Keywords: fasting, food intake, metabolism, refeeding, secreted hormone
Abstract
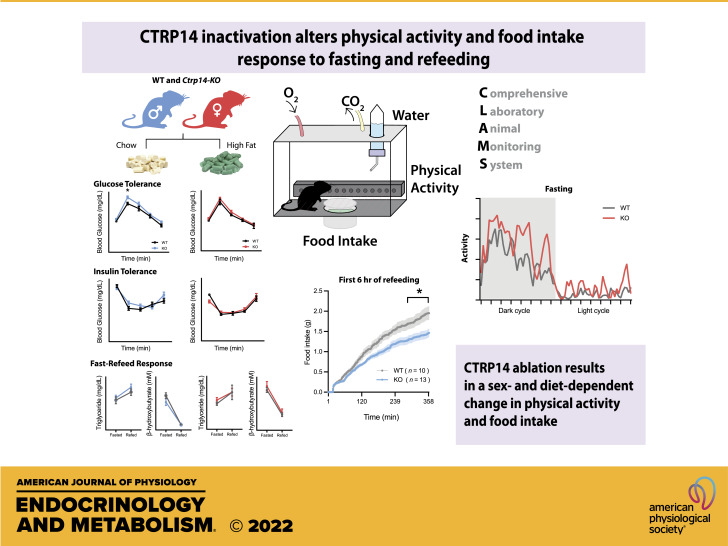
Secreted proteins of the C1q/TNF-related protein (CTRP) family play diverse functions in different organ systems. In the brain, CTRP14/C1QL1 is required for the proper establishment and maintenance of synapses between climbing fibers and cerebellar Purkinje cells. Beyond the central nervous system, the function of CTRP14 is largely unknown. A recent genome-wide association study has implicated CTRP14/C1QL1 as a candidate gene associated with total body fat mass. Here, we explored the potential metabolic roles of CTRP14. We show that Ctrp14 expression in peripheral tissues is dynamically regulated by fasting-refeeding and high-fat feeding. In the chow-fed basal state, Ctrp14 deletion modestly reduces glucose tolerance in knockout (KO) male mice and affects physical activity in a sex- and nutritional state-dependent manner. In the ad libitum fed state, Ctrp14 KO male mice have lower physical activity. In contrast, female KO mice have increased physical activity in the fasted and refed states. In response to an obesogenic diet, CTRP14-deficient mice of either sex gained similar weight and are indistinguishable from wild-type littermates in body composition, lipid profiles, and insulin sensitivity. Ambulatory activity, however, is reduced in Ctrp14 KO male mice. Food intake is also reduced in Ctrp14 KO male mice in the refed period following food deprivation. Meal pattern analyses indicate that decreased caloric intake from fasting to refeeding is due, in part, to smaller meal size. We conclude that CTRP14 is largely dispensable for metabolic homeostasis, but highlight context-dependent and sexually dimorphic metabolic responses of Ctrp14 deletion affecting physical activity and ingestive behaviors.
NEW & NOTEWORTHY CTRP14 is a secreted protein whose function in the peripheral tissues is largely unknown. We show that the expression of Ctrp14 in peripheral tissues is regulated by metabolic and nutritional state. We generated mice lacking CTRP14 and show that CTRP14 deficiency alters physical activity and food intake in response to fasting and refeeding. Our data has provided new and valuable information on the physiological function of CTRP14.
INTRODUCTION
Secreted proteins can function locally as autocrine and paracrine factors, or they can travel via the circulatory system to exert endocrine functions at a distant site. Integrated control of physiology and metabolism is critically dependent on hormone-mediated tissue cross talk (1, 2). To better understand the endocrine control of energy homeostasis, we have previously described a conserved family of secreted proteins, the C1q/TNF-related proteins (CTRP1-15) (3–9). These proteins were identified and cloned based on shared sequence homology to the widely studied insulin-sensitizing hormone, adiponectin. All CTRPs possess the signature globular C1q domain at the C-terminus (10, 11); hence they belong to the larger and growing C1q family (12, 13).
Over the past decade, we have systematically characterized the potential metabolic roles of CTRPs using genetic gain- and loss-of-function mouse models (14–25). These studies have highlighted the important physiological functions of multiple CTRPs in modulating various aspects of glucose and lipid metabolism. We showed that CTRPs influence local and systemic metabolism by three general mechanisms that are nonmutually exclusive: 1) they act directly on liver, skeletal muscle, and adipose tissue to modulate substrate metabolism and insulin sensitivity; 2) they act in the hypothalamus to control food intake, physical activity, and energy expenditure; and 3) they indirectly affect metabolism and insulin action by modulating obesity-linked low-grade inflammation in the adipose tissue and liver.
In the context of metabolism, the potential contribution of CTRP14 (also known as C1QL1 and C1QTNF14) to energy homeostasis has not been explored. The function of CTRP14 in the central nervous system (CNS) and cochlea, however, has recently been established. In the brain, Ctrp14/C1ql1 expression is detected early during embryogenesis (at E13), and in the postnatal brain, it is highly and predominantly expressed by neurons of the inferior olive (26). Recent single-cell RNA sequencing data also indicated hindbrain as the main CNS location of high expression of C1ql1 transcript (27) (mousebrain.org). A biochemical approach was successfully used to identify the adhesion GPCR (brain angiogenesis inhibitor 3; Bai3) as the receptor for C1ql1 (28). Binding of C1ql1 to Bai3 on cerebellar Purkinje cells was shown to be required for the correct formation and maintenance of synapses between climbing fibers from the inferior olive and the cerebellar Purkinje cells (29, 30). Although motor coordination as judged by rotarod test is preserved in C1ql1-deficient mice, loss of C1ql1 adversely affects motor learning as determined by the horizontal optokinetic response (29). Within the sensory system, C1ql1 is found to be robustly expressed in the outer hair cells of the mouse cochlea C1ql1 (31, 32). However, the impact of C1ql1 deficiency on hearing is variable, ranging from minimal/no effect (31) to progressive hearing loss (32).
Aside from the brain, Ctrp14 is also expressed in peripheral tissues (33), albeit at a significantly lower level. Beyond its known function in the CNS and cochlea, the physiological role of CTRP14 in peripheral tissues is largely unknown and unexplored. Interestingly, a recent bivariate genome-wide association study (GWAS) has identified an SNP (rs12150327), located at the 3' end of CTRP14/C1QL1 gene, that is associated with increased hip bone mineral density and decreased total body fat mass (34). This human GWAS study, combined with the documented metabolic functions of many CTRP family members, prompted us to assess the potential metabolic role of CTRP14. Here, we showed that Ctrp14 expression is dynamically regulated by nutritional state. We generated a constitutive knockout (KO) mouse model using the CRISPR-Cas9 deletion method to ascertain whether CTRP14 is required for metabolic homeostasis. We showed that CTRP14 deficiency modestly affects glucose tolerance, and alters physical activity and food intake in response to fasting and refeeding. The effects we observed in Ctrp14 KO mice are dependent on sex and diet. In light of limited information concerning CTRP14/C1QL1 function, our data will be valuable in aiding the further explorations of the physiological role of this highly conserved secreted protein.
MATERIALS AND METHODS
Mouse Model
Eight-week-old mouse tissues from C57BL/6J male mice (The Jackson Laboratory, Bar Harbor, ME) were collected from fasted and refed experiments as we have previously described (35). In brief, for the fasted group, food was removed for 16 h (beginning 10 h into the light cycle), and mice were euthanized 2 h into the light cycle. For the refed group, mice were fasted for 16 h and refed with chow pellets for 3 h before being euthanized. Tissues from C57BL/6J male mice fed a low-fat diet (LFD) or a high-fat diet (HFD) for 12 wk were also collected as we have previously described (35). CRISPR-Cas9 method was used to generate the Ctrp14/C1ql1 knockout (KO) mouse strain at the Transgenic Core facility at Johns Hopkins University School of Medicine. The two gRNA used in generating the KO mice were 5′- CAGCATCACCACGCCCGCGG-3′ and 5′- GCACACCGGGCAGGCGTAGG-3′. Deletion of exon 1 (encoding amino acid 1–199) removed 77% of the entire protein-coding region of the gene, thus ensuring a complete null allele. Ctrp14 KO mice were generated on a C57BL/6J genetic background and subsequently backcrossed to C57BL/6J wild-type (WT) mice for two generations to eliminate any potential off target mutations induced by CRISPR-Cas9. Genotyping primers for WT allele were forward (97F2) 5′- TGGGCACCTGCC GCATGGTGTGCG-3′ and reverse (97R3) 5′- TTGAGCACCTCGTAACCCTCATGAG-3′. The size of the WT band was 367 bp. Genotyping primers for the Ctrp14 KO allele were forward (97F4) 5′- CGCCCCTTACCCGCCGGCATCATTG-3' and reverse (97R5) 5′- CTAGAGCCCTGTGATCTGACTAGTTG-3′. The size of the KO band was 295 bp. The genotyping PCR parameters were as follows: 94°C for 5 min, followed by 10 cycles of 94°C for 10 s, 65°C for 15 s, and 72°C for 30 s, then 25 cycles of 94°C for 10 s, 55°C for 15 s, and 72°C for 30 s, and finally 72°C for 5 min. Due to the presence of GC-rich sequences, 8% DMSO was included in the PCR genotyping reaction. All mice were generated by intercrossing Ctrp14 heterozygous (+/−) mice. Ctrp14 KO (−/−) and WT (+/+) littermate controls were housed in polycarbonate cages on a 12-h light–dark photocycle with ad libitum access to water and food. Mice were fed either a standard chow (Envigo; 2018SX) or a high-fat diet (HFD; 60% kcal derived from fat; Cat. No. D12492, Research Diets, New Brunswick, NJ). Standard chow was provided for 12 wk, beginning at 5 wk of age; HFD was provided for 20 wk, beginning at 12 wk of age. At termination of the study, all mice were fasted for 2 h and euthanized. Tissues were collected, snap-frozen in liquid nitrogen, and kept at −80°C until analysis. All mouse protocols (Protocol no. MO19M48) were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine. All animal experiments were conducted in accordance with the National Institute of Health guidelines and followed the standards established by the Animal Welfare Acts.
Body Composition Analysis
Body composition analyses for total fat, lean mass, and water content were determined using a quantitative magnetic resonance instrument (Echo-MRI-100, Echo Medical Systems, Waco, TX) at the Mouse Phenotyping Core facility at Johns Hopkins University School of Medicine.
