Abstract
The basidiomycetous fungus Nematoloma frowardii produced manganese peroxidase (MnP) as the predominant ligninolytic enzyme during solid-state fermentation (SSF) of wheat straw. The purified enzyme had a molecular mass of 50 kDa and an isoelectric point of 3.2. In addition to MnP, low levels of laccase and lignin peroxidase were detected. Synthetic 14C-ring-labelled lignin (14C-DHP) was efficiently degraded during SSF. Approximately 75% of the initial radioactivity was released as 14CO2, while only 6% was associated with the residual straw material, including the well-developed fungal biomass. On the basis of this finding we concluded that at least partial extracellular mineralization of lignin may have occurred. This conclusion was supported by the fact that we detected high levels of organic acids in the fermented straw (the maximum concentrations in the water phases of the straw cultures were 45 mM malate, 3.5 mM fumarate, and 10 mM oxalate), which rendered MnP effective and therefore made partial direct mineralization of lignin possible. Experiments performed in a cell-free system, which simulated the conditions in the straw cultures, revealed that MnP in fact converted part of the 14C-DHP to 14CO2 (which accounted for up to 8% of the initial radioactivity added) and 14C-labelled water-soluble products (which accounted for 43% of the initial radioactivity) in the presence of natural levels of organic acids (30 mM malate, 5 mM fumarate).
Except for cellulose, lignin is the most abundant biological compound found in nature, yet it is degraded by only a small number of microorganisms, primarily basidiomycetes (white rot fungi) (5, 12, 33). Lignin biodegradation by these fungi has obvious ecological significance and also has promising biotechnological applications (e.g., biopulping and wastewater treatment) (3, 38, 46). White rot fungi produce a variety of extracellular enzymes that are thought to be involved in lignin degradation, the best characterized of which are laccase, lignin peroxidase (LiP), and manganese peroxidase (MnP) (22). These enzymes are capable of forming radicals inside the lignin polymer, which results in destabilization of bonds and finally in the breakdown of the macromolecule (35). In many fungi, MnP is thought to play the crucial role in the primary attack on lignin, because it generates the strong oxidant Mn3+; this oxidant acts as a diffusible redox mediator which attacks certain aromatic moieties of the lignin polymer (6, 22, 61, 62). It has been proposed that another possible mechanism for primary attack on lignin is the mechanism of Pycnoporus cinnabarinus, which lacks MnP and uses laccase-mediated reactions to cleave lignin (11).
Most previous studies of the ligninolytic enzymes of white rot fungi have been carried out with liquid media (22, 47, 60), and there have been only a few studies in which the production and action of these enzymes have been investigated during solid-state fermentation (SSF). MnP and/or laccase was found during SSF of sawdust with Lentinus edodes, Ceriporiopsis subvermispora, or Phanerochaete chrysosporium and also in straw cultures of Pleurotus eryngii and Pleurotus ostreatus (7, 9, 15, 39, 41). Production of LiP during SSF has been observed only during growth of Phlebia radiata and P. chrysosporium on wheat straw and wood pulp, respectively (9, 56). Even less information is available about the formation of organic acids by white rot fungi during growth on lignocellulose. Dicarboxylic or α-hydroxycarboxylic acids are required for MnP activity; these acids act as chelators both for Mn2+ and Mn3+ ions, and in addition, they serve as buffers (36, 62). For the part, the formation of such acids has been investigated with liquid cultures, and oxalic acid has been found to be the main organic acid produced by white rot fungi (37, 55). A number of white rot fungi were recently tested for organic acid production during SSF of wheat straw, and oxalic acid again was the main fungal metabolite of this type (17).
We have recently reported that MnP from the white rot fungi Nematoloma frowardii and P. radiata is capable of depolymerizing synthetic and natural lignin in a cell-free, malonate-buffered reaction system, which results in the formation of water-soluble lignin fragments and CO2 (27, 28). Moreover, MnP from N. frowardii has also been found to mineralize a number of other substances, including humic acids and xenobiotic compounds (24, 25, 49). In the present study, we demonstrated that MnP is the predominant ligninolytic enzyme during SSF of wheat straw with N. frowardii and that this fungus produces sufficient amounts of organic acids so that effective MnP activity can occur.
MATERIALS AND METHODS
Fungus.
The agaric white rot fungus N. frowardii b19 (= DSM 11239 = ATCC 201144) was isolated from fruiting bodies on decaying Nothofagus wood in Bariloche, Argentina (23). Master cultures were subcultured on malt extract agar slants and maintained at 4°C until they were used.
Culture conditions.
SSF was carried out in 250-ml flasks containing 15 g of chopped wheat straw, which was obtained from J. M. Pelayo (SAICA, Zaragoza, Spain). The manganese content of the straw was 11.4 mg kg−1 (204 μM), 70% of which was extractable with water (41a). The straw was sterilized twice by heating it at 121°C for 20 min, and 22.5 mg of glucose in 45 ml of deionized (filter-sterilized) water was added (3 ml of H2O per g of straw). The flasks were inoculated with five agar plugs (diameter, 0.7 cm), closed with stoppers fitted with inlet and outlet tubes for aeration, and incubated at 24°C with a constant flow of water-saturated air (86 ml min−1). Control flasks were incubated without fungus under the same conditions. Fermented straw from three flasks was harvested every 2 days starting 6 days after inoculation and ending 25 days after inoculation.
After harvesting, straw from a culture flask was suspended in 300 ml of deionized water and incubated on a rotary shaker (160 rpm) for 1.5 h, and subsequently it was pressed (500 kPa with N2) and simultaneously washed to separate the extracellular fungal enzymes (56). Then, extracts were filtered through glass fiber filters, and 2-ml portions were used to determine the activities of ligninolytic enzymes and the concentrations of organic acids. The main portions of the liquids were frozen and kept at −20°C prior to protein purification.
Enzyme assays.
MnP activity was determined by a modified method as described by Wariishi et al. (62). Each 1-ml (final volume) reaction mixture contained 50 mM sodium malonate (pH 4.5), 0.5 mM MnCl2, 0.2 mM H2O2, and 5 to 50 μl of straw extract or purified enzyme preparation. The reaction was initiated at 25°C by adding H2O2, and the rate of Mn3+-malonate complex formation was monitored by measuring the increase in absorbance at 270 nm (ɛ270 = 11,590 M cm−1). MnP activity was also measured by using 2,2′-azino-bis(3-ethylthiazoline-6-sulfonate) (ABTS) as the substrate under the conditions described above (26).
