Keywords: exercise hyperemia, heart failure, inflammation, preserved ejection fraction, vasodilation
Abstract
Obesity is now considered a primary comorbidity in heart failure with preserved ejection fraction (HFpEF) pathophysiology, mediated largely by systemic inflammation. Although there is accumulating evidence for a disease-related dysregulation of blood flow during exercise in this patient group, the role of obesity in the hemodynamic response to exercise remains largely unknown. Small muscle mass handgrip (HG) exercise was used to evaluate exercising muscle blood flow in nonobese (BMI < 30 kg/m2, n = 14) and obese (BMI > 30 kg/m2, n = 40) patients with HFpEF. Heart rate (HR), stroke index (SI), cardiac index (CI), mean arterial pressure (MAP), forearm blood flow (FBF), and vascular conductance (FVC) were assessed during progressive intermittent HG exercise [15%–30%–45% maximal voluntary contraction (MVC)]. Blood biomarkers of inflammation [C-reactive protein (CRP) and interleukin-6 (IL-6)] were also determined. Exercising FBF was reduced in obese patients with HFpEF at all work rates (15%: 304 ± 42 vs. 229 ± 15 mL/min; 30%: 402 ± 46 vs. 300 ± 18 mL/min; 45%: 484 ± 55 vs. 380 ± 23 mL/min, nonobese vs. obese, P = 0.025), and was negatively correlated with BMI (R = −0.47, P < 0.01). In contrast, no differences in central hemodynamics (HR, SI, CI, and MAP) were found between groups. Proinflammatory biomarkers were markedly elevated in patients with obesity (CRP: 2,133 ± 418 vs. 4,630 ± 590 ng/mL, P = 0.02; IL-6: 2.9 ± 0.3 vs. 5.2 ± 0.7 pg/mL, nonobese vs. obese, P = 0.04), and both biomarkers were positively correlated with BMI (CRP: R = 0.40, P = 0.03; IL-6: R = 0.57, P < 0.01). Together, these findings demonstrate the presence of obesity and an accompanying milieu of systemic inflammation as important factors in the dysregulation of exercising muscle blood flow in patients with HFpEF.
NEW & NOTEWORTHY Obesity is the primary comorbid condition in HFpEF pathophysiology, but the role of adiposity on the peripheral circulation is not well understood. The present study identified a 30%–40% reduction in forearm blood flow during handgrip exercise, accompanied by a marked elevation in proinflammatory plasma biomarkers, in obese patients with HFpEF compared with their nonobese counterparts. These findings suggest an exaggerated dysregulation in exercising muscle blood flow associated with the obese HFpEF phenotype.
INTRODUCTION
Obesity, defined as a body mass index (BMI) above or equal to 30 kg/m2, is one of the most important risk factors for cardiovascular disease, including heart failure with preserved ejection fraction (HFpEF) (1, 2). In fact, over 80% of patients with HFpEF are overweight or obese (3), contributing to the “constellation of comorbidities” associated with HFpEF, which also includes hypertension, diabetes mellitus, and chronic kidney disease (4). The ubiquitous impact of obesity on cardiac, vascular, and skeletal muscle function is well known (5, 6), and may therefore be a critical contributor to the pathogenesis and clinical course of HFpEF. In fact, “obese HFpEF” is now viewed as a discrete phenotype that includes more severe impairments in renal, autocrine, metabolic, pulmonary, and cardiac function compared with nonobese patients with HFpEF (7, 8), a distinction that may be particularly relevant given the increased interest in determining HFpEF phenogroups to better optimize clinical care in this patient group (9).
Although the role of systemic inflammation in HFpEF etiology originally proposed by Paulus and Tschope (10) is now well recognized, it may be especially germane in the context of the obese HFpEF phenotype. Adipose tissue is metabolically active, producing inflammatory cytokines known as adipokines, which function as an endocrine organ to regulate metabolism (11, 12) and vascular function (13, 14), the latter being particularly significant considering the growing interest in noncardiac, vascular complications of HFpEF. Indeed, the release of adipokines, such as interleukin-6 (IL-6), cause a proinflammatory state that can ultimately affect the vasculature by enhancing reactive oxygen species generation (15) and diminishing nitric oxide bioavailability (16), leading to cardiovascular complications such as atherosclerosis (17). As obesity is associated with endothelial dysfunction, specifically in the microvasculature (18–20), chronic, low-grade inflammation caused by excessive fat cell accumulation in obese patients with HFpEF could promote vascular dysfunction in this patient group. Although there is growing evidence for the prognostic and diagnostic value of proinflammatory biomarkers such as C-reactive protein (CRP) (21) and IL-6 (22), the potential impact of elevated systemic inflammation on blood flow regulation in patients with HFpEF is not well understood.
There is accumulating evidence for disease-related changes in peripheral vascular health that may contribute to exercise intolerance, a hallmark symptom in patients with HFpEF. Indeed, our group has identified a significant reduction in exercising limb blood flow during small muscle mass exercise in both lower (23) and upper (24) extremities in patients with HFpEF compared with controls, an impairment that was accompanied by minimal differences in central hemodynamic responses between groups. Although these studies provided evidence for a clear decrement in “exercise hyperemia” in patients with HFpEF, the role of obesity and inflammation, two prevalent aspects of HFpEF pathophysiology, were not considered. Interestingly, Limberg et al. (25) failed to identify differences in skeletal muscle blood flow during either arm or leg exercise between young healthy lean and obese subjects, suggesting that the presence of obesity, per se, does not appear to alter exercise hyperemia. However, the impact of the obese phenotype on peripheral hemodynamics may be amplified by the comorbidities that accompany HFpEF, as evidenced by studies reporting a reduction in exercising limb blood flow in patients with type-2 diabetes (26), essential hypertension (27), and chronic obstructive lung disease (28).
In light of these previous studies and more recent evidence for the degree of adiposity as an important determinant of overall O2 delivery and usage during exercise in HFpEF (29), this study sought to evaluate central and peripheral hemodynamic responses to exercise, as well as the systemic inflammatory biomarkers CRP and IL-6, among class II and III obese patients with HFpEF compared with nonobese patients with HFpEF. We hypothesized that obese patients with HFpEF would exhibit attenuated exercise hyperemia and increased inflammatory biomarkers compared with their nonobese HFpEF counterparts, and that central hemodynamic responses would not differ between groups. We also anticipated that both exercising muscle blood flow and biomarkers of inflammation would be associated with body mass index (BMI).
