Abstract
Carbon partitioning and residue formation during microbial degradation of polycyclic aromatic hydrocarbons (PAH) in soil and soil-compost mixtures were examined by using [14C]anthracenes labeled at different positions. In native soil 43.8% of [9-14C]anthracene was mineralized by the autochthonous microflora and 45.4% was transformed into bound residues within 176 days. Addition of compost increased the metabolism (67.2% of the anthracene was mineralized) and decreased the residue formation (20.7% of the anthracene was transformed). Thus, the higher organic carbon content after compost was added did not increase the level of residue formation. [14C]anthracene labeled at position 1,2,3,4,4a,5a was metabolized more rapidly and resulted in formation of higher levels of residues (28.5%) by the soil-compost mixture than [14C]anthracene radiolabeled at position C-9 (20.7%). Two phases of residue formation were observed in the experiments. In the first phase the original compound was sequestered in the soil, as indicated by its limited extractability. In the second phase metabolites were incorporated into humic substances after microbial degradation of the PAH (biogenic residue formation). PAH metabolites undergo oxidative coupling to phenolic compounds to form nonhydrolyzable humic substance-like macromolecules. We found indications that monomeric educts are coupled by C-C- or either bonds. Hydrolyzable ester bonds or sorption of the parent compounds plays a minor role in residue formation. Moreover, experiments performed with 14CO2 revealed that residues may arise from CO2 in the soil in amounts typical for anthracene biodegradation. The extent of residue formation depends on the metabolic capacity of the soil microflora and the characteristics of the soil. The position of the 14C label is another important factor which controls mineralization and residue formation from metabolized compounds.
For two decades formation of so-called nonextractable bound residues from 14C-labeled polycyclic aromatic hydrocarbons (PAH) has been observed in investigations of the bioavailability and biodegradability of these compounds in soil (13, 20, 22, 24, 25, 30, 40). However, the formation processes and chemical structures of these residues are still not understood. Remobilization of the parent PAH compounds from bound residues after soil bioremediation techniques are used may result in a continued risk to human health.
Many microorganisms are able to metabolize PAH (9, 17, 53). Several bacteria mineralize PAH that have up to four aromatic rings and use them as a source of carbon and energy. Microbial degradation proceeds by successive cleavage of the aromatic rings. ortho-Hydroxy aromatic acids are formed after degradation of each ring. These metabolites accumulate in some organisms and are also detected in the environment (15, 21, 23, 34, 40). Moreover, PAH are oxidized to phenolic metabolites by cometabolic processes in some microorganisms. Nonspecific oxidation reactions catalyzed by extracellular enzymes of white rot fungi lead to the formation of a variety of quinones and hydroxylated aromatic compounds. In most cases these metabolites are not metabolized further by the organisms and may serve as potential substrates for coupling to the humic soil matrix. The contributions of several PAH metabolites to the formation of bound residues have been described previously (40, 42).
Nonextractable residues are formed in soils during application of pesticides (8, 12, 33, 36). After extraction with organic solvents, some portions of the active compounds remain in the soil. These bound residues can be quantified only by using 14C-labeled parent compounds. However, this labeling technique provides no information about chemical structure. The residues may consist of the parent compound, of biogenic or chemical metabolites, or of labeled carbon assimilated by microorganisms after metabolism of the parent compound. Residues from biomass or highly degraded compounds have no ecotoxicological relevance and are not considered bound residues in terms of the International Union of Pure and Applied Chemistry (IUPAC) definition of pesticides (43). However, bound residues cannot be distinguished from biogenic residues, since the chemical structures of the residues are not known. The chemical reactivity of an active compound or of a metabolite governs the formation of bound residues, whose levels may range from 7 to 90% of the quantity applied (3, 8, 12, 33). Many pesticides are partially degraded, and the metabolites are involved in the formation of bound residues (19, 26, 52). However, the binding types of the metabolites are not known in most cases. By using 15N-labeled substances, covalent bonds of metabolites from phenylamide herbicides to humic substances have been identified by modern 15N nuclear magnetic resonance spectroscopy (54). Functional groups, such as hydroxylic, carboxylic, nitro, amino, or phosphate groups, may contribute to cross-linking reactions with compounds in the soil organic matrix. Phenolic or aniline compounds have the greatest tendency to form bound residues (43).
Generally, microbial degradation of mineral oil compounds and PAH in soil leads to mineralization accompanied by the formation of bound residues. The degradation activity increases after organic additives like compost are added and depends on the presence of the solid soil matrix (37). However, the carbon mass balances of the parent compounds and the CO2 produced reveal that there are considerable losses, which account up to 50% of the carbon applied (30, 49). The aim of the present work was to trace the partitioning of 14C from different labeled anthracenes under various conditions during microbial degradation in soil. The potential of typical PAH metabolites to react in cross-coupling reactions was investigated, and mechanisms of residue formation were evaluated.
MATERIALS AND METHODS
Chemicals.
Unless indicated otherwise, all chemicals were purchased from Merck (Darmstadt, Germany). The following radiochemicals were obtained from Amersham, Braunschweig, Germany: [9-14C]anthracene (specific activity, 559 MBq/mmol; radiochemical purity, >96%) and [1,2,3,4,4a,10a-14C]anthracene (specific activity, 451 MBq/mmol; purity, >98%). [9-14C]anthraquinone (purity, >95%) was prepared from [9-14C]anthracene after oxidation and was isolated by using preparative thin-layer chromatography (TLC).
Soil.
The experiments were carried out with soil samples taken from the Ah horizon of a Luvisol (organic C content, 1%) from an uncontaminated, rural area near Hamburg, Germany. The soil was sieved so that the particle size was <2 mm. The compost used for the experiments was obtained from the Hamburg composting plant (Hamburg, Germany; organic C content, 12.7%; total N content, 1.4%; biomass content, 1.4 g of C/kg; C/N ratio, 9.0) and was sieved so that the particle size was <4 mm. The soil materials used have been described previously (30, 31) and were used for several experiments (10, 40, 41). All of the concentrations given in this paper were based on the dry weight of soil.
Anthracene degradation experiments.
Anthracene degradation experiments were conducted with 2 kg of soil material in continuously aerated 3-liter soil bioreactors (30). The 14C contents in the reactors were measured in the soil and in the waste gas by measuring the sorption of 14CO2 with NaOH and the sorption of volatile metabolites with ethylene glycol monomethyl ether. 14C-labeled anthracene and unlabeled anthracene were dissolved in ethyl acetate and were mixed with the soil material by a method described previously (30). The radioactivity in the soil material was 125 kBq/kg of soil when [9-14C]- or [1,2,3,4,4a,5a-14C]anthracene was used, and the total applied anthracene concentration was 100 mg/kg. Soil and compost were mixed at a ratio of 80:20 (dry weight/dry weight). The final water content was adjusted to 60% of the water holding capacity (∼84%, dry weight). Similar to anthracene degradation, degradation of anthraquinone was examined by using 125 kBq of [9-14C]anthraquinone per kg and a total applied anthraquinone concentration of 100 mg/kg. Soil samples were taken from a mixture of three independent soil profiles (∼30 g) from the reactor column, which were obtained by using a glass pipe connected to a vacuum pump. The contents of the reactors were mixed completely after sampling. The 14C distribution in soil was examined by performing sequential extraction procedures and by combustion (see below). The effects of bacteria or fungi on anthracene degradation and formation of bound residues from anthracene were investigated in previously described batch experiments (31) by using a 250-g soil-compost mixture and [1,2,3,4,4a,5a-14C]anthracene. The activity of fungi was inhibited by cycloheximide (4 g/kg), and bacteria were inhibited by a mixture of tetracycline, ampicillin, and streptomycin (4 g of each per kg of soil). The efficiencies of these doses were determined by incubating soil particles on several agar media.
