Abstract
Topic
To evaluate whether differences exist in systemic complement activation profiles in patients with early to intermediate nonexudative age-related macular degeneration (AMD) or geographic atrophy (GA) compared with control participants without AMD.
Clinical Relevance
Complement inhibition has emerged as a therapeutic strategy for GA, although clinical trials to date have yielded mixed results. Despite these efforts, no clear consensus exists regarding what portions of the complement pathway are dysregulated in AMD or when this dysregulation occurs relative to AMD stage. Although past studies have compared systemic complement activation profiles in patients with AMD versus in control participants without AMD, differences in AMD case definition and differing analytical approaches complicate their interpretation.
Methods
We performed a systematic review by identifying articles from database inception through October 11, 2020, that reported systemic complement activation profiles in patients with early or intermediate nonexudative AMD or GA versus control participants without AMD by searching PubMed, Google Scholar, and Embase. Risk of bias was assessed using a modified Newcastle-Ottawa score.
Results
The 8 reviewed studies included 2131 independent participants. Most studies report significantly higher systemic levels of products associated with complement activation and significantly lower systemic levels of products associated with complement inhibition in patients with early and advanced nonexudative AMD compared with control participants without AMD.
Discussion
Evidence suggests that systemic complement overactivation is a feature of early or intermediate and advanced nonexudative AMD. However, given significant heterogeneity, these findings are not conclusive and warrant further investigation.
Keywords: Age-related macular degeneration, Complement, Geographic atrophy, Systematic review
Abbreviations and Acronyms: AMD, age-related macular degeneration; GA, geographic atrophy
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in older adults and is projected to affect 288 million people by 2040.1 Advanced AMD manifests in 2 forms: advanced nonexudative AMD characterized by geographic atrophy (GA) and irreversible loss of photoreceptors, retinal pigment epithelium, and choriocapillaris; or advanced exudative AMD characterized by choroidal neovascularization. Although exudative AMD historically was responsible for more vision loss compared with nonexudative AMD, therapies that target vascular endothelial growth factor, such as bevacizumab (Avastin), ranibizumab (Lucentis), aflibercept (Eyelea), and brolucizumab (Beovu), have significantly improved outcomes for many patients with exudative AMD.2,3 However, no Food and Drug Administration-approved therapies are available to treat patients with GA or those with exudative AMD who respond to anti-vascular endothelial growth factor therapies, but exhibit late macular atrophy. Although antioxidant and mineral supplementation with the Age-Related Eye Disease Study or Age-Related Eye Disease Study 2 formulations reduces the risk of progression to advanced AMD, secondary analyses suggest that they primarily prevent progression to exudative AMD and do not change risk of progression to GA.4, 5, 6, 7, 8 Thus, a need exists for novel therapeutic strategies to prevent GA and to slow GA progression.
Four landmark studies identified a strong association between AMD and the Y402H common variant of the CFH gene.9, 10, 11, 12 These and numerous subsequent studies have established a clear link between immune dysregulation and AMD pathogenesis,13 which have led to recent studies investigating the possible strategy of complement inhibition to slow GA progression. The initial studies have yielded null results. The phase 2 COMPLETE (Complement Inhibition with Eculizumab for the Treatment of Nonexudative Age-Related Macular Degeneration) study showed no significant change in GA growth rate with systemic C5 inhibition (eculizumab).14 Similarly, although the phase 2 MAHALO study reported a benefit of intravitreal complement factor D inhibition (lampalizumab; 20% reduction in GA growth at 18 months; P = 0.12, meeting a prespecified α value of 0.20),15 the phase 3 CHROMA and SPECTRI trials did not meet their primary end points of showing an effect of lampalizumab on GA growth rate.16
Other studies using different complement inhibition strategies have shown sufficient promise to continue to phase 3 trials. The phase 2 FILLY study reported that monthly intravitreal C3 inhibition (pegcetacoplan) reduced GA growth by 29% (95% confidence interval, 9%–49%) at 18 months.17 Although not yet published, a news release from Apellis reported a significant, albeit modest, effect of pegcetacoplan in the phase 3 OAKS study (ClinicalTrials.gov identifier, NCT03525600), whereas the phase 3 DERBY study (ClinicalTrials.gov identifier, NCT03525613) did not find a statistically significant beneficial effect. The phase 2/3 GATHER1 study reported that intravitreal C5 inhibition (avacincaptad pegol) reduced GA growth by 12 months18; the phase 3 GATHER2 study (ClinicalTrials.gov identifier, NCT04435366) is underway.