METHODS
Subjects
All patients were recruited from the Heart Failure (HF) Clinic at the University of Utah, the Salt Lake City Veterans Affairs Medical Center (VAMC), and the greater Salt Lake City community. Patients were stratified as nonobese (BMI < 30 kg/m2) or obese (BMI ≥ 30 kg/m2). Inclusion criteria for patients with HFpEF were consistent with the TOPCAT trial (30), which are as follows: 1) HF defined by the presence of ≥1 symptom at the time of screening (paroxysmal nocturnal dyspnea, orthopnea, and dyspnea on exertion) and one sign (edema, elevation in jugular venous distention) in the previous 12 mo; 2) left ventricular ejection fraction (LVEF) ≥45%; 3) controlled systolic blood pressure; and 4) either ≥1 hospitalization in the previous 12 mo for which HF was a major component of hospitalization, or B-type natriuretic peptide (BNP) in the previous 60 days ≥100 pg/mL. Exclusion criteria for the HFpEF group included significant valvular heart disease, acute atrial fibrillation, and any orthopedic limitations that would prevent the performance of handgrip exercise. All participants were nonsmokers, and were maintained on guideline-directed pharmacotherapy, with no medications withheld on the experimental study day. All female patients were postmenopausal, devoid of hormone replacement therapy. All procedures were approved by the University of Utah and the Salt Lake City VAMC Institutional Review Boards in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before study participation. Studies were performed at the VA Salt Lake City Geriatric Research, Education, and Clinical Center in the Utah Vascular Research Laboratory.
Protocols
On the experimental day, patients reported to the laboratory ∼8 h postprandial and provided a venous blood sample. Height and weight were measured to calculate body surface area (BSA) using the Haycock method (31) as BSA (m2) = 0.024265 (height, m)0.3964 × (weight, kg)0.5378. Data collection took place in a thermoneutral environment, with participants in the supine position.
Handgrip Exercise
Patients rested in the supine position for ∼20 min before the start of data collection with the right arm abducted at 90°. The elbow joint was extended at heart level to allow patients to perform HG exercise. First, maximal voluntary contraction (MVC) was established by taking the highest value of three maximal contractions using a handgrip dynamometer (Biopac Systems, Goleta, CA). Progressive, intermittent isometric HG exercise was performed at three workloads based on each patient’s respective MVC (15%, 30%, and 45% of MVC, 1 Hz, 3 min per exercise stage), with force output displayed to provide visual feedback.
Ultrasound Doppler
Blood velocity and vessel diameter of the brachial artery were determined using an ultrasound Doppler system (GE Medical Systems, Milwaukee, WI). The brachial artery was insonated approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle. Blood velocity was collected at a Doppler frequency of 5 MHz in high-pulsed repetition frequency mode (2–25 kHz). Sample volume was optimized in relation to vessel diameter and centered within the vessel. Vessel diameter was obtained during end-diastole (corresponding to each R wave documented by the simultaneous ECG signal) using the same transducer at an imaging frequency ranging from 9 to 14 MHz. An angle of insonation of ≤60° (32) was achieved for all measurements. Brachial artery vasodilation was determined offline from end-diastolic, ECG R wave triggered images collected from the doppler using automated edge-detection software (Medical Imaging Applications, Coralville, IA) (33). Ultrasound Doppler measurements were performed continuously, with the last 60 s of each exercise intensity used for the determination of limb blood flow. Forearm blood flow (FBF) was calculated with the formula: FBF (mL·min−1) = [Vmean × π (vessel diameter/2)2 × 60], and forearm vascular conductance (FVC) was calculated as: FVC (mL·min−1·mmHg) = FBF/mean arterial pressure (MAP).
Hemodynamic Variables
Stroke volume (SV) and arterial blood pressure (ABP) were determined noninvasively using photoplethysmography (Finometer, Finapres Medical Systems BV, Amsterdam, the Netherlands). SV was calculated using the Modelflow method, which includes age, sex, height, and weight in its algorithm (Beatscope v 1.1; Finapres Medical Systems BS, Amsterdam, the Netherlands) (34) and has been documented to accurately track SV during a variety of experimental protocols, including exercise (35–37). ABP was measured continuously (Finometer, Finapres Medical Systems BV, Amsterdam, the Netherlands), and mean arterial pressure (MAP) was calculated as MAP (mmHg) = diastolic arterial pressure + (pulse pressure × 0.33). Heart rate was monitored from a standard three-lead electrocardiogram recorded in duplicate on the data acquisition system (Biopac, Goleta, CA) and GE Ultrasound Doppler. To control for body size, stroke index (SI, mL/m2) and cardiac index (CI, L/min/m2) were calculated relative to BSA (m2).
Blood Biomarker Analyses
Blood was sampled in a subset of subjects who consented to a blood draw from the antecubital vein, and plasma and serum samples were stored at −80°C for subsequent analyses. Biomarkers associated with heart failure were assessed (38), including brain natriuretic peptide (BNP), N-terminal-pro hormone BNP (NT-proBNP), suppression of tumorigenicity 2 (ST2), galectin-3, and cystatin C, according to the manufacturer’s instructions (Invitrogen). Serum CRP was measured using a liquid-phase, double-antibody radioimmunoassay. High sensitivity IL-6 concentrations were measured using solid-phase sandwich ELISA kits (kits HS600B, R&D Systems: Minneapolis, MN).
Statistical Analyses
Statistics were performed using commercially available software (SigmaStat 3.10; Systat Software, Point Richmond, CA). Categorical disease and medication characteristics were compared between groups using a χ2 test (α < 0.05). Blood sample characteristics were compared between groups using a two-tailed Student’s t test (α < 0.05). A 2 × 4 repeated-measures ANOVA (α < 0.05) (group, two levels: nonobese vs. obese HFpEF) (workload, 4 levels: rest, 15%, 30%, and 45% of MVC) were performed to determine the hemodynamic responses in nonobese and obese patients with HfpEF during exercise. A 2 × 3 repeated-measures ANOVA (α < 0.05) (group, two levels: nonobese vs. obese HFpEF) (workload, 3 levels: 15%, 30%, and 45% of MVC) were performed to determine the changes in hemodynamic responses in nonobese and obese patients with HFpEF during exercise compared with baseline. The Holm–Sidak method was used for α adjustment and post hoc analysis. Pearson correlations were performed to examine the association between the overall FBF response [slope of brachial artery blood flow (mL/min) across all three workloads (kg)], and CRP, IL-6, and BMI. Subject characteristics are expressed as means ± SD, and all other data are expressed as means ± SE.
RESULTS
Subject Characteristics
Anthropometric data and clinical biomarkers are reported in Table 1. Disease-specific characteristics and pharmacological therapeutic information for both groups are shown in Table 2 with a higher proportion of obese patients with HFpEF using aldosterone antagonists compared with nonobese patients with HFpEF (P = 0.047). Echocardiographic information and circulating biomarkers commonly presented among heart failure severity assessments (38) for patients with HFpEF are provided in Table 3. Nonobese and obese patients with HFpEF were generally well matched including for handgrip strength and HFpEF disease severity, although the obese group had a higher prevalence for diabetes mellitus and were taking an average of four medications versus three medications for nonobese.
Table 1.