LiP activity was measured with veratryl alcohol (vacuum distilled prior to use) (34). Each 1-ml reaction mixture contained 100 mM sodium tartrate (pH 3.0), 1 mM veratryl alcohol, 0.2 mM H2O2, and 50 to 100 μl of enzyme solution. The reaction was started with H2O2, and the formation of veratryl aldehyde was monitored at 310 nm (ɛ310 = 9,300 M−1 cm−1).
Laccase activity was determined by measuring the oxidation of 1 mM ABTS buffered with 100 mM sodium citrate (pH 4.5). Formation of the cation radical of ABTS was monitored at 420 nm (ɛ420 = 36,000 M−1 cm−1) after 20 to 100 μl of straw extract was added (11).
Spectrophotometric measurements were obtained with a UV-visible light spectrophotometer (model UV-160A; Shimadzu, Kyoto, Japan). All enzyme activities detected in the straw extracts were expressed in relation to the initial water contents of the straw cultures (3 ml g−1 = 300%).
Enzyme purification.
After straw extracts (ca. 330 ml from one culture flask) were thawed, they were centrifuged at 12,000 × g for 30 min to remove the precipitates. Then, they were concentrated to ca. 15 ml by ultrafiltration with a 250-ml filter unit equipped with a 10-kDa cutoff filter (Amicon, Beverly, Mass.). Subsequently, the samples were dialyzed by repeated washing with 25 mM acetate buffer (pH 5.5).
Proteins from 23-day-old straw extracts were fractionated by two steps of anion-exchange chromatography performed with Sepharose-Q fast-flow medium (Pharmacia, Uppsala, Sweden) and Mono-Q Sepharose (Pharmacia). In the first step, the column (1.6 by 20 cm) was equilibrated with 25 mM sodium acetate (pH 5.5), and proteins were eluted with a linear 0.05 to 0.3 M NaCl gradient. The elution volume was 250 ml, the flow rate was 1.5 ml min−1, and 1.5-ml fractions were collected. Elution was monitored at 409 nm (heme) and 280 nm (protein). The enzyme activities of MnP, LiP, and laccase were assayed in all fractions, and then they were pooled, concentrated 10-fold, and desalted with a 50-ml Amicon ultrafiltration unit. The pooled ligninolytic enzymes were separated in a second step on a Mono-Q Sepharose column by using a linear 0.05 to 0.3 M NaCl gradient at a flow rate of 1 ml min−1.
MnP characterization.
Purified MnP was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and native isoelectric focusing (IEF)-PAGE (56). Discontinuous gel electrophoresis was carried out by using 10% gels and a Protean II apparatus (Bio-Rad, Richmond, Calif.). Proteins were visualized by silver staining with a Bio-Rad kit, and GIBCO/BRL low-range molecular weight standards were used to calibrate the gel.
IEF was performed by using 0.5-mm-thick polyacrylamide slab gels and a Multiphor II apparatus (Pharmacia). A pH range of 2.4 to 4.7 was obtained by mixing ampholytes (pH ranges, 3.5 to 10.0 and 2.5 to 5.0; Pharmacia). The pH gradient was measured with a Multiphor surface electrode (Pharmacia), and MnP activity was visualized by specific activity staining by using sodium malonate buffer (50 mM, pH 4.5) and phenol red (0.1 g liter−1) or ABTS (0.5 mM) in the presence of 1 mM Mn2+ and 0.5 mM H2O2; the controls did not contain Mn2+ or H2O2 in the staining solution.
Radioactive experiments.
14C-ring-labelled synthetic lignin (14C-DHP) with a molecular mass of 4 to 10 kDa was polymerized from 14C-ring-labelled coniferyl alcohol. The properties of this preparation, including the gel permeation chromatography properties, have been described previously (12, 58). The 14C-DHP was added as a DMF-water suspension (1:20) to 2.5 g of sterilized wheat straw in 100-ml culture flasks, and the final radioactivity was approximately 22,000 dpm. The flasks were each inoculated with three agar plugs, tightly closed with a rubber septum, and incubated at 24°C in the dark. The 14C-labelled volatile organic compounds and 14CO2 that evolved were trapped weekly by bubbling any gas released through two sequential flasks containing Opti-Fluor and Carbosorb/Opti-Fluor (Packard Instrument B.V., Groningen, The Netherlands) (50). Pure oxygen was used for flushing. Radioactivity was measured by liquid scintillation counting with a model 1411 counter (Wallac Oy, Turku, Finland). At the end of cultivation, the straw cultures were each extracted with 20 ml of deionized water and then with 20 ml of dioxane, and radioactivity was detected in the supernatants. The residual straw, including the fungal mycelium, was combusted in a combustion chamber (Junitek Oy, Turku, Finland), and the trapped 14CO2 was quantified. All of the results were expressed as means ± standard deviations based on three replicates.
Mineralization and solubilization (formation of water-soluble 14C-labelled products) of 14C-DHP by purified MnP from straw cultures were investigated in sterile 10-ml reaction tubes tightly closed with rubber septa (24). Since high concentrations of malate and fumarate were detected during the fermentation of wheat straw, these compounds were used as chelator and buffer substances in the in vitro experiments simulating the conditions in the straw cultures on day 12. Each filter-sterilized reaction mixture (total volume, 1 ml) contained 30 mM sodium malate (pH 4.5), 5 mM sodium fumarate (pH 4.5), 1 mM MnCl2, 2 U of MnP, 22,000 dpm of 14C-DHP, and 100 mg of unlabelled DHP per liter. In some experiments, fumarate was omitted. H2O2 was not added to the reaction mixtures, because we recently found that MnP acts effectively in the absence of H2O2 if sufficient amounts of Mn2+ and organic acids are present (26, 28). Samples were incubated at 37°C on a rotary shaker (180 rpm) in the dark and flushed daily with oxygen, and the 14C-labelled organic compounds and 14CO2 were trapped and measured as described above.
High-performance liquid chromatography (HPLC).
