Keywords: aging, exercise, middle cerebral artery blood velocity, pulsatility, V̇o2max
Abstract
There is a positive association between cardiorespiratory fitness and cognitive health, but the interaction between cardiorespiratory fitness and aging on cerebral hemodynamics is unclear. These potential interactions are further influenced by sex differences. The purpose of this study was to determine the sex-specific relationships between cardiorespiratory fitness, age, and cerebral hemodynamics in humans. Measurements of unilateral middle cerebral artery blood velocity (MCAv) and cerebral pulsatility index obtained using transcranial Doppler ultrasound and cardiorespiratory fitness [maximal oxygen consumption (V̇o2max)] obtained from maximal incremental exercise tests were retrieved from study records at three institutions. A total of 153 healthy participants were included in the analysis (age = 42 ± 20 yr, range = 18–83 yr). There was no association between V̇o2max and MCAv in all participants (P = 0.20). The association between V̇o2max and MCAv was positive in women, but no longer significant after age adjustment (univariate: P = 0.01; age-adjusted: P = 0.45). In addition, there was no association between V̇o2max and MCAv in men (univariate: P = 0.25, age-adjusted: P = 0.57). For V̇o2max and cerebral pulsatility index, there were significant negative associations in all participants (P < 0.001), in men (P < 0.001) and women (P < 0.001). This association remained significant when adjusting for age in women only (P = 0.03). In summary, higher cardiorespiratory fitness was associated with a lower cerebral pulsatility index in all participants, and the significance remained only in women when adjusting for age. Future studies are needed to determine the sex-specific impact of cardiorespiratory fitness improvements on cerebrovascular health.
NEW & NOTEWORTHY We present data pooled from three institutions to study the impact of age, sex, and cardiorespiratory fitness on cerebral hemodynamics. Cardiorespiratory fitness was positively associated with middle cerebral artery blood velocity in women, but not in men. Furthermore, cardiorespiratory fitness was inversely associated with cerebral pulsatility index in both men and women, which remained significant in women when adjusting for age. These data suggest a sex-specific impact of cardiorespiratory fitness on resting cerebral hemodynamics.
INTRODUCTION
Cerebral hemodynamics change with advancing age (1–5) and these alterations are important to identify, as they may be related to the pathogenesis of cerebrovascular diseases and dementia (6–11). For example, cerebral blood flow and cerebral blood velocity in the large intracranial arteries generally decrease over the lifespan (1, 12–15), whereas cerebral pulsatility index increases with advancing age (1, 16, 17). High cerebral pulsatility index may be reflective of distal vascular resistance in the brain blood vessels or related to a lack of buffering due to increased large central arterial stiffening (18). Importantly, women have sex-specific risks, etiologies and outcomes of age-related cerebrovascular diseases (19), and the trajectory of age-related changes in cerebral hemodynamics may be sex-specific. We recently reported that the age-related decline in middle cerebral artery blood velocity (MCAv) was greater in women than in men, with the greatest sex-related difference during the 6th decade of life (1).
In addition to sex-specific differences, cardiorespiratory fitness may also impact the trajectory of age-related changes in cerebral hemodynamics. Habitual exercise is beneficial for cognitive health (20), and one of the proposed mechanisms by which habitual exercise may support cognitive health is through improvements in cerebral hemodynamics (21–23). Multiple studies have shown a positive correlation between resting MCAv and maximal oxygen consumption (V̇o2max; 12, 13, 24); however, these findings regarding the effect of cardiorespiratory fitness on cerebral blood flow or cerebral blood velocity have been inconsistent. For example, master’s athletes demonstrated elevated cerebral blood flow only in certain brain areas compared with age-matched sedentary older adults (25). In addition, a recent meta-analysis found that there was no significant effect of higher cardiorespiratory fitness on MCAv, except when the lowest fit and the highest fit groups were compared (26). Importantly, many of the studies regarding the relationship between cardiorespiratory fitness and cerebral hemodynamics do not consider sex or do not include women. Therefore, the purpose of this study was to determine the sex-specific relationships between cardiorespiratory fitness and cerebral hemodynamics in aging humans. We accomplished this by leveraging a large data set of healthy men and women between the ages of 18–83 yr. We hypothesized that there would be a positive association between cardiorespiratory fitness and MCAv and an inverse association between cardiorespiratory fitness and cerebral pulsatility index. In addition, based on our recent report demonstrating the age-related trajectory of MCAv is sex-specific (1), we hypothesized that relationships between aging, cardiorespiratory fitness, and cerebral hemodynamics would also be sex-specific.
MATERIALS AND METHODS
Participants
A data records search from three institutions (University of Kansas Medical Center, Kansas City, Kansas; University of Wisconsin-Madison, Madison, Wisconsin; and Université Laval, Quebec City, Canada) was completed to identify eligible participant data for this study. Aspects of these data have been previously published (1), however, the current manuscript represents an independent analysis on a sub-group of participants. In brief, participants were eligible if they were between the ages of 18–90 yr, were considered healthy based on the cardiovascular disease risk classification from the American College of Sports Medicine (ACSM) criteria (27), and if they did not have a history or evidence of any major cardiovascular, pulmonary, metabolic, or neurological diseases, and had completed both an assessment of resting cerebral hemodynamics and a maximal incremental exercise test to determine cardiorespiratory fitness. Premenopausal females were assessed during days 1–10 of their menstrual cycle or during the nonactive phase if taking oral contraceptives. Detailed inclusion and exclusion criteria have been published elsewhere (28–32). All experimental data collection was approved by the institutional review boards of the respective institutions (University of Kansas Medical Center, University of Wisconsin-Madison, and Université Laval) including written, informed consent obtained from each participant. The data record search was conducted according to data use agreements between the institutions.