Subject characteristics and clinical biomarkers
| Nonobese HFpEF (n = 6 M/8 F) | Obese HFpEF (n = 16 M/24 F) | |
|---|---|---|
| Age, yr | 73 ± 8 | 67 ± 10 |
| Height, cm | 169 ± 11 | 169 ± 12 |
| Weight, kg | 76 ± 16 | 106 ± 23* |
| BMI, kg/m2 | 26 ± 3 | 37 ± 7* |
| BSA, m2 | 1.9 ± 0.3 | 2.3 ± 0.3* |
| MVC, kg | 17 ± 7 | 17 ± 6 |
A Student’s t test was performed between groups (α < 0.05). *P < 0.05 vs. nonobese HFpEF. Means ± SD. BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; F, females; HFpEF, heart failure with preserved ejection fraction; M, males; MVC, maximal voluntary contraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; ST2, suppression of tumorigenicity.
Table 2.
Disease characteristics and medications
| Nonobese HFpEF | Obese HFpEF | |
|---|---|---|
| Disease characteristics, n/% | ||
| NYHA class II | 11/79 | 26/65 |
| NYHA class III | 2/14 | 12/30 |
| NYHA class IV | 0/0 | 2/5 |
| Diabetes | 2/14 | 11/28 |
| COPD | 1/7 | 3/8 |
| CAD | 5/36 | 9/23 |
| Hypertension | 9/64 | 29/73 |
| Hyperlipidemia | 8/57 | 14/35 |
| Medications | ||
| β-Blockers | 7/50 | 22/55 |
| ACEi | 1/7 | 14/35 |
| ARB | 4/29 | 11/28 |
| Loop diuretics | 11/79 | 30/75 |
| Aldosterone antagonists | 7/50 | 33/83* |
| Statin | 9/64 | 26/65 |
| Ca2+ channel blocker | 3/21 | 10/25 |
| Mean Rx types taken ± SE | 3.2 ± 1.2 | 3.8 ± 1.3 |
| Subjects taking Rx, % | 100 | 100 |
A χ2 test was performed between groups (α < 0.05). *P < 0.05 vs. nonobese HFpEF. Means ± SD. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; HFpEF, heart failure with preserved ejection fraction; NYHA class, New York Heart Association functional classification; Rx, prescription.
Table 3.
HFpEF clinical characteristics
| Nonobese HFpEF | Obese HFpEF | Reference Range | |
|---|---|---|---|
| Echocardiography | (n = 5 M/7 F) | (n = 16 M/20 F) | |
| Ejection fraction, % | 63 ± 7 | 63 ± 6 | ≥55 |
| LV IVSD, cm | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.6–1.1 |
| LV PWD, cm | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.6–0.9 |
| LV ID diastole, cm | 4.4 ± 0.8 | 4.6 ± 0.6 | 3.9–5.3 |
| LV ID systole, cm | 3.0 ± 0.7 | 3.0 ± 0.5 | 2.0–4.0 |
| LV FS, % | 32 ± 11 | 35 ± 8 | |
| Peak E wave, cm/s | 98 ± 3 | 99 ± 3 | ≤ 50 |
| Peak A wave, cm/s | 89 ± 4 | 85 ± 4 | |
| E/A ratio | 1.4 ± 0.8 | 1.6 ± 1.4 | 0.6–1.32 |
| E′ lateral wall, cm/s | 8 ± 2 | 8 ± 4 | 13–28 |
| E/E′ lateral ratio | 15 ± 4 | 15 ± 8 | ≤8 |
| Mitral E wave deceleration time, ms | 204 ± 35 | 224 ± 60 | 142–258 |
| Circulating biomarkers | (n = 5 M/5 F) | (n = 8 M/8 F) | |
| NT pro-BNP, ng/mL | 4.92 ± 6.20 | 4.13 ± 6.40 | |
| BNP, pg/mL | 42.0 ± 52.4 | 74.7 ± 68.2 | |
| Galectin-3, ng/mL | 16.6 ± 5.4 | 18.6 ± 4.0 | |
| ST2, ng/mL | 65.4 ± 42.6 | 53.6 ± 31.2 | |
| Cystatin C, ng/mL | 16.4 ± 4.5 | 19.0 ± 5.2 |
Reference values are normal, nonclinical populations. A Student’s t test was performed between groups (α < 0.05). Means ± SD. A wave, peak velocity of late transmitral flow; E wave, peak velocity of early diastolic transmitral flow; E′, peak velocity of early diastolic mitral annular motion; ID, internal dimension; HFpEF, heart failure with preserved ejection fraction; IVSD, interventricular septum thickness at end-diastole; LV, left ventricle; PWD, posterior wall thickness.
Central Hemodynamics
Baseline systolic arterial blood pressure (SBP, nonobese: 147 ± 7; obese: 141 ± 5 mmHg), diastolic arterial blood pressure (DPB, nonobese: 66 ± 4; obese: 59 ± 3 mmHg), mean arterial blood pressure (MAP, nonobese: 97 ± 6; obese: 90 ± 4 mmHg), pulse pressure (PP, nonobese: 81 ± 6; obese: 82 ± 4 mmHg), heart rate (HR, nonobese: 62 ± 2; obese: 64 ± 2 bpm), stroke index (SI, nonobese: 41 ± 4; obese: 54 ± 3 mL/min/m2), and cardiac index (CI, nonobese: 2.5 ± 0.2; obese: 3.3 ± 0.2 L/min/m2) were not different between groups (P > 0.05). During handgrip exercise at 30% and 45% MVC, SBP, DBP, MAP, PP, HR, SI, and CI were not different between groups (P > 0.05). Changes in central hemodynamics during handgrip exercise are presented in Table 4.
Table 4.
Cardiovascular responses to dynamic handgrip exercise
| Relative Handgrip Intensity, %MVC | 15% |
30% |
45% |
|||
|---|---|---|---|---|---|---|
| Nonobese HFpEF | Obese HFpEF | Nonobese HFpEF | Obese HFpEF | Nonobese HFpEF | Obese HFpEF | |
| Systolic arterial blood pressure, ΔmmHg | 6.9 ± 4.8 | 7.6 ± 2.9 | 10.6 ± 4.5 | 14.2 ± 3.5 | 26.3 ± 6.0 | 22.8 ± 4.5 |
| Diastolic arterial blood pressure, ΔmmHg | 9.6 ± 3.0 | 5.2 ± 1.5 | 6.3 ± 2.5 | 5.1 ± 1.5 | 13.8 ± 3.4 | 9.0 ± 2.0 |
| Mean arterial blood pressure, ΔmmHg | 3.2 ± 5.2 | 4.4 ± 1.8 | 5.8 ± 4.6 | 8.2 ± 1.9 | 15.0 ± 5.3 | 13.1 ± 2.8 |
| Pulse pressure, ΔmmHg | 1.9 ± 5.2 | 2.5 ± 2.7 | 8.7 ± 5.4 | 9.1 ± 2.9 | 17.1 ± 5.9 | 13.8 ± 3.2 |
| Heart rate, Δbeats/min | 5.7 ± 1.8 | 3.4 ± 1.2 | 7.4 ± 2.2 | 6.2 ± 1.2 | 11.5 ± 1.8 | 8.7 ± 1.4 |
| Stroke volume, ΔmL/min | 2.5 ± 3.5 | 2.9 ± 3.2 | 3.9 ± 2.8 | 1.4 ± 4.3 | −0.7 ± 5.0 | −6.1 ± 3.3 |
| Stroke index, ΔmL/min/BSA | 1.3 ± 1.7 | 1.0 ± 1.3 | 2.0 ± 1.5 | 0.3 ± 1.7 | −0.3 ± 2.5 | −2.6 ± 1.4 |
| Cardiac output, ΔL/min | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.8 ± 0.4 | 0.6 ± 0.3 |
| Cardiac index, ΔL/min/BSA | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 |
| Brachial artery vascular conductance, ΔmL/min/mmHg | 2.2 ± 0.4 | 1.4 ± 0.1* | 3.1 ± 0.4 | 2.1 ± 0.2* | 3.5 ± 0.5 | 2.7 ± 0.2* |
A 2 × 3 repeated-measures ANOVA (α < 0.05) (group, two levels: nonobese vs. obese HFpEF) (workload, 3 levels: 15%, 30%, and 45% of MVC) were performed to compare the hemodynamic responses in control and HFpEF during exercise. *Group effect, P < 0.05. Means ± SE. BSA, body surface area; HFpEF, heart failure with preserved ejection fraction.
