Abstract
Background
People with cancer are at increased risk of hospitalisation and death following infection with SARS-CoV-2. Therefore, we aimed to conduct one of the first evaluations of vaccine effectiveness against breakthrough SARS-CoV-2 infections in patients with cancer at a population level.
Methods
In this population-based test-negative case-control study of the UK Coronavirus Cancer Evaluation Project (UKCCEP), we extracted data from the UKCCEP registry on all SARS-CoV-2 PCR test results (from the Second Generation Surveillance System), vaccination records (from the National Immunisation Management Service), patient demographics, and cancer records from England, UK, from Dec 8, 2020, to Oct 15, 2021. Adults (aged ≥18 years) with cancer in the UKCCEP registry were identified via Public Health England's Rapid Cancer Registration Dataset between Jan 1, 2018, and April 30, 2021, and comprised the cancer cohort. We constructed a control population cohort from adults with PCR tests in the UKCCEP registry who were not contained within the Rapid Cancer Registration Dataset. The coprimary endpoints were overall vaccine effectiveness against breakthrough infections after the second dose (positive PCR COVID-19 test) and vaccine effectiveness against breakthrough infections at 3–6 months after the second dose in the cancer cohort and control population.
Findings
The cancer cohort comprised 377 194 individuals, of whom 42 882 had breakthrough SARS-CoV-2 infections. The control population consisted of 28 010 955 individuals, of whom 5 748 708 had SARS-CoV-2 breakthrough infections. Overall vaccine effectiveness was 69·8% (95% CI 69·8–69·9) in the control population and 65·5% (65·1–65·9) in the cancer cohort. Vaccine effectiveness at 3–6 months was lower in the cancer cohort (47·0%, 46·3–47·6) than in the control population (61·4%, 61·4–61·5).
Interpretation
COVID-19 vaccination is effective for individuals with cancer, conferring varying levels of protection against breakthrough infections. However, vaccine effectiveness is lower in patients with cancer than in the general population. COVID-19 vaccination for patients with cancer should be used in conjunction with non-pharmacological strategies and community-based antiviral treatment programmes to reduce the risk that COVID-19 poses to patients with cancer.
Funding
University of Oxford, University of Southampton, University of Birmingham, Department of Health and Social Care, and Blood Cancer UK.
Introduction
Global COVID-19 vaccine trials have shown that vaccination decreases the incidence of COVID-19 and its associated complications.1, 2 However, people with cancer are at increased risk of morbidity and mortality from COVID-19.3, 4, 5 A cancer diagnosis or cancer treatment has generally been an exclusion criterion for vaccine trials, leading to a paucity of clear evidence of their benefit and some vaccine hesitancy among patients with cancer.6, 7
Small cohort studies have shown that patients with cancer have an attenuated immune response following COVID-19 vaccination, which could result in lower or absent humoral and cellular responses, compared with groups of healthy volunteers.8, 9, 10, 11, 12 Nevertheless, national and international guidelines recommend vaccinating patients with cancer against COVID-19.13, 14, 15
Considering the wider issue of waning vaccine effectiveness,16, 17 there is a need to clarify the effectiveness of COVID-19 vaccination in patients with cancer and close crucial evidence gaps.18, 19 Therefore, we aimed to conduct one of the first population-based evaluations of COVID-19 vaccine effectiveness in patients with cancer from a real-world health system in England, UK. Our use of the largest cohort of patients with cancer worldwide enabled, to our knowledge, the most comprehensive analysis of the risk that COVID-19 presents to patients with cancer. We describe how cancer subtype, treatment, and patient demographics interact to affect COVID-19 vaccine effectiveness.
Research in context.
Evidence before this study
Using the search terms “coronavirus”, “COVID-19”, “vaccine”, “vaccination”, “cancer”, “effectiveness”, and “efficacy”, we searched PubMed without language restrictions for studies published between database inception and Jan 25, 2022, related to the efficacy or effectiveness of COVID-19 vaccination in patients with cancer. To our knowledge, there are no studies that have described COVID-19 vaccine effectiveness in patients with cancer at a population level. Several studies have described antibody or cellular immune responses following COVID-19 vaccination or SARS-CoV-2 infection. Leticia Monin and colleagues (2021) reported on immune responses to BNT162b2 (Pfizer–BioNtech) in 152 patients with cancer. Fendler and colleagues (2021) reported on immune responses following SARS-CoV-2 infection in 118 patients with cancer. However, no studies have looked at clinical outcome measures, such as the prevention of SARS-CoV-2 infection or COVID-19-related hospitalisation and death, in patients with cancer.
Added value of this study
To our knowledge, this study is one of the first to evaluate COVID-19 vaccine effectiveness in patients with cancer in a real-world health system at a population level in England, UK. We used the largest cohort of patients with cancer globally, enabling the most comprehensive analysis of the risk of COVID-19 to patients with cancer. We found that COVID-19 vaccination is effective in patients with cancer, albeit less so than in the general control population, with evidence of waning vaccine effectiveness at 3–6 months following the second dose. Patients with lymphoma or leukaemia and those who had received a cancer diagnosis or cancer treatment within the past 12 months had lower vaccine effectiveness.
Implications of all the available evidence
The COVID-19 pandemic continues to have a considerable impact on people with cancer. Although COVID-19 vaccination reduces the risk of infection and poor outcomes for the general population, this protection can be heterogenous for patients with cancer, who then remain at increased risk from COVID-19. COVID-19 vaccination for patients with cancer should be used in conjunction with other non-pharmacological strategies, such as behaviour modification and personal protective equipment, and community-based antiviral treatment programmes to reduce the risk that COVID-19 poses to patients with cancer. Such measures will be crucially important as global health-care and cancer care systems adapt to living with COVID-19 as an endemic disease.
