Abstract
Background
The Kato-Katz microscopy technique is the global standard for assessment of soil-transmitted helminth (STH) burden. However, major limitations include its poor sensitivity, requirement for rapid sample processing, and inability to differentiate hookworm species nor detect Strongyloides spp. infections. We assessed the prevalence and intensity of STH species in Solomon Islands by conducting a province-wide survey using quantitative PCR (qPCR) for diagnosis, which can provide much better characterisation of STH burden than microscopy.
Methodology/Principal findings
We conducted a cross-sectional survey in 18 villages in Western Province to detect infections with six STH species and quantify intensity with three. We used linear mixed model regression to identify potential water, sanitation, and hygiene (WASH) and environmental risk factors for infection. We collected stool specimens from 830 village residents. Overall STH prevalence was 63.3% (range 27.5 to 91.5% across villages), led by Necator americanus (54.5% [range 17.5–89.4%]), followed by Ancylostoma ceylanicum (15.5% [range 2.8–45.8%]), Trichuris trichiura (9.1% [range 0–79.2%]), and Strongyloides spp. (3.2% [range 0–29.2%]). Most infections were of light intensity for N. americanus (85.7%) and T. trichiura (90.7%). Owning a household latrine was associated with a lower risk of N. americanus infection (AOR 0.41, 95% CI 0.24–0.68) while greater precipitation was linked to more common T. trichiura infection (AOR 1.14, 95% CI 1.04–1.25).
Conclusion/Significance
In this first large-scale population survey of STH in the Pacific using qPCR, we found evidence that ivermectin should be incorporated into STH control programmes because of the presence of T. trichiura and Strongyloides spp., both of which are poorly responsive to albendazole. Furthermore, One Health strategies are needed for improved A. ceylanicum and Strongyloides spp. control, WASH access and use should be improved to complement deworming programmes, and control efforts should ideally be expanded to entire communities.
Trial registration
ClinicalTrials.gov Australian and New Zealand Clinical Trials Registry ACTRN12618001086257.
Author summary
Routine assessments of the burden of intestinal worm infections rely on microscopy-based diagnostic methods, such as the Kato-Katz technique. However major limitations include its poor sensitivity, requirement for rapid sample processing, and inability to differentiate individual hookworm species and detect Strongyloides spp. infections. It is important to assess the burden of each of these infections to design control approaches beyond the current core strategy of albendazole preventive chemotherapy. Use of qPCR for diagnosis could address these gaps because it can distinguish all relevant intestinal worm species. We completed a province-wide intestinal worm infections prevalence survey using qPCR in Western Province, Solomon Islands. Overall prevalence was extremely high (62.5%). We identified a high burden of Necator americanus (54.5%) and detected Ancylostoma ceylanicum (15.5%), Trichuris trichiura (9.1%), and Strongyloides spp. (3.2%) infections. Increased age and precipitation were associated with higher prevalence of infection, while owning a household latrine was associated with lower odds of N. americanus infection. Our findings support the need to incorporate ivermectin into deworming programmes for Strongyloides spp. and T. trichiura control as these species are poorly responsive to albendazole, implement One Health strategies to address the zoonotic A. ceylanicum and Strongyloides spp., and expand control efforts to reach entire communities. WASH access should also be improved to complement deworming programmes.
Introduction
Infections with soil-transmitted helminths (STHs) including Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis and the hookworms Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum represent the most frequently occurring of all neglected tropical diseases (NTDs). STHs infect an estimated 895 million people worldwide [1] with a disease burden of 1.9 million disability-adjusted life years [2] disproportionately impacting on remote and rural communities in low and middle income countries.
Decisions on the implementation and cessation of STH control programmes rely on findings from epidemiological surveys that assess infection prevalence and intensity. The Kato-Katz microscopy technique, recommended by the World Health Organization (WHO), has been the mainstay for STH detection in these surveys. Its attraction is relative simplicity and low resource requirements. However, major limitations include its poor sensitivity [3,4], requirement for rapid sample processing to avoid hookworm egg degradation, and inability to differentiate the three hookworm species, nor detect Strongyloides spp. infection [4]. As a zoonotic disease, control of A. ceylanicum requires an intersectoral approach, that incorporates One Health strategies as well as preventive chemotherapy [5] particularly in endemic countries in the Asia-Pacific region where high burdens have been documented [6]. Similar considerations apply to S. stercoralis also suggested to be a zoonosis [7], and the subject of new targets introduced by WHO to establish a control programme by 2030 [5] which will need to incorporate ivermectin preventive chemotherapy, as albendazole is not effective for this species [8]. Ivermectin also plays an important role in improving T. trichiura control when used in combination with albendazole [9].
Quantitative real-time polymerase chain reaction (qPCR) is a highly sensitive molecular diagnostic assay that can analyse preserved samples and detect infections with all relevant STH species in stool samples [4]. It has been validated for both STH diagnosis and measuring the infection intensity [3,10,11], and so far primarily used in large-scale surveys in the context of clinical trials [12–15] or evaluation of STH transmission elimination status [16]. In settings with diverse STH species, qPCR is far superior to the Kato-Katz method in its ability to provide the detailed characterisation of STH burden needed to guide interventions.
Surveys in two of the nine Solomon Islands provinces have reported an overall prevalence of up to 52.7%, with hookworm being the most prevalent species [17–19], as determined by copro-microscopy methods [17–21]. These extremely high prevalences may be explained by poor access to water, sanitation, and hygiene (WASH) facilities in many parts of the country, and exposure to environmental conditions that are favourable for STH development and transmission, in the absence of a national STH control programme.
In the context of a trial of two versus one dose(s) of ivermectin administration for scabies, we assessed the prevalence and intensity of STH, including A. ceylanicum, Strongyloides spp., and T. trichiura, in Western Province of Solomon Islands using qPCR. We also aimed to identify WASH and environmental risk factors associated with infection. To our knowledge, this study is the first qPCR prevalence survey of STH infections in the Pacific region and only the second in the world in any low- or middle-income country [12].
Methods
Ethics statement
The survey was embedded within the baseline assessment undertaken for a cluster-randomised control trial of ivermectin mass drug administration (Australian and New Zealand Clinical Trials Registry ACTRN12618001086257), which included impact on STH infections as a secondary outcome. The trial was approved by the Solomon Islands Health Research and Ethics Review Board (HRE005/18) and the Royal Children’s Hospital Human Research Ethics Committee, Melbourne, Australia (38099A).
Written informed consent was provided prior to data collection, including obtaining signed consent from all adults and parents or guardians of children under 18 years old. All residents aged 12 months or more in the study villages were eligible to participate.
Study design and participants
This study took place in Western Province of Solomon Islands. Solomon Islands is an archipelago situated in the Western Pacific region consisting of approximately 695,000 people [22]. Western province is one of nine provinces in the country and is the third most populous with an estimated population of 76,649 people [22]. We conducted a cross-sectional survey of residents of 18 villages in Western Province between May and July 2019. Details of the trial design can be found in the published trial protocol [23]. The 20 villages for the trial were selected randomly from all those in the province with a population size of between 180 to 300 people. For logistical reasons, the STH survey could not be conducted in the first two villages.