Study Procedures
Data retrieved from study records included demographic information [age at study procedures, sex, height, body weight, body mass index (BMI), and cardiovascular risk factors], resting cerebral and cardiovascular hemodynamics [mean arterial pressure (MAP), end-tidal carbon dioxide (ETCO2), unilateral MCAv, and cerebral pulsatility index using the MCA], and cardiorespiratory fitness [maximal oxygen uptake (V̇o2max)]. Study procedures at each institution (University of Kansas Medical Center, University of Wisconsin-Madison, and Université Laval) took place in a quiet and temperature-controlled room (22°C–24°C). Participants arrived at the laboratories after abstention from caffeine for >6 h, exercising for >12 h, and fasting for >4 h before experimental study procedures.
Cerebral Hemodynamics
Cerebral hemodynamic assessments occurred either in a seated or supine position. After a rest period of at least 10 min, cardiovascular and cerebral hemodynamics were recorded for at least 15 s to 90 s. Breath-by-breath ETCO2 was measured using a nasal cannula or a mouthpiece attached to a gas analyzer (BCI Capnocheck 9004, Smiths Medical, Minneapolis, MN; Datex Ohmeda, GE Healthcare, Fairfield, CT; Breezesuite, MedGraphics Corp., Saint Paul, MN). Beat-by-beat MAP was measured using a finger plethysmograph (Finometer Pro/Finometer Nova, Finapres Medical Systems, Amsterdam, Netherlands; Nexfin, Edwards Lifesciences, Irvine, CA; Nexfin, Edwards Lifesciences, Ontario, Canada). Unilateral MCAv was measured using a 2-MHz transcranial Doppler ultrasound probe placed on the left or right transtemporal window and secured with a headband (RobotoC2MD System, Multigon Industries, Yonkers, NY; Neurovision System, Multigon Industries, Yonkers, NY; Spencer Technologies, Redmond, WA; and Doppler Box, Compumedics DWL USA, Inc., San Juan Capistrano, CA).
Cardiorespiratory Fitness
V̇o2max was assessed using an incremental maximal exercise test to exhaustion on either a treadmill or cycle ergometer. During the exercise test, ventilation and gas exchange variables were measured utilizing a metabolic cart (Parvo Medics, Sandy, UT; Breezesuite, MedGraphics Corp., Saint Paul, MN). Standard criteria were used to determine maximal aerobic capacity, including failure to increase oxygen consumption with an increasing workload, a respiratory exchange ratio (RER) of ≥1.10, and a maximum heart rate (HR) value within 10 beats/min of the age-predicted maximum (27) as previously described (28–32). A V̇o2 epoch of 30 s was used as the maximum value, and these criteria was consistent across institutions. All participants met the criteria for an acceptable V̇o2max test.
Data and Statistical Analysis
Data were sampled at 250–1,000 Hz and analyzed offline (National Instruments, Austin, TX; WinDaq DATAQ Instruments, Akron, OH; and AD Instruments, Colorado Springs, CO). Mean MCAv, MAP, and ETCO2 were averaged over the duration of the recording for each participant. ETCO2 data were not collected from 26 participants from one study site. Cerebral pulsatility index was calculated as the difference between systolic and diastolic MCAv divided by mean MCAv [(systolic MCAv − diastolic MCAv)/mean MCAv]. V̇o2max is reported as absolute and relative to body mass.
Statistical analysis was performed in R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria) and SigmaPlot for Windows version 13.0 (Systat Software, San Jose, CA). Participant demographics were compared between men and women using a two-tailed, unpaired students t test. To test the associations between cardiorespiratory fitness and cerebral hemodynamics, multivariate linear models were built. In all cases, cerebral hemodynamics (mean MCAv and cerebral pulsatility index) were considered the dependent variables. In the models, cardiorespiratory fitness was considered V̇o2max relative to body mass. Univariate modeling was used to assess the relationship between cardiorespiratory fitness and cerebral hemodynamics (mean MCAv and cerebral pulsatility index) in all subjects, men only and women only. Age was added to the models to assess the associations while controlling for age. In addition, a model that included age, sex, V̇o2max, and the three-way interaction of age, sex, and V̇o2max, as well as any two-way interactions between the three variables of interest was also built. The interaction models were reduced until significance was found, ensuring to keep age and sex at a minimum. Reduction occurred when variables were not found to be significant. The three-way interaction was first to be removed, followed by any two-way interactions before looking at the main effect. Models were also built to determine the potential influence of exercise modality for the maximal exercise test and body posture for the cerebral hemodynamic assessment. Although body mass is taken into account for relative V̇o2max, BMI may have an influence on our other outcome variables so BMI was assessed as a potential covariate. Similarly, MAP was also assessed as a covariate. Statistical significance was set a priori at P < 0.05.
