While C3and C4trees inhabit similar environments, C4photosynthesis in trees expands the ecological niche relative to that of C3tree species.
Keywords: Biogeography, C4 photosynthesis, Chamaesyce, Euphorbia, euphorbiaceae, trees
Abstract
Previous studies have demonstrated the ecological sorting of herbaceous C3 and C4 species along gradients of precipitation and temperature: C4 herbaceous species typically occupy drier and warmer environments than their C3 relatives. However, it is unclear if this pattern holds true for C4 tree species, which are unique to Euphorbiaceae and found only on the Hawaiian Islands. Here, we combine occurrence data with local environmental and soil datasets to, for the first time, distinguish the ecological factors associated with photosynthetic diversification in the tree life form. These data are presented within a phylogenetic framework. We show that C3 and C4 trees inhabit similar environments, but that C4 photosynthesis expands the ecological niche in trees relative to that of C3 tree species. In particular, when compared with C3 trees, C4 trees moved into higher elevation habitats with characteristically sparse vegetation (and thus greater sunlight) and cooler temperatures, a pattern which contrasts with that of herbaceous species. Understanding the relationship between C4 photosynthesis and ecological niche in tree species has implications for establishing how C4 photosynthesis has, in this rare instance, evolved in trees, and whether this unique combination of traits could be exploited from an engineering perspective.
Introduction
It is widely accepted that the ecological niche of a given plant species is influenced by its photosynthetic efficiency (Black, 1971; Sage et al., 1999; Lundgren et al., 2015). Modifications to the photosynthetic apparatus can increase or decrease efficiency depending on environmental conditions. One such modification that increases carbon, water, and nitrogen use efficiencies is the C4 photosynthetic pathway (Evans, 2013). This pathway largely eliminates the energetically costly process of photorespiration, which occurs when the carbon-fixing enzyme Rubisco catalyses the fixation of oxygen instead of CO2, resulting in the accumulation of toxic by-products that need to be recycled (Bräutigam and Gowik, 2016). Certain environmental conditions are known to increase the rate of photorespiration, including low CO2 concentrations, warmth, bright light, aridity, and salinity (Chollet and Ogren, 1975; Ehleringer et al., 1991; Sage et al., 2018). As a result, species utilizing C4 photosynthesis theoretically perform better than plants using the ancestral C3 pathway in these environments (Pearcy and Ehleringer, 1984). This, at least in part, explains the well-reported differences in global distribution patterns for C3 and C4 species (reviewed in Ehleringer et al., 1997; Christin and Osborne, 2014).
Ecological sorting of photosynthetic types along temperature gradients is particularly apparent in grasses, where C4 grasses are abundant at high temperatures and give way to C3 grasses as temperature declines (Teeri and Stowe, 1976; Bremond et al., 2012; Pau et al., 2013). For eudicot herbaceous species, water availability seems to be the most important determinant of distribution, with C4 forbs being favoured over their C3 counterparts in areas with limited water supply (Stowe and Teeri, 1978; Pyankov et al., 2010). Furthermore, species using the CAM (Crassulacean acid metabolism) photosynthetic pathway have advantages over both C3 and C4 species with respect to their maximum potential water use efficiencies, and frequently dominate in the most arid environments (Orsenigo et al., 1997; Black and Osmond, 2003). However, epiphytic CAM species can also be abundant in tropical rainforests, which have very high levels of precipitation, as it is the epiphytic growth form which drives their water limitation (Whittaker, 1975; Quezada and Gianoli, 2011). The difference in the major environmental predictors of photosynthetic type distribution between C3 and C4 grasses and forbs, as well as the contrasting environments of CAM species highlights the importance of distinguishing growth forms when examining the effect of photosynthetic type on the ecological niche. However, the relationship between photosynthetic type and ecology has not yet been investigated in the tree growth form.
The effects of photosynthetic type on the ecological and geographical distributions of trees may be different from those of herbaceous species for three reasons. First, there are key differences between monocots and eudicots, the plant clades which contain all known C4 species (Sage, 2016). More than half of all the >60 C4 origins have occurred in eudicots, but these lineages account for less than a quarter of all known C4 species (Sage, 2016). Quantum yield (defined as the rate of photosynthesis relative to photon absorption) is generally lower in the eudicots—which includes almost all true tree species—than in the monocots. This results in generally poorer shade tolerance among C4 eudicots compared with monocots that could have implications for their distributions, and thus the relationship between photosynthetic type and ecological niche (Ehleringer et al., 1997). This poor shade tolerance may be augmented by a potentially reduced capacity for exploiting sunflecks in C4 versus C3 plants, although this response does not appear to be consistent or universal given that reduced sunfleck use efficiency is not observed in all species and growth forms of C4 plants (Pearcy et al., 1985; Krall and Pearcy, 1993; discussed in Sage and McKown, 2006; Sage, 2014). Secondly, life history influences ecological niche (Pyankov et al., 2010; Liu et al., 2019). Pyankov et al. (2010) hypothesize that the abundance of C4 annual species compared with C4 perennials reflects the fact that C4 photosynthesis confers the greatest fitness benefit over short periods of time. Therefore, tree species, which have long life spans, may not benefit as much from C4 photosynthesis as short-lived herbaceous species. Furthermore, Liu et al. (2019) demonstrate that the variation in niche descriptors of C3 and C4 subtropical grasses was best explained by differences in life history: annual subtropical grasses (particularly C4 annuals) tend to grow in regions with higher temperatures and lower, more seasonal precipitation compared with perennial grasses. Thirdly, growth form probably influences the ecological niche through functions such as water transport or light competition rather than photosynthetic type. Tall plant species such as trees typically exhibit wider water conduits than shorter plants, and these wider conduits are more vulnerable to embolism under drought or freezing (Olson et al., 2018). This means that the tree state may result in an intrinsically higher degree of vulnerability to drought, and this vulnerability would not necessarily be alleviated by the C4 pathway. This problem may be exacerbated where the C4 state is recently evolved (i.e. a tree evolves C4 or the tree state evolves in a young C4 lineage), as shown by a study of young C4 grass lineages which demonstrates that leaf hydraulic conductance is increased due to the anatomy required by the C4 system, despite the C4 state reducing hydraulic demand, resulting in less negative turgor loss points in young compared with old C4 lineages (Zhou et al., 2020, Preprint). As such, a tall C4 plant such as a tree, particularly one belonging to a young C4 lineage, would theoretically not be able to access more arid environments than their C3 counterparts. This is reflected in the apparent height limitation of the only known C4 trees (found in one of the older C4 eudicot lineages), which are not observed to exceed 10 m in height (Young et al., 2020). Despite these clear differences across life forms, histories, and growth patterning, the ecological sorting of photosynthetic diversity in trees remains largely unexplored.
Evolutionary history is another important factor determining the ecological sorting of photosynthetic diversity. Comparisons between distantly related grass species have often revealed more pronounced differences between the ecological distributions of C3 and C4 species than studies comparing photosynthetic diversity between closely related species or within a single species (Pau et al., 2013; Lundgren et al., 2015). The broad differences identified between C3 and C4 species probably reflect the fact that distribution patterns are not solely determined by the acquisition of C4 photosynthesis and are affected by the ecology and functional traits of the ancestral C3 lineages (Edwards and Still, 2008; Edwards and Smith, 2010). With time, niche specialization may then occur after the initial emergence of C4 physiology, depending on differences in functional traits, resulting in some C4 taxa becoming specialized to environments different from those of their C3 ancestors and generating an apparent niche shift. This means that comparisons between distantly related species of differing photosynthetic type may reveal pronounced differences between C4 and C3 species, but these differences may only be partly driven by photosynthetic type. Furthermore, studies that look at intraspecific photosynthetic diversity offer a closer look at the direct consequences of C4 physiology. Indeed, a study on the intraspecific photosynthetic diversity of the grass Alloteropsis semialata—the only species with both C4 and non-C4 genotypes—showed that C4 photosynthesis actually broadens the ecological niche, rather than shifting it away from that of ancestral C3 lineages (Lundgren et al., 2015). With this in mind, our study set out to determine the ecological sorting of closely related C3 and C4 trees, as well as any influence that evolutionary history may have had on their ecological distributions.
Tree photosynthetic diversity only exists within Euphorbiaceae, a morphologically diverse plant family with C3, C4, and CAM tree species (Table 1; Webster et al., 1975; Webster, 1994; Horn et al., 2012). The Chamaesyce clade of Euphorbia (Euphorbiaceae) is the largest single C4 lineage among the eudicots, with 350 C4 species and containing the only true C4 trees, defined here as tall, perennial, woody life forms with secondary growth and C4 leaves (Pearcy and Troughton, 1975; Yang and Berry, 2011; Young et al., 2020). These C4 trees are endemic to the Hawaiian Islands, where they diversified from a likely herbaceous ancestor that arrived on the islands ~5 million years ago (Yang et al., 2018). This diversification event yielded five C4 trees in present-day Hawaii, which, when combined with the 17 C3 and three CAM Euphorbiaceae tree species currently on these islands (Table 1), makes the Hawaiian Islands the global centre of photosynthetic diversity in trees. The environment on the islands is highly heterogenous, and trees in Euphorbiaceae occupy a range of environments from bright, open scrubland to mesic forest where they experience differing temperatures, precipitation levels, and light availability (Table 1; Sporck, 2011; Sage and Sultmanis, 2016). Hawaiian trees in Euphorbiaceae therefore provide a unique opportunity to compare the ecological niches of tree species with diverse photosynthetic backgrounds from a similar geographic region.
Table 1.