Methods
Study design and data sources
The UK Coronavirus Cancer Evaluation Project (UKCCEP) is a subproject of the UK Coronavirus Cancer Monitoring Project and is the next iteration of the UK's COVID-19 pandemic response to monitor, safeguard, and protect patients with cancer. In this population-based test-negative case-control study, we extracted PCR test results, vaccination records, patient demographics, and cancer records (eg, treatment, stage, and subtype) in England from the UKCCEP registry between Dec 8, 2020 (the start of COVID-19 vaccination in England) and Oct 15, 2021 (the study period). This period of analysis coincided with the second COVID-19 wave in the UK, which was principally driven by the delta variant (B.1.617.2).20
Patient-level COVID-19 PCR test results, including from community and hospital testing, were obtained for UKCCEP from the Second Generation Surveillance System. National Health Service (NHS) England and NHS Test and Trace use PCR testing for those with symptoms of COVID-19 and lateral flow testing (also known as antigen-detecting rapid diagnostic testing) for the identification of asymptomatic cases. During the study period, confirmatory PCR testing was mandated for individuals testing positive on lateral flow tests. In the NHS, infection and prevention control measures in secondary care required COVID-19 PCR testing of asymptomatic patients before many procedures or treatments. Vaccination records for the UKCCEP registry were obtained from the National Immunisation Management Service. All COVID-19 vaccines licensed in England were considered.
The number of COVID-19 contacts was obtained from individuals who had supplied information as part of the Contact Tracing and Advice Service, which records information about the number of interpersonal contacts before infection or following exposure to COVID-19. Data on COVID-19-related hospitalisation and death were extracted from the Secondary Use Statistics dataset between Dec 8, 2020, and Oct 15, 2021.
From those who had SARS-CoV-2 PCR testing in the Second Generation Surveillance System, we identified adults (aged ≥18 years) with cancer to comprise our cancer cohort via Public Health England's Rapid Cancer Registration Dataset between Jan 1, 2018, and April 30, 2021. This date range was selected to better represent individuals with active cancer, excluding those with a more historical diagnosis. The national Rapid Cancer Registration Dataset includes information about receipt of radiotherapy and systemic anticancer treatments, which is an umbrella term of cancer treatments, including cytotoxic (chemotherapy), targeted, immunotherapy, or hormonal treatments. We constructed a control population cohort from adults (aged ≥18 years) with PCR tests in the Second Generation Surveillance System who were not contained within the Rapid Cancer Registration Dataset, excluding those with active cancer. Data linkage between the Second Generation Surveillance System, the National Immunisation Management Service, the Contact Tracing and Advice Service, and the Rapid Cancer Registration Dataset required exact matching of NHS identification numbers.
This study was designed as a public health surveillance analysis to support rapid clinical decision making during the pandemic in accordance with the UK Policy Framework for Health and Social Care Research. The project was supported by the Department of Health and Social Care, with ethical approval from the Health Research Authority (20/WA/0181), and patient consent was waived.
Statistical analysis
The coprimary outcomes of the study were overall vaccine effectiveness (defined relative to breakthrough infections [positive PCR test] following the second dose of COVID-19 vaccine during the period of assessment) and vaccine effectiveness against breakthrough infections at 3–6 months after the second dose. A test-negative case-control method was used to estimate vaccine effectiveness in the cancer cohort and the control population.
Test-negative case-control studies have high concordance with findings from randomised clinical trials and are a standardised measure of vaccine effectiveness for phase 4 surveillance studies.21, 22 Within the test-negative case-control study design, exposure was defined as any positive PCR test result within the study period. Vaccine effectiveness was calculated with the test-negative case-control method formula: 1 minus the ratio of PCR-positive vaccinated to PCR-positive unvaccinated individuals divided by the ratio of PCR-negative vaccinated to PCR-negative unvaccinated individuals. Each datapoint corresponds to a single PCR test and higher vaccine effectiveness would be shown if there were lower numbers of vaccinated individuals among those who had positive tests than among those who had negative tests. The negative tests act as an internal control, comprising individuals who might have symptoms from non-COVID-19 causes. This design addresses challenges that are often present in observational studies, such as differences in health-seeking behaviours or access to testing. Vaccine manufacturers were combined in our evaluation because the focus of our study was a description of vaccine effectiveness and waning in the cancer cohort relative to the control population. Additionally, vaccine effectiveness according to different manufacturers is relatively well described in the literature.1, 2
Predefined subgroup analyses of overall vaccine effectiveness were done in the cancer cohort by vaccine type (BNT162b2 [Pfizer–BioNtech], ChAdOx1 nCov-19 [AZD1222; AstraZeneca], or mixed and other), cancer type (solid organ vs haematological) and subtype (as determined by codes from the tenth revision of the International Classification of Diseases), cancer stage, date of cancer diagnosis (≤12 months vs >12 months relative to data cutoff), and receipt of systemic anticancer cancer treatment or radiotherapy (none vs any and received ≤12 months ago vs received >12 months ago relative to data cutoff). Within the cancer cohort, exploratory multivariable logistic regression with the Wald test was used to describe vaccine effectiveness (overall and at 3–6 months) in the aforementioned predefined subgroups, excluding vaccine type, and was adjusted for the clinically important covariates of age, sex, ethnicity, and Index of Multiple Deprivation (determined by geographical location),23 which might have acted as confounders, effect modifiers, or both for analysing vaccine effectiveness. Further prespecified exploratory analyses of cancer subtypes, receipt of radiotherapy or systemic anticancer treatment, and time of diagnosis (≤12 months vs >12 months relative to data cutoff) were done to identify whether any subgroups were more likely to develop waning vaccine effectiveness at 3–6 months following multivariable correction. Waning vaccine effectiveness was defined as the change in percentage points between vaccine effectiveness over the study period subtracted from vaccine effectiveness at 3–6 months. Wald test z values were used to assess statistical significance.
Variables were either binary (sex, cancer treatments, cancer types, time from diagnosis, PCR status, outcomes and vaccination status) or grouped (age, ethnicity, Index of Multiple Deprivation, cancer subtypes, and stage), with age categorised in 10-year age bands (18–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, 80–89 years, and ≥90 years) in accordance with a previous vaccine effectiveness study.21 We used information from the Contact Tracing and Advice Service for post-hoc analyses of patient behaviour by patient age band and cancer stage. Contacts included both household and non-household contacts. The mean numbers of contacts and SDs were calculated for each subgroup.
Steps were taken to reduce bias at several study stages, including robust adherence to the data analysis plan, minimising selection bias, and ensuring that the full dataset was reviewed and interpretations were approved by multiple consortium authors. Participants with missing or not specified data were excluded from our analyses.
