Abstract
Abscission is the second stage of cytokinesis. Cep55, a coiled-coil protein, is thought to recruit ESCRTs to the midbody to complete abscission. However, recent studies of Cep55 knockout mice reveal that most cells can complete abscission without Cep55. More work is needed to understand abscission mechanisms in different cell types.
Keywords: Cep55, ESCRT, abscission, cytokinesis, midbody, mouse
Cytokinesis has two stages: furrowing and abscission. Furrow ingression compacts the central spindle microtubules inside the intercellular bridge, forming a midbody with a central bulge and two flanks (Figure 1A). The midbody mediates abscission, the process of narrowing and severing the bridge. Depletion of the coiled-coil protein CEP55 in human cells in vitro caused most cells to fail abscission [1-3]. Further imaging and biochemical data supported a model in which CEP55 is critical for recruiting ESCRT machinery necessary for abscission [4]. However, recent studies on Cep55 knockout mice show that embryos develop, and most cells complete abscission, but brain growth is impaired [5-7]. Here we synthesize these findings and highlight questions about Cep55 and requirements for abscission in diverse cell types.
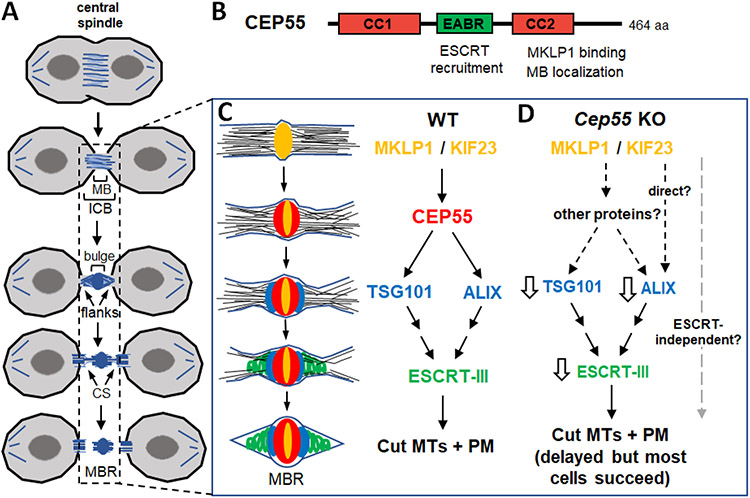
Figure 1. Abscission mechanism with and without the coiled-coil scaffold protein CEP55.
(A) Schematic of cytokinetic abscission. Usually both flanks are severed sequentially, but in some cells only one side may be cut. MB, midbody; ICB, intercellular bridge; CS, constriction site; MBR, midbody remnant.
(B) CEP55 protein domains. CC, coiled coil domains. EABR, ESCRT and ALIX-binding region. The EABR has a single binding site for ESCRT-1/TSG101 or ALIX. The C-terminus binds kinesin MKLP1/KIF23 and is required for localization to the midbody.
(C) Working model of abscission with midbody schematic and molecular pathway for ESCRT cascade in wild type (WT). MKLP1/KIF23 (yellow) crosslinks microtubules at center of midbody, and recruits CEP55 (red). CEP55 then recruits the ESCRT-1 TSG101 (blue) and ALIX (blue) which form rings adjacent to CEP55. TSG101 and ALIX recruit ESCRT-III components (green) in parallel pathways. ESCRT-III forms filaments that gradually narrow the midbody at the constriction sites to mediate abscission. Microtubules must be removed and plasma membrane remodeled. MBR, midbody remnant; MT, microtubules; PM, plasma membrane.
(D) In Cep55 knockout (KO) mouse midbodies, Tsg101, Alix, and ESCRT-III can still be recruited, but are reduced (white arrows), and abscission is delayed but completed in most cells. It is unclear whether ESCRTs can be recruited directly by MKLP1/KIF23, or by other proteins, and/or whether abscission can complete without ESCRTs in some cells.
The working model for abscission and the role of Cep55
The midbody is enriched for hundreds of proteins, many of which mediate or regulate abscission [4, 8]. As the midbody matures, the flanks thin, and constriction sites form on each flank adjacent to the central bulge. Some cell types may cut only one flank, but scission usually occurs sequentially at constriction sites on both sides (Figure 1A) [9]. The midbody remnant is internalized or released extracellularly, and may influence proliferation or stemness [9].
Studies from many labs built a mechanistic model of abscission [4]. It requires disassembly of microtubules as well as plasma membrane remodeling at the constriction sites. These happen concurrently and require ESCRT (endosomal sorting complexes required for transport) machinery, a conserved membrane remodeling system used in diverse cellular processes [4]. Assembly of ESCRT machinery proceeds as a recruitment cascade via site-specific targeting factors. ESCRT-III components assemble into spiral filaments that gradually remodel and constrict the membrane [4].