Specimen collection
A local health promotion team conducted community awareness visits approximately one month prior to the commencement of the study. One day prior to stool collection, trained field team members visited villages to seek permission from village leaders to conduct research. Following approval, the team informed residents of the study either through household visits or community meetings at a central location, provided verbal instructions on how to provide a stool sample and offered the opportunity to ask questions. Each household member was given a stool collection kit containing a 70 ml plastic faeces specimen jar, gloves, a study information sheet, and written instructions on providing a sample. Residents were asked to self-collect a fresh stool sample the following day and drop it off the same day to a central location, ensuring they did so before administration of ivermectin, which was also planned to take place that day. Participants were also asked to participate in a WASH questionnaire administered by local field staff after samples were dropped off. A single aliquot of stool (3g) per participant was fixed in 5% w/v potassium dichromate upon receipt and kept at room temperature for at least 4 weeks due to limited access to electricity and a refrigerator in the field, until reaching the University of Melbourne (UoM) lab where they were kept at at 4°C until DNA extraction. This resulted in embryonation of STH eggs, which was confirmed with microscopic examination of a random subset of positive samples at the UoM.
Quantitative PCR analysis
All samples were couriered to the University of Melbourne for qPCR analysis. Genomic DNA was extracted from a single 200 mg aliquot of each stool sample using a Maxwell RSC Pure Food GMO and Authentication Kit, Promega (Promega Corporation, US) as per manufacturer’s instructions, with the following modifications: an additional bead-beating step with 400μl CTAB Buffer using a FastPrep-24 5G Instrument (MP Biomedicals) and 0.5mm Zirconia/Silica beads (Daintree Scientific, AUS). The full DNA extraction and bead-beating protocol was published elsewhere [3]. Following DNA extraction, samples underwent two Taq Man probe-based quadraplex real-time qPCR assays in duplicate to diagnose STH infections with six species (N. americanus, A. ceylanicum, A. duodenale, T. trichiura, Strongyloides spp., A. lumbricoides) and quantify intensity of infection with three species (N. americanus, T. trichiura, A. lumbricoides) [3,11,24,25]. The first assay was performed to enumerate A. lumbricoides and T. trichiura and detect Strongyloides spp. using EHV-4 as an internal qPCR control; and the second was performed to enumerate N. americanus, A. duodenale, and A. ceylanicum, using human 16S mitochondrial rRNA as an internal qPCR and DNA extraction control. Nuclease-free water was used as negative control. The sequences of primers and probes and PCR conditions were based on published information [3,10,11,24–30] and are summarised in Table A in S1 Text. The cycling conditions for both assays consisted of the following parameters: denaturation at 95°C for 2 min, followed by 40 cycles of 15 sec at 95°C and annealing at 60°C for 1 min, with no extension phase. All samples with Ct values in duplicate were deemed positive. The full qPCR protocol was published elsewhere [3].
To convert qPCR-derived cycle threshold (Ct) values into eggs per gram (epg) of stool, we used the following linear regression equations derived for embryonated eggs using methods described previously [3]: A. lumbricoides epg = 10((Ct-30.048)/-3.2804); T. trichiura epg = 10((Ct-31.888)/-4.048); N. americanus epg = 10((Ct-32.657)/-3.878). Briefly, these conversion equations were produced based on faecal seeding experiments where parasite-free human faeces were spiked with a serial dilution of known quantities of eggs purified from human and pig faeces that were allowed to embryonate in a 28°C incubator for at least 4 weeks to mirror the embryonated state of the field samples [3]. Water was also added to the incubator to mirror the hot and humid conditions of a tropical setting. Triplicate qPCR assays were performed on the spiked samples then Log10 transformations of original epg were plotted against Ct values to produce linear regression equations that predicted egg count from Ct values for each species [3]. The epg values estimated from the conversion formulae were classified into one of three infection intensity classes (light, moderate, heavy) according to WHO recommended thresholds [31]. Conversion equations were not produced for A. ceylanicum as we were unable to obtain eggs needed for the seeding experiments.
Demographic, WASH, and environmental data collection
Demographic and WASH data were collected through an individual, self-reported questionnaire. The WASH section consisted of 12 questions that assessed access to water sources and sanitation facilities, and hygiene behaviours, at the individual and household level.
Global Positioning System coordinates were collected at the village level. Environmental variables were obtained through publicly available sources reporting remotely-sensed data including temperature, precipitation, elevation, soil composition, vegetation, and landcover type geo-referenced at the village level. A summary of the environmental variables used in this analysis, their spatial resolution, temporal resolution, temporal extent, and source are provided in Table 1.
Table 1. Environmental variables extracted for the current analysis.
| Environmental variable | Parameter | Spatial resolution | Temporal resolution and extent | Variable source |
|---|---|---|---|---|
| Ambient temperature (°C) |
|
1 km | Monthly averages from 1970–2000 | Worldclim1 |
|
Precipitation (cm) |
|
1 km | Monthly total averages from 1970–2000 | Worldclim1 |
| Elevation (metres) | Mean elevation in metres | 30 m | March 2000 | NASA ASTER on Terra satellite2 |
| Slope (degrees) | Mean slope in degrees | 30 m | March 2000 | NASA ASTER on Terra satellite2 |
| Vegetation (NDVI, EVI) | Mean vegetation as measured by NDVI & EVI | 250 m | 16-day averages across 2019 | NASA MODIS on Terra satellite3 |
| Landcover (Using IGBP land classification scheme) | Most occurring land cover type in village | 500 m | Yearly average from 2018 | NASA MODIS on Terra & Aqua satellites4 |
| Soil pH | Mean soil water pH on the top 5cm of the soil | 250 m | Yearly averages from1901–2016 | International Soil Reference & Information Centre (ISRIC) Soilgrids5 |
| Soil texture | Proportion of soil clay, silt, and sand content on the top 5cm of the soil | 250 m | Yearly averages from 1901–2016 | ISRIC Soilgrids5 |
1Worldclim Version 2.0,
2Data package ASTGTM Version 3,
3Data package MOD13Q1 Version 6,
4Data package MCD12Q1 Version 6,
5SoilGrids250m Version 2.0
Environmental data were processed and extracted using QGIS version 3.10 (Open Source Geospatial Foundation Project, Chicago). Specified spatial zones (buffers) were individually shaped, sized, and positioned to capture the entirety of each village. The mean of the raster cells (matrix of pixels containing data) within buffer zones were extracted for the variables temperature, precipitation, elevation, vegetation (Normalised Difference Vegetation Index [NDVI], Enhanced Vegetation Index [EVI]), and soil pH. Slope data were extrapolated from a digital elevation model in QGIS. For landcover, the modal raster cell value was used to classify villages according to the International Geosphere-Biosphere Programme classification system [32] Soil texture was classified using the United States Department of Agriculture system [33] based on the relative proportions of clay, sand, and silt present on the top 5 cm. Soil pH had a narrow range of pH (5.20–5.50) so was dichotomised into 2 classes based on median (5.30).