In further post-hoc analyses, we examined COVID-19 hospitalisation (defined as admission to hospital from 1 day before to 14 days after a positive PCR test) and COVID-19 death (death occurring up to 28 days after a positive PCR test) in the cancer cohort overall and at 3–6 months after the second vaccine dose. These analyses were added to translate the documented positive PCR test into more meaningful clinical outcome measures and provide additional clinical insight.
95% CIs were calculated by Wilson score intervals without continuity correction. Analyses were done in R (version 4.0.3) with epiDisplay (version 3.5.0.1).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
During the study period from Dec 8, 2020, to Oct 15, 2021, 77 399 018 COVID-19 PCR tests for 28 010 955 individuals were done. 491 007 PCR tests were excluded because they were void and 4 084 667 were excluded because they contained no or invalid NHS identifiers. 1 712 728 PCR tests were done for 377 194 individuals identified in the Rapid Cancer Registration Dataset. The cancer cohort comprised 377 194 individuals who had 56 102 positive PCR tests, corresponding to 42 882 individuals infected with breakthrough SARS-CoV-2. The control population consisted of 28 010 955 individuals, of whom 5 748 708 had SARS-CoV-2 breakthrough infections. Baseline characteristics of test-positive cases and test-negative controls in both the cancer and control cohorts are shown in table 1 .
Table 1.
Baseline characteristics of the cancer cohort and control population
|
Cancer cohort |
Control population |
||||||
|---|---|---|---|---|---|---|---|
| All (n=1 712 728) | PCR positive (n=56 102) | PCR negative (n=1 656 626) | All (n=75 686 290) | PCR positive (n=5 808 432) | PCR negative (n=69 877 858) | ||
| Age, years | 69 (58–78) | 68 (56–77) | 69 (58–78) | 45 (29–61) | 34 (20–51) | 46 (30–62) | |
| Sex | |||||||
| Female | 862 169 (50·34%) | 27 266 (48·60%) | 834 903 (50·40%) | 45 991 583 (60·77%) | 3 033 061 (52·22%) | 42 958 522 (61·48%) | |
| Male | 850 559 (49·66%) | 28 836 (51·40%) | 821 723 (49·60%) | 29 637 195 (39·16%) | 2 775 160 (47·78%) | 26 862 035 (38·44%) | |
| Other or unknown | 0 | 0 | 0 | 57 512 (0·08%) | 211 (<0·01%) | 57 301 (0·08%) | |
| Ethnicity | |||||||
| White or White British | 1 533 034 (89·51%) | 47 856 (85·30%) | 1 485 178 (89·65%) | 55 551 500 (73·40%) | 2 869 777 (49·41%) | 52 681 723 (75·39%) | |
| Asian or Asian British | 70 859 (4·14%) | 3245 (5·78%) | 67 614 (4·08%) | 5 022 431 (6·64%) | 359 812 (6·19%) | 4 662 619 (6·67%) | |
| Black or Black British | 50 063 (2·92%) | 2051 (3·66%) | 48 012 (2·90%) | 2 611 003 (3·45%) | 102 911 (1·77%) | 2 508 092 (3·59%) | |
| Mixed or other ethnic group | 15 885 (0·93%) | 617 (1·10%) | 15 268 (0·92%) | 1 267 826 (1·68%) | 55 454 (0·95%) | 1 212 372 (1·73%) | |
| Unknown | 42 887 (2·50%) | 2333 (4·16%) | 40 554 (2·45%) | 11 233 530 (14·84%) | 2 420 478 (41·67%) | 8 813 052 (12·61%) | |
| Index of Multiple Deprivation | |||||||
| 1 | 129 287 (7·55%) | 4280 (7·63%) | 125 007 (7·55%) | 5 735 964 (7·58%) | 364 776 (6·28%) | 5 371 188 (7·69%) | |
| 2 | 134 427 (7·85%) | 4390 (7·83%) | 130 037 (7·85%) | 6 073 257 (8·02%) | 388 336 (6·69%) | 5 684 921 (8·14%) | |
| 3 | 143 823 (8·40%) | 4715 (8·40%) | 139 108 (8·40%) | 6 252 170 (8·26%) | 396 746 (6·83%) | 5 855 424 (8·38%) | |
| 4 | 151 891 (8·87%) | 4339 (7·73%) | 147 552 (8·91%) | 6 351 129 (8·39%) | 391 737 (6·74%) | 5 959 392 (8·53%) | |
| 5 | 157 359 (9·19%) | 4106 (7·32%) | 153 253 (9·25%) | 6 296 906 (8·32%) | 382 963 (6·59%) | 5 913 943 (8·46%) | |
| 6 | 163 835 (9·57%) | 4371 (7·79%) | 159 464 (9·63%) | 6 319 149 (8·35%) | 380 069 (6·54%) | 5 939 080 (8·50%) | |
| 7 | 168 024 (9·81%) | 4450 (7·93%) | 163 574 (9·87%) | 6 103 357 (8·06%) | 369 624 (6·36%) | 5 733 733 (8·21%) | |
| 8 | 166 879 (9·74%) | 4178 (7·45%) | 162 701 (9·82%) | 6 102 705 (8·06%) | 377 014 (6·49%) | 5 725 691 (8·19%) | |
| 9 | 168 813 (9·86%) | 4178 (7·45%) | 164 635 (9·94%) | 5 958 016 (7·87%) | 368 232 (6·34%) | 5 589 784 (8·00%) | |
| 10 | 160 864 (9·39%) | 3913 (6·97%) | 156 951 (9·47%) | 5 731 492 (7·57%) | 350 993 (6·04%) | 5 380 499 (7·70%) | |
| Unknown | 167 526 (9·78%) | 13 182 (23·50%) | 154 344 (9·32%) | 14 762 145 (19·50%) | 2 037 942 (35·09%) | 12 724 203 (18·21%) | |
Data are median (IQR) or n (%).
