Background:
We evaluated data from all patients in the Netherlands who underwent endovascular treatment for acute ischemic stroke in the past 3.5 years, to identify nationwide trends in time to treatment and procedural success, and assess their effect on clinical outcomes.
Methods:
We included patients with proximal occlusions of the anterior circulation from the second and first cohorts of the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) Registry (March 2014 to June 2016; June 2016 to November 2017, respectively). We compared workflow times and rates of successful reperfusion (defined as an extended Thrombolysis in Cerebral Infarction score of 2B-3) between cohorts and chronological quartiles (all included patients stratified in chronological quartiles of intervention dates to create equally sized groups over the study period). Multivariable ordinal logistic regression was used to assess differences in the primary outcome (ordinal modified Rankin Scale at 90 days).
Results:
Baseline characteristics were similar between cohorts (second cohort n=1692, first cohort n=1488) except for higher age, poorer collaterals, and less signs of early ischemia on computed tomography in the second cohort. Time from stroke onset to groin puncture and reperfusion were shorter in the second cohort (median 185 versus 210 minutes; P<0.001 and 236 versus 270 minutes; P<0.001, respectively). Successful reperfusion was achieved more often in the second than in the first cohort (72% versus 66%; P<0.001). Functional outcome significantly improved (adjusted common odds ratio 1.23 [95% CI, 1.07–1.40]). This effect was attenuated by adjustment for time from onset to reperfusion (adjusted common odds ratio, 1.12 [95% CI, 0.98–1.28]) and successful reperfusion (adjusted common odds ratio, 1.13 [95% CI, 0.99–1.30]). Outcomes were consistent in the analysis per chronological quartile.
Conclusions:
Clinical outcomes after endovascular treatment for acute ischemic stroke in routine clinical practice have improved over the past years, likely resulting from improved workflow times and higher successful reperfusion rates.
Keywords: groin odds, ratio, puncture, registry, reperfusion
Endovascular treatment (EVT) has drastically improved functional outcomes in patients with acute ischemic stroke because of proximal anterior circulation occlusions and is now standard of care.1–3 Treatment outcomes from clinical trials proved to be reproducible in clinical practice.4–8 Since the introduction of EVT as standard of care, efforts have been focused on further improving patient outcomes, with decreasing time to treatment and improving reperfusion grades as major targets. Both are strongly associated with functional outcome: in daily practice, every hour of treatment delay is associated with a reduced chance of functional independence by ≈5%.9,10 Procedural results are expected to improve over time as stroke teams gain more experience, new treatment approaches and materials are tested and implemented,11–13 and attention for interventionist training increases.14 However, no longitudinal studies have reported on whether the past years’ large-scale efforts to improve workflow times and procedural results in everyday practice lead to measurably better patient outcomes in the whole treated population.
In the Netherlands, the MR CLEAN (Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) Registry included all patients with ischemic stroke treated with EVT from the final patient included in the MR CLEAN.4,15 We compared the results of the second MR CLEAN Registry cohort to those of first, which were previously published,4 to evaluate trends in patient characteristics, workflow times, and interventional results over the past 3.5 years, and their association with clinical outcomes.
Methods
Design
The MR CLEAN Registry is a prospective, nationwide, observational cohort study in all Dutch interventional stroke centers, including all patients treated with EVT for acute ischemic stroke. Registration started immediately after the final MR CLEAN trial inclusion in March 2014.15 The study design and methods have been described in detail in the publication of the results of the first cohort (March 16, 2014 to June 15, 2016).4 In the present study, the second cohort comprising patients registered between June 16, 2016 and November 1, 2017 was analyzed, and results were compared with the first cohort. Methods for patient enrolment, study design, data management, and study procedures were the same for both cohorts.4
The MR CLEAN Registry study protocol was evaluated by the ethics committee of the Erasmus MC University Medical Centre, Rotterdam, the Netherlands (MEC-2014-235), and permission to carry out the study as a registry was granted. The protocol was subsequently approved by the research board of each participating center. All authors had full access to all the study data. Source data will not be made available because of legislatory issues on patient privacy, but detailed analytic methods and study materials, including log files of statistical analyses, will be made available to other researchers on request to the first author. Results were reported in adherence to the RECORD statement guidelines.