Statistical analysis
To take account of the cluster sampling by village, mixed-effects generalised linear models were used to estimate infection prevalence for each species and to examine differences by sex and age. Post-hoc power analysis indicated that a sample size of 810 was sufficient to detect 52.7% prevalence with any STH (derived from the most recent survey [20]) across 18 clusters with 90% power, 5% margin of error, and design effect of 3.0 to account for cluster sampling.
We used the mixed effects methods to also examine WASH and environmental risk factors associated with infection for each species. The model building procedure was based on previous risk factor analyses [12,34]. A series of univariable regressions were first completed for each variable, with variables being retained for the next step of the analysis if their p value was less than 0.20 on the Wald test. Retained variables were grouped into theoretically relevant domains with each then subjected to a series of multivariable regressions with sex and age group entered as covariates, and variables retained if their p value was less than 0.10 on the Wald test. They were then tested for multicollinearity using variance inflation factors (VIFs), with VIF>5 being used as the criterion for violating collinearity. Issues with collinearity were resolved using the Akaike Information Criterion (AIC) wherein variables with lower AICs were retained as this indicated greater predictive performance. Finally, a backward stepwise elimination variable selection procedure was used until all variables in the model (except sex and age group) had p values of less than 0.05 on the Wald test. All base models contained sex and age group as covariates, and household and village as random effect terms. Adjusted odds ratios (AORs) and incidence rate ratios (IRRs) derived from final models and their 95% confidence intervals (CIs) are reported herein. The significance level for final models was set at p<0.05. Analyses were completed using Stata version 14.2 (Stata Corporation, College Station, Texas).
Results
The 18 study villages had an estimated total population of 4920 based on information provided by village leaders. A total of 2392 stool collection kits were distributed, covering 48.6% of the population. Overall, 830 participants provided a stool sample, corresponding to a 34.7% response rate and 16.9% coverage of the population. Of those who provided a sample, 73.5% (n = 619) also completed the WASH questionnaire.
Among the 830 participants, 57.6% were female and the mean age was 21.7 years (SD 20.5). Responses to the WASH questionnaire indicated that 93.5% of participants practiced open defecation, 20.4% lived in dwellings with a household latrine, 67.9% had access to drinking water from an improved water source, and 15.7% always wore shoes outside. The mean annual temperature was 26.9°C (range 26.6–27.2 across villages) and annual precipitation 341.8 cm (range 321.8–353.7). Soil pH levels were in the range 5.20 to 5.30 for 9 villages comprising 41.7% of participants whereas the remaining 58.3% were from villages that had soil pH in the range 5.30 to 5.50. A full summary of descriptive statistics for WASH and environmental variables is provided in Tables B and C in S1 Text.
STH infection prevalence and intensity
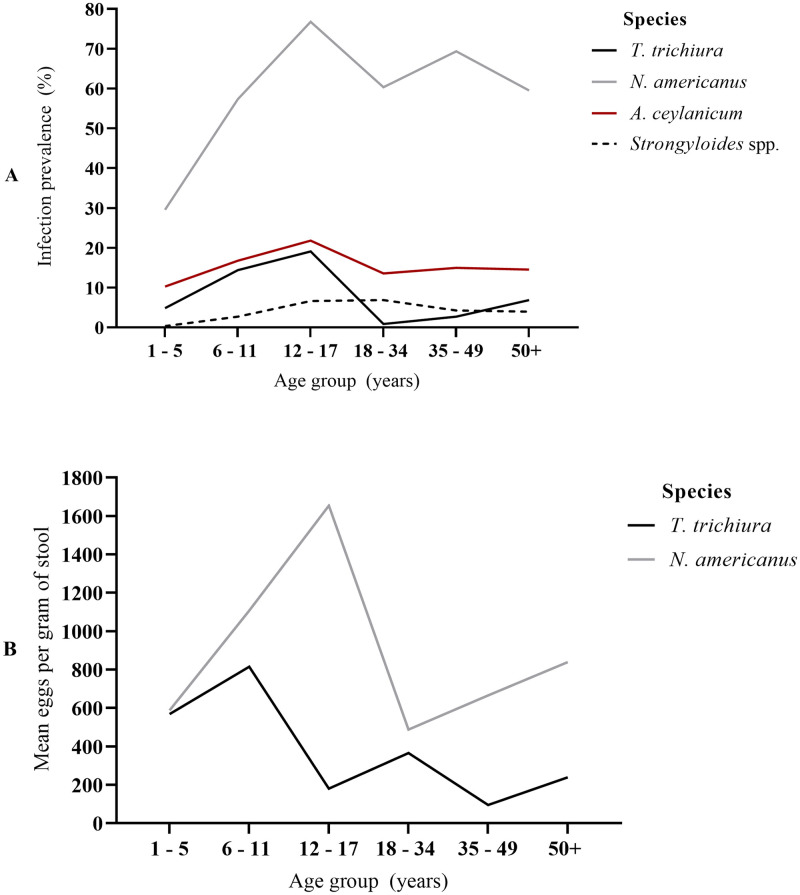
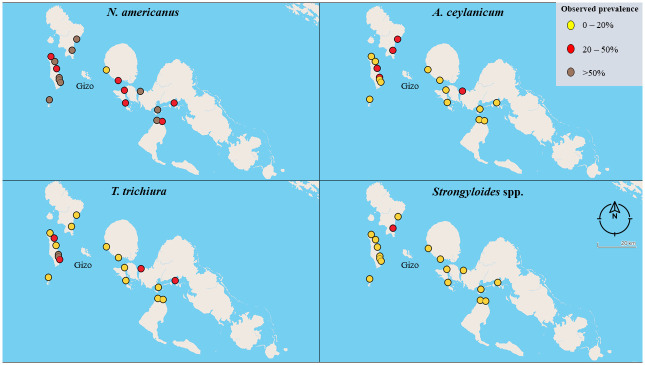
The overall prevalence of infection with any STH was 63.3%, ranging from 27.5 to 91.5% across villages (see Tables 2 and 3 and Fig 1). Most common were infections with N. americanus (54.5%, range 0–79.2%), followed by A. ceylanicum (15.5%, 2.8–45.8%), T. trichiura (9.0%, 0–79.2%]) and Strongyloides spp. (3.2% [range 0–29.2%]). A. lumbricoides infection was rare, at 0.08% (range 0–16.7%). Relative to those who were infected, most infections were of light intensity for A. lumbricoides (66.7%), T. trichiura (90.7%), and N. americanus (85.7%). Relative to the entire sample, the prevalence of moderate-to-heavy intensity infections were as follows: A. lumbricoides (0.2%), T. trichiura (1.9%), and N. americanus (7.6%). Regarding the geographic distribution of infections for each species, we observed that the prevalence of N. americanus infections was above 20% in 94.4% of villages. A total of 33.3% of villages had T. trichiura prevalence of above 20%, 27.8% of villages for A. ceylanicum, and 5.6% of villages for Strongyloides spp. (see map, Fig 2).
