Photosynthetic chilling tolerance is composed of several physiological mechanisms, underpinned by differing amounts of genetic variation. A holistic, high-throughput approach is needed to improve chilling tolerance of photosynthesis in maize.
Keywords: Breeding, chilling stress, chilling tolerance, cold stress, cold tolerance, genetics, maize, photosynthesis, quantitative trait loci (QTL), spectroscopy
Abstract
Chilling tolerance is necessary for crops to thrive in temperate regions where cold snaps and lower baseline temperatures place limits on life processes; this is particularly true for crops of tropical origin such as maize. Photosynthesis is often adversely affected by chilling stress, yet the maintenance of photosynthesis is essential for healthy growth and development, and most crucially for yield. In this review, we describe the physiological basis for enhancing chilling tolerance of photosynthesis in maize by examining nine key responses to chilling stress. We synthesize current knowledge of genetic variation for photosynthetic chilling tolerance in maize with respect to each of these traits and summarize the extent to which genetic mapping and candidate genes have been used to understand the genomic regions underpinning chilling tolerance. Finally, we provide perspectives on the future of breeding for photosynthetic chilling tolerance in maize. We advocate for holistic and high-throughput approaches to screen for chilling tolerance of photosynthesis in research and breeding programmes in order to develop resilient crops for the future.
Introduction
Temperature is a key determinant of plant species distribution (Osmond et al., 1987; Nievola et al., 2017), and our planet is experiencing a rise in the frequency and severity of extreme temperature events (IPCC, 2018). At the same time, the world’s population is increasing rapidly, demanding a concomitant increase in global food production which will depend in part upon improved photosynthesis (Ort et al., 2015; Simkin et al., 2019). Whilst populations are stable or decreasing in many countries that grow maize, improving photosynthesis is nevertheless of relevance for maintaining crop yields in the context of temperature stresses exacerbated by climate change. In cereal crops, reproduction is the most temperature-sensitive growth stage (Yoshida et al., 1981), making temperature stress a critical limitation on yield and therefore of direct relevance for food production; plant establishment and vegetative growth are also susceptible to temperature stress. Chilling temperature stress in particular is a strong limiting factor on plant growth and survival in temperate regions, where it is the primary stress impacting germination as well as affecting subsequent growth and development including crop production (Revilla et al., 2005; Sanghera et al., 2011). Chilling tolerance, including chilling tolerance of photosynthesis, is therefore essential if plants are to survive and even to thrive in such conditions. Improving our understanding of photosynthetic chilling tolerance in crop plants is thus both critical and timely for maintaining and increasing food production to support our growing global population.
Stress affects gene expression, metabolism, physiology, and morphology (Krasensky and Jonak, 2012). Chilling tolerance involves physiological or morphological adaptations to combat chilling stress, in contrast to chilling avoidance which is achieved by seed or vegetative dormancy (Revilla et al., 2005). Chilling tolerance can occur at different time scales, which may be broadly arranged into three categories. The longest of these is adaptation to chilling stress, which occurs when plants have evolved to deal with perennially cold conditions; one example is evergreen trees down-regulating photosynthesis (Savitch et al., 2002). Next, in contrast to evolutionary adaptation, acclimation to chilling stress occurs when plants are grown under cold conditions that they do not necessarily always experience; chilling-tolerant species are those which are able to acclimate to cold temperatures (Ensminger et al., 2006). This acclimation involves the employment of survival strategies that are not constitutively expressed under all growth conditions, in response to a chronic chilling stress that persists for much of the season. Finally, on the shortest time scale, tolerance to acute chilling stress describes resilience to cold snaps—short periods of unexpected or unseasonal cold weather to which plants may not already be acclimated (Hüner et al., 2016). This review focuses on chilling tolerance in maize (Zea mays L.), a species in which the chilling response has been much studied in order to facilitate the growth of this important crop in temperate regions. Maize is the most grown cereal crop in the world, making its temperature response a critical aspect of global food security. Since maize is not adapted to deal with low temperatures, we consider the responses of maize to chronic and acute chilling stresses caused by cool seasons or cold snaps, respectively.
Plant species which originated in tropical regions are often especially sensitive to chilling stress (Sanghera et al., 2011), and maize is no exception (Miedema, 1982). We define chilling stress as the presence of suboptimal cool temperatures above 0 °C. Chilling and freezing temperatures impose stress in different ways: chilling stress imposes a direct temperature stress whilst freezing stress, which occurs at sub-zero temperatures, causes osmotic stress via the dehydration of cells when extracellular ice crystals are formed (Hincha and Zuther, 2020); the two stresses are both genetically and physiologically distinct (Revilla et al., 2005). The specific temperatures causing chilling stress vary between species, as well as between different growth stages and different organs of the plant (Revilla et al., 2005). For example, roots are especially sensitive to chilling stress, and restrictions on root growth can lead to downstream effects such as a reduced supply of water and nutrients later in development. The seed imbibition and photosynthetic initiation phases are the most chilling-sensitive phases within the seed germination and seedling growth period (Revilla et al., 2005). Chilling stress can occur at temperatures ranging from 0 to 15 °C (Revilla et al., 2005; Zhu et al., 2007; Sanghera et al., 2011; Miura and Furumoto, 2013), and temperatures within this range are used for experimental work imposing chilling stress on maize (Hu et al., 2017; Frascaroli and Revilla, 2019).
Maize originated in the tropics, but has been adapted to a range of climates. European varieties of maize, such as varieties in the ‘Flint’ race, can display greater chilling tolerance than those of tropical origin. Indeed, Flint lines are often used in northern European maize breeding to provide chilling tolerance (Riva-Roveda et al., 2016). In temperate regions, where maize production has been increasing for several decades (Fracheboud et al., 1999; Frascaroli and Revilla, 2019), early planting increases plant biomass and reduces exposure to drought and parasites, and the associated canopy coverage decreases competition with weeds. However, early planting also increases seedling stress from chilling and disease. Overall, maize establishment is more difficult in temperate regions (Jompuk et al., 2005).
Chilling stress in maize is already considered to occur at temperatures below 10–15 °C (Hu et al., 2017; Frascaroli and Revilla, 2019). Generally speaking, temperatures below 15 °C slow growth, with this threshold increasing to 20 °C in more established plants, while temperatures below 5 °C cause further cell and tissue damage, and injury to seeds and seedlings (Frascaroli and Revilla, 2019). Temperatures below 10 °C badly affect maize germination (Janowiak et al., 2002) and photosynthesis (Foyer et al., 2002), although it should be noted that maize varieties display significant variation in chilling tolerance, as discussed below. In an agricultural setting, severe chilling stress can occur below 8 °C and maize should therefore ideally be sown when temperatures exceed this threshold (Sobkowiak et al., 2014, 2016; Jończyk et al., 2017).
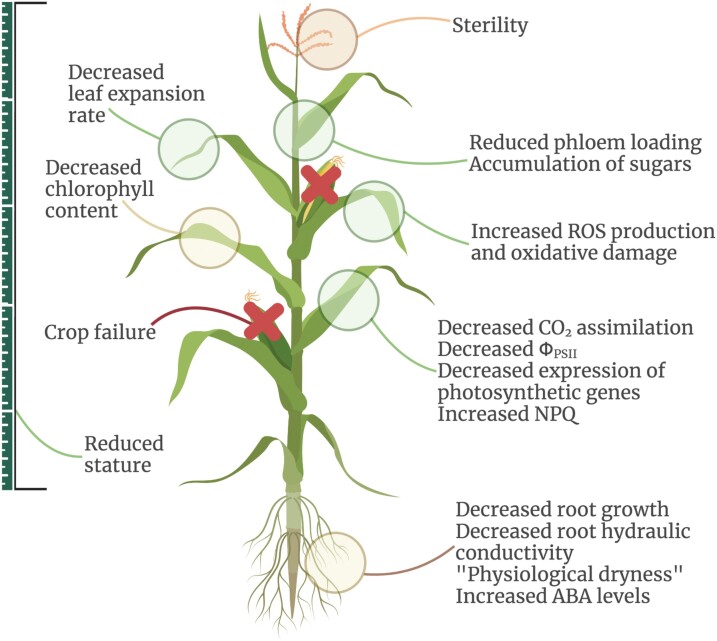
Chilling stress has multiple effects on plant morphology and function (Fig. 1). Chilling reduces plant establishment, growth, and reproduction, and leads to wilting, chlorosis, and necrosis (Revilla et al., 2005). Chlorosis can be linked to cell membrane disruption (Miedema, 1982); properties of the cell wall and membrane are important for chilling tolerance (Sanghera et al., 2011; Frascaroli and Revilla, 2019). Chilling stress affects metabolism, root growth and morphology, leaf area, number of days to emergence, germination rate, chlorophyll, and the efficiency of PSII, ΦPSII (Janowiak et al., 2002; Hund et al., 2007; Frascaroli and Revilla, 2019). Cell membrane disruption, chlorophyll bleaching, and decreased ΦPSII contribute to lowered rates of photosynthesis, impacting growth and productivity.
Fig. 1.
Effects of chilling stress on maize plants. The impacts of chilling temperatures on maize physiology and morphology can be observed across a range of key traits. Growth slows down or ceases entirely, which can be observed in decreased root growth, leaf expansion, and overall plant stature. The negative impact of chilling on the root system leads to decreased hydraulic conductance and partially mirrors drought stress responses, such as, for example, elevated abscisic acid (ABA) levels. Chilling also strongly impacts photosynthetic performance, which can be observed in decreases in CO2 assimilation, PSII operating efficiency (ΦPSII), and down-regulation of photosynthetic genes; this can be further compounded by the accumulation of sugars due to decreased phloem loading. In addition, photoprotection via non-photochemical quenching (NPQ) is up-regulated to mitigate the imbalance between light-dependent and independent reactions; nevertheless chilling enhances the accumulation of reactive oxygen species (ROS) as well as the breakdown of chlorophyll. Finally, chilling around the generative stages can strongly impact yield via male sterility and expansion of the anthesis–silking interval, leading to crop failure. Created with BioRender.com.
Chilling tolerance is a complex polygenic trait (Tokuhisa and Browse, 1999; Thomashow, 2001) and its genetic regulation is not well understood (Frascaroli and Revilla, 2019). Chilling tolerance in maize is regulated independently at different growth stages (Hodges et al., 1997; Revilla et al., 2000) and, furthermore, there are interactions between genotype and environment (Fracheboud et al., 2004; Revilla et al., 2005; Presterl et al., 2007). Genes involved in chilling tolerance may be identified through transcriptomic, proteomic, or genomic approaches (Frascaroli and Revilla, 2019). Variation in traits of interest may be mapped to the genome using genomic markers such as single nucleotide polymorphisms (SNPs) or quantitative trait loci (QTL). SNPs are particularly useful for performing genome-wide association studies (GWAS) which can identify QTL and increase the resolution of QTL mapping (Hu et al., 2017; Frascaroli and Revilla, 2019; Li et al., 2021). Genetic mapping performed using SNPs can also be used for marker-assisted selection and to identify candidate genes (Miculan et al., 2021; Waqas et al., 2021). Once identified, candidate genes relating to physiological traits of interest may be classified according to functional characteristics using Gene Ontology (GO) terms; GO term annotations are now available for all of the protein-coding genes in maize (Wimalanathan et al., 2018).
In this review, we examine the physiology of photosynthetic chilling tolerance in maize, genetic variation in photosynthetic chilling tolerance, and the genetic elements underpinning this variation, in order to address the question: Can we improve the chilling tolerance of maize photosynthesis through breeding?
Physiology of photosynthetic chilling tolerance
In order to survive a period of chilling stress, plants must adjust their physiological processes to minimize damage. Maize plants display a range of chilling responses (Fig. 1), and these occur on different time scales. In this section, we examine the major physiological responses to chilling stress in maize, including both immediate and longer term responses, which enable plants to react to acute and chronic chilling stress. Some responses are indicative of protective mechanisms that mitigate the effects of chilling stress, whilst other responses reveal that damage has already occurred. We outline three categories of physiological response to chilling stress, organized according to the time scales in which they have been reported to occur: photosynthetic responses; photoprotective responses; and signalling and developmental responses. Finally, to conclude this section, we consider the most appropriate physiological measurements for screening natural genetic variation in photosynthetic chilling tolerance.
