Abstract
Introduction:
Point-of-care (POC) tests for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are urgently needed to control the STI epidemic in order to offer patients an immediate diagnoses and accurate treatment before they leave a clinical encounter and thus reduce transmission and sequelae. Nucleic acid amplification tests (NAATs) have increased sensitivity and specificity, but very few POC assays can provide results of such tests within the usual time of the patient visit.
Areas covered:
This review describes the technology and performance characteristics of the binx health io® [Boston, MA] (binx io) CT/NG assay, a new rapid molecular POC assay. The assay is compared to other available molecular POC tests. We also describe the importance of time to results and assay performance for this POC assay.
Expert opinion:
The binx io CT/NG assay offers the ability to incorporate the use of POC tests to identify and immediately treat chlamydia and gonococcal infections into the clinical visit, which will provide improved outcomes for patients. Additional implementation studies are needed to optimize the adoption of this new test.
Keywords: Chlamydia trachomatis, Molecular Diagnostics, Neisseria gonorrhoeae, Point of Care testing, Sexually Transmitted Diseases, binx health
1. Introduction
Chlamydia trachomatis and Neisseria gonorrhoeae are sexually transmitted infections (STIs), which comprise the two most commonly reported notifiable infectious diseases in the United Sates (US) with steadily increasing rates according to the US Centers for Disease Control and Prevention (CDC) [1]. The World Health Organization (WHO) estimates that these two pathogens account for 127 and 87 million cases, respectively, per year worldwide [2,3]. This translates into nearly 600,000 new cases per day, which strongly supports the need for a paradigm shift in STI control. Control efforts range from symptoms-based epidemiologic treatment with no diagnostic test support in resource-constrained settings to laboratory-based screening of symptomatic and asymptomatic persons using molecular technologies. It is important to remember that resource constraints are not exclusively found in low- and middle-income countries (LMIC) but may reflect resource distribution and geographic limitations in any setting. Currently, as nations are focusing exceptional resources toward the control of the COVID-19 pandemic, in many settings, STI services are available only to symptomatic patients, and these patients are often managed syndromically based on regional epidemiology due to supply and staff shortages for STI testing.
Epidemiologic, or syndromic, management of treatable STI is the only available standard of care in many settings across the globe. Due to common symptoms shared by several pathogens, over-treatment is frequent in the attempt to cure any pathogen that might be present [4]. For example, women with discharge are most commonly treated for chlamydia, gonorrhea, and trichomonas since any of these infections can cause cervical or vaginal discharge. This approach may be associated with increasing potential for antimicrobial-resistant gonococcal strains [5]. Furthermore, by definition, this management strategy fails to treat any asymptomatic infections since people without symptoms are not evaluated. Estimates of the frequency of asymptomatic infection range from 30% to 55% and 20% to 40% in women and men [6,7], respectively. Therefore, syndromic management is estimated to treat fewer than 30% of cases worldwide [4]. For many decades, the WHO has been calling for improved diagnostic solutions, which has resulted in the development of several point-of-care (POC) tests for chlamydia. However, these tests are, for the most part, antigen detection assays that rely on lateral flow immunochemistry (LFI) and have varied but generally unacceptably poor performance [4,8,9].
Where resources to support laboratory medicine exist, there are many laboratory technologies for the detection of chlamydia and gonorrhea including microscopy, culture and antigen-based assays. The consensus is that molecular tests, in particular nucleic acid amplification tests (NAAT), offer substantial advantages over these other assays. This is due in large part to lower limits of detection (LOD); the ability to use a single specimen to test for both pathogens; the ability to detect organisms that are not easy to cultivate, such as chlamydia; and the ability to collect minimally invasive samples that may have lower organism load [10]. Numerous laboratory-based molecular assays have been in use for over two decades in high resource settings. These solutions include fully automated, high-throughput solutions [11–15],; smaller volume semi-automated bench-top instruments [16–19]; and, instrument agnostic platforms with semi-manual nucleic acid extraction and amplicon detection processes [20]. However, these solutions still require transport to a testing laboratory, time to run assays and transmittal of reports. Therefore, a delay of anywhere from 1–7 days may be seen between the time of sample collection and result availability. This leads to delayed time to treatment or often complete loss to follow-up (LTFU). Either of these outcomes may increase risk of i) potential transmission events, ii) pelvic inflammatory disease (PID) in women [21], iii) adverse outcomes during pregnancy [22], iv) tubal factor infertility [23,24] and/or v) transmission or acquisition of HIV [5,25]. Thus, rapid molecular assays, especially those that can be utilized in near-patient or POC settings are clearly needed. In this review, the characteristics of the binx health [Boston, MA, formerly Atlas Genetics (Trowbridge, UK,) until October 2018] io® (binx io) CT/NG assay, a new rapid molecular POC assay, are described and compared to other molecular POC assays that are currently available.
