Abstract
Background
Conditional Cash Transfer Programs have been developed in Latin America in response to poverty and marked social inequalities on the continent. In Brazil, the Bolsa Familia Program (BFP) was implemented to alleviate poverty and improve living conditions, health, and education for socioeconomically vulnerable populations. However, the effect of this intervention on maternal and child health is not well understood.
Methods
We will evaluate the effect of BFP on maternal and child outcomes: 1. Birth weight; 2. Preterm birth; 3. Maternal mortality; and 4. Child growth. Dynamic retrospective cohort data from the 100 Million Brazilian Cohort (2001 to 2015) will be linked to three different databases: Live Birth Information System (2004 to 2015); Mortality Information System (2011 to 2015); and Food and Nutritional Surveillance System (2008 to 2017). The definition of exposure to the BFP varies according to the outcome studied. Those who never received the benefit until the outcome or until the end of the follow-up will be defined as not exposed. The effects of BFP on maternal and child outcomes will be estimated by a combination of propensity score-based methods and weighted logistic regressions. The analyses will be further stratified to reflect changes in the benefit entitlement before and after 2012.
Discussion
Harnessing a large linked administrative cohort allows us to assess the effect of the BFP on maternal and child health, while considering a wide range of explanatory and confounding variables.
Background
Poverty and social inequality have been identified as major social causes of poor health, requiring public policies and strategies to eradicate poverty and improve the most vulnerable populations’ living and health conditions [1–3]. Despite the advances observed on maternal and child health in the last decades, the slow decline in maternal mortality and the persistence of adverse outcomes, such as preterm birth (PTB), low birth weight (LBW), and child malnutrition, especially among the low and -middle-income countries (LMIC), hinder the achievement of the Sustainable Development Goals (SDGs) [4–8]. In Brazil, the maternal mortality ratio was 59.7 per 1,000 live births in 2015 (a 57% decline in 25 years), and the national prevalence of PTB and LBW were respectively 11.1%, and 8.4% [9]. Malnutrition estimates in children under five years old enrolled in the Bolsa Familia Program (BFP), a Brazilian Conditional Cash Transfer (CCT), showed a high prevalence of stunting (12.7%) and overweight/obesity (18.4%) in 2014 [10].
CCTs have been adopted as a strategy to promote maternal and child health [11–13]. Programs focused on combating immediate and future poverty may improve access to health, education, social assistance, employment, and income [14]. The BFP is one of the oldest and largest CCTs in the world, with over 13.2 million beneficiary families, corresponding to 96% coverage of the country’s poor households (estimates for February 2020) [15]. While Brazil was one of the pioneers in implementing the CCT in Latin America, there is still little evidence on the effect of BFP on maternal and child outcomes, especially from studies using robust methods and large-scale individual-level data with an extended period of follow-up [11, 12, 14]. Understanding the health and health equity impacts of social policies is important to inform policymaking, including decisions about ongoing investment in these schemes [12, 14, 16, 17].
The most important contribution of the proposed research will be developing robust evidence of the effect of the BFP on maternal and child outcomes, using a cohort which allow us assessing more robust statistical analyzes in the general population and separately for specific subpopulations.
Methods
Primary objective, study design, and overall population
We aim to evaluate the effect of BFP on maternal and child outcomes in the 100 Million Brazilian Cohort [18]. The main objective of the Cohort is to enable the study of the social determinants and the effects of social policies and programs on the different aspects of health in Brazil [18]. It is a dynamic retrospective cohort, the population of which is derived from more than 114 million individual records from the Single Registry for Federal Government’s Social Programs (CadÚnico). The cohort contains administrative records from CadÚnico and the BFP Payroll. CadÚnico identifies and characterizes low-income households and registration is required in order to receive any Federal Government’s social programs, such as the BFP [19]. The Cohort allows us to extract socioeconomic information from the individual, the household, and data related to receiving the benefit. The detailed variables and databases to be used are shown in Table 1.
Table 1. Structure and main components of the 100 Million Brazilian Cohort, sources of data, and relevant variables.
| Components | Data source | Period | Number of Records | Relevant variables |
|---|---|---|---|---|
| Cohort Baseline | Single Registry (CadÚnico) | 2001 to 2015 | 114,008,317 | Socioeconomic and demographic conditions (information on family dynamics, childcare arrangements, parental employment, income, housing family formation, dissolution, social programs information, household characteristics). |
| Intervention (Exposure) | Bolsa Familia Program (BFP) | 2004 to 2015 | 27,376,582 | Start and end of data receipt of benefit, total value by family, and number of months received. |
| Outcomes | Live Birth Information System (SINASC) | 2001 to 2015 | 44,485,274 | Characteristics of the newborn (sex, Apgar score in the 1 and 5 minutes, birth weight, presence of an abnormality, congenital anomalies identified at birth), characteristics of the mother (age, marital status, education, race, place of residence), characteristics of pregnancy and delivery (number of previous pregnancies of live births, stillbirth or abortion, gestational age, place of birth, type of delivery, number of fetuses, number of prenatal visits, month that started prenatal). Some variables such as the month in which the woman started prenatal care and gestational age (continuous) are only available for the period from 2011 to 2015. |
| Outcomes | Mortality Information System (SIM) | 2000 to 2015 | 17,829,111 | Type of death, date of death, date of birth, sex, race, education, duration of the pregnancy, single or multiple pregnancies, type of delivery, age of mother, gestational age, birth weight, and death cause. |
| Outcomes | Food and Nutrition Surveillance System (SISVAN) | 2008 to 2017 | 307,245,508 | Date of birth, age, sex, race/ethnicity, traditional communities, anthropometric data (weight and height), measurement date, presence of chronic diseases (diabetes and cardiovascular diseases), and deficiencies and complications (diarrhea and anemia). |
The primary objective will be achieved by linking the Cohort (2001 to 2015) and data from (i) the Live Birth Information System (SINASC) (2004 to 2015); (ii) the Mortality Information System (SIM) (2011 to 2015); and (iii) the Food and Nutrition Surveillance System (SISVAN) (2008 to 2017). We will use CIDACS Record Linkage (CIDACS-RL) to link the databases [20]. The linkage procedures are common for the 100 Million Cohort studies and consist of two stages. The first will be a deterministic linkage, and the second will be based on the similarity index. More detailed information can be consulted in previous publications [21, 22]. The CIDACS-RL is a tool for linking individual records based on identifiers: name, gender, age or date of birth, mother’s name, and the municipality of residence [22]. All linking procedures will be performed at CIDACS (Center for Data Integration and Knowledge for Health, Fiocruz) [23] in a strict data protection environment and complying with ethical and legal standards [24].