Photosynthetic responses to chilling: carbon assimilation
Maize carries out C4 photosynthesis, which involves a biochemical carbon-concentrating mechanism that helps to increase photosynthetic efficiency, especially under hot and dry conditions. Atmospheric CO2 equilibrates with bicarbonate and is firstly fixed—via phosphoenolpyruvate carboxylase (PEPC; BRENDA: EC 4.1.1.31)—into the 4-carbon metabolite oxalo-acetate in the mesophyll. Oxalo-acetate is converted to malate which diffuses along a concentration gradient inwards from the mesophyll to the bundle sheath cells. In the chloroplasts of the bundle sheath cells where Rubisco (BRENDA: EC 4.1.1.39) is compartmentalized, decarboxylation of malate mediated by NADP-malic enzyme (BRENDA: EC 1.1.1.40) releases CO2 while reducing NADP+ to NADPH. This carbon-concentrating mechanism augments the CO2:O2 ratio and thus increases the efficacy of ribulose bisphospate (RuBP) carboxylation by Rubisco in the Calvin–Benson–Bassham cycle by competitive inhibition of RuBP oxygenation.
The initial physiological responses to chilling stress in maize are related to carbon assimilation. Firstly, the capacity and rate of net CO2 assimilation decrease (Fig. 2) when plants are temporarily exposed to chilling stress. This can already be observed after 2 h exposure to 4 °C chilling stress and was more pronounced after a longer chilling stress of 16 h (Ying et al., 2002), as well as being observed after a chilling stress of 6 h (Aguilera et al., 1999). In both of these studies, measurements of CO2 assimilation were made during a recovery period following the chilling stress period. The decrease in net CO2 assimilation rate is a highly sustained response, which has been reported in many studies after 1 d (Dwyer and Tollenaar, 1989; Ying et al., 2000; Aroca et al., 2001; Riva-Roveda et al., 2016), 2–3 d (Ying et al., 2000), and 8 d of chilling stress (Lee et al., 2002). The measurements by Dwyer and Tollenaar and those by Ying et al. were carried out during recovery after chilling stress, whilst the other studies performed measurements during the chilling stress treatment, indicating that measurements both during and after a chilling stress period may be used to measure decreased CO2 assimilation that occurs during chilling and persists during recovery. A decrease in CO2 assimilation rate was also reported in several studies which imposed a chilling stress for the duration of the experimental period (Nie and Baker, 1991; Kingston-Smith et al., 1999; Ying et al., 2002; Fracheboud et al., 2004; Zaidi et al., 2010; Rodríguez et al., 2014).
Fig. 2.
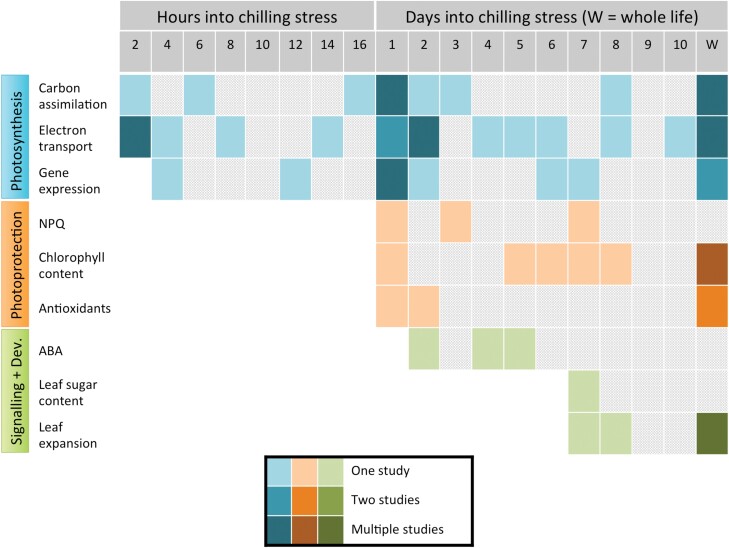
Timeline of maize responses to chilling stress for nine physiological variables. Variables are grouped in three categories: photosynthetic responses in blue, photoprotective responses in orange, and signalling and developmental responses in green. Grey hatching indicates the projected time range during which the response is expected to occur, with confirmed time points indicated by coloured boxes. The darker the colour, the greater the number of studies reviewed here that reported the trend at any given time point. Studies included here do not necessarily include genetic variation, but must demonstrate the relevant response to chilling stress in at least one maize line. Many studies reveal effects following a treatment lasting the duration of the experiment, denoted by ‘W’ for the whole experimental life-span. NPQ, non-photochemical quenching; ABA, abscisic acid.
Various mechanisms may contribute to the sustained decrease in CO2 assimilation including reduced enzyme activity, collapse of metabolic gradients between mesophyll and bundle sheath cells, damage to the photosystems, and increased diffusive limitations to CO2 uptake. Photosynthetic enzyme activities are often reduced under chilling stress (Avila et al., 2018). The activities of fructose bisphosphatase (BRENDA: EC 3.1.3.11), Rubisco, and PEPC decrease in chilled maize leaves (Kingston-Smith et al., 1997). At cooler temperatures, the speed of atomic movement and the rate of collisions decrease; many enzymatic processes are attuned to operate best within a range of optimal temperatures and will therefore perform relatively poorly outside of the relevant range. Rubisco has been speculated to be especially limiting in chilling conditions in C4 species, since C4 plants contain less Rubisco and because Rubisco is operating closer to its maximum capacity due to the high concentration of CO2 created by the carbon-concentrating mechanism (Sage and McKown, 2006). Furthermore, enhanced degradation of photosynthetic gene products under chilling stress reduces the amounts of enzymes in the leaf: protein breakdown is increased at low temperatures (reviewed by Sales et al., 2021). Specifically, the photosynthetic enzymes pyruvate, phosphate dikinase (PPDK; BRENDA: EC 2.7.9.1), PEPC, and Rubisco break down more easily under chilling conditions in C4 species (Kingston-Smith et al., 1997; Du et al., 1999; Chinthapalli et al., 2003). This increased lability means that greater enzyme synthesis is required to maintain a given activity, and therefore decreases the overall enzyme activity across the leaf. The amounts and activities of enzymes can also trade off against one another as part of the chilling stress response. For example, a decrease in Rubisco content coupled with an observed increase in Rubisco activation state may indicate an up-regulation of activation in order to compensate for the lower enzyme content observed during a chronic chilling stress experiment in maize (Kingston-Smith et al., 1999).
While stomatal conductance is usually not a strong constraint to photosynthesis in maize, it also decreases strongly under chilling conditions (Lee et al., 2002), which may enhance the diffusional limitation to CO2 uptake. However, since CO2 assimilation and stomatal conductance are strongly coordinated, the stomatal closure response is more likely to be a reflection of the chilling-induced decreases in CO2 assimilation.
Photosynthetic responses to chilling: electron transport
As well as a decrease in net CO2 assimilation, chilling stress causes a decrease in the operating efficiency of PSII in the light (ΦPSII), which is derived from measurements of chlorophyll fluorescence (Maxwell and Johnson, 2000; Baker, 2008). Down-regulation of ΦPSII in response to chilling occurs in parallel with changes in CO2 assimilation, being observed as early as 2 h into chilling stress (Fig. 2), measured both directly during chilling stress (Fracheboud et al., 2002) and via a decrease in the maximum quantum efficiency of PSII photochemistry, Fv/Fm, determined during recovery following 2 h of chilling (Ying et al., 2002). Decreases in Fv/Fm were also reported at 2, 4, and 8 h into a chilling stress period, with greater decreases observed as time progressed (Dolstra et al., 1994). The down-regulation of ΦPSII has also been reported a few hours after the imposition of chilling stress (Sobkowiak et al., 2014); after 1 d of chilling stress (Sobkowiak et al., 2014, 2016), and after 2 d (Janowiak et al., 2002; Sobkowiak et al., 2014; Urrutia et al., 2021), 4 d (Urrutia et al., 2021), 5 d (Sobkowiak et al., 2014), 6 d (Urrutia et al., 2021), 8 d (Lee et al., 2002), and 10 d (Kościelniak et al., 2005). Each of these results was obtained during the period of chilling stress, although the study by Janowiak et al. also included measurements made during a recovery period which are not reported here. In the case of the measurements by Kościelniak et al., the chilling stress was even augmented at the time of measurement, with measurements made at 6 °C following a period of 10 d at 15 °C. As has been demonstrated for the CO2 assimilation rate, the chilling-induced decrease in ΦPSII persists during prolonged periods of chilling stress, being reported by studies imposing chilling stress for the duration of the experiment (Fracheboud et al., 1999; Kingston-Smith et al., 1999, 2004; Hund et al., 2007).
Balancing ΦPSII with CO2 assimilation enables plants to maintain an appropriate energy balance, regulated by redox and pH changes as well as calcium signalling initiated by changes in plasma membrane fluidity (Ensminger et al., 2006). CO2 assimilation and ΦPSII are correlated, but the relationship between them is not always constant. For example, the relationship between CO2 assimilation and ΦPSII can change under chilling conditions, with higher values of ΦPSII relative to CO2 assimilation (Fryer et al., 1998). However, this is not always the case, with other studies reporting a more sustained relationship between CO2 assimilation and ΦPSII during chilling stress (Kingston-Smith et al., 1997; Foyer et al., 2002), particularly when irradiance is stable (Earl and Tollenaar, 1998). PSII is chronologically the first of two photosystems in linear photosynthetic electron transport, which produces ATP and reductant (NADPH) for subsequent use in the C4 acid shuttle and the Calvin–Benson–Bassham cycle to assimilate CO2 into carbohydrates. PSII is typically thought to be more susceptible to chilling stress than PSI (Kočová et al., 2009). The PSII reaction centre protein D1 is easily damaged, leading to photoinhibition and reduced rates of photosynthesis; this can occur in chilling conditions, particularly when irradiance is high (Farage and Long, 1987). However, PSI is also easily damaged under chilling conditions and sharp fluctuations in light intensity (Kono et al., 2014).
Down-regulation of photosynthetic electron transport may not just be a result of run-away damage to the photosystems under chilling conditions. Instead, reversible down-regulation of PSII activity via non-photochemical quenching (NPQ), or more long term via halting the D1 protein repair cycle, may also be initiated under chilling conditions in order to balance carbon sources and sinks and to reduce the potentially damaging effects of excessive light energy and concomitant production of reactive oxygen species (ROS; Ensminger et al., 2006). Chilling stress reduces the metabolic sink, and photosynthesis must respond in order to maintain an appropriate carbon source:sink balance which is essential for maintaining healthy growth (Ensminger et al., 2006; Burnett et al., 2016; White et al., 2016). Evidence for this hypothesis comes from other plant species including evergreens and Arabidopsis, in which chilling acclimation led to alterations to the thylakoid membrane, sucrose synthesis enzyme expression, and Calvin cycle enzyme expression, all of which have been identified as balancing regulators of the carbon source and sink enabling photosynthetic acclimation to chilling stress (Hüner et al., 1998, 2003; Stitt and Hurry, 2002). In maize, expression of a sucrose synthase increased in response to chilling stress (Urrutia et al., 2021), as has been seen in Arabidopsis (Stitt and Hurry, 2002), and down-regulation of the expression of photosynthetic enzymes is also observed, as we outline in the following section.