2. Assay technology
The binx io assay is a cartridge-based system that provides a sample to result solution for the detection of chlamydial and gonococcal infections in men and women with or without symptoms [Figure 1]. Vaginal swabs or male urine specimens are placed in the kit-specific transport tubes containing lysis buffer and stored at room temperature for up to 24 hours or refrigerated for up to 7 days prior to testing. Samples are transferred to test cartridges using a fixed-volume transept. The sample and cartridge barcodes are scanned into the instrument, and the user confirms the desired test and presses ‘start.’ Following sample collection, all steps until starting the instrument take less than 1 min in total. From this point forward, there is no manual intervention [26]. The processes that occur in the cartridge are illustrated in Figure 2. DNA extraction occurs on an adsorbent matrix, which is then washed to remove potential interfering substances or amplification inhibitors. Microfluidic pressure is used to move the sample through the chambers of the cartridge, which rehydrates reagents in the process, and results in amplification of target sequences using PCR. Amplified product in the detection chamber is bound to labeled probes and the double stranded DNA is enzymatically cleaved to release ferrocene-labeled mononucleotides, which generate differential pulse voltammetry [Figure 3]. Due to the small mass of the mononucleotide, the ferrocene-labeled moieties are concentrated on the detection probe. Using different ferrocene derivatives allows multiplexing due to the variation in redox potentials that allows specific detection of signal [26,27].
Figure 1.

The process of performing the binx io test from left to right: patient or clinician collects a sample, the sample is added, using a fixed volume transept, to the cartridge that is then placed in the io instrument, after ~30 min results of positive or negative are available.
Figure 2.
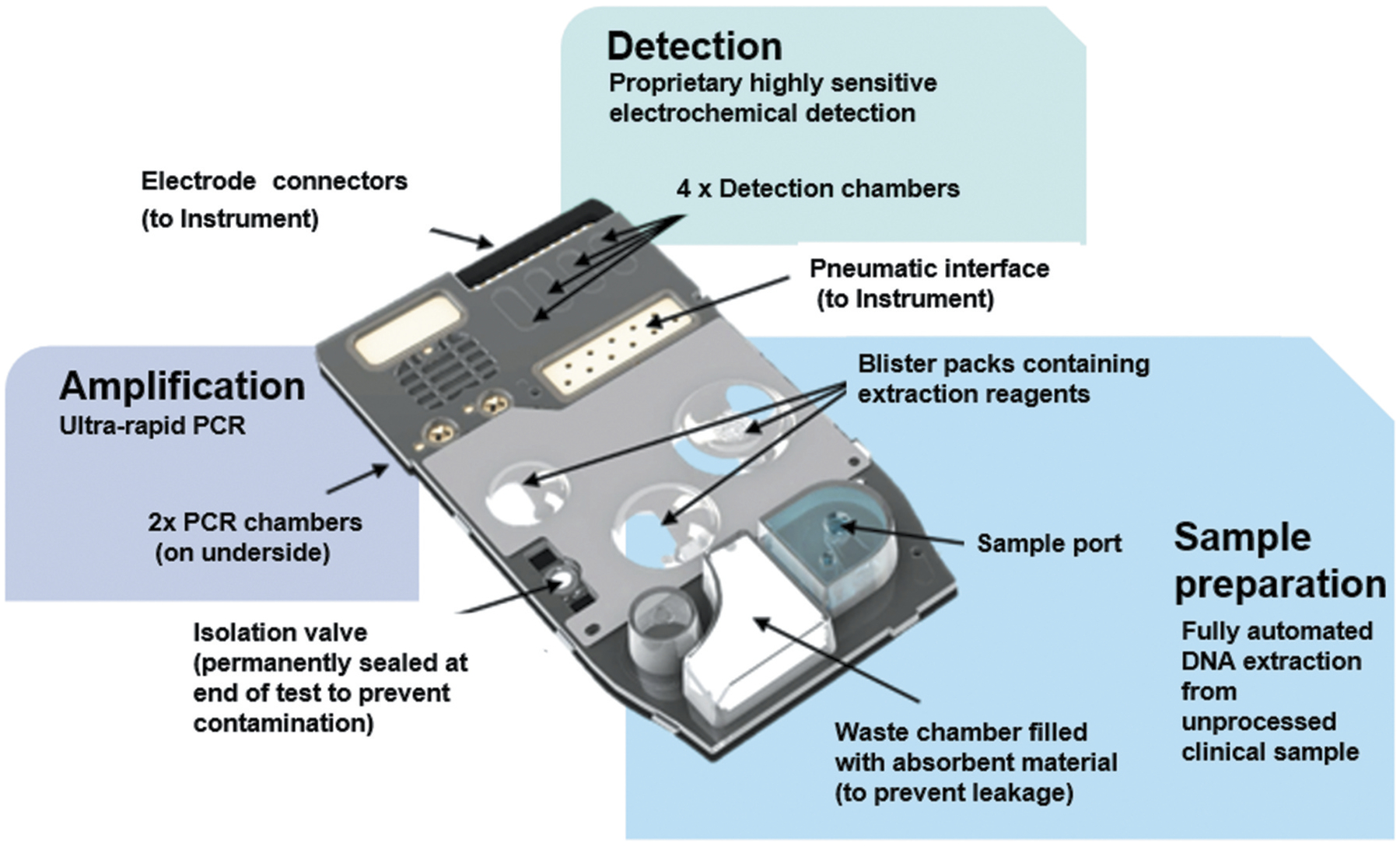
The chambers for sample preparation, amplification and detection inside the cartridge and the placement of reagents and features of the cartridge are shown in detail.