Complete Blood Count Analysis
A complete blood count on blood samples was performed at the Pathology Phenotyping Core at Johns Hopkins University School of Medicine. Tail vein blood was collected using EDTA-coated blood collection tubes (Sarstedt, Nümbrecht, Germany) and analyzed using Procyte Dx analyzer (IDEXX Laboratories, Westbrook, ME).
Indirect Calorimetry
Chow- or HFD-fed WT and Ctrp14 KO male and female mice were used for simultaneous assessments of daily body weight change, food intake (corrected for spillage), physical activity, and whole body metabolic profile in an open flow indirect calorimeter (Comprehensive Laboratory Animal Monitoring System, CLAMS; Columbus Instruments, Columbus, OH) as previously described (36). In brief, data were collected for 3 days to confirm mice were acclimatized to the calorimetry chambers (indicated by stable body weights, food intakes, and diurnal metabolic patterns), and data were analyzed from the fourth day. Rates of oxygen consumption (V̇o2; mL·kg−1·h−1) and carbon dioxide production (V̇co2; mL·kg−1·h−1) in each chamber were measured every 24 min throughout the studies. Respiratory exchange ratio (RER = V̇co2/ V̇o2) was calculated by CLAMS software (v. 4.02) to estimate relative oxidation of carbohydrates (RER = 1.0) versus fats (RER = 0.7), not accounting for protein oxidation. Energy expenditure (EE) was calculated as EE= V̇o2 × [3.815 + (1.232 × RER)] and normalized to lean mass. Physical activities were measured by infrared beam breaks in the metabolic chamber. Average metabolic values were calculated per subject and averaged across subjects for statistical analysis by Student’s t test. Meal pattern data were analyzed for average meal frequency and meal size; a meal was defined as being at least 0.04 g and having a postmeal and intermeal interval of at least 10 min, as previously described (24, 37). A food intake event was considered a meal only when both criteria were met. Intermeal interval was defined as time between consecutive meals. Satiety ratio was defined as intermeal interval divided by meal size (min/g).
Glucose and Insulin Tolerance Tests
For glucose tolerance tests (GTTs), mice were fasted for 6 h before glucose injection. Glucose (Sigma, St. Louis, MO) was reconstituted in saline (0.9 g NaCl/L) and injected intraperitoneally at 1 mg/g body weight. Blood glucose was measured at 0, 15, 30, 60, and 120 min after glucose injection using a glucometer (NovaMax Plus, Billerica, MA). For insulin tolerance tests (ITTs), food was removed 2 h before insulin injection. Insulin was diluted in saline and injected intraperitoneally at 1.0 U/kg body weight. Blood glucose was measured at 0, 15, 30, 60, and 90 min after insulin injection using a glucometer (NovaMax Plus).
Blood and Tissue Chemistry Analysis
Tail vein blood samples were allowed to clot on ice and then centrifuged for 10 min at 10,000 g. Serum samples were stored at −80°C until analyzed. Serum triglycerides (TG) and cholesterol were measured according to the manufacturer’s instructions using an Infinity kit (Thermo Fisher Scientific, Middletown, VA). Nonesterified free fatty acids (NEFAs) were measured using a Wako kit (Wako Chemicals, Richmond, VA). Serum β-hydroxybutyrate (ketone) concentrations were measured with a StanBio Liquicolor kit (StanBio Laboratory, Boerne, TX). Serum insulin levels were measured by ELISA according to the manufacturer’s instructions (Crystal Chem, Elk Grove Village, IL; Cat. No. 90080).
Quantitative Real-Time PCR Analysis
Total RNA was isolated from tissues using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Purified RNA was reverse transcribed using an iScript cDNA Synthesis Kit (Bio-Rad). Real-time quantitative PCR analysis was performed on a CFX Connect Real-Time System (Bio-Rad) using iTaq Universal SYBR Green Supermix (Bio-Rad) as per the manufacturer’s instructions. Data were normalized to the stable housekeeping gene 36B4 (encoding the acidic ribosomal phosphoprotein P0) and expressed as relative mRNA levels using the ΔΔCt method (38). Fold change data were log-transformed to ensure normal distribution and statistics were performed. Real-time qPCR primers used were as follows: Ctrp14 forward (m14-F2), 5′- GTCCTTAGCCTGCGTCCAAC-3′ and reverse (m14-R2), 5′- GCTTTGTGTTCAGCGGAGG-3′. A second set of primers was used to independently confirm the total absence of Ctrp14 transcript in the brain: Ctrp14 forward (m14-F3), 5′- ACCAGTATGTGGGCAGACCTCTG-3′ and reverse (m14-R3), 5′- ATCCGAGTAGATGATGAAGCCAG-3′.
Statistical Analyses
All results are expressed as means ± standard error (SE). Statistical analysis was performed with Prism 8 software (GraphPad Software, San Diego, CA). Data were analyzed with two-tailed Student’s t tests or by repeated-measures ANOVA. For two-way ANOVA, we performed Bonferroni post hoc tests. P < 0.05 was considered statistically significant.
RESULTS
CTRP14 Is Regulated by Metabolic and Nutritional State in Peripheral Tissues
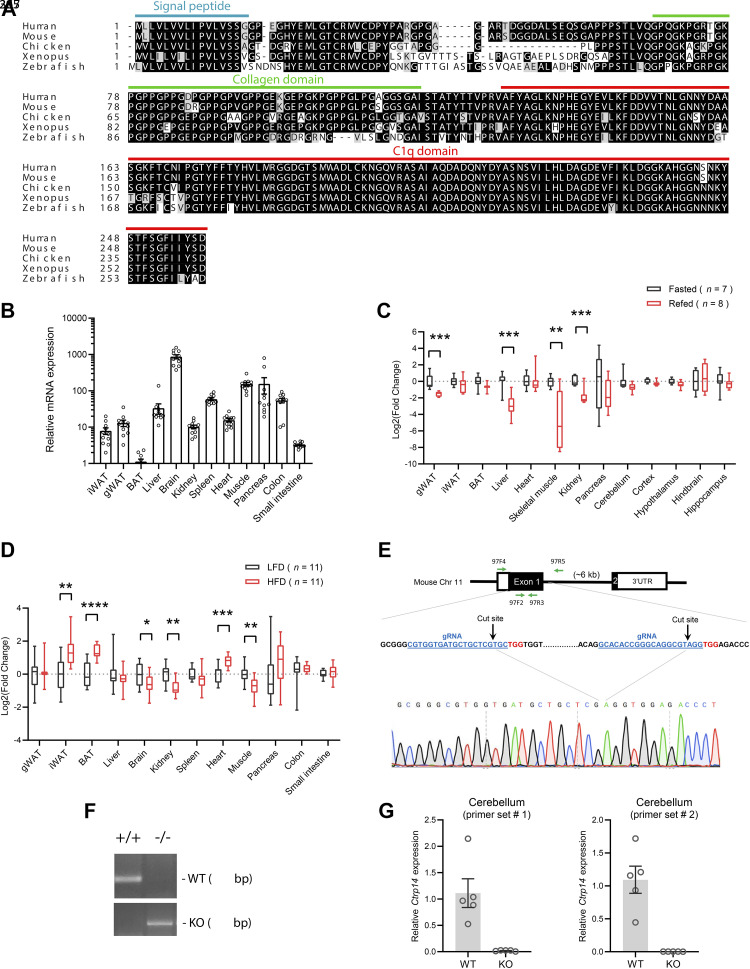
Like most of the C1q family members, CTRP14 is highly conserved across divergent vertebrate species (Fig. 1A). Full-length human and mouse CTRP14 proteins only differ by four amino acids. Our mouse tissue expression analysis confirmed that brain has the highest expression of Ctrp14 transcript (Fig. 1B), consistent with previous report (26). Although expressed at a significantly lower level relative to the brain, Ctrp14 transcript is nevertheless expressed in major metabolic tissues such as the liver, skeletal muscle, pancreas, and white adipose tissue (Fig. 1B). The expression of metabolically relevant genes is often dynamically regulated by changes in energetic states of the animal. To assess the potential metabolic role of CTRP14, we first examined whether its expression is altered by fasting and refeeding. Although CTRP14 has a prominent role in the cerebellum/hindbrain (29, 30), its expression in various brain regions (cortex, hypothalamus, hindbrain, hippocampus) was not changed by fasting and refeeding (Fig. 1C). In contrast, refeeding after an overnight fast significantly suppressed Ctrp14 expression in the visceral (gonadal) white adipose tissue, liver, skeletal muscle, and kidney (Fig. 1C), whereas there were no changes in Ctrp14 expression during the fast itself. These results prompted us to assess whether Ctrp14 expression differs between lean and obese mice. Relative to lean mice fed a matched control low-fat diet (LFD), Ctrp14 expression in the obese mice fed a high-fat diet (HFD) was upregulated in the subcutaneous (inguinal) white adipose tissue, brown adipose tissue, and heart, and downregulated in the brain, kidney, and skeletal muscle (Fig. 1D). These data indicate that Ctrp14 expression is regulated according to metabolic and nutritional states in peripheral tissues, suggestive of potential metabolic roles.
Figure 1.
Expression of Ctrp14 in peripheral tissues is nutritionally regulated. A: sequence alignments of human (NP_006679), mouse (NP_035925.2), chicken (Gallus gallus; XP_040548241), frog (Xenopus tropicalis; NP_001186529), and zebrafish (Danio rerio; XP_683865.2) CTRP14/C1QL1. The signal peptide, collagen domain with its characteristic Gly-X-Y repeat, and globular C1q domain are indicated. Identical amino acids are shaded in black, and similar amino acids are shaded in gray. B: expression of Ctrp14 across different mouse tissues. C: Ctrp14 expression in mouse tissues in response to overnight (16 h) fast and 3 h refeeding (following an overnight fast). D: Ctrp14 expression in mouse tissue in response to a 12-wk period of control low-fat diet (LFD) or a high-fat diet (HFD). E: CRISPR-Cas9 method used to generate a null allele of Ctrp14. The protein-coding region of exon 1 that encodes amino acids 1–199 (75% of the entire protein) is deleted. The guide RNA (gRNA) sequences are underlined and colored in blue. The Cas9 cut sites are indicated by black arrows. The two sets of genotyping primers (97F2/97R3 for WT allele; 97F4/97R5 for KO allele) are indicated by green arrows. The sequencing chromatogram result indicate the precise deletion of exon 1. F: representative PCR genotyping result for WT (+/+) and KO (−/−) mice. G: real-time PCR analysis confirming the absence of Ctrp14 transcript in the cerebellum KO mice. All data are presented as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001. KO, knockout; WT, wild type.
