Abstract
Background
Neospora caninum (N. caninum) is known to be a major cause of reproductive failure in cattle herds around the world. Therefore, the current comprehensive study was performed to estimate the global prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses.
Methods
In this study, PubMed, ScienceDirect, Web of Science, Scopus, and ProQuest databases were systematically searched for relevant studies up until November 4, 2021. Pooled prevalence and corresponding 95% confidence intervals (CI) were estimated using a random effect model. Other analyzes performed on the data of this study include sensitivity analysis, publication bias test, and quality assessment.
Results
The final analyses included 71 studies conducted on 2965 abortive cattle and 4805 aborted fetuses. The overall prevalence rates of N. caninum infection in bovines that had an abortion were 47% and 1% using serological and molecular methods. Furthermore, overall prevalence rates of N. caninum infection in bovine aborted fetuses globally were 35% (95% CI: 8%–62%) and 43% (95% CI: 35%–52%) using serological and molecular methods.
Conclusions
The results of this study showed the high prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses. It is hoped that the results of this study will help prevent abortion in bovines around the world and encourage further studies to determine the impact of this parasite on the occurrence of abortion that may help reduce the economic damage caused by abortion worldwide.
Introduction
Abortion is the delivery of an immature fetus (alive or dead) before the end of pregnancy, which occurs as a result of the failure of pregnancy control mechanisms [1]. Infectious agents such as bacteria, viruses, fungi, and protozoa can play an important role in abortion. Among protozoa, Neospora caninum (N. caninum) is the most common cause of reproductive failure in bovines [2]. Bovines can become infected horizontally via the ingestion of feed and water contaminated with sporulated oocysts shed by dogs as the definitive hosts or vertically (transplacentally) by the transmission of the parasite from a dam to a fetus, which is considered the main route of infection in cattle [3, 4]. Endogenous transplacental transmission is due to the recrudescence of the infection during pregnancy in a persistently infected dam, whereas, exogenous transplacental transmission occurs after the initial infection of the pregnant dam following the ingestion of sporulated oocysts [5, 6]. Overall, N. caninum infection in non-pregnant cattle is latent and asymptomatic. Nevertheless, in pregnant cattle, primary infection or recrudescence may lead to abortion, the birth of a weak calf, or the birth of a clinically normal but chronically infected calf [7, 8]. Various factors such as the virulence of N. caninum, routes of parasite transmission (vertical or horizontal), type of infection (primary infection, recrudescence, and reinfection), immunological competence of the mother, and stage of pregnancy in which the dam is infected can play a key role in determining infection outcome [8]. Abortion is the most important clinical sign of neosporosis and the majority of the cases occur sporadically, endemically, or epidemically in the sixth month of pregnancy. The rate of congenital transmission is 50–95% and plays an important role in keeping the parasite within the herds [9, 10]. Despite extensive studies on N. caninum infection, the pathogenesis of N. caninum-induced abortion is complex and still not well understood. Also, N. caninum is one of the main constraints to the livestock industry that can lead causes to calve loss, possible loss of milk yield, male infertility, as well as costs associated with establishing the diagnosis of the disease [11–13]. Therefore, given that abortion in bovines is a serious problem and causes significant economic losses to the dairy industry around the world, the main objective of this study was to provide data about the prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses by molecular, serological, immunohistochemical (IHC), and histopathological methods worldwide.
Methods
Study design and protocol registration
This extensive research was reported in accordance with the items reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (S1 Checklist) [14]. The details of the protocol were registered in PROSPERO with the registration number CRD42020216694.
Search strategy
To evaluate the global prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses, the literature search was conducted for relevant papers in 5 English-language databases (PubMed, ScienceDirect, Web of Science, Scopus, and ProQuest) until November 4, 2021, using a combination of keywords related to (“Neospora caninum” OR neosporosis) AND (abortion OR miscarriage OR “reproductive failure” OR “fetal loss”) AND (livestock OR ruminant OR cattle OR bovine OR cow). The references of all the original articles in this study were reviewed so that a relevant article would not be missed. All the retrieved articles were saved in EndNote (version X9) to manage the references.
Inclusion and exclusion criteria
Studies meeting the following criteria were considered eligible: cross-sectional and short communication studies investigating the prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses with different diagnostic methods (serological, molecular, IHC, and histopathological), full-text articles available online in English language without limitations regarding publication date. Articles examining the relationship between abortion and N. caninum, studies examining the prevalence of N. caninum in bovines with more than one abortion, case-control studies, review articles, systematic review and meta-analysis articles, dissertations, conference papers, book chapters, experimental studies, and papers with unclear result sections were excluded from this systematic review and meta-analysis.
Study selection and data extraction
The initial records obtained during databases searching were imported directly to Endnote X9 software. Following the removal of duplicates, two trained researchers independently evaluated titles, abstracts, and full texts. In the event of a dispute, another author arbitrated and resolved any disagreements. In the next step, the required information was extracted for each study including the name of the first author, publication year, place of study, type of samples, diagnostic methods, sample size (the number of examined animals), results of serological, molecular, IHC, and histopathological methods (number of positive samples). In order to extract data on bovine aborted fetuses, the number of aborted fetuses was included in the study, not the number of samples that were evaluated from different organs of a fetus. To extract data related to the serum of bovines that had an abortion, maternal serum or serum of dam was included in the study, and in cases where both samples were presented in the study, only maternal serum was included and if in one study, maternal serum for half of the samples and serum of dam for the other half was evaluated, the total results of maternal serum and serum of dam were analyzed together. When more than one diagnostic method was used in articles on aborted cattle, the results of the enzyme-linked immunosorbent assay (ELISA) test were analyzed because most studies used the ELISA method. However, in aborted fetuses, because most studies used the indirect immunofluorescence assay (IFA) method, the results of this test were analyzed.
Quality assessment
The quality of articles was assessed using the Newcastle-Ottawa Scale (NOS) [15]. This quality scale ranges from 0 to 9 points, and higher scores indicate better quality studies. As a result, articles of acceptable quality (≥3 for each study) were included in this study.
Statistical analysis
The present meta-analysis was carried out using Stata version 14 (Stata Corp, College Station, TX, USA). Pooled prevalence and 95% confidence intervals (CI) were estimated using the random-effects model. Also, the I-squared test was applied to evaluate the heterogeneity index; I-squared values of lower than 25%, 25–50%, and higher than 50% were considered as low, moderate, and high heterogeneity, respectively [16]. The publication bias was examined by Egger’s test. Furthermore, the current study benefited from sensitivity analyses of articles. In this study, subgroup analysis was conducted based on diagnostic methods.
Results
Identification and selection of studies
Our preliminary search of five databases yielded 2512 articles, 1717 articles remained after duplicate removal. Following an initial screening based on titles and abstracts, 1526 studies were excluded. In the next step, the remaining 191 full-text articles were assessed. Finally, 71 of these articles were entered into the meta-analysis with respect to the inclusion/exclusion criteria (Fig 1). Information and characteristics of the investigated articles are presented in Tables 1 and 2.
Fig 1. Flow diagram of the study design process.
Table 1. Description of the studies included the prevalence of N. caninum in bovines that had an abortion.
| Id | First author (Publication year) | Place of study | Sample | Method | Sample size (n) | Serological results n (%) | Cut off | Molecular results n (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Reichel and Drake, 1996 [17] | New Zealand | Serum | ELISA and IFA | 76 | 27 (35.52) | 1: 200 | -- |
| 2 | Buxton et al., 1997 [18] | Scotland | Serum | IFA | 465 | 81 (17.4) | 1: 512 ≤ | -- |
| 3 | Campero et al., 1998 [19] | Argentina | Serum | IFA | 9 | 8 (88.88) | 1: 800 | -- |
| 4 | Cox et al., 1998 [20] | New Zealand | Serum | IFA | 11 | 9 (81.81) | -- | -- |
| 5 | Venturini et al., 1999 [21] | Argentina | Serum | IFA, NAT, and ELISA | 189 | 122 (64.55) | 1: 800 | -- |
| 6 | Pitel et al., 2001 [22] | France | Serum | ELISA | 163 | 48 (29.45) | 1: 100 | -- |
| 7 | Morales et al., 2001 [23] | Mexico | Serum | ELISA | 32 | 29 (90.62) | -- | -- |
| 8 | De Meerschman et al., 2002 [24] | Belgium | Serum | IFA | 163 | 33 (20.24) | ≥ 1: 25 | -- |
| 9 | Václavek et al., 2003 [25] | Czech Republic | Serum | ELISA and IFA | 463 | 18 (3.9) | ≥ 1: 640 | -- |
| 10 | Sadrebazzaz et al., 2004 [26] | Iran | Serum | IFA | 139 | 27 (19.42) | 1: 200 | -- |
| 11 | López‐Gatius et al., 2004 [27] | Spain | Serum | ELISA | 38 | 29 (76.31) | -- | -- |
| 12 | Hall et al., 2005 [28] | Australia | Serum | ELISA | 8 | 2 (25) | -- | -- |
| 13 | Santos et al., 2005 [29] | Brazil | Serum | IFA | 35 | 5 (14.28) | ≥ 200 | -- |
| 14 | McInnes et al., 2006 [30] | Australia | Serum | IFA, ELISA, and nested-PCR | 42 | 37 (88.10) | -- | 13 (30.95) |
| 15 | Sadrebazzaz et al., 2007 [31] | Iran | Serum | IFA | 12 | 6 (50) | 1: 200 | -- |
| 16 | Zhang et al., 2007 [32] | China | Serum | ELISA | 16 | 12 (75) | -- | -- |
| 17 | Yao et al., 2009 [33] | China | Serum | ELISA and nested PCR | 20 | 8 (40) | -- | 0/20 (0) |
| 18 | Basso et al., 2010 [34] | Germany | Serum | ELISA | 43 | 38 (88.37) | -- | -- |
| 19 | Nematollahi et al., 2011 [35] | Iran | Serum | ELISA and dot-ELISA | 32 | ELISA: 7 (21.87) and dot-ELISA: 5 (15.62) | -- | -- |
| 20 | Shabbir et al., 2011 [36] | Pakistan | Serum | ELISA | 141 | 66 (46.8) | -- | -- |
| 21 | Ghalmi et al., 2011 [37] | Algeria | Serum | IFA | 5 | 4 (80) | > 1: 200 | -- |
| 22 | Yang et al., 2012 [38] | China | Serum | ELISA | 80 | 28 (35) | -- | -- |
| 23 | Nematollahi et al., 2013 [39] | Iran | Serum | ELISA | 76 | 14 (18.42) | -- | -- |
| 24 | Razmi et al., 2013 [40] | Iran | Serum | ELISA | 200 | 38 (19) | -- | -- |
| 25 | Şuteu et al., 2013 [41] | Romania | Serum | ELISA | 9 | 5 (55.55) | 1: 100 | -- |
| 26 | Gavrilović et al., 2013 [42] | Serbia | Serum | ELISA | 27 | 7 (25.93) | -- | -- |
| 27 | Gharekhani, 2014 [43] | Iran | Serum | ELISA | 85 | 55 (64.70) | -- | -- |
| 28 | Špilovská et al., 2015 [44] | Slovak Republic | Serum | ELISA | 4 | 3 (75) | -- | -- |
| 29 | de Macedo et al., 2017 [45] | Brazil | Serum | ELISA and PCR | 41 | 21 (51.2) | 1: 100 | 0 (0) |
| 30 | Serrano-Martínez et al., 2019 [46] | Peru | Serum | ELISA | 219 | 102 (46.6) | -- | -- |
| 31 | Perotta et al., 2021 [47] | Brazil | Serum | No data | 73 | 44 (60.27) | -- | -- |
| 32 | Köse et al., 2021 [48] | Turkey | Serum | ELISA | 49 | 8 (16.33) | -- | -- |
ELISA: enzyme-linked immunosorbent assay, IFA: indirect immunofluorescence assay, NAT: N. caninum agglutination test, PCR: polymerase chain reaction, and Nested-PCR: nested-polymerase chain reaction.