Incubation of soil under a 14CO2 atmosphere.
To estimate the contribution of CO2 to the formation of bound residues during microbial degradation of anthracene, 250-g portions of a soil-compost mixture were incubated in 1.5-liter vessels with 14CO2 in the absence of light. Oxygen was provided with a flexible gas bag (capacity, 5 liters), which was connected to the culture vessel by a canula and a silicon septum. The quantity of CO2 added was similar to the amount produced by ∼70% mineralization of anthracene in the reactor experiments. Tubes containing 1.4 mmol of NaHCO3 and 6.2 kBq of [14C]NaHCO3 (∼700 mmol of organic C) were put into each culture vessel. After the vessels were closed, concentrated H3PO4 was added dropwise to the tubes by using a syringe with a long canula inserted through the silicon septum. The acid was added until the solid carbonate was completely converted to CO2. After 90 days of incubation, the gas atmosphere in the vessels was evacuated by passing two vessels to absorb the residual 14CO2 with 4 N KOH. The amount of radioactivity bound to the soil matrix was determined after the soil was extracted by procedures similar to the procedures used in the other experiments. A similar experiment was conducted after the microorganisms in the soil were inactivated in a CHCl3 atmosphere for 24 h. This procedure repressed the respiration in the soil to values that were less than 0.5% of the values obtained for native soil. The residue formation by carbonates in the native soil-compost mixture was analyzed after acidification of 0.5 g of soil with 5 ml of concentrated H2SO4 in a serum flask at 80°C. The 100-ml vessel was purged with decarbonated air (2 liters/h), and the released 14CO2 was trapped for 1 h with Carbosorb (Packard, Frankfurt, Germany). The incorporation of 14CO2 into the organic soil matrix was determined by measuring oxidation of the matrix. The acidified soil suspension was supplemented with 100 mg of FeSO4 · 7H2O, and a 30% H2O2 solution was added dropwise until the total amount added was 20 ml. The solution was boiled for 3 h, and the evolved CO2 in the exhaust gas was trapped in Carbosorb until the solution became translucent and slightly yellow. The residues in the inorganic solution after oxidation were assessed as 14CO2 fixation in clay minerals and silicates.
Formation of humic substance-like macromolecules from PAH metabolites.
PAH metabolites were polymerized in 1-liter Erlenmeyer flasks containing 750 ml of H2O and 7.5 ml of phosphate buffer (1.3 g of KH2PO4 per liter, 2.1 g of Na2HPO4 per liter; pH 7.0) at 28°C in the dark. The reaction was started by adding the following bivalent metal cations (per liter): 325 μg of Mn2+ (MnSO4 · H2O), 40 μg of Fe2+ (FeSO4 · 7 H2O), and 46 μg of Zn2+ (as ZnSO4 · 7 H2O). The metabolites added were 50 mg of catechol per liter, 50 mg of 1-hydroxy-2-naphthoic acid per liter, 50 mg of 3-hydroxy-2-naphthoic acid per liter, 10 mg of 1-naphthol per liter, 2.5 mg of 9-hydroxyphenanthrene per liter, 2.5 mg of 9,10-anthraquinone per liter, and 2.5 mg of 9,10-phenanthrenquinone per liter. Naphthol, hydroxyphenanthrene, and the quinones that were hardly soluble in water were added in acetone solutions. Brown precipitates were observed in the reaction mixtures after 1 to 2 days. Each suspension was separated by ultrafiltration with Diaflow ultrafiltration membranes (Amicon, Witten, Germany) with the following molecular weight exclusion values: type YMIO, >10,000; type YM3, >3,000; type YMI, >1,000; and type YC05, >500. The absorption spectra of the fractions were determined with a Lambda 2 photometer (Perkin-Elmer, Überlingen, Germany). The amounts of residual monomeric parent substances were determined by TLC and high-performance liquid chromatography (HPLC).
Analytical procedures.
Three grams of soil was added to a Hungate tube (Bellco International, Feltham, United Kingdom), and then 3 ml of ethyl acetate was added (10). The tube was closed and extracted for 30 min by ultrasonication. After centrifugation, aliquots of the extracts obtained were analyzed. To extract PAH still bound to the soil matrix after extraction with organic solvents, alkaline hydrolysis in a methanol-NaOH solution was performed (10). The amounts of nonextractable bound residues were calculated after two extractions by determining the difference between the total radioactivity in the soil and the radioactivity in the extracts. The accuracy of these calculations was checked for several samples by combustion of the extracted soil material. The radioactivity was quantified by β-scintillation counting after 1-ml portions of the extracts were added to 10-ml portions of a toluene scintillation cocktail containing 8.25 g of 2,5-diphenyloxazol, 0.25 g of 1,4-bis(5-phenyl-2-oxazolyl)-benzene, and 1,000 ml of toluene. The activities of the samples obtained from the alkaline hydrolysis procedure and of NaOH were quantified by using a scintillation cocktail containing 450 ml of toluene scintillation cocktail, 450 ml of Triton X-100, and 100 ml of methanol. To separate fulvic acids, humic acids, and humines, preextracted unhydrolyzed soil samples (3 g) were mixed with 12 to 13 ml of 0.5 M NaOH (37). After mixing for 15 h, the samples were centrifuged. The precipitates contained the insoluble humines, and the supernatants contained humic and fulvic acids. Five milliliters of each supernatant was acidified with concentrated hydrochloric acid (pH <1) to precipitate the humic acid fraction. The resulting solution contained the fulvic acid fraction. The precipitated humic acids were redissolved in 5 ml of 0.5 M NaOH. The partitioning of the radioactivity into these fractions was determined by combustion or by liquid scintillation counting. The standard deviations in these analyses ranged between 5 and 9%.
Total 14C radioactivity was analyzed by combustion of 0.5 g of soil with a model Bio-Oxidizer OX 500 apparatus (Zinsser Analytik, Frankfurt, Germany). The samples were completely oxidized to 14CO2, which was absorbed with scintillation cocktail containing 1,250 ml of Quickszint 212 (Zinsser Analytik), 750 ml of ethanolamine, and 750 ml of methanol. The levels of recovery in the combustion experiments ranged from 97 to 102%. The standard deviations for triplicate analyses were 3 to 9%. 14C radioactivity was quantified by β-scintillation counting with a model LSC 1410 spectrometer (Wallac, Turku, Finland). Triplicate analyses were performed with external standardization, quenching, and chemoluminescence correction. The overall standard deviations for 14C recovery ranged from 7 to 10% (combustion of the soil, 3 to 9%; extraction, 1.5 to 4%; and determination of 14CO2, 1.8 to 3%).