RESULTS
Participant Demographics
Participant demographics are displayed in Table 1. There were 153 unique data sets included in the analysis. The number of participants in each age group are shown in Table 1. There were 37 premenopausal women, 0 perimenopausal women, and 37 postmenopausal women (absence of menstrual cycle for at least 1 yr). Of the premenopausal women, 16 were taking oral contraceptives. The majority of the participants did their V̇o2max test on a cycle ergometer (men = 90% and women = 82%) compared with a treadmill. The majority of participants did their cerebral hemodynamic assessment in the supine position (men = 57% and women = 68%) compared with a seated position. On average, the women in the study were older than the men. Men were taller, weighed more, and had a greater BMI compared with women. Men also had a higher V̇o2max compared with women. Table 2 displays cerebral hemodynamics. Women had a greater mean MCAv compared with men. There were no sex differences in the cerebral pulsatility index.
Table 1.
Participant demographics
| Variables | All Subjects (n = 153) | Men (n = 79) | Women (n = 74) | P Value Men vs. Women |
|---|---|---|---|---|
| Age, yr | 42 ± 20 | 38 ± 19 | 46 ± 21 | 0.02 |
| 18–30 | n = 70 | n = 41 | n = 29 | |
| 31–40 | n = 24 | n = 16 | n = 8 | |
| 41–50 | n = 0 | n = 0 | n = 0 | |
| 51–60 | n = 12 | n = 3 | n = 9 | |
| 61–70 | n = 27 | n = 11 | n = 16 | |
| 71–80 | n = 18 | n = 7 | n = 11 | |
| 81–90 | n = 2 | n = 1 | n = 1 | |
| Height, cm | 171 ± 9 | 177 ± 11 | 165 ± 6 | <0.001 |
| Body weight, kg | 70 ± 12 | 78 ± 11 | 63 ± 8 | <0.001 |
| Body mass index, kg/m2 | 24 ± 3 | 25 ± 3 | 23 ± 2 | <0.001 |
| Absolute V̇o2max, L/min | 2.7 ± 1.0 | 3.3 ± 0.9 | 2.1 ± 0.6 | <0.001 |
| Relative V̇o2max, mL/kg/min | 38 ± 12 | 43 ± 13 | 34 ± 10 | <0.001 |
Participant demographic data are means ± SD or participant count for each age range. Cardiorespiratory fitness (V̇o2max) was measured during a maximal incremental exercise test to exhaustion. V̇o2max, maximal oxygen consumption.
Table 2.
Cerebral hemodynamics
| Variables | All Subjects (n = 153) | Men (n = 79) | Women (n = 74) | P Value Men vs. Women |
|---|---|---|---|---|
| End-tidal carbon dioxide (n = 127) | 39 ± 6 | 39 ± 6 | 39 ± 6 | 0.66 |
| Mean arterial pressure, mmHg | 89 ± 11 | 90 ± 11 | 88 ± 10 | 0.18 |
| MCAv, cm/s | 61 ± 15 | 57 ± 13 | 64 ± 16 | <0.01 |
| Cerebral pulsatility index | 0.77 ± 0.20 | 0.79 ± 0.21 | 0.75 ± 0.18 | 0.30 |
Cerebral hemodynamic data are means ± SD. Cerebral hemodynamic variables, end-tidal carbon dioxide, and mean arterial pressure were measured at rest. Cerebral pulsatility index was measured in the middle cerebral artery. MCAv, middle cerebral artery blood velocity.
Associations between Cerebral Hemodynamics and Cardiorespiratory Fitness
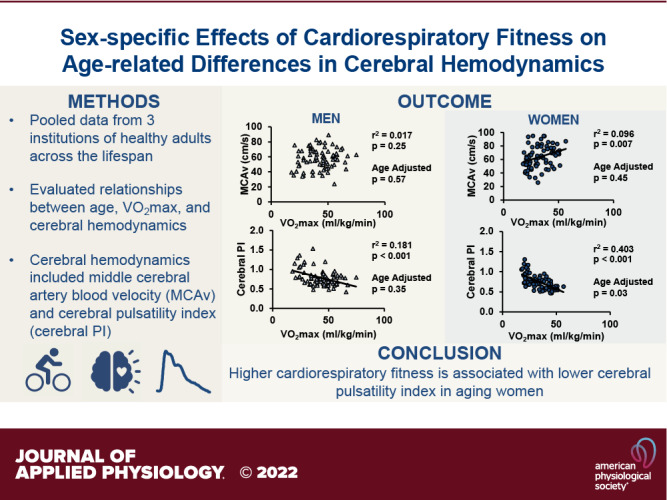
There were no effects of V̇o2max mode or body position on cardiorespiratory fitness, MCAv, or cerebral pulsatility index (P > 0.05). Figure 1 shows the univariate associations between cardiorespiratory fitness and mean MCAv. There were no significant associations between V̇o2max and mean MCAv in all participants (intercept: β = 55.62, SE = 4.03, P < 0.001; V̇o2max: β = 0.13, SE = 0.01, P = 0.20) and in men only (intercept: β = 51.24, SE = 5.43, P < 0.001; V̇o2max: β = 0.14, SE = 0.12, P = 0.25). However, there was a significant, positive association between V̇o2max and MCAv in women only (intercept: β = 47.47, SE = 6.30, P < 0.001; V̇o2max: β = 0.50, SE = 0.18, P = 0.01). Table 3 shows the associations between V̇o2max and mean MCAv adjusted for age. There was a negative association between V̇o2max and MCAv in all participants after adjusting for age. However, there were no significant age-adjusted associations in men only or in women only. BMI and MAP were not significant contributors to any of the models that had MCAv as an outcome.