Peripheral Hemodynamics
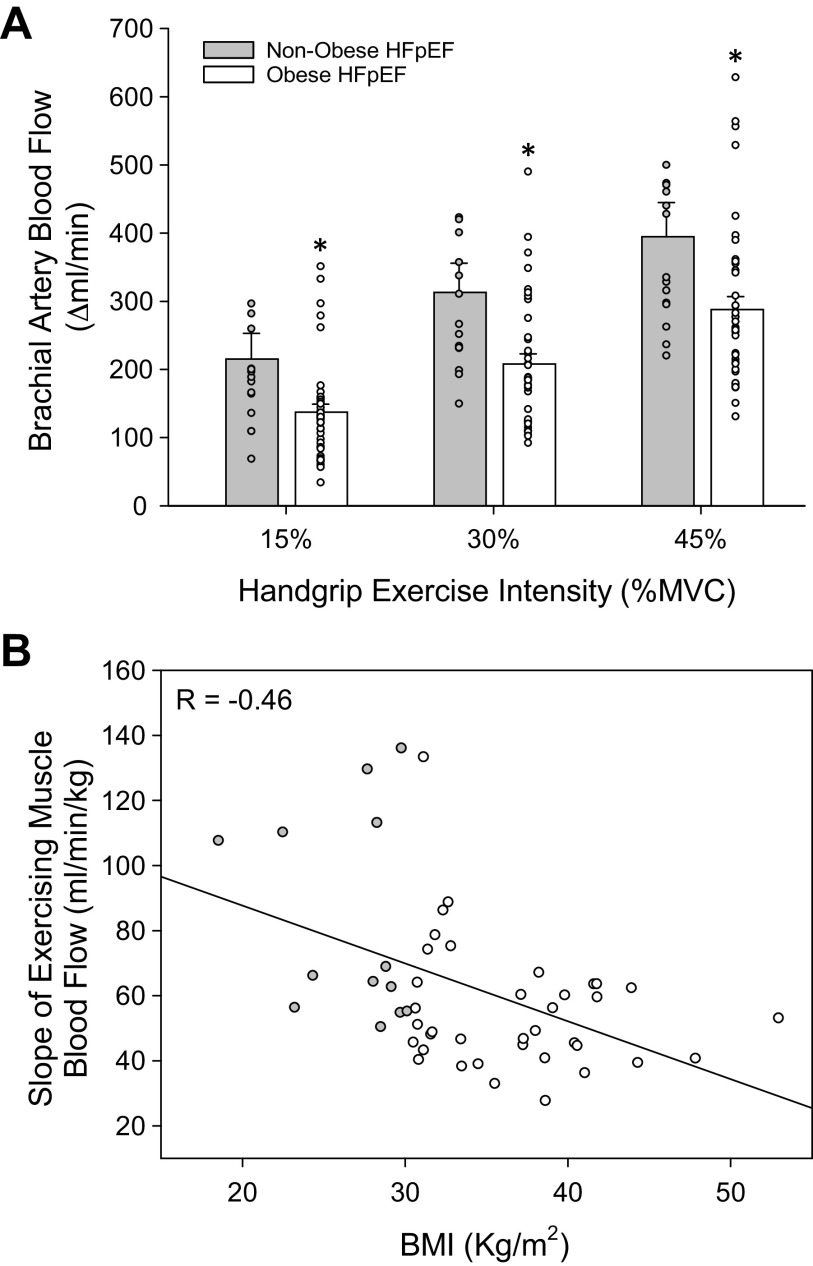
Brachial artery diameter was similar between groups at rest (nonobese: 0.47 ± 0.03, obese: 0.45 ± 0.01 cm), and both nonobese (15%: 0.49 ± 0.03, 30%: 0.49 ± 0.03, 45%: 0.45 ± 0.06 mL/min/mmHg) and obese (15%: 0.46 ± 0.01, 30%: 0.46 ± 0.01, 45%: 0.47 ± 0.01 cm) groups similarly increased brachial artery diameter throughout exercise. Brachial artery blood flow was similar between groups at baseline (nonobese: 89 ± 8, obese: 92 ± 7 mL/min). There was a significant interaction effect for brachial artery blood flow between groups and exercise intensity (P < 0.05). Brachial artery FBF progressively increased across all stages of handgrip exercise for both groups, but was significantly reduced in obese (15%: 229 ± 15, 30%: 300 ± 18, 45%: 380 ± 23 mL/min) compared with nonobese (15%: 304 ± 42, 30%: 402 ± 46, 45%: 484 ± 55 mL/min) patients with HFpEF across all exercise stages (P < 0.05). The change in FBF was also significantly reduced at all stages of exercise in obese patients with HFpEF (Fig. 1A). There was a negative correlation between the slopes of overall FBF response and BMI (Pearson correlation coefficient = −0.456, P < 0.001, n = 50). However, no association was found between the slope of the overall FBF response and CRP (Pearson correlation coefficient = 0.041, P = 0.8, n = 28) or IL-6 (Pearson correlation coefficient = −0.277, P = 0.2, n = 31). Brachial artery FVC was not different between groups at baseline (nonobese: 1.0 ± 0.1, obese: 1.1 ± 0.1 mL/min/mmHg). There was a significant interaction effect for FVC between groups and exercise intensity (P < 0.05). Brachial artery FVC increased across all stages of handgrip exercise for both groups, and was elevated at all stages of exercise in nonobese (15%: 3.1 ± 0.4, 30%: 4.0 ± 0.5, 45%: 4.5 ± 0.6 mL/min/mmHg) compared with obese (15%: 2.5 ± 0.2, 30%: 3.2 ± 0.2, 45%: 3.8 ± 0.2 mL/min/mmHg) patients with HFpEF. The change in brachial artery FVC was also significantly reduced at all exercise stages in obese compared with nonobese patients with HFpEF (Table 4).