Organic acids in the straw extracts were analyzed by using a model HP 1090 liquid chromatograph (Hewlett-Packard, Waldbronn, Germany) equipped with an UltraSep ES FS column (Knauer, Gross-Umstadt, Germany) (24, 26). Phosphoric acid (10 mM) was used as the solvent at a flow rate of 0.55 ml min−1, and chromatograms were recorded at 210 nm. Authentic standards consisting of various organic acids were used for calibration. The concentrations of organic acids were expressed in relation to the initial water contents of the straw cultures.
CZE.
Capillary zone electrophoresis (CZE) analysis was used to quantify oxalic acid in the straw extracts (17). This procedure was performed with a model HP 3D CE system equipped with a diode array detector (Hewlett-Packard). Indirect detection at 300 nm was used, and the reference wavelength was 200 nm. A fused silica capillary column (inside diameter, 50 μm; outside diameter, 360 μm; length, 80 cm) was purchased from Composite Metal Services Ltd. (Worcester, United Kingdom). The voltage applied was −25 kV, and the capillary temperature was maintained at 10°C. Samples were injected by applying 50 × 105 mPa of pressure for 4 s. The buffer solution used was 5 mM potassium hydrogen phthalate supplemented with 0.5 mM cetyltrimethylammonium bromide (pH 6) (Hewlett-Packard). Peaks were identified by adding commercially available oxalic acid (17).
RESULTS
Enzyme activities.
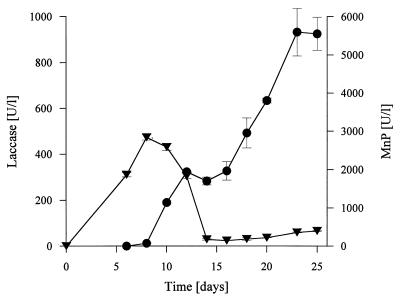
High levels of MnP activity and lower levels of laccase activity were detected in extracts of wheat straw fermented with N. frowardii. Figure 1 shows the time courses for laccase and MnP activities during SSF. Laccase activity appeared first, and the maximum level of activity was observed on day 10, after which the level of activity decreased rapidly, although low levels of laccase activity were detected until the end of incubation. MnP was first detected in the straw extracts after 10 days of fermentation, and a local maximum level of activity occurred on day 12. After this, the MnP activity stagnated, but the level of activity increased again after 16 days of incubation until the end of the experiment. When the laccase substrate ABTS was used as an additional substrate in the MnP assay, the levels of activity were always about 30% lower than the levels of activity obtained in the Mn2+ assay. In each case, however, the maximum level of MnP activity was considerably higher than the maximum level of laccase activity in the straw extracts; the maximum level of MnP activity, which was detected by monitoring the formation of Mn(III)-malonate complexes, was 5,600 U liter−1; the respective MnP activity level for the oxidation of ABTS was 3,700 U liter−1. Laccase reached a maximum activity level of 450 U liter−1 with ABTS as the substrate. LiP activity was not detected in the straw extracts by the veratryl alcohol method either because of the presence of inhibitors or because of color interference by aromatic straw depolymerization products or both. The same phenomenon was observed in previous studies in which lignocelluloses were used as growth substrates for white rot fungi (9, 56).
FIG. 1.
Activities of MnP (●) and laccase (▾) during SSF of wheat straw with N. frowardii. Enzyme activities are expressed in relation to the water content of the straw (in units per liter). Each data point is the mean enzyme activity value for three extracted flasks; the bars indicate standard deviations.
Purification of MnP.
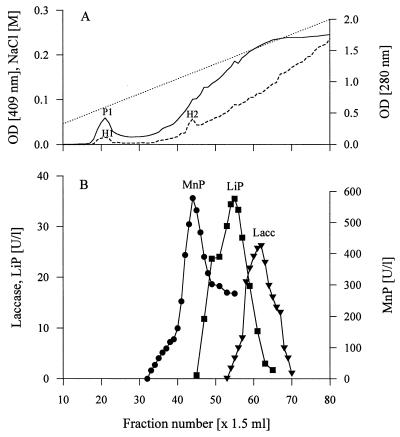
The 23-day-old straw extracts were used to purify MnP, because they contained the highest levels of enzyme activity. Although colored degradation products from straw were partially removed by the ultrafiltration procedure, the concentrated samples had still an intense brownish color. A high level of MnP activity and a low level of laccase activity were observed in the concentrate, but LiP activity was not detected. During the purification procedure, most of the colored compounds were bound to the Sepharose-Q, but they were partially eluted from the column as the concentration of salt in the gradient increased. These compounds interfered with monitoring absorbance at both 280 and 409 nm, which resulted in a constant increase in absorbance during elution (Fig. 2). Thus, the protein profile had only one distinct peak (peak P1) at 280 nm, a corresponding heme peak (peak H1), and another small heme peak (peak H2). Our determination of the enzyme activities in the fractions revealed that MnP, laccase, and LiP were present (Fig. 2). No activity was found to be associated with peaks P1 and H1, but the small heme peak, peak H2, corresponded to a high level of MnP activity. In contrast to the results obtained with the concentrated crude extract, LiP activity was detected after purification on Sepharose-Q at a level similar to the laccase activity level; nevertheless, the level of LiP activity was low compared with the level of MnP activity. Removing the colored straw degradation products during anion-exchange chromatography probably made detection of LiP by the veratryl alcohol method possible.
FIG. 2.
Separation of extracellular proteins from 23-day-old cultures of N. frowardii grown on wheat straw by anion-exchange chromatography on Sepharose-Q. (A) —, absorbance at 280 nm (OD [280 nm]); - - - , absorbance at 409 nm (OD [409 nm]); …, salt gradient (0.05 to 0.30 M NaCl). (B) Enzyme activities. Lacc, laccase.
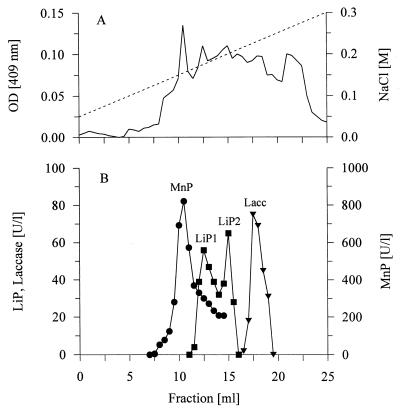
The separation of the pooled ligninolytic activities on Mono-Q Sepharose was similar to the separation on Sepharose-Q, and MnP eluted as a single peak, although a tailing effect was observed (Fig. 3). LiP formed two activity peaks (peak LiP1 and LiP2) after separation on Mono-Q Sepharose, but no further increase in the total activity level was observed. Laccase eluted from the column as one small activity peak after MnP and LiP eluted.