Despite these significant efforts to develop complement inhibition as a therapeutic strategy for GA, no clear consensus exists regarding what portions of the complement pathway are dysregulated in AMD or when this dysregulation occurs relative to AMD stage. Although past studies have quantified systemic complement levels in patients with AMD compared with control participants without AMD, differences in AMD case definition and differing analytical approaches complicate the interpretation of these studies. The purpose of this systematic review was to examine the available evidence to determine whether differences exist in systemic complement activation or inhibition in patients with early to intermediate nonexudative AMD or GA versus control participants without AMD. These findings will not only improve our understanding of the cellular and molecular pathways underlying AMD pathogenesis, but will also inform the future development of novel therapies for GA.
Methods
Protocol and Registration
The review protocol was not registered before publication. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.19 The requirement for informed consent was waived because of the retrospective nature of the study. All research adhered to the tenets of the Declaration of Helsinki.
Search Methods for Identifying Studies, Study Selection, and Eligibility Criteria for Considering Studies
We performed a comprehensive literature search using PubMed, Embase, and Google Scholar on October 11, 2020. The full electronic search syntax used in PubMed was: macular degeneration [MESH terms] OR age related macular degeneration [all fields] OR AMD [all fields] AND complement [all fields]; the syntax used in Embase was: (‘age related macular degeneration’/exp OR ‘age related macular degeneration’ OR ‘amd’/exp OR amd OR ‘macular degeneration’/exp OR ‘macular degeneration’) AND (‘complement’/exp OR complement) AND ‘article’/it; and the syntax used in Google Scholar was: allintitle: “age related macular degeneration” OR AMD OR “macular degeneration” AND complement. For all 3 databases, we used no restrictions based on publication date or article type. This search identified a total of 1627 articles after duplicates were removed. One of the authors (J.B.L.) reviewed the title and abstracts from each of these manuscripts, omitting those written in a language other than English, those clearly not relevant to the present study, and review articles. No controversial cases required adjudication. We then obtained the full texts of remaining studies and assessed them for inclusion. We also reviewed the references of each included article to identify other potential articles for inclusion. We repeated the comprehensive literature search as detailed above on January 7, 2022, which did not identify any additional manuscripts for inclusion.
Data Collection
We included all studies, regardless of study design, that reported quantitative values for at least 1 complement protein and compared either patients with nonexudative AMD by any classification methodology versus control participants without AMD or patients with either central or noncentral GA versus control participants without AMD. We did not require the quantification of complement proteins to be the primary aim of the study. Because we were interested in systemic complement activation patterns in nonexudative AMD and in GA, we excluded any study that did not differentiate between AMD subtype or that included multiple types of AMD (exudative AMD and GA) in a single comparison group, because these studies used inappropriate case definition for our research question. Although some of the included studies were performed by the same groups, no indication was found within the full text that they originated from the same specific patients.
Risk of Bias Assessment
The risk of bias in individual studies was analyzed at the study level, using a modified Newcastle-Ottawa scale (Appendix A). Newcastle-Ottawa scale scores of 7 or more were considered to be of high quality with low risk of bias. One of the authors (J.B.L.) performed the risk assessment, which was confirmed independently by another author (D.G.V.).
