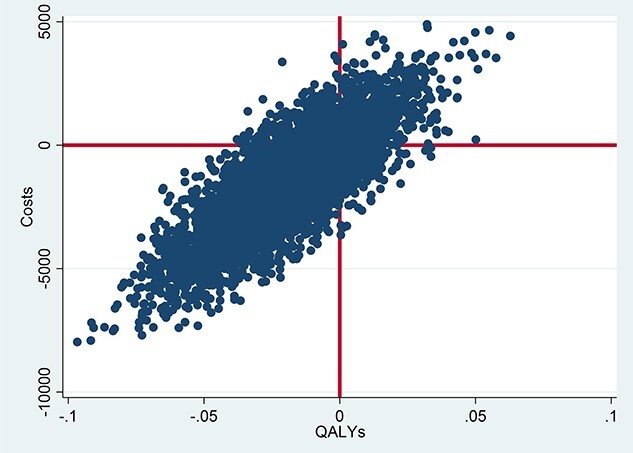
Cost-effectiveness plane
Discussion
This economic evaluation examined the cost-effectiveness of an educational intervention to reduce residents’ PIM use in assisted living facilities. Our results indicate that, compared with TAU, this educational intervention was estimated to be less costly and less effective in terms of QALYs. One interpretation here is that cost-effectiveness is dependent on the decision-makers’ WTA. However, the differences between costs and QALYs were not statistically significant.
Previously, the educational intervention of this study was shown to reduce PIM use and enhance HRQoL [25]. Outcome measures most adopted in earlier studies were PIM use and QoL; impact on QALYs received less attention [19–24]. We found that PIM use reduction did not seem to translate into improvements in QALYs. This finding is consistent with that of a previous study by Gillespie et al. [22], who observed that improvements in PIM use translated into neither QALY gains nor reductions in costs.
QALYs are recognized to have some limitations, although it is claimed to be a common metric that can be applied to any healthcare activity where decision-makers try to maximise health outcomes [37, 38]. It has been argued that it is unsuitable for allocating resources particularly in end-of-life care. Preference-based measures of health valued using death as an anchor point might be inconsequential in a patient group in which death is expected imminently, and potentially desired [39].
Measuring general HRQoL in patients with severe cognitive impairment is complicated, and it has been suggested that both patient- and proxy-reported outcomes should be included to measure the effects of an intervention [40]. In this study, most HRQoL responses were provided by the closest proxy. Thorough validation studies of 15D have shown that the reliability between the proxy and the participant is good and the instrument can be completed by the closest proxy [31, 40]. In addition, other dimensions of QoL, such as social relations and spirituality, may become more important to individuals at the end of life than health status, and HRQoL metrics are unable to measure these dimensions [41]. Mortality among our participants was very high. At 12 months, 33% of the residents in the intervention group had died compared with 22% of participants in the control group [25]. This might explain our finding that HRQoL declined more slowly in the intervention group but QALYs per patient were lower in the intervention group compared with TAU.
Our results differ from the findings of earlier economic evaluations of educational interventions that observed the interventions as being more effective and less costly [19, 20]. However, the study populations and outcome measures differ. For example, Ballard et al. [20] included older people with dementia living in nursing homes, but only those alive at the end of the follow-up. Sanyal et al. [19] included only community-dwelling people. On the other hand, the intergroup differences diminished in our sensitivity analysis with the population alive at the end of the follow-up. This drop indicates that differences in costs and QALYs were mostly dependent on mortality, and not on the intervention itself.
Our results are subject to some other sources of uncertainties. First, costs and QALYs, as well as ICER, had wide CIs and the differences between the groups are not statistically significant. In addition, the widely spread cost-effectiveness plane established the possibility that there is no difference between the arms.
Second, old age and morbidity were associated with a high mortality rate. At baseline, compared with the control, the intervention group had lower HRQoL, higher morbidity and a higher proportion using PIMs. Overall, the intervention group was frailer at baseline. From all the baseline characteristics, only HRQoL and age were correlated with the outcome measures. We tested the effects of all the characteristics on the results, and methods appropriate for cRCT economic evaluations helped reduce bias caused by the study design [35, 36]. It is still possible that there are some non-observable individual covariates, for example social relations. Third, because costs were calculated only during the follow-up, baseline costs for both groups were assumed to be zero. Therefore, costs were divided by the person-years. In addition, costs for residents’ healthcare service use were lacking complete details, and societal costs were not included.
WTA is typically used to indicate the minimum monetary amount required to forgo the health benefit from implementing the intervention. For the educational intervention to be cost-effective, it could well be that a decision-maker would require that the intervention would be more effective or achieve bigger savings compared with the control group. Earlier contingent valuation studies have found that WTA might also exceed WTP in healthcare; they have also proffered explanations for the disparity [34, 42, 43]. Therefore, the results of this study need to be treated with caution.