Table 2. Cluster-adjusted STH prevalence by species, stratified by sex and age group.
| Study sample n (%) |
Any STH % (95% CI) |
A. lumbricoides % (95% CI) |
T. trichiura % (95% CI) |
N. americanus % (95% CI) |
A. ceylanicum % (95% CI) |
A. duodenale % (95% CI) |
Strongyloides spp. % (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 352 (42.41) |
66.07 (53.69–78.46) |
0.09 (0–0.49) |
8.25 (0–16.95) |
58.50 (45.29–71.71) |
13.87 (9.23–18.50) |
0 | 4.27 (0.47–8.06) |
| Female | 478 (57.59) |
61.37 (49.17–73.57) |
0.10 (0–0.51) |
8.02 (0.30–16.00) |
51.64 (39.89–63.39) |
16.49 (10.91–22.06) |
0 | 2.87 (0.18–5.57) |
| Age (years) | ||||||||
| 1–5 | 212 (25.54) |
41.31 (28.55–54.08) |
0.12 (0–0.74) |
4.83 (0–11.71) |
29.53 (18.68–40.37) |
10.27 (4.61–15.94) |
0 | 0.31 (0–1.45) |
| 6–11 | 201 (24.22) |
69.75 (51.33–88.18) |
0.50 (0–1.47) |
14.36 (0–29.61) |
57.34 (37.36–77.32) |
16.74 (9.72–23.76) |
0 | 2.68 (0–6.25) |
| 12–17 | 89 (10.72) |
87.85 (69.60–100) |
2.24 (0–6.75) |
19.07 (0–45.81) |
76.77 (57.71–95.82) |
21.77 (7.18–36.34) |
0 | 6.64 (0–17.06) |
| 18–34 | 99 (11.93) |
71.07 (56.39–85.75) |
0 | 0.87 (0–4.31) |
60.32 (47.62–73.03) |
13.53 (3.50–23.56) |
0 | 6.87 (0–14.92) |
| 35–49 | 108 (13.01) |
76.58 (60.81–92.35) |
0 | 2.67 (0–9.41) |
69.36 (52.10–86.61) |
14.96 (5.96–23.96) |
0 | 4.24 (0–11.04) |
| ≥50 | 121 (14.58) |
61.98 (53.33–70.63) |
0 | 6.85 (0.30–13.41) |
59.50 (50.76–68.25) |
14.51 (5.53–23.49) |
0 | 3.69 (0–9.01) |
| Total | 830 |
63.34
(52.13–74.55) |
0.08
(0–0.36) |
9.08
(1.27–16.90) |
54.51
(43.37–65.64) |
15.47
(10.82–20.11) |
0 |
3.17
(0.59–5.76) |
Table 3. Infection intensity of A. lumbricoides, T. trichiura, and N. americanus as measured by WHO recommended thresholds and mean eggs per gram (epg) of stool.
| Infection intensity class | EPG | |||||
|---|---|---|---|---|---|---|
| Prevalence n (%) |
Light†a % (95% CI) |
Moderate†b % (95% CI) |
Heavy†c % (95% CI) |
Mean (SD) |
Range | |
| A. lumbricoides | 6 (0.08) | 66.67 (14.86–95.81) |
16.67 (0.91–81.39) |
16.67 (0.91–81.39) |
54, 264 (117,180.20) |
1–291, 868 |
| T. trichiura | 172 (9.08) | 90.70 (85.29–94.25) |
9.30 (5.75–14.71) |
0 | 478 (1248) |
1–8412 |
| N. americanus | 460 (54.51) | 85.65 (82.03–88.64) |
8.43 (6.16–11.14) |
5.92 (4.06–8.57) |
920 (1827) |
1–12,456 |
†Proportion of the population determined to be infected;
aThresholds for light intensity infections: A. lumbricoides (1–4999 epg), T. trichiura (1–999 epg), N. americanus (1–1999 epg);
bThresholds for moderate intensity infections: A. lumbricoides (5000–49,999 epg), T. trichiura (1000–9999 epg), N. americanus (2000–3999 epg);
cThresholds for heavy intensity infections: A. lumbricoides (≥50,000 epg), T. trichiura (≥10,000 epg), N. americanus (≥4000 epg).
Fig 1. Age- infection profiles by STH species.
(A) Prevalence, (B) Intensity as measured by mean eggs per gram of stool. Exact values including 95% confidence intervals and standard deviations are shown in Table 2 (prevalence) and Table E in S1 Text (intensity). A. lumbricoides infections excluded due to few positive cases.
Fig 2. Map showing the observed prevalence of infections with N. americanus, A. ceylanicum, T. trichiura, and Strongyloides spp. across 18 villages in Western Province.
In the analysis of risk factors (Tables 4 and 5), males were more likely to be infected with N. americanus, than females (AOR 1.63, 95% CI 1.13–2.34, p = 0.008), and there was a pattern of increasing risk of infection with age relative to young children aged 1–5 years (6–11 years [AOR 5.16, 95% CI 3.04–8.77, p<0.001]; 12–17 years [AOR 9.55, 95% CI 4.63–19.68, p<0.001]; 18–34 years [AOR 5.79, 95% CI 3.04–11.01, p<0.001]; 35–49 years [AOR 6.91, 95% CI 3.66–13.02, p<0.001]; ≥50 years [AOR 6.32, 95% CI 3.43–11.64, p<0.001]). For T. trichiura, there was no detectable difference in infection prevalence by sex but we observed increasing prevalence by age within children (6–11 years; AOR 3.0, 95% CI 1.49–6.01, p = 0.002) and adolescents (12–17 years; AOR 5.99, 95% CI 2.56–14.00, p<0.001), compared young children (see Table 5). There were no detectable differences in Strongyloides spp. infection prevalence by sex, but again higher levels in adolescents (AOR 10.11, 95% CI 2.58–39.60, p = 0.001) and adults (18–34 years, AOR 5.08, 95% CI 1.17–22.05) than young children. There was no evidence of differences in prevalence by sex or age for A. ceylanicum. For infection intensity, there was a pattern of increasing N. americanus egg counts with age within in adolescents (IRR 4.04, 95% CI 2.11–7.71, p<0.001) and older adults (≥50 years; IRR 2.55, 95% CI 1.28–5.06, p = 0.007), compared to young children (Table E in S1 Text). There were no detectable differences in T. trichiura infection intensity by age or sex. Demographic differences in A. lumbricoides infections were not examined due to few positive cases (n = 6).