Generation of Ctrp14-Null Mice
We employed a loss-of-function mouse model to address whether CTRP14 is required for metabolic homeostasis in vivo. A complete null allele of Ctrp14 was generated by the deletion of exon 1 using CRISPR-Cas9 method (Fig. 1E). The entire protein-coding region of exon 1, encompassing amino acids 1–199 (∼75% of the entire protein), was removed, thus ensuring that no functional protein is produced. Sequencing across the deleted region confirmed the exact predicted cut sites of Cas9 (Fig. 1E). PCR genotyping indicated the successful generation of WT (+/+) and homozygous KO (−/−) mice (Fig. 1F). In mice, brain (in particular the cerebellum) expresses the highest level of Ctrp14. As expected, targeted deletion of exon 1 resulted in the complete absence of Ctrp14 transcript in the cerebellum of KO mice (Fig. 1G).
Male Mice Lacking CTRP14 Develop Mild Glucose Intolerance
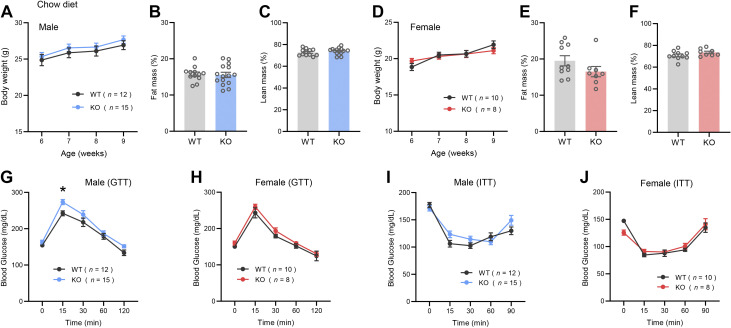
Under the basal state, chow-fed Ctrp14 KO mice of either sex did not differ in body weight and body composition from WT littermates (Fig. 2, A–F). We asked whether CTRP14 is required for proper disposal of glucose in response to acute glucose loading. As indicated by the glucose tolerance tests, the kinetics of glucose clearance was slower in KO male mice (Fig. 2G), though this effect was mild. In contrast, female KO mice were indistinguishable from WT littermates in glucose tolerance tests (Fig. 2H). Direct assessment of insulin sensitivity by insulin tolerance tests did not reveal any significant difference between genotypes of either sex (Fig. 2, I and J). These data indicate that CTRP14 deficiency impaired glucose tolerance modestly and in a sex-dependent manner.
Figure 2.
Chow-fed male mice lacking Ctrp14 develop mild glucose intolerance. A: body weights of chow-fed Ctrp14 KO male and WT littermates over time (WT, n = 12; KO, n = 15). B and C: body composition analysis of % fat mass (relative to body weight) and % lean mass of WT and KO male mice. D: body weights of chow-fed Ctrp14 KO and WT female mice over time (WT, n = 10; KO, n = 8). E and F: body composition analysis of % fat mass and % lean mass of WT and KO female mice. G and H: blood glucose levels during glucose tolerance tests (GTTs) in WT and KO male and female mice. I and J: blood glucose levels during insulin tolerance tests (ITTs) in WT and KO male and female mice. All data are presented as means ± SE. *P < 0.05. KO, knockout; WT, wild type.
CTRP14 Deficiency Does Not Alter Body Weight and Serum Metabolite Response to Fasting and Refeeding
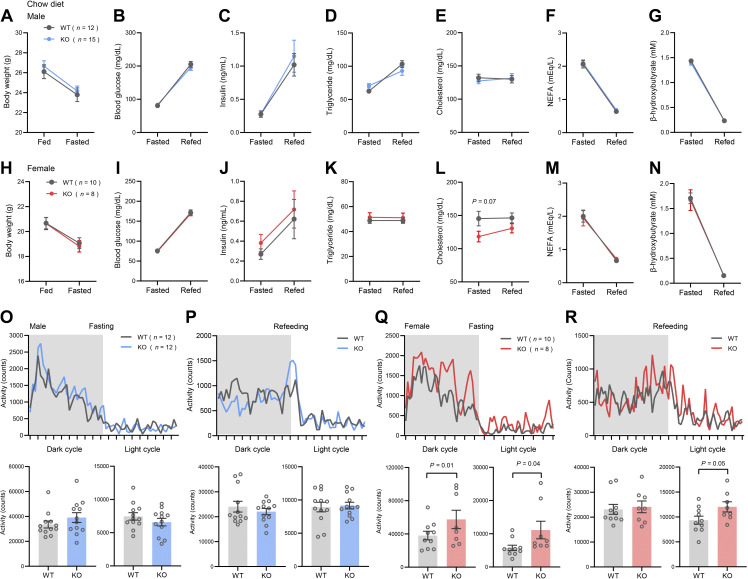
Because Ctrp14 expression is modulated by fasting and refeeding (Fig. 1C), we assessed the impact of CTRP14 deficiency on fasting-refeeding response. Overnight (16 h) food deprivation resulted in similar weight loss between genotypes of either sex (Fig. 3, A and H). The rise of blood glucose and insulin in the first 2 h of refeeding after an overnight fast was also similar between WT and KO mice of either sex (Fig. 3, B, C, I, and J). Likewise, the directions and magnitudes of change for other serum metabolites (triglyceride, cholesterol, nonesterified free fatty acid, and β-hydroxybutyrate) did not differ between WT and KO mice of either sex (Fig. 2, D–G, and K–N). Despite Ctrp14 expression being dynamically regulated by refeeding after food deprivation in WT mice, its complete loss does not appear to affect serum metabolite responses to fasting and refeeding in chow-fed Ctrp14 KO mice.
Figure 3.
Loss of CTRP14 affects physical activity but not serum metabolite levels in response to fasting and refeeding. Changes in body weight (A), blood glucose (B), serum insulin (C), triglyceride (D), cholesterol (E), nonesterified free fatty acids (NEFAs; F), and β-hydroxybutyrate (G) in WT and KO male mice in response to an overnight (16 h) fast followed by 2 h of refeeding. Changes in body weight (H), blood glucose (I), serum insulin (J), triglyceride (K), cholesterol (L), nonesterified free fatty acids (NEFAs; M), and β-hydroxybutyrate (N) in WT and KO female mice in response to an overnight fast followed by 2 h of refeeding. Twenty-four hour tracing of total physical activity during fasting and refeeding in WT and KO male (O and P) and female (Q and R) mice. Bar graphs indicate cumulative physical activity in the dark and light cycle. All data are presented as means ± SE. KO, knockout; WT, wild type.
Loss of CTRP14 Alters Physical Activity and Energy Expenditure in Response to Fasting and Refeeding
Next, we addressed whether CTRP4 deficiency has an impact on organismal response to fasting and refeeding. We used indirect calorimetry method to assess whole body metabolic parameters in chow-fed WT and Ctrp14 KO mice in three different metabolic states across the circadian cycle (Table 1). In the ad libitum fed state, Ctrp14 KO male mice had lower ambulatory activity in the active dark cycle (Table 1; P = 0.055). Food intake, oxygen consumption rate (V̇o2), respiratory exchange ratio (RER), and energy expenditure (EE) were not different between male WT and KO mice. Although ad libitum food intake in the light cycle tended to be higher in female KO mice (P = 0.073), other parameters were not different between female WT and KO mice in the ad libitum-fed state. During food deprivation (24 h food removal), male KO mice had significantly lower RER in the initial 12 h of active dark cycle (Table 1; P = 0.027), indicative of a greater reliance on fat as a preferred oxidative substrate than in WT mice. Other parameters (V̇o2, energy expenditure, and physical activity) were not significantly different between WT and KO male mice. In contrast to male mice (Fig. 3, O and P), fasted female KO mice have significantly higher total physical activity in the light and dark cycle (Table 1 and Fig. 3Q), whereas other parameters were not significantly different from WT littermates. In the 24-h refeeding period following food deprivation, male KO mice had significantly higher energy expenditure in the light cycle (Table 1; P = 0.001). In contrast, during refeeding, female KO mice had higher RER in the light cycle (Table 1; P = 0.043), indicating lesser reliance than WT on fat as the main oxidative substrate. In addition, female KO mice also had higher total physical activity in the light cycle during the refeeding period (Fig. 3R). Thus, contingent on the circadian cycle, CTRP14 deficiency sex dependently affects physical activity, energy expenditure, and respiratory exchange ratio in response to fasting and refeeding.
Table 1.