Included Patients
Patients undergoing arterial puncture with the intention to perform EVT for acute ischemic stroke were included in the MR CLEAN Registry. For the current analysis, we included patients with occlusions of the internal carotid artery, internal carotid artery terminus, middle (M1/M2) cerebral artery, or anterior (A1/A2) cerebral artery on baseline angiography imaging, who were aged 18 years or older, and were treated in a MR CLEAN center,15 to ensure comparability with the first cohort. Because of the limited number of patients undergoing arterial puncture >6.5 hours after symptom onset in both cohorts (n=38 and n=61, respectively), the unknown selection criteria to treat these patients in a time when late-window EVT was not standard of care, and to ensure a comparable analysis between cohorts and time periods, a maximum onset to groin puncture time of 6.5 hours was defined for inclusion in the current study. We performed a separate additional analysis including patients treated beyond 6.5 hours (see Statistical methods). Current Dutch guidelines do not advise any restrictions for EVT based on age, Alberta Stroke Program Early CT Score (ASPECTS), collateral grading score, or carotid tandem lesions.16
Intervention
EVT consisted of arterial puncture, catheterization, and thrombus removal using stent-retriever thrombectomy, aspiration, or both, with or without administration of intraarterial thrombolytics. Interventional device choice was left to the discretion of the treating interventionist. We also included patients in whom no thrombectomy attempt was done despite the intention to treat with EVT, because of, for example, inaccessibility of the intracranial vasculature or spontaneous or intravenous alteplase-induced lysis of the original thrombus.
Imaging
An independent core laboratory assessed baseline noncontrast computed tomography (NCCT) and computed tomography angiography images, digital subtraction angiography (DSA) images of the intervention, and follow-up NCCT images acquired in case of a suspected hemorrhage or clinical deterioration. All observers were blinded to clinical data except for symptom side and were provided with instructions and relevant definitions.
ASPECTS, occlusion location, clot burden score, and collateral grading score were assessed on baseline NCCT or computed tomography angiography.17,18 Reperfusion was graded with the extended Thrombolysis in Cerebral Infarction score on DSA.19 Successful reperfusion was defined as extended Thrombolysis in Cerebral Infarction score 2B-3 on the final DSA run. Only DSA images with final runs including anteroposterior and lateral views could receive a score of 2B or higher. If the final posttreatment DSA run had only one view, a maximum score of 2A could be assigned. Intracranial hemorrhage was assessed on follow-up NCCT according to the Heidelberg criteria.20
Outcomes
Our primary outcome measure was functional outcome assessed with the modified Rankin Scale (mRS) at 90 days.21 The mRS was scored by trained (research) nurses, based on telephonic or in-person interviews. Secondary outcome measures were neurological deficit at 24 to 48 hours after stroke onset, evaluated by means of the National Institutes of Health Stroke Scale (NIHSS),22 and symptomatic intracranial hemorrhage. Intracranial hemorrhage was considered symptomatic if a patient died or showed clinical deterioration of ≥4 points on the NIHSS, and deterioration was deemed related to the intracranial hemorrhage on follow-up imaging by the serious adverse events committee.20 Other safety measures included mortality and stroke progression defined as clinical deterioration of ≥4 points on the NIHSS, not explained by intracranial hemorrhage on follow-up NCCT. Procedural complications (vasospasm, vessel dissection or perforation, presence of distal thrombi, and new clots in a different vascular territory) were scored by core laboratory members on DSA.
Statistical Methods
Baseline characteristics of both cohorts were compared with the Mann-Whitney U test for continuous variables, and χ2 or Fisher exact test for categorical variables. To assess changes in outcomes and clinical or interventional factors over time, results of the second and first cohort were compared. In addition, to further evaluate changes over time, both cohorts were stratified into chronological quartiles of intervention dates to create equally sized groups of patients treated over the entire study period. Ordinal logistic regression was used to compare mRS distributions between the cohorts and between the chronological quartiles (shift analysis). To evaluate the relation between changes in outcomes and clinical or interventional differences over the quartiles and cohorts, we used the following 5 regression models. Cohort or quartile number was included as independent variable of interest in all models. Model 1 (base model) was adjusted for age and sex only. Model 2 was adjusted for additional prognostic variables (NIHSS at baseline, prestroke mRS, previous stroke, diabetes, baseline systolic blood pressure, intravenous alteplase administration, ASPECTS, location of occlusion on computed tomography angiography, and collateral score).23 In model 3, time from onset to reperfusion was added to model 2. In model 4, the extended Thrombolysis in Cerebral Infarction score was added to model 2. In model 5, all parameters were included.
We used linear regression to assess differences in neurological deficit (NIHSS) at 24 to 48 hours, with adjustments as described above, and logistic regression to assess differences in the occurrence of symptomatic intracranial hemorrhage. Additional analyses were performed for patients undergoing arterial puncture >6.5 hours after symptom onset, patients with internal carotid artery(-terminus) or M1 occlusions, and patients with complete DSA images.
Missing data were imputed for the regression analyses only, using pooled data of 5 imputed data sets. Multiple imputation was performed with chained equations techniques using the following variables: age, baseline NIHSS, glucose level, diabetes, previous myocardial infarction, previous stroke, hypercholesterolemia, atrial fibrillation, use of antiplatelet drugs, statins, anticoagulants or antihypertensive drugs, prestroke mRS score, baseline systolic blood pressure, baseline ASPECTS, occlusion location, collateral status, time from symptom onset to start of EVT, time from symptom onset to successful reperfusion or last contrast bolus, extended Thrombolysis in Cerebral Infarction score at the end of the intervention, NIHSS after 24 to 48 hours, and poststroke mRS score.4,24,25 All analyses were performed with R statistical software (version 3.4.2).