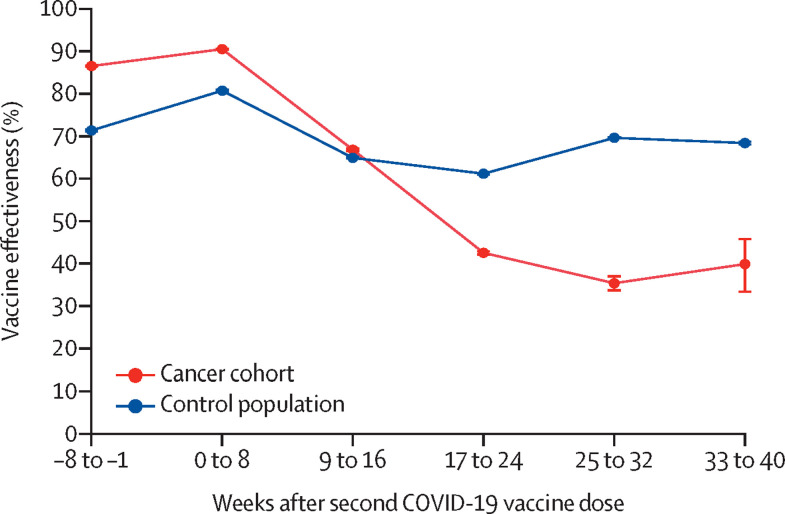
Overall vaccine effectiveness following the second vaccine dose against COVID-19 during the study period was 69·8% (95% CI 69·8–69·9) in the control population and 65·5% (65·1–65·9) in the cancer cohort. Vaccine effectiveness at 3–6 months after the second dose was lower in the cancer cohort (47·0%, 95% CI 46·3–47·6) than in the control population (61·4%, 61·4–61·5). Waning vaccine effectiveness in the cancer cohort reached its lowest point at 24–32 weeks following administration of the second vaccine dose (figure 1 ; appendix p 6).
Figure 1.
Vaccine effectiveness over time after the second COVID-19 vaccine dose in the cancer cohort versus the control population
The error bars represent 95% CIs.
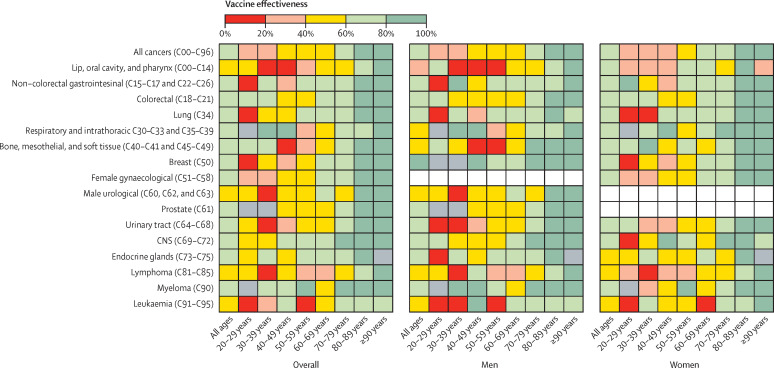
To ascertain whether predefined subgroups within the cancer cohort showed greater differences in vaccine effectiveness against breakthrough infections, exploratory analyses were done (table 2 ; figure 2 ; appendix p 2). In the cancer cohort, vaccine effectiveness was higher in individuals (n=123 060) who had been vaccinated with two doses of BNT162b2 (72·1%, 95% CI 71·6–72·7) than in individuals (n=157 138) who had received two doses of ChAdOx1 nCov-19 (59·0%, 58·5–59·6; table 2).
Table 2.
Number of PCR positive and negative test results and vaccine effectiveness in cancer cohort subgroups
| Overall vaccine effectiveness |
Vaccine effectiveness at 3–6 months |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed (PCR positive) |
Not exposed (PCR negative) |
Vaccine effectiveness (95% CI) | Exposed (PCR positive) |
Not exposed (PCR negative) |
Vaccine effectiveness (95% CI) | ||||||||
| Vaccinated (two doses) | Unvaccinated | Vaccinated (two doses) | Unvaccinated | Vaccinated (two doses) | Unvaccinated | Vaccinated (two doses) | Unvaccinated | ||||||
| All patients with cancer | 18 292 | 31 649 | 780 054 | 465 982 | 65·5% (65·1–65·9) | 12 513 | 31 649 | 347 414 | 465 982 | 47·0% (46·3–47·6) | |||
| Cancer stage | |||||||||||||
| Stage 1 | 3748 | 4678 | 139 476 | 60 749 | 65·1% (64·4–65·8) | 2551 | 4678 | 64 551 | 60 749 | 48·7% (47·2–50·1) | |||
| Stage 2 | 2532 | 3387 | 104 254 | 50 455 | 63·8% (62·9–64·8) | 1755 | 3387 | 46 566 | 50 455 | 43·9% (42·2–45·5) | |||
| Stage 3 | 2203 | 3649 | 109 286 | 58 389 | 67·7% (66·7–68·8) | 1569 | 3649 | 48 642 | 58 389 | 48·4% (46·6–50·1) | |||
| Stage 4 | 966 | 3115 | 69 574 | 47 760 | 78·7% (77·5–79·9) | 674 | 3115 | 30 209 | 47 760 | 65·8% (63·7–67·8) | |||
| Other or unknown | 8843 | 16 820 | 357 464 | 248 629 | NA | 5964 | 16 820 | 157 446 | 248 629 | NA | |||
| Vaccine name or manufacturer (doses 1 and 2) | |||||||||||||
| BNT162b2 (Pfizer–BioNtech) | 7050 | 31 649 | 372 674 | 465 982 | 72·1% (71·6–72·7) | 4667 | 31 649 | 167 336 | 465 982 | 58·9% (58·0–59·9) | |||
| ChAdOx1 nCoV-19 (AstraZeneca) | 11 192 | 31 649 | 402 308 | 465 982 | 59·0% (58·5–59·6) | 7828 | 31 649 | 177 512 | 465 982 | 35·1% (34·1–36·1) | |||
| Mixed (Pfizer–BioNtech and AstraZeneca) or other | 50 | 0 | 5072 | 0 | NA | 18 | 0 | 2566 | 0 | NA | |||
| Cancer diagnosis and treatment | |||||||||||||
| Time of diagnosis | |||||||||||||
| ≤12 months before data cutoff | 2807 | 8286 | 162 082 | 164 729 | 65·6% (64·5–66·6) | 1778 | 8286 | 63 335 | 164 729 | 44·2% (42·2–46·1) | |||
| >12 months before data cutoff | 15 485 | 23 363 | 617 972 | 301 253 | 67·7% (67·3–68·1) | 10 735 | 23 363 | 284 079 | 301 253 | 51·3% (50·6–51·9) | |||
| Systemic anticancer therapy | |||||||||||||
| Yes | 4633 | 9024 | 208 369 | 158 293 | 61·0% (60·1–61·9) | 3328 | 9024 | 92 068 | 158 293 | 36·6% (35·1–38·0) | |||