Number of occurrences (n), photosynthetic type (C3, C2, C4, or CAM), and life form of species in Euphorbiaceae occurring on the Hawaiian Islands
| Species | n | Photosynthetic type | δ 13C a | Reference | Life form (WCSP b) | Environmental data from herbarium record c |
|---|---|---|---|---|---|---|
| Tree | ||||||
| Euphorbia atrococca | 6 | C4 | –12.40 | Pearcy and Troughton (1975) | phan | Disturbed lowland forest |
| Euphorbia celastroides | 43 | C4 | –13.21 | Pearcy and Troughton (1975), Horn et al. (2014) | phan | Highly variable: grassy or rocky windswept slopes to open forest to bog |
| Euphorbia herbstii | 4 | C4 | –12.80 | Pearcy and Troughton (1975) d | phan | Forested slopes, deep shade |
| Euphorbia olowaluana | 12 | C4 | –14.40 | Pearcy and Troughton (1975) | phan | Dry forest, lava field |
| Euphorbia rockii | 4 | C4 | –12.95 | Pearcy and Troughton (1975) | phan | Open bog, open rainforest on hilltop, moist rainforest, exposed ridge, wooded stream bank, high elevations |
| Acalypha wilkesiana | 7 | C3 | NA | nanophan/phan | ||
| Aleurites moluccanus | 107 | C3 | NA | phan | ||
| Claoxylon sandwicense | 18 | C3 | NA | Robichaux and Pearcy (1980) | nanophan/phan | Wooded slope, dry forest |
| Cnidoscolus aconitifolius | 1 | C3 | NA | nanophan/phan | ||
| Croton guatemalensis | 1 | C3 | NA | phan | ||
| Euphorbia cotinifolia | 1 | C3 | –23.93 | Horn et al. (2014) | nanophan/phan | |
| Euphorbia haeleeleana | 3 | C3 | NA | Morden et al. (2014) e | phan | Mesic forest |
| Euphorbia leucocephala | 2 | C3 | –27.58 | Horn et al. (2014) | nanophan/phan | |
| Hevea brasiliensis | 1 | C3 | –28.90 | Martinelli et al. (1998) | phan | |
| Homalanthus populifolius | 3 | C3 | NA | nanophan/phan | ||
| Hura crepitans | 1 | C3 | –26.67 | Horn et al. (2014) | phan | |
| Jatropha integerrima | 16 | C3 | NA | nanophan/phan | ||
| Jatropha multifida | 2 | C3 | NA | nanophan/phan | ||
| Macaranga mappa | 18 | C3 | NA | phan | ||
| Macaranga tanarius | 21 | C3 | NA | phan | ||
| Manihot carthaginensis | 5 | C3 | NA | nanophan/phan | ||
| Vernicia fordii | 1 | C3 | NA | phan | ||
| Euphorbia lactea | 4 | CAM | –14.79 | Horn et al. (2014), Mason et al. (2015) | succ nanophan/phan | |
| Euphorbia tirucalli | 10 | CAM/C3 | –16.45 | Horn et al. (2014), Bender (1971), Bender et al. (1973) | succ nanophan/phan | |
| Jatropha curcas | 3 | CAM/C3 | NA | Winter and Holtum (2015) | nanophan/phan | |
| Shrub | ||||||
| Euphorbia arnottiana | 2 | C4 | –13.40 | Pearcy and Troughton (1975), Yang et al., 2018 | cham | |
| Euphorbia atoto | 1 | C4 | –12.4 | Pearcy and Troughton (1975) | cham/nanophan | |
| Euphorbia clusiifolia | 2 | C4 | –12.20 | Pearcy and Troughton (1975) | – | |
| Euphorbia degeneri | 7 | C4 | –12.9 | Pearcy and Troughton (1975) | – | |
| Euphorbia eleanoriae | 1 | C4 | NA | Yang et al. (2018) | – | |
| Euphorbia halemanui | 6 | C4 | NA | Yang et al. (2018) | – | |
| Euphorbia kuwaleana | 1 | C4 | NA | Yang et al. (2018) | phan | Grassy hilltop, volcanic rock |
| Euphorbia multiformis | 13 | C4 | –12.60 | Pearcy and Troughton (1975), Yang et al. (2018) | – | |
| Euphorbia remyi | 7 | C4 | –13.10 | Pearcy and Troughton (1975) | – | |
| Euphorbia skottsbergii | 7 | C4 | –12.00 | Pearcy and Troughton (1975) | – | |
| Euphorbia sparsiflora | 2 | C4 | NA | Yang et al. (2018) | – | |
| Acalypha hispida | 6 | C3 | NA | nanophan | ||
| Codiaeum variegatum | 23 | C3 | NA | nanophan/phan | ||
| Jatropha podagrica | 11 | C3 | NA | nanophan | ||
| Manihot esculenta | 4 | C3 | NA | De Souza et al. (2017) f | nanophan/phan | |
| Ricinus communis | 82 | C3 | –30.2 | Bender (1971) | nanophan/phan | Side of valley |
| Euphorbia milii | 2 | CAM/C2 | –21.70 | Horn et al. (2014), Herrera (2013) | cham/nanophan | |
| Euphorbia tithymaloides | 2 | CAM/C3 | –23.94 | Horn et al. (2014), Ramachandra et al. (2003) | nanophan | |
| Herb | ||||||
| Euphorbia hirta | 71 | C4 | –13.08 | Horn et al. (2014), Yang and Berry (2011) | ther | |
| Euphorbia hypericifolia | 48 | C4 | NA | Yang and Berry (2011) | ther/cham | |
| Euphorbia hyssopifolia | 9 | C4 | NA | Yang and Berry (2011) | ther | |
| Euphorbia ophthalmica | 3 | C4 | NA | Yang and Berry (2011) | ther | |
| Euphorbia prostrata | 38 | C4 | NA | Yang and Berry (2011) | ther | |
| Euphorbia serpens | 3 | C4 | NA | Yang and Berry (2011) | ther | |
| Euphorbia thymifolia | 22 | C4 | NA | Yang and Berry (2011) | ther/hel | |
| Euphorbia heterophylla | 22 | C3 | –29.67 | Horn et al. (2014), Ramachandra and Das (1986) | ther | |
| Euphorbia peplus | 1 | C3 | –33.32 | Horn et al. (2014) | ther | |
a δ13C data (averaged where multiple measurements were found) are included where available in the literature, NA = not available.
b Life form is obtained from the Kew World Checklist of Selected Plant Families (WCSP) and species designated as one or a combination of phanerophyte (phan, small and large trees), nanophanerophyte (nanophan, shrub), succulent (succ), chamaephyte (cham, woody or herbaceous perennial), therophyte (ther, annual), or helophyte (hel, herbaceous species with roots in water/saturated soil). These life form designations were used in conjunction with information from the citations to group species into trees, shrubs, and herbs.
c Environmental data for selected tree and shrub species were recorded from herbarium datasheets available online at GBIF.
d Named here as E. forbesii.
e Secondary source naming E. haeleeleana as C3.
f Low level CAM, possibly shifts to CAM under drought.
Here, we combine geographical occurrence and environmental datasets in a phylogenetic framework to examine the phylogeography of photosynthetic diversity in trees in order to elucidate the geographical and environmental factors that are permissive for photosynthetic innovation in trees. Based on our current knowledge of the sorting of photosynthetic diversity among herbaceous species, we hypothesize that C4 trees occur in drier, hotter, and more open environments than their C3 counterparts (Table 2), with the differences in water availability acting as the most important determinant of distribution as for herbaceous eudicots. However, as discussed above, these environmental differences may be less pronounced than those for herbaceous species given the theoretically reduced benefits of C4 photosynthesis over long life spans and in the tree growth form. With only three CAM tree species which occur in small numbers on the Hawaiian Islands, we were unable to perform robust analyses on the phylogeography of this photosynthetic type but instead report on its distribution in relation to C3 and C4 trees in our study.
Table 2.
Environmental parameters hypothesized to differ by photosynthetic type in trees
| Type of parameter | Parameter | Hypothesis | Supporting reference a |
|---|---|---|---|
| Climatic | Minimum Temperature of Coldest Month | C4 trees are found in areas with lower minimum monthly temperatures than C3 trees | Edwards and Smith (2010) |
| Temperature of the Wettest Quarter (Growing Season) | C4 trees are found in areas with higher growing season temperatures than C3 trees | Teeri and Stowe (1976), Vogel et al. (1986) | |
| Precipitation of Driest Month | C4 trees are found in areas with lower minimum monthly precipitation levels than C3 trees | Pyankov et al. (2010) | |
| Precipitation Seasonality | C4 trees are found in areas with more seasonal precipitation than C3 trees | Edwards and Smith (2010) | |
| Solar Radiation | C4 trees are found in areas with higher solar radiation than C3 trees | Ehleringer (1978) | |
| Climatic Water Deficit | C4 trees are found in areas with higher climatic water deficits than C3 trees | Witwickiet al. (2016) | |
| Ecological | Vegetation Cover | C4 trees are found in areas with lower vegetation cover than C3 trees | Pau et al. (2013), Still et al. (2014) |
a Supporting references are studies showing environmental distributions for C3 and C4 herbaceous species.
Materials and methods
Geographic distribution data for Euphorbiaceae species
Occurrence data for all species in Euphorbiaceae on the Hawaiian Islands were downloaded from the Global Biodiversity Information Facility (https://www.gbif.org, https://doi.org/10.15468/dl.bc6bus) and the Botanical Information and Ecology Network (BIEN; https://bien.nceas.ucsb.edu/bien/). Data were imported into R using the readr and BIEN packages in R, respectively (Maitner et al., 2018; Wickham et al., 2018; R Core Team, 2020). These combined datasets yielded 1509 occurrence records for 78 species.
Occurrence data were then cleaned in R to remove any records that potentially contained inaccurate or unreliable information. First, all latitude and longitude data were rounded to two decimal places, and records with incomplete coordinate, species, or country data were removed. Taxa names were updated to currently accepted names where appropriate. Secondly, any records that were identified by GBIF as having at least one of the issues listed in Supplementary Table S1 were removed. Thirdly, data were processed using the CoordinateCleaner package in R to flag records with coordinates corresponding to country capitals or centroids, the GBIF headquarters, or another biodiversity institution; records with equal, zero, or invalid values for latitude and longitude; and records with coordinates that were not on land (Zizka et al., 2019). A total of 277 flagged records (summarized in Supplementary Fig. S1) were removed. Finally, duplicate records and records associated with botanic gardens, private gardens, and arboretums were removed. Upon completion of these cleaning steps, 690 occurrence records for 52 species remained (Supplementary Fig. S2).
