Abstract
We tested a novel colorimetric toxicity test, based on inhibition of β-galactosidase activity in the yeast Kluyveromyces marxianus, for sensitivity to a range of mycotoxins. A variety of trichothecene mycotoxins could be detected. The order of toxicity established with this bioassay was verrucarin A > roridin A > T-2 toxin > diacetoxyscirpenol > HT-2 toxin > acetyl T-2 toxin > neosolaniol > fusarenon X > T-2 triol > scirpentriol > nivalenol > deoxynivalenol > T-2 tetraol. The sensitivity of detection was high, with the most potent trichothecene tested, verrucarin A, having a 50% effective concentration (concentration of toxin causing 50% inhibition) of 2 ng/ml. Other mycotoxins (cyclopiazonic acid, fumonisin B1, ochratoxin A, patulin, sterigmatocystin, tenuazonic acid, and zearalenone) could not be detected at up to 10 μg/ml, nor could aflatoxins B1 and M1 be detected at concentrations up to 25 μg/ml. This test should be useful for trichothecene detection and for studies of relevant interactions—both between trichothecenes themselves and between trichothecenes and other food constituents.
Bioassays have become increasingly useful for mycotoxin detection (20, 21) as a precursor to chemical analysis. Bioassays provide a rapid means for screening samples and allow the analyst to make an informed decision when selecting a more detailed chemical analysis procedure (2). Kluyveromyces marxianus (GK1005) is particularly sensitive to the trichothecene mycotoxins (17). This yeast has been used in disk diffusion bioassays (15) and a conductimetric bioassay (4) for the detection of trichothecene mycotoxins.
We recently developed a colorimetric bioassay that uses the inhibition of expression of β-galactosidase as a toxicity indicator (5, 6). With a colorimetric substrate used for the β-galactosidase, toxicity is registered by the K. marxianus cultures remaining yellow, rather than turning blue-green, allowing both visual and spectrophotometric detection. Our objectives were (i) to evaluate this technique for various mycotoxins, (ii) to establish dose-response relationships for a group of trichothecene mycotoxins with a range of different substituents, and (iii) to substantiate the usefulness of this bioassay in mycotoxin detection and investigation.
MATERIALS AND METHODS
Organism and media.
K. marxianus GK1005 was obtained from the Ministry of Food and Fisheries, London, United Kingdom. The yeast was routinely maintained and grown on 1% (wt/vol) yeast extract, 1% (wt/vol) bacteriological peptone, and 2% (wt/vol) glucose (YPG), solidified when required with 2% (wt/vol) agar. Cultures for inoculation of the bioassay were prepared by adding a single colony from an agar plate to 50 ml of YPG-50 liquid medium in a 250-ml flask and incubating this mixture in a rotary incubator for 16 h at 35°C and 200 rpm. (YPG-50 medium contained 1% [wt/vol] yeast extract, 1% [wt/vol] bacteriological peptone, and 50 mM glucose.) For the bioassay procedure, YPG-50 was supplemented from a stock solution of polymyxin B sulfate (PMBS) (ICN Biomedicals, Ltd., Thame, Oxfordshire, United Kingdom) to give a final bioassay PMBS concentration of 15 μg/ml. Stock solutions of PMBS were prepared in water, filter-sterilized, and kept no more than 1 day.
Mycotoxin standards.
