Abstract
Methanotrophic and nitrifying bacteria are both able to oxidize CH4 as well as NH4+. To date it is not possible to estimate the relative contribution of methanotrophs to nitrification and that of nitrifiers to CH4 oxidation and thus to assess their roles in N and C cycling in soils and sediments. This study presents new options for discrimination between the activities of methanotrophs and nitrifiers, based on the competitive inhibitor CH3F and on recovery after inhibition with C2H2. By using rice plant soil as a model system, it was possible to selectively inactivate methanotrophs in soil slurries at a CH4/CH3F/NH4+ molar ratio of 0.1:1:18. This ratio of CH3F to NH4+ did not affect ammonia oxidation, but methane oxidation was inhibited completely. By using the same model system, it could be shown that after 24 h of exposure to C2H2 (1,000 parts per million volume), methanotrophs recovered within 24 h while nitrifiers stayed inactive for at least 3 days. This gave an “assay window” of 48 h when only methanotrophs were active. Applying both assays to model microcosms planted with rice plants demonstrated a major contribution of methanotrophs to nitrification in the rhizosphere, while the contribution of nitrifiers to CH4 oxidation was insignificant.
Methane- and ammonia-oxidizing bacteria play major roles in the global carbon and nitrogen cycles. These bacteria convert the most reduced carbon and nitrogen compounds (CH4 and NH4+) to oxidized forms (CO2 and NO2−). Apart from their primary substrates, CH4 and NH4+, both groups of bacteria need oxygen for growth and energy generation. Hence, habitats with oxic and anoxic conditions in close proximity, such as the rhizospheres of plants in flooded soils and sediments, are ideal for their persistence. Wetland plants supply their respiring roots with oxygen by means of an aerenchymatous tissue which also facilitates the exchange of other gases such as CH4, N2O, and N2 among the atmosphere, shoots, and roots (2, 7). Some of the oxygen is released by the roots into the surrounding soil, thus creating oxic areas within an otherwise anoxic habitat. Indeed, methanotrophs (12, 20) as well as nitrifiers (3, 10, 18, 39) have been shown to profit from the presence of wetland plants, with distinct impacts on nutrient cycling. Due to oxygen release by wetland plants, a substantial part (10 to 90%) of the CH4 potentially emitted can be oxidized by methane-oxidizing bacteria and thus retained in the system as biomass carbon or as CO2 (12, 13, 15, 30, 41, 44). Nevertheless, enough CH4 is still being emitted from natural wetlands and rice paddies to make them prominent sources of atmospheric CH4, accounting for 30% of global emission (34, 42). Because CH4 is involved in global warming (14), knowledge of the CH4 sinks in rice soils and of the controlling factors and organisms involved, and thus of methane- and ammonia-oxidizing bacteria, is essential in order to develop mitigation strategies. This knowledge is still sparse.
Both methane- and ammonia-oxidizing bacteria can act as sinks for CH4 in rice soils. Due to the homology of the key enzymes methane monooxygenase (MMO) and ammonia monooxygenase, methanotrophs as well as ammonia oxidizers can oxidize both CH4 and NH4+, as well as a variety of other substrate analogues (5). However, direct evidence for nitrification by methanotrophs (36, 47) and for methane oxidation by nitrifiers (26, 45) has been given only for pure cultures. In natural wetlands and rice plant soils this has never been studied, while in a few other natural systems this has been demonstrated only indirectly by means of the inhibitor-sensitive 14CH4/14CO oxidation ratio, which is higher for methanotrophs than for nitrifiers (27). By using this technique, CH4 oxidation was assigned to nitrifiers in agricultural and forest soils (43), whereas methanotrophs were found to be dominant in the thermocline of a mesotrophic lake (6). Several inhibitors (e.g., C2H2, CH3F, dimethylether, allylsulfide, allylthiourea, dicyandiamide, picolinic acid, and difluoromethane) have been evaluated for their potential to selectively knock out one group of bacteria without affecting the other (6, 32, 33, 37, 40). However, only allylsulfide (40) and picolinic acid (32) showed potential for discrimination, although neither was able to discriminate 100%.
The purpose of this study was to develop and evaluate new methods to estimate the relative contributions of methanotrophs to NH4+ oxidation and of nitrifiers to CH4 oxidation in soil planted with rice plants. Two approaches, based on a temporary inactivation of one of the two groups, were used. Methylfluoride, being a competitive inhibitor (24, 37) of CH4 and NH4+ oxidation, will be inhibitory only in a certain concentration ratio to CH4 or NH4+, respectively. We assessed the possibility of finding a CH3F/CH4/NH4+ ratio which excludes CH4 oxidation but allows for NH4+ oxidation. The second approach was analogous to a method for discriminating nitrifier and denitrifier production of NO and N2O (28), based on the differential recovery after exposure to C2H2.
MATERIALS AND METHODS
Soil and field site.
The soil used in all experiments was sampled from a rice plant field of the Istituto Sperimentale per la Cerealicoltura in Vercelli (Italy) in the spring of 1997 and was stored after air drying. The soil type and rice plant field management practice have been described earlier (42). Prior to use the soil was crushed and sieved (mesh size, 2 mm).
Effects of C2H2, CH3F, and picolinic acid. (i) Inhibition of CH4 oxidation.
To induce CH4 oxidation in the dried rice plant soil, slurries were prepared by mixing 5-g amounts of soil with 20 ml of demineralized water in 150-ml flasks closed with rubber stoppers (ratio of gas volume to liquid volume, 5:1). After the flasks were flushed with synthetic air (21% O2 in N2) for 15 min, 2.5 ml of pure CH4 (99.995% pure; Messer Griesheim, Siegen, Germany) was added to give a mixing ratio of 20,000 parts per million volume (ppmv). The flasks were incubated at 25°C in the dark on a gyratory shaker (100 rpm) for 3 days, after which all CH4 was consumed, as confirmed by gas chromatographic (GC) analyses. After the preincubation period, the flasks were flushed again with synthetic air for 15 min, after which 1.25 ml of CH4 was added to give a final mixing ratio of 10,000 ppmv (∼10 μM). In the case of the gaseous inhibitors C2H2 (99.6% pure; Messer Griesheim) and CH3F (>98% pure; Fluorochem, Old Glossop, Derbyshire, United Kingdom), triplicate flasks were supplemented with amounts resulting in mixing ratios of 0, 0.1, 1, 10, 100, 1,000, and 10,000 ppmv. The total amounts of gas present and the concentrations of the gases in the liquid phase were calculated by using Bunsen coefficients at 25°C (0.03 for CH4 [16], 0.934 for C2H2 [16], and 0.99 for CH3F [19]) and the gas and liquid volumes of the flasks. Before C2H2 was added, it was purified by passage through 5 N NaOH–5 N H2SO4 (22). In the case of picolinic acid, 19 ml instead of 20 ml of demineralized water was added to the soil. To these slurries, 1 ml of stock solution was added to reach final concentrations of 0, 10, 100, 500, 1,000, and 10,000 μM. After addition of the inhibitor, the flasks were incubated at 25°C in the dark on a gyratory shaker (250 rpm). CH4, C2H2, and CH3F mixing ratios in the headspaces were monitored during 24 h. Gas samples (100 μl) were taken and injected into a GC by using pressure lock syringes (Precision Sampling Corp., Baton Rouge, La.). CH4 oxidation rates were calculated from linear regressions applied to the data of the first 8 h of the assay (r2 > 0.95).