Figure 3.
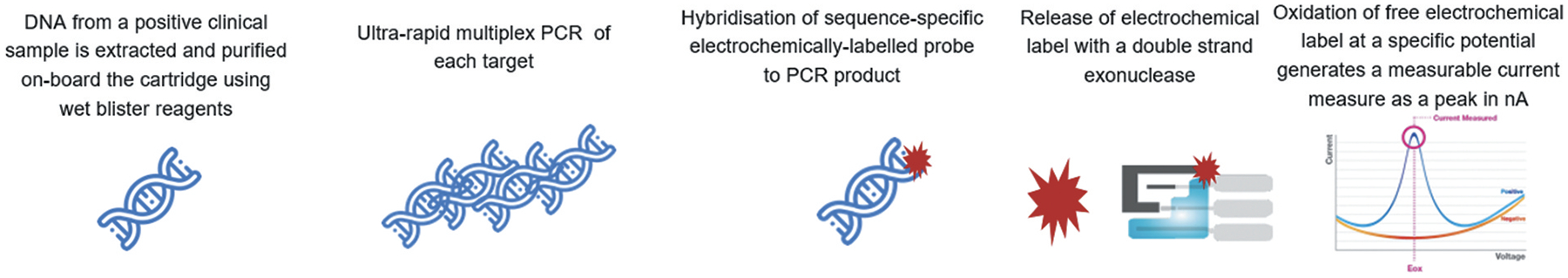
The chemistry of electrochemical detection utilized by the io system are shown.
The binx io assay, originally called the Atlas Genetics CT/NG assay, was first developed as a chlamydia stand-alone test and then adapted to include detection of N. gonorrhoeae DNA. Detection of C. trachomatis DNA relies on a single chromosomal gene sequence for primer binding and a sequence interior to the primers for the probe. The target region covers both the traditional fifteen serovars as well as the Swedish variant [28], which has a plasmid mutation that impacts some older diagnostic assays. The assay utilizes two chromosomal gene targets as primers for the gonorrhea assay. Both sets of primers are required to generate positive signals in order for a sample to be classified by the instrument software as positive for gonorrhea. This scheme is intended to be self-confirming and avoid the potential for false positive results that arise from closely related Neisseria, and other, species. The assay contains an internal control sequence the must amplify to demonstrate that negative results are not due to inhibitory substances. The assay does not include a sample adequacy control that determines whether human DNA is in the specimen.
The results appear on the instrument screen and can be linked to electronic medical records that use compatible communication systems. The system can operate on 110–220 V power supplies and requires no external software (i.e., it can be run without being attached to an external computer). Training for new users requires approximately 5 min, and the recommended maintenance entails cleaning the exterior of the instrument on a monthly basis.
3. Cost-effectiveness and workflow
Cost-effectiveness of POC testing has been modeled from a public health perspective and from a patient cost perspective. In modeling prevention of new cases and of sequelae, including PID, Rönn and colleagues predicted that a chlamydia POC test with sensitivity 10% lower than a lab-based assay might actually reduce the prevalence of chlamydia by 5% and cases of PID by >5,000 when the LTFU is ≥20% and the proportion of infections treated immediately (i.e., patients wait for the result while in clinic) is ≥60% [21]. Furthermore, studies in the United Kingdom (UK) have estimated that if the cost to run the test is approximately 25 USD (£20), the test could be cost savings due to the increase in the proportion of people tested [29]. In both models, these estimates were strongly dependent on the LTFU rate under the current laboratory-based testing paradigm and the proportion of people willing to wait for their POC test results to facilitate rapid and accurate treatment. Widdice et al. demonstrated that among adolescents studied in the Midwestern US, 61% were willing to wait to be treated, expressing hypothetical willingness to wait up to 20 min, while 26% were willing to wait up to 40 min for results to be treated before leaving clinic [30]. In a study performed in a real-world clinical setting in the UK, when using a 90- min test (median time to results following completion of other clinical activities was 46 min), only 21.4% of patients actually waited for their results prior to leaving clinic. However, since results were available via SMS, time to treatment was reduced, on average, to 2 days [31]. In contrast, when using the binx io system in a US university student health center, the median time to results following the completion of clinic visits was under 11 min and 88/106 (83%) of students were willing to wait for their result based on the actual time remaining, despite their class schedules and other time constraints [32]. Thus, the binx io test may be expected to have high potential for being a cost-savings tool for controlling STIs given its performance in clinical settings.
The true cost ramifications associated with the binx io assay are unknown as the pricing for the instrument and the cost of cartridges have not yet been released. However, the efficiency of the assay from a workflow perspective can provide context within which to evaluate this platform. The assay comes with a collection device, a fixed-volume transept and the cartridge. In the future, when other binx io assays may become available, the only change will be the analyte-specific cartridge, which will increase test menu capacity with very little, or no training. The cartridge does currently require cold storage before use and if samples are stored, which is not the intention with a POC test, they are stable at 2–8°C for up to 7 days. The instrument itself is small, requiring no more than 1 sq. ft. (< 900 cm2) of bench space. During clinical studies, the instruments proved to be robust, as some were moved between testing sites with no mechanical problem arising from the transportation. Therefore, very limited costs related to service requirements should be expected, but may need to be studied.