PAH were quantified by HPLC by using a high-pressure gradient mixture, a model AS 360 autosampler, a model PS 322 pump, and a model SFM 25 fluorescence detector (Kontron, Neufarrn, Germany). The PAH were separated on a type RP 18-PAH column (ET 150/4; NUCLEOSIL 100-5C18 PAH; 5 μm; Macherey and Nagel, Düren, Germany) at 23°C. The column was first eluted at a flow rate of 1 ml/min with a 70% methanol–water mixture for 6 min. For the next 19 min a linear gradient ending in 100% methanol was used. After 25 min the column was eluted with 100% methanol up to 47 min. Fluorescence was detected up to 16.2 min with an excitation wavelength of 265 nm and an emission wavelength of 350 nm, up to 28.1 min with an excitation wavelength of 265 nm and an emission wavelength of 430 nm, and up to 47 min with an excitation wavelength of 295 nm and an emission wavelength of 460 nm. Metabolites of the [14C]PAH were determined by TLC and by radio-HPLC. Peak- or time-controlled collection of samples of the HPLC effluent in scintillation vessels was possible after the detector was connected to a model SF 2120 sampling unit (Advantec, Dublin, Calif.). TLC analyses were performed under the following conditions: PAH, 1-hydroxyphenanthrene, 9,10-phenanthrenequinone, and 9,10-anthraquinone were separated on high-performance TLC Silica Gel G 60F254 (Merck) with toluene; and catechol, 1-naphthol, and hydroxynaphthoic acids were analyzed on high-performance TLC RP 18F254 (Merck) with methanol-water (70:30 or 50:50, vol/vol).
RESULTS
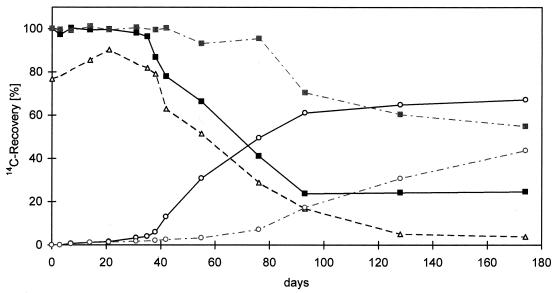
Since preliminary soil experiments showed that CO2 was formed much more slowly than PAH were depleted, we performed mass balance experiments in soil bioreactors to examine microbial degradation of [9-14C]anthracene in soil-compost mixtures. The compound was degraded by the microflora and 14CO2 was formed after a lag phase of 35 days. The amount of extractable radioactivity in the soil was 3.9% of the amount applied, whereas 67.2% of the radioactivity was liberated as 14CO2 after 176 days. CO2 formation was stable after 128 days (Fig. 1 and Table 1). The amount of residual radioactivity in the soil, which was not extractable after the extraction procedures and alkaline hydrolysis were performed, was 20.7% of the amount applied. No metabolism or increased formation of bound residues was observed in similar experiments performed with sterilized soil (31), and almost all of the activity extracted was represented by anthracene.
FIG. 1.
Carbon distribution during microbial degradation of [9-14C]anthracene in a soil-compost mixture (dark lines) and in native soil (light lines). Symbols: ■, total soil; ○, mineralization (CO2); ▵, extractable radioactivity.
TABLE 1.
Distribution of 14C after microbial degradation of different labeled anthracenes and anthraquinone in native soil and a soil-compost-mixture after 176 days
| Parameter | % of applied radioactivity from:
|
|||
|---|---|---|---|---|
| [9-14C]anthracene
|
[9-14C]anthraquinone in soil-compost mixturea | [1,2,3,4,4a,5a-14C]anthracene in soil-compost mixtureab | ||
| Native soil | Soil-compost mixturea | |||
| Total | 98.7 | 91.9 | 95.3 | 94.6 |
| CO2 | 43.8 | 67.2 | 80.2 | 62.4 |
| Soil | 54.9 | 24.6 | 15.1 | 32.2 |
| Extraction + alkaline hydrolysis | 9.5 | 3.9 | 5.0 | 3.7 |
| Bound residues | 45.4 | 20.7c | 10.1 | 28.5 |
Soil-compost mixture (80:20, dry weight/dry weight).
Data obtained after 159 days.
This value represents 25.5 Bq/g of soil.
The culture containing native soil and no compost degraded [9-14C]anthracene much more slowly (Fig. 1). Degradation started after a lag phase of 75 days with slow formation of 14CO2. The amount of extractable radioactivity decreased to a final concentration of 9.5% after 176 days (data not shown). Despite mineralization of 43.8% of the anthracene, the nonextractable bound residues accounted for 45.4% of the radioactivity, and the value was twice as high for the soil-compost mixture (Table 1). The contribution of the parent [14C]anthracene to the total activity in the extracts (9.5%) was 2.9% of the amount applied, whereas the contribution was <1% when compost was added. Most of the radioactivity in the extracts could not be evaluated further. No specific metabolites were identified by TLC or by HPLC of the ethyl acetate extracts; the background levels were high, which indicated that the 14C label was distributed among a large number of compounds (data not shown).
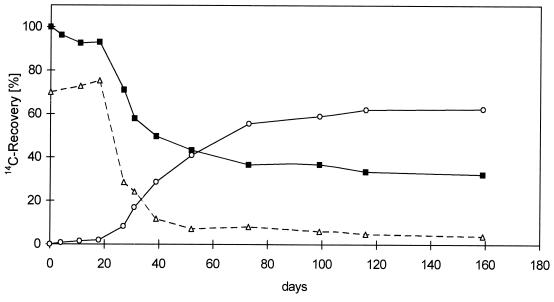
In addition, we examined the metabolism and formation of bound residues of anthracene with different labels in the soil-compost mixture. Under similar culture conditions, the metabolism of [9-14C]anthracene (Fig. 1) was significantly slower than the metabolism of [1,2,3,4,4a,5a-14C]anthracene (Fig. 2). The formation of CO2 from [1,2,3,4,4a,5a-14C]anthracene began after a lag phase of 15 days, compared to a lag phase of 35 days in the case of [9-14C]anthracene. The mineralization phase occurred at the same time in both experiments (90 to 100 days). However, the levels of extractable activity in the experiment performed with [1,2,3,4,4a,5a-[14C]anthracene decreased rapidly within 40 days, whereas more than 100 days were needed to reach similar concentrations in the extracts in the [9-14C]anthracene experiment. The total level of mineralization of [9-14C]anthracene (67.2%) was slightly higher than the total level of mineralization of [1,2,3,4,4a,5a-14C]anthracene (62.4%), whereas the nonextractable bound residues accounted for 20.7% of the [9-14C]anthracene and 28.5% of the [1,2,3,4,4a,5a-14C]anthracene (Table 1). This indicates that the transformation and carbon partitioning of the A-ring label were significantly different from the transformation and carbon partitioning at the C-9 position. A higher level of residues was observed with elevated metabolism of [1,2,3,4,4a,5a-14C]anthracene in the soil-compost mixture.
FIG. 2.
Carbon distribution during microbial degradation of [1,2,3,4,4a,10a-14C]anthracene in a soil-compost mixture. Symbols: ■, total soil; ○, mineralization (CO2); ▵, extractable radioactivity.
No microorganisms able to grow on anthracene were detected in native soil or compost by plate count methods (29). To examine which groups of microorganisms actually metabolized the compounds and formed residues in soil, the soil-compost mixture was incubated with [1,2,3,4,4a,5a-14C]anthracene and bacteria or fungi were selectively inhibited. Bacteria were inhibited by a mixture of ampicillin, streptomycin, and tetracycline, and fungi were inhibited by cycloheximide (Table 2). After inhibition of fungi, slower metabolism and increased formation of bound residues were observed compared to the uninhibited control. After inhibition of bacteria, no metabolism occurred. This indicated that bacteria made the major contribution to the metabolism of anthracene. Nearly all of the extractable radioactivity in this experiment was represented by anthracene. Thus, the observed formation of nonextractable residues in the inhibited cultures had to be due to sorption of the parent compound, which was the dominant mechanism of residue formation. Sorption of the parent compound was also observed in the first 15 to 30 days in other soil cultures when there was no inhibition.
TABLE 2.