(ii) Inhibition of NH4+ oxidation.
NH4+ oxidation in the dried rice plant soil was induced in soil slurries by preincubation for 7 days. For this purpose 20-g amounts of dry soil were transferred to 500-ml flasks equipped with septa at the bottoms for withdrawal of slurry samples. A total of 0.15 g of CaCO3 and 85 ml of assay medium containing 0.33 g of (NH4)2SO4/liter, 0.027 g of KH2PO4/liter, and 0.14 g of K2HPO4/liter were added (ratio of gas volume to liquid volume, 5:1). The flasks were closed with silicone septa and incubated horizontally on a gyratory shaker (120 rpm) at 25°C. After this preincubation period, the flasks were flushed with nitrogen for 1 h, followed by anoxic incubation for 24 h. During this time the initial amounts of NO3− and NO2− were reduced by denitrification. After this treatment the flasks were opened and flushed with pressurized air for 5 min. The flasks were closed again, and the respective amounts of the various inhibitors were added as described above. During the subsequent oxic incubation, nitrification activity was monitored by withdrawing 1-ml subsamples at regular intervals during 24 h. The slurry samples were centrifuged (13,800 × g, 4°C, 15 min), and the supernatant was stored at −20°C for later analysis of NH4+, NO2−, and NO3−. For treatments with C2H2 or CH3F, the mixing ratios of these gases were determined as described above. Potential NH4+ oxidation activities were calculated from linear regressions of the NO3−-plus-NO2− concentration during the first 24 h of incubation (r2 > 0.95).
(iii) Recovery of CH4 and NH4+ oxidation after 24-h exposure to C2H2 and CH3F.
After 24 h of incubation in the presence of the gaseous inhibitors, the flasks were opened and shaken on a gyratory shaker for 30 min. While they were shaken, the flasks were flushed with pressurized air. The flasks were then closed again and shaken vigorously by hand, followed by immediate flushing with air. This was repeated three times for each bottle, after which new stoppers were used to seal the bottles. New stoppers were used because the stoppers already exposed to C2H2 or CH3F release amounts of these gases which they have absorbed during exposure. The absence of C2H2 and CH3F was checked by GC. When the inhibitors could no longer be detected, the flasks were incubated again as described above. Recovery of CH4 and NH4+ oxidation was monitored as described above.
CH4 and NH4+ oxidation in the rice plant rhizosphere. (i) Operation of the model system and growth conditions.
As a model system, compartmented microcosms were used as described in detail by Bodelier et al. (9). In the center of each of these cylindrical stainless steel microcosms (height by diameter, 12 by 9 cm) a perforated steel cylinder (height by diameter, 12 by 4 cm), covered on the inside with nylon gauze (mesh size, 30 μm), served to separate the rooted from the nonrooted soil. The microcosms were filled with 700 g of dry rice plant soil, subsequently flooded with demineralized water, and incubated for 1 week in a growth chamber (Conviron CMS 3244; Controlled Environments Limited, Winnipeg, Manitoba, Canada) at 25°C and 70% relative humidity in the dark. After 1 week, one rice seedling (Oryza sativa cv. Roma, type japonica), which had been germinated on wet filter paper at 25°C in the greenhouse, was planted in the root compartment of each microcosm. The planted microcosms were incubated in the growth chamber for 12 weeks at 70% relative humidity and illuminated in a cycle of 12 h of light and 12 h of dark at a photosynthetic active radiation (PAR) of 450 microeinsteins · m−2 · s−1 and a temperature regimen of 20°C at night and 25°C in the day. The surface of the soil was always covered with 2 cm of demineralized water and shaded with aluminum foil to reduce warming of the microcosms due to illumination. Temperatures of ambient air and temperatures in the soil (5 cm) during the daytime varied between 25 and 28°C, as measured with thermistor probes connected to a data logger (DL2e; Delta-T Devices Ltd., Cambridge, United Kingdom). The microcosms were fertilized weekly with (NH4)HPO4 by syringe injection of 0.84 mmol of N in 10 ml of H2O, corresponding to a total application of 260 kg of N · ha−1.
(ii) Porewater sampling.
In order to monitor CH4, NH4+, NO3−, NO2−, and pH, weekly porewater samples were taken from the root and nonroot compartments of the microcosms by means of Rhizon soil solution samplers (Eijkelkamp, Giesbeek, The Netherlands) as described by Bodelier et al. (9). Evacuated Venoject blood-collecting tubes, which had been flushed with nitrogen to remove residual methane, were mounted on the sampling devices for sample collection. After sampling, the pressure in the tubes was adjusted to atmospheric pressure by addition of ambient air, after which the tubes were shaken vigorously. Subsequently, CH4 was withdrawn from the headspace and analyzed as described below. After the pH was measured, 1 ml of porewater was centrifuged (13,800 × g, 4°C, 15 min) and stored at −20°C for further analysis.
(iii) Harvest and preparation of soil slurries.
After 12 weeks the microcosms were harvested, and the soil from the root and nonroot compartments was treated as follows. Prior to processing of the soil, the upper 2 to 3 cm of both compartments were removed and excluded from further analysis, because this soil layer receives oxygen from the overlying water and not only from the rice plant root. The complete root compartment with roots was transferred to a beaker containing 240 ml of demineralized water. The resulting suspension of rhizosphere soil contained approximately 0.25 g of dry soil per ml. The soil from the nonroot compartment was completely transferred to a glass beaker and mixed. One hundred sixty grams of this soil was suspended in 240 ml of demineralized water. These slurries were used to determine potential CH4 oxidation. For potential NH4+ oxidation, competitive exclusion, and differential recovery assays, 150 ml of these slurries was mixed with 150 ml of a (NH4)2SO4 solution containing 6.23 mM NH4+. This NH4+ concentration was chosen in order to reach a desired concentration of 2 mM in the slurry, accounting for the endogenous ammonium already present and for the adsorption of ammonium to the soil particles (35% of added ammonium), which was determined in a preharvest experiment with soil from identical microcosms (data not shown).