Figure 1.

This figure shows the association between middle cerebral artery blood velocity (MCAv) and cardiorespiratory fitness (V̇o2max) in all participants (n = 153, men = 79, women = 74; A), men only (n = 79; B) and women only (n = 74; C). Men are shown in triangles and women are shown in circles. The coefficient of determination and P values of the univariate linear models are reported above each graph. A solid line indicates a significant association. The data are not adjusted for age. Age adjusted data are in Table 3. V̇o2max, maximal oxygen consumption.
Table 3.
Multiple linear regression model including age, V̇o2max, and MCAv
| Variables | Estimate | Standard Error | P Value | |
|---|---|---|---|---|
| All Participants | Intercept | 87.00 | 7.32 | <0.001 |
| V̇o2max | −0.279 | 0.124 | 0.03 | |
| Age | −0.373 | 0.075 | <0.001 | |
| Men | Intercept | 70.15 | 9.129 | <0.001 |
| V̇o2max | −0.084 | 0.147 | 0.57 | |
| Age | −0.241 | 0.095 | 0.01 | |
| Women | Intercept | 90.93 | 12.48 | <0.001 |
| V̇o2max | −0.180 | 0.238 | 0.45 | |
| Age | −0.452 | 0.115 | <0.001 |
Multiple linear regression model of mean middle cerebral artery blood velocity (MCAv) as the dependent variable with age and V̇o2max as the independent variables in all participants (n = 153), men only (n = 79) and women only (n = 74). V̇o2max, maximal oxygen consumption.
Figure 2 shows the univariate associations between cardiorespiratory fitness and cerebral pulsatility index. There were significant negative associations between V̇o2max and cerebral pulsatility index in all participants (intercept: β = 1.040, SE = 0.047, P < 0.001; V̇o2max: β = −0.007, SE = 0.001, P < 0.001), in men only (intercept: β = 1.097, SE = 0.078, P < 0.001; V̇o2max: β = −0.007, SE = 0.002, P < 0.001), and in women only (intercept: β = 1.136, SE = 0.057, P < 0.001; V̇o2max: β = −0.011, SE = 0.002, P < 0.001). Table 4 shows the associations between V̇o2max and cerebral pulsatility index adjusted for age. There were no significant age-adjusted associations in all participants or in men only. There were also no significant age-adjusted associations in men when including BMI (intercept: β = 1.091, SE = 0.254, P < 0.001; V̇o2max: β = −0.004, SE = 0.002, P = 0.083; age: β = 0.006, SE = 0.001, P < 0.001; BMI: β = −0.011, SE = 0.007, P = 0.040) or MAP (intercept: β = 1.104, SE = 0.190, P < 0.001; V̇o2max: β = −0.002, SE = 0.002, P = 0.398; age: β = 0.006, SE = 0.001, P < 0.001; MAP: β = −0.005, SE = 0.007, P = 0.003) as a covariate. However, there was a significant age-adjusted inverse association between V̇o2max and cerebral pulsatility index in women only. In women, BMI and MAP were not significant contributors to the model with cerebral pulsatility index as the outcome.
Figure 2.

This figure shows the association between middle cerebral artery (MCA) pulsatility index and cardiorespiratory fitness (V̇o2max) in all participants (n = 153, men = 79, women = 74; A), men only (n = 79; B) and women only (n = 74; C). Men are shown in triangles and women are shown in circles. The coefficient of determination and P values of the univariate linear models are reported above each graph. A solid line indicates a significant association. The data are not adjusted for age. Age adjusted data are in Table 4. V̇o2max, maximal oxygen consumption.
Table 4.
Multiple linear regression model including Age, V̇o2max, and cerebral pulsatility index
| Variables | Estimate | Standard Error | P Value | |
|---|---|---|---|---|
| All Participants | Intercept | 0.576 | 0.082 | <0.001 |
| V̇o2max | −0.001 | 0.001 | 0.49 | |
| Age | 0.006 | 0.001 | <0.001 | |
| Men | Intercept | 0.643 | 0.121 | <0.001 |
| V̇o2max | −0.002 | 0.002 | 0.35 | |
| Age | 0.006 | 0.001 | <0.001 | |
| Women | Intercept | 0.721 | 0.112 | <0.001 |
| V̇o2max | −0.005 | 0.002 | 0.03 | |
| Age | 0.004 | 0.001 | <0.001 |
Multiple linear regression models of cerebral pulsatility index as the dependent variable with age and V̇o2max as the independent variables in all participants (n = 153), men only (n = 79) and women only (n = 74). Cerebral pulsatility index was measured in the middle cerebral artery. V̇o2max, maximal oxygen consumption.
The interaction effects of age, sex, and V̇o2max on MCAv and cerebral pulsatility index were also evaluated. There were no significant two-way interaction effects (age and V̇o2max, V̇o2max and sex, or age and sex) or three-way interaction effects (age, sex, and V̇o2max) on mean MCAv (data not shown). In addition, there were no significant two-way interaction effects (age and V̇o2max, V̇o2max and sex, or age and sex) or three-way interaction effects (age, sex, and V̇o2max) on cerebral pulsatility index (data not shown).