Distribution of 14C after microbial degradation of anthracene in a soil-compost mixture (after 174 days) in the presence of antibiotics and after incubation under a 14CO2 atmosphere (after 90 days)
| Parameter | % of applied radioactivity froma:
|
||||
|---|---|---|---|---|---|
| [1,2,3,4,4a,5a-14C]anthracene
|
14CO2
|
||||
| No inhibition | Inhibition by fungicidesb | Inhibition by bactericidesc | Native soil-compost mixture | Soil-compost mixture inactivated with CHCl3d | |
| Total | 93.3 | 90.4 | >95 | 99.8 | 98.7 |
| CO2 | 62.3 | 21.5 | <1 | 4.6e | 11.3e |
| Soil | 31.0 | 68.9 | 95 | 95.2f | 87.4 |
| Extraction + alkaline hydrolysis | 3.1 | 28.1 | 57.2 | 9.4 | 44.2 |
| Bound residues | 27.9 | 43.3 | 37.8 | 85.8 | 43.2 |
All experiments were performed with the soil-compost mixture (80:20, dry weight/dry weight).
Cycloheximide was added to the soil-compost mixture.
Ampicillin, streptomycin, and tetracycline were added to the soil-compost mixture.
The microflora was inhibited by fumigation with CHCl3.
Amount of 14CO2 recovered (the amount initially applied was 24.8 Bq/g of soil).
Acid-labile carbonates contained 31% of the 14C, 67% was incorporated into the organic matrix, and <2% was in clay and silicates.
In addition, degradation of 9,10-anthraquinone in the soil-compost mixture was examined since this compound is the initial oxidation product formed from anthracene during nonspecific oxidation with exoenzymes of ligninolytic fungi (11). If metabolism of anthracene and residue formation in soil occur via transformation to anthraquinone, similar amounts of residues should be obtained with the two compounds. However, the amount residues formed from [9-14C]anthraquinone (10.1%) was much smaller than the amount of residues formed from [9-14C]anthracene (20.7%) or from [1,2,3,4,4a,5a-14C]anthracene (28.5%) (Table 1). 14CO2 was formed from anthraquinone at higher rates than it was formed from anthracene, and the level of mineralization with anthraquinone was 80.2%, compared to 67.2% with [9-14C]anthracene. This indicated that extracellular enzymes of ligninolytic litter-decaying fungi did not significantly contribute to anthracene metabolism in the soil.
To investigate the incorporation of 14CO2 derived from mineralization of labeled anthracene as a possible mechanism of residue formation, the soil-compost mixture was incubated with 14CO2 in the absence of light. The total CO2 concentration used (5.6 mmol/kg) was equivalent to ∼70% of the mineralized anthracene used in our other degradation experiments. After 90 days of incubation, most of the 14CO2 applied (95.2%) was found in the matrix of the soil-compost mixture, and the nonextractable activity accounted for 85.8% of the activity (Table 2). The absolute amount of residue activity (21.3 Bq/g) was similar to the amount of residue activity that was observed during biodegradation of anthracene (25.2 Bq/g) (Table 1). Inhibition of microbial activity by fumigation with CHCl3 (which resulted in respiration that was less than 0.5% of the native soil respiration) decreased the level of immobilization to 40% of the applied 14CO2, which indicated that there was a microbial contribution to the CO2 fixation in the soil. Only some of the CO2 fixation (31%) in the native soil mixture was due to the formation of carbonates, whereas 67% of the CO2 was incorporated into the organic matrix. A minor amount (<2%) was fixed in the clay or silicates (see Materials and Methods). However, the total amount of 14CO2 fixed represented only 4.8 mmol of C (∼0.17%) for a soil carbon matrix containing about 2,830 mmol of C per kg. Although the mechanism of nonphotosynthetic CO2 fixation is not understood yet, the results show that CO2 may have contributed significantly to the formation of residues in the soil experiments described here.
The nonextractable residues were examined to determine their partitioning in different fractions of the organic soil matrix. The radioactivities of the different soil fractions were normalized to the total residual activity of the soil (100%), and the relative contribution of each fraction was determined (Table 3). Degradation of [9-14C]anthracene in native soil led to speciation of the bound activity into fulvic acids (28%), humic acids (33%), and nonsoluble humines (23%). In the soil-compost mixture the major activity was found in humines (29%) and humic acids (38%), and minor activity was found in fulvic acids (18%). The higher level of mineralization in the soil-compost mixture may indicate that increased metabolism led to increased humification of the residual 14C. Compared to the degradation of [9-14C]anthracene, the experiment performed with 14CO2 revealed some striking similarities in the distribution of radioactivity in the different soil fractions. Only slightly greater partitioning to humic acids and humates was observed. This may be an additional indication that fixation of 14CO2 may play a significant role in the formation of residues. Much greater extractability of the radiolabel was observed only with inactivated soil material, and this soil material exhibited a distribution pattern different from the distribution patterns of the other soil cultures.
TABLE 3.
Distribution of 14C in soil extracts and humic substances
| Fraction | % of total radioactivity in soila
|
|||
|---|---|---|---|---|
| [9-14C]anthracene
|
14CO2c
|
|||
| Soil | Soil-compost mixtureb | Soil-compost mixtureb | Inactivated soil-compost mixturebd | |
| Extraction + alkaline hydrolysis | 16 | 15 | 9 | 44 |
| Fulvic acids | 28 | 18 | 18 | 16 |
| Humic acids | 33 | 38 | 38 | 12 |
| Humines | 23 | 29 | 35 | 28 |
In the [9-14C]anthracene experiments the total radioactivities in the soil and the soil-compost mixture were 76.5 and 30.8 Bg/g of soil, respectively, and in the 14CO2 experiments the total radioactivities in the soil-compost mixture and the inactivated soil-compost mixture were 23.2 and 22.0 Bg/g of soil, respectively.
Soil-compost mixture (80:20, dry weight/dry weight).
The amount of 14CO2 applied was 24.8 Bq/g of soil.
The soil-compost mixture was fumigated with CHCl3.
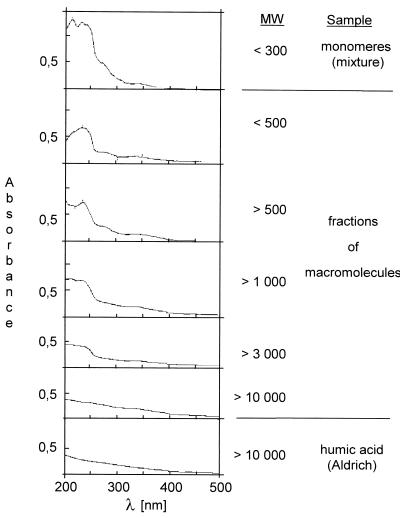
At higher pH values, phenolic compounds tend to polymerize to form macromolecules with humic substance-like characteristics by autoxidation processes (3, 50). The question is whether oxidized PAH metabolites take part in these reactions under neutral conditions in soil. We incubated the PAH metabolites catechol, 1-hydroxy-2- and 2-hydroxy-3-naphthoic acids, 9-hydroxyphenanthrene, 1-naphthol, 9,10-anthraquinone, and 9,10-phenanthrene-quinone in phosphate buffer (pH 7). After we added catalytic amounts of bivalent cations like Fe, Mn, and Zn, which are usually present in soils (5, 48, 58), brown precipitates were formed in the reaction mixture within 1 to 2 days. The molecular weights of the macromolecular products were estimated by separating the products with ultrafiltration membranes. Increasing brown color was observed in the fractions with increasing molecular weights greater than 1,000. The main parts of the products were found in the >10,000-dalton fraction. The dark color was caused by the formation of macromolecular substances which were soluble in alkali and precipitated at pH 1. As the molecular sizes increased, the absorption spectra of the various fractions lost their UV absorption maxima at 200 to 300 nm, which are characteristic of aromatic monomers (Fig. 3). The high-molecular-weight fraction produced nondifferentiated spectra which are typical of humic acids (58). The results implied that macromolecules built of PAH metabolites had characteristics of humic acids.