Table 4. Risk factors associated with N. americanus, A. ceylanicum, and undifferentiated hookworm infection.
|
N. americanus (n = 460) |
A. ceylanicum (n = 140) |
Hookworm (undifferentiated) (n = 489) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor variable | aOR | 95% CI | P value | aOR | 95% CI | P value | aOR | 95% CI | P value |
| Male sexa | 1.63 | 1.13–2.34 | 0.008 | 0.77 | 0.51–1.16 | 0.212 | 1.59 | 1.11–2.26 | <0.001 |
| Age group 6–11 yearsb | 5.16 | 3.04–8.77 | <0.001 | 1.78 | 0.99–3.20 | 0.084 | 4.05 | 2.45–6.70 | <0.001 |
| Age group 12–17 yearsb | 9.55 | 4.63–19.68 | <0.001 | 2.77 | 1.39–5.51 | 0.004 | 7.12 | 3.54–14.34 | <0.001 |
| Age group 18–34 yearsb | 5.79 | 3.04–11.01 | <0.001 | 1.34 | 0.65–2.76 | 0.435 | 5.09 | 2.72–9.51 | <0.001 |
| Age group 35–49 yearsb | 6.91 | 3.66–13.02 | <0.001 | 1.31 | 0.65–2.65 | 0.449 | 5.39 | 2.93–9.94 | <0.001 |
| Age group ≥50 yearsb | 6.32 | 3.43–11.64 | <0.001 | 1.34 | 0.67–2.66 | 0.405 | 4.31 | 2.43–7.64 | <0.001 |
| N. americanus co-infection | - | - | - | 4.58 | 2.58–8.14 | <0.001 | - | - | - |
| A. ceylanicum co-infection | 4.36 | 2.36–8.06 | <0.001 | - | - | - | - | - | - |
| T. trichiura co-infection | 2.79 | 1.39–5.60 | 0.004 | - | - | - | 3.36 | 1.64–6.89 | 0.001 |
| Strongyloides spp. co-infection | - | - | - | 3.19 | 1.39–7.34 | 0.006 | - | - | - |
| Main drinking water is from improved water sourcec* | - | - | - | 2.71 | 1.41–5.24 | 0.003 | - | - | - |
| Has toilet/latrine in household | 0.41 | 0.24–0.68 | <0.001 | - | - | - | 0.45 | 0.27–0.74 | 0.002 |
| Soil pH (pH >5.30–5.50)d | 2.92 | 1.29–6.60 | 0.010 | - | - | - | 2.85 | 1.27–6.37 | 0.011 |
“95% CI” denotes 95% confidence interval, “aOR” denotes adjusted odds ratio.
These results were derived from a model building procedure where variables were removed from the analysis if they did not meet the criterion p value at each stage of the analysis. Variables with blank cells indicate that these were removed in an earlier stage. Tables F-K in S1 Text summarises the p values associated with each variable at each stage of the model building procedure.
Reference categories:
aFemale sex;
bAge group 1–5 years;
cMain drinking water is from unimproved source;
dSoil pH 5.20–5.30.
*Responses were collapsed into 2 response options in accordance with the WHO and United Nations International Children’s Emergency Fund (UNICEF) Joint Monitoring Programme (JMP) for Water Supply and Sanitation definitions of “improved” (public tap/standpipe, protected spring, rainwater) and “unimproved” (unprotected spring, unprotected well) drinking water sources. Sex and age group were entered as covariates in the model.
Table 5. Risk factors associated with T. trichiura, Strongyloides spp., and undifferentiated STH infection.
|
T. trichiura (n = 172) |
Strongyloides spp. (n = 49) |
Any STH (n = 519) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factor variables | aOR | 95% CI | P value | aOR | 95% CI | P value | aOR | 95% CI | P value |
| Male sexa | 1.12 | 0.68–1.85 | 0.643 | 1.86 | 0.86 0 4.03 | 0.098 | 1.49 | 1.03–2.17 | 0.033 |
| Age group 6–11 yearsb | 3.00 | 1.49–6.01 | 0.002 | 2.57 | 0.68–9.69 | 0.162 | 4.04 | 2.39–6.83 | <0.001 |
| Age group 12–17 yearsb | 5.99 | 2.56–14.00 | <0.001 | 10.11 | 2.58–36.60 | 0.001 | 6.82 | 3.24–14.34 | <0.001 |
| Age group 18–34 yearsb | 1.31 | 0.56–3.09 | 0.530 | 5.08 | 1.17–22.05 | 0.030 | 4.73 | 2.47–9.08 | <0.001 |
| Age group 35–49 yearsb | 0.98 | 0.43–2.23 | 0.960 | 4.00 | 0.95–16.90 | 0.059 | 5.31 | 2.77–10.15 | <0.001 |
| Age group ≥50 yearsb | 0.84 | 0.31–2.26 | 0.729 | 4.00 | 0.87–18.31 | 0.074 | 3.60 | 2.01–6.44 | <0.001 |
| N. americanus co-infection | 3.39 | 1.66–6.91 | <0.001 | 4.42 | 1.32–14.75 | 0.013 | - | - | - |
| A. ceylanicum co-infection | - | - | - | - | - | - | - | - | - |
| T. trichiura co-infection | - | - | - | 3.00 | 1.18–7.62 | 0.026 | - | - | - |
| Strongyloides spp. co-infection | - | - | - | - | - | - | - | - | - |
| Wears shoes outside c | |||||||||
| Sometimes | 0.86 | 0.41–1.79 | 0.686 | - | - | - | - | - | - |
| Always | 0.19 | 0.05–0.71 | 0.013 | - | - | - | - | - | - |
| Has toilet/latrine in household | - | - | - | - | - | - | 0.40 | 0.24–0.68 | 0.001 |
| Annual precipitation (cm) | 1.14 | 1.04–1.25 | 0.008 | - | - | - | - | - | - |
| Soil pH (pH >5.30–5.50)d | - | - | - | 7.47 | 1.90–29.33 | 0.004 | 3.91 | 1.71–8.97 | 0.001 |
“95% CI” denotes 95% confidence interval, “aOR” denotes adjusted odds ratio. These results were derived from a model building procedure where variables were removed from the analysis if they did not meet the criterion p value at each stage of the analysis. Variables with blank cells indicate that these were removed in an earlier stage. Tables F-K in S1 Text summarises the p values associated with each variable at each stage of the model building procedure.
Reference categories:
aFemale sex;
bAge group 1–5 years;
cNever wears shoes outside;
dSoil pH 5.20–5.30.
Sex and age group were entered as covariates in the model.
WASH and environmental risk factors
As shown in Table 4, participants who reported owning a household toilet/latrine had lower odds of infection with N. americanus than those who reported not owning a toilet (AOR 0.41, 95% CI 0.24–0.68, p<0.001). Participants from villages with less acidic soil were 3 times more likely to have an infection than those from villages with more acidic soil (AOR 2.92, 95% CI 1.29–6.60, p = 0.010). Co-infection with A. ceylanicum (AOR 4.36, 95% CI 2.36–8.06, p<0.001) and T. trichiura (AOR 2.79, 95% CI 1.39–5.60, p = 0.004) were associated with a higher odds of N. americanus infection.
Participants whose main drinking water source was from an improved source had higher odds of A. ceylanicum infection than those whose water was from an unimproved source (AOR 2.71, 95% CI 1.41–5.24, p = 0.003). Co-infection with N. americanus (AOR 4.58, 95% CI 2.58–8.14, p<0.001) and Strongyloides spp. (AOR 3.19, 95% CI 1.39–7.34, p = 0.006) were associated with a higher prevalence of A. ceylanicum infection.