(iv) Potential CH4 and NH4+ oxidation in soil slurries.
The potential CH4 oxidation activities of root and nonroot compartments of four replicate microcosms were determined as described above. Seventy milliliters of slurry was transferred to 500-ml flasks and assayed as described above for the nitrification assays. The flasks were supplemented with 10,000 ppmv of CH4 and incubated at 25°C on a gyratory shaker (120 rpm). For the potential NH4+ oxidation, 70 ml of slurry [supplemented with (NH4)2SO4 as described above] was used and incubated immediately under the conditions described for the CH4 oxidation. Further assay procedures were as already described.
(v) Potential CH4 and NH4+ oxidation associated with rice plant roots.
The potential CH4 oxidation activities of rice plant roots were determined by using 5 g of fresh root material incubated in 150-ml flasks closed with rubber stoppers. After flushing with synthetic air for 15 min, 1.5 ml of CH4 (10,000 ppmv) was added. The flasks were incubated statically at 25°C in the dark. The CH4 mixing ratio was monitored as already described. Potential NH4+ oxidation activity associated with rice plant roots was determined by incubating 5 g of fresh root material together with 50 ml of assay medium (see “Inhibition of NH4+ oxidation” above) in a 250-ml Erlenmeyer flask. Flasks were incubated on a gyratory shaker (100 rpm) at 25°C in the dark. Samples were taken at regular intervals and were processed as described for the slurry samples from the potential NH4+ oxidation assay.
(vi) CEA.
For the competitive exclusion assay using CH3F (CEA), the slurries which were diluted with (NH4)2SO4 solution were used. Seventy milliliters of slurry from the root and nonroot compartments of four replicate microcosms was transferred to 500-ml assay flasks with no CH4 addition or with the addition of CH4 (10,000 ppmv) alone or of CH4 (10,000 ppmv) plus CH3F (300 ppmv). The latter treatment gives rise to a CH4/CH3F/NH4+ dissolved molar ratio of 0.1:1:18. In all assay bottles CH4, NH4+, NO2−, and NO3− were monitored as described above.
(vii) Differential recovery assay using C2H2 (DRA).
Seventy-milliliter amounts of the NH4+-supplemented slurries from the root and nonroot compartments of four replicate microcosms were transferred to 500-ml assay flasks, and CH4 (10,000 ppmv) was added. The flasks were incubated as described above, and CH4 and NH4+ oxidation was monitored for 24 h, after which C2H2 (1,000 ppmv) was added. After 24 h of exposure the C2H2 was removed as described above. After removal of C2H2, the flasks were supplemented again with CH4. During the following 2 days, the recovery of CH4 and NH4+ oxidation was monitored.
(viii) Numbers of methanotrophs.
The numbers of methanotrophs in soil from the root and nonroot compartments, as well as those associated with the rice plant roots, were determined by the most probable number (MPN) method according to Gilbert and Frenzel (20). Soil slurries and root suspensions were serially diluted in microtiter plates containing ammonium-mineral salts medium. The plates were incubated for 4 weeks at 25°C in gastight jars containing 20% CH4 in air. Inoculated plates without CH4 served as controls. Wells which were turbid were considered positive.
Analyses. (i) Gas analyses.
In inhibitor experiments with preincubated rice plant soil, CH4 was analyzed on an SRI GC (SRI Instruments, Torrance, Calif.) equipped with a flame ionization detector (FID) and a Hayesep D column (length, 2 m; 80/100 mesh). Helium was used as the carrier gas (flow rate, 20 ml · min−1), and synthetic air (250 ml · min−1) and H2 (20 ml · min−1) were used as burning gases. The oven temperature was 80°C. C2H2 and CH3F in the inhibitor experiments with preincubated soil, as well as C2H2, CH3F, and CH4 in the microcosm experiment and CH4 in the weekly porewater samples, were analyzed with an SRI GC equipped with an FID and a Porapak N column (length, 2 m; 80/400 mesh). N2 was used as the carrier gas (20 ml · min−1), and synthetic air (222 ml · min−1) and H2 (20 ml · min−1) were used as burning gases. The oven temperature was 60°C. Calibration was performed at each sampling event by triplicate injection of 1,000 ppmv of CH4 in N2 (Messer Griesheim). C2H2 and CH3F standards were prepared by adding defined amounts to serum bottles of known volumes.
(ii) Slurry and porewater analyses.
The concentrations of NH4+, NO2−, and NO3− in the slurry samples were analyzed colorimetrically with a Technicon Traacs 800 autoanalyzer (Technicon Instrument Corp., Tarrytown, N.Y.). NH4+ in the weekly porewater samples was analyzed by ion chromatography using a high-pressure liquid chromatograph (HPLC) equipped with an LCA (Sykam, Gilching, Germany) A14 column and ascorbic acid-oxalic acid as the eluent. NO2− and NO3− were also analyzed by ion chromatography on an HPLC equipped with an LCA KSP column and Na2CO3 as the eluent.
RESULTS
Effects of C2H2, CH3F, and picolinic acid on CH4 and NH4+ oxidation.
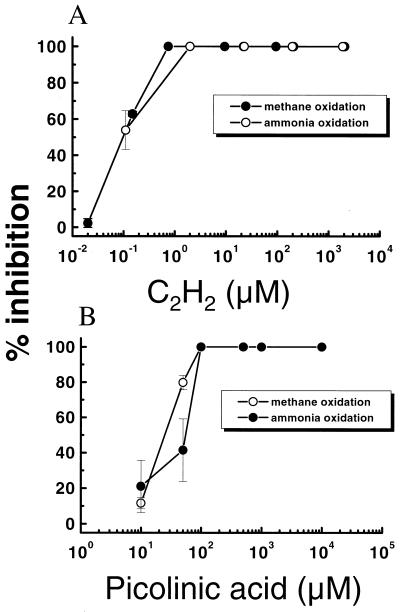
From Fig. 1 it is evident that both methanotrophs and nitrifiers were equally sensitive to C2H2 and picolinic acid. Methanotrophs as well as nitrifiers were completely inhibited at concentrations of 1 μM C2H2 (∼10 ppmv) or 100 μM picolinic acid. Even at picolinic acid concentrations of 10 μM, CH4 and NH4+ oxidation rates were still reduced by 11 and 20%, respectively.