FIG. 3.
Spectra of humic acid and fractions of macromolecules built of microbial PAH metabolites by chemical oxidation in the presence of bivalent metal cations. MW, molecular weight.
After 26 days of reaction less than 0.5% of the applied 1-hydroxy-2-naphthoic acid and less than 0.1% of the 2-hydroxy-3-naphthoic acid were extracted from the macromolecular compounds. Catechol could not be determined, whereas anthraquinone and phenanthrene-quinone were extracted nearly completely. It is assumed that quinoid compounds play an important role in radical reactions during the formation of humic substances (50). However, these compounds were obviously not included in the reactions. In the absence of catechol, only naphthoic acids polymerized under the conditions used. In all cases, the reaction occurred only in the presence of bivalent metal cations. To investigate the contribution of macromolecules formed by complexation with Fe or Mn cations, we examined disintegration after addition of a strong complexing agent. However, no alteration of the macromolecules was observed after EDTA was added. Gel chromatographic analyses of the macromolecules revealed molecular weights of 2,000, which were only slightly less than the molecular weights of commercially available humic acids (2,200; Sigma-Aldrich). When the macromolecules were preextracted with organic solvents, less than 0.9% of the educts were recovered by hydrolysis or pyrolysis. Moreover, traces of phenanthrene and naphthalene (about 1 mg/g) were present after pyrolysis. 13C nuclear magnetic resonance analyses revealed, in addition to the aromatic signals (116, 124, and 126 ppm), characteristic signals in the carboxylic range (140 to 160 ppm), which corresponded to 15% of the carbon of the macromolecules. This value was significantly higher than value obtained for the parent mixture of monomers (data not shown). Polymerization of the PAH metabolites was also initiated by laccases and peroxidases in filtrates of cultures of the white rot fungi Pleurotus ostreatus Z15 and Trametes villosa 165. However, the yields of these reactions were significantly smaller due to oxidation of the substrates. The results indicate that PAH metabolites like hydroxynaphthoic acids and hydroxylated compounds may take part in biotic and abiotic formation of humic macromolecules in soil to a significant extent.
DISCUSSION
In this work we determined mass balances for 14C partitioning in soil after complete microbial degradation of anthracene, and we obtained information about the relevant processes of formation of bound residues during biodegradation of this PAH. Only a few complete mass balances for [14C]PAH biodegradation and residue formation in soil have been described previously (Table 4). The levels of anthracene mineralization were significantly lower in previous studies than in this study. In the present study, the high levels of mineralization were caused by the addition of compost and by its specific microflora, as discussed previously (29). The parent compound anthracene was completely transformed, and the radioactivity in the extracts was distributed nonspecifically to various unidentified substances. In most other studies, especially studies performed with contaminated soils, significant amounts of the parent compounds were extractable from the soil at the end of the experiments (6, 13, 14, 28, 35, 39, 57). After microbial degradation of 14C-labeled PAH, significant portions of the label could not be extracted from native or contaminated soils and could not be mobilized even by rigid extraction methods. The amounts of the residues were usually overestimated, since sequential extraction methods were used only in some cases (10, 30, 31, 40). Most of the data that have been published are data for anthracene, and the effects of different conditions on carbon partitioning during microbial degradation can be demonstrated for this compound (Table 4). In the case of [9-14C]anthracene dissolved in diesel fuel added to the soil, only a small amount of residue was formed, and no degradation was observed in native or sterilized soil. After compost was added to this soil, a large portion of the anthracene was mineralized, and 42% of the applied radioactivity was found in the residues (30, 31). The same soil-compost mixture contained only 21% residues after anthracene was added without diesel fuel, indicating that oil matrices may increase residue formation in contaminated soils.
TABLE 4.
Mineralization and formation of nonextractable residues during microbial degradation of 14C-labeled PAH in soils and sediment microcosms
| Compound | Length of incubation (days) | CO2 (%) | Nonextractable residues (%) | Reference |
|---|---|---|---|---|
| [1-14C]naphthalene | 2 | 51 | 27 | 24a |
| 56 | 60-70 | 5-8 | 22 | |
| 120 | 56 | 19 | 25a | |
| [9-14C]phenanthrene | 21 | 38 | 36 | 7a |
| [9-14C]anthracene | 7 | 4 | 23 | 24a |
| 148 | 25-5 | 35-74 | 13a | |
| 120 | 22 | 65 | 25a | |
| 103 | 24 | 42 | 30b | |
| 176 | 67 (44) | 21 (45) | This studyc | |
| [9-14C]anthraquinone | 176 | 80 | 10 | This study |
| [1,2,3,4,4a,5a-14C]anthracene | 159 | 62 | 28 | This study |
| [3-14C]fluoranthene | 117 | 63 | 19 | 45 |
| [4,5,8,10-14C]pyrene | 64 | 52-16 | 13-45 | 14a |
| 63 | 27-48 | 25-28 | 44 | |
| [7-14C]benzo[a]pyrene | 150 | 48 | 24 | 28 |
Soil from contaminated sites was used.
Anthracene was added with diesel fuel.
See Table 1: soil-compost mixture (native soil).
The amount of organic carbon in soil containing compost is much larger than the amount of organic carbon in native soil. Thus, it can be assumed that the organic matrix may act as an additional binding substrate, as expected based on PAH sorption to humic substances in aqueous phases. However, we demonstrated that the bound residues formed after supplementation of the soil with compost resulted in significantly less formation of bound residues from [9-14C]anthracene and increased mineralization. Obviously, organic substrates like compost, which was degraded to a significant extent during incubation with soil (31), have only a small effect on residue formation and may decrease sequestration of the parent compounds in soil particles. Thus, the metabolic potential of the microflora is an important factor controlling the formation of bound residues during microbial degradation.
Anthraquinone is the primary oxidation product obtained from anthracene during nonspecific oxidation with exoenzymes of ligninolytic fungi (11). Analyses of microbial degradation of anthraquinone showed that extracellular enzymes of ligninolytic and litter-decaying fungi were not involved in anthracene transformation. Fungi play a minor role during mineralization of anthracene in soil, since selective inhibition of fungi resulted in decreased mineralization and increased formation of residues. The major activity is the bacterial activity. No metabolism was observed after inhibition of bacteria, and nearly all of the radioactivity in the extracts was in the parent anthracene. Therefore, sorption of the parent compound had to be the main mechanism of residue formation in this culture. Both cultures containing antibiotics formed more nonextractable residues than the active microflora formed. This indicates that greater residue formation by sorption occurred when metabolism was inhibited. Nonmetabolized PAH may be incorporated into the soil matrix by diffusion and sorption into particles (20, 27, 32) or into a xenobiotic matrix like coal particles (57). However, these mechanisms of residue formation primarily reduce the bioavailability of the compounds, and the major amount remains extractable. In general, the possible mechanisms of residue formation from [14C]PAH depend on the compounds (parent PAH, metabolites, biomass, or CO2) and on the binding matrix (organic matrix, inorganic soil matrix, or xenobiotic matrix). The following three mechanisms for binding of PAH or metabolites are possible: sorption, physical entrapment, and covalent bonds. Sorption is considered a reversible process that is driven by weak interactions (46) and leads to relatively labile associations of xenobiotics compounds and the organic matrix (18). However, the forces may be additive and may cause the adsorption-desorption hysteresis of PAH which is sometimes observed (27). Physical entrapment may occur if cavities of macromolecules are closed by altering the macromolecules. Covalent bonds, like ester-, ether-, C-C-, or C-N- bonds, are much more stable and are linked by chemical reactions. Covalent bonds require specific reactivity of the compounds involved or activation by enzymes or by chemical oxidation reactions and result in the loss of the chemical identities of bound molecules.