Results
Patient Characteristics
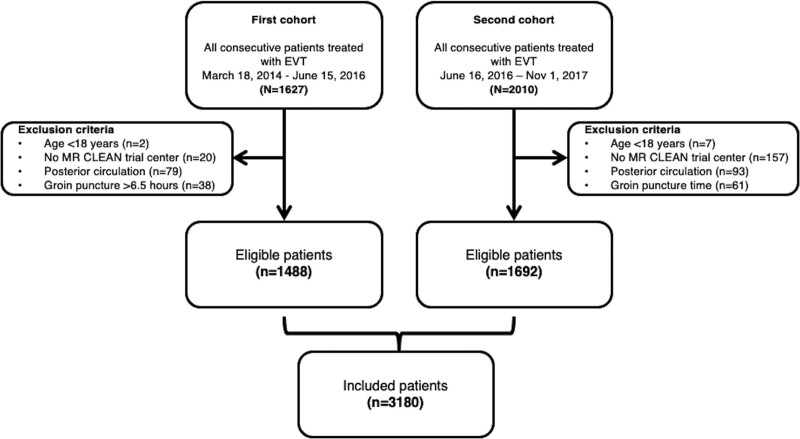
Of 1627 patients in the first cohort, 1488 were included in this analysis (Figure 1). In the second cohort, 2010 patients were registered, of whom 1692 were included (Figure 1). The combined dataset consisted of 3180 patients, and the number of EVT-treated patients was stable over recent years (Figure S1). The first chronological quartile of included patients (n=795) was treated from March 16, 2014 to October 26, 2015, the second quartile (n=797) from October 27, 2015 to July 18, 2016, the third quartile (n=793) from July 19, 2016 to March 24, 2016, and the fourth quartile (n=795) from March 25, 2017 to November 1, 2017. Compared with the first cohort, patients in the second cohort were older (median age 73 versus 71 years; P<0.01); had poorer collaterals (P<0.01); less often had ASPECTS score <6 (5.9% versus 11.0%; P<0.01); more often had occlusions in the M2 segment of the middle cerebral artery (17.6% versus 12.1%; P<0.01); and were less frequently treated with intravenous alteplase (75.0% versus 78.0%; P=0.05) (Table 1). Baseline characteristics per chronological quartile are shown in Table S1.
Figure 1.
Flowchart of included patients. First cohort: March 2014 to June 2016; second cohort: June 2016 to November 2017. EVT indicates endovascular thrombectomy; and MR CLEAN, Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands.
Table 1.
Baseline Characteristics of the First and Second Cohort of the MR CLEAN Registry
Workflow Time Intervals
In general, time from stroke onset to groin puncture and stroke onset to reperfusion or last contrast bolus was shorter in the second cohort (180 versus 208 minutes; P<0.001, and 233 versus 267 minutes; P<0.01, respectively) (Table 2; Figure S1). Time from symptom onset to interventional hospital door did not differ significantly (median 134 versus 132 minutes; P=0.67), but the time from interventional hospital door to arterial puncture was shorter in the second cohort (median 52 versus 69 minutes; P<0.01). The number of transfer patients versus patients directly admitted to an intervention hospital was similar in both cohorts. Findings per chronological quartile were similar (Table S2).
Table 2.
Workflow and Interventional Aspects in 2 Cohorts of the MR CLEAN Registry
Interventional Aspects
Total procedure time was shorter in the second cohort (median 54 versus 63 minutes, P<0.001). EVT was less frequently done under general anesthesia, and aspiration was more often used as a first-line approach in the second cohort than in the first (23.7% versus 27.4%; P=0.03 and 35.1% versus 17.0%; P<0.01, respectively; Table 2). Successful reperfusion was achieved more often in the second cohort (76.7% versus 68.7% for cases with complete runs; 65.7% versus 57.7% for all cases; both P<0.01). DSA images included complete final runs with anteroposterior and lateral views in 89.3% of cases in the second, versus 85.4% in the first cohort (P<0.01). Procedural complication rates did not differ between cohorts, except for a lower rate of vasospasm in the second cohort (14.5% versus 39.3%; P<0.01). Table S2 shows interventional details per chronological quartile.