DISCUSSION
Our purpose was to determine the sex-specific relationships between age, cardiorespiratory fitness, and cerebral hemodynamics. This study leveraged data from three institutions that included over 150 men and women across the adult lifespan who underwent maximal exercise testing and resting cerebral hemodynamic assessment using transcranial Doppler ultrasound. We observed a positive association between MCAv and cardiorespiratory fitness in women; however, this association was no longer significant when we adjusted for age. We did not observe any associations between MCAv and cardiorespiratory fitness in men. In addition, in support of our hypothesis, there was an inverse association between cardiorespiratory fitness and cerebral pulsatility index in men and women, and this association remained significant when adjusting for age in women. Taken together, these findings suggest that the influence of cardiorespiratory fitness on resting cerebral hemodynamics across the adult lifespan is sex specific. Future studies should include more women in the perimenopausal age range. Our study highlights the complexity and importance of consideration of the sexes when evaluating the impact of cardiorespiratory fitness on cerebrovascular health.
Our previous work demonstrated that the age-related decline in MCAv and the age-related rise in cerebral pulsatility index are greater in women compared with men (1). The results from the present study suggest that, in women, higher cardiorespiratory fitness may protect against the deleterious effects of aging on resting cerebral hemodynamics. This is particularly important during the 6th decade of life, when the rate of decline in MCAv is significantly greater in women compared with men (1). Our work suggests that the cerebral pulsatility index in women may be an aspect of cerebral hemodynamics that is particularly sensitive to the impact of cardiorespiratory fitness. It is possible that habitual aerobic exercise reduces the arterial stiffness in the large central and extracranial arteries, thereby lessening the translation of pulsatility into the brain and lowering the cerebral pulsatility index (18, 33). In fact, numerous studies have noted that age-related changes in central arterial stiffness are attenuated in aerobic exercise-trained individuals and associated with cardiorespiratory fitness (34–36). It is also possible that the increase in shear stress from high cardiac outputs obtained during exercise could reduce cerebral vascular resistance, reflected as a lower cerebral pulsatility index in the large intracranial arteries. Therefore, reducing cerebral pulsatility index may be a mechanism by which cardiorespiratory fitness, or habitual aerobic exercise, benefits cerebrovascular health in women. This may not be the case with all exercise modes, as resistance training may increase arterial stiffening (37, 38); however, the effects of resistance exercise on arterial stiffness have primarily been studied only in men.
Despite reports that women have an accelerated rate of change in cerebral hemodynamics with aging (1, 14, 15), there have been very few studies evaluating the impact of cardiorespiratory fitness on cerebral hemodynamics in women. Some studies have shown a positive influence of cardiorespiratory fitness or habitual exercise on cerebral hemodynamics in women, whereas others have shown no effect. For example, V̇o2max was a predictor of cerebrovascular conductance in a cross-sectional study of women aged 50–90 yr (39). Also, 8 wk of aerobic exercise (40) and 12 wk of cycling exercise (41) in postmenopausal women increased resting MCAv. In addition, in young women aged 19–26 yr, those who performed 50 min of aerobic exercise 3–5 days/wk for at least a year had lower ophthalmic artery pulsatility index compared with sedentary women (42). However, in a cross-sectional study of premenopausal women aged 18–35 yr, there was no relationship between cardiorespiratory fitness and MCAv, whereas there was a positive relationship between cardiorespiratory fitness and the MCAv response to CO2 (cerebrovascular reactivity; 43). Furthermore, dynamic cerebral autoregulation was attenuated in premenopausal women who were moderately fit compared with sedentary women, suggesting that cardiorespiratory fitness may not have a beneficial impact on dynamic cerebral autoregulation in young women (30). Taken together, these findings suggest that the effect of cardiorespiratory fitness on cerebral hemodynamics in women is complex and depends on the age of the women included in the study, as well as the cerebral hemodynamic variables being measured. The results from the present study add to the existing literature by including women at different cardiorespiratory fitness levels from across the lifespan as well as including multiple cerebral hemodynamic variables.
In contrast with our hypothesis, we did not observe a positive association between cardiorespiratory fitness and MCAv in men. This finding is dissimilar with previous work in young men (13, 43), older men (13), and in men across the lifespan (12). The reason for our finding of a lack of relationship between cardiorespiratory fitness and MCAv in men is unclear. It is possible that we were unable to see a beneficial impact of cardiorespiratory fitness on cerebrovascular health in healthy men because we only included cerebral hemodynamic variables at rest. Stressing the cerebrovascular system by including measurements of cerebrovascular reactivity to CO2, dynamic cerebral autoregulation, or the cerebral blood velocity response to acute aerobic exercise may have elicited different results (44). This was also highlighted in a recent editorial regarding our recent publication showing sex-specific differences in cerebral hemodynamics across the lifespan (45). Another consideration is that in the study by Ainslie et al. (12), half of the participants were endurance exercise-trained in that they performed vigorous aerobic endurance exercise more than 4 times/wk and were competing in local road running or cycling races. In the present study, the participants were healthy adults without underlying disease and had a range of cardiorespiratory fitness levels, suggesting not all of them were recreational athletes. This might suggest that in healthy adults with a range of cardiorespiratory fitness levels, the impact of cardiorespiratory fitness on the cerebral hemodynamic profile may be more apparent on the cerebral pulsatility index rather than resting cerebral blood velocity. It is also possible that we have observed a greater impact of cardiorespiratory fitness on cerebral hemodynamics in women because we had a greater number of postmenopausal women compared with age-matched men in our study, representing a greater portion of women with advanced vascular age. Future studies with larger sample sizes could evaluate the effect of cardiorespiratory fitness on cerebral hemodynamics in men and women using small age ranges, for example by each decade. These findings highlight the complexities in the relationships between aging, cardiorespiratory fitness, and cerebral hemodynamics.