Mycotoxins (Sigma-Aldrich Chemical Company, Ltd., Poole, Dorset, United Kingdom) were diluted in spectroscopy-grade methanol at, typically, 0.1 mg/ml. Absolute concentrations were verified by UV absorbance. In the initial experiments, we used aflatoxin B1 (AFB1), aflatoxin M1, (AFM1), citrinin (CIT), cyclopiazonic acid (CPA), deoxynivalenol (DON), diacetoxyscirpenol (DAS), fumonisin B1 (FB1), ochratoxin A (OTA), patulin (PAT), roridin A (ROR), sterigmatocystin (STG), T-2 toxin (T-2), tenuazonic acid (TEN), verrucarin A (VER), and zearalenone (ZEA). Each mycotoxin standard was tested at final assay concentrations of 10 μg/ml to 0.1 ng/ml (serial 10-fold dilutions); a 25-μg/ml test concentration also was included for AFB1 and AFM1. For the trichothecene structure-activity study, we used acetyl T-2 (AcT-2), DON, DAS, fusarenon X (FUS), HT-2 toxin (HT-2), neosolaniol (NEO), nivalenol (NIV), ROR, scirpentriol (SCR), T-2 tetraol (TET), T-2 triol (TRI), T-2, VER, at final assay concentrations of 25 μg/ml to 0.1 ng/ml.
Assay procedure.
One hundred thirty-six microliters of PMBS-supplemented YPG-50 medium was added to the wells of a microtiter plate. Eight microliters of mycotoxin stock solution or methanol (control wells) was added, followed by 16 μl of yeast inoculum, to yield an initial cell density of 2 × 108 cells/ml. Blank wells contained 152 μl of medium and 8 μl of methanol. Plates were mixed, and cell density was determined; the plates were sealed with Mylar plate sealers (ICN Biomedicals, Ltd.) and incubated in a plate shaker (Wesbart Ltd., Billinghurst, West Sussex, United Kingdom) at 35°C for the duration of the assay. Cell density was monitored throughout the assay. When the control wells reached stationary phase (∼10 h, with an A560 of ca. 1.2), the cultures were assayed for β-galactosidase activity.
Determination of cell density.
Cell density was determined by measuring A560 with a Titertek Multiscan Plus Mk II microtiter plate reader (Labsystems, Ltd., Basingstoke, Hampshire, United Kingdom) connected to an Amstrad microcomputer and Titresoft 1.01 software (Labsystems). A560 was calibrated by direct hemocytometer counts, and 1 A560 unit corresponded to 1.1 × 109 cells/ml.
Determination of β-galactosidase activity.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (Calbiochem Novobiochem, Ltd., Beeston, Nottinghamshire, United Kingdom) was dissolved in dimethylformamide (DMF) at 100 mg/ml and stored in the dark at −20°C. This stock solution was used to prepare a working solution of 20 mg of X-Gal per ml in aqueous DMF (2 parts water to 3 parts DMF) immediately before each assay. Cells were permeabilized by the addition of 5 μl of 0.1% (wt/vol) sodium dodecyl sulfate and 3 μl of chloroform to each well. Eight microliters of the X-Gal working solution was then added to each well, and the plates were incubated at 35°C in the plate shaker for 20 min. Finally, the plates were read on the microtiter plate reader by using a test filter at 666 nm and a reference filter at 560 nm.
Construction and use of dose-response curves.
Dose-response curves were constructed for the inhibition of growth and for β-galactosidase activity. The percentage of inhibition of a given end point was determined by comparison with that of the methanol controls. For each toxin concentration, at least two replicate wells were used, and for the methanol controls, at least 12 replicates were used. Three parameters were calculated by using the dose-response curves: (i) the no-effect level (NEC), i.e. the highest concentration of toxin at which no inhibition was detected, (ii) the 50% effective concentration (EC50 [the concentration of toxin at which 50% inhibition was observed]), and (iii) the MIC (i.e., the lowest concentration of toxin at which 100% inhibition was detected).
RESULTS
Detection of mycotoxins by the colorimetric bioassay.
Initially we evaluated 14 mycotoxins known as natural contaminants of foods or feeds or previously reported to be toxic to K. marxianus (10, 11, 14). Of the 14 mycotoxins tested, only five trichothecenes could be detected by the colorimetric yeast bioassay: DON (25 μg/ml), DAS (1 μg/ml), ROR (1 μg/ml), T-2 (1 μg/ml), and VER (0.1 μg/ml). None of the nontrichothecene mycotoxins were detected, including CPA, FB1, OTA, PAT, STG, TEN, and ZEA, the latter at up to 10 μg/ml, and AFB1 and AFM1 at up to 25 μg/ml.