Most of the nonextractable bound residues which are formed during microbial degradation of PAH result from incorporation of PAH metabolites. When the parent compounds are sequestered, similar residue formation with the different labeled anthracenes should be expected. However, elevated mineralization rates were observed with [1,2,3,4,4a,5a-14C]anthracene instead of [9-14C]anthracene, and larger amounts were incorporated into the residues under similar conditions. This can only be explained by metabolism, since the availabilities of different C positions in the anthracene molecule are not the same for the anabolic and catabolic processes of soil microorganisms. Another indication that residues are formed from metabolites is the fact that large portions of the radioactivity were extractable before relevant amounts of PAH were degraded. Therefore, formation of microbial metabolites resulting in reactive functional groups in the PAH molecules seems to be essential for decreasing extractability. Oxidation of PAH results in phenolic compounds or aryl radicals, which have high potential for coupling to the organic soil matrix (3). The extent of bound residue formation depends, therefore, on the metabolic potential of the soil microflora and the characteristics of the soil. The position of the 14C label is another important factor which controls the formation of residues from metabolized compounds. The time courses of bound residue formation in the experiments were based on two main processes. Immediately after the PAH were added, diffusion and sequestration of the parent compounds into soil pores occurred (20, 32). After this microbial degradation of the compounds was accompanied by incorporation of metabolites into the organic soil matrix.
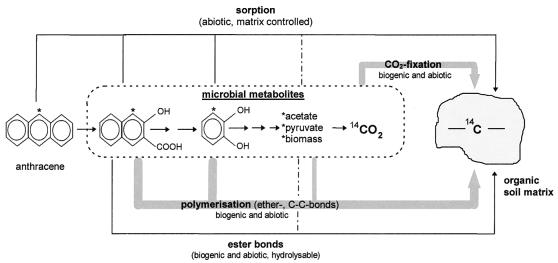
Only limited data concerning the molecular structures of bound residues obtained from PAH are available. In our previous soil studies performed under similar conditions, 0.3% of the PAH applied was extractable after 200 days. Hydrolysis of the soil organic matter released 1.3% of the sorbed or entrapped parent compounds, which were mainly associated with fulvic and humic acids (40). Microbial PAH metabolites like hydroxynaphthoic acids, 9-fluorenone-1-carboxylic acid, 4-phenanthroic acid, and (in other experiments) o-phthalic acid were recovered only after hydrolysis of the soil material. However, the amount obtained represented only 0.5% of the PAH applied. Portions of the metabolites were proven to be covalently bound by ester bonds (40). Only traces of sequestered parent PAH compounds were found in the extracted soil, as determined by pyrolysis gas chromatography-mass spectrometry (41). Overall, only a small amount of the bound residues observed in the experiments could be traced back to PAH or to known metabolites. Thus, the structures of most of the residues were unknown. The high level of stability of the residues suggests that the metabolites were coupled to the organic matrix by ether-, C-C-, or multivalent bonds. This suggestion supports the hypothesis that covalently bound metabolites become integral parts of humic matter, which cannot be further identified (3, 12). Hydroxynaphthoic acids and several hydroxylated PAH metabolites are able to undergo oxidative coupling reactions with compounds in natural organic matter or with themselves and form macromolecules with physical characteristics of humic acids (such as alkaline solubility, precipitation by acids, molecular weights, and nonspecific absorption spectra). These processes may contribute substantially to the formation of bound residues. The formation of macromolecules was initiated by bivalent metal cations which are common in soils (5, 48, 58). Radical-forming extracellular enzymes of ligninolytic fungi are also thought to be involved in generating such macromolecular networks (2, 4, 5, 47). The high levels of stability of these macromolecules also suggest that the metabolites are linked by ether-, C-C-, or multivalent bonds. Some of the nonextractable residues present after mineralizing degradation of anthracene may also be explained by 14CO2 fixation, which accounted for ∼0.2% of the soil carbon content. A minor portion of the residue formed from 14CO2 resulted from fixation of carbonates, but the major portion was due to carbon incorporation into the organic soil matrix, which depended on the activity of the soil microflora. Another indication that CO2 fixation is relevant is based on the partitioning of the radiolabel in humines and humic and fulvic acids, which was similar to partitioning in the residues derived from anthracene. The processes that cause CO2 fixation are still not known. However, our results provide clear evidence that the extent of residue formation by certain PAH metabolites in the humic matrix is smaller than usually presumed. Based on the data presented above, we suggest a scheme for residue formation during biodegradation in soil (Fig. 4). Although no detailed mass balances showing the carbon flow via PAH metabolites or CO2 fixation in the residue fraction are available, we concluded from the amounts transformed that both processes significantly contribute to residue formation. Residue formation by sequestering of the parent compound or by fixation of metabolites that can be remobilized by hydrolysis does not play a major role. In addition, residue formation by intermediate incorporation into biomass and subsequent humification of cell compounds is a general mechanism if the carbon is assimilated during biodegradation. However, no radiolabel was observed in the biomass after degradation of [9-14C]anthracene in previous soil experiments (30). This observation may be explained by the results of experiments performed with pure cultures of Sphingomonas paucimobilis. Position 9 of 13C-labeled anthracene was not transferred into the biomass, although the organism was able to grow on anthracene as a sole source of carbon and energy (unpublished data). Thus, the larger amount of bound residues in the case of [1,2,3,4,4a,5a-14C]anthracene may account for the transformation of the A-ring carbon into residues via biomass. Bound residues derived from highly metabolized PAH do not represent bound residues and should be considered biogenic residues in the sense of humification. Biogenic residues are commonly found in natural soil environments after microbial degradation of natural substances. A total of 30 to 40% of the 14C from 14C-labeled plant material remained in the soil after incubation for 1 year, and this 14C contributed to the formation of humic substances (1, 16, 51).
FIG. 4.
Products of microbial degradation of anthracene and estimated contribution of these products (thick lines) to the formation of nonextractable residues in the organic soil matrix.
Without any evidence of carbon transformation from xenobiotic compounds to natural compounds, the differentiation between bound residues and biogenic residues or humification products is only a theoretical concept. However, it can be generally assumed that bound residues are less toxic, less bioavailable, and less mobile than the free parent compounds (3, 4, 56). Some authors have proposed that enhanced formation of nonextractable residues from xenobiotic compounds should be used as a technique to decrease the toxic potential and bioavailability at contaminated sites (2, 3, 55). However, for critical assessment of such techniques further investigations of residue remobilization under environmental worst-case conditions, of long-term stability, and of structural assignments are necessary.
ACKNOWLEDGMENTS
We thank R. Stegmann for providing the soil bioreactors and K. Spaude for excellent technical assistance.
Parts of this work were supported by grant 1480909 from the German Ministry of Education and Research (BMBF) and by grants from the Deutsche Forschungsgemeinschaft (DFG) within the interdisciplinary research project “Remediation of contaminated soil” (grant SFB 188, project D6).