There are a few limitations of this study to consider. We assessed aging in a cross-sectional manner by leveraging data from three institutions. A longitudinal study evaluating this number of adults across the lifespan would be ideal to address our research question but has high cost and low feasibility. Cerebral hemodynamics were evaluated at rest using transcranial Doppler ultrasound. We are unable to determine the vessel size using this monitoring modality. MCAv can be reflective of cerebral blood flow as long as the diameter of the MCA is constant. There is no evidence of changes in MCA diameter at rest, although it has been reported that MCA diameter and cross-sectional area change in response to perturbations in arterial CO2, especially in young adults (46–48). The MCA was assessed unilaterally, therefore, there are potential global or regional flow differences that were not captured. In addition, measuring a perturbation to cerebral hemodynamics such as cerebrovascular reactivity to CO2, dynamic cerebral autoregulation, or the cerebrovascular response to exercise, along with other cerebral hemodynamic variables related to blood pressure such as cerebrovascular conductance index or cerebrovascular resistance index, may provide additional information regarding the impact of cardiorespiratory fitness on cerebral hemodynamics. Furthermore, because MAP was a significant contributor to the model in men for cerebral pulsatility index, future studies can further assess the impact of MAP on cerebral pulsatility index. By leveraging data from multiple institutions, information was obtained from participants with a wide range of ages (18–83 yr) and cardiorespiratory fitness levels. However, there is a critical age gap in our data in the 5th decade of life, which typically corresponds with perimenopause in women. Many studies from our pooled data set had inclusion criteria that women must either be premenopausal or postmenopausal for at least 1 yr. Studies moving forward should characterize cerebral hemodynamics during the perimenopause timeframe in women and age-matched men. Although data were obtained at multiple institutions assessing cardiorespiratory fitness using two different exercise modes (cycle ergometer or treadmill) and collection of cerebral hemodynamics in two different body postures (seated or supine), there were no effects of exercise testing mode or body position on MCAv or cerebral pulsatility index (P > 0.05). Furthermore, we were unable to capture physical activity and exercise participation in addition to cardiorespiratory fitness. Some participants were taking medications including oral contraceptives (n = 16), statins (n = 11), and thyroid medications (n = 7). The impact of these medications on cerebral hemodynamics is unclear at this time.
In summary, in a large, pooled data set of over 150 participants, higher cardiorespiratory fitness was associated with higher MCAv and lower cerebral pulsatility index in women. The relationship between cardiorespiratory fitness and cerebral pulsatility index in women remained significant after adjusting for age. We did not observe an association between MCAv and cardiorespiratory fitness in men. These results suggest that cerebral pulsatility index may be an aspect of cerebral hemodynamics that is particularly sensitive to the impact of cardiorespiratory fitness in women. Our findings also highlight the complexity and importance of considering aging and the sexes when evaluating the impact of cardiorespiratory fitness on cerebrovascular health. As the incidence and outcomes of cerebrovascular diseases are sex-specific, future studies can determine the sex-specific impacts of improvements in cardiorespiratory fitness and cerebral hemodynamics on cerebrovascular health. In addition, future studies should aim to also include more women in the perimenopausal age range.
GRANTS
This investigation was supported by the Hilldale Undergraduate Research Fellowship from the University of Wisconsin-Madison (to N.P.Z.) and the National Institutes of Health Ruth L. Kirschstein National Research Service Award T32’s from the National Heart Lung and Blood Institute to the University of Wisconsin–Madison Cardiovascular Research Center (HL007936 to K.B.M.). S. E. Aaron was supported by NCATS awarded to the University of Kansas Clinical and Translational Science Institute (#TL1TR002368). P. Brassard’s contributing studies were supported by the Ministère de l’Éducation, du Loisir et du Sport du Québec and the foundation of the Institut Universitaire de Cardiologie et de Pneumologie de Québec. S. A. Billinger’s contributing studies were supported in part by the American Heart Association Grant 16GRNT30450008, the NIH K01-HD-067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Wohlgemuth Faculty Scholar Award. Additional support was provided by the University of Kansas Alzheimer’s Disease Center (P30 AG-035982, P30 AG-072973), the Institutional Clinical and Translational Science Award, NIH/NCATS Grant No. UL1TR000001, NIH NIA R01 AG-043962, and the Landon Center on Aging endowments. J. N. Barnes’s contributing studies were supported in part by the NIH Grant HL118154 and the Wisconsin Alumni Research Foundation. Additional support was provided by The Leo and Anne Albert Charitable Trust.