Table 2. Characteristics of the included studies for prevalence of N. caninum in the bovine aborted fetuses.
| Id | First author (Publication year) | Place of study | Sample | Methods | Sample size (n) | Serological results n (%) | Molecular results n (%) | Histopathology and IHC results n (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Thilsted and Dubey, 1989 [49] | USA | Tissue specimens from multiple fetal organs | Histopathology and IHC | 9 | -- | -- | Histopathology: 7/9 (77.77) and IHC: 3/9 (33.33) |
| 2 | Barr et al., 1991 [50] | USA | Brain | IHC | 86 | -- | -- | IHC: 72/86 (83.72) |
| 3 | Conrad et al., 1993a [51] | USA | Brain | Histopathology and IHC | 2 | -- | -- | Histopathology: 2/2 (100) and IHC: 2/2 (100) |
| 4 | Ogino et al., 199) [52] | Japan | Brain | Histopathology and IHC | 115 | -- | -- | Histopathology: 3/115 (2.60) and IHC: 2/115 (1.74) |
| 5 | Nietfeld et al., 1992 [53] | USA | Brain, heart, lung, liver, kidney, placenta, and skeletal muscle | Histopathology and IHC | 664 | -- | -- | Histopathology: 25/664 (3.76) and IHC: 21/664 (3.16) |
| 6 | Jardine and Last, 1995 [54] | South Africa | Brain and myocardium | Histopathology and IHC | 144 | -- | -- | Histopathology: 2/144 (1.39) and IHC: 2/144 (1.39) |
| 7 | Obendorf et al., 1995 [55] | USA | Brain, heart, kidney, liver, and lung | Histopathology and IHC | 11 | -- | -- | Histopathology: 11/11 (100) and IHC: 3/11 (27.27) |
| 8 | Jamaluddin et al., 1996 [56] | USA | Placenta, fetal tissues, and uterine fluid | Histopathology | 595 | -- | -- | Histopathology: 71/595 (11.93) |
| 9 | McAllister et al., 1996 [57] | USA | Brain | Histopathology and IHC | 8 | -- | -- | Histopathology: 8/8 (100) and IHC: 7/8 (90) |
| 10 | Buxton et al., 2002 [7] | Scotland | Serum | IFA | 547 | 87 (15.9) | -- | -- |
| 11 | Campero et al., 1998 [19] | Argentina | Brain, heart, lung, liver, adrenal glands, spleen, kidney, thymus, and skeletal muscle | Histopathology and IHC | 2 | -- | -- | Histopathology: 2/2 (100) and IHC: 2/2 (100) |
| 12 | Perez et al., 1998 [58] | Costa Rica | Tissue | IHC | 6 | -- | -- | IHC: 1/6 (16.66) |
| 13 | Gottstein et al., 1998 [59] | Switzerland | Brain and fetal heart blood or body cavity fluid samples | Histopathology, IFA, ELISA, and PCR | 83 | 7 (8.43) | 24 (28.91) | Histopathology: 18/24 (75) |
| 14 | Moen et al., 1998 [60] | Netherlands | Brain, heart, and liver | Histopathology and IHC | 51 | -- | -- | Histopathology: 50/51 (98.03) and IHC: 40/51 (78.43) |
| 15 | Hattel et al., 1998 [61] | USA | Brain, heart, placenta, kidney, liver, and skeletal muscle | Histopathology | 688 | -- | -- | Histopathology: 34/688 (4.94) |
| 16 | Baszler et al., 1999 [62] | USA | Brain, heart, kidney, liver, lung, spleen, and placenta | Histopathology, IHC, and PCR | 61 | -- | 30 (49.18) | Histopathology: 34/61 (55.73) and IHC 26/61 (42.62) |
| 17 | Venturini et al., 1999 [21] | Argentina | Brain and serum | Histopathology, IFA, agglutination test, and ELISA | 104 | 21 (20.19) | -- | Histopathology: 7/8 (87.5) |
| 18 | González et al., 1999 [63] | Spain | Brain and fetal fluids | Histopathology, IHC, and IFA | 81 | 32/63 (50.79) | -- | Histopathology: 36/81 (44.44) and IHC: 25/34 (73.53) |
| 19 | Slotved et al., 1999 [64] | Denmark | Fetal fluids | Histopathology, IHC, ELISA, and IFA | 32 | 14 (43.75) | -- | Histopathology: 14/32 (43.75) and IHC: 14/32 (43.75) |
| 20 | Wouda et al., 1999 [65] | Netherlands | Brain, heart, and liver | Histopathology | 305 | -- | -- | Histopathology: 221/305 (72.46) |
| 21 | Atkinson et al., 2000 [66] | New South Wales | Fetal tissues | Histopathology | 12 | -- | -- | Histopathology: 8/12 (66.66) |
| 22 | Pitel et al., 2001 [22] | France | Brain | PCR | 104 | -- | 22 (21.15) | -- |
| 23 | Morales et al., 2001 [67] | Mexico | Brain, myocardium, diaphragmatic muscle, liver, lung, kidney, and spleen | Histopathology and IHC | 211 | -- | -- | Histopathology: 73/211 (34.6) and IHC: 41/53 (77.36) |
| 24 | Morales et al., 2001 [23] | Mexico | Tissue | Histopathology and IHC | 32 | -- | -- | Histopathology: 22/32 (68.75) and IHC: 17/21 (81) |
| 25 | Collantes-Fernández et al., 2002 [68] | Spain | Brain | Histopathology, real-time PCR, and nested-PCR | 12 | -- | 9 (75) | Histopathology: 6/12 (50) |
| 26 | Kim et al., 2002 [69] | Korea | Brain, heart, lung, liver, spleen, kidney, spinal cord, skeletal muscle, stomach, and small and large intestines | Histopathology, IHC, IFA, and PCR | 180 | 38 (21.11) | 34/45 (75.55) | Histopathology: 45/180 (25) and IHC: 38/45 (84.44) |
| 27 | Corbellini et al., 2002 [70] | Brazil | Brain, heart, lung, liver, kidney, and skeletal muscle | Histopathology and IHC | 46 | -- | -- | Histopathology: 22/46 (47.83) and IHC: 18/22 (81.81) |
| 28 | De Meerschman et al., 2002 [24] | Belgium | Brain, heart, liver, and serum | Histopathology, IHC, and IFA | 224 | 10/166 (6.02) | -- | Histopathology: 17/224 (7.59) and IHC: 12/17 (70.59) |
| 29 | Campero et al., 2003 [71] | Argentina | Brain, heart, lung, liver, adrenal glands, spleen, kidney, thymus, and skeletal muscle | Histopathology and IHC | 288 | -- | -- | Histopathology: 43/288 (14.93) and IHC: 26/43 (60.46) |
| 30 | Pereira-Bueno et al., 2003 [72] | Spain | Brain, heart, and fetal sera or thoracic fluids | Histopathology, IHC, IFA, ELISA, and PCR | 80 | 6/56 (10.7) | 9/59 (15.3) | Histopathology: 25/80 (31.3) and IHC: 7/13 (53.8) |
| 31 | Boger and Hattel, 2003 [73] | USA | Adrenal gland, brain, heart, intestine, kidney, liver, lung, lymph node, placenta, spleen, skeletal muscle, and thymus | Histopathology and IHC | 144 | -- | -- | Histopathology: 65/144 (45.14) and IHC: 12/144 (8.33) |
| 32 | Kashiwazaki et al., 2004 [74] | Uruguay | Brain | IHC | 2 | -- | -- | IHC: 2/2 (100) |
| 33 | López‐Gatius et al., 2004 [27] | Spain | Brain | Histopathology, IHC, and PCR | 2 | -- | 2 (100) | Histopathology: 2/2 (100) and IHC: 2/2 (100) |
| 34 | Habibi et al., 2005 [75] | Iran | Brain | Semi-nested PCR | 6 | -- | 4 (66.66) | -- |
| 35 | Khodakaram-Tafti and Ikede, 2005 [76] | Canada | Brain and heart | Histopathology and IHC | 10 | -- | -- | Histopathology: 5/10 (50) and IHC: 5/10 (50) |
| 36 | Hall et al., 2005 [28] | Australia | Placenta | Histopathology | 7 | -- | -- | Histopathology: 1/7 (14.28) |
| 37 | Santos et al., 2005 [29] | Brazil | Fetal tissues | IHC | 5 | -- | -- | IHC: 5/5 (100) |
| 38 | Collantes-Fernández et al., 2006 [77] | Spain | Brain, heart, liver, kidney, and lung | Nested-PCR | 220 | -- | 72 (32.7) | Histopathology: 18/24 (75) |
| 39 | Corbellini et al., 2006 [78] | Brazil | Brain and/or muscle (cardiac and skeletal), liver, lung, and kidney | Histopathology and IHC | 161 | -- | -- | Histopathology: 37/161 (22.98) and IHC: 34/37 (91.89) |
| 40 | McInnes et al., 2006 [30] | Australia | Fetal tissues and serum | Histopathology, IFA, ELISA, and nested-PCR | 42 | -- | 21/42 (50) | Histopathology: 9/19 (47.36) |
| 41 | Medina et al., 2006 [79] | Mexico | Brain | Histopathology and nested-PCR | 44 | -- | 35 (79.54) | Histopathology: 20/44 (45.45) |
| 42 | Razmi et al., 2007 [80] | Iran | Brain | Histopathology, IHC, and PCR | 100 | -- | 13 (13) | Histopathology: 12/53 (22.64) and IHC: 3/53 (5.66) |