Indirect calorimetry analyses of chow-fed male (10 wk old) and female (10 wk old) Ctrp14 KO mice and WT littermates
| WT | KO | P | WT | KO | P | WT | KO | P | |
|---|---|---|---|---|---|---|---|---|---|
| Male, chow diet | Ad libitum, dark cycle | Ad libitum, light cycle | Ad libitum, 24 h | ||||||
| n | 12 | 12 | 12 | 12 | 12 | 12 | |||
| Body weight, g | 26.88 ± 0.46 | 27.49 ± 0.47 | 0.381 | ||||||
| Food intake, kcal | 11.05 ± 0.49 | 9.68 ± 0.54 | 0.095 | 3.08 ± 0.41 | 3.19 ± 0.34 | 0.837 | 13.97 ± 0.70 | 12.88 ± 0.67 | 0.275 |
| V̇o2, mL/kgLM/h | 4,331 ± 151 | 4,291 ± 92 | 0.820 | 3,630 ± 127 | 3,560 ± 55 | 0.621 | 3,982 ± 136 | 3,923 ± 69 | 0.702 |
| V̇co2, mL/kgLM/h | 4,340 ± 156 | 4,207 ± 97 | 0.479 | 3,351 ± 143 | 3,187 ± 73 | 0.323 | 3,848 ± 144 | 3,695 ± 76 | 0.357 |
| RER, V̇co2/V̇o2 | 1.002 ± 0.012 | 0.980 ± 0.005 | 0.101 | 0.921 ± 0.016 | 0.894 ± 0.009 | 0.154 | 0.962 ± 0.013 | 0.936 ± 0.005 | 0.084 |
| EE, kcal/kgLM/h | 21.87 ± 0.76 | 21.55 ± 0.46 | 0.725 | 17.97 ± 0.65 | 17.51 ± 0.29 | 0.523 | 19.93 ± 0.69 | 19.52 ± 0.35 | 0.599 |
| Total activity, beam breaks | 28,562 ± 2,123 | 25,206 ± 1,727 | 0.233 | 8,449 ± 769 | 8,409 ± 482 | 0.965 | 37,011 ± 2,688 | 33,615 ± 1,869 | 0.310 |
| Ambulatory activity, counts | 16,641 ± 2,172 | 11,758 ± 1,045 | 0.055 | 3,895 ± 552 | 3,027 ± 246 | 0.165 | 20,536 ± 2,670 | 14,785 ± 1,164 | 0.061 |
| Fasting, dark cycle | Fasting, light cycle | Fasting, 24 h | |||||||
| Body weight, g | 24.19 ± 0.35 | 23.97 ± 0.40 | 0.690 | ||||||
| Food intake, kcal | 0 | 0 | 0 | 0 | 0 | 0 | – | ||
| V̇o2, mL/kgLM/h | 3,577 ± 134 | 3,561 ± 88 | 0.920 | 2,821 ± 116 | 2,682 ± 48 | 0.282 | 3,196 ± 123 | 3,119 ± 62 | 0.579 |
| V̇co2, mL/kgLM/h | 2,949 ± 137 | 2,809 ± 76 | 0.382 | 2,211 ± 123 | 2,025 ± 47 | 0.173 | 2,577 ± 129 | 2,415 ± 58 | 0.263 |
| RER, V̇co2/V̇o2 | 0.818 ± 0.014 | 0.781 ± 0.004 | 0.027 | 0.778 ± 0.015 | 0.750 ± 0.007 | 0.121 | 0.798 ± 0.014 | 0.766 ± 0.005 | 0.056 |
| EE, kcal/kgLM/h | 17.28 ± 0.67 | 17.04 ± 0.42 | 0.771 | 13.49 ± 0.59 | 12.73 ± 0.24 | 0.246 | 15.37 ± 0.62 | 14.87 ± 0.30 | 0.480 |
| Total activity, beam breaks | 33,526 ± 2,796 | 39,068 ± 3,949 | 0.264 | 7,458 ± 560 | 6,590 ± 567 | 0.287 | 40,985 ± 3,223 | 45,658 ± 4,447 | 0.404 |
| Ambulatory activity, counts | 21,049 ± 2,108 | 23,110 ± 2,734 | 0.556 | 3,539 ± 381 | 2,766 ± 313 | 0.131 | 24,588 ± 2,417 | 25,876 ± 3,014 | 0.742 |
| Refeed, dark cycle | Refeed, light cycle | Refeed, 24 h | |||||||
| Body weight, g | 26.61 ± 0.28 | 26.97 ± 0.53 | 0.283 | ||||||
| Food intake, kcal | 13.12 ± 1.42 | 14.29 ± 0.57 | 0.456 | 3.77 ± 0.42 | 4.40 ± 0.28 | 0.238 | 18.75 ± 0.84 | 18.69 ± 0.76 | 0.961 |
| V̇o2, mL/kgLM/h | 4,101 ± 155 | 4,004 ± 87 | 0.592 | 3,417 ± 130 | 3,426 ± 52 | 0.947 | 3,766 ± 141 | 3,723 ± 65 | 0.783 |
| V̇co2, mL/kgLM/h | 4,130 ± 135 | 4,072 ± 94 | 0.729 | 3,507 ± 120 | 3,567 ± 72 | 0.675 | 3,825 ± 125 | 3,826 ± 72 | 0.995 |
| RER, V̇co2/V̇o2 | 1.010 ± 0.010 | 1.016 ± 0.004 | 0.548 | 1.029 ± 0.015 | 1.042 ± 0.010 | 0.505 | 1.019 ± 0.012 | 1.029 ± 0.006 | 0.050 |
| EE, kcal/kgLM/h | 20.73 ± 0.75 | 20.29 ± 0.44 | 0.620 | 16.17 ± 0.19 | 17.46 ± 0.28 | 0.001 | 19.08 ± 0.68 | 18.91 ± 0.33 | 0.832 |
| Total activity, beam breaks | 24,028 ± 2,152 | 22,048 ± 1,257 | 0.435 | 8,932 ± 721 | 9,156 ± 502 | 0.801 | 32,961 ± 2,654 | 31,204 ± 1,370 | 0.562 |
| Ambulatory activity, counts | 13,746 ± 1,886 | 10,334 ± 915 | 0.118 | 4,297 ± 627 | 3,857 ± 342 | 0.544 | 18,044 ± 2,430 | 14,191 ± 966 | 0.155 |
| Female, chow diet | Ad libitum, dark cycle | Ad libitum, light cycle | Ad libitum, 24 h | ||||||
| n | 10 | 8 | 10 | 8 | 10 | 8 | |||
| Body weight, g | 21.39 ± 0.57 | 20.90 ± 0.40 | 0.524 | ||||||
| Food intake, kcal | 11.23 ± 0.86 | 11.00 ± 1.24 | 0.874 | 2.40 ± 0.31 | 3.36 ± 0.39 | 0.073 | 13.64 ± 0.93 | 14.36 ± 1.49 | 0.670 |
| V̇o2, mL/kgLM/h | 5,040 ± 122 | 5,159 ± 103 | 0.481 | 4,327 ± 106 | 4,266 ± 143 | 0.731 | 4,683 ± 109 | 4,712 ± 121 | 0.860 |
| V̇co2, mL/kgLM/h | 5,024 ± 101 | 5,142 ± 124 | 0.470 | 3,812 ± 109 | 3,823 ± 178 | 0.956 | 4,418 ± 98 | 4,483 ± 149 | 0.715 |
| RER, V̇co2/V̇o2 | 0.996 ± 0.009 | 0.994 ± 0.016 | 0.907 | 0.878 ± 0.008 | 0.890 ± 0.014 | 0.466 | 0.937 ± 0.006 | 0.942 ± 0.013 | 0.737 |
| EE, kcal/kgLM/h | 25.41 ± 0.58 | 26.01 ± 0.51 | 0.465 | 21.20 ± 0.53 | 20.98 ± 0.76 | 0.811 | 23.31 ± 0.53 | 23.50 ± 0.63 | 0.820 |
| Total activity, beam breaks | 32,941 ± 4,974 | 42,623 ± 4,201 | 0.169 | 9,759 ± 820 | 12,216 ± 1,036 | 0.077 | 42,700 ± 5,678 | 54,839 ± 4,612 | 0.129 |
| Ambulatory activity, counts | 19,513 ± 4,780 | 24,138 ± 3,277 | 0.460 | 4,041 ± 862 | 5,022 ± 542 | 0.378 | 23,555 ± 5,607 | 29,160 ± 3,518 | 0.437 |
| Fasting, dark cycle | Fasting, light cycle | Fasting, 24 h | |||||||
| Body weight, g | 19.52 ± 0.53 | 18.19 ± 0.35 | 0.057 | ||||||
| Food intake, kcal | 0 | 0 | 0 | 0 | 0 | 0 | |||
| V̇o2, mL/kgLM/h | 4,214 ± 111 | 4,213 ± 121 | 0.993 | 3,099 ± 143 | 2,979 ± 118 | 0.540 | 3,656 ± 119 | 3,597 ± 103 | 0.720 |
| V̇co2, mL/kgLM/h | 3,435 ± 177 | 3,326 ± 97 | 0.623 | 2,389 ± 176 | 2,230 ± 82 | 0.460 | 2,912 ± 174 | 2,779 ± 75 | 0.531 |
| RER, V̇co2/V̇o2 | 0.806 ± 0.025 | 0.783 ± 0.004 | 0.440 | 0.763 ± 0.019 | 0.751 ± 0.008 | 0.600 | 0.785 ± 0.022 | 0.767 ± 0.006 | 0.503 |
| EE, kcal/kgLM/h | 20.31 ± 0.62 | 20.17 ± 0.58 | 0.874 | 14.76 ± 0.76 | 14.11 ± 0.54 | 0.515 | 17.53 ± 0.66 | 17.14 ± 0.48 | 0.656 |
| Total activity, beam breaks | 38,085 ± 5,147 | 57,446 ± 11,031 | 0.108 | 5,744 ± 811 | 11,125 ± 2,640 | 0.048 | 43,829 ± 5,311 | 68,571 ± 13,205 | 0.078 |
| Ambulatory activity, counts | 24,260 ± 4,251 | 37,151 ± 8,387 | 0.164 | 2,176 ± 369 | 6,025 ± 1,922 | 0.043 | 26,437 ± 4,365 | 43,176 ± 9,939 | 0.116 |
| Refeed, dark cycle | Refeed, light cycle | Refeed, 24 h | |||||||
| Body weight, g | 21.56 ± 0.56 | 26.69 ± 0.44 | 0.246 | ||||||
| Food intake, kcal | 16.18 ± 1.02 | 14.87 ± 1.01 | 0.407 | 3.92 ± 0.39 | 4.42 ± 0.32 | 0.380 | 20.11 ± 1.14 | 19.30 ± 1.29 | 0.664 |
| V̇o2, mL/kgLM/h | 4,544 ± 128 | 4,554 ± 93 | 0.952 | 4,022 ± 119 | 4,127 ± 107 | 0.533 | 4,293 ± 121 | 4,349 ± 97 | 0.734 |
| V̇co2, mL/kgLM/h | 4,623 ± 149 | 4,706 ± 100 | 0.667 | 4,018 ± 178 | 4,344 ± 128 | 0.177 | 4,333 ± 157 | 4,532 ± 109 | 0.336 |
| RER, V̇co2/V̇o2 | 1.015 ± 0.007 | 1.032 ± 0.009 | 0.155 | 0.994 ± 0.021 | 1.051 ± 0.012 | 0.043 | 1.005 ± 0.013 | 1.041 ± 0.010 | 0.056 |
| EE, kcal/kgLM/h | 23.03 ± 0.67 | 23.17 ± 0.47 | 0.872 | 20.29 ± 0.66 | 21.09 ± 0.56 | 0.386 | 21.71 ± 0.65 | 22.17 ± 0.49 | 0.599 |
| Total activity, beam breaks | 23,122 ± 1,981 | 24,112 ± 2,354 | 0.750 | 9,347 ± 805 | 12,025 ± 1,015 | 0.052 | 32,470 ± 2,336 | 36,138 ± 3,261 | 0.362 |
| Ambulatory activity, counts | 12,469 ± 2,304 | 12,226 ± 1,561 | 0.935 | 4,001 ± 605 | 5,334 ± 680 | 0.162 | 16,470 ± 2,813 | 17,560 ± 2,148 | 0.771 |
All data are presented as means ± SE. All significant data (P < 0.05) are underlined and bolded. EE, energy expenditure; KO, knockout; RER, respiratory exchange ratio; V̇co2, rate of carbon dioxide production; V̇o2, rate of oxygen consumption; WT, wild type.