REFERENCES
- 1.Baldock J A, Oades J M, Vassallo A M, Wilson M A. Incorporation of uniformly labelled 13C-glucose carbon into the organic fraction of a soil. Carbon balance and CP/MAS-13C-NMR measurements. Soil Biol Biochem. 1989;27:725–746. [Google Scholar]
- 2.Berry D F, Boyd S A. Decontamination of soil through enhanced formation of bound residues. Environ Sci Technol. 1985;19:1132–1133. doi: 10.1021/es00141a020. [DOI] [PubMed] [Google Scholar]
- 3.Bollag J-M, Loll M J. Incorporation of xenobiotics into soil humus. Experientia. 1983;39:1221–1231. doi: 10.1007/BF01990359. [DOI] [PubMed] [Google Scholar]
- 4.Bollag J-M, Shuttleworth K L, Anderson D H. Laccase-mediated detoxification of phenolic compounds. Appl Environ Microbiol. 1988;54:3086–3091. doi: 10.1128/aem.54.12.3086-3091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollag J-M, Myers C, Pal S, Huang P M. The role of abiotic and biotic catalysts in the transformation of phenolic compounds. In: Huang P M, Berthelin J, Bollag J-M, McGhill W B, Page A L, editors. Environmental impact of soil component interactions. Boca Raton, Fla: CRC Lewis Publishers; 1995. pp. 299–309. [Google Scholar]
- 6.Bossert I, Kachel M W, Bartha R. Fate of hydrocarbons during oily sludge disposal in soil. Appl Environ Microbiol. 1984;47:763–767. doi: 10.1128/aem.47.4.763-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brotkorb T S, Legge R L. Enhanced biodegradation of phenanthrene in oil tar-contaminated soils supplemented with Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3117–3121. doi: 10.1128/aem.58.9.3117-3121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderbank A. The occurrence and significance of bound pesticide residues in soil. Rev Environ Contam Toxicol. 1989;108:71–102. [Google Scholar]
- 9.Cerniglia C E, Sutherland J B, Crow S A. Fungal metabolism of aromatic hydrocarbons. In: Winkelmann G, editor. Microbial degradation of natural products. Weinheim, Germany: VCH; 1992. pp. 193–217. [Google Scholar]
- 10.Eschenbach A, Kästner M, Bierl R, Schaefer G, Mahro B. Evaluation of a new and more effective method to extract polycyclic aromatic hydrocarbons from soil samples. Chemosphere. 1994;28:683–692. [Google Scholar]
- 11.Field J A, de Jong E, Costa G F, deBont J A M. Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Appl Environ Microbiol. 1992;58:2219–2226. doi: 10.1128/aem.58.7.2219-2226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Führ F. Nonextractable pesticide residues in soil. In: Greenhalgh R, Roberts T R, editors. Pesticide science and biotechnology. Oxford, United Kingdom: Blackwell’s Scientific Publications; 1987. pp. 381–389. [Google Scholar]
- 13.Goodin J D, Weber M D. Persistence and fate of anthracene and benzo(a)pyrene in municipal sludge treated soil. J Environ Qual. 1995;24:271–278. [Google Scholar]
- 14.Grosser R J, Warshawsky D, Vestal R. Indigenous and enhanced mineralization of pyrene, benzo[a]pyrene, and carbazole in soils. Appl Environ Microbiol. 1991;57:3462–3469. doi: 10.1128/aem.57.12.3462-3469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerin W F, Jones G E. Two stage mineralization of phenanthrene by estuarine enrichment cultures. Appl Environ Microbiol. 1988;54:929–936. doi: 10.1128/aem.54.4.929-936.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider K. Proceedings of the International Symposium on Advances in Bioactive Natural Product Chemistry. Seoul, Korea: The Korean Agriculture Chemical Society; 1990. Humus and its significance as a bioactive soil component; pp. 99–131. [Google Scholar]
- 17.Hammel K E. Organopollutant degradation by ligninolytic fungi. In: Young L Y, Cerniglia K E, editors. Microbial transformation and degradation of toxic organic chemicals. Wiley Series in Ecological and Applied Microbiology. New York, N.Y: Wiley-Liss Inc.; 1995. pp. 331–346. [Google Scholar]
- 18.Hassett H J, Banwart W L. The sorption of nonpolar organics by soils and sediments. In: Sahwney B L, Brown K, editors. Reactions and movement of organic chemicals in soils. Special Publication no. 22. Soil Science Society of America, Inc. Madison, Wis: American Society of Agronomy, Inc.; 1989. pp. 31–44. [Google Scholar]
- 19.Hatcher P G, Bortiatynski J M, Minard R D, Dec J, Bollag J-M. Use of high-resolution 13C-NMR to examine the enzymatic covalent binding of 13C-labeled 2,4-dichlorophenol to humic substance. Environ Sci Technol. 1993;27:2098–2103. [Google Scholar]
- 20.Hatzinger P B, Alexander M. Effect of ageing of chemicals in soil on their biodegradability and extractability. Environ Sci Technol. 1995;29:537–545. doi: 10.1021/es00002a033. [DOI] [PubMed] [Google Scholar]
- 21.Heitkamp M A, Cerniglia C E. Polycyclic aromatic hydrocarbon degradation by a Mycobacterium sp. in microcosms containing sediment and water from a pristine ecosystem. Appl Environ Microbiol. 1989;55:1968–1973. doi: 10.1128/aem.55.8.1968-1973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heitkamp M A, Freeman J P, Cerniglia C E. Naphthalene biodegradation in environmental microcosms: estimates of degradation rates and characterization of metabolites. Appl Environ Microbiol. 1987;53:129–136. doi: 10.1128/aem.53.1.129-136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heitkamp M A, Freeman J P, Miller D W, Cerniglia C E. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl Environ Microbiol. 1988;54:2556–2565. doi: 10.1128/aem.54.10.2556-2565.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbes S E, Schwall L R. Microbial transformation of polycyclic aromatic hydrocarbons in pristine and petroleum-contaminated sediments. Appl Environ Microbiol. 1978;35:306–316. doi: 10.1128/aem.35.2.306-316.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosler K R, Bulman T L, Fowlie P J A. Der Verbleib von Naphtalin, Anthracen und Benz(a)pyren im Boden bei einem für die Behandlung von Raffinerieabfällen genutztem Gelände. In: Wolf K, van den Brink W J, Colon F J, editors. Altlastensanierung ’88. Dordrecht, The Netherlands: Kluwer Academic Publisher; 1988. pp. 111–113. [Google Scholar]
- 26.Hsu T S, Bartha R. Hydrolysable and nonhydrolysable 3,4-dichloroaniline humus complexes and their respective rates of biodegradation. J Agric Food Chem. 1976;24:118–122. doi: 10.1021/jf60203a021. [DOI] [PubMed] [Google Scholar]
- 27.Kan A T, Fu G, Tomson M B. Adsorption/desorption hysteresis in organic pollutant and soil/sediment interaction. Environ Sci Technol. 1994;28:859–867. doi: 10.1021/es00054a017. [DOI] [PubMed] [Google Scholar]