| 43 | Reitt et al., 2007 [81] | Switzerland | Brain | Real-time PCR and IHC | 223 | -- | 36/76 (47.36) | IHC: 4/223 (1.79) |
| 44 | Sadrebazzaz et al., 2007 [31] | Iran | Fetal sera and fluids and brain | Histopathology, IFA, and semi nested-PCR | 12 | 5 (41.66) | 4 (33) | Histopathology: 3/12 (25) |
| 45 | Zhang et al., 2007 [32] | China | Brain, liver, kidney, heart, lung, muscle, and spleen | Histology, IHC, and PCR | 12 | -- | 4 (33.33) | Histopathology: 1/2 (50) and IHC: 1/2 (50) |
| 46 | Pabón et al., 2007 [82] | Spain | Brain | Histopathology and PCR | 7 | -- | 6 (85.71) | Histopathology: 6/7 (85.71) |
| 47 | Pescador et al., 2007 [83] | Brazil | Brain, heart, lung, liver, kidney, and skeletal muscle | Histopathology and IHC | 258 | -- | -- | Histopathology: 89/258 (34.49) and IHC: 55/258 (21.31) |
| 48 | Escamilla et al., 2007 [84] | Mexico | Lung, myocardium, liver, and kidney | Histopathology | 16 | -- | -- | Histopathology: 10/16 (62.5) |
| 49 | Moore et al., 2008 [85] | Argentina | Fetal fluids, brain, heart, liver, muscle, and placenta | Histopathology, IHC, IFA, and nested-PCR | 666 | 31/55 (56.4) | 34/70 (48.5) | Histopathology: 70/666 (10.5) and IHC: 49/70 (70) |
| 50 | Yao et al., 2009 [33] | China | Brain, heart, lung, liver, spleen, kidney, and skeletal muscle | Nested PCR | 26 | -- | 15 (57.7) | -- |
| 51 | Yildiz et al., 2009 [86] | Turkey | Heart, liver, lung, brain, and lymph nodes | Histopathology and IHC | 55 | -- | -- | Histopathology: 6/55 (10.90) and IHC: -- |
| 52 | Salehi et al., 2009 [87] | Iran | Brain and placenta | Histopathology and nested-PCR | 19 | -- | 17 (89.47) | Histopathology: 19/19 (100) |
| 53 | Sánchez et al., 2009 [88] | Mexico | Brain | Histopathology, IHC, and PCR | 48 | -- | NC5: 12/29 (41.37) and ITS1: 15/29 (51.72) | Histopathology: 29/48 (60.41) and IHC: 21/29 (72.41) |
| 54 | Cabral et al., 2009 [89] | Brazil | Brain, heart, kidney, liver, lung, spleen, thymus, and placenta | Histopathology, IHC, and nested-PCR | 105 | -- | 23 (21.90) | Histopathology: 75/105 (71.43) and IHC: 9/105 (8.6) |
| 55 | Razmi et al., 2010 [90] | Iran | Brain and fetal fluids | IHC, ELISA, and PCR | 151 | 15 (9.93) | 18 (11.92) | IHC: 6/52 (11.54) |
| 56 | Basso et al., 2010 [34] | Germany | Brain | PCR | 20 | -- | 18 (90) | -- |
| 57 | Suteu et al., 2010 [91] | Romania | Brain and heart | Histopathology and PCR | 9 | -- | 3 (33.33) | Histopathology: 0/9 (0) |
| 58 | Ghalmi et al., 2011 [37] | Algeria | Brain | Histopathology, PCR, and real-time PCR | 5 | -- | 3 (60) | Histopathology: 1/5 (20) |
| 59 | Tramuta et al., 2011 [92] | Italy | Abomasal content, brain, lung, spleen, liver, kidney, and muscle | Multiplex PCR | 50 | -- | 7 (14) | -- |
| 60 | dos Santos DS, 2011 [93] | Brazil | Central nervous system, heart, skeletal muscle, liver, lung, kidney, spleen, thymus, lymph nodes, ovary, testicle, uterus, and ear skin | Histopathology, IHC, and PCR | 24 | -- | 5 (20.83) | Histopathology: 8/24 (33.33) and IHC: 3/24 (12.5) |
| 61 | Yang et al., 2012 [38] | China | Brain | Nested-PCR | 80 | -- | 25 (31.3) | -- |
| 62 | Suteu et al., 2012 [94] | Romania | Brain and heart | PCR | 21 | -- | 8 (38.09) | -- |
| 63 | Nematollahi et al., 2013 [39] | Iran | Brain, spinal cord, placenta, liver, and heart | Histopathology and PCR | 14 | -- | 6 (42.86) | Histopathology: 14/14 (100) |
| 64 | Razmi et al., 2013 [40] | Iran | Brain | PCR | 200 | -- | 23 (11.5) | -- |
| 65 | Suteu et al., 2013 [41] | Romania | Brain and heart | Histopathology, IHC, and PCR | 9 | -- | 4 (44.44) | Histopathology: 9/9 (100) and IHC: 2/9 (22.22) |
| 66 | Kamali et al., 2014 [95] | Iran | Brain | Histopathology and PCR | 395 | -- | 179 (45.31) | Histopathology: 16/56 (28.57) |
| 67 | Spilovska et al., 2015 [44] | Slovak Republic | Brain and serum | ELISA and PCR | 4 | 3 (75) | 3 (75) | -- |
| 68 | Salehi et al., 2015 [96] | Iran | Brain | Nested-PCR | 16 | -- | 12 (75) | -- |
| 69 | Medina-Esparza et al., 2016 [97] | Mexico | Brain | Nested-PCR | 63 | -- | 27 (42.86) | -- |
| 70 | Ozkaraca et al., 2017 [98] | Turkey | Brain, myocardium, liver, lung, kidney, spleen, and thymus | IHC and Duplex PCR | 102 | -- | 26 (25.49) | IHC: 18/102 (17.64) |
| 71 | de Macedo et al., 2017 [45] | Brazil | Blood, intrathoracic fluid, brain, heart, liver, and lung | Histopathology, IHC, ELISA, and PCR | 41 | 8/30 (26.7) | 14/36 (38.8) | Histopathology: 29/36 (80.55) and IHC: 9/36 (25) |
| 72 | Kaveh et al., 2017 [99] | Iran | Brain, kidney, spleen, liver, and lung | PCR and RT-PCR | 128 | -- | 39 (30.47) | -- |
| 73 | Qian et al., 2017 [100] | China | Brain, heart, lung, liver, spleen, kidney, and skeletal muscle | Nested-PCR | 7 | -- | 4 (57.14) | -- |
| 74 | Diaz Cao et al., 2018 [101] | Spain | Brain | Real-time PCR | 25 | -- | 2 (8) | -- |
| 75 | Tian et al., 2018 [102] | China | Fetal tissues | LF-RPA and nested-PCR | 75 | -- | LF-RPA: 18 (24) and nested PCR: 17 (22.6) | -- |
| 76 | Snak et al., 2018 [103] | Brazil | Fetal tissues | PCR | 17 | -- | 9 (52.94) | -- |
| 77 | Moroni et al., 2018 [104] | Chile | Brain and optic nerve | Histopathology, IHC, and PCR | 296 | -- | 31 (10.5) | Histopathology: 44/296 (14.9) and IHC: 27/44 (61.36) |
| 78 | Bartley et al., 2019 [105] | Scotland | Brain, heart, and placenta | Nested-PCR | 455 | -- | 82 (18.02) | -- |
| 79 | Acici et al., 2019 [106] | Turkey | Brain, spleen, liver, lung, amniotic fluid, and fetal membranes | Real-time PCR | 88 | -- | 43 (48.9) | -- |
| 80 | Mahajan et al., 2020 [107] | India | Heart, liver, and brain | Histopathology and IHC | 13 | -- | -- | Histopathology: 1/13 (7.69) and IHC: 1/13 (7.69) |
| 81 | Amouei et al., 2019 [108] | Iran | Brain | Nested-PCR | 9 | -- | 2 (22.2) | -- |
| 82 | Serrano-Martínez et al., 2019 [46] | Peru | Fetal tissues and serum | Histopathology, ELISA, and nested-PCR | 68 | 10 (14.70) | 11 (16.17) | Histopathology: 5/68 (7.35) |
| 83 | Villa et al., 2021 [109] | Italy | Brain, lung, and liver | Real-time quantitative PCR | 198 | -- | 55 (27.8) | -- |
| 84 | Salehi et al., 2021 [110] | Iran | Brain | Nested-PCR | 78 | -- | 16 (20.5) | -- |
| 85 | Perotta et al., 2021 [47] | Brazil | Serum, peritoneal and pleural fluids, brain, heart, lung, liver, spleen, thymus, kidney, and skeletal muscle | Histopathology, IFA, and nested-PCR | 5 | 5 (100) | 1/1 (100) | Histopathology: 1/1 (100) |
| 86 | Dorsch et al., 2021 [111] | Argentina | Thoracic-abdominal fluids, brain, cerebellum, spinal cord, heart, lungs, thymus, tongue, skeletal muscle, spleen, abomasum, intestine, liver, kidney, and adrenal glands | Histopathology, IHC, IFA, and nested-PCR | 758 | 59/99 (59.6) | 96/106 (90.6) | Histopathology: 107/758 (14.12) and IHC: 30/62 (48.39) |
| 87 | El-Alfy et al., 2021 [112] | Japan | Brain | Nested-PCR | 5 | -- | 5 (100) | -- |
IHC: immunohistochemistry, IFA: indirect immunofluorescence assay, ELISA: enzyme-linked immunosorbent assay, PCR: polymerase chain reaction, Real-time PCR: real-time polymerase chain reaction, Nested-PCR: nested-polymerase chain reaction, RT-PCR: reverse transcription polymerase chain reaction, and LF-RPA assay: lateral flow strips- recombinase polymerase amplification.