The Bolsa Familia Program (BFP)
We describe the policy in accordance with the TIDieR-PHP reporting guideline [25]. The checklist consists of nine items and helps researchers to describe the characteristics of population health and policy interventions. The BFP was implemented from a national decree in 2004, with eligibility criteria (poverty and extreme poverty cutoff points) and incorporation of benefits that varied over time [26–31]. The cut-off points and the eligibility criteria are shown in Table 2. The selection of households eligible for the BFP occurs through enrollment in the CadÚnico [26, 31]. Households served by the BFP receive a monthly cash benefit through a withdrawal card issued by the Caixa Econômica Federal [32].
Table 2. Changes in the eligibility criteria and inclusion of new groups of beneficiaries.
| Year | Extreme poverty* (R$) | Poverty* (R$) | Inclusion of new groups (varying benefits) |
|---|---|---|---|
| 2004 | 50.00 | 100.00 | No change |
| 2006 | 60.00 | 120.00 | No change |
| 2009 | 70.00 | 140.00 | Concession of benefits to households with adolescents aged 16–17 years enrolled in education institutions |
| 2012 | No change | No change | Concession of benefits to households with children aged zero to six. Concession of varying benefits to pregnant women and nursing mothers |
| 2014 | 77.00 | 154.00 | No change |
| 2016 | 85.00 | 170.00 | No change |
| 2018 | 89.00 | 178.00 | No change |
* Household units with a per capita household income less than or equal to the mentioned value.
The BFP is equipped with fraud prevention control mechanisms, with public access to beneficiaries’ individual data over the internet and semiannual comparison of CadÚnico’s enrolled data with other databases [21]. The suspension of households from BFP can occur due to failure to update the registration information, no longer fitting the profile (eligibility criteria), and noncompliance with conditionalities [32]. The program’s conditionalities are geared to participation in education, health, and social assistance. In the field of health, conditionalities include actions, such as immunization, prenatal care, and child growth monitoring [26, 31].
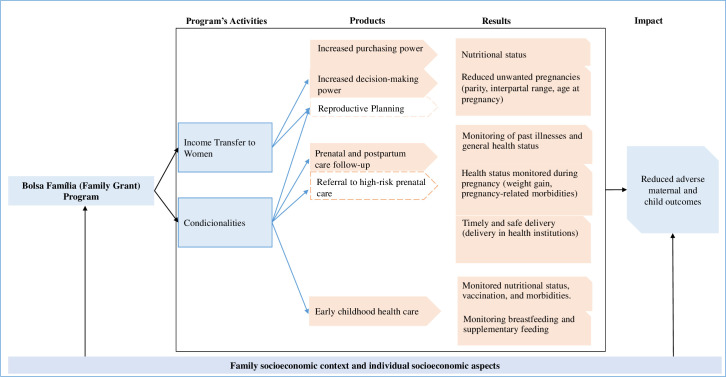
Logic models
We created a logic model to describe the hypothesized mechanisms through which the BFP might affect maternal and child outcomes (Fig 1). The socioeconomic characteristics can influence both the receipt of the benefit and maternal and child health outcomes [33–39]. Characteristics of particular relevance include targeting monetary resources preferentially to women and the fulfillment of conditionalities. Despite not being a guarantee, the BFP may increases women’s decision-making power [40], has the potential to transform women into heads of households with responsibility for directing the money received. The transfer of income to women can have a more immediate effect on maternal and child health outcomes, with female empowerment, the allocation of money for the purchase of food, and the use of health services [41–46].
Fig 1. Logical model of the impact of the Bolsa Familia Program (BFP) in reducing adverse maternal and child outcomes.
On the other hand, BFP also requires the fulfillment of conditionalities, using services during pregnancy, puerperium, and early childhood [26, 31]. Using health services is an important determinant of maternal and child outcomes [4, 47–54] because it can have an immediate effect on these outcomes, with immunization, nutritional counseling and preventive behaviors during prenatal care, monitoring of comorbidities, and connected to the place of birth [47, 55–57]. The reduction of adverse maternal and child outcomes depends on joint efforts that ensure access to quality health services and lower social inequalities [12, 16, 17, 34, 35, 58].
Secondary objectives, study population, definition of exposure, and outcomes
The definitions of outcomes, study populations, and exposure to BFP will be presented separately (detailed information in Chart 1, as supplementary material), according to the objectives:
assess the effect of BFP on birth weight, small and large for gestational age (SGA/LGA) and on preterm births
evaluate the effect of BFP on maternal mortality
assess the effect of BFP on child malnutrition.
i) Birth weight, SGA, LGA, and preterm birth
Study population. The study will include baseline data from the “100 Million Brazilian Cohort” linked to SINASC (Table 1). The study population will consist of the first and the second live birth of women registered in the cohort baseline, from 2004 to 2015, with ages ranging from 10 to 49 years. The study population for the SGA, LGA and PTB refers to 2012 to 2015 period, due to the inclusion gestational age as a continuous variable in 2011.