- 28.Kanaly R, Bartha R, Fogel S, Findlay M. Biodegradation of [14C]benzo[a]pyrene added in crude oil to uncontaminated soil. Appl Environ Microbiol. 1997;63:4511–4515. doi: 10.1128/aem.63.11.4511-4515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kästner M, Breuer-Jammali M, Mahro B. Enumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons. Appl Microbiol Biotechnol. 1994;41:267–273. [Google Scholar]
- 30.Kästner M, Lotter S, Heerenklage J, Breuer-Jammali M, Stegmann R, Mahro B. Fate of 14C-labeled anthracene and hexadecane in compost manured soil. Appl Microbiol Biotechnol. 1995;43:1128–1135. doi: 10.1007/BF00166937. [DOI] [PubMed] [Google Scholar]
- 31.Kästner M, Mahro B. Microbial degradation of polycyclic aromatic hydrocarbons in soils affected by the organic matrix of compost. Appl Microbiol Biotechnol. 1996;46:668–675. doi: 10.1007/BF00172501. [DOI] [PubMed] [Google Scholar]
- 32.Kelsey J W, Alexander M. Declining bioavailability and inappropriate estimation of risk of persistent compounds. Environ Toxicol Chem. 1997;16:582–585. [Google Scholar]
- 33.Khan S U. Bound pesticide residues in soil and plants. Residue Rev. 1982;84:1–25. doi: 10.1007/978-1-4612-5756-1_1. [DOI] [PubMed] [Google Scholar]
- 34.Mahaffey W R, Gibson D T, Cerniglia C E. Bacterial oxidation of chemical carcinogens: formation of polycyclic aromatic acids from benz(a)anthracene. Appl Environ Microbiol. 1988;54:2415–2423. doi: 10.1128/aem.54.10.2415-2423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manilal V B, Alexander M. Factors affecting the microbial degradation of phenanthrene in soil. Appl Microbiol Biotechnol. 1991;35:401–405. doi: 10.1007/BF00172733. [DOI] [PubMed] [Google Scholar]
- 36.Mansour M, Scheunert I, Korte F. Fate of persistent organic compounds in soil and water. NATO Adv Study Inst Ser Ser A Life Sci. 1993;32:111–139. [Google Scholar]
- 37.McCarthy P, Malcolm R L, Clapp C E, Bloom P R. An introduction to soil humic substances. In: McCarthy P, Malcolm R L, Clapp C E, Bloom P R, editors. Humic substances in soil and crop sciences. Vol. 1. Madison, Wis: American Society of Agronomy Inc.; 1990. pp. 1–12. [Google Scholar]
- 38.Means J G, Wood S G, Hassett J J, Banwart W L. Sorption of polynuclear aromatic hydrocarbons by sediments and soils. Environ Sci Technol. 1980;14:1524–1528. doi: 10.1021/es60172a005. [DOI] [PubMed] [Google Scholar]
- 39.Nordlohne L, Eschenbach A, Wienberg R, Mahro B, Kästner M. Versuche in Kleinreaktoren und Batchversuche mit Zudotierung 14C-markierter Schadstoffe. Teilprojekt 4, Wissenschaftliches Untersuchungsprogramm “Veringstraße” (Verbundvorhaben): Sanierungsbegleitende Untersuchung zur Stoffbilanz und zur Metabolitenbildung bei der Durchführung eines Weißfäule-Mietenverfahrens zur Reinigung des PAK-kontaminierten Bodens von dem Schadensfall Veringstraße 2: Abschlußberichte. Hamburg, Germany: Umweltbehörde der Hansestadt Hamburg; 1995. [Google Scholar]
- 40.Richnow H H, Seifert R, Hefter J, Kästner M, Mahro B, Michaelis W. Metabolites of xenobiotica and mineral oil constituents linked to macromolecular organic matter in polluted environments. Org Geochem. 1994;22:671–681. [Google Scholar]
- 41.Richnow H H, Seifert R, Kästner M, Mahro B, Horsfield B, Tiedgen U, Böhm S, Michaelis W. Rapid screening of PAH-residues in bioremediated soils. Chemosphere. 1995;31:3991–3999. [Google Scholar]
- 42.Richnow H H, Seifert R, Hefter J, Link M, Francke W, Schaefer G, Michaelis W. Organic pollutants associated with macromolecular soil organic matter—a mode of binding. Org Geochem. 1997;26:745–758. [Google Scholar]
- 43.Roberts T R. Nonextractable pesticide residues in soil and plants. IUPAC reports on pesticides. Pure Appl Chem. 1984;56:945–956. [Google Scholar]
- 44.Sack U, Fritsche W. Enhancement of pyrene mineralization in soil by wood-decaying fungi. FEMS Microbiol Ecol. 1997;22:77–83. [Google Scholar]
- 45.Schnöder F, Mittelstaedt W, Führ F. Das Verhalten von Benzo(a)pyren und Fluoranthen in einer Parabraunerde—Lysimeter und Abbaustudien. In: Weigert B, editor. Biologischer Abbau von polyzyklischen aromatischen Kohlenwasserstoffen. Schriftenreihe Biologische Abwasserreinigung 4, SFB 193. Berlin, Germany: Technische Universität Berlin; 1994. pp. 217–230. [Google Scholar]
- 46.Senesi N. Organic pollutant migration in soils as affected by soil organic matter. Molecular and mechanistic aspects. NATO Adv Study Inst Ser Ser A Life Sci. 1993;32:47–74. [Google Scholar]
- 47.Shannon M J R, Bartha R. Immobilization of leachable toxic soil pollutants by using oxidative enzymes. Appl Environ Microbiol. 1988;54:1719–1723. doi: 10.1128/aem.54.7.1719-1723.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shindo H. Significance of Mn(IV) and Fe(III) oxides in the synthesis of humic acids from phenolic compounds. In: Senesi N, Miano T M, editors. Humic substances in the global environment and implications on human health. Amsterdam, The Netherlands: Elsevier Science B. V.; 1994. pp. 361–366. [Google Scholar]
- 49.Stegmann R, Lotter S, Heerenklage J. Biological treatment of oil-contaminated soils in bioreactors. In: Hinchee R E, Olfenbuttel R F, editors. On-site bioreclamation. Boston, Mass: Butterworth-Heinemann; 1991. pp. 188–208. [Google Scholar]
- 50.Stevenson F J. Humus chemistry: genesis, composition, reactions. 2nd ed. New York, N.Y: Wiley; 1994. [Google Scholar]
- 51.Stott D E, Kassim G, Jarrell W M, Martin J P, Haider K. Stabilization and incorporation into biomass of specific plant carbons during biodegradation in soil. Plant Soil. 1983;70:15–26. [Google Scholar]
- 52.Stott D E, Martin J P, Focht D D, Haider K. Biodegradation, stabilization in humus, and incorporation into soil biomass of 2,4-D and chlorocatechol carbons. Soil Sci Soc Am J. 1983;47:66–70. [Google Scholar]
- 53.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss Inc.; 1995. pp. 269–306. [Google Scholar]
- 54.Thorn K A, Pettigrew P J, Goldenberg W S, Weber E J. Covalent binding of aniline to humic substances. 2. 15NMR studies on nucleophilic addition reactions. Environ Sci Technol. 1996;30:2764–2774. [Google Scholar]
- 55.Verstraete W, Devliegher W. Formation of non-bioavailable organic residues in soil: perspectives for site remediation. Biodegradation. 1996;7:471–485. [Google Scholar]
- 56.Wang X, Yu X, Bartha R. Effect of bioremediation on polycyclic aromatic hydrocarbon residues in soil. Environ Sci Technol. 1990;24:1086–1089. [Google Scholar]
- 57.Weißenfels W D, Klewer H J, Langhoff J. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: influence on biodegradability and biotoxicity. Appl Microbiol Biotechnol. 1992;36:689–696. doi: 10.1007/BF00183251. [DOI] [PubMed] [Google Scholar]
- 58.Ziechmann W. Humic substances. Mannheim, Germany: BI-Wissenschaftsverlag; 1994. [Google Scholar]