General characteristics of the included studies
The publication date of the studied articles was from 1989 to 2021, and all articles were cross-sectional and short communication studies. Overall, there were 26 studies (Spain = 7, Romania = 3, Switzerland = 2, Netherlands = 2, Scotland = 2, Italy = 2, Denmark = 1, France = 1, New South Wales = 1, Serbia = 1, Czech Republic = 1, Belgium = 1, Germany = 1, and Slovak Republic = 1) in Europe, 29 studies (Iran = 15, China = 5, Turkey = 4, Japan = 2, India = 1, Pakistan = 1, and Korea = 1) in Asia, 2 studies (South Africa = 1 and Algeria = 1) in Africa, 35 studies (USA = 10, Brazil = 9, Mexico = 6, Argentina = 5, Costa Rica = 1, Uruguay = 1, Canada = 1, Chile = 1, and Peru = 1) in America and 4 studies (New Zealand = 2 and Australia = 2) in Australia/Oceania. The most common diagnostic tests of serology and molecular utilized in the studies to examine the serum samples of bovines that had an abortion and serum or brain samples of aborted fetuses were the ELISA and polymerase chain reaction (PCR). Some studies have used more than one diagnostic method for N. caninum infection (Tables 1 and 2).
In addition, the quality assessment of studies with the NOS checklist showed that the articles included in this meta-analysis are of acceptable quality. S1 Table shows the quality scores of various eligible studies.
Prevalence of N. caninum infection in bovines that had an abortion
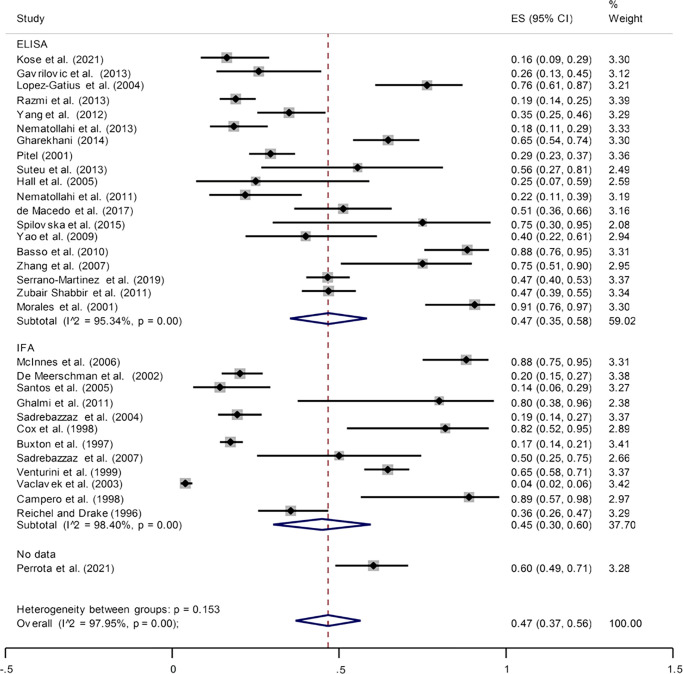
A total of 2965 and 103 bovines that had an abortion were evaluated for the prevalence of N. caninum, out of which 941 and 13 cases were positive using serological and molecular methods in different geographical locations worldwide. The results indicated that the rate of prevalence of N. caninum infection was 47% (95% CI: 37%–56%) and 1% (95% CI: -1%–3%) using serological and molecular methods. Heterogeneity were significant in different studies (I2 = 89.35%, p = 0.000 and I2 = 97.95%, p = 0.000) (Fig 2 and S1 Fig). Egger’s regression test showed that publication bias exerted a significant influence on the prevalence of N. caninum infection in bovines that had an abortion (p = 0.001) (S2 Fig). The pooled prevalence rates of N. caninum infection in bovines that had an abortion according to the diagnostic methods of ELISA and IFA were determined to be 47% (95% CI: 35%–58%) and 45% (95% CI: 30%–60%), respectively. One study did not mention the type of serology test and the prevalence was 60% (95% CI: 49%–71%) [47]. The results of the subgroup analysis revealed that the effect of assessment of the detection methods on the heterogeneity of studies was not statistically significant (p = 0.533). The results of the sensitivity analysis test showed no significant effect of deleting an article with overall results (S3 Fig).
Fig 2. The reported seroprevalence rate of anti- N. caninum antibodies in bovines that had an abortion by serological methods.
Prevalence of N. caninum infection in bovine aborted fetuses
Among databases searched, a total of 1655 bovine aborted fetuses were examined for the seroprevalence rate of the antibodies against N. caninum, out of which 351 cases were seropositive using several serological methods. The overall seroprevalence of the antibodies against N. caninum in bovine aborted fetuses based on the random effect model was calculated at 35% (95% CI: 8%–62%). I-squared statistics showed a high heterogeneity among the studies (I2 = 99.77%, p = 0.000) (Fig 3). Egger’s test was used to determine the publication bias and the results showed no publication bias on the overall prevalence estimate (p = 0.125) (S4 Fig). Based on the meta-analysis, the prevalence of N. caninum infection in the bovine aborted fetuses based on the diagnostic methods of IFA and ELISA was estimated to be 36% (95% CI: 5%-68%) and 20% (95% CI: 8%− 31%), respectively. The results of the subgroup analysis showed that the effect of diagnostic methods on the heterogeneity of studies was not statistically significant (p = 0.595). In addition, the results of the sensitivity analysis showed that the overall estimate did not change with the removal of each study (S5 Fig).
Fig 3. The pooled seroprevalence rate of anti- N. caninum antibodies in the bovine aborted fetuses.
A total number of 52 eligible studies examined 3888 samples from bovine aborted fetuses, out of which 1219 cases were positive using molecular methods. The global pooled prevalence of N. caninum infection in bovine aborted fetuses using molecular methods was estimated at 43% (95% CI: 35%–52%) (I2 = 98.01%, p = 0.00) (Fig 4). The publication bias was significant based on the results of Egger’s test (p = 0.000) using molecular methods (S6 Fig). Based on the meta-analysis, the prevalence of N. caninum infection in the bovine aborted fetuses based on the diagnostic methods of PCR, nested PCR, and others was estimated to be 41% (95% CI: 31%-51%), 50% (95% CI: 33%–67%), and 31% (95% CI: 20%− 42%), respectively. Results of subgroup analysis based on diagnostic methods indicated that the effect of diagnostic methods on the heterogeneity of studies was not statistically significant (p = 0.336). In the sensitivity analysis test, the effect of omission of each study on the overall result of the study was evaluated. The findings of this test indicated the stability of the results of the study. In addition, in three articles, 6826 and 2721 samples were examined by histopathology and IHC methods; 1518 and 674 cases were positive for N. caninum (22.24% and 24.77% positive for neosporosis) (Table 2).
Fig 4. The prevalence of N. caninum infection in the bovine aborted fetuses using molecular methods.
Discussion
N. caninum was identified as the main cause of abortion in cattle [49], which is one of the most important economic diseases. Hence, in this systematic review and meta-analysis study, the prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses was investigated by molecular, serological, IHC, and histopathological methods. Diagnosis of N. caninum abortion may be inconclusive for the following reasons: 1) expensive and sometimes difficult to diagnose, 2) lack of access to fetus and placenta, especially for beef cattle, and 3) using the serology method alone [45]. Identification of compatible histological lesions, detection of parasites in fetal tissues by PCR or IHC, and detection of specific antibodies in fetal fluids and maternal serum are the diagnostic criteria for N. caninum–induced abortion [2].