Structure-activity relationships among the trichothecene mycotoxins.
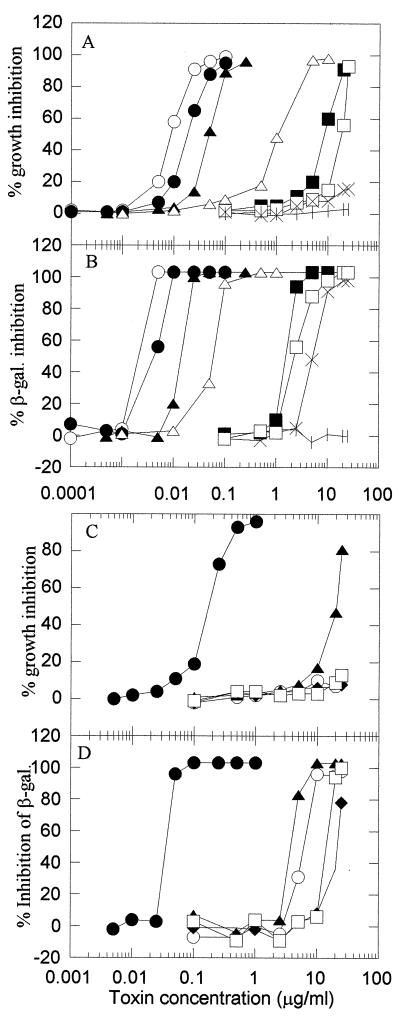
Thirteen mycotoxins were used to determine if structure-activity relationships existed within the trichothecene group of mycotoxins. Dose-response curves (Fig. 1) were used to estimate the NEC, EC50, and MIC of each trichothecene. The curves provide six different estimates of toxicity for each compound (Table 1). For the most potent toxins (VER, ROR, T-2, DAS, HT-2, and AcT-2), all six evaluations gave the same relative order of toxicity. For the less potent toxins, MIC and EC50s for inhibition of growth (Table 1) sometimes could not be determined, which made the exact order of toxicity impossible to establish on this basis. However, the β-galactosidase assay was more sensitive than the growth assay (Table 1), and an unambiguous order of toxicity could be determined by using EC50s for the inhibition of β-galactosidase activity. This order was VER > ROR > T-2 > DAS > HT-2 > AcT-2 > neosolanio (NEO) > FUS > TRI > scirpentriol (SCR) > nivalenol (NIV) > DON > TET.
FIG. 1.
Inhibition of growth and β-galactosidase activity in K. marxianus by trichothecene mycotoxins. Standard deviations for all data points are <10% the value of the point. (A and B) Inhibition of growth (A) and β-galactosidase activity (B) of K. marxianus by VER (○), ROR (●), T-2 (▴), HT-2 (▵), AcT-2 (■), NEO (□), TRI (X), and TET (I). (C and D) Inhibition of growth (C) and β-galactosidase activity (D) of K. marxianus by DAS (●), SCR (○), FUS (▴), NIV (⧫), and DON (□).
TABLE 1.