The remaining records were then designated as herb, shrub, or tree based on life form information in the World Checklist of Selected Plant Families (WCSP, 2021; Table 1). For Hawaiian Euphorbia, Yang et al. (2018) was used to supplement the life form data from the WCSP. Occurrence records were also designated as having C3, C4, C2, or CAM photosynthetic type based on the information available in the literature (Table 1; Supplementary Fig. S3). All C4 Hawaiian species were identified using Yang et al. (2018) and Yang and Berry (2011). Five CAM species were identified to occur on the Hawaiian Islands, including three CAM tree species, Jatropha curcas, Euphorbia lactea, and Euphorbia tirucalli (Mies et al., 1996; Mason et al., 2015; Winter and Holtum, 2015). All other records, including those whose photosynthetic type could not be found in the literature, were designated as C3 (Table 1). Where available from the literature, δ13C stable isotope values are recorded in Table 1 to support these photosynthetic type designations, with values greater (i.e. less negative) than –15 per mille indicating likely C4 biochemistry.
Environmental factors
Biogeographical parameters hypothesized to be associated with the sorting of photosynthetic types were selected for our analyses, including geographical, climatic, and ecological factors (Table 2). Elevation data for occurrence records in Hawaii were obtained from the Shuttle Radar Topography Mission (STRM) digital elevation model (DEM) at 1 arc-second (~30 m) spatial resolution via the rgbif package in R (Farr et al., 2007; Chamberlain et al., 2022). Environmental and ecological data were obtained at ~250 m resolution from the Online Rainfall Atlas of Hawaii and Climate of Hawaii (Giambelluca et al., 2013, 2014). Monthly precipitation and temperature data were processed using the bioclim2 function from the climates package in R to recreate the standard 19 bioclimatic variables (VanDerWal et al., 2014). Climatic water deficit (CWD) was calculated as potential evapotranspiration minus actual evapotranspiration (Stephenson, 1998). C3 and C4 tree data were plotted onto the Whittaker biomes using the plotbiomes package in R (Stefan and Levin, 2019). Soil data for the Hawaiian Islands were obtained from the Hawaii Soil Atlas (http://gis.ctahr.hawaii.edu/SoilAtlas) at ~670 m spatial resolution and imported into ArcGIS Pro (https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview). Data were obtained for the following soil variables: fertility (including soil class and cation exchange capacity), order, organic matter content, pH, shrink–swell potential (soil stability), and water permeability. Soil data for coordinates of interest were then extracted via the Spatial Join function from the Analysis toolbox.
Phylogeny
A dated phylogenetic tree for Euphorbiaceae was generated using previously published data. Three plastid regions (trnK–matK, rbcL, and trnL–trnF) were targeted as they were the best represented for the required taxa. Initially, the trnK–matK and rbcL dataset of Tokuoka (2007) was used, adding additional taxa and trnL–trnF sequence information where available. Each gene was aligned with MAFFT v.7.017 (Katoh et al., 2002) and concatenated into a single alignment. The final alignment was 4507 bp long and included 112 species (see Supplementary Table S2 for a summary of sequence data used and accession numbers; see Supplementary Fig. S4 for a phylogenetic tree with all 112 species). A dated phylogeny was inferred using BEAST2 v2.6.3 (Bouckaert et al., 2014) through the Cyberinfrastructure for Phylogenetic Research (CIPRES) Science Gateway V.3.3. To date the tree, a secondary calibration point from Horn et al. (2014) was used, fixing the divergence time between Euphorbia herbstii and Hura crepitans at 64.6076 million years ago (SD=0.0001). Three different analyses were run with 100 000 000 generations, sampling a tree every 1000 generations, a Yule speciation process, a relaxed log-normal clock, and the GTR+G model. The convergence of all three runs was verified using Tracer v. 1.6.0 (Drummond and Rambaut, 2007), after which a burn-in period of 10% was set. All trees were concatenated, and a maximum credibility tree inferred mapping median ages onto the nodes.
Statistical analyses
Statistical analyses were conducted in R version 4.0.4 (R Core Team, 2020). CAM species were excluded from the analyses owing to the small sample size (0 herb, 4 shrub, and 15 tree occurrence data points). Analyses were conducted primarily on data for C3 and C4 trees. Data for all C3 and C4 life forms, including herb and shrub occurrence data, were analysed separately from the tree data (Supplementary Figs S5, S6). Soil data and environmental data were analysed separately from each other due to soil data only being available for a subset (61%) of the occurrence data points (Supplementary Fig. S7).
To explain any ecological variation between trees of C3 and C4 photosynthetic types, principal component analyses (PCAs) were conducted using the FactoMineR package (Le et al., 2008). Predictor variables that were hypothesized to influence the sorting of photosynthetic types were chosen for the analysis (Table 2), including minimum temperature of the coldest month, temperature of the wettest quarter (proxy for growing season temperature), precipitation seasonality, precipitation of the driest month, CWD, solar radiation, and vegetation cover (i.e. to indicate shaded habitats). Leave-one-out cross-validation was used to estimate the number of dimensions for the PCAs. All variables were scaled to unit variance before fitting. The first PCA was performed on 268 occurrence points representing 22 tree species, comprising 17 C3 species (n=202) and five C4 species (n=66). The 95% confidence ellipses were calculated for each group.
A second PCA was conducted on environmental data for C3 and C4 herbaceous (n=21, n=178, respectively), shrub (n=119, n=47), and tree (n=202, n=66) occurrence points to examine the ecological differences between C3 and C4 individuals of all life forms (Supplementary Fig. S5). Data for woody species (i.e. trees and shrubs) were considered together in order to examine the ecological differences between woody and non-woody species. The continuous soil variables from the soil dataset were included in a third PCA. These included cation exchange capacity (CEC), organic matter content (OM), pH, shrink–swell potential (soil stability), and water permeability (Ksat). Soil data for C3 and C4 tree (n=134, n=29) occurrence points were included in this analysis (Supplementary Fig. S7). In all cases, all variables were scaled to unit variance before fitting.
To identify potential correlation between environmental and geographical distances (i.e. whether C3 and C4 trees have expanded their environmental range as they have expanded their geographical range across the Hawaiian Islands), Mantel permutation tests were conducted using the ape package (Paradis and Schliep, 2019). Geographic distances between points were extracted and assembled into a matrix using the earth.dist function in the fossil package (Matthew, 2011), and then compared with environmental distances (Euclidian distance in the space formed by the first three axes of the PCA) to test for statistical associations between matrices of environmental and geographical data. Linear regression analysis was carried out by fitting a linear model to each of the C3 and C4 datasets, with the slope of this regression representing the environmental change per unit of geographical expansion.
To distinguish the role that evolutionary history may have played in photosynthetic type sorting across these six environmental variables, phylogenetic generalized least squares (PGLS) analyses were performed using the pgls function in the caper package in R (Orme et al., 2018). A comparative dataset was assembled from species mean data for each of the seven environmental variables used in the PCA, the first three principal components (PCs) of the PCA, and the species phylogeny. This dataset included a variance–covariance (VCV) matrix that represents species’ phylogenetic relationships to one another. A PGLS model was then fitted to each of the seven environmental variables and three PCs in the dataset with photosynthetic type as the categorical predictor. Pagel’s lambda (λ) was calculated to estimate the strength of the phylogenetic signal in the mean response of each species on a scale of zero (no phylogenetic dependence) to one (perfect phylogenetic dependence). ANOVA was then performed on each model.
Results
Geographical and environmental distributions of C3 and C4 trees overlap
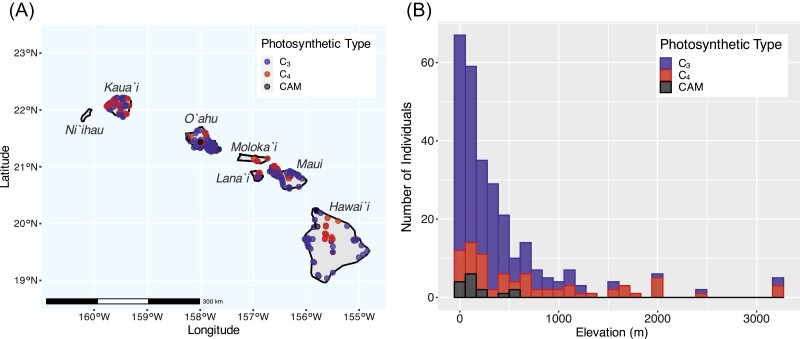
C3, C4, and CAM Euphorbiaceae trees have largely overlapping geographical distributions across the Hawaiian Islands (Fig. 1A). Based on occurrence data available in the BIEN and GBIF databases, both C3 and C4 trees in Euphorbiaceae occur on the islands of Kaua`i, O`ahu, Lana`i, Maui, and Hawai`i. Only a single Euphorb tree species, Euphorbia celastroides, occurs on Molokaa`i. In fact, E. celastroides is the only C4 tree species recorded on each of Molokaa`i, Lana`i, and Maui, and has the most widespread distribution of any of the C4 trees. Only one island—Ni`ihau—has no record of Euphorb trees. There are also three CAM tree species in Euphorbiaceae on the Hawaiian Islands (Jatropha curcas, Euphorbia lactea, and E. tirucalli), which occur on Maui, O`ahu, and Hawai`i (Fig. 1A). Trees in Euphorbiaceae occur across a broad range of elevations across the Islands, from –5 m to 3212 m (Fig. 1B). C3 trees occupy this entire range of elevations, but are largely skewed toward the lower elevations, with a median value of 170 m. C4 trees are found at elevations of 6 m to 3212 m, and are more evenly distributed across this range than their C3 counterparts, with a median elevation of 526 m.
Fig. 1.