Results
Study Characteristics
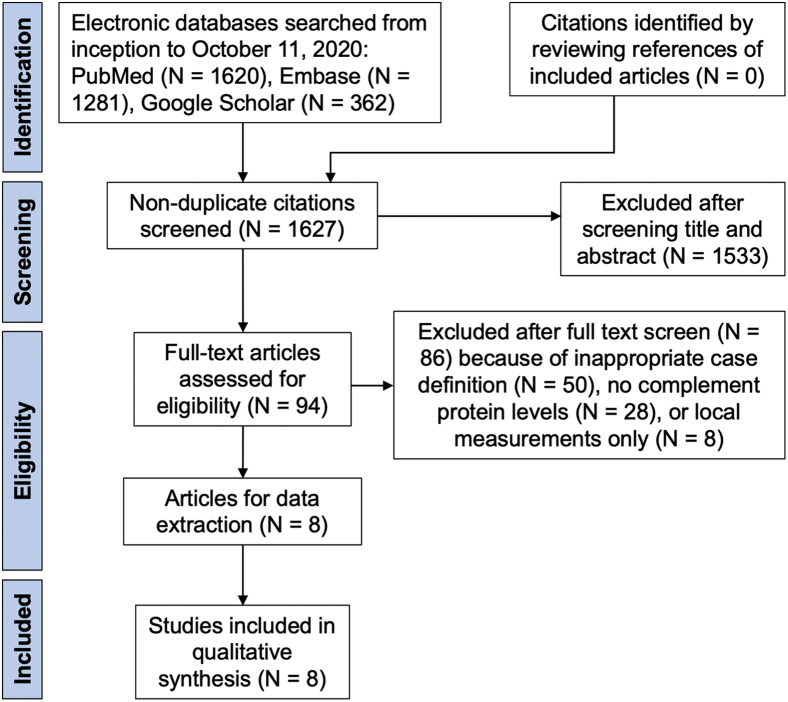
Our search strategy yielded 1627 articles; after omitting studies not in English, review articles, and those that were clearly not relevant to the present systematic review, we reviewed the full texts of 94 articles (Fig 1). Authors were not contacted for further clarification. Of the 94 articles that were reviewed in full, 50 articles (53%) were excluded because of ineligible case definitions (e.g., not distinguishing between nonexudative and exudative AMD); 28 studies (30%) were excluded because they did not report systemic complement protein levels; and 8 studies (9%) were excluded because they reported local (e.g., aqueous humor, vitreous humor) rather than systemic complement levels.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram showing citations identified, included, and excluded with reasons for exclusions.
In total, the 8 studies reviewed herein included 2131 independent patients20, 21, 22, 23, 24, 25, 26, 27 (Table 1). Two studies (25%) were cross-sectional; 2 studies (25%) were hybrid studies that combined data of patients from the observational (pretreatment) phase of a phase 2 randomized clinical trial and a separate cross-sectional study; and 4 studies (50%) were case-control studies that used a subset of patients from AMD registries or databases. Most studies (63%) originated from the United States. The included studies used different AMD classification criteria, including those from the International ARM (Age-Related Maculopathy) Epidemiological Study Group, the Clinical Age-Related Maculopathy Staging system, the Age-Related Eye Disease Study severity scale, or investigator-defined clinical criteria (Table 1).
Table 1.
Characteristics of Studies Included in Systematic Review
| Study (Country) | Year | Study Design | No. of Participants |
Control Group | Age-Related Macular Degeneration Classification Methodology | ||
|---|---|---|---|---|---|---|---|
| Nonexudative Age-Related Macular Degeneration | Geographic Atrophy | Without Age-Related Macular Degeneration | |||||
| Sivaprasad et al (England) | 2007 | Cross-sectional | 42 | 0 | 38 | Likely clinic based; healthy without AMD | International ARM Epidemiological Study Group |
| Reynolds et al (USA) | 2009 | Case-control from registry | 0 | 58 | 60 | Registry based; no AMD, CARMS grade 1 | Clinical Age-Related Maculopathy Staging |
| Machalińska et al (Poland) | 2009 | Cross-sectional | 30 | 0 | 30 | Clinic based; no AMD | Study-specific clinical definition |
| Lashkari et al (USA) | 2018 | Hybrid | 41 | 37 | 33 | Clinic based; no AMD, AREDS stage 0 | Age-Related Eye Disease Study |
| Lynch et al22 (USA) | 2020b | Case-control from registry | 0 | 46 | 27 | Registry based; cataract control participants without AMD | Study-specific clinical definition |
| Lynch et al21 (USA) | 2020a | Case-control from registry | 109 | 0 | 65 | Registry based; cataract control participants without AMD | Study-specific clinical definition |
| Lashkari et al (USA) | 2020 | Hybrid | 24 | 37 | 33 | Clinic based; no AMD, AREDS stage 0 | Age-Related Eye Disease Study |
| Heesterbeek et al (Europe) | 2020 | Case-control from database | 414 | 62 | 945 | Genetic database based; no AMD | Study-specific clinical definition |
AMD = age-related macular degeneration; AREDS = Age-Related Eye Disease Study; ARM = age-related maculopathy; CARMS = Clinical Age-Related Maculopathy Staging.