In this systematic review and meta-analysis study, 57 papers performed the histopathological evaluations based on observation of characteristic or compatible lesions with N. caninum infection, and no analysis was performed on them. According to the results of the included articles, the prevalence of N. caninum infection in bovine aborted fetuses by histopathology was 22.24%. Since many factors can play a role in abortion, determining the cause is often difficult. Abortions usually show no gross lesions or clinical signs in the fetus, and a history of abortion rarely provides convincing clues to the cause [38]. However, histopathological examination of the aborted fetus and isolation or culture of pathogens are common methods for routine diagnostic examination of materials submitted [38]. The cell culture system is laborious, time-consuming, and relatively low sensitive [38]. Histopathological examination of the fetus is essential for a definitive diagnosis. Nevertheless, histological examinations of tissues from autolyzed fetuses are not possible [113]. Ideally, the entire fetus should be sent, but if this is not possible, samples from the brain, heart, and liver should be examined for histopathological changes and body fluids or serum for serological evaluation. The fetal brain is more damaged than other organs, but the heart and liver are also commonly affected [3]. Focal encephalitis is the most significant lesion that is associated with necrosis and nonsuppurative inflammation particularly, especially in the brain and to a lesser extent in the cord [3]. As the lesion progresses, necrotic areas may be replaced by macrophages, and the glial cells that cause the lesion appear as discrete granuloma [114]. In addition, other techniques, such as IHC, are used to show parasites associated with lesions in aborted fetal tissues. IHC is a relatively insensitive technique for detecting the parasite in host tissues due to the low quality of the fetal tissue (autolyzed, mummified, or macerated) and low parasite numbers that may lead to false negatives [62, 115]. In this study, 2721 samples were evaluated for the presence of N. caninum in fetal tissues, of which 674 were positive (24.77% positive for neosporosis) by the IHC method. Serology is another method used to reliably diagnose N. caninum-related abortion problems, but it alone is not enough [39]. Tests such as IFA and ELISA are used for serological diagnosis of neosporosis. IFA is the gold standard for the serological diagnosis of N. caninum infection and is highly specific. Despite numerous common antigens, there is no evidence of cross-reaction between N. caninum and T. gondii [116]. However, indirect ELISA indicates the possibility of cross-reactivity between the sera of animals infected with N. caninum, T. gondii, or Sarcocystis species and leads to false-positive results [116]. Positive results of serological tests indicate infection of the animal with N. caninum, but in the case of abortion, serological tests cannot provide a definitive diagnosis. To confirm the diagnosis, fetal tissues should be examined for the presence of specific lesions, tissue cysts, and tachyzoites [117]. Overall, this meta-analysis demonstrated that seroprevalence of N. caninum infection is 35% in the aborted fetuses of cattle using serological tests. Also, the prevalence of N. caninum infection in the bovine aborted fetuses using different molecular tests was obtained at 43%. The use of molecular techniques, such as PCR, is useful for the diagnosis of neosporosis in bovines. PCR is a very specific and sensitive technique for the detection of small numbers of parasites in tissue and the ability to amplify small amounts of N. caninum DNA in a larger quantity of tissue [108, 115]. However, DNA detection in aborted fetuses is not sufficient to confirm that N. caninum is responsible for reproductive failure because other abortifacient factors may also play a potential role in abortion [106]. Although PCR is one of the most accurate and widely used molecular methods to study the global prevalence of N. caninum infection in aborting bovines and lost fetuses, it is best to use PCR and IHC tests simultaneously to increase the success of the definitive diagnosis of neosporosis.
In this study, the pooled prevalence rate of N. caninum infection in bovines that had an abortion was 47% and 1% by serological and molecular methods. Given that the seroprevalence of N. caninum in cattle is high and the cattle that abort the infected fetus is probably seropositive. Therefore, the maternal serological examination is useful to rule out N. caninum-associated abortion [36].
N. caninum causes heavy economic losses in livestock, particularly cattle, which are economically the most important host of natural N. caninum infections [105]. One of the major effects of infection in cows is abortion, in some geographical areas up to 42.5% of abortions are caused by N. caninum. In general, the economic impact of neosporosis has several aspects, including losses directly caused by the disease, the costs related to disease prevention, and the value of fetuses lost. The main output of a herd is its products, such as calf, milk, and meat. Indirect costs include costs such as professional help, re-breeding of cows, increased lactation time, decreased production of milk and dairy products, and early replacement of infected animals [11, 118]. In one study, costs of the disease in the New Zealand beef industry were estimated at an average of US $1.1 million due to abortion or infection and in the US, it is estimated that neosporosis costs the dairy industry US $546.3 million annually [119].
In this systematic study, heterogeneity was significant (I2 > 50). Geographical factors of each region, differences in the ages of the animals in the different studies, differences in sampling, the study of various tissues to estimate the prevalence in the included studies, and a variety of detection methods can be reasons for high heterogeneity. The lack of evaluation of various associated factors in the eligible studies can be considered a basic gap. The number of bovine aborted fetuses sent to the laboratory was relatively small in some studies, which may limit the ability of the results to generalize. Also, this small number can lead to wide confidence intervals. Another limitation is that this study used only articles published in English language, and articles related to other languages were excluded and this can be one of the reasons for publication bias. To the best of our knowledge, this is the first review that systematically assesses the studies on the prevalence of N. caninum infection in bovines that had an abortion and aborted fetuses. The results of the meta-analysis demonstrated a high prevalence of neosporosis in bovines that had an abortion and aborted fetuses throughout the world. According to the study, N. caninum infection could be considered a potential risk factor for reproductive failure in bovines worldwide. These findings provide a better picture of the epidemiology of N. caninum among bovines that had an abortion and aborted fetuses and may be useful for improving prevention and control strategies in the future as well as helping to reduce significant economic losses to the livestock industry.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This article is an approved plan from Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran (number: 8558). The code of ethics of this plan is (IR.MAZUMS.REC.1399.783).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Shaapan RM. The common zoonotic protozoal diseases causing abortion. J Parasit Dis. 2016;40(4):1116–29. doi: 10.1007/s12639-015-0661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey J, Schares G. Diagnosis of bovine neosporosis. Vet Parasitol. 2006;140(1–2):1–34. doi: 10.1016/j.vetpar.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41(1):1. doi: 10.3347/kjp.2003.41.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAllister MM, Björkman C, Anderson-Sprecher R, Rogers DG. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J Am Vet Med Assoc. 2000;217(6):881–7. doi: 10.2460/javma.2000.217.881 [DOI] [PubMed] [Google Scholar]
- 5.Trees AJ, Williams DJ. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21(12):558–61. doi: 10.1016/j.pt.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Wouda W. Neosporosis: Biology, transmission and clinical signs. Protozoal abortion in farm ruminants Wallingford, England CAB international. 2007:46–53. [Google Scholar]
- 7.Buxton D, McAllister MM, Dubey JP. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18(12):546–52. doi: 10.1016/s1471-4922(02)02414-5 [DOI] [PubMed] [Google Scholar]
- 8.Innes EA, Andrianarivo AG, Björkman C, Williams DJ, Conrad PA. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 2002;18(11):497–504. doi: 10.1016/s1471-4922(02)02372-3 [DOI] [PubMed] [Google Scholar]