Outcomes
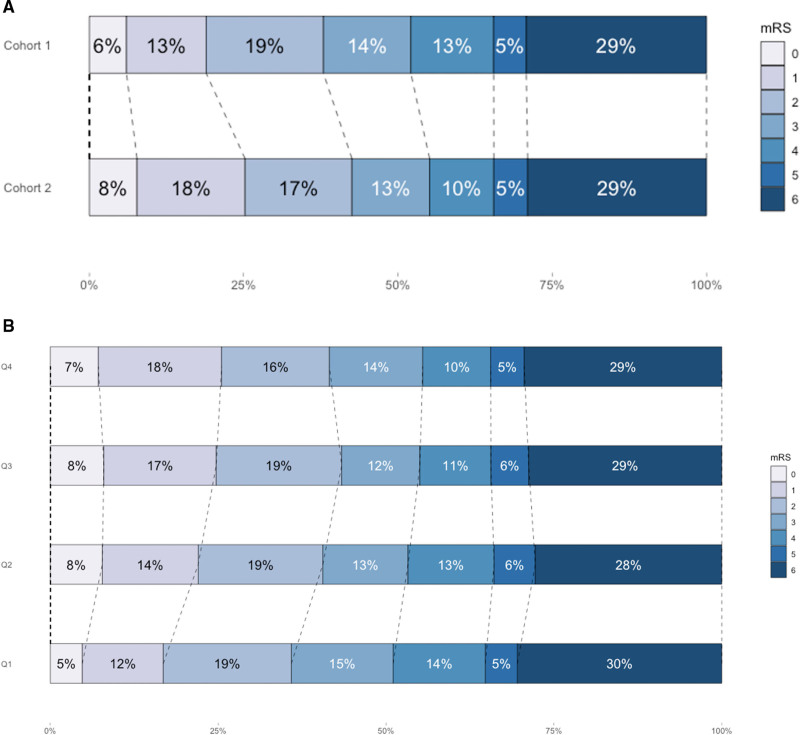
Functional outcomes of patients in the second cohort were better than of those in the first cohort (adjusted common odds ratio [acOR], 1.26 [95% CI, 1.11–1.43]) (Table 3; Figure 2A). In addition, more patients were functionally independent at 90 days in the second cohort (42.6% versus 37.9%; P=0.01; Table 4). After adjustments for either time from onset to reperfusion or for reperfusion grade, the effect was attenuated and lost statistical significance (acOR, 1.02 [95% CI, 0.88–1.17] and acOR, 1.11 [95% CI, 0.96–1.28], respectively). The additional analyses showed that results were consistent for patients presenting after 6.5 hours of symptom onset, with internal carotid artery(-terminus) and M1-occlusions only, and with complete posttreatment DSA images (Table S3; Figures S3 and S4).
Table 3.
Adjusted Comparison of Outcomes Between First (March 2014 to June 2016) and Second (June 2016 to November 2017) Cohort and Chronological Quartiles of the MR CLEAN Registry
Figure 2.
Modified Rankin Scale (mRS) scores at 90 d. A, First cohort (March 2014 to June 2016, n=1488) vs second cohort of the MR CLEAN Registry (June 2016 to November 2017, n=1692). Shift toward better functional outcomes in cohort 2 (age and sex adjusted common odds ratio [acOR], 1.26 [95% CI, 1.11–1.43]). B, Outcomes per chronological quartile of treated patients over time (Q1 n=795, Q2 n=797, Q3 n=793, Q4 n=795; age and sex adjusted acOR, 1.13 [95% CI, 1.06–1.19]). Q indicates quartile.
In the analysis per chronological quartile, similar results were found. Functional outcome improved over time (acOR, 1.13 [95% CI, 1.06–1.19]) (Table 3 and Figure 2B). Statistical significance was lost after adjustment for time from stroke onset (acOR, 0.99 [95% CI, 0.93–1.06]), reperfusion grade (acOR, 1.04 [95% CI, 0.97–1.10]), or both (acOR, 0.97 [95% CI, 0.91–1.10]). Outcomes per quartile are shown in Table S4.
NIHSS at 24 to 48 hours after stroke was lower in the second cohort (median 10 points [interquartile range, 4–16]) than in the first cohort (median 11 points [interquartile range, 4–18]). The greater improvement in neurological deficit also lost significance after adjustment for time from onset to reperfusion or reperfusion grade, both in the per-cohort and per-quartile analyses (Table 3). No statistically significant change in occurrence of symptomatic intracranial hemorrhage (5.8% versus 5.9%; P=0.903) and mortality (27.3% versus 26.7%; P=0.703) was observed (Tables 3 and 4).
Table 4.
Clinical and Safety Outcomes
Discussion
Clinical outcomes after EVT in routine clinical practice in the Netherlands improved over a period of 3 and a half years, largely attributable to faster in-hospital workflow times and higher reperfusion rates. Particularly, in-hospital workflow times decreased: despite a longer time from stroke onset to door of the first hospital in the second cohort, total time from onset to arterial puncture was reduced. The endovascular procedure was more often successful in the second cohort, also when only evaluating patients with complete DSA imaging. Because adjustment for workflow times and reperfusion success attenuated the effect of cohort number on patients’ functional outcome, the observed improvement in outcomes is most likely attributable to these in-hospital workflow and interventional improvements.