4. Assay performance
Another parameter of the potential impact of an assay is the sensitivity of the assay under consideration. In some hypothetical models, the importance of the same day test and treat, which is possible when using a POC test, suggested that even assays with limited sensitivity (as low as 65%) could result in a greater proportion of infections being treated than when using a highly sensitive lab-based NAAT due to LTFU [33,34]. However, for measureable population level impact, higher sensitivity is desirable [21]. Although the binx CT/NG assay is not yet being widely marketed, performance data are available from developmental and research studies and from the US clinical study.
4.1. Analytical performance
The binx io assay was originally developed as a chlamydia only (CT-only) test due to the limited prevalence of N. gonorrhoeae in the UK and Europe. Analytical data for the CT-only assay utilized dilutions of C. trachomatis organisms, which demonstrated an LOD of 25 organisms in transport medium introduced into the cartridge [26]. In this early evaluation, the CT-only assay using residual vaginal swab samples that had previously been tested demonstrated 98.0% positive agreement and 98.1% negative agreement with the BD ProbeTec ET system (BD Diagnostics, Sparks, MD) [26].
Further analytical work was performed for the CT/NG assay in preparation for the submission for review by the US Food and Drug Administration (FDA) and is described in the instructions for use package insert. [Available from authors upon request] For chlamydia, the CT/NG assay was able to reach an LOD of ≤ 6 infection forming units (IFU)/mL in vaginal matrix and ≤ 7 IFU/mL in male urine. Inclusivity of all 15 traditional serovars as well as the Swedish Variant was confirmed in both vaginal matrix and male urine. For gonorrhea, the LOD in colony-forming units (CFU)/mL was determined to be ≤ 3CFU/mL for both vaginal matrix and male urine. Inclusivity for gonorrhea was assessed using a panel of 30 gonococcal isolates. Exclusivity, or lack of reaction with closely related species or organisms commonly found in the sample types utilized for the CT/NG assay, was demonstrated using a panel of 62 species (genomic sequences or whole organisms depending on availability). No cross-reactivity was observed in these experiments.
4.2. Clinical performance
In clinical studies of the CT-only assay, the performance using vaginal swabs was quite good with sensitivity ranging from 92.9 to 96.1% and specificity from 97.7 to 98.8% [30,35]. The largest evaluation to date of the CT/NG assay was the multi-site clinical study designed to obtain data for FDA review. In this study, participants were enrolled at STD, obstetrics and gynecology (OB/GYN) and family planning clinics throughout the US [35]. During the study, almost 95% of testing was performed by clinical staff with no laboratory training. binx io results were compared to a composite infection standard (CIS) using three commercially available, laboratory-based chlamydia/gonorrhea molecular diagnostic assays where infection status was defined based on at least 2/3 comparator assay results. Thus if ≥2 results were positive, the participant was classified as infected while if ≥2 results were negative, the participant was categorized as uninfected. For the single instance where one result was positive, one was negative and the third was invalid, the participant data were excluded from analysis. Samples from 1,523 women and 922 men were evaluated with overall chlamydia positivity (based on the CIS) ranging from 8.5–13.0% for women and men, respectively, and gonorrhea positivity of 3.0–8.0% for women and men, respectively. One sample tested on the binx platform had a final result of invalid due to inhibition of amplification. The sensitivity and specificity estimates are shown in Table 1.
Table 1.
Performance of the binx health io® CT and CT/NG Assays.
| Assay | Population | N (% positive) | Sensitivity n/N (95% CI) | Specificity n/N (95% CI) | Comparator Assay/s | Reference |
|---|---|---|---|---|---|---|
| CT-only | Women attending Reproductive/Sexual Health Clinics | 709 (7.2%) | 49/51 96.1% (86.5–99.5%) |
643/658 97.7% (96.3–98.7%) |
BD Chlamydia Qx | Harding-Esch [35] |
| CT-only | Women attending Adolescent Health Clinic | 284 (9.9%) | 26/28 92.9& (83.3–100%) |
253/256 98.8% (97.5–100%) |
Hologic Aptima Combo 2 (AC2) | Widdice [31]. |
| CT/NG (Chlamydia Performance) | Women attending STI, OB/GYN and Family Planning Clinics | 1523 (8.5%) | 124/129 96.1% (91.2–98.3%) |
1381/1394 99.1% (98.4–99.5%) |
BD Chlamydia Qx/BD Gonorrhea Qx Hologic AC2 Roche cobas4800 CT/NG | Van Der Pol [36] |
| CT/NG (Gonorrhea Performance) | Women attending STI, OB/GYN and Family Planning Clinics | 1523 (3.0%) | 45/45 100% (92.1–100%) |
1476/1478 99.9% (99.5–100%) |
BD Chlamydia Qx/BD Gonorrhea Qx Hologic AC2 Roche cobas4800 CT/NG | Van Der Pol [36] |
| CT/NG (Chlamydia Performance) | Men attending STI and Family Planning Clinics | 922 (13.0%) | 111/120 92.5% (86.4–96.0%) |
796/802 99.3% (98.4–99.7%) |
BD Chlamydia Qx/BD Gonorrhea Qx Hologic AC2 Roche cobas4800 CT/NG | Van Der Pol [36] |
| CT/NG (Gonorrhea Performance) | Men attending STI and Family Planning Clinics | 922 (8.0%) | 72/74 97.3% (90.7–99.3%) |
848/848 100% (99.5–100%) |
BD Chlamydia Qx/BD Gonorrhea Qx Hologic AC2 Roche cobas4800 CT/NG | Van Der Pol [36] |
5. Alternative molecular POC diagnostic solutions
Despite the long-recognized need for molecular POC assays for the detection of STIs, there are currently only two assays, binx io and Cepheid Xpert (Cepheid, Sunnyvale CA), with both CE-IVD and FDA-approved status for use with male and female samples. The Xpert CT/NG assay is easy to use although currently only approved in the US for use by laboratorians. This assay has successfully been used by clinical staff in studies conducted in Australia [36]. The Xpert platform has the advantage of an additional test that is available for detection of Trichomonas vaginalis (TV) DNA.