Geographical and topographical distribution of photosynthetic diversity in trees in Euphorbiaceae across the Hawaiian Islands. (A) Individual occurrence points and (B) a histogram of elevations are shown for C3 (blue, n=202), C4 (red, n=66), and CAM (black, n=15) trees, representing 17, 5, and 3 species, respectively.
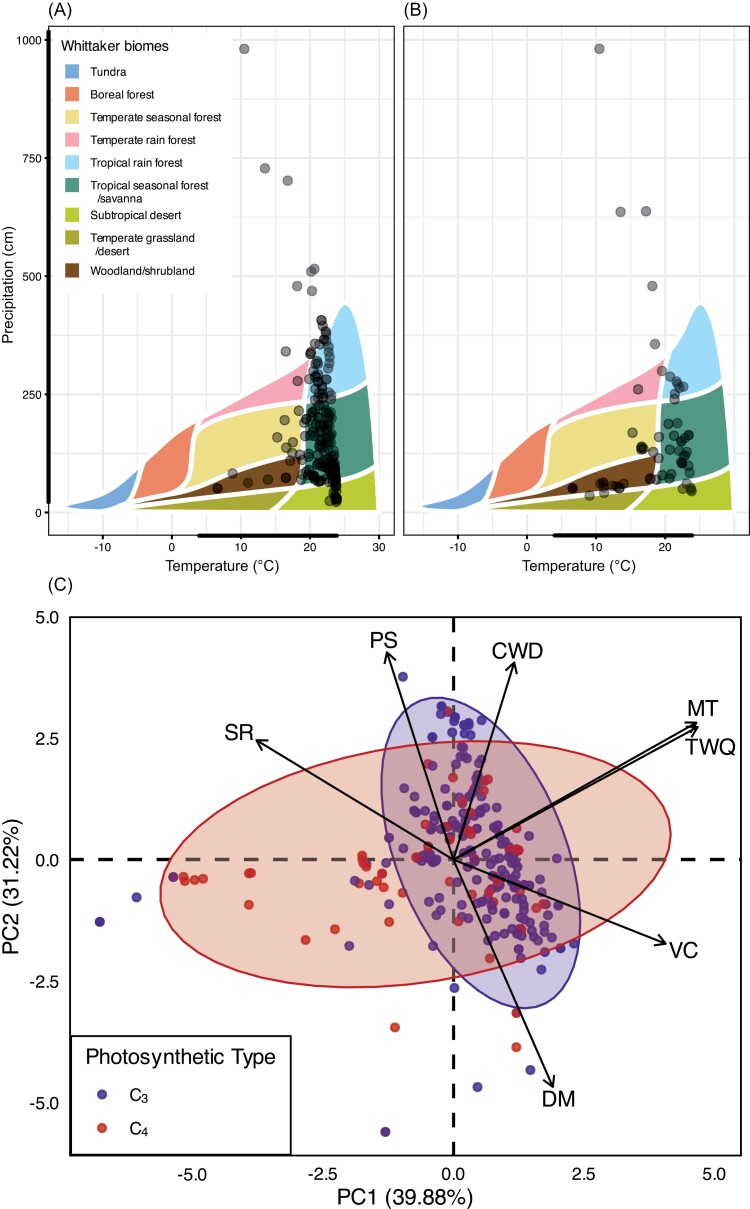
Overall, trees in Euphorbiaceae are broadly distributed across precipitation regimes that span the range found across the Hawaiian Islands (Fig. 2A, B). C3 trees occur in areas with as little as 232 mm mean annual precipitation (MAP) on the northwest coast of Hawai`i and as high as 9010 mm MAP in Kaua`i. Similarly, C4 trees have been recorded on the south slopes of Mauna Kea in Hawai`i with only 356 mm MAP, but also in Kaua`i with MAP up to 9010 mm. The distribution of Euphorbiaceae trees across temperatures was narrower, but also reflected that of the Hawaiian Islands (Fig. 2A, B). Mean annual temperature (MAT) ranged from 6.6 to 23.8 °C, with the majority of trees occurring within a much narrower range of 18–24 °C. While C3 and C4 trees inhabited similar temperature spaces, C3 trees were more skewed towards the upper end of the temperature range compared with C4 trees. According to the Whittaker Biomes theory (Whittaker, 1975), these MAP and MAT ranges suggest that Hawaiian Euphorb trees are most commonly found within tropical rainforest and tropical seasonal forest/savanna biomes. C3 and C4 trees are similarly distributed across these biomes (Fig. 2A, B).
Fig. 2.
Ecological distributions of C3 and C4 trees across the Hawaiian Islands. (A) C3 and (B) C4 trees plotted on the Whittaker Biomes. The thick black bar on each axis shows the range of values for mean annual temperature and precipitation found on the Hawaiian Islands according to the Climate of Hawaii and Rainfall Atlas of Hawaii (Giambelluca et al., 2013, 2014). (C) Principal component analysis of seven environmental variables for C3 (blue) and C4 (red) trees. Arrows show the loading of each of the seven variables. Abbreviations are as follows: MT, minimum temperature of the coldest month; TWQ, temperature of the wettest quarter; DM, precipitation of the driest month; PS, precipitation seasonality; SR, solar radiation; VC, vegetation cover; CWD, climatic water deficit. The 95% confidence ellipses were calculated for each group.
C4 photosynthesis expands the ecological range of trees in Euphorbiaceae on the Hawaiian Islands
We performed three PCAs to determine which environmental and soil variables most strongly distinguish the distributions of C3 and C4 individuals on the Hawaiian Islands. The first PCA incorporated the seven environmental explanatory variables that we hypothesized would differ in C3 and C4 trees based on the published literature on monocots and herbaceous eudicots (Table 2). This generated three PCs which captured nearly 85% (39.88% PC1, 31.22% PC2, and 13.31% PC3) of the variation across the dataset (Fig. 2C; Supplementary Fig. S8). Wettest quarter temperature, minimum coldest month temperature, vegetation cover, and solar radiation were most strongly correlated with PC1, while precipitation seasonality, precipitation of the driest month, and CWD were most strongly correlated with PC2 (Fig. 2C; Supplementary Table S3). The two temperature variables showed strong positive correlation with each other in the PCA space, while the pair of precipitation variables showed strong negative correlation with each other, as did vegetation cover and solar radiation. There is a large degree of overlap between the C3 and C4 groups in the PCA space, with the 95% confidence ellipse for the C3 group almost completely contained within that of the C4 group. The C3 and C4 groups occupied a similar range of values on PC2 but the C4 group had a much broader distribution than the C3 group on PC1 with a skew towards lower PC1 values. This indicates that C4 trees occupy a broader range of ecological niches than C3 trees, and that environments with low temperature and vegetation cover, and high levels of solar radiation are more often occupied by C4 trees than their C3 counterparts.
To determine whether the difference in ecological space between C3 and C4 trees was larger or smaller than that of closely related herbaceous and shrub life forms, we performed a second PCA on the same seven environmental variables but including all growth forms (herb, shrub, and tree) (Supplementary Figs S5, S6). PC1 accounted for 36.18% of the variation in the dataset, while PC2 accounted for 34.23% (Supplementary Fig. S5). The results of this analysis show that, similarly to trees, C4 shrubs occupy a broader range of ecological niches than C3 shrubs (Supplementary Fig. S5C). The niche-broadening effect is even more pronounced in shrubs than in trees, and is driven by all of the seven environmental variables. As such, when considered together, C4 woody species (i.e. shrubs and trees) generally occupy a greater ecological niche than their C3 counterparts (Supplementary Fig. S5E). Conversely, C3 and C4 non-woody species (i.e. herbs) in this family have a smaller degree of ecological difference than shrubs and trees (shown by their more similar distributions in the PCA space), but C4 herbs have a slightly broader range of values for PC2 (and to a lesser extent PC1) compared with C3 herbs (Supplementary Fig S5B). PC2 is most strongly correlated with solar radiation, vegetation cover, and precipitation of the driest month (Supplementary Table S4), so this suggests that C4 herbs occupy brighter, more open, drier environments than their C3 counterparts.
The third PCA incorporated all the continuous soil variables available from the Hawaii Soil Atlas for C3 and C4 tree species (Supplementary Fig. S7). PC1 accounted for 35.7% of the variation in the dataset, while PC2 accounted for 29.95%. The variables of OM, Ksat, and pH were most strongly correlated with PC1, while CEC was most strongly correlated with PC2 (Supplementary Table S5). There was almost complete overlap of the 95% confidence ellipses for the C3 and C4 groups in the PCA space (Supplementary Fig. S6), indicating that soil variables did not differ greatly between the C3 and C4 trees in this dataset.
C4 trees expanded their ecological range more than C3 trees as they dispersed across the Islands
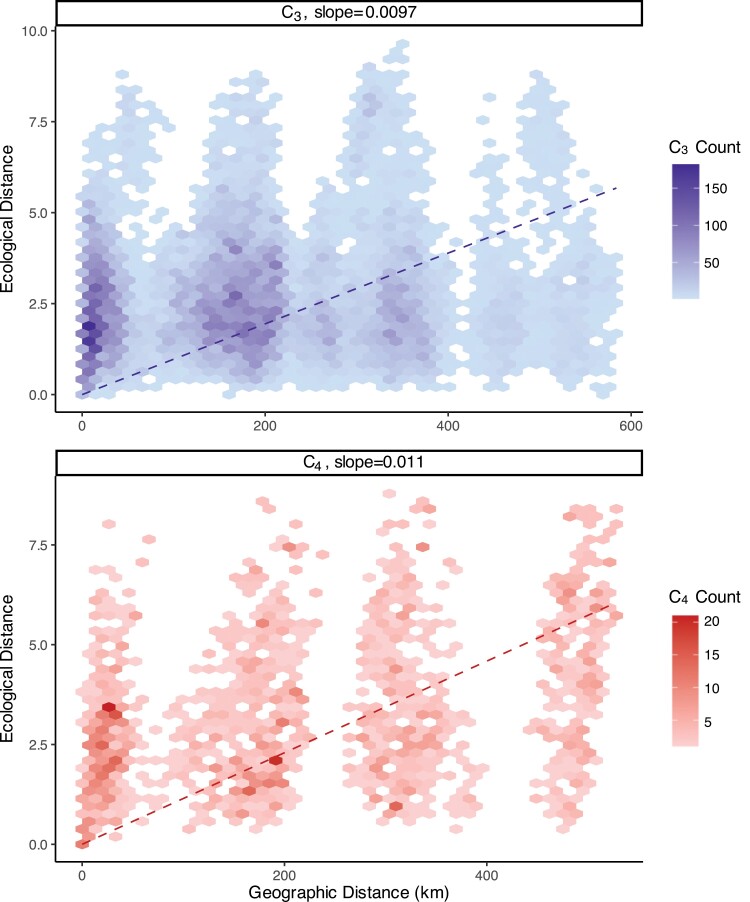
We used Mantel permutation tests to examine the association between ecological and geographical expansion in C3 and C4 trees. The results of the Mantel tests show a positive correlation between ecological and geographical expansion for both C3 (P=0.019) and C4 (P<0.001) trees. This was confirmed by linear regression analysis, which showed that the regression line for the C4 group had a slightly steeper slope (0.011) than the C3 group (0.0097), indicating that C4 trees expanded their ecological niche more for a given degree of geographic dispersal compared with C3 trees (Fig. 3). This is possibly associated with a small number of C4 trees accessing higher elevation environments, where they are disproportionately abundant relative to C3 trees (Fig. 1B).