Quality Assessment
To analyze risk of bias in the included studies, we used a modified Newcastle-Ottawa scale (Appendix A). All 8 studies showed a low risk of bias, given Newcastle-Ottawa scale scores of 7 or more (Supplemental Table 1). Five of the 8 studies (63%) did not include a statement regarding whether the outcome measurement was performed blinded to the group to which the samples belonged.
Complement in Early or Intermediate Nonexudative Age-Related Macular Degeneration
The earliest published study comparing patients with nonexudative AMD and control participants without AMD was from 2007 by Sivaprasad et al.20 In a cross-sectional study design, they found higher levels of C3a-desArg in patients with nonexudative AMD (median, 52.6 ng/ml; range, 2.8–198.1 ng/ml) compared with control participants without AMD (median, 40.3 ng/ml; range, 6.1–81.7 ng/ml). C3a-desArg is produced during complement activation and thus is considered a general marker of complement activation. In contrast, a subsequent cross-sectional study by Machalińska et al23 found no statistically significant difference in plasma C3a-desArg in patients with dry AMD versus control participants (P = 0.54). Of note, these 2 studies used different AMD classification criteria: the former used that of the International ARM Epidemiological Study Group, whereas the latter used their own clinical criteria. Of note, both studies were also relatively small (n = 30–42 per group).
Almost 1 decade later, Lynch et al21 published a larger case-control study, comparing patients with intermediate AMD (n = 109) with control participants without AMD (n = 65). This study examined multiple complement factors and found significant differences in the levels of multiple complement components. Of interest, multiple components known to be associated with complement activation, including C3a, Ba, and C5a, were found to be significantly elevated in patients with intermediate AMD versus control participants, whereas other components known to be associated with complement inhibition, including iC3b/C3b, CFH, and CFI, were found to be significantly decreased. Lashkari et al24,25 published 2 companion articles in which they quantified the levels of complement inhibition proteins in patients with nonexudative AMD versus control participants without AMD and found no significant differences in CFI or CFH levels.
The largest case-control study to date was published by Heesterbeek et al27 with 797 patients with AMD and 945 control participants without AMD. They found a significantly higher C3d to C3 ratio (a marker of complement activation) in patients with intermediate AMD versus control participants, but not in patients with early AMD versus control participants. They used their own AMD classification criteria, whereby early AMD was defined as more than 10 small (<63 μm in diameter) drusen with pigmentary abnormalities or 1 to 14 intermediate drusen (63–125 μm in diameter) and intermediate AMD was defined as more than 1 large (>125 μm in diameter) drusen, 15 or more intermediate drusen, or presence of GA, but not in the central macula. Table 2 summarizes these studies on complement activation profiles in patients with early to intermediate nonexudative AMD versus control participants without AMD.
Table 2.
Complement Activation Profiles in Patients with Early to Intermediate Age-Related Macular Degeneration versus Control Participants without Age-Related Macular Degeneration
| Variable | Control Participants without Age-Related Macular Degeneration | Patients with Early to Intermediate Nonexudative Age-Related Macular Degeneration |
|---|---|---|
| Sivaprasad et al | ||
| C3a-desArg∗ | 40.3 (6.1-81.7) ng/ml | 52.6 (2.8-198.1) ng/ml |
| Machalińska et al | ||
| C3a-desArg† | 445 (389-525) ng/ml | 459 (412-518) ng/ml |
| Lynch et al21 | ||
| C3a† | 50 (41-62) ng/ml | 61 (50-76) ng/ml |
| Ba† | 597 (522-709) ng/ml | 722 (617-876) ng/ml |
| C5a† | 564 (448-692) pg/ml | 703 (575-793) pg/ml |
| iC3b/C3b† | 882 (657-1119) ng/ml | 654 (561-834) ng/ml |
| CFH† | 221 (195-251) μg/ml | 198 (177-226) μg/ml |
| CFI† | 31 (28-34) μg/ml | 26 (22-29) μg/ml |
| Lashkari et al24 | ||
| CFH‡ | 585.2 (17.8) μg/ml | 551.3 (16.2) μg/ml |
| Lashkari et al25 | ||
| CFI§ | 25.9 (1.0) μg/ml | 24.2 (1.0) μg/ml‖ |
| Heesterbeek et al | ||
| Log C3d/C3¶ | 1.40 | 1.57# |
Median (range).