Photosynthetic responses to chilling: gene expression
Following soon after the down-regulation of CO2 assimilation and ΦPSII is a down-regulation of photosynthetic gene expression (Fig. 2). While not as rapid as the decreases in CO2 assimilation and PSII operating efficiency, down-regulation of photosynthetic gene expression (i.e. the abundance of photosynthesis-related transcripts) has been reported as early as 4 h after the beginning of chilling stress (Li et al., 2019), after 12 h in another study (Yu et al., 2021), and after 1 d of chilling stress in several studies (Trzcinska-Danielewicz et al., 2009; Zhang et al., 2009; Jończyk et al., 2017; Avila et al., 2018; Banović Đeri et al., 2021). A decrease in photosynthetic protein accumulation occurs soon after, reported after 2 d of chilling stress (Urrutia et al., 2021). This decrease may be caused by the transcriptional or translational down-regulation of photosynthetic genes leading to a reduction in protein synthesis; by the damage and breakdown of photosynthetic proteins discussed above; by damage to the cellular machinery responsible for the synthesis and repair of proteins; or by a combination of these factors. Similarly to the other photosynthetic responses detailed in this section, the down-regulation of the expression of genes involved in photosynthesis persists during longer periods of chilling stress, being reported after 6 d (Szalai et al., 2018), 7 d (Riva-Roveda et al., 2016), and in long-term studies of chilling stress (Nie and Baker, 1991; Kingston-Smith et al., 1999). This sustained response of down-regulation of gene expression contributes to the sustained low rates of photosynthesis observed over long periods of chilling stress.
Photoprotective responses to chilling: NPQ, chlorophyll content, and reactive oxygen species
Since enzymatic reactions are more strongly affected than the photochemical electron transfer processes on the thylakoid membrane, chilling can lead to over-reduction of electron transfer components, and can promote production of damaging ROS. As a result, exposure to chilling tends to induce photoprotective responses to mitigate these issues. Three potentially photoprotective responses can already be seen after 1 d of chilling stress in chilling-tolerant maize plants: increased levels of NPQ, a decrease in chlorophyll content, and an alteration in antioxidant enzymes or oxidative damage (Fig. 2). These responses should be interpreted with caution, as each of these potentially photoprotective mechanisms may also be a reflection of damage caused by chilling stress.
NPQ is a compound term that encompasses a range of different non-photochemical quenching mechanisms to dissipate excitation energy in the light-harvesting antennae (reviewed by Malnoë, 2018). Some forms of NPQ are readily reversible such as energy-dependent quenching (qE), which is primarily controlled by the pH of the thylakoid lumen. In contrast, photoinactivation of the PSII reaction centre protein D1 gives rise to a sustained photoinhibitory qI-type quenching, namely quenching which leads to a long-term depression of the quantum yield of CO2 fixation. Unlike qE which may be activated or deactivated within minutes, qI is not rapidly reversible as it requires molecular repair. A decrease in Fv/Fm after dark-acclimation indicates the presence of photoinhibition (Fracheboud et al., 1999). Increases in NPQ have been observed after 1 d of chilling stress in some maize lines (Fig. 2), and may continue after an additional 2 d or 6 d depending on the line (Riva-Roveda et al., 2016; measurements made during chilling stress). Further resolving the different forms of NPQ that are specifically up-regulated in response to chilling will be important for elucidating the photoprotective or photoinhibitory nature of these responses.
A strong decrease in leaf chlorophyll content can often be observed in young maize plants grown under suboptimal temperature. This phenotype may be a manifestation of excessive oxidative damage to chlorophylls in the light-harvesting antennae leading to photobleaching, but may also form part of a reorganization and restructuring of the light-harvesting capacity as a photoprotective response to chilling stress (Ensminger et al., 2006). A decrease in chlorophyll content can already be observed after 1 d of chilling stress (Avila et al., 2018). This effect has also been reported after 5 d (Aroca et al., 2001), 6 d (Szalai et al., 2018), 7 d (Riva-Roveda et al., 2016), and after 8 d (Lee et al., 2002; Fig. 2). These measurements were all performed during the chilling stress period, although Aroca et al. (2001) also included a subsequent recovery period, not reported here. Furthermore, a decrease in chlorophyll content is a highly sustained response to chilling stress, with multiple studies reporting decreased chlorophyll content after a chilling stress that was imposed for the whole life of the maize plants prior to measurement, suggesting that the potential for acclimation may be limited (Nie and Baker, 1991; Kingston-Smith et al., 1999; Fracheboud et al., 2004; Hund et al., 2007; Rodríguez et al., 2008).
Closely intertwined with chilling effects on leaf chlorophyll content, alterations in antioxidant capacity also manifest after 1 d of chilling stress (Fig. 2). Increased antioxidant enzyme activities were found in a chilling-tolerant maize variety (Aroca et al., 2001), whereas the antioxidant molecule ascorbic acid decreased after 30 h of chilling stress in chilling-sensitive sweet-corn seedlings (Xiang et al., 2020), both measured during chilling stress. Alterations to antioxidant capacity can be very persistent in response to long-term chilling. Increases in several antioxidant enzyme activities were observed across a range of maize genotypes in response to 26 d of chilling stress. In this case, superoxide dismutase, ascorbate peroxidase, and glutathione reductase all showed increased activity, whilst the response of catalase activity was dependent on the genotype (Kočová et al., 2009). These changes in anti-oxidant capacity may impact accumulation of ROS. For example, increased hydrogen peroxide levels were observed in leaves exposed to 14 °C (Kingston-Smith et al., 1999), which may reflect enhanced oxidative stress levels. In maize, the localization patterns of antioxidant enzymes between bundle sheath and mesophyll tissue (Doulis et al., 1997) increase the propensity for oxidative damage (Kingston-Smith et al., 1999; Foyer et al., 2002). Reduced metabolite transport between the bundle sheath and mesophyll tissues under chilling conditions increases oxidative stress in the bundle sheath, since antioxidant enzymes are primarily localized in the mesophyll (Kingston-Smith and Foyer, 2000).
Signalling and developmental responses to chilling: ABA, leaf sugar content, and leaf expansion
Lastly, we outline three responses related to signalling and development that occur in response to chilling stress (Fig. 2). The fastest of these three is an increase in the level of abscisic acid (ABA) which was already observable after 2 d as well as after 4 d and 5 d of chilling stress, and correlates with chilling tolerance (Capell and Dörffling, 1993; Janowiak and Dörffling, 1996; Janowiak et al., 2002), and has also been confirmed under field conditions (Janowiak et al., 2003). It is well known that ABA is involved in the response to drought stress and exhibits crosstalk with several metabolic and regulatory pathways (Ensminger et al., 2006; Sreenivasulu et al., 2012; Munemasa et al., 2015; Sah et al., 2016; Zhu, 2016). Guard cells are subject to ABA regulation, which stimulates stomatal closure during drought. In chilling stress conditions, ABA may therefore contribute to a sustained decrease in stomatal conductance to CO2 such as has been reported by Lee et al. (2002).
While increased ABA levels can occur relatively rapidly, a longer term response to chilling stress can be seen in the leaf soluble sugar content, which has been reported to increase after 7 d of chilling, measured during the chilling stress period (Riva-Roveda et al., 2016). This increase could be a result of a decrease in phloem loading, due to chilling-induced restrictions on transport (Krapp and Stitt, 1995; Ainsworth and Bush, 2011). Alternatively, the increase in foliar sugar content may be a physiological response to maintain turgor pressure when water transfer from the roots is impaired by chilling stress. The accumulation of foliar sugars initiates negative feedback repression of photosynthesis (Krapp and Stitt, 1995; Smeekens et al., 2010), which may contribute to the sustained reduction in net CO2 assimilation discussed above.
Finally, long-term exposure to chilling leads to a pronounced reduction in growth rate, which can be observed very clearly in a decline of leaf expansion rate. This common phenotype is often included in studies examining plants over multiple days of chilling (e.g. Riva-Roveda et al., 2016). The slowing of leaf expansion and appearance rate can be striking. For example, the time taken to reach the leaf 8 stage (the growth stage at which leaf 8 is the most recent fully expanded leaf, where leaf 8 is the eighth leaf to appear on the plant) was tripled after 8 d of chilling stress at 15/13 °C at the leaf 7 stage compared with plants grown under control conditions (Lee et al., 2002). To account for the decreased rate of leaf expansion under long-term chilling conditions, many studies compare control and chilling-treated plants at the same developmental stage rather than at the same time point (Fracheboud et al., 1999, 2002, 2004). However, this can give rise to extreme age differences between treatment and control groups, where the chilling-treated plants can sometimes take twice as long to reach the same developmental stage (Rodríguez et al., 2008). Whilst increases in foliar ABA and soluble sugars have not yet been demonstrated to be sustained effects, a decrease in leaf expansion rate is clearly a persistent effect during chilling stress.
Screening for chilling stress responses
Considering the nine responses outlined in this section (Fig. 2), the four most studied components of the physiological response to chilling stress are consistent between studies focused on exploring effects of chilling on physiological processes and studies focused on examining genetic variation in chilling tolerance. These four components are the three ‘photosynthesis’ parameters, and chlorophyll content. However, the degree to which each parameter is used varies between physiology- and genetics-focused studies. Assessing the studies of the photosynthetic chilling response in maize reviewed here, in physiological studies net CO2 assimilation rate is the most frequently studied parameter, followed by photosynthetic gene expression, ΦPSII, and chlorophyll content. In contrast, in genetics-focused studies, this order is reversed, with chlorophyll content being the most frequently studied parameter, followed by ΦPSII, photosynthetic gene expression, and net CO2 assimilation. In both types of study, the remaining five responses (NPQ, antioxidant enzymes or antioxidant damage, ABA, leaf sugar content, and leaf expansion) are used relatively less frequently.
The different emphasis on each of the three photosynthesis parameters and chlorophyll content between physiology- and genetics-focused studies reflect the different priorities of the two types of study. For studies of genetic variation, chlorophyll content and ΦPSII provide rapid, relatively high-throughput proxies for chilling stress which are useful for screening large populations and carrying out genetic mapping, whilst measurements of the net CO2 assimilation rate are less high-throughput but provide more physiological detail and are therefore favoured by studies focusing on the physiological responses of maize to chilling stress. Regarding the proxies for photosynthetic chilling tolerance favoured by genetics-focused studies, we note that chlorophyll fluorescence is a particularly valuable screening tool (Fracheboud et al., 1999; Baker, 2008). Specifically, ΦPSII provides a useful means of distinguishing between chilling-tolerant and chilling-susceptible lines, and has been used in initial breeding attempts to enhance chilling tolerance (Fracheboud et al., 1999). Fluorescence measurements are non-destructive, facilitating repeated measurements during an experimental time course. Chlorophyll content may be measured destructively using pigment analysis following extraction in solvent, but may also be estimated non-destructively from transmittance at a few specific wavelengths with a SPAD meter or more elaborate spectrometry (Avila et al., 2018), both providing great rapidity and the ability to repeat measurements throughout a time course compared with biochemical pigment analysis. A major advantage of chlorophyll fluorescence over chlorophyll content is the versatility and available diversity of fluorescence measurements. Depending on the instrument and protocol used, a measurement of a few minutes may suffice to provide Fv/Fm, ΦPSII, and NPQ.
However, since both ΦPSII and chlorophyll content may be decreased during stress for protective reasons or due to photodamage, it is advantageous to include a metabolic component such as net CO2 assimilation rate or leaf sugar content in parallel to allow more robust conclusions about the nature of the chilling response to be drawn. The time scale of the response is also relevant: short-term down-regulation of ΦPSII or initiation of NPQ could be a photoprotective response, whilst long-term differences in ΦPSII between genotypes are more likely to indicate variation in the capacity for sustained photosynthesis under chilling conditions and may therefore reveal chilling tolerance or susceptibility.
Genetic variation in chilling tolerance
Having established the primary physiological responses to chilling stress in maize, we now examine the evidence for genetic variation within maize germplasm across these responses. Our focus is on naturally occurring genetic variation, which provides a useful pool of resources for breeding plants with greater chilling tolerance of photosynthesis (Faralli and Lawson, 2020). Evidence for genetic variation in photosynthetic chilling tolerance can become apparent whenever lines with contrasting chilling tolerance are studied. Studies containing two—or a few—lines may be used to identify differentially expressed genes (DEGs) in response to chilling between tolerant and susceptible lines. In contrast, large populations with sufficient phenotypic variation and tractable genotypic variation are needed for the identification of QTL or SNPs that significantly correlate with variation in chilling tolerance.