- 9.Wouda W, Moen AR, Visser IJ, van Knapen F. Bovine fetal neosporosis: a comparison of epizootic and sporadic abortion cases and different age classes with regard to lesion severity and immunohistochemical identification of organisms in brain, heart, and liver. J Vet Diagn Invest. 1997;9(2):180–5. doi: 10.1177/104063879700900212 [DOI] [PubMed] [Google Scholar]
- 10.Schares G, Peters M, Wurm R, Bärwald A, Conraths FJ. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet Parasitol. 1998;80(2):87–98. doi: 10.1016/s0304-4017(98)00195-2 [DOI] [PubMed] [Google Scholar]
- 11.Thurmond MC, Hietala SK, Blanchard PC. Herd-based diagnosis of Neospora caninum-induced endemic and epidemic abortion in cows and evidence for congenital and postnatal transmission. J Vet Diagn Invest. 1997;9(1):44–9. doi: 10.1177/104063879700900108 [DOI] [PubMed] [Google Scholar]
- 12.Nasir A, Parveen Z, Shah M, Rashid M. Seroprevalence of brucellosis in animals at government and private livestock farms in Punjab. Pak Vet J. 2004;24(3):144–6. [Google Scholar]
- 13.Asmare K, Asfaw Y, Gelaye E, Ayelet G. Brucellosis in extensive management system of Zebu cattle in Sidama Zone, Southern Ethiopia. Afr J Agric Res. 2010;5(3):257–63. 10.5897/AJAR09.045 [DOI] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 17.Reichel M, Drake J. The diagnosis of Neospora abortions in cattle. N Z Vet J. 1996;44(4):151–4. doi: 10.1080/00480169.1996.35960 [DOI] [PubMed] [Google Scholar]
- 18.Buxton D, Caldow G, Maley S, Marks J, Innes E. Neosporosis and bovine abortion in Scotland. Vet Rec. 1997;141(25):649–51. 10.1136/vr.141.25.649 [DOI] [PubMed] [Google Scholar]
- 19.Campero CM, Anderson ML, Conosciuto G, Odrozola H, Bretschneider G, Poso MA. Neospora caninum-associated abortion in a dairy herd in Argentina. Vet Rec. 1998;143(8):228–9. doi: 10.1136/vr.143.8.228 [DOI] [PubMed] [Google Scholar]
- 20.Cox B, Reichel M, Griffiths L. Serology of a Neospora abortion outbreak on a dairy farm in New Zealand: a case study. N Z Vet J. 1998;46(1):28–31. doi: 10.1080/00480169.1998.36046 [DOI] [PubMed] [Google Scholar]
- 21.Venturini M, Venturini L, Bacigalupe D, Machuca M, Echaide I, Basso W, et al. Neospora caninum infections in bovine foetuses and dairy cows with abortions in Argentina. Int J Parasitol. 1999;29(10):1705–8. doi: 10.1016/s0020-7519(99)00143-5 [DOI] [PubMed] [Google Scholar]
- 22.Pitel P-H, Pronost S, Chatagnon G, Tainturier D, Fortier G, Ballet J-J. Neosporosis in bovine dairy herds from the west of France: detection of Neospora caninum DNA in aborted fetuses, seroepidemiology of N. caninum in cattle and dogs. Vet Parasitol. 2001;102(4):269–77. doi: 10.1016/s0304-4017(01)00544-1 [DOI] [PubMed] [Google Scholar]
- 23.Morales Trigo Francisco, barra Froylan Eduardo Puente, Santacruz M. Seroprevalence study of bovine neosporosis in Mexico. J Vet Diagn Invest. 2001;13(5):413–5. doi: 10.1177/104063870101300508 [DOI] [PubMed] [Google Scholar]
- 24.De Meerschman F, Speybroeck N, Berkvens D, Rettigner C, Focant C, Leclipteux T, et al. Fetal infection with Neospora caninum in dairy and beef cattle in Belgium. Theriogenology. 2002;58(5):933–45. doi: 10.1016/s0093-691x(02)00934-2 [DOI] [PubMed] [Google Scholar]
- 25.Václavek P, Koudela B, Modrý D, Sedlák K. Seroprevalence of Neospora caninum in aborting dairy cattle in the Czech Republic. Vet Parasitol. 2003;115(3):239–45. doi: 10.1016/s0304-4017(03)00215-2 [DOI] [PubMed] [Google Scholar]
- 26.Sadrebazzaz A, Haddadzadeh H, Esmailnia K, Habibi G, Vojgani M, Hashemifesharaki R. Serological prevalence of Neospora caninum in healthy and aborted dairy cattle in Mashhad, Iran. Vet Parasitol. 2004;124(3–4):201–4. doi: 10.1016/j.vetpar.2004.06.027 [DOI] [PubMed] [Google Scholar]
- 27.López‐Gatius F, López‐Béjar M, Murugavel K, Pabón M, Ferrer D, Almería S. Neospora‐associated abortion episode over a 1‐year period in a dairy herd in north‐east Spain. J Vet Med B Infect Dis Vet Public Health. 2004;51(7):348–52. doi: 10.1111/j.1439-0450.2004.00779.x [DOI] [PubMed] [Google Scholar]
- 28.Hall C, Reichel M, Ellis J. Neospora abortions in dairy cattle: diagnosis, mode of transmission and control. Vet Parasitol. 2005;128(3–4):231–41. doi: 10.1016/j.vetpar.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 29.Santos A, Navarro I, Bracarense A, Freire R, Marana E, Ogawa L, et al. Dairy cow abortion associated with Neospora caninum and other infectious agents. Arq Bras Med Vet Zootec. 2005;57:545–7. 10.1590/S0102-09352005000400017 [DOI] [Google Scholar]
- 30.McInnes LM, Ryan UM, O’Handley R, Sager H, Forshaw D, Palmer DG. Diagnostic significance of Neospora caninum DNA detected by PCR in cattle serum. Vet Parasitol. 2006;142(3–4):207–13. doi: 10.1016/j.vetpar.2006.07.013 [DOI] [PubMed] [Google Scholar]
- 31.Sadrebazzaz A, Habibi G, Haddadzadeh H, Ashrafi J. Evaluation of bovine abortion associated with Neospora caninum by different diagnostic techniques in Mashhad, Iran. Parasitol res. 2007;100(6):1257–60. doi: 10.1007/s00436-006-0417-3 [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Deng C, Liu Q, Liu J, Wang M, Tian K, et al. First identification of Neospora caninum infection in aborted bovine foetuses in China. Vet Parasitol. 2007;149(1–2):72–6. doi: 10.1016/j.vetpar.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 33.Yao L, Yang N, Liu Q, Wang M, Zhang W, Qian W, et al. Detection of Neospora caninum in aborted bovine fetuses and dam blood samples by nested PCR and ELISA and seroprevalence in Beijing and Tianjin, China. Parasitology. 2009;136(11):1251–6. doi: 10.1017/S0031182009990813 [DOI] [PubMed] [Google Scholar]
- 34.Basso W, Schares S, Minke L, Bärwald A, Maksimov A, Peters M, et al. Microsatellite typing and avidity analysis suggest a common source of infection in herds with epidemic Neospora caninum-associated bovine abortion. Vet Parasitol. 2010;173(1–2):24–31. doi: 10.1016/j.vetpar.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 35.Nematollahi, Jozani J, Neda Z. Adaptation of Dot-ELISA for serodiagnosis of Neospora caninum infestation in aborted cows. Glob Vet. 2011;7(2):149–52. [Google Scholar]
- 36.Shabbir MZ, Nazir MM, Maqbool A, Lateef M, Shabbir MAB, Ahmad A, et al. Seroprevalence of Neospora caninum and Brucella abortus in dairy cattle herds with high abortion rates. J Parasitol. 2011;97(4):740–2. doi: 10.1645/GE-2734.1 [DOI] [PubMed] [Google Scholar]
- 37.Ghalmi F, China B, Kaidi R, Losson B. Neospora caninum is associated with abortion in Algerian cattle. J of Parasitol. 2011;97(6):1121–4. doi: 10.1645/GE-2861.1 [DOI] [PubMed] [Google Scholar]
- 38.Yang N, Cui X, Qian W, Yu S, Liu Q. Survey of nine abortifacient infectious agents in aborted bovine fetuses from dairy farms in Beijing, China, by PCR. Acta Vet Hung. 2012;60(1):83–92. doi: 10.1556/AVet.2012.007 [DOI] [PubMed] [Google Scholar]
- 39.Nematollahi A, Moghaddam G, Jaafari R, Helan JA, Norouzi M. Study on outbreak of Neospora caninum-associated abortion in dairy cows in Tabriz (Northwest Iran) by serological, molecular and histopathologic methods. Asian Pac J Trop Med. 2013;6(12):942–6. doi: 10.1016/S1995-7645(13)60168-6 [DOI] [PubMed] [Google Scholar]
- 40.Razmi G, Zarae H, Norbakhsh MF, Naseri Z. Estimating the rate of transplacental transmission of Neospora caninum to aborted fetuses in seropositive dams in Mashhad area, Iran. Iran J Vet Med. 2013;7(4):253–6. [Google Scholar]
- 41.Şuteu O, Paştiu A, Györke A, Borza G, Ardelean A, Cozma V. A survey of Neospora caninum-associated abortion in dairy cattle of Romania. Sci Parasitol. 2013;14:139–46. [Google Scholar]
- 42.Gavrilović P, Živulj A, Todorović I, Jovanović M, Parunović J. Investigation of importance of Neospora caninum in aetiology of abortion in dairy cows in Serbia. Rev Med Vet. 2013;164:100–4. [Google Scholar]
- 43.Gharekhani J. Seroprevalence of Neospora caninum and Toxoplasma gondii infections in aborted cattle in Hamedan, Iran. J Adv Vet Anim Res. 2014;1(2):32–5. 10.5455/javar.v1i2p32-35 [DOI] [Google Scholar]
- 44.Špilovská S, Reiterová K, Antolová D. Neospora caninum-associated abortions in Slovak dairy farm. Iran J Parasitol. 2015;10(1):96. [PMC free article] [PubMed] [Google Scholar]
- 45.de Macedo CABd Macedo MFSBd, Miura AC, Taroda A, Cardim ST, Innes EA, et al. Occurrence of abortions induced by Neospora caninum in dairy cattle from Santa Catarina, southern Brazil. Rev Bras Parasitol Vet. 2017;26:292–8. doi: 10.1590/S1984-29612017051 [DOI] [PubMed] [Google Scholar]
- 46.Serrano-Martínez ME, Cisterna CAB, Romero RCE, Huacho MAQ, Bermabé AM, Albornoz LAL. Evaluation of abortions spontaneously induced by Neospora caninum and risk factors in dairy cattle from Lima, Peru. Rev Bras Parasitol Vet. 2019;28:215–20. doi: 10.1590/S1984-29612019026 [DOI] [PubMed] [Google Scholar]
- 47.Perotta JH, Freitas BBd, Marcom NN, Pescador CA, Pereira CC, Locatelli-Dittrich R et al. An abortion storm in dairy cattle associated with neosporosis in southern Brazil. Rev Bras Parasitol Vet. 2021;30. doi: 10.1590/S1984-29612021045 [DOI] [PubMed] [Google Scholar]
- 48.Köse O, Adanır R, Kocamüftüoğlu M, Çetin Y. Investigation of Neospora caninum seroprevalence and association with reproductive problems in cows in Burdur Province of Turkey. Iran J Parasitol. 2021;16(3):386. doi: 10.18502/ijpa.v16i3.7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thilsted JP, Dubey J. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diagn Invest. 1989;1(3):205–9. doi: 10.1177/104063878900100301 [DOI] [PubMed] [Google Scholar]