Multiple births and newborns with congenital anomalies will be excluded to avoid bias, as these conditions are known to be strongly associated with low birth weight and PTB [52, 59–61]. Fetal viability criteria can be applied [62–65]. Regarding birth weight, the inclusion of the first live birth is a strategy to capture the effect of receiving the BFP during the first and the second pregnancy. Ordering the live births will allow us to select/extract the population of interest and obtain previous birth information such as inter-birth interval, low birth weight, and preterm birth.
Since nulliparous women are at increased risk of chronic and acute medical and obstetrical complications leading to preterm birth, we will restrict the population related to PTB to singleton live births whose mothers in reproductive age have at least one child before joining the Cohort. Only the firstborn after enrolment will be included.
Exposure to BFP. The exposure is defined as having started receiving BFP before the birth of their child in the 2004 to 2015 period (or 2012 to 2015 for SGA, LGA and PTB) and did not stop receiving from pregnancy to delivery. Live births of women who did not receive the benefit at any time until delivery will be considered as not exposed.
Outcomes. Birth weight will be considered as (1) birth weight, in grams (continuous variable), and (2) birth weight categorized into very low, low, normal, and high (see Table 3) [66].
Table 3. Description of the outcomes that will be considered in studies by assessing the impact of the Bolsa Familia Program (BFP).
| Objective | Original variables used to construct the outcome | Outcome |
|---|---|---|
| To evaluate the effect of BFP on birth weight, small and large for gestational age and preterm birth | Birth weight in grams | Birth weight in grams (continuous variable) |
| Adequate birth weight (≥2500g) vs. low birth weight (<2500g) | ||
| Adequate weight (2500-3999g) vs. extremely low weight (<1000g), very low weight (1000-1499g), low birth weight (1500-2499g) and macrosomia (≥4000g) | ||
| Weight in grams and Gestational age in full weeks (available from 2011) | Adequate for gestational age (between 10th and 90th percentiles) vs. Small for gestational age (<10th percentile) and Large for gestational age (>90th percentile) | |
| Extreme weights for gestational age: 10th to 90th percentile vs. <3rd percentile; 3rd to 9th percentile, 91st to 97th percentile and >97th percentile | ||
| Gestational age in categories | non-preterm birth (≥37 gestational weeks) vs. preterm birth (<37 gestational weeks) | |
| Non-PTB vs. moderate-to-late PTB (32 to 36 gestational weeks), very PTB (28–31 gestational weeks) e extreme PTB (< 28 gestational weeks) | ||
| To assess the effect of BFP on maternal mortality | Underlying cause of death | Non-death vs. death of a woman during pregnancy or up to 42 days after the end of pregnancy, due to any cause related to or aggravated by the pregnancy, but not due to accidental or incidental causes. |
| Intermediate cause of death | ||
| To assess the effect of BFP on child malnutrition | Length/height in centimeters, age in months, and sex | Height-for-age z-score (HAZ) |
| HAZ ≥ –2 (benchmark) vs. HAZ < –2 (stunting) | ||
| HAZ ≥ –2 (benchmark) vs. HAZ <-3 (severe stunting) and HAZ ≥ –3 to HAZ < –2 (moderate stunting) | ||
| Weight in grams, age in months, and gender | Weight-for-age z-score (WAZ) | |
| WAZ ≥ –2 to ≤ +2 (benchmark) vs. WAZ < –2 (underweight) | ||
| WAZ ≥ –2 to ≤ +2 (benchmark) vs. WAZ < –3 (severe underweight) and WAZ ≥ –3 to < –2 (moderate underweight) | ||
| Weight in grams, length/height in centimeters, and gender | Weight-for-height z-score (WHZ) | |
| WHZ ≥ –2 and ≤ +2 (benchmark) vs. WHZ < –2 (wasting) | ||
| WHZ ≥ –2 and ≤ +2 (benchmark) vs. WHZ < –3 (severe wasting) and WHZ ≥ –3 and < –2 (moderate wasting) | ||
| WHZ ≥ –2 and ≤ +2 (benchmark) vs. WHZ > +2 (overweight/obesity) | ||
| WHZ ≥ –2 and ≤ +2 (benchmark) vs. WHZ > +3 (obesity) and WHZ ≤ +3 to > +2 (overweight) |
Small for Gestational Age will be defined as birth weight according to gestational age and gender below the 10th percentile; Adequate for Gestational Age, between the 10th and 90th percentiles; and Large for Gestational Age, above the 90th percentile [66, 67]. Categories will also be explored, including weight extremes for gestational age (Table 3).
Preterm birth will be defined as 1. PTB (22 to <37 gestational weeks) vs. non-PTB (37 to 42 gestational weeks); and 2. stratified (Table 3), according to the degree of severity [66].
ii) Maternal mortality
Study population. The study will include data from 100 Million Brazilian Cohort linked to SINASC and SIM. The study population will consist of women of reproductive age (10 to 49 years) according the surveillance criteria in Brazil, registered in the Cohort baseline, in their last pregnancy in the 2004 to 2015 period.
Exposure to BFP. The exposure is defined as having started receiving the BFP before or during pregnancy and did not stop receiving the benefit before the outcome or until childbirth. Women who have not received the benefit at any time until childbirth or the puerperium will be considered as not exposed.
Outcome. Maternal death will be defined as the death of women during pregnancy or up to 42 days after the end of pregnancy, due to any cause related to or aggravated by the pregnancy, but not due to accidental or incidental causes. We will evaluate the follow outcome according the International Classification of Diseases–ICD-10: ICD-10 “XV” codes will be considered (Pregnancy, childbirth and the puerperium (O00-O99) to compose cases of maternal death, except for deaths after 42 days, “O96” and “O97”; and other ICD-10 chapters (A34, F53, M83.0, B20 to B24, D39.2, and E23.0) [68].
iii) Child malnutrition
Study population. The study will include data from the 100 Million Brazilian Cohort linked to SISVAN and SINASC. The study population will consist of children aged 0 to 5 years registered in the cohort baseline from 2004 to 2015. Anthropometric information from the last visit in the 2008 to 2017 period will be used.