CTRP14 Deficiency Does Not Affect High-Fat Diet-Induced Obesity and Metabolic Dysfunction
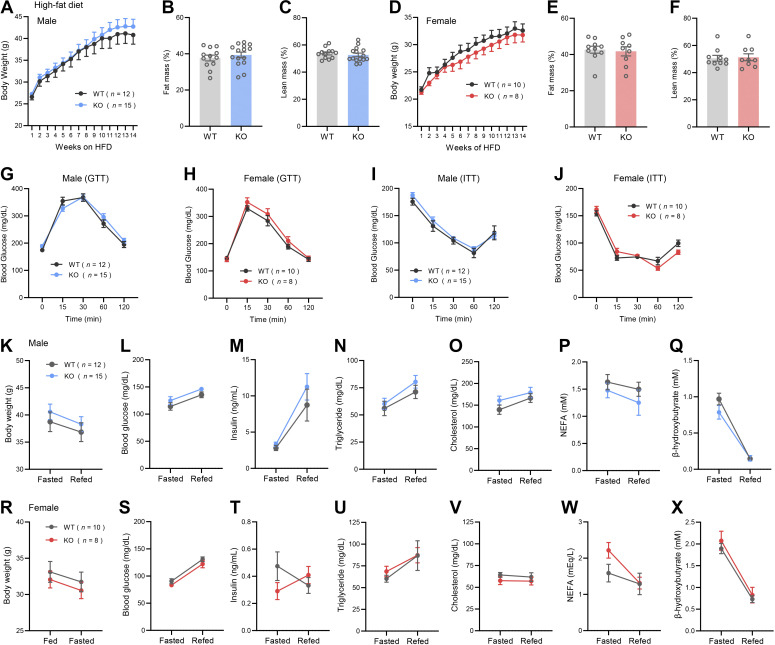
Next, we asked whether Ctrp14 deletion affects the course and outcomes of obesity and metabolic derangements induced by chronic high-fat feeding. When fed an HFD, body weight gain and body composition (fat and lean mass normalized to body weight) of Ctrp14 KO mice of either sex were not significantly different from WT littermates (Fig. 4, A–F). The rate of glucose disposal in response to acute glucose loading (in glucose tolerance tests) was indistinguishable between genotypes of either sex (Fig. 4, G and H). Whole body insulin sensitivity, as judged by insulin-stimulated glucose disposal (insulin tolerance tests), was also not different between genotypes of either sex (Fig. 4, I and J). Although serum metabolite response to fasting and refeeding was not different between chow-fed WT and Ctrp14 KO mice (Fig. 3, A–N), we assessed whether this would be different in the context of diet-induced obese state. In response to an overnight (16 h) fast, WT and Ctrp14 KO mice of either sex lost comparable amount of weight (Fig. 4, L and R). In response to 2 h refeeding after food deprivation, the magnitude and direction of change in blood glucose and serum insulin, triglyceride, cholesterol, NEFA, and β-hydroxybutyrate were also not significantly different between genotypes of either sex (Fig. 4, L–Q, S–X). Organ weights were collected at the end of our high-fat diet study. Neither visceral fat (gWAT), subcutaneous fat (iWAT), liver, heart, or kidney weights (absolute or relative to body weight) was significantly different between genotypes of either sex (Table 2). These data indicate that CTRP14 deficiency does not affect the metabolic outcomes (body weight, organ weight, insulin sensitivity, serum metabolite levels) of diet-induced obesity.
Figure 4.
CTRP14 deficiency does not affect body weight, serum glucose and lipid profiles, and insulin sensitivity in response to diet-induced obesity. A: body weights of Ctrp14 KO male and WT littermates fed HFD over time (WT, n = 12; KO, n = 15). B and C: body composition analysis of % fat mass (relative to body weight) and % lean mass of WT and KO male mice. D: body weights of Ctrp14 KO and WT female mice fed HFD over time (WT, n = 10; KO, n = 8). E and F: body composition analysis of % fat mass and % lean mass of WT and KO female mice. Blood glucose levels during glucose tolerance tests (GTTs) in WT and KO male (G) and female (H) mice. Blood glucose levels during insulin tolerance tests (ITTs) in WT and KO male (I) and female (J) mice. Changes in body weight (K), blood glucose (L), serum insulin (M), triglyceride (N), cholesterol (O), nonesterified free fatty acids (NEFAs; P), and β-hydroxybutyrate (Q) in WT and KO male mice in response to an overnight fast followed by 2 h of refeeding. Changes in body weight (R), blood glucose (S), serum insulin (T), triglyceride (U), cholesterol (V), NEFA (W), and β-hydroxybutyrate (X) in WT and KO female mice in response to an overnight fast followed by 2 h of refeeding. All data are presented as means ± SE. HFD, high-fat diet; KO, knockout; WT, wild type.
Table 2.
Body weights and organ weights of WT and Ctrp14 KO male and female mice at the end of high-fat study
| Variable | Male |
Female |
||||
|---|---|---|---|---|---|---|
| WT | KO | P | WT | KO | P | |
| n | 12 | 15 | 10 | 8 | ||
| Body weight, g | 44.36 ± 1.85 | 45.32 ± 1.57 | 0.695 | 35.17 ± 1.57 | 34.45 ± 1.36 | 0.742 |
| Gonadal fat (gWAT), g | 1.198 ± 0.087 | 1.106 ± 0.043 | 0.329 | 1.250 ± 0.098 | 1.213 ± 0.099 | 0.794 |
| gWAT, % | 2.77 ± 0.25 | 2.49 ± 0.14 | 0.317 | 3.50 ± 0.16 | 3.48 ± 0.17 | 0.923 |
| Inguinal fat (iWAT), g | 1.106 ± 0.105 | 1.241 ± 0.099 | 0.362 | 1.096 ± 0.112 | 1.006 ± 0.108 | 0.581 |
| iWAT, % | 2.43 ± 0.14 | 2.68 ± 0.15 | 0.254 | 3.04 ± 0.19 | 2.87 ± 0.21 | 0.562 |
| Liver weight, g | 1.804 ± 0.204 | 1.783 ± 0.140 | 0.929 | 0.909 ± 0.041 | 0.867 ± 0.038 | 0.485 |
| Liver, % | 3.96 ± 0.28 | 3.86 ± 0.18 | 0.767 | 2.59 ± 0.05 | 2.51 ± 0.05 | 0.398 |
| Heart weight, g | 0.135 ± 0.005 | 0.143 ± 0.005 | 0.278 | 0.102 ± 0.003 | 0.105 ± 0.003 | 0.554 |
| Heart, % | 0.305 ± 0.006 | 0.318 ± 0.008 | 0.271 | 0.293 ± 0.007 | 0.307 ± 0.013 | 0.338 |
| Kidney weight, g | 0.164 ± 0.006 | 0.169 ± 0.007 | 0.604 | 0.123 ± 0.005 | 0.121 ± 0.003 | 0.794 |
| Kidney, % | 0.373 ± 0.013 | 0.378 ± 0.016 | 0.83 | 0.352 ± 0.013 | 0.355 ± 0.014 | 0.884 |
Values are presented as means ± SE. gWAT, gonadal white adipose tissue; iWAT, inguinal white adipose tissue; KO, Ctrp14 knockout; WT, wild type.
Loss of CTRP14 Alters Food Intake and Physical Activity in Mice Fed a High-Fat Diet
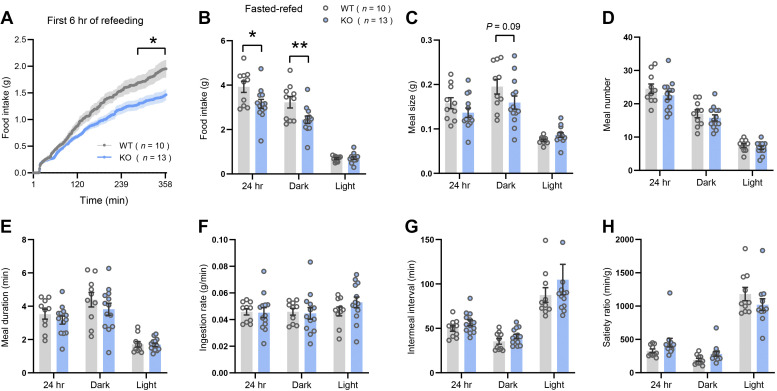
We again used indirect calorimetry to determine the impact of CTRP14 deficiency on whole body energetic response across the circadian cycle in three different metabolic states (Table 3). In the ad libitum fed state, none of the metabolic parameters (food intake, V̇o2, respiratory exchange ratio, physical activity, and energy expenditure) were significantly different between HFD-fed female WT and KO mice (Table 3). In male KO mice, however, ambulatory activity was lower and close to being significant in the dark cycle (P = 0.058) and significantly reduced in the light cycle (P = 0.02) and over the 24 h period (P = 0.037). In the fasted state, the only parameter that was significantly different was the respiratory exchange ratio (RER) of KO female mice in the active dark cycle when compared with WT littermates (P = 0.002). In the refed state following food deprivation, Ctrp14 KO male mice had lower ambulatory activity compared with WT controls over a 24 h period (P = 0.064), mostly driven by reduced ambulatory activity during the light cycle (P = 0.045). Furthermore, Ctrp14 KO male mice in the refed period consumed significantly less food in the active dark cycle compared with WT controls (P = 0.01). Reduced food intake in Ctrp14 KO male mice was already apparent in the first 6 h when food was reintroduced after a 24 h fast (Fig. 5A). Analyses of meal patterns suggested the overall reduction in food intake in KO mice (Fig. 5B) was likely due mainly to smaller meal size (Fig. 5C), with potential contribution of lower meal number, although neither of these measures individually were significantly different by genotype (Fig. 5, C and D), nor were other methods of examining meal patterns (Fig. 5, E–H). In female mice, none of the measured parameters were significantly different between genotypes (Table 3). Thus, in the context of obesity, CTRP14 deficiency alters ambulatory activity, RER, and food intake patterns in a sex-dependent manner.
Table 3.