NEC, EC50, and MIC estimates for inhibition of growth and β-galactosidase activity of K. marxianus by trichothecenesa
| Toxin type | Inhibition (μg/ml) of:
|
Relative toxicity | |||||
|---|---|---|---|---|---|---|---|
| Growth
|
β-Galactosidase induction
|
||||||
| NEC | EC50 | MIC | NEC | EC50 | MIC | ||
| Group 1 (no oxygen function at the C-8 position [R1]) | |||||||
| DAS | 0.005 | 0.18 | 1.0 | 0.0065 | 0.03 | 0.1 | 15 |
| SCR | 0.1 | >25 | >25 | 2.5 | 6.0 | 10 | 3,000 |
| Group 2 (non-keto oxygen function at the C-8 position [R1]) | |||||||
| T-2 | 0.001 | 0.05 | 0.25 | 0.005 | 0.012 | 0.025 | 6 |
| HT-2 | 0.01 | 1 | 5 | 0.01 | 0.07 | 0.5 | 35 |
| AcT-2 | 0.1 | 8.0 | >25 | 0.1 | 1.5 | 5 | 750 |
| NEO | 0.5 | 20 | >25 | 0.5 | 2.0 | 20 | 1,000 |
| TRI | 1.0 | >25 | >25 | 0.8 | 5.0 | 20 | 2,500 |
| TET | >25 | >25 | >25 | >25 | >25 | >25 | >12,500 |
| Group 3 (ketone at the C-8 position [R1]) | |||||||
| FUS | 0.1 | 22 | >25 | 0.7 | 3.5 | 10.0 | 1,750 |
| NIV | 0.1 | >25 | >25 | 4.0 | 14 | >25 | 7,000 |
| DON | 0.1 | >25 | >25 | 4.0 | 21 | 25 | 10,500 |
| Group 4 (macrocyclic trichothecenes) | |||||||
| VER | 0.001 | 0.008 | 0.1 | 0.001 | 0.002 | 0.005 | 1 |
| ROR | 0.001 | 0.018 | 0.25 | 0.001 | 0.004 | 0.01 | 2 |
The trichothecenes are arranged in the four chemical groups of Tamm and Tori (18) and are ranked, within each group, in order of potency. The relative toxicities of the individual toxins are also given, based on their EC50s, with VER being 1.
DISCUSSION
The insensitivity of K. marxianus to the nontrichothecene mycotoxins has been previously noted with disk diffusion assays (10, 11, 15). The apparent insensitivity of K. marxianus to many mycotoxins, contrasting with the good sensitivity to at least some trichothecene mycotoxins, suggests that it might be exploited in a selective bioassay for tricothecenes (11).
Our colorimetric bioassay exhibits as great or greater sensitivity than the other yeast bioassays. For example, considering the most potent trichothecene, VER, the colorimetric bioassay gave a MIC of 5 ng/ml (Table 1) compared with a MIC of 120 ng/ml reported by Schappert et al. (16) for a disk diffusion assay. Expressed slightly differently, the colorimetric bioassay gave an EC50 of 0.32 ng/well, compared with the minimum reported detection level (4-mm inhibition zone diameter) of 5 ng/disk reported by Madhyastha et al. (11) for an optimized disk bioassay. The β-galactosidase–colorimetric end point, which contributes the main novelty of the bioassay used here, is more sensitive than inhibition of cell growth—the end point used in the other yeast bioassays. This sensitivity can be seen when the inhibition of β-galactosidase dose-response curves (Fig. 1B and D) is inflected more sharply and at lower toxin concentrations than the curves for growth inhibition (Fig. 1A and C); the effect is quantitatively displayed as EC50s and MICs (Table 1). The value of the β-galactosidase end point is particularly clear for TRI, which was virtually undetected on the basis of growth inhibition.
The most potent toxins in our assay (Table 1) were VER and ROR, which have a macrocylic ring between the C-6 and C-4 positions and no substituents at the C-3, C-7 and C-8 positions. T-2 was the most potent of the nonmacrocyclic trichothecenes tested, followed by DAS. T-2 and DAS both possess acetoxy groups at the C-4 and C-15 positions, together with a hydroxy group at the C-3 position; potency declines greatly when these groups are absent and/or when keto or hydroxy moieties are at the C-8 position (Table 1). The HT-2 results show that replacement of the C-4 acetoxy (T-2) by a hydroxy (HT-2) causes a modest loss in potency (6-fold), whereas the same substitution at C3 (T-2 changed to AcT-2) causes a much more dramatic potency reduction (over 100-fold). VER, ROR, T-2 and DAS stand out as the most potent of the trichothecenes in our yeast system.