Definition of exposure. Exposure in the studied population will consist of children who started and did not stop receiving the BFP before the last visit (2008 to 2017) to answer the objective of interest. Those not exposed will be the ones who have not received the benefit at any time until the date of the child’s last visit.
Outcome. Nutritional status in children will be computed according to the WHO growth references and cutoff points for standardized height-for-age z-score (HAZ), weight-for-age z-score (WAZ), and weight-for-height z-score (WHZ) [69]. Anthropometric indices will be considered as continuous and categorized measures (Table 3).
Statistical analysis
The effect of BFP on birth weight, preterm birth, maternal mortality, and child growth will be estimated based on propensity score-based methods (PS). The PS can be characterized as the conditional probability of receiving the treatment (to be a BFP beneficiary or not), given its observable characteristics [70]. These methods are different from the others in that they avoid multidimensionality and can be implemented using a control variable, which is the propensity score itself [46].
First, the missing data patterns will be evaluated for the variables considered in the calculation of the PS. Depending on these analyses, the PS calculation can be performed only with complete data or including the missing data as a category in each variable. The PS will be estimated using a logit model with baseline covariables related to receipt of BFP according Chart 1 (supplementary material).
The models will be weighted by the Inverse Probability Treatment Weighting (IPTW) and by the Kernel weights. Balancing will be performed before and after weighting to ensure that the procedure used controlled for the available confounders. Finally, weighted Logistic Regressions and the Average Treatment Effect on Treated (ATT) will be calculated using non-linear and linear models, depending on the analyzed outcome [71, 72].
Robustness analysis for propensity score-based methods
As it is a dynamic cohort, analyses will be considered according to the treatment exposure time. Supplementary analyses will also be carried out with subpopulations with similar lengths of time since entering the cohort to balance the time until the outcome between recipients and controls. Also, analyses will be carried out for municipalities with a higher quality of information from vital statistics and according to the quantiles of coverage of the Family Health Strategy, region of residence and the decentralized (municipal) management index (IGD) of the BFP; and for subpopulations of maternal reproductive age (15 to 49 years or 10 to 49 years) [73, 74] and prenatal care follow-up.
Ethical considerations
The Research Ethics Committee of the Institute of Collective Health, Federal University of Bahia (ICS-UFBA), approved the studies involved in this protocol under Opinion N° CAAE: 41695415.0.0000.5030 on May 30, 2017.
The linkage of the databases will be carried out in a secure environment, following a strict internal information security procedure to ensure data privacy and confidentiality [21]. A non-identified database will be used for the analyses, which can only be accessed by previously authorized researchers, and all steps after obtaining the data will be carried out following the CIDACS information security culture.
Discussion
This study will use propensity score-based methods to assess the BFP effect on maternal and child health outcomes in a large sample of poor and impoverished Brazilian households. The BFP might be expected to result in positive effects in all conditions related to difficulties in accessing health, education, social assistance, employment, and income, thus, improving maternal and child health conditions. The study will follow internationally recognized guidelines for conducting and disseminating the results of impact assessment studies, providing transparency in conducting data analysis, and greater comparability of results [25, 75, 76].
Some limitations must be considered. Information systems can include missing data and lack of relevant information on potential outcome and confounding variables, such as more specialized access and quality of prenatal or postnatal care indicators, which could allow a better understanding of the nuances of the intervention (for example, distance to the clinic or ability and training of health professionals). We will not explore the results of the BFP concerning the amount of the transfers granted. BFP is a binary variable in our study, and nuances related to the amount received and poverty levels will not be explored in this first proposal.
On the other hand, the large-scale data set will allow us to investigate comprehensively and in subpopulations the effects of BFP on maternal and child outcomes. The use of these databases will allow us exploring rarer outcomes with a high level of statistical power. The databases used in this study have national coverage, low under-registration, and some have already documented reliability [59]. Thus, this study will provide a comprehensive and representative analysis of the poor and extremely poor Brazilian population and reinforce the adequacy of these bases for epidemiological investigations [59]. The availability of a cohort with socioeconomic information linked to maternal and child health data provides us with the possibility to assess the effect of the BFP on these outcomes, considering a wide range of explanatory and confounding variables, and enabling the use of methods based on propensity scores.
Dissemination of knowledge
This evaluation of BFP will provide tools and evidence to program management focused on poverty reduction and reduction of adverse outcomes related to maternal and child health. We will disseminate the data in scientific journals, reports, and policy briefings targeting policymakers and civil society.
Supporting information
(DOCX)
List of abbreviations
- ATT
Average Treatment Effect on Treated
- BFP
Bolsa Familia (Family Grant) Program
- CadÚnico
Single Registry for Federal Government’s Social Programs
- CCT
Conditional Cash Transfer Program
- CIDACS
Centre for Data and Knowledge Integration for Health
- CIDACS-RL
CIDACS Record Linkage
- HAZ
Height-for-age z-score
- IGD
Decentralized (municipal) management index of the BFP
- IPTW
Inverse Probability of Treatment Weighting
- LBW
Low birth weight
- LGA
Large for Gestational Age
- LMIC
Low and -middle-income countries
- PTB
Preterm birth
- PS
Propensity Score
- SDG
Sustainable Development Goals
- SGA
Small for Gestational Age
- SIM
Mortality Information System
- SINASC
Live Birth Information System
- SISVAN
Food and Nutrition Surveillance System
- WAZ
Weight-for-age z-score
- WHZ
Weight-for-height z-score
Data Availability
All data will be obtained from Centro de Integração de Dados e Conhecimentos para Saúde (CIDACS). Importantly, restrictions apply to the availability of these data, which will be licensed for exclusive use in the studies, and are thus not publicly available. Upon reasonable request and with the express permission of CIDACS, the authors are willing to make every effort to grant data availability. No data was used or analyzed for this protocol. Data will be included in completed study.