Figure 1.
Exercising muscle blood flow responses. Changes in brachial artery blood flow during handgrip exercise in nonobese and obese patients with HFpEF (A). When the relationship between the overall hyperemic response across all exercise intensities was viewed across the range of BMIs (18–61 kg/m2) among patients with and without obesity, a significant negative correlation was found between the slope of exercising muscle blood flow and BMI (B, Pearson correlation coefficient = −0.456, P < 0.001, n = 50). A 2 × 3 repeated-measures ANOVA (α < 0.05) (group, two levels: nonobese vs. obese HFpEF) (workload, three levels: 15%, 30%, and 45% of MVC) were performed to compare the hemodynamic responses in control and HFpEF during exercise. *Group effect, P < 0.05. Means ± SE. HFpEF, heart failure with preserved ejection fraction; MVC, maximal voluntary contraction.
Circulating Biomarkers
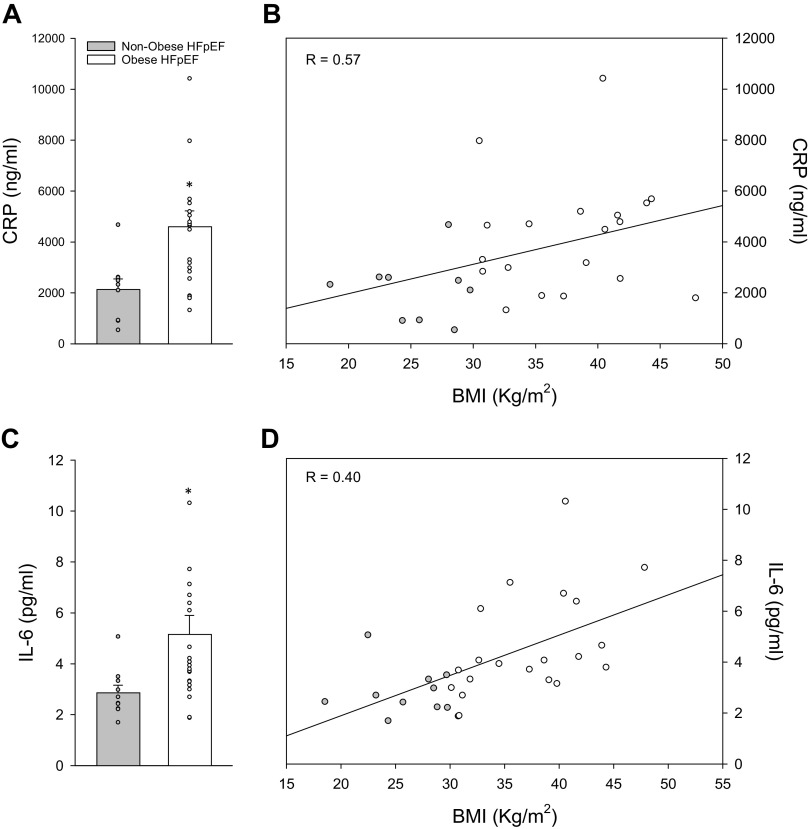
Circulating biomarkers used to characterize the severity of HFpEF, including BNP, NT pro-BNP, ST2, galectin-3, and cystatin C, were not different between groups (P > 0.05) (Table 3). Circulating biomarkers of inflammation are shown in Fig. 2. The inflammatory markers CRP (nonobese, n = 9: 2874 ± 829; obese, n = 19: 4601 ± 620 ng/mL) and IL-6 (nonobese, n = 10: 2.9 ± 0.3; obese, n = 21: 5.2 ± 0.7 pg/mL) were 35% and 46% elevated, respectively, in obese compared with nonobese patients with HFpEF (P < 0.05) (Fig. 2). Significant correlations were found between CRP and BMI (Pearson correlation coefficient = 0.393, P < 0.001, n = 28) and between IL-6 and BMI among combined subject groups (Pearson correlation coefficient = 0.573, P < 0.001, n = 31).
Figure 2.
Circulating inflammatory biomarkers. Both plasma CRP (A) and IL-6 (C) were significantly elevated in obese patients with HFpEF compared with nonobese controls. When these inflammatory biomarkers of inflammation were viewed across the range of BMIs (18–61 kg/m2) among patients with and without obesity, a significant positive correlation was found between both CRP (Pearson correlation coefficient = 0.393, P < 0.001, n = 28; B) and IL-6 (Pearson correlation coefficient = 0.573, P < 0.001, n = 31; D) and BMI values. A Student’s t test was performed between groups (α < 0.05). *Group effect, P < 0.05. Means ± SE. CRP, C-reactive protein; HFpEF, heart failure with preserved ejection fraction; IL-6, interleukin-6.
DISCUSSION
The present study provides significant new insight regarding the impact of obesity on exercising skeletal muscle blood flow in patients with HFpEF. Across a range of handgrip exercise intensities, patients with obesity exhibited 30%–40% attenuation in forearm hyperemia and vascular conductance compared with nonobese patients with HFpEF. The overall hyperemic response to exercise across all work rates was well correlated with BMI, further supporting the detrimental impact of obesity on exercising muscle blood flow. Importantly, the use of the handgrip exercise modality provoked similar changes in central hemodynamics between groups, suggesting that the differential response in skeletal muscle blood flow between patients with and without obesity was not attributable to disease-related changes in cardiac function. We also observed a significant elevation in proinflammatory biomarkers (IL-6 and CRP) in the patient with obesity group and identified a significant correlation between these biomarkers and BMI. Taken together, these findings demonstrate the presence of obesity and an accompanying milieu of systemic inflammation as important factors in the dysregulation of exercising muscle blood flow in patients with HFpEF.
Obesity in HFpEF
Although the HFpEF clinical syndrome has been described as a “constellation of comorbidities,” the specific contribution of obesity to the etiology and clinical progression of patients with HFpEF has been increasingly recognized, such that “obese HFpEF” is now viewed as a distinct phenotype (7). The need for a better understanding of the role of adiposity in HFpEF pathophysiology is underscored by the high prevalence of obesity within the HFpEF patient population (3). Indeed, among over 4,000 patients enrolled in the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial, 83% were either overweight or obese, and the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial has revealed an association between obesity and all-cause mortality (both cardiovascular and noncardiovascular) among patients with HFpEF (1). In fact, the mortality risk associated with increasing BMI is greater among patients with HFpEF than those with heart failure with a reduced ejection fraction (HFrEF) (39), further highlighting the truly unique nature of the obese HFpEF phenotype.