Indirect calorimetry analyses of male and female Ctrp14 KO mice and WT littermates fed a high-fat diet for 14 wk
| WT | KO | P | WT | KO | P | WT | KO | P | |
|---|---|---|---|---|---|---|---|---|---|
| Male, high-fat diet | Ad libitum, dark cycle | Ad libitum, light cycle | Ad libitum, 24 h | ||||||
| n | 11 | 12 | 11 | 12 | 11 | 12 | |||
| Body weight, g | 40.46 ± 1.80 | 42.64 ± 1.66 | 0.384 | ||||||
| Food intake, kcal | 11.77 ± 0.81 | 11.15 ± 0.55 | 0.544 | 4.29 ± 0.20 | 4.63 ± 0.18 | 0.257 | 15.45 ± 0.83 | 15.78 ± 0.58 | 0.740 |
| V̇o2, mL/kgLM/h | 4,858 ± 88 | 4,807 ± 93 | 0.696 | 4,014 ± 68 | 4,075 ± 82 | 0.579 | 4,436 ± 74 | 4,441 ± 82 | 0.968 |
| V̇co2, mL/kgLM/h | 3,998 ± 87 | 3,947 ± 65 | 0.642 | 3,285 ± 61 | 3,335 ± 68 | 0.593 | 3,642 ± 72 | 3,641 ± 62 | 0.994 |
| RER, V̇co2/V̇o2 | 0.822 ± 0.005 | 0.821 ± 0.003 | 0.922 | 0.817 ± 0.003 | 0.818 ± 0.002 | 0.843 | 0.819 ± 0.003 | 0.820 ± 0.002 | 0.982 |
| EE, kcal/kgLM/h | 23.46 ± 0.44 | 23.20 ± 0.43 | 0.682 | 19.36 ± 0.33 | 19.65 ± 0.39 | 0.581 | 21.41 ± 0.37 | 21.42 ± 0.39 | 0.976 |
| Total activity, beam breaks | 22,273 ± 1,937 | 20,462 ± 1,272 | 0.435 | 8,436 ± 910 | 6,767 ± 506 | 0.116 | 30,709 ± 2,806 | 27,230 ± 1,606 | 0.284 |
| Ambulatory activity, counts | 11,370 ± 1,392 | 8,278 ± 751 | 0.058 | 3,639 ± 675 | 1,914 ± 228 | 0.020 | 15,009 ± 2,036 | 10,192 ± 921 | 0.037 |
| Fasting, dark cycle | Fasting, light cycle | Fasting, 24 h | |||||||
| Body weight, g | 38.68 ± 1.69 | 39.82 ± 1.55 | 0.626 | ||||||
| Food intake, kcal | 0 | 0 | 0 | 0 | 0 | 0 | |||
| V̇o2, mL/kgLM/h | 4,416 ± 83 | 4,446 ± 92 | 0.822 | 3,438 ± 64 | 3,471 ± 70 | 0.746 | 3,931 ± 65 | 3,955 ± 80 | 0.824 |
| V̇co2, mL/kgLM/h | 3,395 ± 64 | 3,427 ± 71 | 0.750 | 2,658 ± 47 | 2,678 ± 58 | 0.808 | 3,029 ± 50 | 3,050 ± 63 | 0.808 |
| RER, V̇co2/V̇o2 | 0.768 ± 0.001 | 0.770 ± 0.001 | 0.509 | 0.773 ± 0.001 | 0.771 ± 0.001 | 0.423 | 0.771 ± 0.001 | 0.771 ± 0.001 | 0.978 |
| EE, kcal/kgLM/h | 21.03 ± 0.39 | 21.18 ± 0.44 | 0.807 | 16.39 ± 0.30 | 16.54 ± 0.34 | 0.759 | 18.73 ± 0.31 | 18.84 ± 0.38 | 0.821 |
| Total activity, beam breaks | 31,377 ± 2,159 | 28,022 ± 1,372 | 0.203 | 7,652 ± 578 | 7,877 ± 623 | 0.800 | 39,029 ± 2,603 | 35,900 ± 1,606 | 0.316 |
| Ambulatory activity, counts | 19,455 ± 1,422 | 14,899 ± 1,092 | 0.020 | 3,571 ± 328 | 3,148 ± 377 | 0.425 | 23,026 ± 1,656 | 18,047 ± 1,255 | 0.027 |
| Refeed, dark cycle | Refeed, light cycle | Refeed, 24 h | |||||||
| Body weight, g | 39.99 ± 1.67 | 41.12 ± 1.57 | 0.631 | ||||||
| Food intake, kcal | 17.09 ± 1.21 | 13.08 ± 0.69 | 0.010 | 3.95 ± 0.23 | 4.03 ± 0.28 | 0.849 | 20.46 ± 1.27 | 17.11 ± 0.84 | 0.041 |
| V̇o2, mL/kgLM/h | 4,877 ± 85 | 4,805 ± 77 | 0.547 | 3,936 ± 63 | 3,941 ± 69 | 0.963 | 4,415 ± 72 | 4,381 ± 70 | 0.750 |
| V̇co2, mL/kgLM/h | 3,958 ± 86 | 3,903 ± 53 | 0.596 | 3,299 ± 60 | 3,295 ± 52 | 0.957 | 3,634 ± 72 | 3,605 ± 50 | 0.743 |
| RER, V̇co2/V̇o2 | 0.811 ± 0.004 | 0.812 ± 0.004 | 0.796 | 0.838 ± 0.004 | 0.836 ± 0.003 | 0.719 | 0.824 ± 0.004 | 0.824 ± 0.003 | 0.972 |
| EE, kcal/kgLM/h | 23.48 ± 0.43 | 23.14 ± 0.35 | 0.554 | 19.08 ± 0.31 | 19.09 ± 0.32 | 0.908 | 21.32 ± 0.36 | 21.15 ± 0.33 | 0.747 |
| Total activity, beam breaks | 26,323 ± 1,271 | 25,459 ± 1,390 | 0.662 | 7,599 ± 530 | 7,041 ± 452 | 0.440 | 33,923 ± 1,562 | 32,500 ± 1,708 | 0.559 |
| Ambulatory activity, counts | 14,272 ± 1,378 | 11,449 ± 863 | 0.096 | 3,450 ± 453 | 2,341 ± 260 | 0.045 | 17,722 ± 1,725 | 13,791 ± 1,044 | 0.064 |
| Female, high-fat diet | Ad libitum, dark cycle | Ad libitum, light cycle | Ad libitum, 24 h | ||||||
| n | 10 | 8 | 10 | 8 | 10 | 8 | |||
| Body weight, g | 31.51 ± 1.13 | 31.38 ± 1.21 | 0.938 | ||||||
| Food intake, kcal | 9.23 ± 0.79 | 11.27 ± 1.44 | 0.210 | 2.61 ± 0.32 | 2.85 ± 0.24 | 0.577 | 11.85 ± 0.87 | 14.13 ± 1.42 | 0.174 |
| V̇o2, mL/kgLM/h | 5,769 ± 126 | 5,613 ± 161 | 0.451 | 4,956 ± 140 | 4,697 ± 139 | 0.215 | 5,363 ± 131 | 5,155 ± 149 | 0.310 |
| V̇co2, mL/kgLM/h | 4,765 ± 114 | 4,683 ± 140 | 0.653 | 4,039 ± 107 | 3,866 ± 113 | 0.288 | 4,402 ± 107 | 4,275 ± 125 | 0.450 |
| RER, V̇co2/V̇o2 | 0.825 ± 0.006 | 0.833 ± 0.005 | 0.383 | 0.814 ± 0.004 | 0.822 ± 0.002 | 0.162 | 0.819 ± 0.005 | 0.827 ± 0.003 | 0.244 |
| EE, kcal/kgLM/h | 27.88 ± 0.618 | 27.18 ± 0.78 | 0.489 | 23.88 ± 0.668 | 22.68 ± 0.67 | 0.228 | 25.88 ± 0.62 | 24.93 ± 0.72 | 0.334 |
| Total activity, beam breaks | 24,346 ± 3,097 | 31,071 ± 2,038 | 0.106 | 8,700 ± 842 | 10,086 ± 846 | 0.268 | 33,046 ± 3,831 | 41,157 ± 2,838 | 0.123 |
| Ambulatory activity, counts | 11,669 ± 1,894 | 15,065 ± 1,234 | 0.175 | 2,895 ± 305 | 3,308 ± 367 | 0.395 | 14,565 ± 2,168 | 18,374 ± 1,559 | 0.193 |
| Fasting, dark cycle | Fasting, light cycle | Fasting, 24 h | |||||||
| Body weight, g | 24.15 ± 4.09 | 26.26 ± 3.90 | 0.718 | ||||||
| Food intake, kcal | 0 | 0 | 0 | 0 | 0 | 0 | |||
| V̇o2, mL/kgLM/h | 5,425 ± 157 | 5,158 ± 166 | 0.264 | 4,314 ± 161 | 4,110 ± 180 | 0.411 | 4,870 ± 156 | 4,634 ± 171 | 0.325 |
| V̇co2, mL/kgLM/h | 4,181 ± 113 | 4,017 ± 127 | 0.350 | 3,336 ± 123 | 3,193 ± 146 | 0.461 | 3,759 ± 115 | 3,605 ± 135 | 0.397 |
| RER, V̇co2/V̇o2 | 0.770 ± 0.001 | 0.778 ± 0.001 | 0.002 | 0.773 ± 0.003 | 0.775 ± 0.002 | 0.565 | 0.771 ± 0.001 | 0.777 ± 0.001 | 0.061 |
| EE, kcal/kgLM/h | 25.85 ± 0.74 | 24.62 ± 0.79 | 0.279 | 20.57 ± 0.76 | 19.61 ± 0.86 | 0.421 | 23.21 ± 0.73 | 22.12 ± 0.81 | 0.338 |
| Total activity, beam breaks | 26,463 ± 4,383 | 35,042 ± 2,967 | 0.144 | 8,319 ± 1,077 | 8,997 ± 626 | 0.618 | 34,782 ± 5,281 | 44,039 ± 3,137 | 0.177 |
| Ambulatory activity, counts | 14,195 ± 2,848 | 19,487 ± 2,025 | 0.169 | 3,107 ± 497 | 3,371 ± 343 | 0.683 | 17,302 ± 3,253 | 22,859 ± 2,034 | 0.192 |
| Refeed, dark cycle | Refeed, light cycle | Refeed, 24 h | |||||||
| Body weight, g | 30.21 ± 1.10 | 30.11 ± 1.20 | 0.951 | ||||||
| Food intake, kcal | 10.77 ± 0.62 | 11.77 ± 0.91 | 0.368 | 2.92 ± 0.35 | 3.34 ± 0.38 | 0.437 | 13.69 ± 0.62 | 15.11 ± 1.01 | 0.233 |
| V̇o2, mL/kgLM/h | 5,587 ± 121 | 5,391 ± 195 | 0.388 | 4,849 ± 155 | 4,695 ± 133 | 0.476 | 5,226 ± 135 | 5,052 ± 161 | 0.418 |
| V̇co2, mL/kgLM/h | 4,624 ± 90 | 4,536 ± 145 | 0.598 | 4,046 ± 126 | 3,968 ± 111 | 0.656 | 4,341 ± 106 | 4,259 ± 123 | 0.620 |
| RER, V̇co2/V̇o2 | 0.827 ± 0.005 | 0.842 ± 0.005 | 0.096 | 0.833 ± 0.005 | 0.844 ± 0.004 | 0.167 | 0.830 ± 0.005 | 0.843 ± 0.004 | 0.114 |
| EE, kcal/kgLM/h | 27.01 ± 0.57 | 26.15 ± 0.92 | 0.423 | 23.48 ± 0.74 | 22.80 ± 0.64 | 0.510 | 25.28 ± 0.64 | 24.52 ± 0.76 | 0.453 |
| Total activity, beam breaks | 23,413 ± 2,312 | 28,207 ± 1,264 | 0.110 | 8,933 ± 923 | 11,089 ± 1,712 | 0.258 | 3,2347 ± 2,971 | 39,297 ± 2,503 | 0.102 |
| Ambulatory activity, counts | 10,531 ± 1,316 | 12,989 ± 767 | 0.151 | 2,961 ± 277 | 4,184 ± 959 | 0.196 | 13,492 ± 1,473 | 17,174 ± 1,386 | 0.093 |
All data are presented as means ± SE. All significant data (P < 0.05) are underlined and bolded. EE, energy expenditure; KO, knockout; RER, respiratory exchange ratio; V̇co2, rate of carbon dioxide production; V̇o2, rate of oxygen consumption; WT, wild type.