| No | 13 659 | 22 625 | 571 685 | 307 689 | 67·5% (67·1–67·9) | 9185 | 22 625 | 255 346 | 307 689 | 51·1% (50·4–51·8) | |||
| Received ≤12 months before data cutoff | 3061 | 6509 | 144 513 | 121 632 | 60·4% (59·3–61·5) | 2152 | 6509 | 62 253 | 121 632 | 35·4% (33·5–37·3) | |||
| Received >12 months before data cutof | 1572 | 2515 | 63 856 | 36 661 | 64·1% (62·8–65·4) | 1176 | 2515 | 29 815 | 36 661 | 42·5% (40·4–44·6) | |||
| Radiotherapy | |||||||||||||
| Yes | 2576 | 4591 | 114 754 | 82 298 | 59·8% (58·6–60·9) | 1823 | 4591 | 51 564 | 82 298 | 36·6% (34·7–38·5) | |||
| No | 15 716 | 27 058 | 665 300 | 383 684 | 66·5% (66·1–66·9) | 10 690 | 27 058 | 295 850 | 383 684 | 48·8% (48·1–49·4) | |||
| Received ≤12 months before data cutoff | 911 | 2230 | 49 023 | 50 364 | 58·0% (56·0–60·0) | 657 | 2230 | 21 194 | 50 364 | 30·0% (26·2–33·7) | |||
| Received >12 months before data cutoff | 1665 | 2361 | 65 731 | 31 934 | 65·7% (64·6–66·9) | 1166 | 2361 | 30 370 | 31 934 | 48·1% (46·1–50·1) | |||
| Type of malignancy | |||||||||||||
| Solid organ malignancy | 15 070 | 26 203 | 685 675 | 390 844 | 67·2% (66·8–67·6) | 10 245 | 26 203 | 304 288 | 390 844 | 49·8% (49·1–50·5) | |||
| Haematological malignancy | 3222 | 5446 | 94 379 | 75 138 | 52·9% (51·7–54·1) | 2268 | 5446 | 43 126 | 75 138 | 27·4% (25·6–29·3) | |||
| Cancer subtype | |||||||||||||
| Lip, oral cavity, and pharynx (C00–C14) | 441 | 684 | 16 718 | 13 798 | 46·8% (43·5–50·2) | 297 | 684 | 7353 | 13 798 | 18·5% (12·9–24·2) | |||
| Non-colorectal gastrointestinal (C15–C17 and C22–C26) | 921 | 2698 | 61 577 | 45 563 | 74·7% (73·3–76·2) | 596 | 2698 | 25 495 | 45 563 | 60·5% (58·0–62·9) | |||
| Colorectal gastrointestinal (C18–C21) | 2031 | 3740 | 114 874 | 63 005 | 70·2% (69·2–71·2) | 1399 | 3740 | 49 974 | 63 005 | 52·8% (51·1–54·6) | |||
| Lung (C34) | 1228 | 3344 | 70 528 | 49 068 | 74·5% (73·2–75·7) | 820 | 3344 | 31 250 | 49 068 | 61·5% (59·4–63·5) | |||
| Respiratory and intrathoracic organs (C30–C33 and C35–C39) | 161 | 359 | 7376 | 5840 | 64·5% (59·9–68·9) | 123 | 359 | 3304 | 5840 | 39·4% (32·0–46·6) | |||
| Bone, mesothelial, and soft tissue (C40–C41 and C45–C49) | 283 | 637 | 14 976 | 13 091 | 61·2% (57·5–64·7) | 185 | 637 | 6203 | 13 091 | 38·7% (32·2–44·9) | |||
| Breast (C50) | 3774 | 4877 | 147 465 | 70 606 | 62·9% (62·2–63·7) | 2568 | 4877 | 66 651 | 70 606 | 44·2% (42·8–45·6) | |||
| Female gynaecological (C51–C58) | 1095 | 2067 | 52 094 | 33 122 | 66·3% (64·7–67·9) | 709 | 2067 | 23 001 | 33 122 | 50·6% (48·0–53·2) | |||
| Male urological (C60, C62, and C63) | 234 | 428 | 5328 | 4759 | 51·2% (46·5–55·8) | 133 | 428 | 2294 | 4759 | 35·5% (28·2–42·6) | |||
| Prostate (C61) | 3093 | 3867 | 108 522 | 39 592 | 70·8% (70·1–71·5) | 2178 | 3867 | 50 373 | 39 592 | 55·7% (54·3–57·2) | |||
| Urinary tract (C64–C68) | 1372 | 2223 | 70 547 | 34 539 | 69·8% (68·6–71·0) | 968 | 2223 | 31 654 | 34 539 | 52·5% (50·4–54·6) | |||
| CNS (C69–C72) | 186 | 789 | 8127 | 11 991 | 65·2% (61·6–69·0) | 117 | 789 | 3506 | 11 991 | 49·3% (41·9–56·0) | |||
| Endocrine glands (C73–C75) | 251 | 490 | 7543 | 5870 | 60·1% (56·2–64·0) | 152 | 490 | 3230 | 5870 | 43·6% (41·9–56·0) | |||
| Lymphoma (C81–C85) | 1806 | 2427 | 37 107 | 27 855 | 44·1% (42·5–45·8) | 1277 | 2427 | 16 811 | 27 855 | 12·8% (10·4–15·3) | |||
| Myeloma (C90) | 472 | 918 | 29 545 | 12 921 | 77·5% (75·8–79·2) | 345 | 918 | 13 458 | 12 921 | 63·9% (60·7–67·0) | |||
| Leukaemia (C91–C95) | 809 | 1954 | 24 555 | 32 581 | 45·1% (42·5–47·6) | 554 | 1954 | 11 333 | 32 581 | 18·5% (13·9–23·0) | |||
| Other | 135 | 147 | 3172 | 1781 | NA | 92 | 147 | 1524 | 1781 | NA | |||
NA=not applicable.
Figure 2.