Participants who reported always wearing shoes outside were significantly less likely to have T. trichiura infection (AOR 0.19, 95% CI 0.05–0.71, p = 0.013). Greater annual precipitation was associated with marginally higher odds of infection (AOR 1.14, 95% CI 1.04–1.25, p = 0.008), as was co-infection with N. americanus (AOR 3.39, 95% CI 1.66–6.91, p = 0.001).
We did not detect any statistically significant associations between WASH variables and Strongyloides spp. infection (Table 5). Participants from villages with less acidic soil were 7 times more likely to have Strongyloides spp. infections than those from villages with more acidic soil (AOR 7.47, 95% CI 1.90–29.33, p = 0.004).
Discussion
In this study, to our knowledge, we completed the first STH prevalence survey in a low- or middle-income country of the Pacific region that used qPCR, enabling the assessment of Strongyloides spp. and individual hookworm species. Previous large-scale STH epidemiological surveys to use qPCR as a stand-alone diagnostic tool include three conducted in Timor-Leste in the context of trials [12–15] and one in Japan to confirm STH transmission elimination [16]. We found that the overall STH prevalence (63.3%) is the highest reported in the country, with previous microscopy-based studies documenting prevalence of up to 52.7% [17–21]. Consistent with two previous surveys using microscopy [17,18], the leading species present was hookworm. T. trichiura, Strongyloides spp. and the zoonotic A. ceylanicum were also abundant. Most infections were of light intensity for N. americanus, T. trichiura, and A. lumbricoides.
Solomon Islands follows WHO recommendations for STH control wherein school-based albendazole preventive chemotherapy is provided, so far only within the capital city, Honiara. Guidelines specify treatment to groups at highest risk of morbidity, including pre-school and school-aged children, in communities with prevalence above 20%, with the aim of reducing the prevalence of moderate-to-heavy intensity infections to less than 2% [5]. Our findings indicate that STH burden in Solomon Islands is well above these thresholds, highlighting an urgent need to expand deworming to reach all provinces.
However, our findings also show that the drug-based treatment approach alone is unlikely to control STHs, for several reasons. A. ceylanicum is a zoonoses and is a predominant hookworm of domestic dogs and cats in the Asia Pacific region [35]. Strongyloides spp., for which WHO recently introduced control targets to be attained by 2030 [5], is also a potential zoonosis comprising two distinct genetic clades, one restricted to dogs and another infecting humans, non-human primates, dogs and cats [7,36]. Zoonotic transmission therefore needs to be addressed through One Health strategies. Canine and feline population control through desexing programs and treating community dogs and cats with macrocyclic lactone based anthelmintics have been proposed as potentially effective control measures [6,37]. Another barrier is the limited efficacy of albendazole against both Strongyloides spp. [8] and T. trichiura [9]. The co-administration of albendazole and ivermectin has superior efficacy for T. trichiura [9] and ivermectin monotherapy is highly effective against S. stercoralis [8]. STH control in Solomon Islands provinces where these species are endemic would therefore be strengthened through the co-administration of albendazole and ivermectin, a strategy that would also provide integrated control of other co-endemic NTDs, including scabies [38].
It is important to note that risk of infection for N. americanus, T. trichiura, and Strongyloides spp., and greater N. americanus infection intensity, increased with age. Although school-based treatment programs will benefit children, the considerable adult reservoir in the population suggests that treatment should ideally be expanded to entire communities, perhaps integrating with mass drug administration programmes targeting other NTDs.
Overall, only few WASH and environmental factors emerged as significant predictors of STH infection, possibly due to the presence of homogenously poor WASH access/conditions and limited variation in the environmental data across a small geographical area. We found that owning a household latrine was associated with a lower prevalence of N. americanus infections, probably because of reduced exposure to contaminated faecal matter [39]. Higher annual precipitation was associated with marginally increased odds of T. trichiura infection, likely due to moist soil conditions favourable to survival and development of STH eggs and larvae [12]. Precipitation has emerged as a risk factor for other species [12,34]. We found that less acidic soil was associated with a higher risk of infection with N. americanus and Strongyloides spp. consistent with in-vitro findings on the optimal pH being 6.0 [40]. Given some evidence suggesting that STH eggs cannot survive in highly alkaloid conditions (pH>12) [41], the use of lime (a common agricultural practice to improve crop yields) has been suggested as a potential tool for STH control [34], although this hypothesis has not been tested.
An unexpected finding was that individuals who reported always wearing shoes outside had reduced odds of T. trichiura infection. While shoe-wearing can confer protection against species that enter by percutaneous penetration, T. trichiura transmission occurs via the faecal-oral route. More likely, increased shoe-wearing behaviour might reflect higher socioeconomic status, which may be a proxy of an unmeasured variable that protects against T. trichiura exposure. Surprisingly, participants whose drinking water was from an improved source had increased odds of infection with A. ceylanicum. This might be due to increased exposure to A. ceylanicum larvae near improved water sources deposited by infected dogs in communities. We did not collect data on STH burden in dogs or contamination of the environment with dog faeces, a key gap for future research to address.
Limitations of this study should be considered. The WHO recommended thresholds for defining infection intensity were created using infection intensity measurements derived from the Kato-Katz technique. While there have been studies, including this one, deriving epg from Ct values [3,10], additional studies are needed to further validate this approach and standardize the assessment of infection intensity using qPCR. There are also several important practical issues to be considered when deciding whether qPCR should be used, given its perceived higher cost and need for specialised equipment and trained personnel when compared to microscopy [42]. Several strategies to address these issues have been proposed, such as sample pooling [43], production of PCR equipment in low- and middle-income countries [44], and transfer of technology including training personnel [44]. Moreover, while we opted to use logistic regression for our environmental analysis, which allows us to adjust for clustering effects, this may have limited our ability to identify environmental risk factors given that regression-based techniques do not allow intercorrelated predictor variables to be included in the same model. Recent evidence suggests that alternative statistical approaches, such as Bayesian networks, may better identify risk factors when predictors are intercorrelated [45].
In conclusion, by using qPCR in a province-wide epidemiological survey in Solomon Islands, we were able to comprehensively assess the burden of all STH species including Strongyloides spp. and individual hookworm species. This enabled us to identify opportunities to strengthen STH control in Solomon Islands, particularly incorporating ivermectin preventive chemotherapy into deworming programmes, adopting a One Health framework, and expanding STH control to entire communities. Improving WASH use and access could complement deworming programmes by protecting against exposure pathways.
Supporting information
Table A in S1 Text. Sequences of primers and probes used for quantitative polymerase chain reaction. Table B in S1 Text. WASH characteristics of study population (N = 619). Table C in S1 Text. Environmental characteristics of study population (N = 830). Table D in S1 Text. Unadjusted STH prevalence by species, stratified by sex and age group. Table E in S1 Text. Mean eggs per gram (epg) of stool and incidence rate ratios (IRRs) by sex and age group for T. trichiura and N. americanus infections. Table F in S1 Text. Results of model building steps for N. americanus model. Table G in S1 Text. Results of model building steps for A. ceylanicum model. Table H in S1 Text. Results of model building steps for T. trichiura model. Table I in S1 Text. Results of model building steps for Strongyloides spp. model Table J in S1 Text. Results of model building steps for hookworm (undifferentiated) model Table K in S1 Text. Results of model building steps for STH (undifferentiated) model.