Figure 5.
Increased food intake in HFD-fed Ctrp14 KO male mice during the refed period following food deprivation. A: food intake in the first 6 h when food was reintroduced after a 24 h food removal. B: total food intake in the light and dark cycle, as well as, over the 24-h period. Meal size (C), meal number (D), meal duration (E), ingestive rate (F), intermeal interval (G), and satiety ratio (H) in WT (n = 10) and KO (n = 13) male mice. HFD, high-fat diet; KO, knockout; WT, wild type.
CTRP14 Deficiency Elevates Lymphocyte Counts in Diet-Induced Obese Female Mice
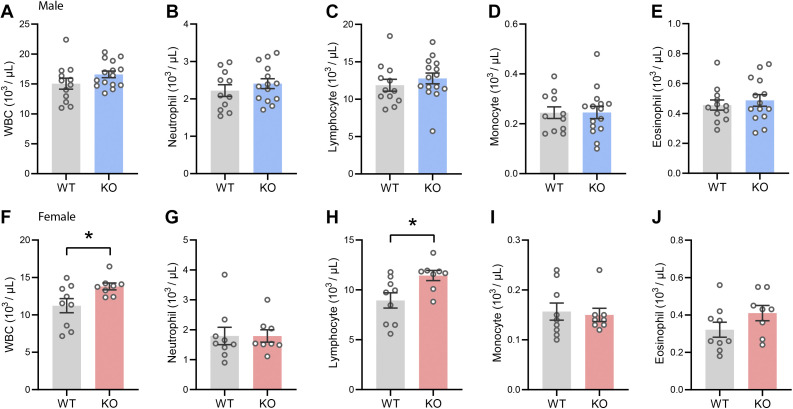
Obesity is known to be associated with local and systemic low-grade inflammation (39). We therefore also determined blood cell composition to assess if CTRP14 deficiency affects the immune profiles in the KO animals. We noted that total white blood cell (WBC) and lymphocyte counts were significantly elevated in Ctrp14 KO female, but not male, mice fed an HFD (Fig. 6). This effect was diet-dependent. In chow-fed lean mice, total WBC, neutrophil, lymphocyte, monocyte, and eosinophil counts were not different between genotypes of either sex (data not shown). These results indicate sexually dimorphic and diet-dependent changes in circulating immune profiles associated with CTRP14 deficiency.
Figure 6.
CTRP14 deficiency is associated with elevated white blood cell and lymphocyte counts in KO female mice fed an HFD. A–E: total white blood cell (WBC), neutrophil, lymphocyte, monocyte, and eosinophil counts in WT (n = 12) and KO (n = 15) male mice fed an HFD. F–J: total white blood cell (WBC), neutrophil, lymphocyte, monocyte, and eosinophil counts in WT (n = 9) and KO (n = 8) female mice fed an HFD. HFD, high-fat diet; KO, knockout; WT, wild type.
DISCUSSION
In the present study, we investigated the potential metabolic function of CTRP14 and provided detailed analyses on the impact of CTRP14 deficiency on whole body energy balance and physiological response to fasting and refeeding. We noted sexually dimorphic metabolic responses to Ctrp14 deletion that are context- and diet-dependent. Under the basal state when mice are fed a standard chow diet, the contributions of CTRP14 to metabolic homeostasis are modest and limited. Specifically, we observed the following in our KO mice relative to WT littermates: 1) male KO mice exhibit mild glucose intolerance relative to WT littermates but no apparent changes in whole body insulin sensitivity; 2) male KO mice tended to have lower ambulatory activity in the active dark cycle; 3) male KO mice have a modest but significantly elevated energy expenditure in the light cycle during the refeeding period following food deprivation; 4) female KO mice have increased physical activity in the light cycle during fasting and refeeding; and 5) female KO mice have higher RER in the light cycle during the refeeding period, indicating greater carbohydrate oxidation.
In the context of obesity and metabolic dysregulation induced by HFD, the contributions of CTRP14 to metabolic outcomes are also limited and appear to be largely dispensable. The limited phenotypes we observed are also context- and sex-dependent. Specifically, we noted the following in our HFD-fed KO mice relative to WT controls: 1) female KO mice have elevated RER in the dark cycle during fasting, indicating lesser reliance on fat oxidation during the first phase of fasting than WT mice; 2) in the ad libitum-fed state, male KO mice have significantly reduced ambulatory activity; 3) in the refed period, male KO mice partially regain this decrease in ambulatory activity; 4) in the refed period following food deprivation, male KO mice also have reduced caloric intake, due in part to smaller meal size; 5) female KO mice have elevated white blood cell and lymphocyte counts. The significance of this observation as it relates to metabolism, however, is presently unclear since the KO female mice are largely indistinguishable from WT controls.
Despite the dynamic regulation of Ctrp14 expression in peripheral tissues in response to altered energetic states, its complete loss appears to have minimal impact at the organismal level in terms of whole body energy balance, glucose and lipid metabolism, insulin sensitivity, and metabolite response to fasting-refeeding cycle. Based on all the available data obtained from our genetic loss-of-function mouse model, we conclude that CTRP14’s role is mainly limited to regulating specific behavioral responses (e.g., physical activity and food intake) to fasting and refeeding. Both physical activity across the circadian cycle and food intake patterns are known to be centrally regulated (40, 41). Recent single-cell RNA sequencing reveals the predominant expression of Ctrp14/C1q1 in the excitatory neurons of the hindbrain (27), with little to no expression in other anatomical regions of the brain, including the hypothalamus. Although orexigenic and anorexigenic neurons within the hypothalamus are well known for regulating food intake and appetite (41), other brain regions, such as the nucleus tractus solitaries (NTS) of the hindbrain that expresses several key neuropeptide receptors (e.g., GLP-1R and leptin receptor) also play important roles in regulating ingestive physiology (42–44). However, unlike the hypothalamus and the NTS of hindbrain, the role of cerebellum—where Ctrp14 is robustly expressed—in food intake regulation is much less appreciated (45–47). Receptor for the satiety hormone (leptin) and the insulin-responsive glucose transporter (GLUT4) are known to be expressed and nutritionally regulated in the cerebellum (48–50). Furthermore, the cerebellum can signal to the hypothalamus to control food intake (51–54). The role of cerebellum in food intake regulation appears to be conserved in humans (55–57). Given that hindbrain is the main site of Ctrp14 expression (27), the food intake phenotype we observed in Ctrp14 KO male mice in response to fasting and refeeding may be due to altered neural activity within the hindbrain/cerebellum.
Sexually dimorphic responses to metabolic perturbations have been extensively documented (58, 59). Thus, it is not surprising that physiological response to Ctrp14 deletion in the basal (chow-fed) and obese (HFD-fed) states is sex-dependent. Although it is generally assumed that sex hormones are responsible for sexually dimorphic metabolic outcomes, it is not always clear cut and straightforward to demonstrate, especially if the sex-dependent phenotypes are mild. In general, removal of the gonads (e.g., ovariectomy and castration) is required to show that sex hormones are indeed underlying the observed phenotypes in genetic mouse models. Given the modest effects seen in the Ctrp14 KO male and female mice, we did not attempt to uncover the mechanism responsible for the sexually dimorphic phenotypes. This is a limitation of the present study. Nonetheless, our study once again underscored the importance of sex as a biological determinant of phenotypic outcomes and reinforced the need to include both sexes, whenever possible, in preclinical animal models (60).
One major caveat of the present study is the use of constitutive whole body KO mice. We therefore could not rule out the possibility that the mild metabolic phenotypes seen in Ctrp14 KO mice is due to developmental compensation. Although our extensive analyses of the constitutive Ctrp14 KO mice yielded largely negative data, this information is valuable in guiding future studies aimed at uncovering additional physiological functions of CTRP14. In summary, unlike many other related CTRP family members with salutary metabolic functions, CTRP14 is largely dispensable for systemic energy balance, and its role in modulating metabolic physiology appears to be limited to normal fasting-refeeding response.
GRANTS
This work was supported by the National Institutes of Health Grant DK084171 (to G.W.W.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.C.S. and G.W.W. conceived and designed research; D.C.S., C.X., S.A., and G.W.W. performed experiments; D.C.S., C.X., S.A., and G.W.W. analyzed data; D.C.S., S.A., and G.W.W. interpreted results of experiments; D.C.S., C.X., and G.W.W. prepared figures; D.C.S. and G.W.W. drafted manuscript; D.C.S., C.X., S.A., and G.W.W. edited and revised manuscript.