Heatmap showing overall vaccine effectiveness after the second dose and the interaction of patient age, sex, and cancer diagnosis
Grey boxes denote insufficient data; white boxes denote inapplicable sections.
Cancer subtype analysis identified that vaccine effectiveness (overall and at 3–6 months) was lower among patients with haematological malignancies than among those with solid organ malignancies, driven principally by those with a diagnosis of lymphoma or leukaemia (table 2; figure 2; appendix p 2). By contrast, we observed that overall and 3–6-month vaccine effectiveness in the myeloma subgroup was high (table 2). Among the solid cancers, vaccine effectiveness was lowest in those with head and neck malignancies (lip, oral cavity, and pharynx; table 2, appendix p 3).
Patients who received systemic anticancer therapy or radiotherapy had a lower vaccine effectiveness overall and at 3–6 months compared with those who had not received these types of treatment (table 2). Patients who received systemic anticancer treatments or radiotherapy within 12 months of data cutoff versus more than 12 months had lower vaccine effectiveness at 3–6 months (table 2). Patients with a more recent diagnosis (≤12 months relative to data cutoff) had a lower vaccine effectiveness at 3–6 months than those with an older diagnosis (>12 months relative to data cutoff; table 2). For every cancer stage, vaccine effectiveness at 3–6 months was lower than overall vaccine effectiveness (table 2).
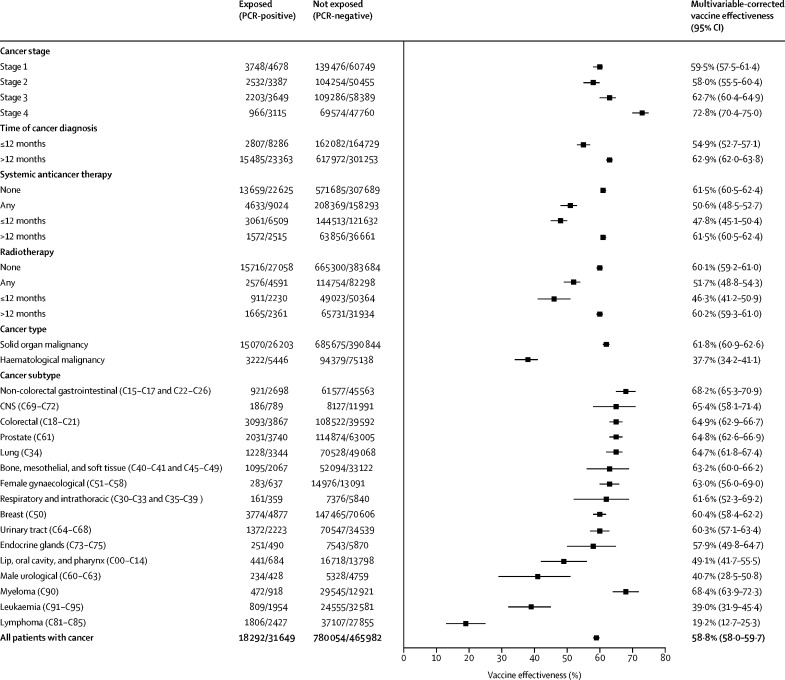
To examine clinically relevant covariates that might drive these differences in the cancer cohort, a multivariable logistic regression model was fitted to adjust for the effects of the age, sex, Index of Multiple Deprivation, and ethnicity (figure 3 ; appendix p 7). At 3–6 months, vaccine effectiveness was significantly lower for those who had received systemic anticancer treatments at any time or within the last 12 months, radiotherapy at any time or within the last 12 months, or a cancer diagnosis within the last 12 months compared with those who had not, but was not different between those with versus without haematological malignancies (appendix p 7).
Figure 3.
Forest plot showing multivariable-corrected overall vaccine effectiveness among predefined cancer subgroups
The error bars represent 95% CIs. Regression models were fitted for the clinically relevant covariates of age, sex, Index of Multiple Deprivation, and ethnicity.
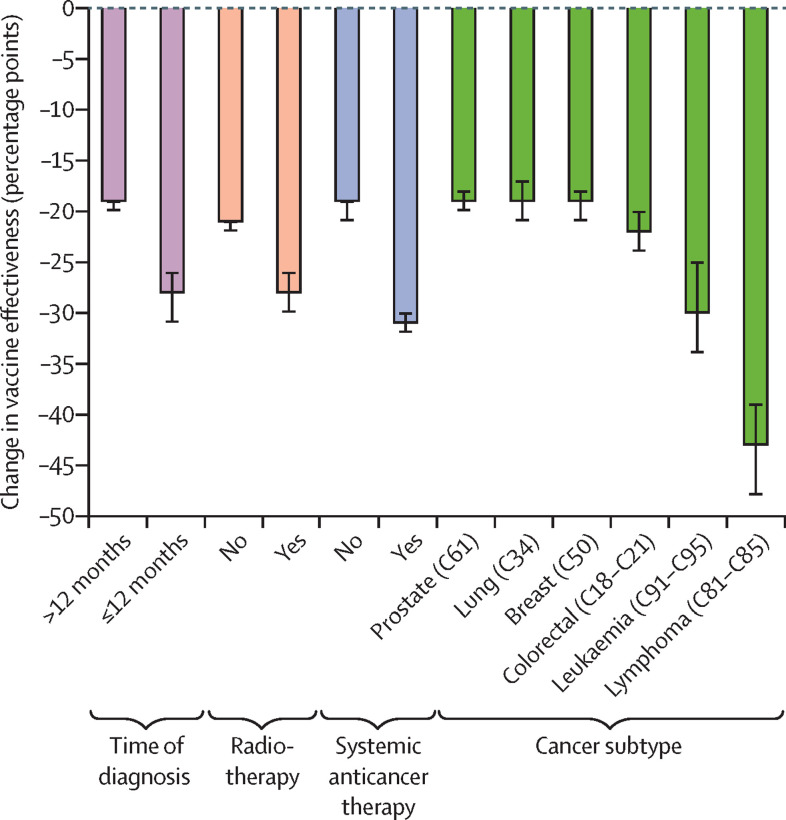
In the adjusted multivariable logistic regression, patients with stage 4 cancers versus all other stages and those aged 70 years or older versus those younger than 70 years had reduced frequencies of breakthrough infections and higher vaccine effectiveness (Figure 2, Figure 3). To investigate whether this result might be due to variations in patient behaviour, we did an exploratory post-hoc analysis in which we linked the cancer cohort to the Contact Tracing and Advice Service dataset. We found that patients with stage 4 cancer had fewer mean contacts than those with stage 1 cancer (1·32 [SD 4·36] vs 2·04 [7·76]) and that the mean number of contacts was lower for patients older than 70 years compared with those younger than 70 years (appendix pp 4, 8). We identified evidence of an inverse relationship between age group and the number of contacts (appendix pp 4, 8). The greatest levels of waning vaccine effectiveness were observed in those with a diagnosis of lymphoma or leukaemia, in those who were diagnosed within 12 months of data cutoff, and in those who had received systemic anticancer treatments or radiotherapy (figure 4 ; appendix p 5).