Fig. 3.
Comparison of geographical and ecological distances for C3 and C4 trees across the Hawaiian Islands. Ecological and geographical distances were obtained for pairs of C3 (blue) and C4 (red) individuals. Regression lines forced to the origin were identified for each of the C3 and C4 groups, and the slopes calculated.
Evolutionary history does not strongly influence C3 and C4 tree distributions
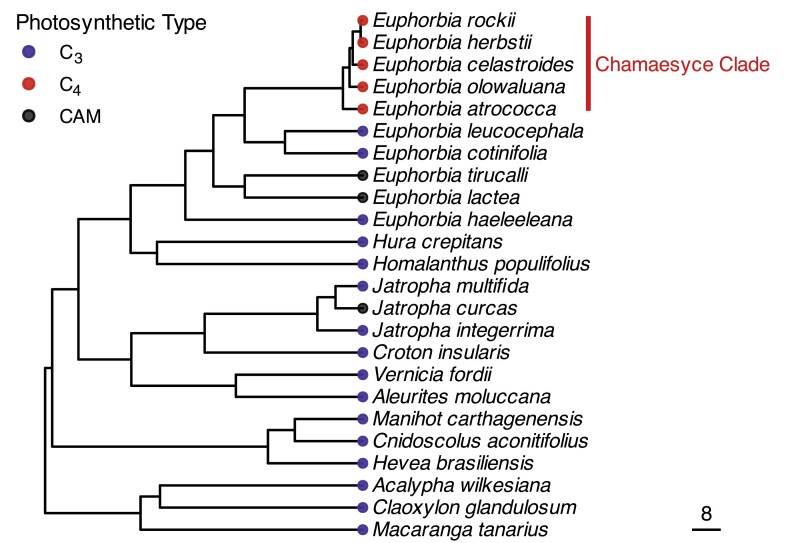
We generated a phylogeny of Euphorbiaceae trees found on the Hawaiian Islands using previously published sequence data (Fig. 4). Our phylogeny showed that C4 tree species on the Hawaiian Islands form a monophyletic group, consistent with previous phylogenies [Fig. 4; Yang et al., 2018 (posterior probability=1, bootstrap=100)]. This is expected given that the Hawaiian Chamaesyce radiation was previously reported as being from a single common ancestor. We used the phylogeny to perform PGLS analyses on each environmental variable and the first three PCs of the first PCA (which included only tree species) to support the findings of the PCA and determine the role of evolutionary history in C3 and C4 tree environmental distributions. These analyses showed no influence of evolutionary history in six of the seven environmental variables and two of the three PCs, and that none of the environmental variables or PCs showed significant differences between photosynthetic types after accounting for any phylogenetic signal (Table 3). The temperature variables did, however, show marginally significant differences between photosynthetic types. The PGLS showed evidence of strong phylogenetic signal in CWD and PC2 (λ=0.954 and λ=1.000, respectively); however, these variables did not show significant differences between photosynthetic type.
Fig. 4.
Phylogeny of Hawaiian Euphorbiaceae trees. Evolutionary relationships between C3 (blue, n=16), C4 (red, n=5), and CAM (black, n=3) Hawaiian Euphorbiaceae trees are presented in a pruned maximum credibility tree inferred from the BEAST analysis of a dataset representing 112 species in Euphorbiaceae. The C3 species Macaranga mappa is missing from this tree due to an absence of available published nucleotide data. The Hawaiian species Claoxylon sandwicense is represented by the congeneric species C. glandulosum. The Hawaiian species Croton guatemalensis is represented by the congeneric species C. insularis.
Table 3.
Results of phylogenetic generalized least squares (PGLS) analysis and ANOVA for the effects of photosynthetic type on environmental variables and principal components (PCs) for C3 and C4 trees on the Hawaiian Islands.
| Parameter a | Unit | C3 min | C3 max | C3 mean | C3 SD | C4 min | C4 max | C4 mean | C4 SD | λ b | F c | P c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum Temperature of the Coldest Month | °C | 0.410 | 17.941 | 15.649 | 2.983 | 0.410 | 17.939 | 12.477 | 5.137 | 0.000 | 3.971 | 0.061 |
| Temperature of the Wettest Quarter (Growing Season) | °C | 4.710 | 24.565 | 20.326 | 3.030 | 4.710 | 23.106 | 17.203 | 5.103 | 0.000 | 3.763 | 0.067 |
| Precipitation of the Driest Month | mm | 0.808 | 611.644 | 93.195 | 90.269 | 4.556 | 611.644 | 82.623 | 107.192 | 0.000 | 0.002 | 0.968 |
| Precipitation Seasonality | – | 0.101 | 1.058 | 0.356 | 0.196 | 0.128 | 0.774 | 0.371 | 0.161 | 0.000 | 0.058 | 0.812 |
| Yearly Average Solar Radiation | W m–2 | 148.715 | 290.951 | 202.409 | 26.206 | 152.292 | 290.951 | 216.390 | 30.964 | 0.057 | 0.285 | 0.600 |
| Yearly Average Vegetation Cover Fraction | – | 0.048 | 1.000 | 0.736 | 0.205 | 0.048 | 0.991 | 0.674 | 0.272 | 0.000 | 0.043 | 0.839 |
| Climatic Water Deficit | mm | 504.600 | 6620.157 | 2665,233 | 1502.165 | 504.600 | 4784.676 | 2072.157 | 951.226 | 0.954 | 0.008 | 0.930 |
| PC1 | – | –6.774 | 2.311 | 0.290 | 1.285 | –6.774 | 1.720 | –0.993 | 2.301 | 0.000 | 2.421 | 0.136 |
| PC2 | – | –5.603 | 3.764 | 0.132 | 1.526 | –5.603 | 3.058 | –0.287 | 1.366 | 1.000 | 0.484 | 0.495 |
| PC3 | – | –2.422 | 2.216 | 0.053 | 1.009 | –2.091 | 1.383 | –0.111 | 0.882 | 0.000 | 0.248 | 0.624 |
a Minimum (min), maximum (max), mean, and SD are given for each of seven environmental variables and three PCs for C3 (n=184 individuals, 16 species) and C4 (n=66 individuals, five species) trees.
b Pagel’s lambda (λ) was obtained from the PGLS and estimates the variance due to phylogenetic sources.
c F- and P-values were obtained from the ANOVA. Marginally significant results (P<0.1) are italicized. No variable remained significant, marginal or otherwise, after a Bonferroni correction.
All values are given to three decimal places.
Discussion
C3 and C4 trees have more similar geographical and environmental distributions than C3 and C4 herbaceous species
The results presented here suggest that C3 and C4 tree species across the Hawaiian Islands inhabit similar geographical and environmental spaces. There has been no significant niche shift after the single origin of the C4 pathway in this clade, as indicated by the paucity of phylogenetic signal in the data. Where phylogenetic signal was present, in CWD and PC2, this was not associated with a significant difference between the two photosynthetic types. This suggests that this phylogenetic structuring is present across the whole family and is not driven by a niche shift associated with the evolution of C4 photosynthesis.
The similar ecological distributions of C3 and C4 tree species contrasts with previous studies on herbaceous species from other families and geographical regions, which show clear differences between the ecological niches of C3 and C4 grasses and forbs (Teeri and Stowe, 1976; Stowe and Teeri, 1978; Pyankov et al., 2010; Bremond et al., 2012; Pau et al., 2013). Given that all true angiosperm trees are eudicots, and, in C3 versus C4 eudicots, aridity is an important determinant of distribution (more so than temperature, which is the primary determinant for monocots), we hypothesized that aridity would be the primary environmental determinant distinguishing the distributions of C3 and C4 trees. However, we found no difference in the driest month precipitation, precipitation seasonality, or CWD between C3 and C4 trees, despite highly variable precipitation levels across the Hawaiian Islands (Fig. 2; Table 3). In fact, precipitation variables seemed to be the least important factors in determining C4 tree distribution, with solar radiation, vegetation cover, and temperature driving differences in distributions between the C3 and C4 groups in the PCA space (Fig. 2). Soil variables were notably similar between the C3 and C4 groups; however, this is consistent with a previous study on the biogeographic controls on C3 and C4 grass distributions (Griffith et al., 2015).