Reflecting on the nine physiological responses identified in the previous section, several studies have looked at gene expression changes in conjunction with chilling treatments in tolerant and susceptible maize lines, and candidate genes have been identified for chilling-related variation in CO2 assimilation rate, ΦPSII, photosynthetic gene expression, chlorophyll content, antioxidant capacity, leaf sugar content, and morphology related to leaf expansion (summarized in Table 1), but not for NPQ or ABA. In addition, several studies have used chilling-related variation in CO2 assimilation rate, ΦPSII, photosynthetic gene expression, NPQ, and chlorophyll across mapping populations to identify QTL for each of these traits. SNPs significantly correlated with variation in CO2 assimilation, ΦPSII, chlorophyll, and morphology related to leaf expansion have also been identified (Table 1). In contrast, we could not find any studies where genetic mapping was used for variation in antioxidant capacity, ROS accumulation and oxidative damage, ABA, or leaf sugar content in response to chilling (Table 1). Genetics-focused studies of photosynthetic chilling tolerance typically measure CO2 assimilation, ΦPSII, photosynthetic gene expression, and chlorophyll content. Considering these four traits, some general trends emerge in studies that have examined genetic variation in two or more genotypes (Table 1). Overall, decreases in CO2 assimilation, ΦPSII, and chlorophyll content are generally less pronounced in chilling-tolerant genotypes compared with chilling-sensitive genotypes, indicating that lower values of ΦPSII and chlorophyll content may be more likely to reflect the result of photodamage rather than photoprotection in chilling-sensitive lines.
Table 1.
Genetic mapping and candidate genes for nine physiological responses to chilling stress in maize
| Study | Genetic variation | Genetic mapping | Candidate genes |
|---|---|---|---|
| CO 2 assimilation rate | |||
| F2:3 population from chilling-tolerant (ETH-DH7) and chilling-sensitive (ETH-DL3) lines. 15/13 °C for whole life following establishment; measured leaf 3 (Fracheboud et al., 2004) | Yes | Yes—QTL for carbon exchange rate (a measurement of CO2 assimilation)a | No |
| 233 RILs from drought-tolerant (Ac7643) and drought-susceptible (Ac7729/TZSRW) lines. 15/13 °C for whole life following establishment; measured leaf 3 (Fracheboud et al., 2002) | Yes | Yes—QTL for CO2 fixation; eight regions with QTL for photosynthetic traits; pericentromeric region of chromosome 3 a key locationb | No |
| 226 F2:3 families from ETH-DH7×ETH-DL3 and 168 F2:4 from Lo964×Lo1016 (different chilling tolerance at germination and different root morphology). 15/13 °C for 14 d following establishment; measured leaf 3 (Hund et al., 2005) | Yes | Yes—QTL for carbon exchange rate | No |
| 282 inbred lines. 8 °C at germination (Hu et al., 2017) | Carbon exchange rate not measured directly | Yes—SNPs related to carbon exchange rate in other studies | Yes—identified 18 candidate genes in totalc |
| 49 inbred lines. 15/13 °C at 7 leaf stage, measured leaf 8 (Lee et al., 2002) | Yes | No | No |
| PSII operating efficiency (ΦPSII) | |||
| F2:3 population from ETH-DH7×ETH-DL3. Early and late sowing in the field provided chilling treatment (Jompuk et al., 2005) | Yes | Yes—QTL for ΦPSII located on chromosomes 2, 4, 6, 8, 9 (most prominent on 6) | No |
| Population from chilling sensitive×tolerant inbred lines. 14/8 °C for the duration of the experiment. (Rodríguez et al., 2014) | Yes | Yes—two QTL for maintenance of ΦPSII in chilling stressd | No |
| Fracheboud et al. (2004) | Yes | Yes—QTL for ΦPSII | No |
| Fracheboud et al. (2002) | Yes | Yes—QTL for ΦPSII | No |
| Hund et al. (2005) | Yes | Yes—QTL for ΦPSII, located on different chromosomes in the different populations | No |
| 168 F2:4 families from Lo964×Lo1016 (see above). 15/13 °C for the duration of the experiment; measured at first leaf stage (Hund et al., 2004) | Yes | Yes—four QTL for ΦPSII | A locus for ΦPSII was identified |
| One chilling-tolerant and one chilling-sensitive line. (ETH-DH7 and ETH-DL3). 8/6 °C imposed for 14 h at third leaf stage (Sobkowiak et al., 2014) | Yes | Yes—DEGs adjacent to QTL for chlorophyll fluorescence | Yes—overall, identified 66 genes responding differently between lines (DEGs) |
| Lee et al. (2002) | Yes | No | No |
| Two panels: 306 Dent lines and 292 Flint lines. 14/8 °C for duration of experiment (Revilla et al., 2016) | Yes | Yes—two SNPs for ΦPSII in chilling stress in Flint population (chromosomes 1, 4); QTL for ΦPSII. Overall, more QTL for chilling tolerance were identified in the Flint panel |
Yes—performed GWAS and identified candidate genes |
| Three breeding groups, total 375 inbred lines. 16/13 °C. (Strigens et al., 2013) | Yes—significant differences in ϕPSII between the breeding groups | Yes—identified three QTL for ΦPSII (two under chilling stress, one only under optimal conditions) | No |
| Photosynthetic gene expression | |||
| Sobkowiak et al. (2014) | Yes | Yes—DEGs adjacent to QTL for C4 enzymes | Yes (see above) |
| One chilling-tolerant (S68911) and two chilling-sensitive lines (S160 and S50676). 14/12 °C for 4 d then 8/6 °C for 4 d at third leaf stage (Sobkowiak et al., 2016) | Yes | No | Yes—GO enrichment identified photosynthetic genes |
| Two unrelated inbred lines: CG60, CG102. 14/2 °C for 3 d at second leaf stage; measured after 1 d chilling (Avila et al., 2018) | Yes | No | Yes—GO term analysis identified photosynthetic genes down-regulated in chilling stress |
| Four stress-sensitive ‘Lancaster’ lines, four tolerant lines. 6/4 °C for 24 h at fourth leaf stage (Banović Đeri et al., 2021) | Yes | No | Yes—seven DEGs including photosynthetic genes. Differential expression between genotypes and treatment/control and between genotypes |
| One chilling-tolerant (M54), one chilling-sensitive (753F) line. 4 °C chilling stress for up to 24 h at fourth leaf stage (Li et al., 2019) | Yes | No | Yes—chilling stress affected photosynthetic genes |
| One chilling-tolerant (B144), one-chilling sensitive (Q319) line. 5 °C chilling stress for 12 h or 24 h at third leaf stage (Yu et al., 2021) | Yes | No | Yes—up-regulation of the D1 protein psb29 after 24 h (following initial decrease at 12 h) enabled B144 to protect PSII from photooxidation |
| Non-photochemical quenching (NPQ) | |||
| Fracheboud et al. (2002) | Yes | Yes—QTL for xanthophylls | No |
| A chilling-sensitive inbred line (A661) and B73. 15 °C for the duration of the experiment (Rodríguez et al., 2013) | Yes—lower xanthophylls in A661 | No | No |
| Chlorophyll content | |||
| 302 RILs from B73×Mo17. 14/8 °C for the experiment duration; measured after 30 d (Rodríguez et al., 2008) | Yes—measured chlorophyll using optical scale | Yes—QTL identified on chromosomes 3 and 6, under chilling conditions onlye | QTL on chromosome 6 may correspond to luteus11 locus |
| Fracheboud et al. (2004) | Yes | QTL identified on chromosome 3 | No |
| Fracheboud et al. (2002) | Yes | Yes—QTL for chlorophyll | No |
| Hund et al. (2005) | Yes | Yes—QTL for chlorophyll | No |
| Hu et al. (2017) | Chlorophyll not measured directly | Yes—SNPs related to chlorophyll in other studies | Yes—see above |
| Two populations of field×sweet corn (B73×P39, 179 RILs; B73×IL14 h, 213 RILs). 14/10 °C for the experiment duration (Allam et al., 2016) | Yes | Yes—QTL for chlorophyll content | No |
| Hund et al. (2004) | Yes | Yes—seven QTL for chlorophyll | No |
| 76 accessions. 10/8 °C for whole life, measured at fourth leaf stage (Bano et al., 2015) | Yes | No | No |
| Sobkowiak et al. (2014) | Chlorophyll not measured directly | Yes—DEGs adjacent to QTL for chlorophyll content | Yes—see above |
| Lee et al. (2002) | Yes | No | No |
| Jompuk et al. (2005) | Yes | Yes—six QTL on chromosomes 1, 2, 3, 4, 10 in early-sown plants; four QTL in late-sown plantsf | No |
| Avila et al. (2018) | Yes | No | Differential expression of chloroplast genes under chilling stress |
| Rodríguez et al. (2013) | Yes—lower chlorophyll and higher chlorophyllase activity in A661 | Yes—QTL on chromosome 2 for chilling-induced albinismg | Yes—a putative gene in chlorophyll biosynthesis, and a chlorophyll-binding protein |
| Revilla et al. (2016) | Yes | Yes—two SNPs for chlorophyll in chilling stress in Dent population (chromosomes 1, 4) | Yes |
| Antioxidant enzymes, or oxidative damage | |||
| Association panel of 125 inbred lines. 6.4 °C for 7 d at third leaf stage (Huang et al., 2013) | Not measured directly | No | Candidate genes in five categories including one for antifreeze and H2O2 removal |
| Sobkowiak et al. (2014) | Not measured directly | Yes—DEGs adjacent to QTL related to antioxidant levels | Genes for antioxidant systems identified |
| Tolerant (S68911) and sensitive (B73) inbred lines. 14/10 °C for the duration of the experiment, measured at early growth stages (Jończyket al., 2021) | Not measured directly, but transcriptomic data suggest greater ROS scavenging in S68911 in chilling conditions | No | No; examined stress-response motifs and chromatin accessibility, related to chilling tolerance in the tolerant line which switched from growth to defence |
| Abscisic acid (ABA) | |||
| Jończyk et al. (2021) | Not measured directly, but transcriptomic data suggest greater ABA synthesis in tolerant line in chilling conditions | No | No—but see above |
| Leaf sugar content | |||
| Tolerant (S68911) and sensitive (S160) inbred lines. 14/12 °C for 28 h at third leaf stage (Bilska-Kos et al., 2016) | Yes—decreased phloem loading in sensitive line was observed; this leads to increased leaf sugars (not measured) | No | Yes—expression of genes involved in phloem loading |
| Leaf expansion | |||
| Huang et al. (2013) | Yes | Yes—SNPs for shoot length identified | Yes—13 genes involved in biosynthesis, metabolism, cell division, and growth |
Synthesis of studies including more than one genotype and measuring physiological responses to chilling stress. Studies are grouped according to the order of responses presented in Fig. 2. When describing each study, only chilling temperatures are included; control temperatures are omitted for brevity. Studies are listed under each applicable category but only described at the first instance.
a QTL for a range of traits explained between 37% and 54% of the phenotypic variance in this study.
b QTL explained up to 20% of phenotypic variance in this study.
c Of these 18 genes, 10 were supported by other studies and three were novel.
d These two QTL explained 19% and 6% of phenotypic variance.
e The QTL on chromosome 6, probably at the end of bin 6.03, is located near to—and may be the same as—the QTL at bin 6.04 in the IBM2 2005 Neighbors 6 map, identified by Fracheboud et al. (2004). These may correspond to the luteus11 locus which affects leaf colour (Rodríguez et al., 2008).
f A QTL related to leaf greenness on chromosome 3 was identified as being the same as a previously identified QTL related to photosynthesis, in a population derived from the same parent lines (Fracheboud et al., 2004). Of the four QTL in late-sown plants, three were common with the early-sown plants.
g This QTL explains 14% of phenotypic variation in chilling-induced albinism.