Median (interquartile range).
Mean (standard error).
Geometric mean (standard error).
Value taken from Age-Related Eye Disease Study stage III group.
Mean, no measurement of dispersion provided.
Value taken from intermediate group.
Complement in Advanced Nonexudative Age-Related Macular Degeneration
The earliest study examining complement activation in patients with GA compared with control participants without AMD was in 2009 by Reynolds et al.26 They measured levels of multiple complement components and found significantly higher levels of components associated with activation, including C3a, C5a, and Bb, in patients with GA versus control participants without AMD and significantly lower levels of CFH, a factor associated with complement inhibition. In a similar case-control study performed by Lynch et al,22 similar elevations of the complement activation products C3a and sC5b-9 were found in patients with GA compared with control participants. Finally, a recent study by Heesterbeek et al27 found a significantly higher C3d to C3 ratio (a marker of complement activation) in patients with central GA versus control participants. In contrast, Lashkari et al24,25 measured levels of the complement inhibition proteins CFI and CFH and found no differences between patients with GA versus control participants without AMD. Table 3 summarizes these studies of complement activation profiles in patients with GA versus control participants without AMD.
Table 3.
Complement Activation Profiles in Patients with Geographic Atrophy versus Control Participants without Age-Related Macular Degeneration
| Variable | Control Participants without Age-Related Macular Degeneration | Patients with Geographic Atrophy |
|---|---|---|
| Reynolds et al | ||
| C3a∗ | 1498 (768-2154) ng/ml | 1567 (959-2899) ng/ml |
| C5a∗ | 14 (8-20) ng/ml | 17 (9-21) ng/ml |
| Bb∗ | 0.8 (0.5-1.4) μg/ml | 1.0 (0.6-2.0) μg/ml |
| CFH∗ | 312 (253-420) μg/ml | 289 (231-417) μg/ml |
| Lynch et al22 | ||
| C3a† | 34 (20-75) ng/ml | 43.5 (30-93) ng/ml |
| sC5b-9† | 141 (87-220) ng/ml | 164 (100-319) ng/ml |
| Heesterbeek et al | ||
| Log C3d/C3‡ | 1.40 | 1.57 |
| Lashkari et al24 | ||
| CFH§ | 585.2 (17.8) μg/ml | 613.4 (20.6) μg/ml |
| Lashkari et al25 | ||
| CFI‖ | 25.9 (1.0) μg/ml | 25.6 (0.9) μg/ml |
Median (10th–90th percentile).
Median (range).
Mean, no measurement of dispersion provided.
Mean (standard error).
Geometric mean (standard error).
Discussion
In this systematic review, we identified all published studies comparing systemic levels of complement proteins in patients with early to intermediate nonexudative AMD or GA versus control participants without AMD. Cumulatively, evidence suggests that systemic complement common final pathway activation is increased and systemic complement inhibition is decreased in patients with nonexudative AMD and patients with GA versus control participants without AMD (Fig 2). However, given the substantial heterogeneity between existing studies in terms of study design, AMD case definition, and the specific complement proteins measured, these findings are not conclusive. Moreover, many studies thus far have been relatively small with limited statistical power. These points are especially important because no phase 3 clinical trials examining the usefulness of complement inhibition strategies for slowing GA progression have published significant peer-reviewed results to date. Larger studies with more precise case definitions based on standard clinical criteria and more sophisticated analysis of specific parts of the complement pathway are necessary to identify the ideal therapeutic targets and optimal patient populations who may benefit the most from such therapies.
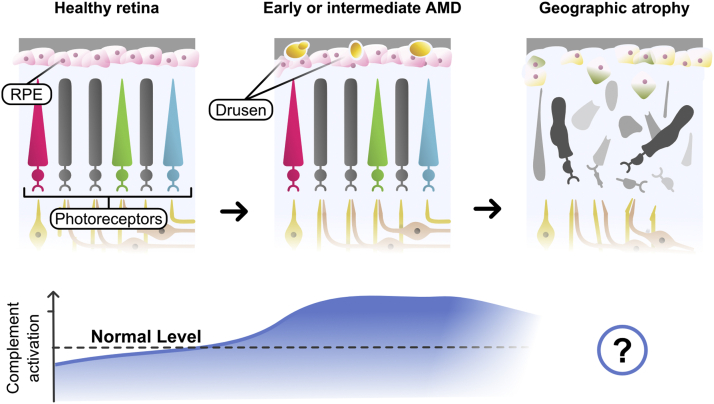
Figure 2.