Previous research has been restricted to short-term effectiveness of interventions, but evidence is lacking regarding the sustainability of implementation. This educational intervention has demonstrated a positive impact on PIM use, which however appears to diminish at 12 months [25]. This might partly stem from nursing staff turnover, as training was not provided on a continuous basis. In addition, not all nurses in the intervention group participated in these sessions. A higher level of participation would have increased the intervention costs, but it might have gained better effectiveness in the intervention group.
The educational intervention could be considered as quite minimal and also feasible, and intervention costs were only around €30 per participant. To achieve sustainable effectiveness in implementation, educational intervention could be organised on a more continuous basis targeted for nurses and physicians. In practice, nurses play a key role in identifying medical-related problems in assisted living facilities whereas physicians make the final decision about medications based on assessing the risks and benefits.
This economic evaluation indicates that the educational intervention was estimated to be less costly and less effective in terms of QALYs compared with TAU. The reduction in PIMs did not seem to translate into improvements in QALYs although HRQoL declined more slowly in the intervention arm. Our study illustrates the apparent difference in HRQoL and QALY in a very frail long-term care population close to death. This emphasises that further research into the impact of reducing PIM use on health outcomes is needed.
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by University Pharmacy, Research Grant 01/2019. This study was supported by the Päivikki and Sakari Sohlberg Foundation and Helsinki University Hospital of Helsinki. They played no role in the design, execution, analysis, interpretation of data or writing.
References
- 1. Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med 1991; 151: 1825–32. [PubMed] [Google Scholar]
- 2. Wallace E, McDowell R, Bennett K, Fahey T, Smith SM. impact of potentially inappropriate prescribing on adverse drug events, health related quality of life and emergency hospital attendance in older people attending general practice: a prospective cohort study. J Gerontol: Ser A 2017; 72: 271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schöttker B. The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc 2016; 18: 211–20. [DOI] [PubMed] [Google Scholar]
- 4. Hyttinen V, Jyrkkä J, Valtonen H. A systematic review of the impact of potentially inappropriate medication on health care utilization and costs among older adults. Med Care 2016; 54: 950–64. [DOI] [PubMed] [Google Scholar]
- 5. Hyttinen V, Jyrkkä J, Saastamoinen LK, Vartiainen AK, Valtonen H. The association of potentially inappropriate medication use on health outcomes and hospital costs in community-dwelling older persons: a longitudinal 12-year study. Eur J Health Econ 2019; 20: 233–43. [DOI] [PubMed] [Google Scholar]
- 6. Harrison SL, Kouladjian L, O’Donnell MRet al. Costs of potentially inappropriate medication use in residential aged care facilities. BMC Geriatr 2018; 18: 9. 10.1186/s12877-018-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan SG, Hunt J, Rioux J, Proulx J, Weymann D, Tannenbaum C. Frequency and cost of potentially inappropriate prescribing for older adults: a cross-sectional study. CMAJ Open 2016; 4: E346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol 2015; 71: 1415–27. [DOI] [PubMed] [Google Scholar]
- 9. Morin L, Laroche ML, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 2016; 17: 862. 10.1016/j.jamda.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 10. Davidoff AJ, Miller GE, Sarpong EM, Yang E, Brandt N, Fick DM. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers criteria. J Am Geriatr Soc 2015; 63: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lau DT, Kasper JD, Potter DEB, Lyles A. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res 2004; 39: 1257–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos NS, Marengo LL, Moraes FS, Barberato-Filho S. Interventions to reduce the prescription of inappropriate medicines in older patients. Rev Saude Publica 2019; 53: 7. 10.11606/S1518-8787.2019053000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loganathan M, Singh S, Franklin BD, Bottle A, Majeed A. Interventions to optimize prescribing in care homes: systematic review. Age Ageing 2011; 40: 150–62. [DOI] [PubMed] [Google Scholar]
- 14. Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging 2009; 26: 1013–28. [DOI] [PubMed] [Google Scholar]
- 15. O’Connor MN, Gallagher P, O’Mahony P. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging 2012; 29: 437–52. [DOI] [PubMed] [Google Scholar]
- 16. Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies—a synthesis of systematic review findings. J Eval Clin Pract 2018; 14: 888–97. [DOI] [PubMed] [Google Scholar]
- 17. Hoomans T, Ament AJHA, Evers SMAA, Severens JL. Implementing guidelines into clinical practice: what is the value? J Eval Clin Pract 2011; 17: 606–14. [DOI] [PubMed] [Google Scholar]