The time to result of the Xpert assay is approximately 90 min, and this may represents an unacceptable wait time, particularly in urban settings, for receiving results during the initial visit. In a study of the logistics of implementation of the Xpert system in a health clinic setting, while most patients were not willing to wait for result, the overall time to treatment was reduced to a median of 2 days from a median of 8 days [37]. Thus, the Xpert offers great public health benefits associated with improved clinical management, and we can expect even greater benefits to be observed following implementation of the binx io system because of the shorter time to results. Two other molecular POC assays are available with either CE-IVD clearance [Table 2] or are currently being evaluated by the FDA. The Randox STI Array, which runs on a Bosch Healthcare Solutions Vivalytic Analyzer has CE-IVD status and tests for a panel of pathogens including C. trachomatis, N. gonorrhoeae, T. vaginalis, Mycoplasma genitalium (MG), Ureaplasma urealyticum (UU), Mycoplasma hominis (MH), Herpes Simplex Virus (HSV) types 1 and 2, Haemophilus ducreyi (HD), Treponema pallidum (TP) [38,39]. Unfortunately, the members of this panel (the first four are recognized sexually transmitted pathogens, the next two (UU and MH) are likely commensal organisms and the last three are associated with genital ulcer disease) may be too diverse for use with a single sample such as a vaginal swab or urine. Finally, the Visby Medical Sexual Health Test [40,41] can detect chlamydia, gonorrhea and trichomonas in a multiplex assay that is simple and rapid with inexpensive components. The assay does not require an instrument to run and provides results in >30 min. Data regarding the performance of this test were generated using vaginal swabs and are currently under evaluation at the FDA; no male testing is offered at this point [40,41].
Table 2.
Attributes of Currently Available Point-of-Care Molecular Assays.
| Assay | Availability | Targets | Sensitivity | Time to Results | Ease of Use | Ability to link to electronic medical record |
|---|---|---|---|---|---|---|
| binx io | CE-IVD mark and FDA approval | CT/NG | Very Good | 30 min | Non-laboratorian | Yes |
| Cepheid GeneXpert | CE-IVD mark and FDA approval | CT/NG with separate TV available | Excellent | 90 min | Non-laboratorian* | Yes |
| Randox run on Bosch Healthcare Vivalytic | CE-IVD | CT/NG/TV/MG/UU/MH/HD/TP/HSV | Unknown | 30 min | Laboratorian | Unknown |
| Visby (formerly Click) | In FDA clearance process | CT/NG/TV Female specimen only | Very Good | 30 min | Non-laboratorian - may be appropriate for home-use eventually | Yes |
This test does not have US FDA clearance for use by non-laboratorians
6. Expert opinion
The binx io CT/NG assay is the first molecular POC assay with sufficiently short running time to have the potential for significantly impacting STI control practice. The test is simple enough that it was performed almost exclusively by non-laboratorians, including nurses and administrative staff, during the multi-site US clinical trial. The assay has recently received CLIA-waived status, which provides US regulatory approval for use by non-laboratorians. By adapting to a sample-first specimen collection process where the testing can begin immediately following registration, the results may, in many settings, be available to the health care provider prior to patient interactions thus facilitating a discussion about the meaning of the results, as well as making accurate treatment decisions possible before the patients leave the clinic. Managing use of antimicrobials rather than relying on epidemiologic factors and clinical observations is an important strategy in antimicrobial stewardship efforts.
Over the last two decades, laboratory-based NAATs have substantially improved our ability to detect STIs, and this technology is now being successfully adapted for use at the point of care or in near-patient settings. For such POC tests to be truly effective and result in improved accuracy of treatment, the time to results needs to be rapid and the qualifications and training for testing staff needs to be minimal. Cost effectiveness analyses and modeling can be useful in directing POC test adoption, but these studies rely heavily on the assumptions utilized, which include sensitivity, time to results, LTFU rates, time to treatment, and willingness to wait for results. It is clear that studies of adoption and implementation are needed in order to help define the optimal clinic flow and POC testing processes in a variety of settings [31,42,43]. Value-based decision making, where all stakeholders have input, is helpful in adoption of POC tests and should be carefully considered prior to implementation [44,45]. Adoption of POC tests in LMIC settings will require special consideration, since cost, infrastructure requirements (e.g., refrigeration, electricity. etc.), and performance complexity are important barriers to overcome. In some settings, adoption of less sensitive and cheaper tests must be considered as a viable alternative [46,47]. In any setting where the binx io or any POC tests are adopted, we strongly recommend routine testing of quality controls (QC) samples, which can consist of previously positive and negative samples or purchased QC materials. External quality assurance and operator proficiency testing should be performed in accordance with local or national regulations governing medical testing.