DISCLAIMERS
The contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Patrice Brassard is an editor of Journal of Applied Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
P.B., S.A.B., and J.N.B. conceived and designed research; N.P.Z., K.B.M., A.J.H., L.L., S.E.A., P.B., S.A.B., and J.N.B. performed experiments; N.P.Z., K.B.M., R.D.Z., and J.N.B. analyzed data; N.P.Z., K.B.M., R.D.Z., P.B., S.A.B., and J.N.B. interpreted results of experiments; N.P.Z. and K.B.M. prepared figures; N.P.Z., K.B.M., and J.N.B. drafted manuscript; N.P.Z., K.B.M., R.D.Z., A.J.H., P.B., S.A.B., and J.N.B. edited and revised manuscript; N.P.Z., K.B.M., R.D.Z., A.J.H., L.L., S.E.A., P.B., S.A.B., and J.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the study participants for their time and efforts.
REFERENCES
- 1.Alwatban MR, Aaron SE, Kaufman CS, Barnes JN, Brassard P, Ward JL, Miller KB, Howery AJ, Labrecque L, Billinger SA. Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. J Appl Physiol (1985) 130: 1675–1683, 2021. doi: 10.1152/japplphysiol.00926.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 209: 667–674, 1998. doi: 10.1148/radiology.209.3.9844657. [DOI] [PubMed] [Google Scholar]
- 3.Demirkaya S, Uluc K, Bek S, Vural O. Normal blood flow velocities of basal cerebral arteries decrease with advancing age: a transcranial Doppler sonography study. Tohoku J Exp Med 214: 145–149, 2008. doi: 10.1620/tjem.214.145. [DOI] [PubMed] [Google Scholar]
- 4.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure, and normal values. J Clin Invest 27: 476–483, 1948. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Jones T, Healy MJR, Gibbs JM, Wise RJS, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RSJ. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 15: 934–943, 2016. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beishon L, Haunton VJ, Panerai RB, Robinson TG. Cerebral hemodynamics in mild cognitive impairment: a systematic review. J Alzheimers Dis 59: 369–385, 2017. doi: 10.3233/JAD-170181. [DOI] [PubMed] [Google Scholar]
- 8.Breteler MMB, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, Hofman A. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 44: 1246–1252, 1994. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 9.Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18: 419–434, 2017. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roher AE, Garami Z, Tyas SL, Maarouf CL, Kokjohn TA, Belohlavek M, Vedders LJ, Connor D, Sabbagh MN, Beach TG, Emmerling MR. Transcranial Doppler ultrasound blood flow velocity and pulsatility index as systemic indicators for Alzheimer’s disease. Alzheimers Dement 7: 445–455, 2011. doi: 10.1016/j.jalz.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Piero VD, Lenzi GL. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial doppler study. Eur Neurol 58: 84–89, 2007. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 12.Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJA, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586: 4005–4010, 2008. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 14.Bakker SLM, de Leeuw F-E, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral haemodynamics in the elderly: the rotterdam study. Neuroepidemiology 23: 178–184, 2004. doi: 10.1159/000078503. [DOI] [PubMed] [Google Scholar]
- 15.Tegeler CH, Crutchfield K, Katsnelson M, Kim J, Tang R, Passmore Griffin L, Rundek T, Evans G. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging 23: 466–472, 2013. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 16.Lefferts WK, DeBlois JP, Augustine JA, Keller AP, Heffernan KS. Age, sex, and the vascular contributors to cerebral pulsatility and pulsatile damping. J Appl Physiol (1985) 129: 1092–1101, 2020. doi: 10.1152/japplphysiol.00500.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Carlberg B, Eklund A, Malm J. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab 36: 1519–1527, 2016. doi: 10.1177/0271678X16629486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fico BG, Miller KB, Rivera-Rivera LA, Corkery AT, Pearson AG, Eisenmann NA, Howery AJ, Rowley HA, Johnson KM, Johnson SC, Wieben O, Barnes JN. The impact of aging on the association between aortic stiffness and cerebral pulsatility index. Front Cardiovasc Med 9: 821151, 2022. doi: 10.3389/fcvm.2022.821151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, Joyner MJ, Shuster LT, Rocca WA. Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ 4: 6, 2013. doi: 10.1186/2042-6410-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE; For 2018 Physical Activity Guidelines Advisory Committee. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 51: 1242–1251, 2019. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes JN, Pearson AG, Corkery AT, Eisenmann NA, Miller KB. Exercise, arterial stiffness, and cerebral vascular function: potential impact on brain health. J Int Neuropsychol Soc 27: 761–775, 2021. doi: 10.1017/S1355617721000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furby HV, Warnert EA, Marley CJ, Bailey DM, Wise RG. Cardiorespiratory fitness is associated with increased middle cerebral arterial compliance and decreased cerebral blood flow in young healthy adults: a pulsed ASL MRI study. J Cereb Blood Flow Metab 40: 1879–1889, 2020. doi: 10.1177/0271678X19865449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarumi T, Ayaz Khan M, Liu J, Tseng BY, Tseng BM, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. [Erratum in J Cereb Blood Flow Metab 34: 1255, 2014]. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes JN, Taylor JL, Kluck BN, Johnson CP, Joyner MJ. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J Appl Physiol (1985) 114: 1383–1387, 2013. doi: 10.1152/japplphysiol.01258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J Magn Reson Imaging 38: 1177–1183, 2013. doi: 10.1002/jmri.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EC, Pizzey FK, Askew CD, Mielke GI, Ainslie PN, Coombes JS, Bailey TG. Effects of cardiorespiratory fitness and exercise training on cerebrovascular blood flow and reactivity: a systematic review with meta-analyses. Am J Physiol Heart Circ Physiol 321: H59–H76, 2021. doi: 10.1152/ajpheart.00880.2020. [DOI] [PubMed] [Google Scholar]