FIG. 1.
Effects of C2H2 (A) and picolinic acid (B) on CH4 and NH4+ oxidation in preincubated rice plant soil slurries. Each value is the arithmetic mean from three replicate assays ± standard deviation (SD). The percent inhibition is relative to the activity without the presence of the inhibitor. The control activities for CH4 oxidation were 0.85 ± 0.04 and 0.92 ± 0.02 μmol · g (dry weight)−1 · h−1 for C2H2 and picolinic acid, respectively. Corresponding NH4+ oxidation rates were 30.98 ± 9.41 and 30.27 ± 2.31 nmol of NO3− plus NO2− · g (dry weight)−1 · h−1.
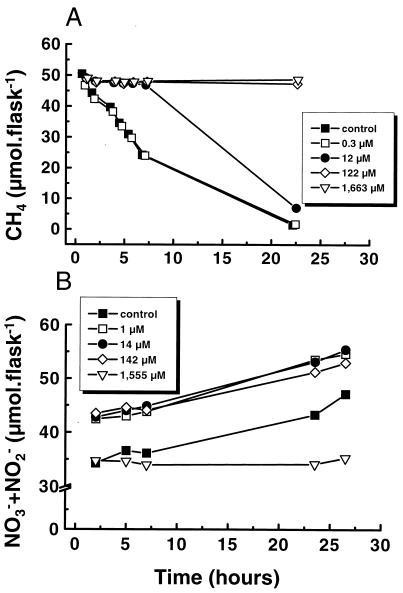
CH4 oxidation at a concentration of 10 μM (10,000 ppmv) CH4 was completely inhibited by the competitive inhibitor CH3F at a concentration of 12 μM (∼100 ppmv) (Fig. 2A). However, this inhibition lasted for only 7 h, due to the oxidation of CH3F. At a concentration of 122 μM (∼1,000 ppmv), CH3F inhibited CH4 oxidation completely for at least 24 h. In the presence of 2.78 mM NH4+, ammonia-oxidizing bacteria were not affected by the addition of as much as 142 μM CH3F (Fig. 2B). Only the addition of 10,000 ppmv (1,555 μM) of CH3F inhibited NH4+ oxidation. Hence, using a CH4/CH3F/NH4+ molar ratio of 0.1:1:18 enables the “competitive exclusion” of methanotrophic activity while preserving the activity of NH4+ oxidizers. Differentiation of CH4 and NH4+ oxidation by methanotrophs and nitrifiers in this way is referred to below as the CEA.
FIG. 2.
Effects of different CH3F mixing ratios on CH4 oxidation at a concentration of 10 μM CH4 (A) and on NH4+ oxidation at a concentration of 2.68 mM NH4+ (B), in preincubated rice plant soil slurries. Each value is the arithmetic mean from three replicate assays. CH3F concentrations are given in the keys.
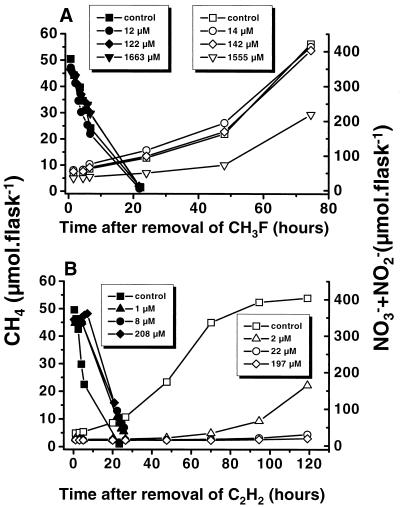
Recovery of CH4 and NH4+ oxidation after 24-h exposure to CH3F or C2H2.
After the removal of CH3F, methanotrophs immediately resumed activity at the same rate as that prior to inhibition (Fig. 3A), indicating the competitive nature and thus the reversibility of the inhibition. The ammonia oxidizers also resumed activity after inhibition with 10,000 ppmv of CH3F but did not reach the initial level within the next 50 h of incubation (Fig. 3A). After inhibition with C2H2, CH4 oxidation recovered fully within 10 to 15 h irrespective of the level of C2H2 (Fig. 3B). Recovery of the ammonia oxidizers took substantially longer and depended on the C2H2 concentration used (Fig. 3B). After exposure to 10 ppmv (∼2 μM) it took 24 h, and with 1,000 ppmv (∼197 μM) it took more than 3 days, before nitrification resumed. Hence, the DRA provides a “window” of at least 48 h in which to monitor CH4 and NH4+ oxidation by methanotrophs only.
FIG. 3.
Recovery of CH4 (solid symbols) and NH4+ (open symbols) oxidation after 24 h of exposure to different mixing ratios of CH3F (A) or C2H2 (B). Each value represents the arithmetic mean from three replicate assays. CH3F and C2H2 concentrations are given in the keys.
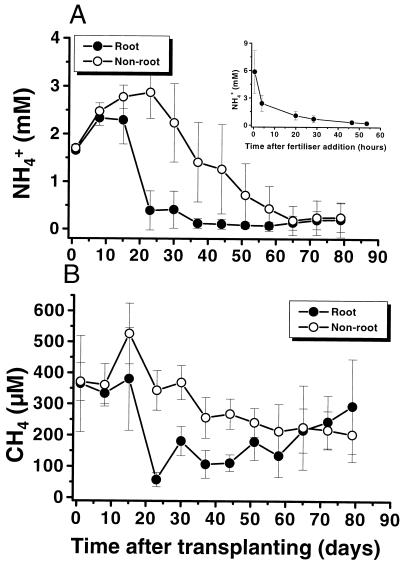
CH4 and NH4+ oxidation in microcosms planted with rice plants. (i) Availability of CH4 and NH4+ in the porewater.
Porewater NH4+ concentrations in the rhizospheres of the rice plant microcosms dropped below 0.5 mM at 25 days after transplanting (Fig. 4A). In this period the plants grew exponentially, as determined from total leaf length and plant height (data not shown). During the remainder of the experimental period, NH4+ levels of 100 to 200 μM were recorded. After 65 days the NH4+ concentration was at the same low level in both the root and nonroot compartments despite regular fertilization. Porewater samples were always taken prior to fertilizer addition. The inset in Fig. 4A shows NH4+ dynamics in the root compartments after one of the weekly fertilizer additions, i.e., 44 days after transplanting. During a period of 20 h, NH4+ availability was between 1 and 6 mM, and it stabilized at 0.5 mM 50 h after fertilizer addition. NO3− and NO2− were never detected in the weekly porewater samples (detection limit, 1 to 5 μM).