- 50.Barr B, Anderson ML, Dubey J, Conrad PA. Neospora-like protozoal infections associated with bovine abortions. Vet pathol. 1991;28(2):110–6. doi: 10.1177/030098589102800202 [DOI] [PubMed] [Google Scholar]
- 51.Conrad P, Barr B, Sverlow K, Anderson M, Daft B, Kinde H, et al. In vitro isolation and characterization of a Neospora sp. from aborted bovine foetuses. Parasitology. 1993;106(3):239–49. doi: 10.1017/s0031182000075065 [DOI] [PubMed] [Google Scholar]
- 52.Ogino H, Watanabe E, Watanabe S, Agawa H, Narita M, Haritani M, et al. Neosporosis in the aborted fetus and newborn calf. J Comp Pathol. 1992;107(2):231–7. doi: 10.1016/0021-9975(92)90039-w [DOI] [PubMed] [Google Scholar]
- 53.Nietfeld JC, Dubey J, Anderson ML, Libal MC, Yaeger MJ, Neiger RD. Neospora-like protozoan infection as a cause of abortion in dairy cattle. J Vet Diagn Invest. 1992;4(2):223–6. doi: 10.1177/104063879200400228 [DOI] [PubMed] [Google Scholar]
- 54.Jardine J, Last R. The prevalence of neosporosis in aborted bovine foetuses submitted to the Allerton regional veterinary laboratory. Onderstepoort J Vet Res. 1995. [PubMed] [Google Scholar]
- 55.Obendorf D, Murray N, Veldhuis G, Munday B, Dubey J. Abortion caused by neosporosis in cattle. Aust Vet J. 1995;72(3):117–8. doi: 10.1111/j.1751-0813.1995.tb15025.x [DOI] [PubMed] [Google Scholar]
- 56.Jamaluddin AA, Case JT, Hird DW, Blanchard PC, Peauroi JR, Anderson ML. Dairy cattle abortion in California: evaluation of diagnostic laboratory data. J Vet Diagn Invest. 1996;8(2):210–8. doi: 10.1177/104063879600800211 [DOI] [PubMed] [Google Scholar]
- 57.McAllister MM, Huffman E, Hietala SK, Conrad PA, Anderson ML, Salman MD. Evidence suggesting a point source exposure in an outbreak of bovine abortion due to neosporosis. J Vet Diagn Invest. 1996;8(3):355–7. doi: 10.1177/104063879600800313 [DOI] [PubMed] [Google Scholar]
- 58.Perez E, Gonzalez O, Dolz G, Morales J, Barr B, Conrad PA. First report of bovine neosporosis in dairy cattle in Costa Rica. Vet Rec. 1998;142(19):520–1. doi: 10.1136/vr.142.19.520 [DOI] [PubMed] [Google Scholar]
- 59.Gottstein B, Hentrich B, Wyss R, Thür B, Busato A, Stärk K, et al. Molecular and immunodiagnostic investigations on bovine neosporosis in Switzerland. Int J Parasitol. 1998;28(4):679–91. doi: 10.1016/s0020-7519(98)00006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moen A, Wouda W, Mul M, Graat E, Van Werven T. Increased risk of abortion following Neospora caninum abortion outbreaks: a retrospective and prospective cohort study in four dairy herds. Theriogenology. 1998;49(7):1301–9. doi: 10.1016/S0093-691X(98)00077-6 [DOI] [PubMed] [Google Scholar]
- 61.Hattel A, Castro M, Gummo J, Weinstock D, Reed J, Dubey J. Neosporosis-associated bovine abortion in Pennsylvania. Vet parasitol. 1998;74(2–4):307–13. doi: 10.1016/s0304-4017(97)00158-1 [DOI] [PubMed] [Google Scholar]
- 62.Baszler TV, Gay LJ, Long MT, Mathison BA. Detection by PCR of Neospora caninum in fetal tissues from spontaneous bovine abortions. J Clin Microbiol. 1999;37(12):4059–64. doi: 10.1128/JCM.37.12.4059-4064.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González L, Buxton D, Atxaerandio R, Aduriz G, Maley S, Marco J, et al. Bovine abortion associated with Neospora caninum in northern Spain. Vet Rec. 1999;144(6):145–50. doi: 10.1136/vr.144.6.145 [DOI] [PubMed] [Google Scholar]
- 64.Slotved H-C, Jensen L, Lind P. Comparison of the IFAT and Iscom-ELISA response in bovine foetuses with Neospora caninum infection. Int J Parasitol. 1999;29(8):1165–74. doi: 10.1016/s0020-7519(99)00095-8 [DOI] [PubMed] [Google Scholar]
- 65.Wouda W, Bartels C, Moen A. Characteristics of Neospora caninum-associated abortion storms in dairy herds in The Netherlands (1995 to1997). Theriogenology. 1999;52(2):233–45. doi: 10.1016/s0093-691x(99)00125-9 [DOI] [PubMed] [Google Scholar]
- 66.Atkinson R, Cook R, Reddacliff L, Rothwell J, Broady K, Harper P, et al. Seroprevalence of Neospora caninum infection following an abortion outbreak in a dairy cattle herd. Aust Vet J. 2000;78(4):262–6. doi: 10.1111/j.1751-0813.2000.tb11752.x [DOI] [PubMed] [Google Scholar]
- 67.Morales E, Trigo F, Ibarra F, Puente E, Santacruz M. Neosporosis in Mexican dairy herds: lesions and immunohistochemical detection of Neospora caninum in fetuses. J Comp Pathol. 2001;125(1):58–63. doi: 10.1053/jcpa.2001.0477 [DOI] [PubMed] [Google Scholar]
- 68.Collantes-Fernández E, Zaballos Á, Álvarez-García G, Ortega-Mora LM. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J Clin Microbiol. 2002;40(4):1194–8. doi: 10.1128/JCM.40.4.1194-1198.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J-H, Lee J-K, Lee B-C, Park B-K, Yoo H-S, Hwang W-S, et al. Diagnostic survey of bovine abortion in Korea: with special emphasis on Neospora caninum. J Vet Med Sci. 2002;64(12):1123–7. doi: 10.1292/jvms.64.1123 [DOI] [PubMed] [Google Scholar]
- 70.Corbellini L, Driemeier D, Cruz C, Gondim LFP, Wald V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol. 2002;103(3):195–202. doi: 10.1016/s0304-4017(01)00600-8 [DOI] [PubMed] [Google Scholar]
- 71.Campero CM, Moore D, Odeón AC, Cipolla AL, Odriozola E. Aetiology of bovine abortion in Argentina. Vet Res Commun. 2003;27(5):359–69. doi: 10.1023/a:1024754003432 [DOI] [PubMed] [Google Scholar]
- 72.Pereira-Bueno J, Quintanilla-Gozalo A, Pérez-Pérez V, Espi-Felgueroso A, Alvarez-Garcıa G, Collantes-Fernández E, et al. Evaluation by different diagnostic techniques of bovine abortion associated with Neospora caninum in Spain. Vet Parasitol. 2003;111(2–3):143–52. doi: 10.1016/s0304-4017(02)00361-8 [DOI] [PubMed] [Google Scholar]
- 73.Boger LA, Hattel AL. Additional evaluation of undiagnosed bovine abortion cases may reveal fetal neosporosis. Vet Parasitol. 2003;113(1):1–6. doi: 10.1016/s0304-4017(03)00041-4 [DOI] [PubMed] [Google Scholar]
- 74.Kashiwazaki Y, Gianneechini RE, Lust M, Gil J. Seroepidemiology of neosporosis in dairy cattle in Uruguay. Vet Parasitol. 2004;120(1–2):139–44. doi: 10.1016/j.vetpar.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 75.Habibi GR, Hashemi-Fesharki R, Sadrebazzaz A, Bozorgi S, Bordbar N. Seminested PCR for diagnosis of Neospora caninum infection in cattle. Arch Razi Inst. 2005;59(2):55–64. 10.22092/ari.2005.103813 [DOI] [Google Scholar]
- 76.Khodakaram-Tafti A, Ikede BO. A retrospective study of sporadic bovine abortions, stillbirths, and neonatal abnormalities in Atlantic Canada, from 1990 to 2001. Can Vet J. 2005;46(7):635. [PMC free article] [PubMed] [Google Scholar]
- 77.Collantes-Fernández E, Rodríguez-Bertos A, Arnáiz-Seco I, Moreno B, Aduriz G, Ortega-Mora LM. Influence of the stage of pregnancy on Neospora caninum distribution, parasite loads and lesions in aborted bovine foetuses. Theriogenology. 2006;65(3):629–41. doi: 10.1016/j.theriogenology.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 78.Corbellini LG, Pescador CA, Frantz F, Wunder E, Steffen D, Smith DR, et al. Diagnostic survey of bovine abortion with special reference to Neospora caninum infection: importance, repeated abortion and concurrent infection in aborted fetuses in Southern Brazil. Vet J. 2006;172(1):114–20. doi: 10.1016/j.tvjl.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 79.Medina L, Cruz-Vázquez C, Quezada T, Morales E, García-Vázquez Z. Survey of Neospora caninum infection by nested PCR in aborted fetuses from dairy farms in Aguascalientes, Mexico. Vet Parasitol. 2006;136(3–4):187–91. doi: 10.1016/j.vetpar.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 80.Razmi GR, Maleki M, Farzaneh N, Garoussi MT, Fallah A. First report of Neospora caninum-associated bovine abortion in Mashhad area, Iran. Parasitol Res. 2007;100(4):755–7. doi: 10.1007/s00436-006-0325-6 [DOI] [PubMed] [Google Scholar]
- 81.Reitt K, Hilbe M, Voegtlin A, Corboz L, Haessig M, Pospischil A. Aetiology of bovine abortion in Switzerland from 1986 to 1995–a retrospective study with emphasis on detection of Neospora caninum and Toxoplasma gondii by PCR. J Vet Med A Physiol Pathol Clin Med. 2007;54(1):15–22. doi: 10.1111/j.1439-0442.2007.00913.x [DOI] [PubMed] [Google Scholar]
- 82.Pabón M, López-Gatius F, García-Ispierto I, Bech-Sàbat G, Nogareda C, Almería S. Chronic Neospora caninum infection and repeat abortion in dairy cows: a 3-year study. Vet Parasitol. 2007;147(1–2):40–6. doi: 10.1016/j.vetpar.2007.03.017 [DOI] [PubMed] [Google Scholar]
- 83.Pescador C, Corbellini L, Oliveira E, Raymundo D, Driemeier D. Histopathological and immunohistochemical aspects of Neospora caninum diagnosis in bovine aborted fetuses. Vet Parasitol. 2007;150(1–2):159–63. doi: 10.1016/j.vetpar.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 84.Escamilla HP, Martínez MJJ, Medina CM, Morales SE. Frequency and causes of infectious abortion in a dairy herd in Queretaro, Mexico. Can J Vet Res. 2007;71(4):314. [PMC free article] [PubMed] [Google Scholar]