Figure 4.
Waterfall plot showing multivariable-corrected waning vaccine effectiveness at 3–6 months by key cancer subgroups
The most common solid tumours and haematological malignancies according to Cancer Research UK are shown.
In a post-hoc analysis, we observed that there were higher levels of protection afforded against COVID-19 hospitalisation (84·5%, 95% CI 83·6–85·4) and death (93·5%, 93·0–94·0) than against breakthrough infections in our cancer cohort following the second dose (appendix p 6). Similar to vaccine effectiveness against breakthrough infections, vaccine effectiveness against more severe COVID-19 outcomes waned at 3–6 months (appendix p 6).
Discussion
Patients with cancer initially had high COVID-19 vaccine effectiveness, similar to the control population, but this vaccine effectiveness rapidly waned. Reduced vaccine effectiveness was observed in individuals who had been diagnosed with cancer or had received radiotherapy or systemic anticancer treatments within the preceding 12 months. A diagnosis of lymphoma or leukaemia was also associated with both lower, and more rapidly waning, vaccine effectiveness. Our findings reflect published clinical data from a US cohort of 184 485 patients with cancer and a cohort of 2391 patients with cancer from France.24, 25 Waning of vaccine effectiveness at 3–6 months was less pronounced for the outcomes of COVID-19 hospitalisation or death than for breakthrough infections, although we note that these metrics are a lagged indicator of vaccine effectiveness. Although this study cannot address the mechanisms for this drop in vaccine effectiveness, the findings match those of previous studies that have identified reduced levels of protective antibody and T-cell responses after vaccination in this cohort.8, 10 These patients, especially those with lymphoma and leukaemia, might have a limited capacity to maintain immunological vaccine memory, in many cases as a consequence of cancer treatments that specifically suppress immune responses. For patients in the cancer cohort, the BNT162b2 vaccine resulted in higher levels of vaccine effectiveness than the ChAdOx1 nCov-19 vaccine, in keeping with studies in the general population.21
We found that the absolute difference in vaccine effectiveness against breakthrough infections in people with cancer compared with the control population was 4·3 percentage points. However, at 3–6 months, this difference in vaccine effectiveness widened to 14·4 percentage points, representing a reduction in vaccine effectiveness of nearly a third in patients with cancer. Waning vaccine effectiveness has been described in other studies of COVID-19 vaccines in people without cancer.17, 26 In parallel to this work, an analysis of a UK cohort has identified waning vaccine effectiveness against symptomatic disease of 25 percentage points at week 20 after second-dose vaccination for both BNT162b2 and ChAdOx1 nCov-19 in a clinically extremely vulnerable group, which comprised patients with a range of different medical conditions, including trisomy 21, obesity, post-splenectomy, and cancer.27, 28 Our evaluation had the advantage of being done at the population level, reducing the risk of sampling error, and included larger numbers of patients than any previously published analysis on cancer and COVID-19,29 enabling a more granular cancer subgroup evaluation.
There are some limitations to this analysis. First, we only included patients recorded as having cancer up to April 30, 2021, excluding those who were diagnosed more recently. This restriction is likely to have resulted in underestimation of the reduction in vaccine effectiveness in the cancer cohort, as those who were recently diagnosed were more likely to have been receiving active treatment but will not have been counted among the positive SARS-CoV-2 test results of the cancer cohort. The effect might be additionally compounded by the older median age of the cancer cohort versus the control population; we found that older patients might have had fewer social contacts and therefore fewer potential transmission events. Second, we note that the reduced vaccine effectiveness with radiotherapy might have been driven by concurrent systemic cytotoxic treatment. Third, we are not able to exclude the possibility that the control population might display differences in behaviour compared with patients with cancer. Specifically, there might have been differences in attendance rates for confirmatory PCR following a positive lateral flow test, which might have been exacerbated by patients with cancer being monitored more closely, having tests offered more frequently, and being able to access care more readily. Some of the aforementioned behavioural differences could alter the denominator in test-negative case-control analyses and make it more difficult to make highly certain population inferences. Fourth, we have not corrected our analyses for causes of death other than COVID-19, partly due to the challenges of identifying whether cause of death was due to COVID-19 or associated with COVID-19. Fifth, our analysis comprised patients who had received two doses of COVID-19 vaccine and patients with cancer in England are now routinely offered a third or fourth vaccine booster dose. Sixth, time-to-event analyses were not in the data analysis plan because breakthrough infections occur in waves and vaccination was implemented during several months by age groups. Finally, our analysis also pre-dates the most recent wave of SARS-CoV-2 infection with the omicron variant (B.1.1.529); further follow-up is required to determine whether the same differences in vaccine effectiveness are present between controls and patients with cancer—whether our study is generalisable—in this new situation, although we envisage that findings would be similar.
To conclude, we found that individuals with cancer have demonstrable, albeit impaired, overall vaccine effectiveness against breakthrough infections with SARS-CoV-2. Vaccine effectiveness for those with cancer waned more rapidly than for the control population; this effect was more pronounced in those with haematological malignancies. Put into the wider context of the ongoing emergence of highly transmissible COVID-19 strains, such as omicron, our findings support the global prioritisation and evaluation of vaccination booster types and programmes for people with cancer, including analyses on the impact of different treatments. Patients with cancer should also be encouraged to use non-pharmacological strategies, such as behavioural modifications or personal protective equipment, to prevent transmission when community rates are high; the general population should also be conscious about getting tested before being in contact with high-risk individuals. We have identified groups at high risk of breakthrough infections who can be prioritised for research or pandemic response interventions, early community treatment, or pre-exposure prophylaxis programmes. Such measures will be crucially important as global health-care and cancer care systems adapt to living with COVID-19 as an endemic disease.