Several other registries on EVT for ischemic stroke can be compared with our data.6,26,27 In general, baseline characteristics, workflow times, intravenous thrombolysis administration rates, reperfusion rates, and outcomes are similar. However, no trends over time have been reported in these studies. Reported rates of functional independence at 90 days are higher in other registries (54.8% and 44.1%) than in our first cohort (38.0%), in the presence of more favorable baseline characteristics, but similar to that in our second cohort (42.5%). Furthermore, the reported reperfusion grades in other registries tend to be higher (74.1%–81.7%) than ours. This difference is, in addition to the maximum score of TICI 2A for incomplete post-EVT runs, likely caused by the fact that we included all patients who underwent groin puncture with intention to treat with EVT into our analysis, rather than excluding those where no intracranial access was gained; and strict adjudication by our core laboratory.28 Thus, comparison of data between these reports of different patient populations warrants caution. Throughout the Netherlands, all EVT-performing centers have taken measures to decrease door to treatment times. For example, centers participate in a national quality assurance program and receive yearly feedback on their door-to-needle and door-to-groin times through the Dutch Acute Stroke Audit (DASA) Registry.29 Of the many workflow improvements that were implemented in both intervention and primary stroke centers, examples are specialized acute stroke care teams were trained in several hospitals to enable efficient and parallel workflows, computed tomography angiography images were acquired immediately after NCCT to prevent time lost in logistics, some centers adapted their protocols to local anesthesia,30 and one hospital installed an intervention suite in the emergency department.10
The difference in outcome between the cohorts was also attenuated after adjusting for reperfusion grade, also when restricting the analysis to patients with complete DSA images. Potential causes for improved reperfusion grades could be the introduction of novel interventional techniques and devices,11–13 as well as the increased experience or improved training of individual interventionalists and the entire stroke and interventional teams. Although few studies have investigated stroke teams’ and interventionalists’ training and experience and their effects on outcome, it is likely that they lead to improved reperfusion grades and shorter procedure times.31
The observed improvement is unlikely to have been caused by stricter patient selection only. The number of performed EVT procedures did not decline over time, the proportion of patients with mRS score 5 and 6 stayed the same, and the only baseline differences were higher age, a less frequent history of myocardial infarction, poorer collateral scores, higher ASPECTS, and more distal occlusions (specifically more M2-occlusions) in patients from the second cohort; some of these even indicate a less strict selection. Furthermore, after adjustment for these baseline characteristics, the differences in outcomes between the 2 cohorts remained statistically significant. Despite our encouraging findings, the observation that the proportion of patients with poststroke mRS score 5 and 6 remained constant is humbling. There is still much left to gain in stroke care and outcomes for the patients with the most severe strokes.
Strengths of the current study include analysis of a large number of consecutively treated patients, in a constant set of stroke centers who work in close collaboration with each other to improve dissemination of knowledge and research results. Participation in the MR CLEAN trial and registry was a prerequisite for reimbursement by the major insurance companies, motivating centers to include all EVT-treated patients in the Registry. In addition, no clinical trials on EVT for anterior circulation stroke were active during our study period, limiting selection bias. All patients who underwent arterial puncture were included in the registry, also patients in whom EVT was intended but not possible because of, for example, access problems, giving a realistic image of real-life success rates of EVT. Finally, imaging was assessed by a blinded, independent core laboratory of certified observers, limiting bias in the imaging assessments.
The current study has limitations. First, because we included patients from the moment of groin puncture, patients who were deemed ineligible for EVT by the treating neurologist or interventionist were not registered. Hence, we do not know the number and clinical characteristics of patients who may have been technically eligible for but did not undergo EVT—a selection bias might have occurred. However, since the Dutch guidelines do not advise any cutoff points for EVT eligibility in ASPECTS, collaterals, or age, and the number of patients with unfavorable baseline characteristics was relatively high, we think that the effect of this selection bias is limited. Second, functional outcome was assessed as part of standard care in all stroke patients in the Netherlands, and was not centrally adjudicated. However, these methods did not change compared with the first cohort, so they should not affect cohort comparability. Third, we cannot compare individual effects of all the exact workflow improvements that were made in each participating center from when these were initiated. Therefore, we cannot recommend or discourage specific workflow changes based on the current study. The data presented here apply to the current situation in the Netherlands—extrapolation to other countries with different infrastructure or populations should be done with caution. Finally, the chosen time point to distinguish between the MR CLEAN Registry first and second cohort is based on practical considerations from a data collection point of view. No pivotal practical or scientific event happened to separate the 2 cohorts. However, analysis over chronological quartiles showed consistent results.
The results from the MR CLEAN Registry first and second cohorts show that efforts invested in workflow time and procedural improvement in the Netherlands lead to real-life benefit. More research is needed to assess which specific workflow or interventional improvements will lead to further improved outcomes.
Conclusions
Functional outcomes of EVT-treated patients with acute ischemic stroke have improved over the past 3.5 years. This improvement can at least partly be attributed to improvement in workflow and procedural success. We hope that these results will further inspire everyone involved in acute stroke care to monitor and improve their teams’ performances.
Article Information
Acknowledgments
We acknowledge the effort, time, and contributions of the MR CLEAN Registry investigators (Supplemental Material).