Studies measuring multiple traits across chilling-tolerant and chilling-sensitive genotypes frequently report relationships between physiological traits of interest. These relationships provide information about whether certain responses might indicate photoprotection or photodamage. For example, CO2 assimilation rate, ΦPSII, and chlorophyll content were all much lower in a chilling-sensitive line than in a chilling-tolerant line under prolonged chilling stress in a study by Fracheboud et al. (2004), indicating that reductions in ΦPSII and chlorophyll content are more likely to be related to photodamage rather than protection. Similarly, in a study of two genotypes, there was a greater decrease in ΦPSII under chilling stress in the chilling- sensitive line compared with the chilling-tolerant line (Sobkowiak et al., 2014). Similar relationships were found between CO2 assimilation rate, ΦPSII, and chlorophyll content and chilling tolerance across a diverse collection of inbred lines (Lee et al., 2002), again suggesting that decreased ΦPSII is related to photodamage rather than being a photoprotective response. Additionally, an examination of 19 lines characterized for high or low ΦPSII under chilling stress showed that the ‘high ΦPSII’ lines had a high CO2 assimilation rate, ΦPSII, and chlorophyll content under chilling stress (Hund et al., 2005). Similarly, QTL linked to higher ΦPSII under chilling stress derived from a mapping population originated from the chilling-tolerant parent (Jompuk et al., 2005); and in a phenotypic screen for the effects of long-term chilling, a ‘favourable’ allele was linked to higher ΦPSII (Fracheboud et al., 2002). Taken together, these results indicate strongly that rather than lowering ΦPSII for photoprotection, the maintenance of ΦPSII is an important aspect of photosynthetic resilience to chilling stress. Similarly, most of the ‘favourable’ alleles at the QTL linked to a relatively smaller decrease in chlorophyll content under chilling stress were derived from the chilling-tolerant parent (Jompuk et al., 2005). This indicates that in addition to limiting chilling-induced decreases in ΦPSII, the maintenance of chlorophyll is also advantageous during chilling stress, and suggests that the observed reduction in chlorophyll content may largely reflect photodamage rather than photoprotection.
Whilst the evidence provided by these studies supports the hypothesis that reduced ΦPSII and chlorophyll content are linked to photodamage, repeated measurements made during a prolonged chilling stress also reveal a protective response that may occur as a result of priming. Fracheboud et al. (2002) showed that following an initial decrease in ΦPSII in leaf 1 in response to chilling stress, which is likely to be a result of photodamage, ΦPSII in leaf 3, that was subsequently developed under chilling stress, was also decreased. In this case, the down-regulation of ΦPSII may indeed be part of photoprotective acclimatory responses. Interestingly, priming at a cool temperature prior to the imposition of a more severe chilling stress of 8 °C led to a less pronounced reduction in ΦPSII at 8 °C, compared with plants that had been exposed directly to the 8 °C treatment with no priming (Sobkowiak et al., 2016). This priming was more beneficial in the chilling-tolerant line than in the two chilling-sensitive lines used in the study where the sensitive lines always showed a greater reduction in ΦPSII than the tolerant line.
Regarding the expression of photosynthetic genes, Li et al. (2019) examined transcriptional changes in a chilling-tolerant and a chilling-sensitive maize line. They found that the number of DEGs was much greater in the tolerant line during the first 24 h of chilling stress, with 1665 DEGs after 4 h and 3970 DEGs after 24 h; in the sensitive line there were 547 DEGs after 4 h and 1766 DEGs after 24 h. This may indicate either a more wide-ranging, or a more rapid, response in the tolerant line, although a more prolonged time course would be required to confirm this. Photosynthesis-related genes showed a faster response to chilling stress in the tolerant line, whilst genes related to the light-harvesting complexes decreased after 4 h in both lines, indicating an early photoprotective response. Interestingly, genes related to ΦPSII were down-regulated after 24 h of chilling stress in the chilling-sensitive line only, which suggests that the tolerant line was not dependent on a photoprotective down-regulation of ΦPSII. Indeed, in the sensitive line, a greater decrease in Fv/Fm coupled with an increase in Fo (the minimum fluorescence value measured after dark adaptation) indicated that photoinhibition and photodamage had occurred.
Many studies examining changes in chlorophyll content in response to chilling have identified both QTL and candidate genes, whilst few studies have identified candidate genes relating to the chilling-induced decrease in net CO2 assimilation rate (Table 1). CO2 assimilation is a complex trait, relying upon the amount, activation state, and activity of a range of enzymes, as well as the physiological status of the leaf, such as the status of the photosystems involved in the light reactions, plasmodesmatal conductivity to facilitate metabolite transfer, phloem loading rate, and—although only to a certain extent in C4 species—stomatal aperture. In contrast, chlorophyll content depends primarily on the synthesis and breakdown of chlorophyll, although of course the efficacy of chlorophyll in photosynthesis further depends upon its binding and coordination within the light-harvesting complexes. Because the regulation of chlorophyll content is less complex than the regulation of photosynthesis, it may be more straightforward to use chlorophyll content for the identification of candidate genes to enhance chilling tolerance, rather than using CO2 assimilation or ΦPSII. Candidate genes involved in the regulation of chlorophyll content under chilling stress have been identified, with more studies reporting candidate genes for chlorophyll than any of the other traits, with the exception of gene expression changes under chilling stress (Table 1). In spite of the relative paucity of candidate genes related to net CO2 assimilation or to ΦPSII under chilling conditions, the fact that many studies have identified QTL (or SNPs) for these two traits suggests that it will be possible to establish some candidate genes in the near future. The relative contribution of these QTL to the level of each trait in response to chilling and the persistence of this contribution in different genomic backgrounds and across different environments will be important determinants of their utility in breeding programmes.
Although several of the photosynthetic and photoprotective responses to chilling have already been used for genetic mapping studies, this is not the case for variation in antioxidant capacity in response to chilling. Candidate genes involved in antioxidant capacity were identified both as a result of mapping variation in chilling tolerance indices (Huang et al., 2013) and by making transcriptomic comparisons between tolerant and sensitive lines (Sobkowiak et al., 2014; Jończyk et al., 2021), but their involvement awaits further experimental verification since antioxidant capacity was not directly measured in any of these studies. Following up this work with direct measurements of antioxidant capacity, or with genetic mapping of variation in antioxidant capacity in response to chilling may provide another piece of the puzzle as we move towards a more complete understanding of chilling-tolerant photosynthesis in maize. Interestingly, the accumulation of zeaxanthin was negatively correlated with chilling tolerance in a study of maize genotypes differing in chilling tolerance (Fracheboud et al., 2002). While the accumulation of zeaxanthin is associated with a sustained form of NPQ (qZ; Nilkens et al., 2010), it is also a potent ROS scavenger (Havaux et al., 2007), which leaves two possible explanations for the observed negative relationship. On the one hand, the impact of zeaxanthin on NPQ may depress maize photosynthetic efficiency in response to chilling, as suggested by Fryer et al. (1995). Alternatively, the increased accumulation of zeaxanthin in sensitive genotypes could reflect a greater need for photoprotection in these genotypes. The fact that lower ΦPSII and CO2 assimilation across the sensitive genotypes in Fracheboud et al. (2002) also correlated strongly with proxies for larger light-harvesting antennae, which would increase excitation pressure per PSII reaction centre, would seem most consistent with the second explanation.
Overall, many QTL relating to the physiological components of photosynthetic chilling tolerance in maize have been identified, particularly with respect to CO2 assimilation, ΦPSII, and chlorophyll content. However, it is striking that relatively few candidate genes have been identified when considering the broad range of studies examined in this review (Table 1). This may be due to the fact that many traits are polygenic, meaning that whilst QTL may be readily identified, pinpointing genes of interest that are responsible for the traits in question is altogether more difficult. For example, CO2 assimilation is an emergent property that depends upon a plethora of physiological and molecular players, meaning that a wealth of genes underpins this complex trait. Likewise, candidate genes for ΦPSII are relatively rare and no candidate genes for NPQ have been identified (Table 1). Whilst transcriptomic analysis of photosynthetic gene expression by definition identifies the expression of photosynthesis-related genes, even chlorophyll content—which is a comparatively simple trait related to chilling tolerance of photosynthesis—does not have many associated candidate genes in the studies reviewed here, whilst for leaf sugar content the candidate genes that have been identified are involved in phloem loading rather than being more directly involved in sugar metabolism (Table 1). Finally, some genes relating to antioxidant activity and to leaf expansion have been identified, but none for ABA with respect to chilling tolerance (Table 1). It should also be noted that QTL mapping is much easier than the definitive identification of candidate genes and, since the draft genome of maize was published relatively recently (Schnable et al., 2009), the possibility of identifying candidate genes is rather new in maize compared with model species such as Arabidopsis. Furthermore, many of the studies reviewed here focused on meeting breeding objectives, for which QTL are instrumental but the identification of specific candidate genes is generally not necessary. From a physiological perspective, elucidating the causal sequence for a trait increases the possibility of successfully understanding the underlying mechanism, so studies focused on physiological goals may be more likely to pursue the identification of candidate genes rather than QTL.
Looking to the future, there exists significant diversity in ΦPSII between breeding groups and populations (Strigens et al., 2013), and this could be exploited for the development of chilling-tolerant germplasm. Future studies might investigate the genetic basis of variation in the other physiological traits we have highlighted in this review, and the contribution of this variation to chilling tolerance or susceptibility. The identification of more candidate genes will also be important, as outlined above. Due to the complexity of several responses with respect to photoprotection and damage, the use of experimental time courses in combination with phenotyping across the broader spectrum of physiological responses to chilling as outlined here will be critical for appropriate interpretation and may lead to the identification of more stable QTL and candidate genes.
High-throughput breeding approaches
Having examined the physiological basis for photosynthetic chilling tolerance and the genetic variation for this tolerance revealed in a range of populations and responses, we now return to our central question: Can we improve the chilling tolerance of maize photosynthesis through breeding? Whereas most of the responses to chilling appear to show intraspecific genetic variation in maize, appropriate interpretation of this variation requires determination of several responses in parallel across large populations.
Physiological breeding for improving photosynthetic chilling tolerance
Physiological breeding aims to incorporate physiological trait measurements into breeding programmes (Reynolds and Langridge, 2016). Such measurements can be more time-consuming and labour-intensive, but are valuable for understanding the physiological responses of plants to different stresses, especially when combined with powerful QTL analysis in the breeding context. High-throughput approaches for measuring physiological traits are therefore of great benefit; two such approaches are chlorophyll fluorescence, which has been discussed above, and reflectance spectroscopy. While measurements of ΦPSII using chlorophyll fluorescence may be readily applied in a high-throughput manner (Hund et al., 2005) and can be tailored to specific traits of interest (Maxwell and Johnson, 2000; Baker, 2008; Murchie and Lawson, 2013), several additional techniques to cover more of the nine key responses to chilling in parallel are now available. In particular, reflectance spectroscopy offers another high-throughput approach. A major advantage of this technique is that similar to fluorescence techniques, a rapid measurement (~1 s) enables the simultaneous estimation of a suite of metabolic and physiological parameters of interest via correlative models (Yendrek et al., 2017; Ely et al., 2019; Burnett et al., 2021a, b, c). For example, following the development of training datasets and models which are appropriate for the genotypes and traits of interest, the maximum carboxylation rate of Rubisco (Serbin et al., 2012; Meacham-Hensold et al., 2020), leaf protein and sugar content (Ely et al., 2019), ABA (Burnett et al., 2021b), and chlorophyll content (Yendrek et al., 2017) may all be predicted from a single hyperspectral measurement. Taken together, these parameters provide a more holistic picture of the physiological response to chilling stress and would enable quantification of photoprotective mechanisms as well as foliar damage caused by chilling. Chilling tolerance can trade off against other useful desired traits in maize (Frascaroli and Revilla, 2019); this furthers the requirement for a holistic perspective when breeding for chilling tolerance.