(DOCX)
(DOCX)
Acknowledgments
We are indebted to all the villages and their leaders for allowing for the study to take place, and to all residents who participated in the study. We would like to express our gratitude to the local staff in Solomon Islands for contributing to fieldwork procedures and data collection. We are especially thankful to Erica Lazu, Salote Wickham, Deanne Seppy, and Sharmillah Jack for their support in completing the STH component of the fieldwork reported here.
Data Availability
Some restrictions will apply to the data for this study. All relevant aggregated data are within the paper and its Supporting information files. Individual data cannot be made public in compliance with the protocol approved by the research ethics board in order to respect participant privacy. Researchers may request access to data from Solomon Islands Health Research and Ethics Review Board, https://solomons.gov.sb/contact/, and The Royal Children’s Hospital Human Research Ethics Committee, Melbourne, Australia, rch.ethics@rch.org.au.
Funding Statement
Fieldwork for this research was funded by a Centres of Research Excellence Grant from the National Health and Medical Research Council of Australia (NHMRC) (GNT1153727). https://www.nhmrc.gov.au/ The Regimens of Ivermectin for Scabies Elimination (RISE) trial, in which this study was embedded, is funded by the NHMRC (GNT1127297). https://www.nhmrc.gov.au/ BL is supported by the Australian Government Research Training Program Scholarship. https://www.dese.gov.au/ JK and LR are supported by fellowships from the NHMRC. https://www.nhmrc.gov.au/ AS is supported by a Viertel Senior Medical Research Fellowship and an NHMRC Investigator Grant. http://viertel.org.au/ https://www.nhmrc.gov.au/ RT is supported by the Australian Research Council Future Fellowship. https://www.arc.gov.au/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zendejas-Heredia PA, Colella V, Hii SF, Traub RJ. Comparison of the egg recovery rates and limit of detection for soil-transmitted helminths using the Kato-Katz thick smear, faecal flotation and quantitative real-time PCR in human stool. PLoS Negl Trop Dis. 2021;15(5):e0009395. doi: 10.1371/journal.pntd.0009395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90(3):535–45. doi: 10.4269/ajtmh.13-0268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation (WHO). 2030 targets for soil-transmitted helminthiases control programmes: WHO; 2019.
- 6.Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43(12–13):1009–15. doi: 10.1016/j.ijpara.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Jaleta TG, Zhou S, Bemm FM, Schar F, Khieu V, Muth S, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11(8):e0005752. doi: 10.1371/journal.pntd.0005752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC Jr., Terashima A, Samalvides F, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016(1):CD007745. doi: 10.1002/14651858.CD007745.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke NE, Doi SAR, Wangdi K, Chen Y, Clements ACA, Nery SV. Efficacy of Anthelminthic Drugs and Drug Combinations Against Soil-transmitted Helminths: A Systematic Review and Network Meta-analysis. Clin Infect Dis. 2019;68(1):96–105. doi: 10.1093/cid/ciy423 [DOI] [PubMed] [Google Scholar]
- 10.Bartlett AW, Traub R, Amaral S, Hii SF, Clarke NE, Matthews A, et al. Comparison between Quantitative Polymerase Chain Reaction and Sodium Nitrate Flotation Microscopy in Diagnosing Soil-Transmitted Helminth Infections. The American Journal of Tropical Medicine and Hygiene. 2021;105(5):1210–3. [Google Scholar]
- 11.Hii SF, Senevirathna D, Llewellyn S, Inpankaew T, Odermatt P, Khieu V, et al. Development and Evaluation of a Multiplex Quantitative Real-Time Polymerase Chain Reaction for Hookworm Species in Human Stool. Am J Trop Med Hyg. 2018;99(5):1186–93. doi: 10.4269/ajtmh.18-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell SJ, Nery SV, Wardell R, D’Este CA, Gray DJ, McCarthy JS, et al. Water, Sanitation and Hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR. PLoS Negl Trop Dis. 2017;11(3):e0005393. doi: 10.1371/journal.pntd.0005393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nery SV, Traub RJ, McCarthy JS, Clarke NE, Amaral S, Llewellyn S, et al. WASH for WORMS: A Cluster-Randomized Controlled Trial of the Impact of a Community Integrated Water, Sanitation, and Hygiene and Deworming Intervention on Soil-Transmitted Helminth Infections. Am J Trop Med Hyg. 2019;100(3):750–61. doi: 10.4269/ajtmh.18-0705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell SJ, Nery SV, D’Este CA, Gray DJ, McCarthy JS, Traub RJ, et al. Water, sanitation and hygiene related risk factors for soil-transmitted helminth and Giardia duodenalis infections in rural communities in Timor-Leste. Int J Parasitol. 2016;46(12):771–9. doi: 10.1016/j.ijpara.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Nery SV, Clarke NE, Richardson A, Traub R, McCarthy JS, Gray DJ, et al. Risk factors for infection with soil-transmitted helminths during an integrated community level water, sanitation, and hygiene and deworming intervention in Timor-Leste. Int J Parasitol. 2019;49(5):389–96. doi: 10.1016/j.ijpara.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Pilotte N, Kikuchi M, Means AR, Papaiakovou M, Gonzalez AM, et al. What does soil-transmitted helminth elimination look like? Results from a targeted molecular detection survey in Japan. Parasit Vectors. 2020;13(1):6. doi: 10.1186/s13071-019-3875-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes RG, Sharp DS, Hughes MC, Akau’ola S, Heinsbroek P, Velayudhan R, et al. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. Int J Environ Health Res. 2004;14(3):163–77. doi: 10.1080/0960312042000218589 [DOI] [PubMed] [Google Scholar]
- 18.Harrington H, Bradbury R, Taeka J, Asugeni J, Asugeni V, Igeni T, et al. Prevalence of soil-transmitted helminths in remote villages in East Kwaio, Solomon Islands. Western Pac Surveill Response J. 2015;6(3):51–8. doi: 10.5365/WPSAR.2015.6.1.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JD, Yen CM, Wang JJ, Lin RJ, Chung LY. A school-based soil-transmitted helminths survey in the Guadalcanal Province, the Solomon Islands. Trop Doct. 2021;51(2):167–70. doi: 10.1177/0049475520970055 [DOI] [PubMed] [Google Scholar]
- 20.Bradbury RS, Harrington H, Kekeubata E, Esau D, Esau T, Kilivisi F, et al. High prevalence of ascariasis on two coral atolls in the Solomon Islands. Trans R Soc Trop Med Hyg. 2018;112(4):193–9. doi: 10.1093/trstmh/try041 [DOI] [PubMed] [Google Scholar]
- 21.Bradbury RS, Hii SF, Harrington H, Speare R, Traub R. Ancylostoma ceylanicum Hookworm in the Solomon Islands. Emerg Infect Dis. 2017;23(2):252–7. doi: 10.3201/eid2302.160822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon Islands National Statistics Office. Solomon Islands Population: Solomon Islands Goverment; 2019 https://www.statistics.gov.sb/statistics/social-statistics/population.