Evidence primarily from HeLa cells led to the model that CEP55 is the midbodytargeting factor for ESCRTs, and is required for abscission in all vertebrate cells (CEP55 is absent in invertebrates) [1-4] (Figure 1B, C). Although CEP55 was identified as a centrosome protein, reports differ about centrosome localization [1-3, 5], and no function there was found [1, 3]. During late abscission, CEP55 accumulates in a disk at the midbody center by binding KIF23/MKLP1 [3, 4]. CEP55 binds and recruits ALIX and the ESCRT-I TSG101 [2]. ALIX and TSG101 in turn recruit ESCRT-III components through two parallel pathways [2, 4, 10]. Supporting this model, when Cep55 was >90% depleted from HeLa cells, the percentage of midbodies with detectable recruitment of ALIX, TSG101, or ESCRT-III components was greatly reduced, from over 50% to ~2% [2, 10]. The CEP55-depleted cells had high rates of abscission delays, failures, and binucleation. Cells imaged one day after siRNA treatment proceeded normally through mitosis and furrowing, but had hours-long abscission delays in ~55-90% of cells, versus ~5-15% of controls [1, 3]. In cells fixed two days post-transfection, 24% were at midbody stage and 24% were binucleate, versus 3% each of controls [1], and the binucleate fraction increased many-fold over controls after more days in culture [1-3]. Thus, strong depletion of CEP55 in HeLa cells caused specific severe defects in abscission.
Loss of CEP55 in vivo does not preclude embryo development, but hinders brain growth
The HeLa cell results suggested that CEP55 loss in vivo might block tissue growth. In humans, nonsense mutations in CEP55 result in stillbirths with almost no brain, but other organs relatively spared. Milder mutations are viable but cause microcephaly, intellectual disability, and minor skeletal abnormalities [11]. Thus, proper brain development requires full CEP55 function, but other tissues may not. However, it was not determined how these mutations affected CEP55 protein levels or function.
To test the requirement for Cep55 in cell proliferation and development, three independent groups investigated mouse knockouts of Cep55 [5-7]. They all found homozygous knockouts proceed through development, born with only slightly smaller bodies but disproportionately small brains. Pups die within two weeks. Apoptosis occurs specifically in neural tissues and depletes embryonic neural stem cells (NSCs), but gross brain structure is preserved [5, 6]. Kidney maturation is affected, but other organs appear normal [5-7].
Two groups used the knockouts to examine ESCRT recruitment and abscission in midbodies without Cep55 [5, 6] (Figure 1D). Although Cep55 protein was undetectable, 5-8% of knockout fibroblast and NSC midbodies could still recruit ALIX, TSG101, and ESCRT-III components, compared to ~33% of controls [5, 6]. A quarter of cells in Cep55 knockout embryos’ brains are binucleate, but most are mononucleate. Several analyses in fixed knockout brains suggest abscission delay: altered midbody structure, more NSCs in abscission stage, and twice as many midbody remnants [5]. Direct measurement of NSC abscission by live imaging in brain explants shows midbody microtubule disassembly is indeed delayed in Cep55 knockout NSCs, but usually completes [5]. However, conditional knockout of Cep55 (<1% of control protein level) in cultured NSCs quadruples abscission failure [6]. Thus, Cep55 ensures the speed and success of abscission in NSCs, especially in vitro, but is not absolutely essential.
Cep55 appears less critical for cell divisions and tissue growth outside the brain. Knockout pups had normal eye size and almost normal body size at birth [5, 6]. This is not due to maternal rescue because proteins deposited into the egg by the mother only perdure through pre-implantation development. Primary mouse embryonic fibroblasts (MEFs) cultured from Cep55 knockouts show a ~2-fold increase in binucleate cells, less than the ~10-fold increase in brains [5, 6]. Conditional knockout of Cep55 in the adult intestine (a proliferative tissue) produces no detectable defects or binucleate cells [6]. Thus, NSCs, fibroblasts, and intestine cells differ in their requirements for Cep55. In addition, the brain has a lower tolerance for abscission failures, with a tissue-specific p53 response: p53-dependent apoptosis occurs in knockout binucleate NSCs but not fibroblasts, accounting for part of the brain growth defect [5].
Finally, a third group studying Cep55 knockout proposed a new role for Cep55: cilia disassembly [7]. Previously, Cep55 protein had been detected in centrosomes by some investigators, but centrosome functions were not disrupted after thorough knockdown [1-3]. Newborn Cep55 knockout mice have longer cilia on multiciliated cells of the choroid plexus, and longer primary cilia on renal epithelial cells [7]. Cultured primary knockout MEFs have longer cilia than controls after serum starvation, and reduced cilia disassembly after serum stimulation. Analyses in human cell line RPE1 show Cep55 can localize to the ciliary base, and suggest Cep55 stabilizes Aurora A levels during cilium disassembly [7]. This appears to be an interphase rather than mitotic centrosome role. It is not clear how the cilia phenotypes relate to the cytokinesis defects or microcephaly of the Cep55 knockout, since neither the cilia nor abscission of NSCs in the brain were analyzed.