FIG. 4.
Porewater NH4+ (A) and CH4 (B) concentrations in the root and nonroot compartments of compartmented microcosms planted with rice plants. Each value represents the arithmetic mean (± SD) from four replicate microcosms. The inset in panel A depicts NH4+ concentrations in the root compartment during the first 55 h after fertilizer was added at day 44.
After 1 week of preincubation, the CH4 concentrations in the porewater of both compartments reached values of 300 to 400 μM (Fig. 4B). Concurrently with the drop in NH4+ and with the exponential-growth phase of the plants, the CH4 concentration in the root compartment decreased to 50 μM. Up to day 44, the availability of CH4 in the root compartment was lower than that in the nonroot compartment. This difference disappeared after 53 days, when concentrations between 150 and 300 μM were reached. CH4 concentrations even tended to become higher in the rhizosphere 75 days after transplanting.
(ii) Potential CH4 and NH4+ oxidation rates.
CH4 oxidation in slurries from the root compartment started after a lag of 10 h, while it took 30 h before CH4 was consumed in the assay from the nonroot compartment. Potential activities were calculated from the linear decrease of CH4 following the lag phase. Potential CH4 oxidation in the rhizosphere was significantly higher than that in the nonroot compartment (Table 1). Potential activities associated with the rice plant roots reached a level of 3.68 ± 0.34 μmol of CH4 · g of dry root−1 · h−1. Numbers of methanotrophs determined by MPN were 15 times higher in the root compartment (3.98 × 106 ± 1.96 × 106) than in the nonroot compartment (0.26 × 106 ± 0.06 × 106) and differed significantly (P < 0.05; n = 4; t test). No stimulating effect of rice plants on NH4+ oxidation rates was observed (Table 1). The nitrification rates in root and nonroot compartments did not differ, and nitrification activity associated with rice plant roots was not detected (data not shown). The nitrogen conversion rates in the rhizosphere per gram of dry soil were 3 orders of magnitude lower than the carbon conversion rates of methanotrophs.
TABLE 1.
Potential CH4 and NH4+ oxidation rates and the contribution of methanotrophs to nitrification in soil slurries from the root and nonroot compartments of rice microcosms
| Compartment | CH4 oxidationa (μmol of CH4 · g [dry wt]−1 · h−1) | NH4+ oxidation (nmol of NO3− + NO2− · g [dry wt]−1 · h−1)
|
Contribution of methanotrophs to NH4+ oxidation (% of total activity)
|
|||
|---|---|---|---|---|---|---|
| Without CH4 | With CH4 | With CH4 + CH3F | Without CH4b | With CH4c | ||
| Root | 1.43 ± 0.09*d | 2.99 ± 1.66 | 9.95 ± 0.32* | 1.51 ± 0.82 | 55.74 ± 14.32 | 84.97 ± 7.72 |
| Nonroot | 0.66 ± 0.30 | 2.13 ± 0.58 | 3.09 ± 1.09 | 1.05 ± 0.79 | 44.48 ± 25.90 | 61.69 ± 35.55 |
Without NH4+.
Calculated as [1 − (NH4 oxidation with CH4 plus CH3F/NH4 oxidation without CH4 or CH3F)] × 100.
Calculated as [1 − (NH4 oxidation with CH4 plus CH3F/NH4 oxidation with CH4 alone)] × 100.
Asterisks indicate significant differences between root and nonroot compartments (t test; P > 0.05; n = 4).
(iii) Contribution of methanotrophs and nitrifiers to CH4 and NH4+ oxidation.
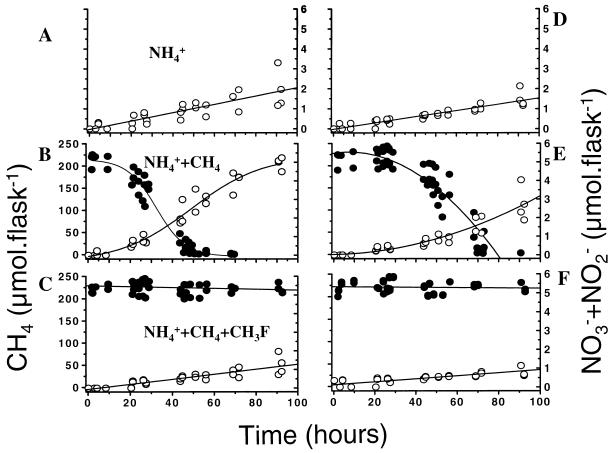
The results of the CEA are displayed in Fig. 5. When CH4 was added to the assay flasks, the oxidation of NH4+ was stimulated and mirrored the CH4 depletion curves for both the root (Fig. 5A and B) and nonroot compartments (Fig. 5D and E). In parallel assays with CH3F, the NH4+ oxidation rates were lower than those in the control (Table 1; Fig. 5C and F). No CH4 was consumed in these flasks. From these data the contribution of methanotrophs to nitrification was calculated by assuming that the rate in the presence of CH3F was the result of nitrifier activity exclusively (Table 1). When no CH4 was added, methanotrophs contributed about 50% to NH4+ oxidation; when CH4 was present, their contributions were 85 and 62% in the root and nonroot compartments, respectively.
FIG. 5.
CEAs using CH3F applied to NH4+ (2 mM)-supplemented slurries from the root (A through C) and nonroot (D through F) compartments of four compartmented microcosms planted with rice plants. The scatter diagram depicts the assay values of all four microcosms. (A and D) NH4+ oxidation in the absence of CH4 and CH3F, putatively caused by the activities of both methanotrophs and nitrifiers. (B and E) Oxidation of CH4 (solid circles) and NH4+ (open circles) in the presence of CH4 (10,000 ppmv), putatively caused by both methanotrophs and nitrifiers. (C and F) Oxidation of CH4 (solid circles) and NH4+ (open circles) in the presence of CH4 (10,000 ppmv) and CH3F (300 ppmv), putatively allowing for activity of nitrifiers only. The respective conversion rates are displayed in Table 1.