Regulation of Skeletal Muscle Blood Flow during Exercise in Obesity and HFpEF
Exercise intolerance is a defining symptom of HFpEF, with cardiac, pulmonary, autonomic, and vascular factors contributing to the multifactorial nature of the disease. Although diastolic dysfunction is a hallmark feature of HFpEF, decrements in cardiac function do not appear to fully explain the degree of exercise intolerance in this patient group (40–45), supporting the possibility of peripheral hemodynamic dysfunction as a contributor to the development of exertional symptoms. Indeed, our group has identified a marked, 15%–20% reduction in exercising leg blood flow in patients with HFpEF compared with healthy controls (23), and more recently, extended these findings to the upper extremity, where a 20%–40% reduction in brachial artery blood flow was observed during handgrip exercise in patients with HFpEF compared with hypertensive controls (24). Interestingly, the hyperemic response during 45% MVC handgrip exercise in the hypertensive control subjects from this previous study (24) appears strikingly similar to our nonobese patients with HFpEF in our current investigation (∼400 ΔmL/min), further highlighting the importance of considering adiposity when evaluating the regulation of exercising blood flow among patients with HFpEF.
The present study thus builds upon this previous work, identifying a clear decrement in brachial artery blood flow across a range of submaximal handgrip exercise intensities in obese patients with HFpEF compared with their nonobese counterparts (Fig. 1A). Interestingly, when the overall hyperemic response to exercise is viewed across the wide range of BMIs included in this study (18–61 kg/m2), a significant negative correlation was observed (Fig. 1B). Although it is recognized that correlation does not prove causation, this relationship does indicate a progressive worsening of vascular control across the spectrum from patients without obesity, with obesity, and with morbid obesity, and thus suggests that BMI may be an important consideration when evaluating disease-related decrements in exercising limb blood flow in this patient group. However, it should be noted that obesity, per se, may not be the sole contributor to this deficit. Indeed, others have reported similar increases in exercising muscle blood flow between young healthy lean and obese subjects (25), though there is clear evidence for a reduction in exercising limb blood flow in a variety of patient groups, including patients with type-2 diabetes (26), essential hypertension (27), and chronic obstructive lung disease (28). Together, these previous findings suggest that multiple comorbid conditions may be required before a dysregulation in exercise hyperemia is evident. Specific to HFpEF, Zamani et al. (29) recently identified a significant correlation between exercising arteriovenous oxygen content difference (ΔAVO2) and adiposity in patients with HFpEF, with increasing fat associated with reduced ΔAVO2, a maladaptation the authors postulate could be related to perfusion of less metabolically active adipocytes, the negative impact of adipose tissue on skeletal muscle metabolism, or the presence of obesity-related anemia. Although this previous study elegantly demonstrated the importance of adiposity to the peripheral determinants of O2 usage, it did not observe any differences in forearm blood flow during handgrip exercise across groups or as a consequence of adiposity, which was likely due to a relatively abbreviated exercise protocol. The present study using multiple submaximal exercise intensities and steady-state hemodynamic measurements thus build upon this recent work, demonstrating a consistent and marked impairment in exercise hyperemia in obese patients with HFpEF.
Central Hemodynamic Responses to Handgrip Exercise in Obesity and HFpEF
Small muscle mass exercise uniquely allows for the evaluation of exercising skeletal muscle blood flow in the absence of disease-related changes in cardiac function compared with whole body exercise, which is particularly important among patients with HFpEF, as chronotropic incompetence is a well-described feature of HFpEF pathophysiology (46, 47). In the present study, a similar increase in HR, SI, and CI was observed during handgrip exercise between obese and nonobese cohorts (Table 4), supporting the concept that the decrement in forearm blood flow (Fig. 1A) during exercise is unlikely due to greater central limitations in patients with obesity. Although it is beyond the scope of the present study, given the similar changes in cardiac output between groups, it is tempting to speculate that the reduction in exercising muscle blood flow observed in obese patients with HFpEF may represent an impairment in the ability to appropriately redistribute blood flow from inactive to active tissues. Indeed, elevations in sympathetic nervous system (SNS) activity (48) and impaired β-adrenergic vasodilation (49) are both well-described aspects of obesity, each of which could contribute to inefficient blood volume redistribution during exercise. Although several mechanisms may be responsible for a change in blood flow distribution with exercise, including local and systemic metabolic factors, the degree to which neurovascular control of blood flow may be altered in HFpEF, and whether this may specifically contribute to the inadequate redistribution of blood flow during exercise in the obese HFpEF phenotype, has yet to be explored in this patient group (50), and therefore represents an intriguing area for further study.
Inflammation and the Obese HFpEF Phenotype
Obesity and inflammation have been heralded as key contributors to HFpEF etiology and subsequent peripheral vascular dysfunction (10). Excess and hypertrophied adipose tissue ultimately undergoes a biological transformation to a proinflammatory state that adversely affects the vascular structure of most visceral organs (51). Adipokines, adipose cell cytokines, play pivotal roles in the adipocyte-cardiovascular balance (52), regulating endothelial function and inflammation. In particular, IL-6 is an adipokine responsible for the release of CRP, an important marker for cardiovascular disease progression (17). In addition to stimulating downstream inflammation, IL-6 may also impair insulin sensitivity (11), giving rise to derangements in skeletal muscle glucose uptake (12) and impaired insulin-induced vasodilation (53), most notably in the microvasculature (54). In fact, a paradoxical shift from insulin-induced skeletal muscle vasodilation to vasoconstriction has been demonstrated among obese individuals (55), which could represent a mechanistic link between obesity, metabolic disease, and the dysregulation of exercising skeletal muscle microvascular blood flow observed in the present study as opposed to conduit vessel macrovascular regulation of the brachial artery (53, 54, 56). Additional studies focused on the role of insulin signaling in vascular control among obese patients with HFpEF are warranted to explore this possibility.
In the present study, we observed a marked elevation in both CRP (Fig. 2A) and IL-6 (Fig. 2C) among obese patients with HFpEF compared to their nonobese counterparts, suggesting that the known presence of systemic inflammation within the general HFpEF population may be exaggerated in the presence of excess adiposity. We also found a significant positive correlation between both CRP (Fig. 2B) and IL-6 (Fig. 2D) across a wide range of BMIs, again indicating a progressively greater proinflammatory state across the spectrum of nonobese, obese, and morbidly obese patients with HFpEF. In an ancillary study of the multicenter Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial that sought to better characterize the obese phenotype of HFpEF, Reddy et al. (57) observed a modest linear correlation (r = 0.26) between BMI and CRP, indicating greater systemic inflammation in obese compared with nonobese patients with HFpEF. However, to our knowledge, this is the first study to explore the relationship between adiposity and IL-6 in patients with HFpEF. Thus, the present study identifying a significant relationship between BMI and both proinflammatory adipokine (IL-6) and cytokine (CRP) biomarkers both confirm and extend this earlier work, providing new evidence in support of the hypothesis that there may be an inflammatory role of excess adipose tissue in patients with the obese HFpEF phenotype.