- 27.Riebe D, Ehrman JK, Liguori G, Magal MAmerican College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription (10th edn.). Philadelphia: Wolters Kluwer, 2018. [Google Scholar]
- 28.Drapeau A, Labrecque L, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Brassard P. Six weeks of high-intensity interval training to exhaustion attenuates dynamic cerebral autoregulation without influencing resting cerebral blood velocity in young fit men. Physiol Rep 7: e14185, 2019. doi: 10.14814/phy2.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Lucas SJE, Bailey DM, Smirl JD, Brassard P. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep 5: e13486, 2017. doi: 10.14814/phy2.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labrecque L, Rahimaly K, Imhoff S, Paquette M, Le Blanc O, Malenfant S, Drapeau A, Smirl JD, Bailey DM, Brassard P. Dynamic cerebral autoregulation is attenuated in young fit women. Physiol Rep 7: e13984, 2019. doi: 10.14814/phy2.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrecque L, Drapeau A, Rahimaly K, Imhoff S, Billaut F, Brassard P. Comparable blood velocity changes in middle and posterior cerebral arteries during and following acute high‐intensity exercise in young fit women. Physiol Rep 8: e14430, 2020. doi: 10.14814/phy2.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller KB, Howery AJ, Harvey RE, Eldridge MW, Barnes JN. Cerebrovascular reactivity and central arterial stiffness in habitually exercising healthy adults. Front Physiol 9: 1096, 2018. doi: 10.3389/fphys.2018.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility – Reykjavik study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998. doi: 10.1161/01.ATV.18.1.127. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000.doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 36.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88: 1456–1462, 1993. doi: 10.1161/01.CIR.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 37.Kawano H, Tanimoto M, Yamamoto K, Sanada K, Gando Y, Tabata I, Higuchi M, Miyachi M. Resistance training in men is associated with increased arterial stiffness and blood pressure but does not adversely affect endothelial function as measured by arterial reactivity to the cold pressor test. Exp Physiol 93: 296–302, 2008. doi: 10.1113/expphysiol.2007.039867. [DOI] [PubMed] [Google Scholar]
- 38.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 110: 2858–2863, 2004. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 39.Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging 31: 2047–2057, 2010. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Akazawa N, Choi Y, Miyaki A, Sugawara J, Ajisaka R, Maeda S. Aerobic exercise training increases cerebral blood flow in postmenopausal women. Artery Res., 6: 124–129, 2012. doi: 10.1016/j.artres.2012.05.003. [DOI] [Google Scholar]
- 41.Bailey TG, Cable NT, Aziz N, Dobson R, Sprung VS, Low DA, Jones H. Exercise training reduces the frequency of menopausal hot flushes by improving thermoregulatory control. Menopause N Y N 23: 708–718, 2016. doi: 10.1097/GME.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 42.Hata K, Hata T, Miyazaki K, Kunishi H, Masuda J. Effect of regular aerobic exercise on cerebrovascular tone in young women. J Ultrasound Med 17: 133–136, 1998. doi: 10.7863/jum.1998.17.2.133. [DOI] [PubMed] [Google Scholar]
- 43.Marley CJ, Brugniaux JV, Davis D, Calverley TA, Owens TS, Stacey BS, Tsukamoto H, Ogoh S, Ainslie PN, Bailey DM. Long-term exercise confers equivalent neuroprotection in females despite lower cardiorespiratory fitness. Neuroscience 427: 58–63, 2020. doi: 10.1016/j.neuroscience.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Brugniaux JV, Marley CJ, Hodson DA, New KJ, Bailey DM. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J Cereb Blood Flow Metab 34: 1873–1876, 2014. doi: 10.1038/jcbfm.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefferts WK, Smith KJ. Let’s talk about sex, let’s talk about pulsatility, let’s talk about all the good things and the bad things of MCAv. J Appl Physiol (1985) 130: 1672–1674, 2021. doi: 10.1152/japplphysiol.00215.2021. [DOI] [PubMed] [Google Scholar]
- 46.Al-Khazraji BK, Shoemaker LN, Gati JS, Szekeres T, Shoemaker JK. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J Cereb Blood Flow Metab 39: 1204–1214, 2019. doi: 10.1177/0271678X18762880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coverdale NS, Badrov MB, Shoemaker JK. Impact of age on cerebrovascular dilation versus reactivity to hypercapnia. J Cereb Blood Flow Metab 37: 344–355, 2017. doi: 10.1177/0271678X15626156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller KB, Howery AJ, Rivera-Rivera LA, Johnson SC, Rowley HA, Wieben O, Barnes JN. Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front Aging Neurosci 11: 281, 2019. doi: 10.3389/fnagi.2019.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]