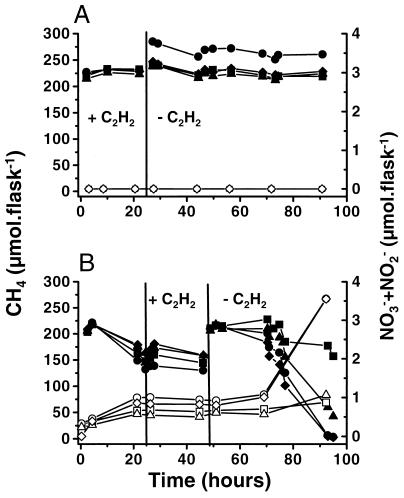
The DRA allows the measurement of CH4 and NH4+ oxidation exclusively associated with methanotrophs in the 48-h period after the removal of C2H2 (see Fig. 3B). In this period methanotrophic nitrification in slurries from rhizosphere soil started immediately after CH4 oxidation had recovered (Fig. 6B). Recovery took place only when the inhibitor was added after the lag phase of the methanotrophs. When C2H2 was added at the start of the assay, no recovery, and hence no CH4 or NH4+ oxidation, took place (Fig. 6A) during the incubation period of 90 h.
FIG. 6.
Differential recovery of CH4 (solid symbols; left axis) and NH4+ (open symbols; right axis) oxidation after 24-h exposure to C2H2 applied to slurries from the root compartments of four replicate microcosms planted with rice plants. (A) C2H2 was added at the start of the incubation period. (B) C2H2 was added 24 h after the start of the incubation period.
DISCUSSION
Evaluation of inhibitor-based discrimination.
Despite the ecological and biogeochemical impacts of methane and ammonia oxidation, especially in agricultural and other wetland soils, the knowledge about both processes and the organisms involved is still far from sufficient. The contributions of methanotrophs to nitrification and of nitrifiers to methane oxidation in these systems have never been assessed properly. To address this matter in various ecosystems, the main approach up to now was to find possible discriminating substances (5). Inhibitors like methylfluoride and dimethylether (37), allylthiourea, dicyandiamide, and allylsulfide (40), monoterpenes (1), and difluoromethane (33) were evaluated. Of all these substances, only picolinic acid and allylsulfide seemed to have a potential for discrimination. We therefore evaluated picolinic acid in our rice plant soil. However, our results were in contrast to those of Megraw and Knowles (32), who found nitrifiers to be less sensitive for this compound. In the rice plant soil, methane and ammonia oxidation were equally sensitive to picolinic acid, with complete inhibition at 100 μM. The contrasting results can be explained only by assuming the presence of more-sensitive bacteria in the rice plant soils or of physicochemical soil parameters which make picolinic acid more effective. Our results suggest that this inhibitor should be reevaluated for every soil type and situation. When using picolinic acid, one should also consider the time course of the experiments, since inhibition with concentrations of <1 mM did not last for more than 20 h, demonstrating the rapid degradation of this compound. However, as much as 10 mM picolinic acid did not affect methanogenesis (unpublished data), demonstrating the potential for use of this compound in flux studies where CH4 oxidation has to be eliminated without affecting methanogenesis. We did not test allylsulfide, which is apparently less inhibitory to methanotrophs than to nitrifiers (40), because 100% discrimination was not possible. Moreover, this compound is insoluble in water, which poses practical problems for its application in field and microcosm studies.
We looked for other options, mainly based on temporary inactivation of one or both groups of bacteria. The CEA is based on the competitive nature of the inhibition by CH3F, which was clearly affirmed by the immediate recovery after removal (Fig. 3A). The inherently different substrate concentrations in potential methane (10 μM CH4) and NH4+ (1 to 5 mM NH4+) oxidation assays allow for selective inactivation of methanotrophs with the same inhibitor concentration. The advantage of this technique is that it is only concentration dependent and will thus work with different methanotrophic and nitrifying species as well as with samples from any environment. It will also work with other competitive inhibitors, for instance, difluoromethane (33). However, one has to take care that the CH3F concentration is high enough to be effective during the entire assay period, because CH3F can be oxidized by both methanotrophs (37) and nitrifiers (24). From our experiments with rice plant soil it could be concluded that CH3F did not inhibit methane oxidation at a CH4/CH3F molar ratio of <2:1 (data not shown). With ammonia oxidizers an 18:1 ratio of NH4+ to CH3F did not inhibit ammonia oxidation at all, while a ratio of <2:1 inhibited it 95% (Fig. 2B). A disadvantage of the CEA is that it will be difficult to apply in situ, because the concentrations of NH4+ and CH4 need to be manipulated.
Our second discriminating approach was based on the ability of recovery after a discrete exposure to C2H2, analogous to the method described by Kester et al. (28). These authors observed recovery of denitrifiers in soil and sediment slurries within 1 day after a 24-h exposure to 1,000 ppmv of C2H2, while nitrification activity resumed after 6 days. We observed the same phenomenon for methanotrophs and nitrifiers, enabling discrimination of the two groups in our rice plant soil. However, because C2H2 is a suicide substrate for both nitrifiers (25) and methanotrophs (38), recovery will require de novo enzyme synthesis (23). This implies that the physiological status of the bacteria at the moment of the assay is decisive for the recovery. From a theoretical point of view it can be argued that methanotrophs will have more “reserves” for a swift recovery than the chemolithoautotrophic ammonia oxidizers, which spent 80% of the energy generated from the oxidation of NH4+ on the fixation and incorporation of CO2 (46). This seems to hold true for our rice plant soils, where the methanotrophs recovered very fast and reached the same activity as that prior to inhibition. However, the facts that NH4+ oxidation in soil from barley fields recovered within 24 h after a 15-h exposure to 100 ppmv of C2H2 (11) and that methane oxidation did not recover in soil after inhibition with C2H2 (33) indicate that the DRA is certainly no routine assay and has to be evaluated for every situation. The study of Bollmann and Conrad (11) points to a better physiological status of the ammonia oxidizers in their oxic agricultural soils, explaining the relatively swift recovery. In the study of Miller et al. (33), methanotrophs did not recover because the C2H2 was applied at the start of the assay, when, according to the controls, the methanotrophs were still in a lag phase. We observed the same phenomenon (Fig. 6). When C2H2 was added after CH4 oxidation was induced, recovery took place. Apparently the presence of C2H2 prior to the induction of enzyme synthesis leads to permanent inactivation. Taking into account that C2H2 irreversibly inhibits the amount of MMO that has already been expressed, the lack of recovery must be due to a block of the initiation of de novo enzyme synthesis. This blockage is relieved when the cells metabolize CH4 prior to inhibition of the MMO. Apparently, the amount of energy synthesized during this initial oxidation is essential for triggering enzyme synthesis after the inhibitor is removed. Another possible explanation may be found in a direct link of the active MMO to the enzyme transcriptional level of enzymes downstream in the CH4 oxidizing pathway.
CH4 and NH4+ oxidation by methanotrophs and nitrifiers in the rhizospheres of rice plants.