Hyperspectral reflectance measurements are rapid and, once equipment has been purchased, the costs per measurement are negligible. Many options are available, including leaf clips for leaf-level measurements and unmanned aerial vehicle (UAV) platforms for screening fields at the plot level. Currently, hyperspectral measurements typically need calibration within each system of interest before they can be used for trait identification. However, it is possible to predict the structural trait leaf mass per unit area (LMA) using reflectance data alone (Serbin et al., 2019), and in the future it will become increasingly feasible to predict traits of interest based on generalized models once models have been trained on wider-ranging datasets and the leaf structural and optical properties have been accounted for. This will significantly augment the utility of hyperspectral reflectance for breeding programmes.
A physiological breeding approach will be instrumental when dealing with multiple complex stresses. Rarely does a single stress occur. Rather, the dynamic field environment can impose stresses in combination, such as heat and drought stress during hot summers, or chilling and high light stress in temperate spring seasons; considering biotic stresses such as pathogens adds a further dimension. Interestingly, plant responses to stresses often overlap or compound each other. For example, a population of 233 maize recombinant inbred lines (RILs) derived from a drought-tolerant and a drought-sensitive parent was subsequently shown to contain a large degree of segregation in chilling tolerance, demonstrating strong overlap between chilling and drought stress tolerance (Fracheboud et al., 2002). Levels of ABA and proline, which are involved in responses to and alleviation of drought stress, have also been shown to be involved in acclimation to chilling stress in maize (Dory et al., 1990; Xin and Li, 1993; Revilla et al., 2005). Chilling temperature stress generates a distinct metabolic and molecular fingerprint, but also leads to responses that are shared with other stresses (Geange et al., 2021). Understanding the hallmark signs of enhanced tolerance to a combination of stresses is essential for breeding maize for an increasingly chaotic and unpredictable future climate.
Breeding for enhanced chilling tolerance must consider crop phenology and target environment
The goal of a breeding programme must be carefully considered when designing experiments destined to inform the selection and development of maize germplasm. Both field and controlled environments have limitations when it comes to conducting chilling stress experiments; combining both approaches, with multiple years and locations, is recommended for understanding and exploiting the true variation in maize chilling tolerance (Frascaroli and Revilla, 2019). The timing of the chilling stress is also important. Breeding chilling-tolerant maize able to withstand long-term chilling temperatures and acclimate to chilling conditions may give a different outcome than breeding maize able to withstand short-term ‘cold snaps’ in otherwise mild conditions. Cold snaps at any stage of growth can impact yield—by reducing germination, slowing vegetative growth and development, or inhibiting reproductive processes. Chilling tolerance does not always increase yield, and indeed there can be a trade-off between yield and stress tolerance (Revilla et al., 2005), although historic maize yield improvement has been shown to be strongly related to enhanced stress tolerance (Tollenaar and Wu, 1999). Successful breeding for chilling tolerance must consider which growth stage is of particular interest and determine which trait or combination of traits to target. Improvements in resource use efficiency are often only revealed when plants are in stressful conditions (Tollenaar and Wu, 1999). In this context, we note that chilling stress at the reproductive stage in maize is relatively understudied, and may be an important area for further research in an increasingly erratic climate.
Transgenic approaches for improving chilling tolerance of photosynthesis
While this review focuses on pre-existing variation in chilling tolerance of photosynthesis in maize, and the genomic regions related to this tolerance which may be utilized in breeding programmes, it is worth noting that genetic modification approaches also offer valuable tools for improving photosynthesis and chilling tolerance. For example, increasing Rubisco and electron transport capacity can improve the photosynthetic performance of C4 plants; Rubisco is predicted to have a greater effect on chilling recovery than other photosynthetic enzymes in the C4 pathway (Sales et al., 2021). The overexpression of Rubisco large and small subunits, in concert with Rubisco Assembly Factor 1 (RAF1), increased maize Rubisco content by >30% (although Rubisco activase is probably a vital factor for translating this increased enzyme content into a proportional increase in photosynthetic activity); this overexpression of Rubisco can speed recovery following chilling stress (Salesse-Smith et al., 2018). Transgenic introduction of chilling-tolerant PPDK into maize lowered the threshold for chilling stress in the extracted enzyme and increased photosynthesis by 23% under chilling conditions of 8 °C (Ohta et al., 2004, 2006) whilst introducing the osmoprotectant molecule glycinebetaine transgenically into maize increased photosynthesis and reduced chilling damage (Quan et al., 2004).
Transgenic work carried out in other species demonstrates useful proofs of concept, although we acknowledge that a detailed discussion of this topic is outside the scope of the present review. For example, the AlSAP gene from the grass Aeluropus littoralis has been successfully expressed in rice where it increased photosynthesis and stress tolerance when plants were exposed to a chilling treatment as well as other abiotic stresses (Ben Saad et al., 2012). Work in Arabidopsis has shown that the CBF/DREB1 transcription factors are important for the chilling response (Miura and Furumoto, 2013), and transgenic CBF/DREB1 transcription factors from Arabidopsis have been used to improve chilling tolerance in tobacco and wheat (Sanghera et al., 2011). Multiple genes, including genes from the CBF/DREB1 family, have been transgenically introduced into rice to increase chilling tolerance, highlighting the complex nature of chilling tolerance and its regulation (da Cruz et al., 2013).
Finally, the activation of latent genes already present within the genome, and a greater understanding of genetic regulatory mechanisms, are important elements of increasing chilling tolerance (Revilla et al., 2005). Transgenic approaches may also be used to investigate the presence and function of genes that already exist within the species of interest. For example, a study overexpressing a stress-responsive binding factor from the Antarctic grass Deschampsia antarctica in rice used RNA-seq to identify a candidate set of genes involved in the rice chilling stress response, putatively regulated by the D. antarctica binding factor (Byun et al., 2018). Finally, gene editing using CRISPR/Cas9 can be used to introduce specific beneficial alleles into germplasm (Waqas et al., 2021).
Expanding allelic diversity for chilling tolerance
Considering conventional breeding methods, broad genetic diversity is important for breeding (Revilla et al., 2005), and this includes diversity encompassing pre-existing variation in photosynthesis (Faralli and Lawson, 2020). Introducing germplasm from varieties or wild crop relatives adapted to high altitude and/or low temperature can aid chilling tolerance of crops (Sanghera and Wani, 2008). In maize, the use of germplasm from different environments of origin is a useful means of increasing allelic diversity for improving chilling tolerance. For example, it was shown that many Mexican highland maize landraces contain several introgressions obtained from a highland subspecies of the wild relative teosinte (Zea mays ssp. mexicana). One of these introgressions, a large chromosome inversion segment, could indeed be linked to increased chilling tolerance and improved photosynthesis under chilling conditions, including increased ΦPSII and increased chlorophyll gene expression (Crow et al., 2020). The use of maize lines developed in temperate regions may also improve chilling tolerance. In a study comparing 598 European inbred lines, several ‘favourable’ alleles for ΦPSII were identified, especially across the European flint lines (Revilla et al., 2016). Local landraces may be used to introduce additional diversity into elite germplasm, but due to their heterozygous nature these are more difficult to use directly for breeding. Recent efforts to create doubled-haploid lines produced from landraces therefore provide a useful resource for understanding and exploiting the genetic and phenotypic diversity available in maize landraces (Hölker et al., 2019).
Finally, it will be important to integrate agronomic and genetic approaches to achieve future food security (McKersie, 2015). Besides breeding for increased resilience, agronomic techniques can be employed to increase chilling tolerance. For example, the application of ‘climate-smart agriculture’ regimes such as altered planting times, the application of exogenous plant growth regulators, and seed coating and seed priming can further help to mitigate the effects of low temperatures (Waqas et al., 2021). Just as priming with a moderate chilling stress can alleviate a severe temperature stress in maize plants (Capell and Dörffling, 1993; Sobkowiak et al., 2016), seed priming has been shown to improve antioxidant levels and growth under chilling stress (Li et al., 2017).
Conclusion
Whilst the relationship between photosynthesis and yield is complex, photosynthesis is a major contributing factor to yield (Sarquís et al., 1998; Simkin et al., 2019) and the chilling tolerance of photosynthesis is an important component of improved performance of maize under chilling temperatures (Dwyer and Tollenaar, 1989; Tollenaar and Wu, 1999). Here we have identified nine traits that are pivotal in the maize chilling response: carbon assimilation; electron transport; the expression of photosynthetic genes; NPQ; chlorophyll content; ROS; ABA; leaf sugar content; and leaf expansion. Since the chilling tolerance of photosynthesis is a complex breeding goal with multiple phenotypic and genotypic components, we advocate for a multi-trait holistic approach that takes specific phenological and geographical considerations into account for successful breeding for chilling tolerance of photosynthesis. Breeding for increased chilling tolerance of photosynthesis by exploiting the substantial natural genetic variation for traits aligned with key chilling responses will improve maize yields in cooler climes and contribute to meeting the significant global food security challenges faced by humankind.
Author contributions
ACB and JK: conceptualization; ACB: writing with input from JK; ACB and JK: editing and approval of the final version.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
ACB and JK were supported by the UK Research and Innovation Future Leaders Fellowship (UKRI-FLF) MR/T042737/1 awarded to JK.