- 85.Moore D, Regidor-Cerrillo J, Morrell E, Poso MA, Cano DB, Leunda MR, et al. The role of Neospora caninum and Toxoplasma gondii in spontaneous bovine abortion in Argentina. Vet Parasitol. 2008;156(3–4):163–7. doi: 10.1016/j.vetpar.2008.06.020 [DOI] [PubMed] [Google Scholar]
- 86.Yildiz K, Kul O, Babur C, Kılıc S, Gazyagcı AN, Celebi B, et al. Seroprevalence of Neospora caninum in dairy cattle ranches with high abortion rate: special emphasis to serologic co-existence with Toxoplasma gondii, Brucella abortus and Listeria monocytogenes. Vet Parasitol. 2009;164(2–4):306–10. doi: 10.1016/j.vetpar.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 87.Salehi N, Haddadzadeh H, Ashrafihelan J, Shayan P, Sadrebazzaz A. Molecular and pathological study of bovine aborted fetuses and placenta from Neospora caninum infected dairy cattle. Iran J Parasitol. 2009;4(3):40–51. [Google Scholar]
- 88.Sánchez G, Banda R, Sahagun R, Ledesma M, Morales S. Comparison between immunohistochemistry and two PCR methods for detection of Neospora caninum in formalin-fixed and paraffin-embedded brain tissue of bovine fetuses. Vet Parasitol. 2009;164(2–4):328–32. doi: 10.1016/j.vetpar.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 89.Cabral AD, Camargo CN, Galleti NTC, Okuda LH, Pituco EM, Del Fava C. Diagnosis of Neospora caninum in bovine fetuses by histology, immunohistochemistry, and nested-PCR. Rev Bras Parasitol Vet. 2009;18(4):14–9. doi: 10.4322/rbpv.01804003 [DOI] [PubMed] [Google Scholar]
- 90.Razmi GR, Zarea H, Naseri Z. A survey of Neospora caninum-associated bovine abortion in large dairy farms of Mashhad, Iran. Parasitol Res. 2010;106(6):1419–23. doi: 10.1007/s00436-010-1820-3 [DOI] [PubMed] [Google Scholar]
- 91.Şuteu O, Titilincu A, Modrý D, Mihalca A, Mircean V, Cozma V. First identification of Neospora caninum by PCR in aborted bovine foetuses in Romania. Parasitol Res. 2010;106(3):719–22. doi: 10.1007/s00436-009-1684-6 [DOI] [PubMed] [Google Scholar]
- 92.Tramuta C, Lacerenza D, Zoppi S, Goria M, Dondo A, Ferroglio E, et al. Development of a set of multiplex standard polymerase chain reaction assays for the identification of infectious agents from aborted bovine clinical samples. J Vet Diagn Invest. 2011;23(4):657–64. doi: 10.1177/1040638711407880 [DOI] [PubMed] [Google Scholar]
- 93.dos Santos DS AM, Varaschin MS, Guimarães AM, Hirsch C. Neospora caninum in bovine fetuses of Minas Gerais, Brazil: genetic characteristics of rDNA. Rev Bras Parasitol Vet. 2011;20:281–8. doi: 10.1590/s1984-29612011000400005 [DOI] [PubMed] [Google Scholar]
- 94.Şuteu O, Paştiu A, Györke A, Cozma V. Molecular detection of Neospora caninum abortion in dairy cattle from different historical regions of Romania. Sci Parasitol. 2012;13(4):159–62. [Google Scholar]
- 95.Kamali A, Seifi HA, Movassaghi AR, Razmi GR, Naseri Z. Histopathological and molecular study of Neospora caninum infection in bovine aborted fetuses. Asian Pac J Trop Biomed. 2014;4(12):990–4. 10.12980/APJTB.4.201414B378 [DOI] [Google Scholar]
- 96.Salehi N, Gottstein B, Haddadzadeh H. Genetic diversity of bovine Neospora caninum determined by microsatellite markers. Parasitol Int. 2015;64(5):357–61. doi: 10.1016/j.parint.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 97.Medina-Esparza L, Regidor-Cerrillo J, García-Ramos D, Álvarez-García G, Benavides J, Ortega-Mora LM, et al. Genetic characterization of Neospora caninum from aborted bovine foetuses in Aguascalientes, Mexico. Vet Parasitol. 2016;228:183–7. doi: 10.1016/j.vetpar.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 98.Ozkaraca M, Irehan B, Parmaksiz A, Ekinci AI, Comakli S. Determination of Neospora caninum and Toxoplasma gondii in aborted bovine foetuses by duplex PCR, immunohistochemistry and immunofluorescence methods. Med Weter. 2017;73(06). [Google Scholar]
- 99.Kaveh A, Merat E, Samani S, Danandeh R, Soltannezhad S. Infectious causes of bovine abortion in Qazvin Province, Iran. Arch Razi Inst. 2017;72(4):225–30. doi: 10.22092/ari.2017.113299 [DOI] [PubMed] [Google Scholar]
- 100.Qian W, Wang T, Yan W, Zhang M, Han L, Xue R, et al. Seroprevalence and first multilocus microsatellite genotyping of Neospora caninum in dairy cattle in Henan, central China. Vet Parasitol. 2017;244:81–4. doi: 10.1016/j.vetpar.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 101.Díaz Cao JMD, Lago AP, Lorenzo GL, Fernández PD, Sández CML, Pelayo MPM, et al. Broadening the diagnosis panel of reproductive pathogens associated with abortion in ruminants. Span J Agric Res. 2018;16(2):17. 10.5424/sjar/2018162-12180 [DOI] [Google Scholar]
- 102.Tian A-L, Elsheikha HM, Zhou D-H, Wu Y-D, Chen M-X, Wang M, et al. A novel recombinase polymerase amplification (RPA) assay for the rapid isothermal detection of Neospora caninum in aborted bovine fetuses. Vet Parasitol. 2018;258:24–9. doi: 10.1016/j.vetpar.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 103.Snak A, Garcia FG, Lara AA, Pena HFJ, Osaki SC. Neospora caninum in properties in the west region of Paraná, Brazil: prevalence and risk factors. Rev Bras Parasitol Vet. 2018;27:51–9. doi: 10.1590/S1984-29612018001 [DOI] [PubMed] [Google Scholar]
- 104.Moroni M, Navarro M, Paredes E, Romero A, Alberdi A, Lischinsky T, et al. Identification of Neospora caninum in aborted bovine fetuses of Southern Chile. Braz J Vet Pathol. 2018. 10.24070/bjvp.1983-0246.v11i2p37-41 [DOI] [Google Scholar]
- 105.Bartley P, Guido S, Mason C, Stevenson H, Chianini F, Carty H, et al. Detection of Neospora caninum DNA in cases of bovine and ovine abortion in the South-West of Scotland. Parasitology. 2019;146(7):979–82. doi: 10.1017/S0031182019000301 [DOI] [PubMed] [Google Scholar]
- 106.Acici M, Bolukbas CS, Pekmezci GZ, Gurler H, Genc O, Gurler AT, et al. A diagnostic survey of Neospora caninum infection in aborted fetuses in the Middle Black Sea Region and Sivas Province, Turkey. Turk J Vet Anim Sci. 2019;43(6):761–6. 10.3906/vet-1908-16 [DOI] [Google Scholar]
- 107.Mahajan V, Banga H, Filia G. Patho-epidemiological and risk factor studies for detection of Neospora-associated abortion in cattle and buffaloes in Punjab, India. Rev Sci Tech. 2020;38(3):801–8. 10.20506/rst.38.3.3027 [DOI] [PubMed] [Google Scholar]
- 108.Amouei A, Sharif M, Sarvi S, Nejad RB, Aghayan SA, Hashemi-Soteh MB, et al. Aetiology of livestock fetal mortality in Mazandaran province, Iran. PeerJ. 2019;6:e5920. eCollection 2019 doi: 10.7717/peerj.5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villa L, Maksimov P, Luttermann C, Tuschy M, Gazzonis AL, Zanzani SA, et al. Spatial distance between sites of sampling associated with genetic variation among Neospora caninum in aborted bovine foetuses from northern Italy. Parasit Vectors. 2021;14(1):1–14. doi: 10.1186/s13071-020-04557-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salehi B, Amouei A, Dodangeh S, Daryani A, Sarvi S, Safari-Kharyeki MR, et al. Molecular identification of Neospora caninum infection in aborted fetuses of sheep, cattle, and goats in Mazandaran Province, Northern Iran. Iran J Parasitol. 2021;16(3):483. doi: 10.18502/ijpa.v16i3.7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dorsch MA, Moore DP, Regidor-Cerrillo J, Scioli MV, Morrell EL, Cantón GJ, et al. Morphometric study of encephalic lesions in aborted bovine fetuses naturally infected by two subpopulations of Neospora caninum. Parasitol Res. 2021;120(8):2995–3000. doi: 10.1007/s00436-021-07248-y [DOI] [PubMed] [Google Scholar]
- 112.El-Alfy E-S, Ohari Y, Shimoda N, Nishikawa Y. Genetic characterization of Neospora caninum from aborted bovine fetuses in Hokkaido, Japan. Infect Genet Evol. 2021;92:104838. doi: 10.1016/j.meegid.2021.104838 [DOI] [PubMed] [Google Scholar]
- 113.Kirkbride CA. Etiologic agents detected in a 10-year study of bovine abortions and stillbirths. J Vet Diagn Invest. 1992;4(2):175–80. doi: 10.1177/104063879200400210 [DOI] [PubMed] [Google Scholar]
- 114.Conrad PA, Sverlow K, Anderson M, Rowe J, BonDurant R, Tuter G, et al. Detection of serum antibody responses in cattle with natural or experimental Neospora infections. J Vet Diagn Invest. 1993;5(4):572–8. doi: 10.1177/104063879300500412 [DOI] [PubMed] [Google Scholar]
- 115.Dubey J. Recent advances in Neospora and neosporosis. Vet Parasitol. 1999;84(3–4):349–67. doi: 10.1016/s0304-4017(99)00044-8 [DOI] [PubMed] [Google Scholar]
- 116.Dubey J, Lindsay D, Adams D, Gay J, Baszler T, Blagburn B, et al. Serologic responses of cattle and other animals infected with Neospora caninum. Am J Vet Res. 1996;57(3):329–36. [PubMed] [Google Scholar]
- 117.Georgieva D, Prelezov P, Koinarski V. Neospora caninum and neosporosis in animals. A review. Bulg J Vet Med. 2006;9(1):1–26. [Google Scholar]
- 118.Hernandez J, Risco C, Donovan A. Association between exposure to Neospora caninum and milk production in dairy cows. J Am Vet Med Assoc. 2001;219(5):632–5. doi: 10.2460/javma.2001.219.632 [DOI] [PubMed] [Google Scholar]
- 119.Reichel MP, Ayanegui-Alcérreca MA, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle–the billion dollar question. Int J Parasitol. 2013;43(2):133–42. doi: 10.1016/j.ijpara.2012.10.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.