There are many possible factors contributing to the similar environmental distributions of C3 and C4 trees. First, the height of trees relative to herbaceous species may play a role, as tall plant height may confer greater embolism vulnerability under drought (Olson et al., 2018), and this inherent limitation may occur regardless of photosynthetic type. However, the C4 trees on the Hawaiian Islands have not been observed to exceed 10 m in height (Pearcy and Troughton, 1975; Sporck, 2011) so this effect is likely to be minor. Second, and more likely, the small geographical area of the Hawaiian Islands (~28 000 km2) may also constrain the degree of environmental differences between C3 and C4 trees. Strong trends in global distribution patterns are observed for C3 and C4 grasses, where the geographical area in question is much larger: global grassland areas comprise ~52.5 million km2 (Sage et al., 1999; White et al., 2000). However, previous studies of C3 and C4 grasses on the Hawaiian Islands have revealed significant differences in environmental distributions (Edwards and Still, 2008; Still et al., 2014), so while differences in ecological niche may be less pronounced in an island environment, they are not necessarily completely obscured. Third, the nature of the climate and soil datasets may mask some of the true variability in environmental niche. In the case of the Hawaiian climate datasets, the data are averaged over multiple years, and the Hawaiian soil atlas data are only available for a limited set of variables and a limited number (61% in this study) of occurrence data points. Notably, the Hawaiian soil atlas lacks data on soil nitrogen content, although soil nitrogen availability is related to some of the other variables included in this analysis such as CEC (which is linked to NH4+ levels) and pH and organic matter content (which are linked to nitrate levels) (Kemmitt et al., 2006). Soil nitrogen availability to plants may also be influenced by temperature, slope, soil aeration, and soil water (Amer and Bartholomew, 1951; Stanford et al., 1975; Kong et al., 2019), all of which vary across the Islands. It is possible that soil nitrogen availability may affect the distribution of the different photosynthetic types in this study given that the C4 pathway increases nitrogen use efficiency (Pearcy and Ehleringer, 1984), but this effect cannot be detected in our analyses. However, this is likely to be a limitation for most studies of this nature, many of which still show clear differences in the ecological niches of C3 and C4 herbaceous species. Finally, life history is probably also significant. Grasses can have both annual and perennial life history strategies, and it is annual grasses (particularly C4 annuals) which grow in the hottest environments with the most seasonal precipitation, whereas perennial grasses tend to grow in regions with lower, more seasonal precipitation (Liu et al., 2019). This indicates that C4 photosynthesis may be more important for carbon accumulation in hot, dry environments for fast-lived grasses, but not as important in longer lived species such as trees.
C4 trees occupy a broad ecological niche on highly heterogenous Islands
Although C3 and C4 trees occupy similar environments, the results of the PCA and linear regression analysis of the environmental versus geographical distances suggest that C4 photosynthesis does have a niche-broadening effect across all growth forms in Euphorbiaceae, with this effect being the most pronounced in woody species (Figs 2, 3; Supplementary Fig. S5). In particular, C4 tree species have expanded their range into environments with characteristically sparse vegetation cover, higher sunlight, and cooler temperatures (Fig. 2C). Reduced vegetation cover, increased solar radiation, and cooler temperatures are all correlated with increasing elevation levels (Supplementary Fig. S9). This suggests that C4 trees have expanded their ecological niche relative to their C3 counterparts as they moved into higher elevation environments. It is worth noting that this niche broadening is not an effect of different sample sizes as there are fewer C4 than C3 tree occurrence points, and broader ecological distributions of C4 species are seen in all life forms in this study, despite differences in relative sample sizes of C3 versus C4 groups. Furthermore, a broadening of the environmental niche associated with C4 photosynthesis is consistent with a previous intraspecific study in grasses (Lundgren et al., 2015). As such, it may be that C4 photosynthesis does influence the niche of woody species on the Hawaiian Islands, but that there has not been sufficient opportunity for niche specialization among C4 tree species to generate an apparent niche shift relative to their C3 counterparts due to their restricted global range. As previously stated, the small geographic range of C4 trees could have acted to limit the environmental conditions that they can access, even though this limitation is minimized by two factors.
First, the trees have dispersed across six of the Hawaiian Islands (Fig. 1), which gives them the opportunity to access the full range of the environments and climates that occur across the Islands (Fig. 2). This dispersal across the Islands has probably been facilitated by the seed characteristics of the ancestor of the C4 Hawaiian Euphorbia, which had small seeds with mucilaginous seed coats that adhered to birds to facilitate dispersal (Price and Wagner, 2004). Some species of Hawaiian Euphorbia have then undergone habitat specialization following dispersal, such as the C4 tree E. rockii, which is a single-island endemic on O`ahu (Yang et al., 2018). Euphorbia rockii has large, non-sticky seeds which may be beneficial for seedling survival in forest understorey habitats, but also have reduced dispersal (Koutnik, 1987; Jordan and Hayden, 1992; Yang et al., 2018). It is worth noting that there are some islands, such as Ni`ihau, that have no record of Euphorb species (Supplementary Fig. S3), which may be a result of limited sampling rather than true absence.
Second, the environments of the Hawaiian Islands are highly heterogenous, which generates large environmental gradients over relatively small geographic distances (Barajas-Barbosa et al., 2020). This underlies the suitability of the Hawaiian Islands for this type of analysis: there is the potential for large ecological niche variation between species in close geographic proximity and, as such, there is a high degree of potential variation in climatic and environmental variables between individuals. Indeed, studies of the ecological differences between C3 and C4 grasses on the Islands have identified trends in ecological distributions that are consistent with globally observed patterns (Pau et al., 2013; Still et al., 2014).
Despite these mitigating factors, it is likely that geographical limitation still acts to constrain the magnitude of potential environmental differences between C3 and C4 trees. As the Hawaiian Islands formed, this generated a high availability and diversity of new niches, which probably facilitated the radiation of the C4Euphorbia (Yang et al., 2018). However, the small and isolated nature of the Hawaiian Islands means that the potential for niche shifts following this initial radiation is likely to be lower than that on a continental land mass. This is reflected in the limited environmental differences that are seen across all growth forms (Supplementary Fig. S5) in Euphorbiaceae on the Hawaiian Islands. Indeed, herbaceous C3 and C4 Hawaiian Euphorbs seem to grow in more similar environments than has previously been observed for their counterparts in other geographical regions (Batanouny et al., 1991).
Euphorbiaceae is a morphologically and photosynthetically diverse plant family
In addition to the geographic limitations of the Hawaiian Islands, there are further complications in this study in conclusively determining growth form and photosynthetic type in Euphorbiaceae.
With respect to growth form, there is no unilaterally accepted definition of a tree: it is not a single phylogenetic grouping, but a life ‘strategy’ that can vary between and within species. For example, Euphorbia celastroides can achieve the tree growth form, but also has varieties with shrubbier, more prostrate growth forms (Sporck, 2011; Yang et al., 2018), and distinguishing between a large shrub and a small tree of the same species is subjective. These intraspecific differences are not resolved within the available occurrence data, so certain individuals identified as trees in this study may have had shrubbier growth forms. However, this issue is mitigated somewhat by the fact that the ecological differences between C3 and C4 shrubs and trees seem to be driven by similar factors, and the niche-broadening effect of C4 photosynthesis is consistent across all woody species (Supplementary Fig. S5).
Furthermore, photosynthetic type has not been conclusively established for all the species in Euphorbiaceae, as determining photosynthetic type can require a combination of carbon isotope discrimination (δ13C), gas exchange, and leaf anatomy data. These data are not available in the literature for all species: for most species, only δ13C data could be obtained. As such, there may be unrecognized photosynthetic diversity within species currently classified as using C3 photosynthesis. This is made more likely by the fact that Euphorbiaceae is a highly photosynthetically diverse plant family, known to include species using C3, C4, C3–C4 intermediate, and CAM modes of photosynthesis, although there are currently no known C3–C4 trees in Euphorbiaceae or otherwise.
Photosynthetic diversity in Euphorbiaceae provides insights into C4 evolution in tree species
The reason for the global rarity of C4 and C3–C4 tree species is an ongoing question in plant ecophysiology and evolution. In order to answer this question, the two potential evolutionary routes to C4 photosynthesis in trees must be assessed (Young et al., 2020).
First, C4 photosynthesis may arise in an existing tree, via a C3–C4 intermediate state. The feasibility of this evolutionary path cannot be confirmed due to the apparent absence of C3–C4 trees from global plant biodiversity. Given the high degree of photosynthetic diversity in woody Euphorbs, the lack of C3–C4 photosynthesis is particularly apparent. Indeed, in Euphorbia alone, there have been at least 17 independent evolutions of a photosynthetic CO2-concentrating mechanism; 16 evolutions of CAM and one of C4 (Horn et al., 2014). The C4 lineage was ancestrally herbaceous, while all 16 evolutions of CAM occurred in ancestrally woody lineages. In view of this, Horn et al. (2014) hypothesize that life history is important in establishing evolutionary trajectory. We hypothesize that biogeographical factors associated with life history traits may also affect the ability of a lineage to evolve C4 photosynthesis; that is, a lower quantum yield in C3–C4 photosynthetically intermediate species (i.e. potential C3–C4 trees) could occlude them from shaded habitats (Monson et al., 1986; Monson and Moore, 1989). This could therefore select against the evolution of intermediate photosynthetic types, and thus C4 photosynthesis, in ancestrally woody forest species, and provides some explanation for the absence of woody C3–C4 species.
Second, a C4 tree species may evolve from an herbaceous C4 ancestor. This was the route taken by the Hawaiian Euphorbia, indicating that this is a feasible evolutionary path (Yang et al., 2018). However, if the transition to the tree state means that the potential benefits of the C4 pathway are diminished or eliminated, this reduces the likelihood of C4 trees emerging via this pathway and persisting in competitive environments, thus providing some explanation for the rarity of C4 trees. The minimal ecological differences between C3 and C4 trees presented here offer some support for this explanation: there may be little to no benefit, or even a cost, to the C4 photosynthetic pathway in trees in terms of carbon or water gain in hotter and/or drier climates. It is possible that C4 photosynthesis has only persisted in trees on the Hawaiian Islands due to the high availability and diversity of new niches that emerged as the Islands formed (which has been shown to be consistent with a high level of species diversity; Sebastian et al., 2012; Barajas-Barbosa et al., 2020), rather than the C4 photosynthetic pathway providing any benefit over the ancestral C3 state. However, it is difficult to fully assess the validity of this conclusion given that the Hawaiian Euphorbia represent a single C4 lineage (one of 34 in the eudicots), and the only C4 lineage to include true trees (Sage, 2016).