FIG. 3.
Purification of pooled ligninolytic activities on a Mono-Q Sepharose column. (A) —, absorbance at 409 nm (OD [409 nm]); - - - , salt gradient. (B) Enzyme activities. Lacc, laccase.
Enzyme characterization.
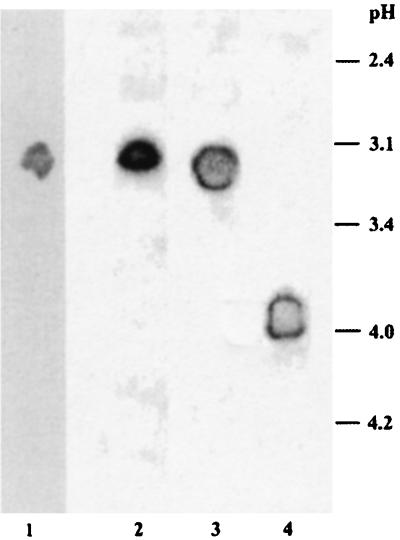
Purified SSF MnP was analyzed by several gel techniques, and its molecular mass and pI were determined. The enzyme produced a single band on the sodium dodecyl sulfate-PAGE gel at a molecular mass of 50 kDa, a value that is higher than the molecular masses of MnP from liquid cultures (42 to 44 kDa). Analysis of IEF-PAGE gels revealed a single, relatively broad band for SSF MnP when both ABTS and phenol red staining were used (Fig. 4). The pI of SSF MnP (3.0 to 3.2) was nearly identical to the pI of MnP2 purified from liquid cultures of N. frowardii (3.1 to 3.3), whereas the pI of MnP1 was slightly higher (3.8 to 4.0) (51).
FIG. 4.
IEF analysis of MnP separated from N. frowardii during SSF of wheat straw (lanes 1 and 2) and IEF analysis of MnP2 (lane 3) and MnP1 (lane 4) obtained from liquid cultures (51). MnP activity was stained with phenol red (lane 1) or ABTS (lanes 2 through 4) in the presence of Mn(II) and H2O2.
Production of organic acids during SSF.
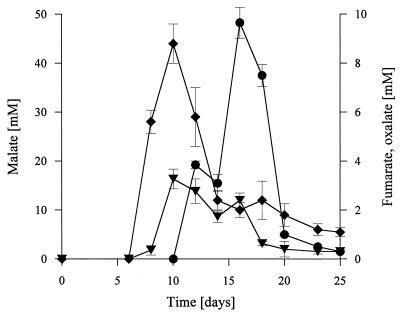
During fermentation of wheat straw, the pH of the extracts decreased from 5.5 to 4.0, indicating that organic acids were produced by the fungus. HPLC analysis revealed the presence of high levels of malate and fumarate. Figure 5 shows the time courses for malate, fumarate, and oxalate concentrations in relation to the water content of the fermented wheat straw. Malate and fumarate were detected after 8 days of incubation prior to production of MnP, and the maximum malate and fumarate concentrations (45 mM malate, 3.5 mM fumarate) were detected on day 10. The concentrations of these compounds decreased rapidly after MnP appeared, but they were detected until the end of the experiment on day 25. The decreases in concentrations were probably due to MnP-Mn3+-catalyzed decomposition of malate and fumarate, similar to the decarboxylation reactions that have been described for oxalate, glyoxylate, and malonate (26, 55). The HPLC analyses revealed that oxalate was formed in addition to malate and fumarate; however, the presence of other compounds, probably originating from straw, prevented a quantitative analysis. Therefore, CZE was used to analyze oxalic acid in the straw samples. Oxalic acid appeared later than malate and fumarate, on day 12, and the maximum concentration of oxalic acid (10 mM) was observed on day 16 (Fig. 5). After this, the oxalate concentration decreased rapidly, probably due to the high level of MnP activity.
FIG. 5.
Accumulation of malate (⧫), fumarate (▾), and oxalate (●) during SSF of wheat straw with N. frowardii. Millimolar concentrations are expressed in relation to the water content of straw. Each data point is the mean organic acid level for three extracted culture flasks; the bars indicate standard deviations.
On the basis of these findings, we concluded that sufficient amounts of organic acids are present during growth of N. frowardii on straw to enable chelation of manganese ions and, consequently, effective MnP activity.
Mineralization of 14C-DHP during growth of N. frowardii on wheat straw.
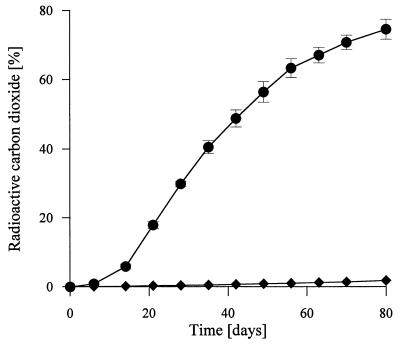
14C-DHP was effectively mineralized during SSF of wheat straw with N. frowardii. Figure 6 shows the time course of 14CO2 evolution over a period of 12 weeks. Mineralization started after 1 week, the maximum rate of mineralization (1.7% 14CO2 per day) was reached rapidly after 2 weeks of incubation, and then the rate was nearly constant for the next 3 weeks, after which the rate decreased slowly. Interestingly, the rate of 14C-DHP mineralization increased simultaneously with the production of MnP and the decrease in accumulated organic acid contents (Fig. 1, 5, and 6), indicating that MnP may be involved in the mineralization process. The balance of radioactivity at the end of incubation showed that ca. 75% of the 14C-DHP was converted into 14CO2 and 13% was converted into 14C-labelled water-soluble compounds; 4% of the radioactivity was extractable with dioxane representing nonconverted 14C-DHP, and 6% was detected as 14CO2 after combustion of the residual straw, including the well-developed fungal biomass (Table 1).
FIG. 6.
Mineralization of 14C-ring-labelled synthetic lignin (22,000 dpm) by N. frowardii during growth on wheat straw (●). ⧫, noninoculated control. The bars indicate standard deviations (n = 3).