Perspectives
The current findings of impaired vascular control within a milieu of systemic inflammation in the obese HFpEF phenotype represent another line of evidence for the potential of obesity as a therapeutic target in the treatment of HFpEF. In one of the most important studies to date in nonpharmacological HFpEF therapeutics, Kitzman et al. (58) identified the benefits of weight loss achieved by caloric restriction, alone and in combination with aerobic exercise training, to improve exercise capacity and reduce biomarkers of inflammation in patients with HFpEF. Given the well-known relationship between limb blood flow and exercise capacity (59), it stands to reason that the marked improvement in functional outcomes in this previous study may have been due, at least in part, to a restoration in vascular control. It is therefore conceivable that the exaggerated dysregulation of exercising muscle blood flow and systemic inflammation observed among obese patients with HFpEF in the present study may be remediable with interventions targeting weight reduction, whether through lifestyle interventions such as diet and cardiac rehabilitation, or more invasive procedures such as bariatric surgery. Future trials focused specifically on how adipose tissue reduction impacts exercising limb blood flow are needed to better define the potential plasticity of obesity-related vascular dysregulation in patients with HFpEF.
Limitations
There are several potential limitations in the current study. By design, no prescribed medications were withheld on study days, which enabled the opportunity to study these patients in a “real‐world” setting. However, we cannot exclude the potential of this approach to influence the observed vascular responses. As the study design did not match for mediation usage or the presence of comorbidities between obese and nonobese groups, we cannot exclude the possibility that these variables contributed to the observed differences in our reported outcome measures. We also recognize that peak oxygen consumption, a gold standard for the determination of exercise tolerance, was not evaluated in the present study. No significant association was found between the overall FBF response and biomarkers of inflammation, which may have been the consequence of a relatively small sample size for this statistical comparison. Finally, we acknowledge that forearm blood flow was not normalized for muscle mass in the present study. Although muscle mass does not appear to be a determinant of exercise hyperemia in young, healthy individuals (60), we recognize that this relationship has not been evaluated in patients with HFpEF.
Conclusions
The present study has identified a marked attenuation in exercise hyperemia and elevated biomarkers of inflammation in obese patients with HFpEF compared with their nonobese HFpEF counterparts. These findings thus identify the incidence of obesity and an accompanying milieu of systemic inflammation as important factors in the dysregulation of exercising muscle blood flow in patients with HFpEF.
GRANTS
The study is funded in part by the National Institutes of Health Grant HL139451 (to K.B.) and the U.S. Department of Veterans Affairs Grants I01RX001311 and I01CX002152 ( to D.W.W.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.R. and D.W.W. conceived and designed research; S.M.R., J.F.L., K.B., J.K.A., and D.W.W. performed experiments; S.M.R., J.F.L., K.B., J.K.A., J.Z., and D.W.W. analyzed data; S.M.R., J.F.L., K.B., J.K.A., C.L.M., J.J.R., L.L.K., and D.W.W. interpreted results of experiments; S.M.R. and D.W.W. prepared figures; S.M.R. and D.W.W. drafted manuscript; S.M.R., J.F.L., K.B., J.K.A., J.Z., C.L.M., J.J.R., L.L.K., and D.W.W. edited and revised manuscript; S.M.R., J.F.L., K.B., J.K.A., J.Z., C.L.M., J.J.R., L.L.K., and D.W.W. approved final version of manuscript.
REFERENCES
- 1.Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 70: 2739–2749, 2017. doi: 10.1016/j.jacc.2017.09.1111. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail 6: 633–639, 2018. doi: 10.1016/j.jchf.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Pe. C. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 4: 324–331, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J, Filippatos G. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail 18: 744–758, 2016. doi: 10.1002/ejhf.600. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 68: 200–203, 2016. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction: an emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev Endocr Metab Disord 14: 29–38, 2013. doi: 10.1007/s11154-012-9231-7. [DOI] [PubMed] [Google Scholar]
- 7.Koutroumpakis E, Kaur R, Taegtmeyer H, Deswal A. Obesity and heart failure with preserved ejection fraction. Heart Fail Clin 17: 345–356, 2021. doi: 10.1016/j.hfc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136: 6–19, 2017. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134: 73–90, 2016. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 11.Sabio G, Davis RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci 35: 490–496, 2010. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan W, Ding L, Gao RY, Liu J, Shang J. Interleukin-6 signal transduction and its role in hepatic lipid metabolic disorders. Cytokine 66: 133–142, 2014. [Erratum in Cytokine 70: 200, 2014]. doi: 10.1016/j.cyto.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Mangalat D, Korbut R. Adipocytokines: novel link between inflammation and vascular function? J Physiol Pharmacol 57: 505–528, 2006. [PubMed] [Google Scholar]
- 15.Volk T, Hensel M, Schuster H, Kox WJ. Secretion of MCP-1 and IL-6 by cytokine stimulated production of reactive oxygen species in endothelial cells. Mol Cell Biochem 206: 105–112, 2000. doi: 10.1023/a:1007059616914. [DOI] [PubMed] [Google Scholar]
- 16.Steyers CM 3rd, Miller FJ. Jr.. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 15: 11324–11349, 2014. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation 14: 375–387, 2007. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- 18.Bagi Z, Feher A, Cassuto J. Microvascular responsiveness in obesity: implications for therapeutic intervention. Br J Pharmacol 165: 544–560, 2012. doi: 10.1111/j.1476-5381.2011.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol 52: 848–856, 2012. doi: 10.1016/j.yjmcc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koller A, Balasko M, Bagi Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Intern Emerg Med 8 suppl 1: S51–S54, 2013. doi: 10.1007/s11739-013-0910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakhani I, Wong MV, Hung JKF, Gong M, Waleed KB, Xia Y, Lee S, Roever L, Liu T, Tse G, Leung KSK, Li KHC. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail Rev 26: 1141–1150, 2021. doi: 10.1007/s10741-020-09927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chia YC, Kieneker LM, van Hassel G, Binnenmars SH, Nolte IM, van Zanden JJ, van der Meer P, Navis G, Voors AA, Bakker SJL, De Borst MH, Eisenga MF. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc 10: e018549, 2021. doi: 10.1161/JAHA.120.018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JF, Barrett-O'Keefe Z, Nelson AD, Garten RS, Ryan JJ, Nativi-Nicolau JN, Richardson RS, Wray DW. Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. Int J Cardiol 211: 14–21, 2016. doi: 10.1016/j.ijcard.2016.02.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratchford SM, Clifton HL, La Salle DT, Broxterman RM, Lee JF, Ryan JJ, Hopkins PN, Wright JB, Trinity JD, Richardson RS, Wray DW. Cardiovascular responses to rhythmic handgrip exercise in heart failure with preserved ejection fraction. J Appl Physiol (1985), 129: 1267–1276 2020. doi: 10.1152/japplphysiol.00468.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limberg JK, De Vita MD, Blain GM, Schrage WG. Muscle blood flow responses to dynamic exercise in young obese humans. J Appl Physiol (1985) 108: 349–355, 2010. doi: 10.1152/japplphysiol.00551.2009. [DOI] [PubMed] [Google Scholar]