Next generation POC assays will likely be multiplexed (e.g., binx health is currently developing a CT/NG/TV/MG assay and developed a SARS-COV2 PCR-CRSPR-based assay in 2020, which received FDA Emergency Use Authorization) and will likely begin to have more rapid time to results. While the SARS-CoV2 pandemic has been disastrous on a global scale, a positive outcome may be the enormous support for rapid development of POC technologies. These technologies may very well be applied to the problem of STI diagnostics more quickly than might have happened in the absence of the pandemic and thus we may realize an unanticipated benefit. New assays must continue to be highly sensitive and specific and focus on the ease of use and biosafety features that will support testing in non-clinical settings. The potential use cases could include testing (not just specimen collection) at schools, health fairs, retail outlets and ultimately in the privacy of one’s own home.
Areas of concern remain that may limit adoption of POC testing. In the US, POC tests are reimbursed by private insurance at rates lower than laboratory-based diagnostics. As a result, many sites, particularly public health settings, may not be able to afford POC tests, and many individuals may not be able to pay for these tests unless the costs are quite low [18]. Other issues that need to be resolved in support of implementation of POC tests are data management and surveillance issues. Chlamydial and gonorrhea are notifiable diseases in the US and much of Europe so linking test results to reporting databases will be important. Often, POC test results do not easily link to electronic medical records and the legal requirement for reporting infections for chlamydia and gonorrhea for surveillance may not be easily accomplished unless the POC test is performed in a healthcare setting. While these concerns are meaningful, tests like the binx io CT/NG assay may play a role in overcoming these issues. The availability of a truly rapid, simple test will increase interest in finding solutions to barriers and moving the field of STI control forward.
Over the coming years, we will see explosive increases in the application of bio-sensing technologies to the detection of infectious pathogens, and this will include agents responsible for STIs and detection of antimicrobial resistance genetic markers. The ability to multiplex will continue to expand, but we need rigorous clinical utility studies that will determine the appropriate members of testing panels, and we need well-designed cost-impact studies that consider the cost of testing in the context of negative outcomes (in addition to infection) averted. Both of these types of studies are expensive and time consuming, and it is unlikely that assay developers/manufacturers will have the resources to conduct such studies for each new product developed. Therefore, collaboration with public health and regulatory agencies will be critical to achieving this goal. Costs should continue to reduce over time, as there will be less expensive components, newer technologies that requires less expensive reagents, and healthy market competition with multiple commercially available choices. The other major advance that we can expect is that the transition from laboratory to near-patient testing will continue logically to self-testing in the privacy of one’s home. Like home pregnancy tests and home HIV tests, the technology is within our grasp and what remains is to make the assays instrument-free, user friendly and cost rational. Obtaining approval for home testing will not be without challenges, but these have been faced in other areas (e.g. self-glucose monitoring), and it can be done in the field of STI control if we have the political will to see it happen. Ultimately, self-testing may have hurdles but has the potential to be an enormous factor in stigma reduction efforts that will benefit all people who will need sexual health services over the course of their lifetime. While laboratory testing will not be eliminated, the available options should allow control programs to vastly expand their reach and finally provide services, including treatment for people who have performed self-tests, to the entire population.
Article Highlights.
The binx health io CT/NG assay is a highly accurate molecular assay that compares well to laboratory-based molecular diagnostics.
The assay can be performed using male urine or female vaginal swabs.
The testing steps (transfer to the cartridge, and inserting the cartridge and running the assay) can be performed by health-care providers who have no laboratory training.
The results are available within 30 min, and the adoption of a sample-first clinic flow facilitates the availability of results before the conclusion of the clinic visit.
The assay supports test and treat in a single visit that is important for accurate treatment.
The future promises more tests on the market with options to detect a greater number of pathogens and/or antimicrobial resistance genetic marker
Funding
This paper was not funded.
Declaration of interest
B. Van Der Pol receives grant funding from: Abbott Molecular, BD Diagnostics, binx health, BioFire Molecular, Cepheid, Hologic, Rheonix, Roche Molecular, and SpeeDx. CA Gaydos receives grant funding from: Hologic, Cepheid, Becton Dickenson, SpeeDx, and Abbott. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 2.World Health Organization. Report on Global Sexually Transmitted Infection Surveillance 2018. 2018.
- 3.Rowley J, Hoorn SV, Korenromp E, et al. Chlamydia, Gonorrhoea, Trichomoniasis and Syphilis: global Prevalence and Incidence Estimates, 2016. Geneva: Bulletin of the World Health Organization; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wi TE, Ndowa FJ, Ferreyra C, et al. , Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J Int AIDS Soc. 2019;22(S6): e25343. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides an overview of the potential impact of POC tests in resource constrained settings.