TABLE 1.
Balance of radioactive carbon (14C) from 14C-ring-labelled synthetic lignin (22,000 dpm) added to wheat straw after SSF with N. frowardii
| Sample | % of 14C in:
|
|||||
|---|---|---|---|---|---|---|
| 14CO2 | 14C-labelled volatile organic compounds | 14C-labelled water-soluble substances | 14C-labelled dioxan-soluble substances | Residual 14Ca | Total | |
| N. frowardii | 75 ± 2.5 | 1.3 ± 0.2 | 13.7 ± 1.7 | 4.2 ± 0.6 | 5.9 ± 1.2 | 100.1 |
| Control | 0.6 ± 0.2 | 0.2 ± 0.05 | 2 ± 0.6 | 76.8 ± 2.2 | 13.6 ± 1.2 | 92.8 |
Radioactivity associated with the residual straw material including the fungal biomass.
Mineralization and solubilization of 14C-DHP by MnP.
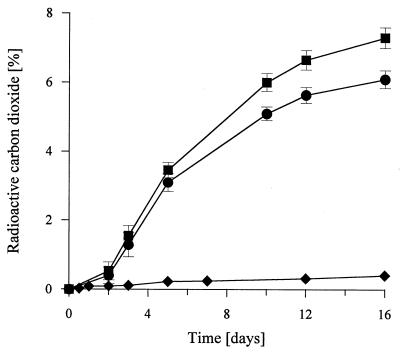
An in vitro reaction system was designed to simulate the conditions in the straw cultures of N. frowardii on the 12th day of cultivation. Using comparable concentrations of malate and fumarate, which were detected during SSF, we examined the ability of purified MnP to mineralize and solubilize 14C-DHP in a cell-free system. Figure 7 shows the time course of 14CO2 evolution from 14C-DHP due to purified MnP in malate- or malate-fumarate-containing reaction systems in the absence of external H2O2. About 7.5% of the initial radioactivity was released as 14CO2 in the malate-fumarate-containing reaction mixture within 16 days, whereas when malate was used alone, only 6% of the 14C-DHP was converted into 14CO2. The double bond in the fumarate molecule probably made the formation of radicals possible, which stimulated lignin degradation in a way similar to the way that has been postulated for radicals derived from unsaturated fatty acids (29). The extent of mineralization was relatively low during the first 2 days of incubation but then increased considerably, and mineralization occurred until the end of the experiment. Controls released less than 0.5% 14CO2. The maximum rate of in vitro mineralization (ca. 1.1% 14CO2 per day) was in the same range as the maximum rate in the fungal straw cultures (1.7% 14CO2 per day).
FIG. 7.
Production of 14CO2 from 14C-DHP by purified N. frowardii MnP in a cell-free system. Each reaction mixture contained 2 U of MnP, 30 mM malate, 5 mM fumarate, 1 mM MnCl2, 100 mg of unlabelled DHP per liter, and 22,000 dpm of 14C-DHP and was incubated on a rotary shaker at 37°C in the dark. The data points are means of three replicates, and the bars indicate standard deviations. Symbols: ■, complete reaction mixture; ●, reaction mixture without fumarate; ⧫, control without MnP.
Analysis of water-soluble radioactivity in the reaction mixture produced similar results. More 14C-DHP was solubilized in the malate-fumarate-containing system (43% ± 2.7%) than in the system containing malate alone (36% ± 3.2%); controls without MnP formed 12% ± 0.9% water-soluble radioactivity. These results demonstrate that in the presence of organic acid concentrations which are naturally produced by N. frowardii during SSF, effective mineralization and solubilization of lignin by MnP occur.
DISCUSSION
The white rot fungus N. frowardii produced MnP as the predominant ligninolytic enzyme during SSF of wheat straw, and by using comparable concentrations of organic acids excreted by the fungus, purified MnP was able to mineralize and solubilize 14C-DHP in a cell-free system. Thus, we demonstrated for the first time that production of MnP and organic acids is directly connected with mineralization of lignin.
Lignocellulose contains high levels of manganese (Mn), which, after calcium, potassium, and magnesium, is the most abundant metal. Mn concentrations up to 150 ppm have been detected in several soft- and hardwoods, and up to 50 ppm of Mn has been detected in wheat straw (13, 44). White rot fungi were found to accumulate Mn as MnO2 during growth on lignocellulose in black regions and flecks that contained more than 100-fold more Mn than sound lignocellulose (4).
MnP activities have been found during SSF of different lignocelluloses (wood, pulp, straw) with white rot fungi. Thus, the corticioid and polyporoid white rot fungi P. chrysosporium, Rigidoporus lignosus, and C. subvermispora grown on wood chips or sawdust produced multiple forms of MnP (9, 16, 41). MnP was also found to be the main ligninolytic enzyme during treatment of kraft pulp with Trametes versicolor, although laccase activity was also present (46, 48). Other authors investigated the activities of ligninolytic enzymes of three white rot fungi (P. chrysosporium, T. versicolor, Coriolopsis polyzona) during growth on wheat straw (59). MnP activity was the dominant enzyme activity during the initial phase of incubation, and the activity profiles of MnP were similar in all three fungi. Five species of the genus Pleurotus produced MnP and laccase as the major lignin-degrading enzymes when they were grown on straw under SSF conditions, and the maximum level of MnP activity was 4 to 10 times higher than the maximum level of laccase activity (7). Later it was demonstrated that in addition to MnP, LiP is secreted during SSF of wheat straw (56), an observation which we also made with N. frowardii, although the level of LiP activity was comparatively low. In their study, Vares et al. used P. radiata, a wood-rotting fungus that belongs to the same family of basidiomycetes (Meruliaceae) as the most investigated white rot fungus, P. chrysosporium (19). The P. radiata MnP isozymes MnPb and MnPa purified from wheat straw cultures each had a molecular mass of 50 kDa and had pI values of 3.4 to 3.9 and 4.9 to 5.3, respectively (56). Unlike P. radiata, our agaric fungus, N. frowardii (a member of the family Strophariaceae), produced only one MnP isozyme, which had a molecular mass of 50 kDa and a relatively low pI (3.0 to 3.2).