Funding Statement
CIDACS received financial support by MCTI / CNPq / MS / SCTIE / Decit / Bill & Melinda Gates Foundation’s Grandes Desafios Brasil – Desenvolvimento Saudável para Todas as Crianças (call number 47/2014) (grant number OPP1142172). CIDACS and the 100 Million cohort received financial support from the Wellcome Trust (grant number 202912/Z/16/Z), the Health Surveillance Secretariat, Ministry of Health, Brazil, Bahia State (Decentralized Execution Term – TED number 159/2019), Research Support Foundation of the State of Bahia (FAPESB) (grant number INT0001/2015), the Research and Project Funding Agency (FINEP) (Notice CT-INFRA - FIOESTAT - Agreement number 04.10.0635.00, reference number 811/10). CIDACS received material support (referring to rooms in Bahia Technology Park in Salvador, state of Bahia) from Secretariat of Science and Technology of the State of Bahia (SECTI) (term of assignment of movable property 048/2018, process number 1430150022698). Individual financial support: IRF received a doctoral scholarship from the Research Support Foundation of the State of Bahia (FAPESB) (grant number BOL2330/2016). ESP is a fellow supported by the Wellcome Trust (grant number 13589/Z/18/Z). SVK acknowledges funding from a NRS Senior Clinical Fellowship (grant number SCAF/15/02). SVK and AHL also receive funding from the Medical Research Council (grant number MC_UU_12017/13) and Scottish Government Chief Scientist Office (grant number SPHSU13). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors received no specific funding for this work.
References
- 1.Marmort M. Social determinants of health inequalities. The Lancet. 2005; 365: 1099–104. [DOI] [PubMed] [Google Scholar]
- 2.Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Reports (Washington, DC: 1974). 2014; 129 Suppl 2(Suppl 2): 19–31. doi: 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreto ML. Health inequalities: a global perspective. Ciência & Saúde Coletiva. 2017;22(7):2097–108. doi: 10.1590/1413-81232017227.02742017 [DOI] [PubMed] [Google Scholar]
- 4.Glassman A, Duran D, Fleisher L, Singer D, Sturke R, Angeles G, et al. Impact of conditional cash transfers on maternal and newborn health. Journal of Health, Population and Nutrition. 2013;31(4 SUPPL.2):S48–S66. [PubMed] [Google Scholar]
- 5.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. The Lancet Global Health. 2019; 7(7): e849–e60. doi: 10.1016/S2214-109X(18)30565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012; 379 (9832): 2162–72. Epub 2012/06/12. doi: 10.1016/s0140-6736(12)60820-4 . [DOI] [PubMed] [Google Scholar]
- 7.Gonçalves H, Barros FC, Buffarini R, Horta BL, Menezes AMB, Barros AJD, et al. Infant nutrition and growth: trends and inequalities in four population-based birth cohorts in Pelotas, Brazil, 1982–2015. International journal of epidemiology. 2019; 48(Supplement_1): i80–i8. doi: 10.1093/ije/dyy233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araújo TSd, Oliveira CSdM, Muniz PT, Silva-Nunes Md, Cardoso MA. Desnutrição infantil em um dos municípios de maior risco nutricional do Brasil: estudo de base populacional na Amazônia Ocidental Brasileira. Revista Brasileira de Epidemiologia. 2016; 19: 554–66. doi: 10.1590/1980-5497201600030007 [DOI] [PubMed] [Google Scholar]
- 9.Leal MdC, Szwarcwald CL, Almeida PVB, Aquino EML, Barreto ML, Barros F, et al. Saúde reprodutiva, materna, neonatal e infantil nos 30 anos do Sistema Único de Saúde (SUS). Ciência & Saúde Coletiva. 2018; 23: 1915–28. [DOI] [PubMed] [Google Scholar]
- 10.Silva NJ, Ribeiro-Silva RC, Rasella D, Alves FJO, Campello T, Fiaccone RL, et al. Shifts towards overweight and double burden of malnutrition among socio-economically vulnerable children: a longitudinal ecological analysis of Brazilian municipalities. Public health nutrition. 2021; 24(15): 4908–17. Epub 2020/11/24. doi: 10.1017/S1368980020004735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paes-Sousa R, Santos LMP, Miazaki ES. Effects of a conditional cash transfer programme on child nutrition in Brazil. Bulletin of the World Health Organization. 2011; 89(7): 496–503. doi: 10.2471/BLT.10.084202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasella D, Aquino R, Santos CAT, Paes-Sousa R, Barreto ML. Effect of a conditional cash transfer programme on childhood mortality: A nationwide analysis of Brazilian municipalities. The Lancet. 2013;382(9886):57–64. doi: 10.1016/S0140-6736(13)60715-1 [DOI] [PubMed] [Google Scholar]
- 13.Rivera JA, Sotres-Alvarez D, Habicht JP, Shamah T, Villalpando S. Impact of the Mexican program for education, health, and nutrition (Progresa) on rates of growth and anemia in infants and young children: a randomized effectiveness study. Jama. 2004; 291(21): 2563–70. Epub 2004/06/03. doi: 10.1001/jama.291.21.2563 . [DOI] [PubMed] [Google Scholar]
- 14.Santos LMP, Paes-Sousa R, Miazagi E, Silva TF, Fonseca AMMd. The Brazilian experience with conditional cash transfers: A successful way to reduce inequity and to improve health inequity. World Conference on Social Determinants of Health; Rio de Janeiro, Brazil: World Health Organization; 2011. Available from: https://dds.cepal.org/redesoc/publication?id=1636.