It is clear that methanotrophs were able to profit from the oxygen release from the rice plants, as reflected by the potential activities and numbers. Gilbert and Frenzel (21) demonstrated the same effect, using a similar compartmented-microcosm approach, with activities and numbers in the same range. Methane oxidation rates associated with our rice plant roots were also in the same order of magnitude as those found with other rice plants (12, 21) and a range of other wetland plants (29). The CH4 availability in the porewater was also comparable to that in artificial rice systems (20, 21), natural rice paddies (35), and natural wetlands (44) and was high enough to give the methanotrophs the opportunity to profit from the oxygen derived from the plants. This was obviously not the case for the nitrifiers. Due to methodological problems, we were not able to assess the number of nitrifiers in our microcosms. However, in comparable microcosms there was no difference in MPN numbers of ammonia oxidizers in rhizospheres and bulk rice plant soils (4). The nitrification potentials in the rhizospheres and in the bulk soils of our microcosms were at very low levels. It is very likely that, just as in some other studies (3, 8, 10, 17), the ammonia oxidizers are outcompeted for NH4+ by the plants. Indeed, NH4+ availability in the rhizosphere in our experiment was low despite the frequent fertilization. Low NH4+ concentrations are common in the rhizospheres of rice plants (21) and other wetland plants (8, 10, 44). A stimulation of nitrate production in the rhizospheres of rice plants was only indirectly demonstrated after fertilization by analysis of denitrification products (see, e.g., references 3 and 39). However, taking our results with the CEA and DRA into account, one can argue about which bacteria are responsible for nitrification in the rhizosphere. We clearly demonstrated that, at least potentially, methanotrophs could be responsible for the nitrification in the rice plant rhizosphere. So far, methanotrophic nitrification in soil was reported only for humisol slurries and subsequent enrichment cultures (31, 32). However, these slurries were enriched for methanotrophs first by incubation with 20% CH4, thereby making reference to the in situ situation difficult. Moreover, discrimination between methane- and ammonia-oxidizing bacteria was assessed by using picolinic acid, which apparently is not always 100% discriminatory. The methanotrophic nitrification in our study was measured in the first 24 h after harvesting of the microcosm by using an assay which discriminates 100%, thus improving the credibility of extrapolations to the in situ situation.
It is very unlikely that ammonia oxidizers contribute significantly to CH4 oxidation in our microcosms. From a theoretical point of view, taking the kinetic properties of CH4 oxidation by pure cultures of ammonia oxidizers and the low nitrification potential in the microcosms into account, a prominent role of nitrifiers in methane oxidation in our rice plant soil is highly unlikely. Additionally, the CH4 oxidation rates after recovery of inhibition with C2H2 should be lower than the rates prior to inhibition if nitrifiers play a significant role in CH4 oxidation. The rates during the first 48 h after recovery, due exclusively to methanotrophs, were not different from the rates before inhibition, which theoretically could have been the result of both nitrifiers and methanotrophs. The only report so far on involvement in methane oxidation of nitrifiers in natural systems originates from fertilized forest soils (43). Steudler et al. (43) used the inhibitor-sensitive 14CH4/14CO oxidation ratio as a criterion for methane oxidation dominated by methanotrophs (ratio of >0.05) or nitrifiers (ratio of <0.05). However, these ratios are based on pure culture studies and are thus also indirect evidence. Hence, measuring the nitrifier methane oxidation in natural systems is still a challenge.
Conclusions.
This study presents new methods for discerning the interactions and overlap of C and N cycling processes, in which different but functionally highly similar bacterial groups are involved. The CEA and DRA are applicable to soil slurries, but similar approaches may be useful for assessing the contributions of methanotrophs and nitrifiers to CH4 and NH4+ turnover in situ. For the rice plant rhizosphere system, however, we can conclude that methanotrophs have the potential to contribute substantially to nitrification, while nitrifiers are probably of little importance for methane oxidation.
ACKNOWLEDGMENTS
We thank Ralf Conrad and Peter Dunfield for the critical reading of the manuscript and Alexandra Runkel and Markus Drescher for their technical assistance. We also appreciate the cooperation of the Centre for Terrestrial Ecology of the Netherlands Institute of Ecology with regard to the autoanalyzer analysis.
This project was financially supported by the EU, project BIO 4 CT 960419, and the DFG, project Fr 1054/1.
REFERENCES
- 1.Amaral J A, Ekins A, Richards S R, Knowles R. Effect of selected monoterpenes on methane oxidation, denitrification, and aerobic metabolism by bacteria in pure culture. Appl Environ Microbiol. 1998;64:520–525. doi: 10.1128/aem.64.2.520-525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong W, Brändle R, Jackson M B. Mechanisms of flood tolerance in plants. Acta Bot Neerl. 1994;43:307–358. [Google Scholar]
- 3.Arth I, Frenzel P, Conrad R. Denitrification coupled to nitrification in the rhizosphere of rice. Soil Biol Biochem. 1998;30:509–515. [Google Scholar]
- 4.Arth, I. 1998. Personal communication.