Production of organic acids, which are thought to be mediators of ligninolytic enzymes (in particular, chelators of Mn3+ generated by MnP), by wood-rotting basidiomycetes has been investigated previously, but most previous studies have been performed with liquid cultures (2, 10, 54) and information about the conditions in lignocellulose is limited (53). In all cases, accumulation of oxalic acid was reported, but only Takao also observed the formation of substantial amounts of other organic acids (malate, fumarate, succinate) by using CaCO3-containing shake cultures of a number of white and brown rot fungi (54). As far as we know, only one report of production of organic acids in a natural substrate of ligninolytic fungi has been published (17). Glakin et al. (17) demonstrated that oxalate was produced during SSF of wheat straw with different white rot fungi (e.g., P. radiata, P. chrysosporium, and C. subvermispora). Due to analytical difficulties (only CZE was used), other organic acids were not detected in the straw extracts. In the present study, this problem was overcome by using an HPLC method for detection of organic acids other than oxalate (24, 26). Using this method, we showed for the first time that high levels of malate and fumarate are produced by a white rot fungus during SSF.
Effective mineralization and solubilization of lignin by white-rot fungi have been demonstrated by using both natural and synthetic 14C-labelled lignins and lignocelluloses (6, 18, 21, 31, 32, 57). So far, the highest level of mineralization of a 14C-labelled lignin was observed with P. radiata, which released up to 71% 14CO2 from 14C-DHP when it was grown in a liquid medium (20). Interestingly, only 3% of the radioactivity was associated with the fungal biomass after 40 days of cultivation. Similar results were obtained with natural 14C-labelled lignins (e.g., lignins from fir or oak; 58 to 61% mineralization; 12 to 13% 14C in the mycelium) (20). Our results obtained with N. frowardii confirmed these results and revealed an even higher level of mineralization (75% of the 14C-DHP) during SSF, while also only a small percentage of the initial radioactivity (6%) was incorporated into the residual straw and the fungal biomass. The rate of lignin mineralization during SSF slowed down later than in liquid cultures, and substantial 14CO2 evolution was observed until the end of cultivation on day 80.
Given the assumption that 14C-labelled organic substances are normally converted intracellularly into 14CO2, the incorporation of such a small amount of radioactivity into the biomass is remarkable. In connection with our other findings, the label distribution provides an additional indication that at least some extracellular mineralization of lignin occurs. A low but significant level of mineralization of water-soluble lignin fragments by filter-sterilized P. chrysosporium culture fluids was reported by Boyle et. al. (6). These authors concluded that certain extracellular enzymes might be responsible for this mineralization. We recently demonstrated that crude MnP and purified MnP from N. frowardii are in fact capable of converting lignin and other aromatic and aliphatic compounds to CO2 in a cell-free reaction system (24–28). The cell-free reaction system routinely contained malonic acid, which is known to be the optimal chelator for Mn3+ formed by MnP (1, 62). Malonic acid, however, was not detected in the cultures of N. frowardii and was secreted only in trace amounts by other white rot fungi (62). Our present results show that malonate can be successfully replaced by the fungal metabolite malate (or malate-fumarate) during direct mineralization of lignin by MnP.
The formation of carboxylic groups or related structures from aromatic rings and the subsequent decarboxylation of these structures by Mn3+ are probably the basis for the MnP-catalyzed mineralization of aromatic compounds (24, 52). It has been reported that phenanthrene and veratryl alcohol are converted to a biphenyl dicarboxylic acid and a lactone, respectively, by MnP (8, 43). Moreover, reactive radicals (e.g., superoxide, carbon-centered radicals, and peroxyl radical), which are formed from organic acids by MnP, might also be involved in the mineralization process (26, 30). Furthermore, we propose that LiP may support the whole degradation process by cleaving bonds of recalcitrant lignin structures (e.g., ether bridges between nonphenolic lignin moieties).
On the basis of the present results, we propose that certain white rot fungi are able to mineralize lignin extracellularly; this proposal does not rule out the possibility that a substantial amount of lignin is also converted intracellularly into CO2. Future investigations will have to clarify to what extent extracellular mineralization occurs under natural conditions and whether similar systems have developed in other wood-rotting fungi.
ACKNOWLEDGMENTS
This study was carried out while M. Hofrichter was on a research leave at the Department of Applied Chemistry and Microbiology, University of Helsinki, and was supported by grant D/97/19017 from the German Academic Exchange Service within the “Hochschulprogramm III von Bund und Ländern,” as well as by grant 0327051D from the German Ministry of Education and Research.
We thank K. Steffen for help with the computer.
REFERENCES
- 1.Aitken M D, Irvine R L. Characterization of reactions catalyzed by manganese peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1990;276:405–414. doi: 10.1016/0003-9861(90)90739-l. [DOI] [PubMed] [Google Scholar]
- 2.Akamatsu Y, Takahashi M, Shimada M. Production of oxalic acid by wood-rotting basidiomycetes grown on low and high nitrogen culture media. Mater Org (Berlin) 1994;28:251–264. [Google Scholar]
- 3.Akhtar M, Blanchette R A, Kirk T K. Fungal delignification and mechanical pulping of wood. In: Eriksson K-E L, editor. Biotechnology in the pulp and paper industry. Berlin, Germany: Springer-Verlag; 1997. pp. 159–195. [Google Scholar]
- 4.Blanchette R A. Manganese accumulation in wood decayed by white rot fungi. Phytopathology. 1984;74:153–160. [Google Scholar]
- 5.Blanchette R A. Delignification by wood-decay fungi. Annu Rev Phytopathol. 1991;29:381–398. [Google Scholar]
- 6.Boyle C D, Kropp B R, Reid I D. Solubilization and mineralization of lignin by white rot fungi. Appl Environ Microbiol. 1992;58:3217–3224. doi: 10.1128/aem.58.10.3217-3224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlat V, Ruel K, Martinez A T, Camarero S, Hatakka A, Vares T, Joseleau J-P. Proceedings of the 7th International Conference on Biotechnology in Pulp and Paper Industry. Montreal, Canada: Canadian Pulp and Paper Association; 1998. The nature of lignin and its distribution in wheat straw affect the patterns of degradation by filamentous fungi; pp. A75–A78. [Google Scholar]
- 8.D’Annibale A, Crestini C, Di Mattia E, Sermanni G G. Veratryl alcohol oxidation by manganese-dependent peroxidase from Lentinus edodes. J Biotechnol. 1996;48:231–239. [Google Scholar]