- 23.Lake SJ, Phelan SL, Engelman D, Sokana O, Nasi T, Boara D, et al. Protocol for a cluster-randomised non-inferiority trial of one versus two doses of ivermectin for the control of scabies using a mass drug administration strategy (the RISE study). BMJ Open. 2020;10(8):e037305. doi: 10.1136/bmjopen-2020-037305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements AC, et al. Application of a Multiplex Quantitative PCR to Assess Prevalence and Intensity Of Intestinal Parasite Infections in a Controlled Clinical Trial. PLoS Negl Trop Dis. 2016;10(1):e0004380. doi: 10.1371/journal.pntd.0004380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103(4):342–6. doi: 10.1016/j.trstmh.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84(2):338–43. doi: 10.4269/ajtmh.2011.10-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, et al. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol. 2013;51(2):472–80. doi: 10.1128/JCM.02658-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. The Lancet. 2018;391(10117):252–65. doi: 10.1016/S0140-6736(17)31930-X [DOI] [PubMed] [Google Scholar]
- 29.Lambert SB, Whiley DM, O’Neill NT, Andrews EC, Canavan FM, Bletchly C, et al. Comparing nose-throat swabs and nasopharyngeal aspirates collected from children with symptoms for respiratory virus identification using real-time polymerase chain reaction. Pediatrics. 2008;122(3):e615–20. doi: 10.1542/peds.2008-0691 [DOI] [PubMed] [Google Scholar]
- 30.Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77(4):685–90. [PubMed] [Google Scholar]
- 31.World Health Organisation (WHO). Helminth control in school-age children: A guide for managers of control programmes (2nd ed). Geneva, Switzerland2011.
- 32.Sulla-Menashe D, Friedl, MA. User guide to collection 6 MODIS land cover (MCD12Q1 and MCD12C1) product 2018 https://icdc.cen.uni-hamburg.de/fileadmin/user_upload/icdc_Dokumente/MODIS/mcd12_user_guide_v6.pdf.
- 33.United States Department of Agriculture (USDA). Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys (2nd ed). Washington, USA: USDA; 1999.
- 34.Wardell R, Clements ACA, Lal A, Summers D, Llewellyn S, Campbell SJ, et al. An environmental assessment and risk map of Ascaris lumbricoides and Necator americanus distributions in Manufahi District, Timor-Leste. PLoS Negl Trop Dis. 2017;11(5):e0005565. doi: 10.1371/journal.pntd.0005565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traub RJ, Zendejas-Heredia PA, Massetti L, Colella V. Zoonotic hookworms of dogs and cats—lessons from the past to inform current knowledge and future directions of research. Int J Parasitol. 2021;51(13–14):1233–41. doi: 10.1016/j.ijpara.2021.10.005 [DOI] [PubMed] [Google Scholar]
- 36.Bradbury RS, Pafco B, Noskova E, Hasegawa H. Strongyloides genotyping: a review of methods and application in public health and population genetics. Int J Parasitol. 2021;51(13–14):1153–66. doi: 10.1016/j.ijpara.2021.10.001 [DOI] [PubMed] [Google Scholar]
- 37.Knaus M, Taweethavonsawat P, Cheesman T, Visser M, Rehbein S. Efficacy of Broadline(R) in cats against induced infections with developing fourth-stage larval and adult Ancylostoma ceylanicum hookworms. Vet Parasitol. 2020;277S:100025. doi: 10.1016/j.vpoa.2020.100025 [DOI] [PubMed] [Google Scholar]
- 38.Romani L, Whitfeld MJ, Koroivueta J, Kama M, Wand H, Tikoduadua L, et al. Mass Drug Administration for Scabies Control in a Population with Endemic Disease. N Engl J Med. 2015;373(24):2305–13. doi: 10.1056/NEJMoa1500987 [DOI] [PubMed] [Google Scholar]
- 39.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620. doi: 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udonsi JK, Atata G. Necator americanus: temperature, pH, light, and larval development, longevity, and desiccation tolerance. Exp Parasitol. 1987;63(2):136–42. doi: 10.1016/0014-4894(87)90154-8 [DOI] [PubMed] [Google Scholar]
- 41.Maya C, Torner-Morales FJ, Lucario ES, Hernandez E, Jimenez B. Viability of six species of larval and non-larval helminth eggs for different conditions of temperature, pH and dryness. Water Res. 2012;46(15):4770–82. doi: 10.1016/j.watres.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 42.Papaiakovou M, Gasser RB, Littlewood DTJ. Quantitative PCR-Based Diagnosis of Soil-Transmitted Helminth Infections: Faecal or Fickle? Trends Parasitol. 2019;35(7):491–500. doi: 10.1016/j.pt.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 43.Papaiakovou M, Wright J, Pilotte N, Chooneea D, Schar F, Truscott JE, et al. Pooling as a strategy for the timely diagnosis of soil-transmitted helminths in stool: value and reproducibility. Parasit Vectors. 2019;12(1):443. doi: 10.1186/s13071-019-3693-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organisation (WHO). Increasing access to diagnostics through technology transfer and local production. Geneva, Switzerland: WHO; 2011.
- 45.Aw JYH, Clarke NE, Mayfield HJ, Lau CL, Richardson A, Vaz Nery S. Novel statistical approaches to identify risk factors for soil-transmitted helminth infection in Timor-Leste. Int J Parasitol. 2021;51(9):729–39. doi: 10.1016/j.ijpara.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A in S1 Text. Sequences of primers and probes used for quantitative polymerase chain reaction. Table B in S1 Text. WASH characteristics of study population (N = 619). Table C in S1 Text. Environmental characteristics of study population (N = 830). Table D in S1 Text. Unadjusted STH prevalence by species, stratified by sex and age group. Table E in S1 Text. Mean eggs per gram (epg) of stool and incidence rate ratios (IRRs) by sex and age group for T. trichiura and N. americanus infections. Table F in S1 Text. Results of model building steps for N. americanus model. Table G in S1 Text. Results of model building steps for A. ceylanicum model. Table H in S1 Text. Results of model building steps for T. trichiura model. Table I in S1 Text. Results of model building steps for Strongyloides spp. model Table J in S1 Text. Results of model building steps for hookworm (undifferentiated) model Table K in S1 Text. Results of model building steps for STH (undifferentiated) model.
(DOCX)
(DOCX)
Data Availability Statement
Some restrictions will apply to the data for this study. All relevant aggregated data are within the paper and its Supporting information files. Individual data cannot be made public in compliance with the protocol approved by the research ethics board in order to respect participant privacy. Researchers may request access to data from Solomon Islands Health Research and Ethics Review Board, https://solomons.gov.sb/contact/, and The Royal Children’s Hospital Human Research Ethics Committee, Melbourne, Australia, rch.ethics@rch.org.au.