The overall results obtained here are in general agreement with those from other investigations. A study using K. marxianus in a disk diffusion assay showed the orders of toxicity to be VER > ROR > T-2 > HT-2 > TRI > TET (16) and T-2 > DAS > HT-2 > AcT-2 > FUS > TRI > NEO > NIV > DON > TET (12). By using the Chlorella growth inhibition assay, AcT-2 and NEO inhibited growth at 1 mg/ml, whereas TET, NIV, and DON had no effect (9). Two studies of trichothecene lymphotoxicity gave results strongly paralleling those from our yeast system: one showed a similar decrease in toxicity with substitution at C-4 (FUS > NIV > DON) (7). The other study highlighted the importance for potency of a hydroxy at C-3, together with acetoxy groups at C-4 and C-15 (T-2 and DAS), and the decrease in toxicity that occurs when these groups are absent and/or when there are keto or hydroxy moieties at C-8 (1). The similarity of the responses of the two systems indicates the potential application of our test to the evaluation of trichothecene lymphotoxicity. Again, measuring inhibition of protein synthesis in cultured Vero (animal) cells, the order of toxicity was shown to be VER > ROR > T-2 > DAS > HT-2 > NEO > FUS > SCR > TRI > DON > AcT-2 > NIV > TET (19). This ranking is very similar to that of our colorimetric bioassay. Our bioassay can also detect some trichothecenes with a sensitivity comparable to chemical methods. For example, the EC50 for T-2 toxin in our assay is approximately 10 ng/ml, compared to 20 to 25 ng per “spot” needed for detection on a thin-layer chromatography plate (3), 10 ng needed for detection by high-performance liquid chromatography (by UV absorption of the p-nitrobenzoate derivative) (3), and approximately 20 ng needed for detection by electron impact-selective ion monitoring-mass spectrometry detection (3).
In summary, our colorimetric bioassay is highly sensitive to a number of trichothecene mycotoxins and showed essentially similar results to, but greater sensitivity than, other yeast (11, 15) and animal tissue culture (7, 8, 13, 19) bioassays. The simplicity, speed, ease of replication, and quantification of results mean this assay is particularly well suited to studies of possible synergistic and antagonistic interactions between trichothecene mycotoxins and between trichothecenes and other toxicants and food components.
ACKNOWLEDGMENTS
This research was supported in part by a SERC-CASE studentship to K.E. and the Department for International Development of the United Kingdom.
We also thank J. Gibbs and M. Nagler for technical advice and assistance and Geoff Cooper, School of Chemical and Life Sciences, for help with computations.
REFERENCES
- 1.Bondy G S, Beremand M N, McCormick S P, Pestka J J. Murine lymphocyte proliferation impaired by substituted neosolaniols and calonectrins—Fusarium metabolites associated with trichothecene biosynthesis. Toxicon. 1991;29:1107–1113. doi: 10.1016/0041-0101(91)90208-9. [DOI] [PubMed] [Google Scholar]
- 2.Coker R D. Mycotoxins and their control: constraints and opportunities. NRI bulletin 73. Chatham, Kent, United Kingdom: Natural Resources Institute; 1997. [Google Scholar]
- 3.Coker, R. D. The chromatography of mycotoxins. In I. Wilson (ed.), Encyclopedia of separation science, in press.