- 18. Roberts SLE, Healey A, Sevdalis N. Use of health economic evaluation in the implementation and improvement science fields—a systematic literature review. Implementation Sci 2019;14: 72. 10.1186/s13012-019-0901-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanyal C, Turner JP, Martin P, Tannenbaum C. Cost-effectiveness of pharmacist-led deprescribing of NSAIDs in community-dwelling older adults. J Am Geriatr Soc 2020; 68: 1090–7. [DOI] [PubMed] [Google Scholar]
- 20. Ballard C, Corbett A, Orrell Met al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLoS Med 2018;5: e1002500. 10.1371/journal.pmed.1002500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher J, O’Sullivan D, McCarthy S, Woods Net al. Structured pharmacist review of medication in older hospitalised patients: a cost-effectiveness analysis. Drugs Aging 2016; 33: 285–94. [DOI] [PubMed] [Google Scholar]
- 22. Gillespie P, Clyne B, Raymakers A, Fahey T, Hughes CM, Smith SM. Reducing potentially inappropriate prescribing for older people in primary care: cost-effectiveness of the OPTI-script intervention. Int J Technol Assess Health Care 2017; 33: 494–503. [DOI] [PubMed] [Google Scholar]
- 23. Patterson SM, Hughes CM, Cardwell C, Lapane KL, Murray AM, Crealey GE. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland Study): a cost-effectiveness analysis. J Am Geriatr Soc 2011; 59: 586–93. [DOI] [PubMed] [Google Scholar]
- 24. Malet-Larrea A, Goyenechea E, Gastelurrutia MAet al. Cost analysis and cost–benefit analysis of a medication review with follow-up service in aged polypharmacy patients. Eur J Health Econ 2017; 18: 1069–78. [DOI] [PubMed] [Google Scholar]
- 25. Pitkälä KH, Juola A-L, Kautiainen H et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc 2014; 15: 892–8. [DOI] [PubMed] [Google Scholar]
- 26. Husereau D, Drummond M, Petrou Set al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluations publication guidelines good reporting practices task force. Value Health 2013; 16: 231–50. [DOI] [PubMed] [Google Scholar]
- 27. Morris JN, Fries BE, Bernabei Ret al. RAI-home care [RAI-HC] Assessment Manual. Version 2.0 Marblehead. MA: Opus Communications, 2000. [Google Scholar]
- 28. Pitkälä KH, Juola A-L, Soini H. Reducing inappropriate, anticholinergic and psychotropic drugs among older residents in assisted living facilities: study protocol for a randomized controlled trial. Trials 2012; 13: 85. 10.1186/1745-6215-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biggs J. Enhancing teaching through constructive alignment. Higher Educ 1996; 32: 347–64. [Google Scholar]
- 30. Dolmans DH, De Grave W, Wolfhagen IH, Vleuten CP. Problem-based learning: future challenges for educational practice and research. Med Educ 2005; 39: 732–41. [DOI] [PubMed] [Google Scholar]
- 31. Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med 2001; 2001, 38: 328–36. [DOI] [PubMed] [Google Scholar]
- 32. Kapiainen S, Väisänen A, Haula T. Terveyden—ja sosiaalihuollon yksikkökustannukset Suomessa vuonna. 2011. Terveyden ja hyvinvoinnin laitos. Raportti 3/2014.
- 33. Official Statistics of Finland (OSF) : Price index of public expenditure [e-publication]. Helsinki: Statistics Finland; [referred: 27.9.2021]. Access method: http://www.stat.fi/til/jmhi/index_en.html [Google Scholar]
- 34. Rotteveel AH, Lambooij MS, Zuithoff NPA, Exel J, Moons KGM, Wit GA. valuing healthcare goods and services: a systematic review and meta-analysis on the WTA–WTP disparity. Pharmacoeconomics 2020; 38: 443–58. [DOI] [PubMed] [Google Scholar]
- 35. Gomes M, Ng ES-W, Grieve R, Nixon R, Carpenter J, Thompson SG. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decis Making 2012; 32: 350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gomes M, Grieve R, Nixon R, Ng ES, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analyses of cluster randomised trials. In: Health Econ, vol. 21, 2012; 1101–18. [DOI] [PubMed] [Google Scholar]
- 37. Briggs A, Calxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press, 2006. [Google Scholar]
- 38. Round J. Is a QALY still a QALY at the end of life? J Health Econ 2012;31: 521–7. [DOI] [PubMed] [Google Scholar]
- 39. Normand C. measuring outcomes in palliative care: limitations of qalys and the road to PalYs. J Pain Symptom Manage 2009; 38: 27–31. [DOI] [PubMed] [Google Scholar]
- 40. Yang F, Dawes P, Leroi I, Gannon B. Measurement tools of resource use and quality of life in clinical trials for dementia or cognitive impairment interventions: a systematically conducted narrative review. Int J Geriatr Psychiatry 2018; 33: e166–76. [DOI] [PubMed] [Google Scholar]
- 41. Hughes, J. Palliative care and the QALY problem. Health Care Anal 2015; 13: 289–301. [DOI] [PubMed] [Google Scholar]
- 42. Severens JL, Brunenberg DEM, Fenwick EAL, O’Brien B, Joore MA. Cost-effectiveness acceptability curves and a reluctance to lose. Pharmacoeconomics 2005; 23: 1207–14. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien BJ, Gertsen K, Willan AR, Faulkner A. Is there a kink in consumers’ threshold value for cost-effectiveness in health care? Health Econ 2002; 11: 175–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