- 9.Datta A, Bettermann A, Kirk T K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991;57:1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutton M V, Evans C S. Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment. Can J Microbiol. 1996;42:881–895. [Google Scholar]
- 11.Eggert C, Temp U, Dean J F D, Eriksson K-E L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson K-E L, Blanchette R A, Ander P. Microbial and enzymatic degradation of wood and wood components. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 13.Fengel D, Wegner G. Wood. Chemistry, ultrastructure, reactions. Berlin, Germany: Walter de Gruyter; 1989. pp. 217–220. [Google Scholar]
- 14.Forrester I T, Grabski A C, Burgess R R, Leatham G F. Manganese, Mn-dependent peroxidases and the biodegradation of lignin. Biochem Biophys Res Commun. 1988;157:992–999. doi: 10.1016/s0006-291x(88)80972-0. [DOI] [PubMed] [Google Scholar]
- 15.Forrester I T, Grabski A C, Mishra C, Kelley B D, Strickland W N, Leatham G F, Burgess R R. Characteristics and N-terminal amino acid sequence of manganese peroxidase purified from Lentinula edodes cultures grown on commercial wood substrate. Appl Microbiol Biotechnol. 1990;33:359–365. doi: 10.1007/BF00164536. [DOI] [PubMed] [Google Scholar]
- 16.Galliano H, Gas G, Seris J L, Boudet A M. Lignin degradation by Rigidoporus lignosus involves synergistic action of two oxidizing enzymes: Mn peroxidase and laccase. Enzyme Microb Technol. 1991;13:478–482. [Google Scholar]
- 17.Glakin S, Vares T, Kalsi M, Hatakka A. Production of organic acids by different white-rot fungi as detected using capillary zone electrophoresis. Biotechnol Techniques. 1998;12:267–271. [Google Scholar]
- 18.Haider K, Trojanowski J. Decomposition of specifically 14C-labelled phenol and dehydropolymers of coniferyl alcohols as model for lignin degradation by soft- and white-rot fungi. Arch Microbiol. 1975;105:33–41. [Google Scholar]
- 19.Haksworth D L, Kirk P M, Sutton B C, Pegler D N. Ainsworth and Bisby’s dictionary of fungi. 8th ed. Oxon, United Kingdom: CAB International; 1995. [Google Scholar]
- 20.Hatakka A, Buswell J A, Pirhonen T I, Uusi-Rauva A K. Degradation of 14C-labelled lignins by white-rot fungi. In: Higuchi T, Chang H M, Kirk T K, editors. Recent advances in lignin biodegradation research. Tokyo, Japan: Uni Publishers Co.; 1983. pp. 176–187. [Google Scholar]
- 21.Hatakka A, Uusi-Rauva A K. Degradation of 14C-labelled poplar wood lignin by selected white-rot fungi. Eur J Appl Microbiol Biotechnol. 1983;17:235–242. [Google Scholar]
- 22.Hatakka A. Ligninolytic enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev. 1994;13:125–135. [Google Scholar]
- 23.Hofrichter M, Fritsche W. Depolymerization of low-rank coal by extracellular fungal enzyme systems. II. The ligninolytic enzymes of the coal-humic-acid-depolymerizing fungus Nematoloma frowardii b19. Appl Microbiol Biotechnol. 1997;47:419–424. [Google Scholar]
- 24.Hofrichter M, Scheibner K, Schneegaß I, Fritsche W. Enzymatic combustion of aromatic and aliphatic compounds by manganese peroxidase from Nematoloma frowardii. Appl Environ Microbiol. 1998;64:399–404. doi: 10.1128/aem.64.2.399-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofrichter M, Scheibner K, Schneegaß I, Ziegenhagen D, Fritsche W. Mineralization of synthetic humic substances by manganese peroxidase from the white-rot fungus Nematoloma frowardii. Appl Microbiol Biotechnol. 1998;49:584–588. [Google Scholar]
- 26.Hofrichter M, Ziegenhagen D, Vares T, Friedrich M, Jäger M G, Fritsche W, Hatakka A. Oxidative decomposition of malonic acid as the basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 1998;434:362–366. doi: 10.1016/s0014-5793(98)01023-0. [DOI] [PubMed] [Google Scholar]
- 27.Hofrichter, M., K. Scheibner, F. Bublitz, I. Schneegaß, D. Ziegenhagen, R. Martens, and W. Fritsche. Depolymerization of straw lignin by manganese peroxidase from Nematoloma frowardii is accompanied by release of carbon dioxide. Holzforschung, in press.
- 28.Hofrichter M, Vares T, Scheibner K, Galkin S, Sipilä J, Hatakka A. Mineralization and solubilization of synthetic lignin (DHP) by manganese peroxidases from Nematoloma frowardii and Phlebia radiata. J Biotechnol. 1999;67:217–228. [Google Scholar]
- 29.Jensen K A, Jr, Bao W, Kawai S, Srebotnik E, Hammel K E. Manganese-dependent cleavage of nonphenolic lignin structures by Ceriporiopsis subvermispora in the absence of lignin peroxidase. Appl Environ Microbiol. 1996;62:3679–3686. doi: 10.1128/aem.62.10.3679-3686.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerem Z, Hadar Y. TAPPI Proceedings, 1997 Biological Sciences Symposium. Atlanta, Ga: TAPPI Press; 1997. The role of manganese in enhanced lignin degradation by Pleurotus ostreatus; pp. 29–33. [Google Scholar]
- 31.Kirk T K, Connors W J, Bleam R D, Hackett W F, Zeikus J G. Preparation and microbial decomposition of synthetic (14C)lignins. Proc Natl Acad Sci USA. 1975;72:2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirk T K, Schultz E, Connors W J, Lorenz L F, Zeikus J G. Influence of culture parameters on lignin metabolism by Phanerochaete chrysosporium. Arch Microbiol. 1978;117:277–285. [Google Scholar]
- 33.Kirk T K, Cowling E B. The chemistry of solid wood. Adv Chem Ser. 1984;207:435–487. [Google Scholar]
- 34.Kirk T K, Croan S, Tien M, Murtagh E, Farrell R L. Production of multiple ligninases by Phanerochaete chrysosporium: effect of selected growth conditions and use of mutant strain. Enzyme Microb Technol. 1986;8:27–32. [Google Scholar]
- 35.Kirk T K, Farrell R L. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 36.Kuan I-C, Johnson K A, Tien M. Kinetic analysis of manganese peroxidase. The reaction with manganese complexes. J Biol Chem. 1993;268:20064–20070. [PubMed] [Google Scholar]
- 37.Kuan I-C, Tien M. Stimulation of Mn peroxidase activity: a possible role for oxalate in lignin biodegradation. Proc Natl Acad Sci USA. 1993;90:1242–1246. doi: 10.1073/pnas.90.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lackner R, Srebotnik E, Messner K. Oxidative degradation of high molecular weight chlorolignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991;178:1092–1098. doi: 10.1016/0006-291x(91)91004-v. [DOI] [PubMed] [Google Scholar]
- 39.Lang E, Nerud F, Novotna E, Zadrazil F, Martens R. Production of ligninolytic exoenzymes and pyrene mineralization by Pleurotus sp. in lignocellulose substrate. Folia Microbiol. 1996;41:489–493. [Google Scholar]
- 40.Leatham G F. The ligninolytic activities of Lentinus edodes and Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 1986;24:51–58. [Google Scholar]
- 41.Lobos S, Larrain J, Salas L, Cullen D, Vicuña R. Isoenzymes of manganese-dependent peroxidase and laccase produced by the lignin degrading basidiomycete Ceriporiopsis subvermispora. Microbiology. 1994;140:2691–2698. doi: 10.1099/00221287-140-10-2691. [DOI] [PubMed] [Google Scholar]
- 41a.Martinez, A. T. 1998. Personal communication.