- 4.Connolly P, Corry J E L. Effect of polymyxin B nonapeptide and polymyxin B sulphate on trichothecene mycotoxin sensitivity of yeast using a conductimetric instrument. Int J Food Microbiol. 1990;10:73–90. doi: 10.1016/0168-1605(90)90010-3. [DOI] [PubMed] [Google Scholar]
- 5.Engler K. Ph.D. thesis. London, United Kingdom: University of Greenwich; 1996. [Google Scholar]
- 6.Engler, K., R. D. Coker, and I. H. Evans. A novel colorimetric yeast bioassay for detecting trichothecene mycotoxins. J. Microbiol. Methods, in press. [DOI] [PubMed]
- 7.Forsell J H, Pestka J J. Relation of 8-ketotrichothecene and zearalenone analog structure to inhibitors of mitogen-induced human lymphocyte blastogenesis. Appl Environ Microbiol. 1985;50:1304–1307. doi: 10.1128/aem.50.5.1304-1307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanelt M, Gareis M, Kollarczik B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia. 1994;128:167–174. doi: 10.1007/BF01138479. [DOI] [PubMed] [Google Scholar]
- 9.Ikawa M, Carr C, Tatsuno T. Trichothecene structure and toxicity to the green alga Chlorella pyrenoidosa. Toxicon. 1985;23:535–537. doi: 10.1016/0041-0101(85)90040-6. [DOI] [PubMed] [Google Scholar]
- 10.Madhyastha M S, Marquardt R R, Masci A, Borsa J, Frohlich A A. Comparison of toxicity of different mycotoxins to several species of bacteria and yeasts: use of Bacillus brevis in a disc diffusion assay. J Food Prot. 1994;57:48–53. doi: 10.4315/0362-028X-57.1.48. [DOI] [PubMed] [Google Scholar]
- 11.Madhyastha M S, Marquardt R R, Frohlich A A, Borsa J. Optimisation of yeast bioassay for trichothecene mycotoxins. J Food Prot. 1994;57:490–495. doi: 10.4315/0362-028X-57.6.490. [DOI] [PubMed] [Google Scholar]
- 12.Madhyastha M S, Marquardt R R, Abramson D. Structure-activity relationships and interactions among trichothecene mycotoxins as assessed by yeast bioassay. Toxicon. 1994;32:1147–1152. doi: 10.1016/0041-0101(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 13.Porcher J M, Lafarge-Frayssinet C, Frayssinet C, Nurie A, Melcion D, Richard-Molard D. Determination of cytotoxicity of trichothecenes in corn by cell culture assay. J Assoc Off Anal Chem. 1987;70:844–849. [PubMed] [Google Scholar]
- 14.Schappert K T, Khachatourians G G. Effect of fusariotoxin T-2 on Saccharomyces cerevisiae and Saccharomyces carlsbergensis. Appl Environ Microbiol. 1983;45:862–867. doi: 10.1128/aem.45.3.862-867.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schappert K T, Khachatourians G G. A yeast bioassay for T2-toxin. J Microbiol Methods. 1984;3:43–46. [Google Scholar]
- 16.Schappert K T, Koshinsky H A, Khachatourians G G. Growth inhibition of yeast by T-2, HT-2, T-2 triol, T-2 tetraol, diacetoxyscirpenol, verrucarol, verrucarin A, and roridin A mycotoxins. J Am Coll Toxicol. 1986;5:181–187. [Google Scholar]
- 17.Sukroongreeung S, Schappert K T, Khatchatourians G G. Survey of sensitivity of twelve yeast genera toward T-2 toxin. Appl Environ Microbiol. 1984;48:416–419. doi: 10.1128/aem.48.2.416-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamm C, Tori M. Trichothecenes. In: Betina V, editor. Mycotoxins. Production, isolation, separation and purification. Amsterdam, The Netherlands: Elsevier Scientific Publications; 1984. pp. 131–182. [Google Scholar]
- 19.Thompson W L, Wannemacher R W. Structure-function relationships of 12,13-epoxytrichothecene mycotoxins in cell culture: comparison to whole animal lethality. Toxicon. 1986;24:985–994. doi: 10.1016/0041-0101(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 20.Watson D H, Lindsay D G. A critical review of biological methods for the detection of fungal toxins in foods and feedstuffs. J Sci Food Agric. 1982;33:59–67. doi: 10.1002/jsfa.2740330112. [DOI] [PubMed] [Google Scholar]
- 21.Yates I E. Bioassay systems and their use in diagnosis of mycotoxicoses. In: Richards J L, Thurston J R, editors. Diagnosis of mycotoxicoses. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 333–381. [Google Scholar]