- 15.Secretaria de Avaliação e Gestão da Informação—SAGI. Quantidade de Famílias beneficiadas pelo Bolsa Família, Estimativa de Famílias Pobres—Censo IBGE 2010, Percentual de cobertura das Famílias beneficiárias do PBF 2020 [cited 2020 08/04/2020]. Available from: https://aplicacoes.mds.gov.br/sagi/vis/data3/data-explorer.php#.
- 16.Boubred F, Pauly V, Romain F, Fond G, Boyer L. The role of neighbourhood socioeconomic status in large for gestational age. PLOS ONE. 2020; 15(6): e0233416. doi: 10.1371/journal.pone.0233416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell EA. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin Obstet Gynecol. 2018; 61(2): 387–99. doi: 10.1097/GRF.0000000000000349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto ML, Ichihara MY, Pescarini JM, Ali MS, Borges GL, Fiaccone RL, et al. Cohort profile: The 100 Million Brazilian Cohort. International journal of epidemiology. 2021: dyab213. doi: 10.1093/ije/dyab213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caixa Econômica Federal. Cadastro Único 2018 [cited 2019 05/11/2019]. Available from: http://www.caixa.gov.br/cadastros/cadastro-unico/Paginas/default.aspx.
- 20.Barbosa GCG, Ali MS, Araujo B, Reis S, Sena S, Ichihara MYT, et al. CIDACS-RL: a novel indexing search and scoring-based record linkage system for huge datasets with high accuracy and scalability. 2020; 20(289). doi: 10.1186/s12911-020-01285-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali MS, Ichihara MY, Lopes LC, Barbosa GCG, Pita R, Carreiro RP, et al. Administrative Data Linkage in Brazil: Potentials for Health Technology Assessment. Front Pharmacol. 2019;10:984-. doi: 10.3389/fphar.2019.00984 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida D, Gorender D, Ichihara MY, Sena S, Menezes L, Barbosa GCG, et al. Examining the quality of record linkage process using nationwide Brazilian administrative databases to build a large birth cohort. BMC medical informatics and decision making. 2020; 20(1): 173. Epub 2020/07/28. doi: 10.1186/s12911-020-01192-0 ; PubMed Central PMCID: PMC7382864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreto ML, Ichihara MY, Almeida BA, Barreto ME, Cabral L, Fiaccone RL, et al. The centre for data and knowledge integration for health (CIDACS): Linking health and social data in Brazil. International Journal of Population Data Science. 2019; 4(2): 1–12. doi: 10.23889/ijpds.v4i2.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harron K, Dibben C, Boyd J, Hjern A, Azimaee M, Barreto ML, et al. Challenges in administrative data linkage for research. Big data & society. 2017; 4(2): 2053951717745678. Epub 2018/11/02. doi: 10.1177/2053951717745678 ; PubMed Central PMCID: PMC6187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell M, Katikireddi SV, Hoffmann T, Armstrong R, Waters E, Craig P. TIDieR-PHP: a reporting guideline for population health and policy interventions. BMJ. 2018; 361: k1079. doi: 10.1136/bmj.k1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasil. Decreto n° 5.209, de 17 de setembro de 2004. Regulamenta a Lei n° 10.836, de 9 de janeiro de 2004, que cria o Programa Bolsa Família, e dá outras providências. Brasil: Diário Oficial da União; 2004. Available from: https://legislacao.presidencia.gov.br/atos/?tipo=DEC&numero=5209&ano=2004&ato=516oXWU5keRpWT36b.
- 27.Brasil. Decreto n° 7.758, de 15 de junho de 2012. Altera o Decreto n° 5.209, de 17 de setembro de 2004, que regulamenta a Lei n° 10.836, de 9 de janeiro de 2004, que cria o Programa Bolsa Família; 2012. Available from: https://legislacao.presidencia.gov.br/atos/?tipo=DEC&numero=7758&ano=2012&ato=1a0kXRq10MVpWT0ee.
- 28.Brasil. Decreto n° 5.749, de 11 de abril de 2006. Altera o caput do art. 18 do Decreto n° 5.209, de 17 de setembro de 2004, dispondo sobre atualizações de valores referenciais para caracterização das situações de pobreza e extrema pobreza no âmbito do Programa Bolsa Família, previstos no art. 2°, §§ 2° e 3°, da Lei n° 10.836, de 9 de janeiro de 2004; 2006 Available from: http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2004/decreto/d5209.htm.
- 29.Decreto n° 10.930, de 2022. Declara a revogação, para os fins do disposto no art. 16 da Lei Complementar n° 95, de 26 de fevereiro de 1998, de decretos normativos; 2022. Available from: http://www.planalto.gov.br/ccivil_03/_Ato2019-2022/2022/Decreto/D10930.htm#art1.
- 30.Decreto n° 6.824, de 2009. Altera o caput do art. 18 do decreto n° 5209 e 17 de setembro de 2004; 2009. Available from: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2009/decreto/D6824.htm.
- 31.Brasil. Decreto n° 8.232, de 30 de abril de 2014. Altera o Decreto n° 5.209, de 17 de setembro de 2004, que regulamenta o Programa Bolsa Família, e o Decreto n° 7.492, de 2 de junho de 2011, que institui o Plano Brasil Sem Miséria. Brasil: Diário Oficial da União; 2014. Available from: https://legislacao.presidencia.gov.br/atos/?tipo=DEC&numero=8232&ano=2014&ato=b28cXUE5UNVpWT048.
- 32.Brasil. Bolsa Família: Ministério do desenvolvimento social; 2019 [cited 2019 11/25/2019]. Available from: http://mds.gov.br/assuntos/bolsa-familia/o-que-e/como-funciona.