References
- Aguilera C, Stirling C, Long S.. 1999. Genotypic variation within Zea mays for susceptibility to and rate of recovery from chill-induced photoinhibition of photosynthesis. Physiologia Plantarum 106, 429–436. [Google Scholar]
- Ainsworth EA, Bush DR.. 2011. Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiology 155, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam M, Revilla P, Djemel A, Tracy WF, Ordás B.. 2016. Identification of QTLs involved in cold tolerance in sweet × field corn. Euphytica 208, 353–365. [Google Scholar]
- Aroca R, Irigoyen JJ, Sánchez-Díaz M.. 2001. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Science 161, 719–726. [Google Scholar]
- Avila LM, Obeidat W, Earl H, Niu X, Hargreaves W, Lukens L.. 2018. Shared and genetically distinct Zea mays transcriptome responses to ongoing and past low temperature exposure. BMC Genomics 19, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113. [DOI] [PubMed] [Google Scholar]
- Bano S, Aslam M, Saleem M, Basra SMA, Aziz K.. 2015. Evaluation of maize accessions under low temperature stress at early growth stages. Journal of Animal and Plant Sciences 25, 392–400. [Google Scholar]
- Banović Đeri B, Božić M, Dudić D, Vićić I, Milivojević M, Ignjatović-Micić D, Samardžić J, Vančetović J, Delić N, Nikolić A.. 2021. Leaf transcriptome analysis of Lancaster versus other heterotic groups’ maize inbred lines revealed different regulation of cold-responsive genes. Journal of Agronomy and Crop Science 1, 13. [Google Scholar]
- Ben Saad R, Fabre D, Mieulet D, Meynard D, Dingkuhn M, Al-Doss A, Guiderdoni E, Hassairi A.. 2012. Expression of the Aeluropus littoralis AlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant, Cell & Environment 35, 626–643. [DOI] [PubMed] [Google Scholar]
- Bilska-Kos A, Grzybowski M, Jończyk M, Sowiński P.. 2016. In situ localization and changes in the expression level of transcripts related to intercellular transport and phloem loading in leaves of maize (Zea mays L.) treated with low temperature. Acta Physiologiae Plantarum 38, 123. [Google Scholar]
- Burnett AC, Rogers A, Rees M, Osborne CP.. 2016. Carbon source–sink limitations differ between two species with contrasting growth strategies. Plant, Cell & Environment 39, 2460–2472. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Anderson J, Davidson KJ, Ely KS, Lamour J, Li Q, Morrison BD, Yang D, Rogers A, Serbin SP.. 2021a. A best-practice guide to predicting plant traits from leaf-level hyperspectral data using partial least squares regression. Journal of Experimental Botany 72, 6175–6189. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Serbin SP, Davidson KJ, Ely KS, Rogers A.. 2021b. Detection of the metabolic response to drought stress using hyperspectral reflectance. Journal of Experimental Botany 72, 6474–6489. [DOI] [PubMed] [Google Scholar]
- Burnett AC, Serbin SP, Rogers A.. 2021c. Source:sink imbalance detected with leaf- and canopy-level spectroscopy in a field-grown crop. Plant, Cell & Environment 44, 2466–2479. [DOI] [PubMed] [Google Scholar]
- Byun MY, Cui LH, Lee J, Park H, Lee A, Kim WT, Lee H.. 2018. Identification of rice genes associated with enhanced cold tolerance by comparative transcriptome analysis with two transgenic rice plants overexpressing DaCBF4 or DaCBF7, isolated from Antarctic flowering plant Deschampsia antarctica. Frontiers in Plant Science 9, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell B, Dörffling K.. 1993. Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiologia Plantarum 88, 638–646. [DOI] [PubMed] [Google Scholar]
- Chinthapalli B, Murmu J, Raghavendra A.. 2003. Dramatic differences in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. Journal of Experimental Botany 54, 707–714. [DOI] [PubMed] [Google Scholar]
- Crow T, Ta J, Nojoomi S, Aguilar-Rangel MR, Rodríguez JVT, Gates D, Rellán-Álvarez R, Sawers R, Runcie D.. 2020. Gene regulatory effects of a large chromosomal inversion in highland maize. PLoS Genetics 16, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz RP, Sperotto RA, Cargnelutti D, Adamski JM, de FreitasTerra T, Fett JP.. 2013. Avoiding damage and achieving cold tolerance in rice plants. Food and Energy Security 2, 96–119. [Google Scholar]
- Dolstra O, Haalstra SR, van der Putten PEL, Schapendonk AHCM.. 1994. Genetic variation for resistance to low-temperature photoinhibition of photosynthesis in maize (Zea mays L.). Euphytica 80, 85–93. [Google Scholar]
- Dory I, Böddi B, Kissimon J, Paldi E.. 1990. Cold stress responses of inbred maize lines with various degrees of cold tolerance. Acta Agronomica Hungaria 39, 309–318. [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH.. 1997. Differential localization of antioxidants in maize leaves. Plant Physiology 114, 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y-C, Nose A, Wasano K.. 1999. Effects of chilling temperature on photosynthetic rates, photosynthetic enzyme activities and metabolite levels in leaves of three sugarcane species. Plant, Cell & Environment 22, 317–324. [Google Scholar]
- Dwyer L, Tollenaar M.. 1989. Genetic improvement in photosynthetic response of hybrid maize cultivars, 1959–1988. Candian Journal of Plant Science 69, 81–91. [Google Scholar]
- Earl HJ, Tollenaar M.. 1998. Relationship between thylakoid electron transport and photosynthetic CO2 uptake in leaves of three maize (Zea mays L.) hybrids. Photosynthesis Research 58, 245–257. [Google Scholar]
- Ely KS, Burnett AC, Lieberman-Cribbin W, Serbin SP, Rogers A.. 2019. Spectroscopy can predict key leaf traits associated with source–sink balance and carbon–nitrogen status. Journal of Experimental Botany 70, 1789–1799. [DOI] [PubMed] [Google Scholar]
- Ensminger I, Busch F, Hüner NPA.. 2006. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiologia Plantarum 126, 28–44. [Google Scholar]
- Farage P, Long S.. 1987. Damage to maize photosynthesis in field during periods when chilling is combined with high photon fluxes. In: Biggens J, ed. Progress in Photosynthesis Research, Vol. IV. Dordrecht: Springer, 139–142. [Google Scholar]
- Faralli M, Lawson T.. 2020. Natural genetic variation in photosynthesis: an untapped resource to increase crop yield potential? The Plant Journal 101, 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Vanacker H, Gomez LD, Harbinson J.. 2002. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiology and Biochemistry 40, 659–668. [Google Scholar]
- Fracheboud Y, Haldimann P, Leipner J, Stamp P.. 1999. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). Journal of Experimental Botany 50, 1533–1540. [Google Scholar]
- Fracheboud Y, Jompuk C, Ribaut JM, Stamp P, Leipner J.. 2004. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Molecular Biology 56, 241–253. [DOI] [PubMed] [Google Scholar]
- Fracheboud Y, Ribaut JM, Vargas M, Messmer R, Stamp P.. 2002. Identification of quantitative trait loci for cold-tolerance of photosynthesis in maize (Zea mays L.). Journal of Experimental Botany 53, 1967–1977. [DOI] [PubMed] [Google Scholar]
- Frascaroli E, Revilla P.. 2019. Genomics of cold tolerance in maize. In: Bennetzen J, Flint-Garcia S, Hirsch C, Tuberosa R, eds. The maize genome. Cham: Springer Nature, 287–304. [Google Scholar]
- Fryer M, Oxborough K, Martin B, Ort DR, Baker N.. 1995. Factors associated with depression of photosynthetic quantum efficiency in maize at low growth temperature. Plant Physiology 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR.. 1998. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiology 116, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geange SR, Arnold PA, Catling AA, et al. 2021. The thermal tolerance of photosynthetic tissues: a global systematic review and agenda for future research. New Phytologist 229, 2497–2513. [DOI] [PubMed] [Google Scholar]
- Havaux M, Dall’Osto L, Bassi R.. 2007. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiology 145, 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha DK, Zuther E.. 2020. Plant cold acclimation and winter survival. In: Hincha DK, Zuther E, eds. Plant cold acclimation. New York: Springer Nature, 1–7. [DOI] [PubMed] [Google Scholar]
- Hodges D, Andrews C, Johnson D, Hamilton R.. 1997. Sensitivity of maize hybrids to chilling and their combining abilities at two developmental stages. Crop Science 37, 850–856. [Google Scholar]
- Hölker AC, Mayer M, Presterl T, Bolduan T, Bauer E, Ordas B, Brauner PC, Ouzunova M, Melchinger AE, Schön CC.. 2019. European maize landraces made accessible for plant breeding and genome-based studies. Theoretical and Applied Genetics 132, 3333–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Li Z, Lu Y, et al. 2017. Genome-wide association study identified multiple genetic loci on chilling resistance during germination in maize. Scientific Reports 7, 10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang J, Li W, Hu W, Duan L, Feng Y, Qiu F, Yue B.. 2013. Genome-wide association analysis of ten chilling tolerance indices at the germination and seedling stages in maize. Journal of Integrative Plant Biology 55, 735–744. [DOI] [PubMed] [Google Scholar]
- Hund A, Fracheboud Y, Soldati A, Frascaroli E, Salvi S, Stamp P.. 2004. QTL controlling root and shoot traits of maize seedlings under cold stress. Theoretical and Applied Genetics 109, 618–629. [DOI] [PubMed] [Google Scholar]
- Hund A, Frascaroli E, Leipner J, Jompuk C, Stamp P, Fracheboud Y.. 2005. Cold tolerance of the photosynthetic apparatus: pleiotropic relationship between photosynthetic performance and specific leaf area of maize seedlings. Molecular Breeding 16, 321–331. [Google Scholar]
- Hund A, Richner W, Soldati A, Fracheboud Y, Stamp P.. 2007. Root morphology and photosynthetic performance of maize inbred lines at low temperature. European Journal of Agronomy 27, 52–61. [Google Scholar]
- Hüner N, Öquist G, Melis A.. 2003. Photostasis in plants, green algae and cyanobacteria: the role of light harvesting antenna complexes. In: Green B, Parson W, eds. Advances in photosynthesis and respiration. Light harvesting antennas in photosynthesis. Dordrecht: Kluwer Academic Publishers, 401–421. [Google Scholar]
- Hüner N, Öquist G, Sarhan F.. 1998. Energy balance and acclimation to light and cold. Trends in Plant Science 3, 224–230. [Google Scholar]
- Hüner NPA, Dahal K, Bode R, Kurepin LV, Ivanov AG.. 2016. Photosynthetic acclimation, vernalization, crop productivity and ‘the grand design of photosynthesis’. Journal of Plant Physiology 203, 29–43. [DOI] [PubMed] [Google Scholar]
- IPCC. 2018. Global warming of 1.5 °C: an IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change. Geneva, Switzerland: IPCC. [Google Scholar]
- Janowiak F, Dörffling K.. 1996. Chilling of maize seedlings: changes in water status and abscisic acid content in ten genotypes differing in chilling tolerance. Journal of Plant Physiology 147, 582–588. [Google Scholar]
- Janowiak F, Luck E, Dörffling K.. 2003. Chilling tolerance of maize seedlings in the field during cold periods in spring is related to chilling-induced increase in abscisic acid level. Journal of Agronomy and Crop Science 189, 156–161. [Google Scholar]
- Janowiak F, Maas B, Dörffling K.. 2002. Importance of abscisic acid for chilling tolerance of maize seedlings. Journal of Plant Physiology 159, 635–643. [Google Scholar]
- Jompuk C, Fracheboud Y, Stamp P, Leipner J.. 2005. Mapping of quantitative trait loci associated with chilling tolerance in maize (Zea mays L.) seedlings grown under field conditions. Journal of Experimental Botany 56, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Jończyk M, Sobkowiak A, Trzcinska-Danielewicz J, Skoneczny M, Solecka D, Fronk J, Sowiński P.. 2017. Global analysis of gene expression in maize leaves treated with low temperature. II. Combined effect of severe cold (8 °C) and circadian rhythm. Plant Molecular Biology 95, 279–302. [DOI] [PubMed] [Google Scholar]
- Jończyk M, Sobkowiak A, Trzcinska-Danielewicz J, Sowiński P.. 2021. Chromatin-level differences elucidate potential determinants of contrasting levels of cold sensitivity in maize lines. Plant Molecular Biology Reporter 39, 335–350. [Google Scholar]
- Kingston-Smith AH, Foyer CH.. 2000. Bundle sheath proteins are more sensitive to oxidative damage than those of the mesophyll in maize leaves exposed to paraquat or low temperatures. Journal of Experimental Botany 51, 123–130. [PubMed] [Google Scholar]
- Kingston-Smith AH, Harbinson J, Foyer CH.. 1999. Acclimation of photosynthesis, H2O2 content and antioxidants in maize (Zea mays) grown at sub-optimal temperatures. Plant, Cell & Environment 22, 1071–1083. [Google Scholar]
- Kingston-Smith A, Harbinson J, Williams J, Foyer C.. 1997. Effect of chilling on carbon assimilation, enzyme activation, and photosynthetic electron transport in the absence of photoinhibition in maize leaves. Plant Physiology 114, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kočová M, Holá D, Wilhelmová N, Rothová O.. 2009. The influence of low-temperature on the photochemical activity of chloroplasts and activity of antioxidant enzymes in maize leaves. Biologia Plantarum 53, 475–483. [Google Scholar]
- Kono M, Noguchi K, Terashima I.. 2014. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant & Cell Physiology 55, 990–1004. [DOI] [PubMed] [Google Scholar]
- Kościelniak J, Janowiak F, Kurczych Z.. 2005. Increase in photosynthesis of maize hybrids (Zea mays L.) at suboptimal temperature (15 °C) by selection of parental lines on the basis of chlorophyll a fluorescence measurements. Photosynthetica 43, 125–134. [Google Scholar]