If indeed it is the case that the C4 pathway provides limited benefit in trees, then this not only helps to explain why C4 photosynthesis is so rare in trees but also provides insight into the value (or lack thereof) of C4 trees from an engineering perspective. If the C4 pathway did allow trees to perform better under hot and/or dry environmental conditions, the engineering of C4 photosynthesis into trees would be a valid target for future-proofing against climate change to secure timber yields or forest carbon sequestration. However, if the C4 pathway does not influence tree ecology in this way, as the results presented here suggest, then this approach would not be beneficial. C4 trees may only be exceptional with respect to their rarity, and not their performance relative to their C3 counterparts.
Conclusions
Our results suggest that C3 and C4 trees in Euphorbiaceae inhabit similar environments across the Hawaiian Islands. This may, in part, be due to the limited geographic range of C4 trees globally, but may also indicate that C4 photosynthesis is not beneficial in trees in terms of accessing hotter and/or drier climates. However, C4 photosynthesis does appear to have a niche-broadening effect in tree species, and C4 trees have expanded their range on the Hawaiian Islands into environments with characteristically sparse vegetation cover, higher sunlight, and cooler temperatures. The high level of photosynthetic and morphological diversity in Euphorbiaceae merits further investigation to conclusively establish (i) how C4 photosynthesis, in this rare case, evolved in trees; (ii) whether there has been evolution of intermediate photosynthetic types in woody species in this family; and (iii) whether these intermediate photosynthetic types may confer any benefit over the C3 state.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Map of occurrence data flagged by CoordinateCleaner.
Fig. S2. Map of occurrence data by data source.
Fig. S3. Map of distributions of Hawaiian herbs, shrubs, and trees by photosynthetic type.
Fig. S4. Complete phylogeny of all species whose data were obtained in this study.
Fig. S5. Principal components 1 and 2 of a principal component analysis (PCA) of environmental variables for all growth forms.
Fig. S6. Principal components 3 and 4 of a principal component analysis (PCA) of environmental variables for all growth forms.
Fig. S7. Principal component analysis (PCA) of soil variables for C3 and C4 trees.
Fig. S8. Histogram of principal component 3 of a principal component analysis (PCA) of environmental variables for C3 and C4 trees.
Fig. S9. Distribution of C3 and C4 trees by elevation and environmental variables correlated with elevation.
Table S1. Issues identified by GBIF which resulted in a record being removed from this analysis
Table S2. Summary of sequence data used and accession numbers.
Table S3. Environmental variables correlated to PCA axes (trees only).
Table S4. Environmental variables correlated to PCA axes (all life forms).
Table S5. Soil variables correlated to PCA axes (trees only).
Acknowledgements
We thank Dylan Childs and Natalie Cooper for useful discussions on the statistical analyses.
Author contributions
SNRY, CJS, and MRL: conceptualization; SNRY: data retrieval and analysis; LTD: generating the phylogeny; HL: consulting on the data analyses; SNRY, LTD, HL, CJS, and MRL: writing.
Conflict of interest
The authors have no conflicts to declare.
Funding
SNRY is funded by a Natural Environment Research Council Envision Doctoral Training Partnership studentship (NE/S007423/1). MRL is funded by a Leverhulme Early Career Fellowship (ECF–2018-302) and UKRI Future Leaders Fellowship (MR/T043970/1). LTD is supported by a Natural Environment Research Council Independent Research Fellowship (NE/T011025/1).
Data availability
The data that were used in this study were all from publicly available datasets which have been fully cited.
References
- Amer FM, Bartholomew WV.. 1951. Influence of oxygen concentration in soil air on nitrification. Soil Science 71, 215–220. [Google Scholar]
- Barajas-Barbosa MP, Weigelt P, Borregaard MK, Keppel G, Kreft H.. 2020. Environmental heterogeneity dynamics drive plant diversity on oceanic islands. Journal of Biogeography 47, 2248–2260. [Google Scholar]
- Batanouny KH, Stichler W, Ziegler H.. 1991. Photosynthetic pathways and ecological distribution of Euphorbia species in Egypt. Oecologia 87, 565–569. [DOI] [PubMed] [Google Scholar]
- Bender MM. 1971. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10, 1239–1244. [Google Scholar]
- Bender MM, Rouhani I, Vines HM, Black CC.. 1973. 13C/12C ratio changes in crassulacean acid metabolism plants. Plant Physiology 52, 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CC. 1971. Ecological implications of dividing plants into groups with distinct photosynthetic production capacities. Advances in Ecological Research 7, 87–114. [Google Scholar]
- Black CC, Osmond CB.. 2003. Crassulacean acid metabolism photosynthesis: ‘working the night shift’. Photosynthesis Research 76, 329–341. [DOI] [PubMed] [Google Scholar]
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard MA, Rambaut A, Drummond AJ.. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10, e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A, Gowik U.. 2016. Photorespiration connects C3 and C4 photosynthesis. Journal of Experimental Botany 67, 2953–2962. [DOI] [PubMed] [Google Scholar]
- Bremond L, Boom A, Favier C.. 2012. Neotropical C3/C4 grass distributions—present, past and future. Global Change Biology 18, 2324–2334. [Google Scholar]
- Chamberlain S, Barve V, McGlinn D, Oldoni D, Desmet P, Geffert L, Ram K.. 2022. rgbif: interface to the Global Biodiversity Information Facility API. https://CRAN.R-project.org/package=rgbif.
- Chollet R, Ogren WL.. 1975. Regulation of photorespiration in C3 and C4 species. The Botanical Review 41, 137–179. [Google Scholar]
- Christin P-A, Osborne CP.. 2014. The evolutionary ecology of C4 plants. New Phytologist 204, 765–781. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Massenburg LN, Jaiswal D, Cheng S, Shekar R, Long SP.. 2017. Rooting for cassava: insights into photosynthesis and associated physiology as a route to improve yield potential. New Phytologist 213, 50–65. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A.. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA.. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences, USA 107, 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ.. 2008. Climate, phylogeny and the ecological distribution of C4 grasses. Ecology Letters 11, 266–276. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR. 1978. Implications of quantum yield differences on the distributions of C3 and C4 grasses. Oecologia 31, 255–267. [DOI] [PubMed] [Google Scholar]
- Ehleringer J, Cerling T, Helliker B.. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Sage RF, Flanagan LB, Pearcy RW.. 1991. Climate change and the evolution of C4 photosynthesis. Trends in Ecology and Evolution 6, 95–99. [DOI] [PubMed] [Google Scholar]
- Evans JR. 2013. Improving photosynthesis. Plant Physiology 162, 1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TG, Rosen PA, Caro E, et al. 2007. The shuttle radar topography mission. Reviews of Geophysics 45, RG2004. [Google Scholar]
- Giambelluca TW, Chen Q, Frazier AG, Price JP, Chen Y-L, Chu P-S, Eischeid JK, Delparte DM.. 2013. Online rainfall atlas of Hawai`i. Bulletin of the American Meteorological Society 94, 313–316. [Google Scholar]
- Giambelluca T, Shuai X, Barnes M, et al. 2014. Evapotranspiration of Hawai`i: Final report submitted to the US Army Corps of Engineers—Honolulu District, and the Commission on Water Resource Management, State of Hawai`i
- Griffith DM, Anderson TM, Osborne CP, Strömberg CAE, Forrestel EJ, Still CJ.. 2015. Biogeographically distinct controls on C3 and C4 grass distributions: merging community and physiological ecology. Global Ecology and Biogeography 24, 304–313. [Google Scholar]
- Herrera A. 2013. Crassulacean acid metabolism-cycling in Euphorbia milii. AoB PLANTS 5, plt0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JW, Van Ee BW, Morawetz JJ, Riina R, Steinmann VW, Berry PE, Wurdack KJ.. 2012. Phylogenetics and the evolution of major structural characters in the giant genus Euphorbia L. (Euphorbiaceae). Molecular Phylogenetics and Evolution 63, 305–326. [DOI] [PubMed] [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ.. 2014. Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68, 3485–3504. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Hayden WJ.. 1992. A survey of mucilaginous testa in Chamaesyce. Collectanea Botanica 21, 79–89. [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmitt SJ, Wright D, Goulding KWT, Jones DL.. 2006. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biology and Biochemistry 38, 898–911. [Google Scholar]
- Kong W, Yao Y, Zhao Z, Qin X, Zhu H, Wei X, Shao M, Wang Z, Bao K, Su M.. 2019. Effects of vegetation and slope aspect on soil nitrogen mineralization during the growing season in sloping lands of the Loess Plateau. Catena 172, 753–763. [Google Scholar]
- Koutnik DL. 1987. A taxonomic revision of the Hawaiian species of the genus Chamaesyce (Euphorbiaceae). Allertonia 4, 331–388. [Google Scholar]
- Krall JP, Pearcy RW.. 1993. Concurrent measurements of oxygen and carbon dioxide exchange during lightflecks in maize (Zea mays L.). Plant Physiology 103, 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S, Josse J, Husson F.. 2008. FactoMineR: a package for multivariate analysis. Journal of Statistical Software 25, 1–18. [Google Scholar]