REFERENCES
- 1.Castillo-Armengol J, Fajas L, Lopez-Mejia IC. Inter-organ communication: a gatekeeper for metabolic health. EMBO Rep 20: e47903, 2019. doi: 10.15252/embr.201947903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metab 1: 1177–1188, 2019. doi: 10.1038/s42255-019-0145-5. [DOI] [PubMed] [Google Scholar]
- 3.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980, 2012. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation of AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 286: 15652–15665, 2011. doi: 10.1074/jbc.M110.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. doi: 10.1074/jbc.M113.458711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf RM, Steele KE, Peterson LA, Zeng X, Jaffe AE, Schweitzer MA, Magnuson TH, Wong GW. C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery. J Clin Endocrinol Metab 101: 2211–2217, 2016. doi: 10.1210/jc.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 15: 111–123, 2014. doi: 10.1007/s11154-013-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ressl S, Vu BK, Vivona S, Martinelli DC, Südhof TC, Brunger AT. Structures of C1q-like proteins reveal unique features among the C1q/TNF superfamily. Structure 23: 688–699, 2015. doi: 10.1016/j.str.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, Reid KBM, Sim RB, Arlaud GJ. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol 25: 551–561, 2004. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Ghai R, Waters P, Roumenina LT, Gadjeva M, Kojouharova MS, Reid KB, Sim RB, Kishore U. C1q and its growing family. Immunobiology 212: 253–266, 2007. doi: 10.1016/j.imbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Lei X, Rodriguez S, Petersen PS, Seldin MM, Bowman CE, Wolfgang MJ, Wong GW. Loss of CTRP5 improves insulin action and hepatic steatosis. Am J Physiol Endocrinol Physiol 310: E1036–E1052, 2016. doi: 10.1152/ajpendo.00010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei X, Seldin MM, Little HC, Choy N, Klonisch T, Wong GW. C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance. J Biol Chem 292: 14836–14850, 2017. doi: 10.1074/jbc.M116.766808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei X, Wong GW. C1q/TNF-related protein 2 (CTRP2) deletion promotes adipose tissue lipolysis and hepatic triglyceride secretion. J Biol Chem 294: 15638–15649, 2019. doi: 10.1074/jbc.RA119.009230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little HC, Rodriguez S, Lei X, Tan SY, Stewart AN, Sahagun A, Sarver DC, Wong GW. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J 33: 8666–8687, 2019. doi: 10.1096/fj.201900520R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen PS, Lei X, Wolf RM, Rodriguez S, Tan SY, Little HC, Schweitzer MA, Magnuson TH, Steele KE, Wong GW. CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress. Am J Physiol Endocrinol Physiol 312: E309–E325, 2017. doi: 10.1152/ajpendo.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One 9: e88535, 2014. doi: 10.1371/journal.pone.0088535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–224, 2013. doi: 10.1152/ajpgi.00102.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R522–R533, 2013. doi: 10.1152/ajpregu.00110.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez S, Lei X, Petersen PS, Tan SY, Little HC, Wong GW. Loss of CTRP1 disrupts glucose and lipid homeostasis. Am J Physiol Endocrinol Physiol 311: E678–E697, 2016. doi: 10.1152/ajpendo.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan SY, Lei X, Little HC, Rodriguez S, Sarver DC, Cao X, Wong GW. CTRP12 ablation differentially affects energy expenditure, body weight, and insulin sensitivity in male and female mice. Am J Physiol Endocrinol Physiol 319: E146–E162, 2020. doi: 10.1152/ajpendo.00533.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Physiol 306: E779–E790, 2014. doi: 10.1152/ajpendo.00593.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahav R, Haim Y, Bhandarkar NS, Levin L, Chalifa-Caspi V, Sarver D, Sahagun A, Maixner N, Kovesh B, Wong GW, Rudich A. CTRP6 rapidly responds to acute nutritional changes, regulating adipose tissue expansion and inflammation in mice. Am J Physiol Endocrinol Physiol 321: E702–E713, 2021. doi: 10.1152/ajpendo.00299.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iijima T, Miura E, Watanabe M, Yuzaki M. Distinct expression of C1q-like family mRNAs in mouse brain and biochemical characterization of their encoded proteins. Eur J Neurosci 31: 1606–1615, 2010. doi: 10.1111/j.1460-9568.2010.07202.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22, 2018. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolliger MF, Martinelli DC, Südhof TC. The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc Natl Acad Sci USA 108: 2534–2539, 2011. doi: 10.1073/pnas.1019577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakegawa W, Mitakidis N, Miura E, Abe M, Matsuda K, Takeo YH, Kohda K, Motohashi J, Takahashi A, Nagao S, Muramatsu S, Watanabe M, Sakimura K, Aricescu AR, Yuzaki M. Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85: 316–329, 2015. doi: 10.1016/j.neuron.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Sigoillot SM, Iyer K, Binda F, González-Calvo I, Talleur M, Vodjdani G, Isope P, Selimi F. The secreted protein C1QL1 and its receptor BAI3 control the synaptic connectivity of excitatory inputs converging on cerebellar Purkinje cells. Cell Rep 10: 820–832, 2015. doi: 10.1016/j.celrep.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Biswas J, Pijewski RS, Makol R, Miramontes TG, Thompson BL, Kresic LC, Burghard AL, Oliver DL, Martinelli DC. C1ql1 is expressed in adult outer hair cells of the cochlea in a tonotopic gradient. PLoS One 16: e0251412, 2021. doi: 10.1371/journal.pone.0251412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi Y, Xiong W, Yu S, Du Z, Qu T, He L, Wei W, Zhang L, Liu K, Li Y, He DZ, Gong S. Deletion of C1ql1 causes hearing loss and abnormal auditory nerve fibers in the mouse cochlea. Front Cell Neurosci 15: 713651, 2021. doi: 10.3389/fncel.2021.713651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348: 648–660, 2015. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei XT, Feng GJ, Zhang H, Xu Q, Ni JJ, Zhao M, Yang XL, Tian Q, Shen H, Hai R, Deng HW, Zhang L, Pei YF. Pleiotropic genomic variants at 17q21.31 associated with bone mineral density and body fat mass: a bivariate genome-wide association analysis. Eur J Hum Genet 29: 553–563, 2021. doi: 10.1038/s41431-020-00727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez S, Stewart AN, Lei X, Cao X, Little HC, Fong V, Sarver DC, Wong GW. PRADC1: a novel metabolic-responsive secretory protein that modulates physical activity and adiposity. FASEB J 33: 14748–14759, 2019. doi: 10.1096/fj.201901279R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarver DC, Stewart AN, Rodriguez S, Little HC, Aja S, Wong GW. Loss of CTRP4 alters adiposity and food intake behaviors in obese mice. Am J Physiol Endocrinol Physiol 319: E1084–E1100, 2020. doi: 10.1152/ajpendo.00448.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 39.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol 11: 81, 2011. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zink AN, Perez-Leighton CE, Kotz CM. The orexin neuropeptide system: physical activity and hypothalamic function throughout the aging process. Front Syst Neurosci 8: 211, 2014. doi: 10.3389/fnsys.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 42.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309, 2012. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011. [Erratum in Cell Metab 23: 745, 2016]. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 121: 2413–2421, 2011. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol 28: 469–478, 2008. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahler P, Guastavino JM, Jacquart G, Strazielle C. An unexpected role of the cerebellum: involvement in nutritional organization. Physiol Behav 54: 1063–1067, 1993. doi: 10.1016/0031-9384(93)90325-a. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza J, Pévet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci 30: 1894–1904, 2010. doi: 10.1523/JNEUROSCI.5855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koros C, Boukouvalas G, Gerozissis K, Kitraki E. Fat diet affects leptin receptor levels in the rat cerebellum. Nutrition 25: 85–87, 2009. doi: 10.1016/j.nut.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 49.Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH. Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol 133: 1–7, 1997. doi: 10.1016/s0303-7207(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 50.El Messari S, Leloup C, Quignon M, Brisorgueil MJ, Penicaud L, Arluison M. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol 399: 492–512, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 51.Li B, Guo CL, Tang J, Zhu JN, Wang JJ. Cerebellar fastigial nuclear inputs and peripheral feeding signals converge on neurons in the dorsomedial hypothalamic nucleus. Neurosignals 17: 132–143, 2009. doi: 10.1159/000197913. [DOI] [PubMed] [Google Scholar]
- 52.Wen YQ, Zhu JN, Zhang YP, Wang JJ. Cerebellar interpositus nuclear inputs impinge on paraventricular neurons of the hypothalamus in rats. Neurosci Lett 370: 25–29, 2004. doi: 10.1016/j.neulet.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 53.Zhu JN, Li HZ, Ding Y, Wang JJ. Cerebellar modulation of feeding-related neurons in rat dorsomedial hypothalamic nucleus. J Neurosci Res 84: 1597–1609, 2006. doi: 10.1002/jnr.21059. [DOI] [PubMed] [Google Scholar]
- 54.Zhu JN, Zhang YP, Song YN, Wang JJ. Cerebellar interpositus nuclear and gastric vagal afferent inputs reach and converge onto glycemia-sensitive neurons of the ventromedial hypothalamic nucleus in rats. Neurosci Res 48: 405–417, 2004. doi: 10.1016/j.neures.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Berman SM, Paz-Filho G, Wong ML, Kohno M, Licinio J, London ED. Effects of leptin deficiency and replacement on cerebellar response to food-related cues. Cerebellum 12: 59–67, 2013. doi: 10.1007/s12311-012-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96: 4569–4574, 1999. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Low AYT, Goldstein N, Gaunt JR, Huang KP, Zainolabidin N, Yip AKK, Carty JRE, Choi JY, Miller AM, Ho HST, Lenherr C, Baltar N, Azim E, Sessions OM, Ch'ng TH, Bruce AS, Martin LE, Halko MA, Brady RO Jr, Holsen LM, Alhadeff AL, Chen AI, Betley JN. Reverse-translational identification of a cerebellar satiation network. Nature 600: 269–273, 2021. doi: 10.1038/s41586-021-04143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 402: 113–119, 2015. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6: 14, 2015. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauvais-Jarvis F, Arnold AP, Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab 25: 1216–1230, 2017. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]