- Krapp A, Stitt M.. 1995. An evaluation of direct and indirect mechanisms for the ‘sink-regulation’ of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195, 313–323. [Google Scholar]
- Krasensky J, Jonak C.. 2012. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EA, Staebler MA, Tollenaar M.. 2002. Genetic variation in physiological discriminators for cold tolerance—early autotrophic phase of maize development. Crop Science 42, 1919–1929. [Google Scholar]
- Li J, Li D, Espinosa CZ, et al. 2021. Genome-wide analyses reveal footprints of divergent selection and popping-related traits in CIMMYT’s maize inbred lines. Journal of Experimental Botany 72, 1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sui N, Lin L, Yang Z, Zhang Y.. 2019. Transcriptomic profiling revealed genes involved in response to cold stress in maize. Functional Plant Biology 46, 830–844. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu J, Gao Y, et al. 2017. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Frontiers in Plant Science 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë A. 2018. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environmental and Experimental Botany 154, 123–133. [Google Scholar]
- Maxwell K, Johnson GN.. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51, 659–668. [DOI] [PubMed] [Google Scholar]
- McKersie B. 2015. Planning for food security in a changing climate. Journal of Experimental Botany 66, 3435–3450. [DOI] [PubMed] [Google Scholar]
- Meacham-Hensold K, Fu P, Wu J, et al. 2020. Plot-level rapid screening for photosynthetic parameters using proximal hyperspectral imaging. Journal of Experimental Botany 71, 2312–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miculan M, Nelissen H, Ben Hassen M, Marroni F, Inzé D, Mario Enrico P, Dell’Acqua M.. 2021. A forward genetics approach integrating genome-wide association study and expression quantitative trait locus mapping to dissect leaf development in maize (Zea mays). The Plant Journal 107, 1056–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema P. 1982. The effects of low temperature on Zea mays. Advances in Agronomy 35, 93–128. [Google Scholar]
- Miura K, Furumoto T.. 2013. Cold signaling and cold response in plants. International Journal of Molecular Sciences 14, 5312–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI.. 2015. Mechanisms of abscisic acid-mediated control of stomatal aperture. Current Opinion in Plant Biology 28, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Lawson T.. 2013. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany 64, 3983–3998. [DOI] [PubMed] [Google Scholar]
- Nie GY, Baker NR.. 1991. Modifications to thylakoid composition during development of maize leaves at low growth temperatures. Plant Physiology 95, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievola C, Carvalho C, Carvalho V, Rodrigues E.. 2017. Rapid responses of plants to temperature changes. Temperature 4, 371–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P.. 2010. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochimica et Biophysica Acta 1797, 466–475. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ishida Y, Usami S.. 2004. Expression of cold-tolerant pyruvate, orthophosphate dikinase cDNA, and heterotetramer formation in transgenic maize plants. Transgenic Research 13, 475–485. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ishida Y, Usami S.. 2006. High-level expression of cold-tolerant pyruvate, orthophosphate dikinase from a genomic clone with site-directed mutations in transgenic maize. Molecular Breeding 18, 29–38. [Google Scholar]
- Ort DR, Merchant SS, Alric J, et al. 2015. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proceedings of the National Academy of Sciences, USA 112, 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Austin M, Berry J, Billings W, Boyer J, Dacey J, Nobel P, Smith S, Winner W.. 1987. Stress physiology and the distribution of plants. BioScience 37, 38–48. [Google Scholar]
- Presterl T, Ouzunova M, Schmidt W, Möller E, Röber F, Knaak C, Ernst K, Westhoff P, Geiger H.. 2007. Quantitative trait loci for early plant vigour of maize grown in chilly environments. Theoretical and Applied Genetics 114, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Quan R, Shang M, Zhang H, Zhao Y, Zhang J.. 2004. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Science 166, 141–149. [Google Scholar]
- Revilla P, Malvar R, Cartea M, Butrón A, Ordás A.. 2000. Inheritance of cold tolerance at emergence and during early season growth in maize. Crop Science 40, 1579–1585. [Google Scholar]
- Revilla P, Butrón A, Cartea M, Malvar R, Ordás A.. 2005. Breeding for cold tolerance. In: Ashraf M, Harris PJC, eds. Abiotic stresses. Plant resistance through breeding and molecular approaches. Boca Raton, FL: CRC Press, 301–398. [Google Scholar]
- Revilla P, Rodríguez VM, Ordás A, et al. 2016. Association mapping for cold tolerance in two large maize inbred panels. BMC Plant Biology 16, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Langridge P.. 2016. Physiological breeding. Current Opinion in Plant Biology 31, 162–171. [DOI] [PubMed] [Google Scholar]
- Riva-Roveda L, Escale B, Giauffret C, Périlleux C.. 2016. Maize plants can enter a standby mode to cope with chilling stress. BMC Plant Biology 16, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez VM, Butrón A, Malvar RA, Ordás A, Revilla P.. 2008. Quantitative trait loci for cold tolerance in the maize IBM population. International Journal of Plant Sciences 169, 551–556. [Google Scholar]
- Rodríguez VM, Butrón A, Rady MOA, Soengas P, Revilla P.. 2014. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Molecular Breeding 33, 363–371. [Google Scholar]
- Rodríguez VM, Velasco P, Garrido JL, Revilla P, Ordás A, Butrón A.. 2013. Genetic regulation of cold-induced albinism in the maize inbred line A661. Journal of Experimental Botany 64, 3657–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, McKown AD.. 2006. Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? Journal of Experimental Botany 57, 303–317. [DOI] [PubMed] [Google Scholar]
- Sah SK, Reddy KR, Li J.. 2016. Abscisic acid and abiotic stress tolerance in crop plants. Frontiers in Plant Science 7, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales CRG, Wang Y, Evers JB, Kromdijk J.. 2021. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. Journal of Experimental Botany 72, 5942–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salesse-Smith CE, Sharwood RE, Busch FA, Kromdijk J, Bardal V, Stern DB.. 2018. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nature Plants 4, 802–810. [DOI] [PubMed] [Google Scholar]
- Sanghera G, Wani S.. 2008. Innovative approaches to enhance genetic potential of rice for higher productivity under temperate conditions of Kashmir. Journal of Plant Science Research 24, 99–113. [Google Scholar]
- Sanghera G, Wani S, Hussain W, Singh N.. 2011. Engineering cold stress tolerance in crop plants. Current Genomics 12, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarquís JI, Gonzalez H, Sánchez De Jiménez E, Dunlap JR.. 1998. Physiological traits associated with mass selection for improved yield in a maize population. Field Crops Research 56, 239–246. [Google Scholar]
- Savitch L, Leonardos E, Krol M, Jansson S, Grodzinski B, Hüner N, Öquist G.. 2002. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant, Cell & Environment 25, 761–771. [Google Scholar]
- Schnable PS, Ware D, Fulton RS, et al. 2009. The B73 maize genome: complexity, diversity and dynamics. Science 326, 1112–1115. [DOI] [PubMed] [Google Scholar]
- Serbin SP, Dillaway DN, Kruger EL, Townsend PA.. 2012. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. Journal of Experimental Botany 63, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbin SP, Wu J, Ely KS, Kruger EL, Townsend PA, Meng R, Wolfe BT, Chlus A, Wang Z, Rogers A.. 2019. From the Arctic to the tropics: multibiome prediction of leaf mass per area using leaf reflectance. New Phytologist 224, 1557–1568. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, López-Calcagno PE, Raines CA.. 2019. Feeding the world: improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany 70, 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S, Ma J, Hanson J, Rolland F.. 2010. Sugar signals and molecular networks controlling plant growth. Current Opinion in Plant Biology 13, 274–279. [DOI] [PubMed] [Google Scholar]
- Sobkowiak A, Jończyk M, Jarochowska E, Biecek P, Trzcinska-Danielewicz J, Leipner J, Fronk J, Sowiński P.. 2014. Genome-wide transcriptomic analysis of response to low temperature reveals candidate genes determining divergent cold-sensitivity of maize inbred lines. Plant Molecular Biology 85, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowiak A, Jończyk M, Adamczyk J, et al. 2016. Molecular foundations of chilling-tolerance of modern maize. BMC Genomics 17, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A.. 2012. Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506, 265–273. [DOI] [PubMed] [Google Scholar]
- Stitt M, Hurry V.. 2002. A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Current Opinion in Plant Biology 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Strigens A, Freitag NM, Gilbert X, Grieder C, Riedelsheimer C, Schrag TA, Messmer R, Melchinger AE.. 2013. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant, Cell & Environment 36, 1871–1887. [DOI] [PubMed] [Google Scholar]
- Szalai G, Majláth I, Pál M, Gondor OK, Rudnóy S, Oláh C, Vanková R, Kalapos B, Janda T.. 2018. Janus-faced nature of light in the cold acclimation processes of maize. Frontiers in Plant Science 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. 2001. So what’s new in the field of plant cold acclimation? Lots! Plant Physiology 125, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhisa J, Browse J.. 1999. Genetic engineering of plant chilling tolerance. In: Setlow JK, ed. Genetic engineering, Vol. 21. Boston: Springer, 79–93. [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Wu J.. 1999. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Science 39, 1597–1604. [Google Scholar]
- Trzcinska-Danielewicz J, Bilska A, Fronk J, Zielenkiewicz P, Jarochowska E, Roszczyk M, Jończyk M, Axentowicz E, Skoneczny M, Sowiński P.. 2009. Global analysis of gene expression in maize leaves treated with low temperature. I. Moderate chilling (14 °C). Plant Science 177, 648–658. [Google Scholar]
- Urrutia M, Blein-Nicolas M, Prigent S, et al. 2021. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant, Cell & Environment 44, 1504–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas MA, Wang X, Zafar SA, Noor MA, Hussain HA, Azher Nawaz M, Farooq M.. 2021. Thermal stresses in maize: effects and management strategies. Plants 10, 2931–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Rogers A, Rees M, Osborne CP.. 2016. How can we make plants grow faster? A source–sink perspective on growth rate. Journal of Experimental Botany 67, 31–45. [DOI] [PubMed] [Google Scholar]
- Wimalanathan K, Friedberg I, Andorf CM, Lawrence-Dill CJ.. 2018. Maize GO Annotation—Methods, Evaluation, and Review (maize-GAMER). Plant Direct 2, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang N, Hu J, Wen T, Brennan MA, Brennan CS, Guo X.. 2020. Effects of temperature stress on the accumulation of ascorbic acid and folates in sweet corn (Zea mays L.) seedlings. Journal of the Science of Food and Agriculture 100, 1694–1701. [DOI] [PubMed] [Google Scholar]
- Xin Z, Li P.. 1993. Relationship between proline and abscisic acid in the induction of chilling tolerance in maize suspension-cultured cells. Plant Physiology 103, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendrek CR, Tomaz T, Montes CM, Cao Y, Morse AM, Brown PJ, McIntyre LM, Leakey ADB, Ainsworth EA.. 2017. High-throughput phenotyping of maize leaf physiological and biochemical traits using hyperspectral reflectance. Plant Physiology 173, 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J, Lee EA, Tollenaar M.. 2000. Response of maize leaf photosynthesis to low temperature during the grain-filling period. Field Crops Research 68, 87–96. [Google Scholar]
- Ying J, Lee EA, Tollenaar M.. 2002. Response of leaf photosynthesis during the grain-filling period of maize to duration of cold exposure, acclimation, and incident PPFD. Crop Science 42, 1164–1172. [Google Scholar]
- Yoshida S, Satake T, Mackill D.. 1981. High temperature stress in rice. IRRI Research Paper Series 67, 1–15. [Google Scholar]
- Yu T, Zhang J, Cao J, et al. 2021. Leaf transcriptomic response mediated by cold stress in two maize inbred lines with contrasting tolerance levels. Genomics 113, 782–794. [DOI] [PubMed] [Google Scholar]
- Zaidi P, Yadav M, Maniselvan P, Khan R, Shadakshari T, Singh R, Pal D.. 2010. Morpho-physiological traits associated with cold stress tolerance in tropical maize (Zea maysL.). Maydica 55, 201–208. [Google Scholar]
- Zhang Y, Fu J, Gu R, Wang J, Chen X, Jia J, Zhang J, Wang G.. 2009. Isolation and analysis of cold stress inducible genes in Zea mays by suppression subtractive hybridization and cDNA macroarray. Plant Molecular Biology Reporter 27, 38–49. [Google Scholar]
- Zhu JK. 2016. Abiotic stress signaling and responses in plants. Cell 167, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Dong C, Zhu J.. 2007. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Current Opinion in Plant Biology 10, 290–295. [DOI] [PubMed] [Google Scholar]