Data sharing
To comply with data privacy laws, data from this study, including individual participant data, are not available for sharing. Data field definition within the data dictionary is available by reasonable request to the corresponding author. The privacy statement for individuals performing COVID-19 testing provided by the Department of Health and Social Care is available at https://www.gov.uk/government/publications/phe-privacy-information/privacy-information.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank the Department of Health and Social Care Test and Trace, the UK Health Security Agency, the University of Oxford, the University of Birmingham, the University of Southampton, and Blood Cancer UK for providing funding and support for this study. The research was supported by the National Institute of Health Research Oxford Biomedical Research Centre. We would also like to acknowledge the work of the National Cancer Research Institute Consumer Forum for initiating this project. This work uses data provided by patients and collected by the NHS as part of their care and support. The views expressed in this Article are those of the authors and not necessarily those of the NHS, the National Institute of Health Research, or the Department of Health and Social Care. We thank our patients and the oncologists, physicians, and health-care staff working tirelessly on the frontlines of the COVID-19 pandemic.
Contributors
LYWL, TS, MCI, ML, MT, ART, HSM, LB, MB, SR, TWR, AP, GM, MM, MWF, TF, and PJ contributed to study design; LYWL, MCI, LB, MB, JC, SR, and MP contributed to data acquisition; LYWL, TS, MCI, LB, and MB accessed and verified the data; LYWL, TS, MCI, ML, MT, ART, HSM, YA-H, MB, LB, AB, ELC, JC, JJC, SK, QG, GI, CH-W, RJH, AJXL, PCL, JKHL, MP, JSP, JRP, VAP, AR, ASR, TMR, TWR, RLR, SR, MHT, IW, SW, TI, SML, GM, MM, AP, MWF, TF, and PJ interpreted the data; and LYWL, TS, MCI, ML, MT, ART, HSM, YA-H, MB, LB, AB, ELC, JC, JJC, SK, QG, GI, CH-W, RJH, AJXL, PCL, JKHL, MP, JSP, JRP, VAP, AR, ASR, TMR, TWR, RLR, SR, MHT, IW, SW, TI, SML, GM, MM, AP, MWF, TF, and PJ wrote the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
NCRI Consumer Forum:
Emma Kinloch, Emily Lam, Gillian Murphy, Malcolm Rhodes, and Kate Robinson
Supplementary Material
References
- 1.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LYW, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal-Garza C, Vaca-Cartagena BF, Becerril-Gaitan A, et al. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7:1242–1244. doi: 10.1001/jamaoncol.2021.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram SA, Caston NE, Andrews CJ, et al. Hesitancy and malignancy: vaccine hesitancy among individuals with cancer. Proc Am Soc Clin Oncol. 2021;39(suppl 28):148. (abstr 148). [Google Scholar]
- 8.Fendler A, Au L, Shepherd STC, et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2:1321–1337. doi: 10.1038/s43018-021-00275-9. [DOI] [PubMed] [Google Scholar]
- 9.Barrière J, Chamorey E, Adjtoutah Z, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madelon N, Lauper K, Breville G, Royo IS, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab954. published online Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer J, Le NS, Mattes D, et al. Evaluation of antibody responses to COVID-19 vaccines among solid tumor and hematologic patients. Cancers (Basel) 2021;13 doi: 10.3390/cancers13174312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JK, Zhang T, Wang AZ, Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14:38. doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaunak N, Nijjar R, Polwart C, Enting D. Clinician frequently asked questions (FAQs) and guidance on COVID-19 vaccine for patients receiving systemic anti-cancer therapy. December, 2021. https://www.ukchemotherapyboard.org/_files/ugd/638ee8_0d39e832a9354f1faaf81f7f302598e6.pdf
- 15.National Comprehensive Cancer Network Recommendations of the National Comprehensive Cancer Network (NCCN) Advisory Committee on COVID-19 vaccination and pre-exposure prophylaxis. 2022. https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v2-0.pdf?sfvrsn=b483da2b_2
- 16.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korompoki E, Gavriatopoulou M, Kontoyiannis DP. COVID-19 vaccines in patients with cancer—a welcome addition, but there is need for optimization. JAMA Oncol. 2021;7:1113–1114. doi: 10.1001/jamaoncol.2021.1218. [DOI] [PubMed] [Google Scholar]
- 19.Kuderer NM, Hill JA, Carpenter PA, Lyman GH. Challenges and opportunities for COVID-19 vaccines in patients with cancer. Cancer Invest. 2021;39:205–213. doi: 10.1080/07357907.2021.1885596. [DOI] [PubMed] [Google Scholar]
- 20.UK Health Security Agency Investigation of SARS-CoV-2 variants: technical briefings. Oct 1, 2021. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings
- 21.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on Covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Housing. Communities & Local Government English indices of deprivation 2019. Sept 26, 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 24.Wu JT-Y, La J, Branch-Elliman W, et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide veterans affairs study. JAMA Oncol. 2022;8:281–286. doi: 10.1001/jamaoncol.2021.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heudel P, Favier B, Solodky ML, et al. Survival and risk of COVID-19 after SARS-COV-2 vaccination in a series of 2391 cancer patients. Eur J Cancer. 2022;165:174–183. doi: 10.1016/j.ejca.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Public Health England PHE: duration of protection of COVID-19 vaccines against clinical disease, 9 September 2021. Sept 14, 2021. https://www.gov.uk/government/publications/phe-duration-of-protection-of-covid-19-vaccines-against-clinical-disease-9-september-2021
- 27.Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Service Who is at high risk from coronavirus (COVID-19) 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/who-is-at-high-risk-from-coronavirus/
- 29.Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 2021;5 doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To comply with data privacy laws, data from this study, including individual participant data, are not available for sharing. Data field definition within the data dictionary is available by reasonable request to the corresponding author. The privacy statement for individuals performing COVID-19 testing provided by the Department of Health and Social Care is available at https://www.gov.uk/government/publications/phe-privacy-information/privacy-information.