Diagram depicting the central conclusion of the systematic review: systemic complement overactivation may be a central feature of early or intermediate age-related macular degeneration (AMD). Although systemic complement overactivation seems to occur in patients with advanced nonexudative AMD, no complement inhibitory strategies thus far have shown efficacy for slowing progression of geographic atrophy. RPE = retinal pigment epithelium. (Illustration commissioned by authors with permission granted by illustrator for publication.)
Another remaining question is whether systemic complement profiles reflect local complement activation profiles in the local retinal environment. Although no study to our knowledge has quantified local complement protein levels in patients with GA, 2 recent studies suggest that local complement common final pathway overactivation indeed exists in early and intermediate AMD, as evidenced by higher levels of complement activation products in aqueous humor.28,29 These findings are consistent with the findings of this systematic review in that they suggest that complement overactivation may be a feature of early AMD.
Despite the possible usefulness of complement inhibition for treating AMD, further studies are necessary to investigate potential unintended consequences of complement inhibition. Animal studies suggest that the complement pathway may not only be important for retinal homeostasis, but may also be protective in models of retinal disease.30, 31, 32, 33 Although not all studies have reached the same conclusions, one study reported that mice lacking C3 or C5 may exhibit increased neovascularization in a laser injury-induced model compared with control mice,34 suggesting that complement deficiency may be proangiogenic. These findings are especially concerning in light of data from the FILLY and GATHER1 trials showing 3- to 17-fold higher rates of conversion to exudative AMD in patients receiving intravitreal complement inhibition compared with those receiving sham injections.17,18
In conclusion, complement overactivation may be present in multiple stages of nonexudative AMD. However, further mechanistic studies are necessary to confirm the usefulness of complement pathway modulation in various stages of nonexudative AMD and GA.
Acknowledgments
The authors thank Danyel Cavazos (Rice University) for help with scientific illustration.
Manuscript no. D-21-00115.
Footnotes
Supplemental material available atwww.ophthalmologyscience.org
Disclosure(s): No relevant financial disclosures.
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): S.S.: Employee – Google LLC
J.W.M.: Board member – Aptiynx, Inc; Consultant – Genentech/Roche, Sunovion, KalVista Pharmaceuticals, Ltd, ONL Therapeutics, LLC, Heidelberg Engineering; Financial support – Lowy Medical Research Institute, Ltd; Patents – ONL Therapeutics, Valeant Pharmaceuticals; Royalties – Massachusetts Eye and Ear/Valeant Pharmaceuticals; Equity owner – Aptinyx, Inc.
D.G.V.: Board member – Olix Pharmaceutical; Consultant – Twenty Twenty
Supported by the VitreoRetinal Surgery Foundation (J.B.L.); the Monte J. Wallace Chair in Retina (D.G.V.); the Ines and Fred Yeatts Retina Research laboratory fund (D.G.V.); the MLS Foundation (D.G.V.); the American Macular Degeneration Foundation (D.G.V.); and the National Institutes of Health, Bethesda, Maryland (grant no.: R01 EY030088 [J.W.M.]). The sponsors and funding organizations had no role in the design or conduct of this research.
Dr Demetrios G. Vavvas, an editorial board member of this journal, was recused from the peer-review process of this article and had no access to information regarding its peer review.