Sources of Funding
This study was funded and carried out by the Erasmus University Medical Centre, Amsterdam University Medical Centers location Academic Medical Center, and Maastricht University Medical Centre. The study was additionally funded by the Applied Scientific Institute for Neuromodulation (Toegepast Wetenschappelijk Instituut voor Neuromodulatie), which played no role in trial design and patient enrollment, nor in data collection, analysis, or writing of the article.
Disclosures
Dr Postma received grants from Siemens, Bayer. Dr Yoo received grants from Medtronic, Ceronovus, Penumbra, Stryker, personal fees from Ceronovus, Penumbra, Vesalio, and equity ownership interest from Insera Therapeutics. Dr van der Lugt received grants from CVON/Dutch Heart Foundation, Brain Foundation, Stryker, Medtronic, Ceronovus, Penumbra. Dr van der Worp received grants from Stryker, personal fees from Bayer, LivaNova. Dr Majoie received grants from CVON/Dutch Heart Foundation, Stryker, European Commission, TWIN, Dutch Health Evaluation Program and is shareholder of Nico.Lab. Dr Dippel received grants from Dutch Heart Foundation, Brain Foundation Netherlands, The Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Sciences & Health, Stryker, Penumbra, Inc, Medtronic, Thrombolytic Science LLC, Ceronovus. Dr Boogaarts received personal fees from Stryker. Dr Coutinho received grants from Boehringer Ingelheim, Bayer, Medtronic. Dr van Doormaal received other view from Stryker). Dr van den Berg received other view from Ceronovus. Dr van Zwam received personal fees from Ceronovus, Stryker. Dr Roos is a shareholder at Nico.Lab. The other authors report no conflicts.
Supplemental Material
Tables S1–S5
Figures S1–S5
MR CLEAN Registry Investigators
RECORD statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- acOR
- adjusted common odds ratio
- ASPECTS
- Alberta Stroke Program Early CT Score
- CTA
- computed tomography angiography
- DSA
- digital subtraction angiography
- EVT
- endovascular treatment
- ICA
- internal carotid artery
- MR CLEAN
- Multicenter Randomized Clinical trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- mRS
- modified Rankin Scale
- NCCT
- noncontrast computed tomography
- NIHSS
- National Institutes of Health Stroke Scale
K.C.J. Compagne and M. Kappelhof contributed equally.
A list of MR CLEAN Registry investigators are listed in the Supplemental Material.
This manuscript was sent to Shelagh Coutts, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.034919.
For Sources of Funding and Disclosures, see page 1871.
Contributor Information
Manon Kappelhof, Email: m.kappelhof@amc.uva.nl.
Wouter H. Hinsenveld, Email: wouter.hinsenveld@mumc.nl.
Josje Brouwer, Email: j.brouwer@amsterdamumc.nl.
Robert-Jan B. Goldhoorn, Email: robertjan.goldhoorn@mumc.nl.
Maarten Uyttenboogaart, Email: m.uyttenboogaart@umcg.nl.
Reinoud P.H. Bokkers, Email: r.p.h.bokkers@umcg.nl.
Wouter J. Schonewille, Email: w.schonewille@antoniusziekenhuis.nl.
Jasper M. Martens, Email: jmmartens@rijnstate.nl.
Jeannette Hofmeijer, Email: jhofmeijer@rijnstate.nl.
H. Bart van der Worp, Email: h.b.vanderworp@umcutrecht.nl.
Rob T.H. Lo, Email: T.H.Lo@umcutrecht.nl.
Koos Keizer, Email: koos_keizer@msn.com.
Lonneke S.F. Yo, Email: lonneke.yo@catharinaziekenhuis.nl.
Geert J. Lycklama à Nijeholt, Email: g.lycklama.a.nijeholt@haaglandenmc.nl.
Heleen M. den Hertog, Email: m.h.den.hertog@isala.nl.
Emiel J.C. Sturm, Email: sturm@dz.nl.
Paul J.A.M. Brouwers, Email: pjam.brouwers@wxs.nl.
Marianne A.A. van Walderveen, Email: M.A.A.van_Walderveen@lumc.nl.
Marieke J.H. Wermer, Email: m.j.h.wermer@lumc.nl.
Sebastiaan F. de Bruijn, Email: s.debruijn@hagaziekenhuis.nl.
Lukas C. van Dijk, Email: Ewoud.vanDijk@radboudumc.nl.
Hieronymus D. Boogaarts, Email: Jeroen.Boogaarts@Radboudumc.nl.
Ewout J. van Dijk, Email: Ewoud.vanDijk@radboudumc.nl.
Julia H. van Tuijl, Email: j.vantuijl@etz.nl.
Jo P.P. Peluso, Email: jo.peluso@uzleuven.be.
Paul L.M. de Kort, Email: p.dekort@etz.nl.
Boudewijn A.A.M. van Hasselt, Email: b.a.a.m.van.hasselt@isala.nl.
Puck S. Fransen, Email: p.s.s.fransen@isala.nl.