- 33.Santos LMP, Guanais F, Porto DL, Morais Neto OLd, Stevens A, Cortez-Escalante JJ, et al. Menor ocorrência de baixo peso ao nascer entre crianças de famílias beneficiárias do programa bolsa família. In: Campello T, Neri MCO, editors. Programa Bolsa Família uma década de inclusão e cidadania. Brasília: Ipea; 2013. Available from: https://www.ipea.gov.br/portal/index.php?option=com_content&id=20408 [Google Scholar]
- 34.Joseph KS, Liston RM, Dodds L, Dahlgren L, Allen AC. Socioeconomic status and perinatal outcomes in a setting with universal access to essential health care services. CMAJ. 2007; 177(6): 583–90. doi: 10.1503/cmaj.061198 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savitz DA, Kaufman JS, Dole N, Siega-Riz AM, Thorp JM Jr., Kaczor DT. Poverty, education, race, and pregnancy outcome. Ethnicity & disease. 2004; 14(3): 322–9. Epub 2004/08/27. . [PubMed] [Google Scholar]
- 36.Steiner N, Wainstock T, Sheiner E, Segal I, Landau D, Walfisch A. Small for gestational age as an independent risk factor for long-term pediatric gastrointestinal morbidity of the offspring. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2019; 32(9): 1407–11. Epub 2017/11/22. doi: 10.1080/14767058.2017.1406473 . [DOI] [PubMed] [Google Scholar]
- 37.Policiano C, Fonseca A, Mendes JM, Clode N, Graça LM. Small-for-gestational-age babies of low-risk term pregnancies: does antenatal detection matter? The Journal of Maternal-Fetal & Neonatal Medicine. 2018; 31(11): 1426–30. doi: 10.1080/14767058.2017.1317741 [DOI] [PubMed] [Google Scholar]
- 38.Madden JV, Flatley CJ, Kumar S. Term small-for-gestational-age infants from low-risk women are at significantly greater risk of adverse neonatal outcomes. American Journal of Obstetrics & Gynecology. 2018; 218(5): 525.e1-.e9. doi: 10.1016/j.ajog.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 39.Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. The Lancet Global Health. 2013; 1(1): e26–e36. doi: 10.1016/S2214-109X(13)70006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Brauw A, Gilligan DO, Hoddinott J, Roy S. The Impact of Bolsa Família on Women’s Decision-Making Power. World Development. 2014; 59: 487–504. 10.1016/j.worlddev.2013.02.003. [DOI] [Google Scholar]
- 41.Ferrario MN. The impacts on family consumption of the Bolsa Família subsidy programme. CEPAL–ECLAC (Economic Comission for Latin America and the Caribbean). 2014; 2014(112): 147–63. doi: 10.18356/5579e867-en Available from: https://www.cepal.org/en/publications/37025-impacts-family-consumption-bolsa-familia-subsidy-programme [DOI] [Google Scholar]
- 42.Duarte GB, Sampaio B, Sampaio Y. Programa Bolsa Família: impacto das transferências sobre os gastos com alimentos em famílias rurais. Revista de Economia e Sociologia Rural [online]. 2009; 47(4): 903–18. Epub 20 Maio 2010. doi: 10.1590/S0103-20032009000400005 [DOI] [Google Scholar]
- 43.Sperandio N, Rodrigues CT, Franceschini SdCC, Priore SE. Impact of the Bolsa Família Program on energy, macronutrient, and micronutrient intakes: Study of the Northeast and Southeast. Revista de Nutrição [online]. 2016; 29(06): 833–44. doi: 10.1590/1678-98652016000600008 [DOI] [Google Scholar]
- 44.Sperandio N, Rodrigues CT, Franceschini SCC, Priore SE. The impact of the bolsa família program on food consumption: A comparative study of the southeast and northeast regions of Brazil. Ciencia e Saude Coletiva. 2017; 22(6): 1771–80. doi: 10.1590/1413-81232017226.25852016 [DOI] [PubMed] [Google Scholar]
- 45.Martins APB, Monteiro CA. Impact of the Bolsa Família program on food availability of low-income Brazilian families: A quasi experimental study. BMC Public Health. 2016; 16(1). doi: 10.1186/s12889-016-3486-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coelho PL, Melo ASSdA. Impacto do Programa “Bolsa Família” sobre a qualidade da dieta das famílias de Pernambuco no Brasil. Ciência & Saúde Coletiva [online]. 2017; 22(2): 393–402. doi: 10.1590/1413-81232017222.13622015 [DOI] [Google Scholar]
- 47.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet. 2013; 382(9890): 427–51. Epub 2013/06/12. doi: 10.1016/S0140-6736(13)60937-X . [DOI] [PubMed] [Google Scholar]
- 48.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bulletin of the World Health Organization. 1987; 65(5): 663–737. Epub 1987/01/01. ; PubMed Central PMCID: PMC2491072. [PMC free article] [PubMed] [Google Scholar]
- 49.Kader M, Perera NK. Socio-economic and nutritional determinants of low birth weight in India. North American journal of medical sciences. 2014; 6(7): 302–8. Epub 2014/08/01. doi: 10.4103/1947-2714.136902 ; PubMed Central PMCID: PMC4114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian pediatrics. 2012; 49(1): 25–8. Epub 2011/07/02. doi: 10.1007/s13312-012-0010-z . [DOI] [PubMed] [Google Scholar]
- 51.Minuci EG, Almeida MF. Birth weight intra-urban differentials in the city of São Paulo. Revista de saude publica. 2009; 43(2): 256–66. Epub 2009/02/20. doi: 10.1590/s0034-89102009005000011 . [DOI] [PubMed] [Google Scholar]
- 52.Woodhouse C, Lopez Camelo J, Wehby GL. A Comparative Analysis of Prenatal Care and Fetal Growth in Eight South American Countries. PLOS ONE. 2014; 9(3): e91292. doi: 10.1371/journal.pone.0091292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barros AJD, Ronsmans C, Axelson H, Loaiza E, Bertoldi AD, França GVA, et al. Equity in maternal, newborn, and child health interventions in Countdown to 2015: a retrospective review of survey data from 54 countries. The Lancet. 2012; 379(9822): 1225–33. doi: 10.1016/S0140-6736(12)60113-5 [DOI] [PubMed] [Google Scholar]