- 5.Chesson HW, Kirkcaldy RD, Gift TL, et al. An illustration of the potential health and economic benefits of combating antibiotic-resistant gonorrhea. Sex Transm Dis. 2018;45(4):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaMontagne DS, Fine DN, Marrazzo JM, et al. Chlamydia trachomatis infection in asymptomatic men. Am J Prev Med. 2003;24 (1):36–42. [DOI] [PubMed] [Google Scholar]
- 7.Lewis J, PJ W. Estimating local chlamydia incidence and prevalence using surveillance data. Epidemiol. 2017;28(4):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst de Cortina S, Cc B, Joseph DD, et al. A systematic review of point of care testing for chlamydia trachomatis, neisseria gonorrhoeae, and trichomonas vaginalis. Infect Dis Obstet Gynecol. 2016;17:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides a detailed overview of rapid, POC assays for chlamydia/gonorrhea including non-molecular methodologies.
- 9.Gaydos C, Hardick J. Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Rev Anti Infect Ther. 2014;12(6):657–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papp J, Schachter J, Gaydos C, et al. Recommendations for the laboratory-based detection of chlamydia trachomatis and neisseria gonorrhoeae — 2014. MMWR. 2014;63(RR–2):1–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Cherkaoui A, Renzi G, Mombelli M, et al. Comparison of analytical performances of the roche cobas 6800 CT/NG assay with the abbott m2000 real time CT/NG assay for detecting chlamydia trachomatis and neisseria gonorrhoeae. J Med Microbiol. 2019;68 (2):197–200. [DOI] [PubMed] [Google Scholar]
- 12.Unemo M, Hansen M, Hadad R, et al. Sensitivity, specificity, inclusivity and exclusivity of the updated aptima combo 2 assay, which provides detection coverage of the new diagnostic-escape chlamydia trachomatis variants. BMC Infect Dis. 2020;20(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Der Pol B, Fife K, Taylor SN, et al. Evaluation of the performance of the cobas CT/NG test for use on the cobas 6800/8800 systems for detection of chlamydia trachomatis and neisseria gonorrhoeae in male and female urogenital samples. J Clin Microbiol. 2019;57(4): e01996–01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Pol B, Hook EW 3rd, Williams JA, et al. Performance of the BD CTQX and GCQX amplified assays on the BD viper LT compared with the BD viper XTR system. Sex Transm Dis. 2015;42(9):521–523. [DOI] [PubMed] [Google Scholar]
- 15.Nye MB, Osiecki J, Lewinski M, et al. Detection of chlamydia trachomatis and neisseria gonorrhoeae with the cobas CT/NG v2.0 test: performance compared with the BD probetec CT Q(X) and GC Q(X) amplified DNA and aptima AC2 assays. Sex Transm Infect. 2019;95 (2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Der Pol B, Torres-Chavolla E, Kodsi S, et al. Clinical Performance of the BD CTGCTV2 assay for the BD max™ system for detection of chlamydia trachomatis, neisseria gonorrhoeae and trichomonas vaginalis infections. Sex Transm Dis. 2021; 48:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Der Pol B, Williams JA, Fuller D, et al. Combined testing for chlamydia, gonorrhea, and trichomonas by use of the BD MAX CT/GC/TV assay with genitourinary specimen types. J Clin Microbiol. 2017;55(1):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow CC, Morris SR, Little SJ, et al. Meta-analysis of the cepheid xpert® CT/NG assay for extragenital detection of chlamydia trachomatis (CT) and neisseria gonorrhoeae (NG) infections. Sex Health. 2019;16(4):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the cepheid CT/NG xpert rapid PCR test for detection of chlamydia trachomatis and neisseria gonorrhoeae. J Clin Microbiol. 2013;51 (6):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Salazar A, Espadafor B, Fuentes-López A, et al. Comparison between aptima assays (hologic) and the allplex STI essential assay (seegene) for the diagnosis of sexually transmitted infections. PloS One. 2019;14(9):e0222439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mm R, Na M, Tl G, et al. , Potential for point-of-care tests to reduce chlamydia-associated burden in the United States: a mathematical modeling analysis. Clin Infect Dis. 2020. ;70(9): 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides detailed mathematical modeling of the impact POC tests will have based on a variety of clinical assumptions.
- 22.Olson-Chen C, Balaram K, Hackney DN, et al. Chlamydia trachomatis and adverse pregnancy outcomes: meta-analysis of patients with and without infection. Matern Child Health J. 2018;22(6):812–821. [DOI] [PubMed] [Google Scholar]
- 23.Arustamyan K, Totoyan E, Karapetyan A, et al. The State of fallopian tubes in women with urogenital chlamydia and infertility. Georgian Med News. 2017;80:268–269. [PubMed] [Google Scholar]
- 24.Reekie J, Donovan B, Guy R, et al. Risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis. 2019;69(9):1621–1623. [DOI] [PubMed] [Google Scholar]
- 25.Buckner LR, Amedee AM, Albritton HL, et al. Chlamydia trachomatis infection of endocervical epithelial cells enhances early HIV transmission events. PLoS One. 2016;11(1):e0146663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dm P, Dp S, Holden J, et al. Evaluation of a novel electrochemical detection method for chlamydia trachomatis: application for point-of-care diagnostics. IEEE Trans Biomed Eng. 2010;58 (3):755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes in detail the chemistry utilized by the binx io system.