Tobien H.C.M.L. Schreuder, Email: t.schreuder@zuyderland.nl.
Roel J.J. Heijboer, Email: rjjheijboer@hotmail.com.
Sjoerd F.M. Jenniskens, Email: Sjoerd.Jenniskens@radboudumc.nl.
Marieke E.S. Sprengers, Email: m.e.sprengers@amsterdamumc.nl.
Elias Ghariq, Email: e.ghariq@haaglandenmc.nl.
Ido R. van den Wijngaard, Email: i.van.den.wijngaard@haaglandenmc.nl.
Stefan D. Roosendaal, Email: stefan.roosendaal@amsterdamumc.nl.
Anton F.J.A. Meijer, Email: Anton.Meijer@radboudumc.nl.
Ludo F.M. Beenen, Email: l.f.beenen@amsterdamumc.nl.
Alida A. Postma, Email: l.jacobi@mumc.nl.
René van den Berg, Email: r.vdberg@amsterdamumc.nl.
Albert J. Yoo, Email: ajyoo74@gmail.com.
Pieter Jan van Doormaal, Email: p.j.vandoormaal@erasmusmc.nl.
Marc P. van Proosdij, Email: m.p.van.proosdij@nwz.nl.
Menno G.M. Krietemeijer, Email: menno.krietemeijer@catharinaziekenhuis.nl.
Dick G. Gerrits, Email: d.gerrits@mst.nl.
Sebastiaan Hammer, Email: s.hammer@hagaziekenhuis.nl.
Jan Albert Vos, Email: j.a.vos@antoniusziekenhuis.nl.
Jelis Boiten, Email: j.boiten@haaglandenmc.nl.
Jonathan M. Coutinho, Email: j.coutinho@amc.uva.nl.
Bart J. Emmer, Email: b.j.emmer@amsterdamumc.nl.
Ad C.G.M. van Es, Email: A.C.G.M.van_Es@LUMC.nl.
Bob Roozenbeek, Email: b.roozenbeek@erasmusmc.nl.
Yvo B.W.E.M. Roos, Email: y.b.roos@amsterdamumc.nl.
Wim H. van Zwam, Email: w.van.zwam@mumc.nl.
Robert J. van Oostenbrugge, Email: r.vanoostenbrugge@mumc.nl.
Charles B.L.M. Majoie, Email: c.b.majoie@amsterdamumc.nl.
Diederik W.J. Dippel, Email: d.dippel@erasmusmc.nl.
Aad van der Lugt, Email: a.vanderlugt@erasmusmc.nl.
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. ; American Heart Association Stroke Council. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4:6–12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen IGH, Mulder MJHL, Goldhoorn RB; MR CLEAN Registry investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wollenweber FA, Tiedt S, Alegiani A, Alber B, Bangard C, Berrouschot J, Bode FJ, Boeckh-Behrens T, Bohner G, Bormann A, et al. Functional outcome following stroke thrombectomy in clinical practice. Stroke. 2019;50:2500–2506. doi: 10.1161/STROKEAHA.119.026005 [DOI] [PubMed] [Google Scholar]
- 6.Alegiani AC, Dorn F, Herzberg M, Wollenweber FA, Kellert L, Siebert E, Nolte CH, von Rennenberg R, Hattingen E, Petzold GC, et al. Systematic evaluation of stroke thrombectomy in clinical practice: The German Stroke Registry Endovascular Treatment. Int J Stroke. 2019;14:372–380. doi: 10.1177/1747493018806199 [DOI] [PubMed] [Google Scholar]
- 7.Mueller-Kronast NH, Zaidat OO, Froehler MT, Jahan R, Aziz-Sultan MA, Klucznik RP, Saver JL, Hellinger FR, Jr, Yavagal DR, Yao TL, et al. ; STRATIS Investigators. Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS Registry. Stroke. 2017;48:2760–2768. doi: 10.1161/STROKEAHA.117.016456 [DOI] [PubMed] [Google Scholar]
- 8.Zerna C, Rogers E, Rabi DM, Demchuk AM, Kamal N, Mann B, Jeerakathil T, Buck B, Shuaib A, Rempel J, et al. Comparative effectiveness of endovascular treatment for acute ischemic stroke: a population-based analysis. J Am Heart Assoc. 2020;9:e014541. doi: 10.1161/JAHA.119.014541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulder MJHL, Jansen IGH, Goldhoorn RB, Venema E, Chalos V, Compagne KCJ, Roozenbeek B, Lingsma HF, Schonewille WJ, van den Wijngaard IR, et al. ; MR CLEAN Registry Investigators. Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN Registry Results. Circulation. 2018;138:232–240. doi: 10.1161/CIRCULATIONAHA.117.032600 [DOI] [PubMed] [Google Scholar]
- 10.Janssen PM, Venema E, Dippel DWJ. Effect of workflow improvements in endovascular stroke treatment. Stroke. 2019;50:665–674. doi: 10.1161/STROKEAHA.118.021633 [DOI] [PubMed] [Google Scholar]
- 11.Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, Spiotta A, Mokin M, Dewan M, Quarfordt S, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6:260–264. doi: 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 12.Ospel JM, Volny O, Jayaraman M, McTaggart R, Goyal M. Optimizing fast first pass complete reperfusion in acute ischemic stroke - the BADDASS approach (BAlloon guiDe with large bore Distal Access catheter with dual aspiration with Stent-retriever as Standard approach). Expert Rev Med Devices. 2019;16:955–963. doi: 10.1080/17434440.2019.1684263 [DOI] [PubMed] [Google Scholar]