- 5.Bèdard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bèdard C, Knowles R. Some properties of methane oxidation in a thermally stratified lake. Can J Fish Aquat Sci. 1997;54:1639–1645. [Google Scholar]
- 7.Blom C W P M, Voesenek L A C J. Flooding: the survival strategies of plants. TREE. 1996;11:290–295. doi: 10.1016/0169-5347(96)10034-3. [DOI] [PubMed] [Google Scholar]
- 8.Bodelier P L E, Duyts H, Blom C W P M, Laanbroek H J. Interactions between nitrifying and denitrifying bacteria in gnotobiotic microcosms planted with the emergent macrophyte Glyceria maxima. FEMS Microbiol Ecol. 1998;25:63–78. [Google Scholar]
- 9.Bodelier P L E, Wijlhuizen A G, Blom C W P M, Laanbroek H J. Effects of photoperiod on growth of and denitrification by Pseudomonas chlororaphis in the root zone of Glyceria maxima, studied in a gnotobiotic microcosm. Plant Soil. 1997;190:91–103. [Google Scholar]
- 10.Bodelier P L E, Libochant J A, Blom C W P M, Laanbroek H J. Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Appl Environ Microbiol. 1996;62:4100–4107. doi: 10.1128/aem.62.11.4100-4107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollmann A, Conrad R. Recovery of nitrification and production of NO and N2O after exposure of soil to acetylene. Biol Fertil Soils. 1997;25:41–46. [Google Scholar]
- 12.Bosse U, Frenzel P. Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryza sativa) Appl Environ Microbiol. 1997;63:1199–1207. doi: 10.1128/aem.63.4.1199-1207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosse U, Frenzel P. Methane emissions from rice microcosms: the balance of production, accumulation and oxidation. Biogeochemistry. 1998;41:199–214. [Google Scholar]
- 14.Conrad R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO) Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denier van der Gon H A C, Neue H U. Oxidation of methane in the rhizosphere of rice plants. Biol Fertil Soils. 1996;22:359–366. [Google Scholar]
- 16.Elsevier Science bv. Encyclopedie des Gaz. Amsterdam, The Netherlands: Elsevier Science bv; 1976. L’air liquide. [Google Scholar]
- 17.Engelaar W H M G, Bodelier P L E, Laanbroek H J, Blom C W P M. Nitrification in the rhizosphere of a flooding-resistant and a flooding-non-resistant Rumex species under drained and waterlogged conditions. FEMS Microbiol Ecol. 1991;86:33–42. [Google Scholar]
- 18.Engelaar W M H G, Symens J C, Laanbroek H J, Blom C W P M. Preservation of nitrifying capacity and nitrate availability in waterlogged soils by radial oxygen loss from roots of wetland plants. Biol Fertil Soils. 1995;20:243–248. [Google Scholar]
- 19.Frenzel P, Bosse U. Methyl fluoride, an inhibitor of methane oxidation and methane production. FEMS Microbiol Ecol. 1996;21:25–36. [Google Scholar]
- 20.Gilbert B, Frenzel P. Methanotrophic bacteria in the rhizosphere of rice microcosms and their effect on porewater methane concentration and methane emission. Biol Fertil Soils. 1995;20:93–100. [Google Scholar]
- 21.Gilbert B, Frenzel P. Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biol Biochem. 1998;30:1903–1916. [Google Scholar]
- 22.Hyman M R, Arp D J. Quantification and removal of some contaminating gases from acetylene used to study gas-utilizing enzymes and microorganisms. Appl Environ Microbiol. 1987;53:298–303. doi: 10.1128/aem.53.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman M R, Arp D J. 14C2H2- and 14CO2-labelling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267:1534–1545. [PubMed] [Google Scholar]
- 24.Hyman M R, Page C L, Arp D J. Oxidation of methylfluoride and dimethyl ether by ammonia monooxygenase in Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:3033–3035. doi: 10.1128/aem.60.8.3033-3035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman M R, Wood P M. Suicidal inactivation and labelling of ammonia monooxygenase by acetylene. Biochem J. 1985;227:719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones R D, Morita R Y. Methane oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983;45:401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones R D, Morita R Y, Griffiths R P. Method for estimating in situ chemolithotrophic ammonium oxidation using carbon monoxide oxidation. Mar Ecol Prog Ser. 1984;17:259–269. [Google Scholar]
- 28.Kester R A, de Boer W, Laanbroek H J. Short exposure to acetylene to distinguish between nitrifier and denitrifier nitrous oxide production in soil and sediment samples. FEMS Microbiol Ecol. 1996;20:111–120. [Google Scholar]
- 29.King G M. Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl Environ Microbiol. 1994;60:3220–3227. doi: 10.1128/aem.60.9.3220-3227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King G M. In situ analysis of methane oxidation associated with the roots and rhizomes of a bur reed, Sparganium eurycarpum, in a Maine wetland. Appl Environ Microbiol. 1996;62:4548–4555. doi: 10.1128/aem.62.12.4548-4555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megraw S R, Knowles R. Methane-dependent nitrate production by a microbial consortium enriched from a cultivated humisol. FEMS Microbiol Ecol. 1989;62:359–366. [Google Scholar]
- 32.Megraw S R, Knowles R. Effect of picolinic acid (2-pyridine carboxylic acid) on the oxidation of methane and ammonia in soil and in liquid culture. Soil Biol Biochem. 1990;22:635–641. [Google Scholar]
- 33.Miller L G, Sasson C, Oremland R S. Difluoromethane, a new improved inhibitor of methanotrophy. Appl Environ Microbiol. 1998;64:4357–4362. doi: 10.1128/aem.64.11.4357-4362.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neue H U. Fluxes of methane from rice fields and potential for mitigation. Soil Use Manag. 1997;13:258–267. [Google Scholar]
- 35.Nouchi I, Hosono T, Aoki K, Minami K. Seasonal variation in methane flux from rice paddies associated with methane concentration in soil water, rice biomass and temperature, and its modelling. Plant Soil. 1994;161:195–208. [Google Scholar]
- 36.O’Neill J G, Wilkinson J F. Oxidation of ammonia by methane-oxidizing bacteria and the effects of ammonia on methane oxidation. J Gen Microbiol. 1977;100:407–412. [Google Scholar]
- 37.Oremland R S, Culbertson C W. Evaluation of methylfluoride and dimethylether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992;58:2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prior S D, Dalton H. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1985;29:105–109. [Google Scholar]
- 39.Reddy K R, Patrick W H, Jr, Lindau C W. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol Oceanogr. 1989;34:1004–1013. [Google Scholar]
- 40.Roy R, Knowles R. Differential inhibition by allylsulfide of nitrification and methane oxidation in freshwater sediment. Appl Environ Microbiol. 1995;61:4278–4283. doi: 10.1128/aem.61.12.4278-4283.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schipper L A, Reddy K R. Determination of methane oxidation in the rhizosphere of Sagittaria lancifolia using methyl fluoride. Soil Sci Soc Am J. 1996;60:611–616. [Google Scholar]
- 42.Schütz H, Holzapfel-Pschorn A, Conrad R, Rennenberg H, Seiler W. A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. J Geophys Res. 1989;94:16405–16416. [Google Scholar]
- 43.Steudler P A, Jones R D, Castro M S, Mellilo J M, Lewis D L. Microbial controls of methane oxidation in temperate forest and agricultural soils. NATO ASI Ser Ser I. 1996;39:69–84. [Google Scholar]
- 44.Van der Nat F J W A, Middelburg J J, van Meteren D, Wielemakers A. Diel methane emission patterns from Scirpus lacustris and Phragmites australis. Biogeochemistry. 1998;42:1–22. [Google Scholar]
- 45.Ward B B. Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus. Arch Microbiol. 1987;147:126–133. [Google Scholar]
- 46.Wood P M. Nitrification as bacterial energy source. Spec Publ Soc Gen Microbiol. 1986;20:39–61. [Google Scholar]
- 47.Yoshinari T. Nitrite and nitrous oxide production by Methylosinus trichosporium. Can J Microbiol. 1984;31:139–144. doi: 10.1139/m85-027. [DOI] [PubMed] [Google Scholar]