- 26.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 27.Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol 590: 1481–1494, 2012. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iepsen UW, Munch GW, Rugbjerg M, Ryrso CK, Secher NH, Hellsten Y, Lange P, Pedersen BK, Thaning P, Mortensen SP. Leg blood flow is impaired during small muscle mass exercise in patients with COPD. J Appl Physiol (1985) 123: 624–631, 2017. doi: 10.1152/japplphysiol.00178.2017. [DOI] [PubMed] [Google Scholar]
- 29.Zamani P, Proto EA, Mazurek JA, Prenner SB, Margulies KB, Townsend RR, Kelly DP, Arany Z, Poole DC, Wagner PD, Chirinos JA. Peripheral determinants of oxygen utilization in heart failure with preserved ejection fraction: central role of adiposity. JACC Basic Transl Sci 5: 211–225, 2020. doi: 10.1016/j.jacbts.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 162: 966–972, 2011. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93: 62–66, 1978. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 32.Logason K, Barlin T, Jonsson ML, Bostrom A, Hardemark HG, Karacagil S. The importance of Doppler angle of insonation on differentiation between 50–69% and 70–99% carotid artery stenosis. Eur J Vasc Endovasc Surg 21: 311–313, 2001. doi: 10.1053/ejvs.2001.1331. [DOI] [PubMed] [Google Scholar]
- 33.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 35.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- 36.de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth 95: 326–331, 2005. doi: 10.1093/bja/aei189. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 38.Gaggin HK, Januzzi JL. Jr.. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta 1832: 2442–2450, 2013. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, Berry JD. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol 69: 1129–1142, 2017. doi: 10.1016/j.jacc.2016.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puntawangkoon C, Kitzman DW, Kritchevsky SB, Hamilton CA, Nicklas B, Leng X, Brubaker PH, Hundley WG. Reduced peripheral arterial blood flow with preserved cardiac output during submaximal bicycle exercise in elderly heart failure. J Cardiovasc Magn Reson 11: 48, 2009. doi: 10.1186/1532-429X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 104: 779–782, 2001. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 42.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105: 1387–1393, 2002. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 43.Burkhoff D, Maurer MS, Packer M. Heart failure with a normal ejection fraction: is it really a disorder of diastolic function? Circulation 107: 656–658, 2003. doi: 10.1161/01.cir.0000053947.82595.03. [DOI] [PubMed] [Google Scholar]
- 44.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49: 198–207, 2007. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 45.Solomon SD, Verma A, Desai A, Hassanein A, Izzo J, Oparil S, Lacourciere Y, Lee J, Seifu Y, Hilkert RJ, Rocha R, Pitt B; Exforge Intensive Control of Hypertension to Evaluate Efficacy in Diastolic Dysfunction I. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension 55: 241–248, 2010. doi: 10.1161/HYPERTENSIONAHA.109.138529. [DOI] [PubMed] [Google Scholar]
- 46.Na S, Clark H, Brubaker P, Witte KK, Jamil H, Gierula J, Patel HC, Pearson MJ. Effects of chronotropic incompetence on exercise capacity in people with heart failure versus age-matched controls. Heart Fail Rev 27: 795–809, 2022. doi: 10.1007/s10741-021-10081-1. [DOI] [PubMed] [Google Scholar]
- 47.Wolsk E, Kaye D, Komtebedde J, Shah SJ, Borlaug BA, Burkhoff D, Kitzman DW, Lam CSP, van Veldhuisen DJ, Ponikowski P, Petrie MC, Hassager C, Moller JE, Gustafsson F. Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail 7: 321–332, 2019. doi: 10.1016/j.jchf.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Grassi G, Biffi A, Seravalle G, Trevano FQ, Dell’Oro R, Corrao G, Mancia G. Sympathetic neural overdrive in the obese and overweight state. Hypertension 74: 349–358, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12885. [DOI] [PubMed] [Google Scholar]
- 49.Sivitz WI, Wayson SM, Bayless ML, Sinkey CA, Haynes WG. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J Diabetes Complications 21: 149–157, 2007. doi: 10.1016/j.jdiacomp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Toschi-Dias E, Rondon M, Cogliati C, Paolocci N, Tobaldini E, Montano N. Contribution of autonomic reflexes to the hyperadrenergic state in heart failure. Front Neurosci 11: 162, 2017. doi: 10.3389/fnins.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia G, Jia Y, Sowers JR. Contribution of maladaptive adipose tissue expansion to development of cardiovascular disease. Compr Physiol 7: 253–262, 2016. doi: 10.1002/cphy.c160014. [DOI] [PubMed] [Google Scholar]
- 52.Freitas Lima LC, Braga VA, do S, de Franca Silva M, Cruz JC, Sousa Santos SH, de Oliveira Monteiro MM, Balarini CM. Adipokines, diabetes and atherosclerosis: an inflammatory association. Front Physiol 6: 304, 2015. doi: 10.3389/fphys.2015.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallsten K, Yki-Jarvinen H, Peltoniemi P, Oikonen V, Takala T, Kemppainen J, Laine H, Bergman J, Bolli GB, Knuuti J, Nuutila P. Insulin- and exercise-stimulated skeletal muscle blood flow and glucose uptake in obese men. Obes Res 11: 257–265, 2003. doi: 10.1038/oby.2003.39. [DOI] [PubMed] [Google Scholar]
- 54.Meijer RI, Serne EH, Korkmaz HI, van der Peet DL, de Boer MP, Niessen HW, van Hinsbergh VW, Yudkin JS, Smulders YM, Eringa EC. Insulin-induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 58: 1907–1915, 2015. doi: 10.1007/s00125-015-3606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olver TD, Hazell TJ, Hamilton CD, Shoemaker JK, Lemon PW. Impaired superficial femoral artery vasodilation and leg blood flow in young obese women following an oral glucose tolerance test. Appl Physiol Nutr Metab 37: 176–183, 2012. doi: 10.1139/h11-148. [DOI] [PubMed] [Google Scholar]
- 57.Reddy YNV, Lewis GD, Shah SJ, Obokata M, Abou-Ezzedine OF, Fudim M, Sun JL, Chakraborty H, McNulty S, LeWinter MM, Mann DL, Stevenson LW, Redfield MM, Borlaug BA. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX Trial Ancillary Study. Mayo Clin Proc 94: 1199–1209, 2019. doi: 10.1016/j.mayocp.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 58.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 315: 36–46, 2016. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poole DC, Behnke BJ, Musch TI. The role of vascular function on exercise capacity in health and disease. J Physiol 599: 889–910, 2021. doi: 10.1113/JP278931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garten RS, Groot HJ, Rossman MJ, Gifford JR, Richardson RS. The role of muscle mass in exercise-induced hyperemia. J Appl Physiol (1985) 116: 1204–1209, 2014. doi: 10.1152/japplphysiol.00103.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]