- 54.Kusuma D, Cohen J, McConnell M, Berman P. Can cash transfers improve determinants of maternal mortality? Evidence from the household and community programs in Indonesia. Soc Sci Med. 2016; 163: 10–20. doi: 10.1016/j.socscimed.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 55.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014; 2(6): e323–e33. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 56.Shei A, Costa F, Reis MG, Ko AI. The impact of Brazil’s Bolsa Família conditional cash transfer program on children’s health care utilization and health outcomes. BMC Int Health Hum Rights. 2014; 14:10-. doi: 10.1186/1472-698X-14-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade MV, Chein F, Souza LRd, Puig-Junoy J. Income transfer policies and the impacts on the immunization of children: the Bolsa Família Program. Cadernos de Saúde Pública. 2012; 28: 1347–58. doi: 10.1590/s0102-311x2012000700013 [DOI] [PubMed] [Google Scholar]
- 58.Redding S, Conrey E, Porter K, Paulson J, Hughes K, Redding M. Pathways Community Care Coordination in Low Birth Weight Prevention. Maternal and Child Health Journal. 2014; 19(3): 643–50. doi: 10.1007/s10995-014-1554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC pregnancy and childbirth. 2013; 13: 242. Epub 2013/12/29. doi: 10.1186/1471-2393-13-242 ; PubMed Central PMCID: PMC3877972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li CY, Sung FC. Socio-economic inequalities in low-birth weight, full-term babies from singleton pregnancies in Taiwan. Public health. 2008; 122(3): 243–50. Epub 2007/09/11. doi: 10.1016/j.puhe.2007.05.011 . [DOI] [PubMed] [Google Scholar]
- 61.Wehby GL, Murray JC, Castilla EE, Lopez-Camelo JS, Ohsfeldt RL. Prenatal care effectiveness and utilization in Brazil. Health Policy and Planning. 2009; 24(3): 175–88. doi: 10.1093/heapol/czp005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mercer BM. Periviable Birth and the Shifting Limit of Viability. Clinics in perinatology. 2017; 44(2): 283–6. Epub 2017/05/10. doi: 10.1016/j.clp.2017.02.002 . [DOI] [PubMed] [Google Scholar]
- 63.Upadhyay K, Pourcyrous M, Dhanireddy R, Talati AJ. Outcomes of neonates with birth weight 500 g: a 20-year experience. Journal Of Perinatology. 2015; 35: 768. doi: 10.1038/jp.2015.44 [DOI] [PubMed] [Google Scholar]
- 64.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of Infants Born at Periviable Gestational Ages. Clin Perinatol. 2017; 44(2): 287–303. Epub 03/22. doi: 10.1016/j.clp.2017.01.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ecker JL, Kaimal A, Mercer BM, Blackwell SC, deRegnier RAO, Farrell RM, et al. Periviable birth: Interim update. American Journal of Obstetrics & Gynecology. 2016; 215(2): B2–B12.e1. doi: 10.1016/j.ajog.2016.04.017 [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization. Certain conditions originating in the perinatal period. 2019 [cited 2019-06-12]. In: International statistical classification of diseases and related health problems for mortality and morbidity statistics [Internet]. World Health Organization. 11th. [cited 2019-06-12]. Available from: https://icd.who.int/.
- 67.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384(9946): 857–68. Epub 2014/09/12. doi: 10.1016/S0140-6736(14)60932-6 . [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization. International statistical classification of diseases and related health problems: tenth revision. 2nd. ed. Geneva; 2004. Available from: https://www.who.int/standards/classifications/classification-of-diseases [PubMed]
- 69.World Health Organization. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. In: Organization WH, editor. Geneva; 2006. p. 312. Available from: https://www.who.int/publications/i/item/924154693X.
- 70.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. Journal of the American Statistical Association. 1984; 79(387): 516–24. doi: 10.2307/2288398 [DOI] [Google Scholar]
- 71.Becker SO, Ichino A. Estimation of Average Treatment Effects Based on Propensity Scores. The Stata Journal. 2002; 2(4): 358–77. doi: 10.1177/1536867X0200200403 [DOI] [Google Scholar]
- 72.Imbens GW. Nonparametric Estimation of Average Treatment Effects under Exogeneity: A Review. The Review of Economics and Statistics. 2004; 86(1): 4–29. [Google Scholar]
- 73.World Health Organization. WHO guidance for measuring maternal mortality from a census. World Health Organization; 2013. p. 82. Available from: https://apps.who.int/iris/bitstream/handle/10665/87982/9789241506113_eng.pdf.
- 74.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Análise de Situação em Saúde. Guia de vigilância epidemiológica do óbito materno / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Análise de Situação em Saúde.–Brasília: Ministério da Saúde, 2009. 84 p.: il.–(Série A. Normas e Manuais Técnicos). ISBN 978-85-334-1616-1. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_epidem_obito_materno.pdf.
- 75.Malta M, Cardoso LO, Bastos FI, Magnanini MM, Silva CM. STROBE initiative: guidelines on reporting observational studies. Revista de saude publica. 2010; 44(3): 559–65. Epub 2010/06/16. doi: 10.1590/s0034-89102010000300021 . [DOI] [PubMed] [Google Scholar]
- 76.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS medicine. 2015; 12(10): e1001885. Epub 2015/10/07. doi: 10.1371/journal.pmed.1001885 ; PubMed Central PMCID: PMC4595218 conflicts of interest to declare. [DOI] [PMC free article] [PubMed] [Google Scholar]