HUMAN SUBJECTS: No human subjects were included in this study. The requirement for informed consent was waived because of the retrospective nature of the study. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Lin, Serghiou, Vavvas
Analysis and interpretation: Lin, Serghiou, Miller, Vavvas
Data collection: Lin, Serghiou
Obtained funding: Lin, Vavvas, Miller; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Lin, Serghiou, Miller, Vavvas
Supplementary Data
References
- 1.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Sene A., Chin-Yee D., Apte R.S. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med. 2015;21:43–51. doi: 10.1016/j.molmed.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J.B., Tsubota K., Apte R.S. A glimpse at the aging eye. Aging Mech Dis. 2016;2:16003. doi: 10.1038/npjamd.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew E.Y., Clemons T.E., SanGiovanni J.P., et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report no. 3. JAMA Ophthalmol. 2014;132:142. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 6.Vavvas D.G., Small K.W., Awh C.C., et al. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc Natl Acad Sci U S A. 2018;115:E696–E704. doi: 10.1073/pnas.1718059115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew E.Y., Clemons T.E., Agrón E., et al. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013;120:1604–1611.e4. doi: 10.1016/j.ophtha.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R.J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines J.L. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 11.Edwards A.O. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 12.Zareparsi S., Branham K.E.H., Li M., et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J.W., Bagheri S., Vavvas D.G. Advances in age-related macular degeneration understanding and therapy. US Ophthalmic Re. 2017;10:119. doi: 10.17925/USOR.2017.10.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehoshua Z., Alexandre de Amorim Garcia Filho C., Nunes R.P., et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaspan B.L., Williams D.F., Holz F.G., et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf1443. [DOI] [PubMed] [Google Scholar]
- 16.Holz F.G., Sadda S.R., Busbee B., et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666. doi: 10.1001/jamaophthalmol.2018.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao D.S., Grossi F.V., El Mehdi D., et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2020;127:186–195. doi: 10.1016/j.ophtha.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe G.J., Westby K., Csaky K.G., et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration. Ophthalmology. 2021;128(4):576–586. doi: 10.1016/j.ophtha.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J., Bossuyt P., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXiv. 2020 doi: 10.1186/s13643-021-01626-4. https://osf.io/v7gm2 Available at: Accessed 03.02.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivaprasad S., Adewoyin T., Bailey T.A., et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125:515–519. doi: 10.1001/archopht.125.4.515. [DOI] [PubMed] [Google Scholar]
- 21.Lynch A.M., Palestine A.G., Wagner B.D., et al. Complement factors and reticular pseudodrusen in intermediate age-related macular degeneration staged by multimodal imaging. BMJ Open Ophthalmol. 2020;5 doi: 10.1136/bmjophth-2019-000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch A.M., Mandava N., Patnaik J.L., et al. Systemic activation of the complement system in patients with advanced age-related macular degeneration. Eur J Ophthalmol. 2020;30:1061–1068. doi: 10.1177/1120672119857896. [DOI] [PubMed] [Google Scholar]
- 23.Machalińska A., Dziedziejko V., Mozolewska-Piotrowska K., et al. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42:54–59. doi: 10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 24.Lashkari K., Teague G.C., Beattie U., et al. Plasma biomarkers of the amyloid pathway are associated with geographic atrophy secondary to age-related macular degeneration. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lashkari K., Teague G., Chen H., et al. A monoclonal antibody targeting amyloid β (Aβ) restores complement factor I bioactivity: potential implications in age-related macular degeneration and Alzheimer’s disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds R., Hartnett M.E., Atkinson J.P., et al. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heesterbeek T.J., Lechanteur Y.T.E., Lorés-Motta L., et al. Complement activation levels are related to disease stage in AMD. Invest Ophthalmol Vis Sci. 2020;61:18. doi: 10.1167/iovs.61.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitnilska V., Enders P., Cursiefen C., et al. Association of imaging biomarkers and local activation of complement in aqueous humor of patients with early forms of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2021;259(3):623–632. doi: 10.1007/s00417-020-04910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altay L., Sitnilska V., Schick T., et al. Early local activation of complement in aqueous humour of patients with age-related macular degeneration. Eye (Lond) 2019;33:1859–1864. doi: 10.1038/s41433-019-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M., Zou W., Peachey N.S., et al. A novel role of complement in retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7684–7692. doi: 10.1167/iovs.12-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoh Kam J., Lenassi E., Malik T.H., et al. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am J Pathol. 2013;183:480–492. doi: 10.1016/j.ajpath.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Mukai R., Okunuki Y., Husain D., et al. The complement system is critical in maintaining retinal integrity during aging. Front Aging Neurosci. 2018;10:15. doi: 10.3389/fnagi.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman S.M., Ma W., Wang X., et al. C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J Exp Med. 2019;216:1925–1943. doi: 10.1084/jem.20190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poor S.H., Qiu Y., Fassbender E.S., et al. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Invest Ophthalmol Vis Sci. 2014;55:6525. doi: 10.1167/iovs.14-15067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.