- 13.Peker A, Arsava EM, Topçuoğlu MA, Arat A. Catch Plus thrombectomy device in acute stroke: initial evaluation. J Neurointerv Surg. 2017;9:1214–1218. doi: 10.1136/neurintsurg-2016-012760 [DOI] [PubMed] [Google Scholar]
- 14.Liebig T, Holtmannspötter M, Crossley R, Lindkvist J, Henn P, Lönn L, Gallagher AG. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239–e242. doi: 10.1161/STROKEAHA.118.021089 [DOI] [PubMed] [Google Scholar]
- 15.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 16.Federatie Medisch Specialisten, Nederlandse Vereniging voor Neurologie. Richtlijnen database: Endovasculaire trombectomie (EVT) bij herseninfarct 2019 [Internet]. The Netherlands; c2020. Accessed September 23, 2021. https://richtlijnendatabase.nl/richtlijn/herseninfarct_en_hersenbloeding/reperfusietherapie_voor_acute_herseninfarct/endovasculaire_trombectomie_evt_bij_herseninfarct.html.
- 17.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 18.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, Demchuk AM. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6:83–86. doi: 10.1136/neurintsurg-2013-010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 22.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–2226. doi: 10.1161/01.str.25.11.2220 [DOI] [PubMed] [Google Scholar]
- 23.Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, Berkhemer OA, Emmer BJ, Roos YBWEM, Majoie CBLM, et al. Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017;357:j1710. doi: 10.1136/bmj.j1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moons KG, Donders RA, Stijnen T, Harrell FE, Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101. doi: 10.1016/j.jclinepi.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 25.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 26.Köcher M, Šaňák D, Zapletalová J, Cihlář F, Czerný D, Černík D, Duras P, Endrych L, Herzig R, Lacman J, et al. Mechanical thrombectomy for acute ischemic stroke in Czech Republic: Technical Results from the Year 2016. Cardiovasc Intervent Radiol. 2018;41:1901–1908. doi: 10.1007/s00270-018-2068-z [DOI] [PubMed] [Google Scholar]
- 27.Weisenburger-Lile D, Blanc R, Kyheng M, Desilles JP, Labreuche J, Piotin M, Mazighi M, Consoli A, Lapergue B, Gory B; on behalf of the Endovascular Treatment in Ischemic Stroke Investigators. Direct admission versus secondary transfer for acute stroke patients treated with intravenous thrombolysis and thrombectomy: insights from the Endovascular Treatment in Ischemic Stroke Registry. Cerebrovasc Dis. 2019;47:112–120. doi: 10.1159/000499112 [DOI] [PubMed] [Google Scholar]
- 28.Zhang G, Treurniet KM, Jansen IGH, Emmer BJ, van den Berg R, Marquering HA, Uyttenboogaart M, Jenniskens SFM, Roos YBWEM, van Doormaal PJ, et al. ; MR CLEAN Registry Investigators. Operator versus core lab adjudication of reperfusion after endovascular treatment of acute ischemic stroke. Stroke. 2018;49:2376–2382. doi: 10.1161/STROKEAHA.118.022031 [DOI] [PubMed] [Google Scholar]
- 29.Kuhrij LS, Wouters MW, van den Berg-Vos RM, de Leeuw FE, Nederkoorn PJ. The dutch acute stroke audit: benchmarking acute stroke care in the Netherlands. Eur Stroke J. 2018;3:361–368. doi: 10.1177/2396987318787695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldhoorn RB, Bernsen MLE, Hofmeijer J, Martens JM, Lingsma HF, Dippel DWJ, van der Lugt A, Buhre WFFA, Roos YBWEM, Majoie CBLM, et al. ; MR CLEAN Registry Investigators. Anesthetic management during endovascular treatment of acute ischemic stroke in the MR CLEAN Registry. Neurology. 2020;94:e97–e106. doi: 10.1212/WNL.0000000000008674 [DOI] [PubMed] [Google Scholar]
- 31.Lavine SD, Cockroft K, Hoh B, Bambakidis N, Khalessi AA, Woo H, Riina H, Siddiqui A, Hirsch JA, Chong W, et al. Training guidelines for endovascular stroke intervention: an international multi-society consensus document. Neuroradiology. 2016;58:537–541. doi: 10.1007/s00234-016-1667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.