Unanswered questions about abscission failure or success in the absence of Cep55
It is unclear why the phenotypes after Cep55 loss differ greatly by cell type. Abscission was severely disrupted by Cep55 depletion in HeLa cells and dissociated NSCs. But in the gestating mouse embryo, even in the brain, abscission can usually be completed without Cep55, despite significantly reduced ESCRTs in the midbodies. These contrasts reveal the limitations of generalizing an abscission model from cell lines to developing tissues. A variety of factors could contribute to these phenotypic disparities, including physical forces on the midbody, distinct demands on the abscission machinery, and differential abilities to compensate.
The relative success of cell division in Cep55 knockouts versus depleted HeLa cells suggests that abscission might be more robust in vivo than in vitro. Dividing cells experience different external forces in a tissue than on a coverslip. Indeed, a recent study showed soft microenvironments protect cells from abscission failure compared to stiff microenvironments [12].
Some cells may place higher demands on abscission machinery. Tumors and cancerous cell lines like HeLa may depend on high levels of Cep55 and ESCRTs to proliferate rapidly. Developing mammalian brain NSCs must produce billions of neurons within a short time, so one may speculate that evolution of Cep55 in vertebrates helped build bigger brains.
Do some cells compensate for Cep55 loss with a Cep55-independent pathway for midbody ESCRT recruitment? C. elegans and Drosophila lack Cep55, yet still complete ESCRT-mediated abscission. In Drosophila, the MKLP1 homologue Pavarotti directly recruits ALIX [13]. The ALIX binding sequence in Pavarotti is conserved in MKLP1, providing a hypothetical workaround [4]. Possible candidates for alternative pathways include ESCRT-targeting factors for other membrane scission events. In cells that recruit ESCRTs to the midbody without Cep55, is recruitment simply reduced or delayed, or is filament assembly or constriction abnormal? Quantitative high resolution imaging of ESCRT recruitment kinetics and filament structures without Cep55 could answer these questions.
Some cells might compensate for Cep55 loss using ESCRT-independent abscission. There is evidence for this in some cell types. In C. elegans, depletion of TSG-101, which inhibits recruitment of ESCRT-III, causes abscission delay and defective midbody membrane removal, but the midbody is still severed [14]. In mouse tail fibroblasts, > 95% knockdown of CHMP4B did not affect abscission, despite causing 65% failure in cultured NSCs [6]. Perhaps other abscission machinery, like vesicle trafficking or cytoskeleton, might provide a backup, if less efficient, mechanism than ESCRTs. One earlier model proposed abscission occurs through fusion of many vesicles at the midbody [15]. Actin polymers and myosin activity are required at late abscission in C. elegans, but it is unclear if they can mediate abscission or only stabilize the midbody to enable ESCRT function [14]. Many experiments using diverse approaches are needed to refine models for CEP55 function and abscission mechanisms in diverse cell types and tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fabbro M et al. (2005) Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell 9 (4), 477–88. [DOI] [PubMed] [Google Scholar]
- 2.Morita E et al. (2007) Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26 (19), 4215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao WM et al. (2006) Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell 17 (9), 3881–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoten CL and Carlton JG (2018) ESCRT-dependent control of membrane remodelling during cell division. Semin Cell Dev Biol 74, 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little JN, McNeely KM et al. (2021) Loss of Coiled-Coil Protein Cep55 Impairs Neural Stem Cell Abscission and Results in p53-Dependent Apoptosis in Developing Cortex. J Neurosci 41 (15), 3444–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tedeschi A et al. (2020) Cep55 promotes cytokinesis of neural progenitors but is dispensable for most mammalian cell divisions. Nat Commun 11 (1), 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y-C et al. (2021) CEP55 promotes cilia disassembly through stabilizing Aurora A kinase. J Cell Biol 220 (2), e202003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addi C et al. (2020) The Flemmingsome reveals an ESCRT-to-membrane coupling via ALIX/syntenin/syndecan-4 required for completion of cytokinesis. Nat Commun 11 (1), 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeely KC and Dwyer ND (2020) Cytokinesis and post-abscission midbody remnants are regulated during mammalian brain development. Proc Natl Acad Sci U S A 117 (17), 9584–9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christ L et al. (2016) ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J Cell Biol 212 (5), 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrie E et al. (2020) Expanding the spectrum of CEP55-associated disease to viable phenotypes. Am J Med Genet A 182 (5), 1201–1208. [DOI] [PubMed] [Google Scholar]
- 12.Rabie E et al. (2021) Substratum stiffness signals through integrin-linked kinase and β1-integrin to regulate midbody proteins and abscission during EMT. Mol Biol Cell mbcE21020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lie-Jensen A et al. (2019) Centralspindlin Recruits ALIX to the Midbody during Cytokinetic Abscission in Drosophila via a Mechanism Analogous to Virus Budding. Curr Biol 29 (20), 3538–3548 e7. [DOI] [PubMed] [Google Scholar]
- 14.Konig J et al. (2017) Membrane remodeling during embryonic abscission in Caenorhabditis elegans. J Cell Biol 216 (5), 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skop A et al. (2001) Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol 11 (10), 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]