- Liu H, Taylor SH, Xu Q, Lin Y, Hou H, Wu G, Ye Q.. 2019. Life history is a key factor explaining functional trait diversity among subtropical grasses, and its influence differs between C3 and C4 species. Journal of Experimental Botany 70, 1567–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren MR, Besnard G, Ripley BS, et al. 2015. Photosynthetic innovation broadens the niche within a single species. Ecology Letters 18, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Maitner BS, Boyle B, Casler N, et al. 2018. The bien r package: atool to access the Botanical Information and Ecology Network (BIEN) database. Methods in Ecology and Evolution 9, 373–379. [Google Scholar]
- Martinelli LA, Almeida S, Brown IF, Moreira MZ, Victoria RL, Sternberg LSL, Ferreira CAC, Thomas WW.. 1998. Stable carbon isotope ratio of tree leaves, boles and fine litter in a tropical forest in Rondonia, Brazil. Oecologia 114, 170–179. [DOI] [PubMed] [Google Scholar]
- Mason PM, Glover K, Smith JAC, Willis KJ, Woods J, Thompson IP.. 2015. The potential of CAM crops as a globally significant bioenergy resource: moving from ‘fuel or food’ to ‘fuel and more food’. Energy and Environmental Science 8, 2320–2329. [Google Scholar]
- Matthew JV. 2011. Fossil: palaeoecological and palaeogeographical analysis tools. Palaeontologia Electronica 14, 16. [Google Scholar]
- Mies B, Jiménez MS, Morales D.. 1996. Ecophysiology and distribution of the endemic leafless spurge Euphorbia aphylla and the introduced E. tirucalli (Euphorbiaceae, Euphorbia sect. Tirucalli) in the Canary Islands. Plant Systematics and Evolution 202, 27–36. [Google Scholar]
- Monson RK, Moore BD.. 1989. On the significance of C3–C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant, Cell & Environment 12, 689–699. [Google Scholar]
- Monson RK, Moore BD, Ku MSB, Edwards GE.. 1986. Co-function of C3- and C4-photosynthetic pathways in C3, C4 and C3–C4 intermediate Flaveria species. Planta 168, 493–502. [DOI] [PubMed] [Google Scholar]
- Morden CW, Hiramoto T, Yorkston M.. 2014. Genetic diversification among populations of the endangered Hawaiian endemic Euphorbia kuwaleana (Euphorbiaceae). Pacific Science 68, 75–83. [Google Scholar]
- Olson ME, Soriano D, Rosell JA, et al. 2018. Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences, USA 115, 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W.. 2018. caper: comparative analyses of phylogenetics and evolution in R. R package version 1.0.1. https://CRAN.R-project.org/package=caper
- Orsenigo M, Patrignani G, Rascio N.. 1997. Ecophysiology of C3, C4 and CAM plants. In: Pessarakli M, ed. Handbook of photosynthesis. New York: Marcel Dekker,1–25. [Google Scholar]
- Paradis E, Schliep K.. 2019. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. [DOI] [PubMed] [Google Scholar]
- Pau S, Edwards EJ, Still CJ.. 2013. Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Global Change Biology 19, 184–196. [DOI] [PubMed] [Google Scholar]
- Pearcy RW, Ehleringer JR.. 1984. Comparative ecophysiology of C3 and C4 plants. Plant, Cell & Environment 7, 1–13. [Google Scholar]
- Pearcy RW, Osteryoung K, Calkin HW.. 1985. Photosynthetic responses to dynamic light environments by Hawaiian trees. Plant Physiology 79, 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW, Troughton J.. 1975. C4 photosynthesis in tree form Euphorbia species from Hawaiian rainforest sites. Plant Physiology 55, 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JP, Wagner WL.. 2004. Speciation in Hawaiian angiosperm lineages: cause, consequence, and mode. Evolution 58, 2185–2200. [DOI] [PubMed] [Google Scholar]
- Pyankov VI, Ziegler H, Akhani H, Deigele C, Lüttge U.. 2010. European plants with C4 photosynthesis: geographical and taxonomic distribution and relations to climate parameters. Botanical Journal of the Linnean Society 163, 283–304. [Google Scholar]
- Quezada IM, Gianoli E.. 2011. Crassulacean acid metabolism photosynthesis in Bromeliaceae: an evolutionary key innovation. Biological Journal of the Linnean Society 104, 480–486. [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramachandra RA, Das VSR.. 1986. Correlation between biomass production and net photosynthetic rates and kinetic properties of RuBP carboxylase in certain C3 plants. Biomass 10, 157–164. [Google Scholar]
- Ramachandra RA, Sundar D, Gnanam A.. 2003. Photosynthetic flexibility in Pedilanthus tithymaloides Poit, a CAM plant. Journal of Plant Physiology 160, 75–80. [DOI] [PubMed] [Google Scholar]
- Robichaux RH, Pearcy RW.. 1980. Photosynthetic responses of C3 and C4 species from cool shaded habitats in Hawaii. Oecologia 47, 106–109. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2014. Stopping the leaks: new insights into C4 photosynthesis at low light. Plant, Cell & Environment 37, 1037–1041. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2016. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67, 4039–4056. [DOI] [PubMed] [Google Scholar]
- Sage RF, McKown AD.. 2006. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany 57, 303–317. [DOI] [PubMed] [Google Scholar]
- Sage RF, Monson RK, Ehleringer JR, Adachi S, Pearcy RW.. 2018. Some like it hot: the physiological ecology of C4 plant evolution. Oecologia 187, 941–966. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sultmanis S.. 2016. Why are there no C4 forests? Journal of Plant Physiology 203, 55–68. [DOI] [PubMed] [Google Scholar]
- Sage RF, Wedin DA, Li M.. 1999. The biogeography of C4 photosynthesis: patterns and controlling factors. In: Sage RF, Monson RK, eds. C4 plant biology. Academic Press, 313–373. [Google Scholar]
- Sebastian P, Schaefer H, Lira R, Telford IRH, Renner SS.. 2012. Radiation following long-distance dispersal: the contributions of time, opportunity and diaspore morphology in Sicyos (Cucurbitaceae). Journal of Biogeography 39, 1427–1438. [Google Scholar]
- Sporck MJ. 2011. The Hawaiian C4 Euphorbia adaptive radiation: an ecophysiological approach to understanding leaf trait diversification. PhD thesis, University of Hawaii.
- Stanford G, Frere MH, Vander Pol RA.. 1975. Effect of fluctuating temperatures on soil nitrogen mineralisation. Soil Science 119, 222–226. [Google Scholar]
- Ștefan V, Levin S.. 2019. plotbiomes: Plot Whittaker biomes with ggplot2. R package version 0.0.0.9001. https://github.com/valentinitnelav/plotbiomes
- Stephenson NL. 1998. Actual evapotranspiration and deficit: biologically meaningful correlates of vegetation distribution across spatial scales. Journal of Biogeography 25, 855–870. [Google Scholar]
- Still CJ, Pau S, Edwards EJ.. 2014. Land surface skin temperature captures thermal environments of C3 and C4 grasses. Global Ecology and Biogeography 23, 286–296. [Google Scholar]
- Stowe LG, Teeri JA.. 1978. The geographic distribution of C4 species of the dicotyledonae in relation to climate. The American Naturalist 112, 609–623. [Google Scholar]
- Teeri JA, Stowe LG.. 1976. Climatic patterns and the distribution of C4 grasses in North America. Oecologia 23, 1–12. [DOI] [PubMed] [Google Scholar]
- Tokuoka T. 2007. Molecular phylogenetic analysis of Euphorbiaceae sensu stricto based on plastid and nuclear DNA sequences and ovule and seed character evolution. Journal of Plant Research 120, 511–522. [DOI] [PubMed] [Google Scholar]
- VanDerWal J, Beaumont L, Zimmerman N, Lorch P, Blodgett D.. 2014. climates: methods for working with weather & climate. https://rdrr.io/github/jjvanderwal/climates/
- Vogel JC, Fuls A, Danin A.. 1986. Geographical and environmental distribution of C3 and C4 grasses in the Sinai, Negev, and Judean deserts. Oecologia 70, 258–265. [DOI] [PubMed] [Google Scholar]
- WCSP. 2021. World checklist of selected plant families. Facilitated by the Royal Botanic Gardens, Kew. http://wcsp.science.kew.org/ Retrieved 10 September 2021.
- Webster GL. 1994. Classification of the Euphorbiaceae. Annals of the Missouri Botanical Garden 81, 3. [Google Scholar]
- Webster GL, Brown WV, Smith BN.. 1975. Systematics of photosynthetic carbon fixation pathways in Euphorbia. Taxon 24, 27–33. [Google Scholar]
- White R, Murray S, Rohweder M, Prince SD, Thompson KM.. 2000. Grassland ecosystems. Washington, DC: World Resources Institute. [Google Scholar]
- Whittaker RH. 1975. Communities and ecosystems. New York: MacMillan Publishing Company, Inc. [Google Scholar]
- Wickham H, Hester J, Francois R.. 2018. readr: read rectangular text data. https://cran.r-project.org/web/packages/readr/index.html
- Winter K, Holtum JAM.. 2015. Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas. Functional Plant Biology 42, 711–717. [DOI] [PubMed] [Google Scholar]
- Witwicki DL, Munson SM, Thoma DP.. 2016. Effects of climate and water balance across grasslands of varying C3 and C4 grass cover. Ecosphere 7, 1–19. [Google Scholar]
- Yang Y, Berry PE.. 2011. Phylogenetics of the Chamaesyce clade (Euphorbia, Euphorbiaceae): reticulate evolution and long-distance dispersal in a prominent C4 lineage. American Journal of Botany 98, 1486–1503. [DOI] [PubMed] [Google Scholar]
- Yang Y, Morden CW, Sporck-Koehler MJ, Sack L, Wagner WL, Berry PE, Ya Yang C.. 2018. Repeated range expansion and niche shift in a volcanic hotspot archipelago: radiation of C4 Hawaiian Euphorbia subgenus Chamaesyce (Euphorbiaceae). Ecology and Evolution 8, 8523–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SNR, Sack L, Sporck-Koehler MJ, Lundgren MR.. 2020. Why is C4 photosynthesis so rare in trees? Journal of Experimental Botany 71, 4629–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Akçay E, Edwards E, Helliker B.. 2020. The legacy of C4 evolution in the hydraulics of C3 and C4 grasses. bioRxiv doi: 10.1101/2020.05.14.097030. [Preprint]. [Google Scholar]
- Zizka A, Silvestro D, Andermann T, et al. 2019. CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods in Ecology and Evolution 10, 744–751. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that were used in this study were all from publicly available datasets which have been fully cited.