- 27.Trotter M, Borst N, Thewes R, et al. Electrochemical DNA sensing–principles, commercial systems, and applications. Biosens Bioelectron. 2020;154:112069. [DOI] [PubMed] [Google Scholar]
- 28..Niemi S, Hiltunen-Back E, Puolakkainen M. et al. Chlamydia trachomatis genotypes and the Swedish new variant among urogenital chlamydia trachomatis strains in Finland. Infect Dis Obstet Gynecol. 2011; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huntington SE, Burns RM, Harding-Esch E, et al. , Modelling-based evaluation of the costs, benefits and cost-effectiveness of multi-pathogen point-of-care tests for sexually transmitted infections in symptomatic genitourinary medicine clinic attendees. BMJ Open. 2018. ;8(9): e020394. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides udeful cost modeling for practices in the UK.
- 30.Widdice LE, Hsieh Y-H, Silver B, et al. Performance of the atlas rapid test for chlamydia trachomatis and women’s attitudes toward point-of-care testing. Sex Transm Dis. 2018;45(11):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Em H-E, Av N, Hegazi A, et al. , Impact of deploying multiple point-of-care tests with a ‘Sample First’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect. 2017. ;93(6): 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes real-world implementation of the Xpert CT/GC assay and the ability to treat patients during a clinical visit.
- 32.Gettinger J, Van WN, Daniels B, et al. , Patients are willing to wait for rapid sexually transmitted infection results in a University student health clinic. Sex Transm Dis. 2020. ;47(1): 67–69. [DOI] [PubMed] [Google Scholar]; • Describes real world experiences with willingness to wait for actual time remaining when using the binx io system.
- 33.Gift T, Pate M, Hook E, et al. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for hlamydia trachomatis. Sex Transm. Dis 1999;26 (4):232–240. [DOI] [PubMed] [Google Scholar]
- 34.Harding-Esch EM, Cousins EC, Chow S-L, et al. A 30-min Nucleic Acid amplification point-of-care test for genital Chlamydia trachomatis infection in women: a prospective. Multi-Center Study of Diagnostic Accuracy. ebiomed. 2018;28:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Der Pol B, Sn T, Mena L, et al. , Evaluation of the performance of a point-of-care test for chlamydia and gonorrhea. JAMA Network Open. 2020. ;3(5): e204819–e204819. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides data from the largest study to date comparing the binx io CT/NG assay to laboratory based diagnostics.
- 36.Lm C, RJ G, Sn T, et al. Molecular test for chlamydia and gonorrhoea used at point of care in remote primary healthcare settings: a diagnostic test evaluation. Sex Transm Infect. 2018;94(5):340–345. [DOI] [PubMed] [Google Scholar]
- 37.Wingrove I, McOwan A, Nwokolo N, et al. Diagnostics within the clinic to test for gonorrhoea and chlamydia reduces the time to treatment: a service evaluation. Sex Transm Infect. 2014;90(6):474. [DOI] [PubMed] [Google Scholar]
- 38.Meyer T, Buder S. The laboratory diagnosis of neisseria gonorrhoeae: current testing and future demands. Pathog. 2020;9(2):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mm M. The point-of-care diagnostic landscape for sexually transmitted infections (STIs).[cited 2020 Oct 20]. Available from: https://www.who.int/reproductivehealth/topics/rtis/Diagnostic-Landscape-for-STIs-2019.pdf.; • Provides a glimpse of assays in development.
- 40.Bristow CC, Wierzbicki M, Sarno M, et al. Performance of a single use rapid point-of-care pcr device for the detection of neisseria gonorrhoeae, chlamydia trachomatis and trichomonas vaginalis. Sex Trans Dis. 2020;47(S2):S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris SR, Bristow CC, Wierzbicki MR, et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of neisseria gonorrhoeae, chlamydia trachomatis, and trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2020;5:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Der Pol B. Making the most of point-of-care testing for sexually transmitted diseases. Clin Infec Dis. 2019;70(9):1824–1825. [DOI] [PubMed] [Google Scholar]
- 43.Fisk KM, Derouin A, Holm G, et al. Getting it right: the impact of point-of-care testing for gonorrhea and chlamydia in the urgent care setting. J Nurse Pract. 2020;16(5):388–393. [Google Scholar]
- 44.Korte BJ, Rompalo A, Manabe YC, et al. Overcoming challenges with the adoption of point-of-care testing: from Technology push and clinical needs to value propositions. Point Care. 2020;19(3):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Provides a framework for studies of adoption and implementation.
- 45.Pai NP, Vadnais C, Denkinger C, et al. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low-and middle-income Countries. PLoS Med. 2012;9(9):e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart J, Bukusi E, Celum C, et al. Sexually transmitted infections among African women: an opportunity for combination sexually transmitted infection/HIV prevention. AIDS. 2020;34 (5):651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]