- 42.Michel F C, Jr, Dass S B, Grulke E A, Reddy C A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of Kraft bleach plant effluent. Appl Environ Microbiol. 1991;57:2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moen M A, Hammel K E. Lipid peroxidation by the manganese peroxidase of Phanerochaete chrysosporium is the basis for phenathrene oxidation by the intact fungus. Appl Environ Microbiol. 1994;60:1956–1961. doi: 10.1128/aem.60.6.1956-1961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahkala K, Mela T, Hakkola H, Järvi A, Virkajärvi P. Production and use of agrofibre in Finland. Part I. Production of agrofibre crops. Agronomy and varieties. Jokioinen, Finland: Maatalouden tutkimus keskus; 1996. [Google Scholar]
- 45.Paice M G, Bourbonnais R, Archibald F S, Jurasek L. Manganese peroxidase, produced by Trametes versicolor during pulp bleaching, demethylates and delignifies kraft pulp. Appl Environ Microbiol. 1993;59:260–265. doi: 10.1128/aem.59.1.260-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paice M G, Archibald F S, Bourbonnais R, Reid I D, Renaud S. TAPPI Proceedings, 1997 Biological Sciences Symposium. Atlanta, Ga: TAPPI Press; 1997. Manganese peroxidase catalyzed bleaching of Kraft pulp; pp. 343–345. [Google Scholar]
- 47.Paláez F, Martínez M J, Martínez A T. Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol Res. 1995;99:37–42. [Google Scholar]
- 48.Reid I D. Fate of residual lignin during delignification of Kraft pulp by Trametes versicolor. Appl Environ Microbiol. 1998;64:2117–2125. doi: 10.1128/aem.64.6.2117-2125.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sack U, Hofrichter M, Fritsche W. Degradation of PAHs by manganese peroxidase of Nematoloma frowardii. FEMS Microbiol Lett. 1997;152:227–334. doi: 10.1111/j.1574-6968.1997.tb10432.x. [DOI] [PubMed] [Google Scholar]
- 50.Scheibner K, Hofrichter M, Herre A, Michels J, Fritsche W. Screening for fungi effectively mineralizing 2,4,6-trinitrotoluene. Appl Microbiol Biotechnol. 1997;47:452–457. doi: 10.1007/s002530050955. [DOI] [PubMed] [Google Scholar]
- 51.Schneegaß I, Hofrichter M, Scheibner K, Fritsche W. Purification of the main manganese peroxidase isoenzyme MnP2 from the white-rot fungus Nematoloma frowardii. Appl Microbiol Biotechnol. 1997;48:602–605. [Google Scholar]
- 52.Shimada M, Ma D B, Akamatsu Y, Hattori T. A proposed role of oxalic acid in wood decay systems of wood-rotting basidiomycetes. FEMS Microbiol Rev. 1994;13:285–295. [Google Scholar]
- 53.Shimada M, Akamatsu Y, Tokimatsu T, Mii K, Hattori T. Possible biochemical roles of oxalic acid as a low molecular weight compound involved in brown-rot and white-rot decays. J Biotechnol. 1997;53:103–113. [Google Scholar]
- 54.Takao S. Organic acid production by basidiomycetes. I. Screening of acid-producing strains. Eur J Appl Microbiol. 1965;13:732–737. doi: 10.1128/am.13.5.732-737.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urzúa U, Larrondo L F, Lobos S, Larrain J, Vicuña R. Oxidation reactions catalyzed by manganese peroxidase isoenzymes from Ceriporiopsis subvermispora. FEBS Lett. 1995;371:132–136. doi: 10.1016/0014-5793(95)00874-9. [DOI] [PubMed] [Google Scholar]
- 56.Vares T, Kalsi M, Hatakka A. Lignin peroxidases, manganese peroxidases, and other ligninolytic enzymes produced by Phlebia radiata during solid-state fermentation of wheat straw. Appl Environ Microbiol. 1995;61:3515–3520. doi: 10.1128/aem.61.10.3515-3520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vares T, Hatakka A. Lignin-degrading activity and ligninolytic enzymes of different white-rot fungi: effects of manganese and malonate. Can J Bot. 1996;75:61–71. [Google Scholar]
- 58.Vares T, Niemenmaa O, Hatakka A. Secretion of ligninolytic enzymes and mineralization of 14C-labelled synthetic lignin by three Phlebia tremellosa strains. Appl Environ Microbiol. 1994;60:569–575. doi: 10.1128/aem.60.2.569-575.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vyas B R M, Volc J, Sasek V. Ligninolytic enzymes of selected white rot fungi cultivated on wheat straw. Folia Microbiol. 1994;39:235–240. [Google Scholar]
- 60.Waldner R, Leisola M S A, Fiechter A. Comparison of ligninolytic activities of selected white-rot fungi. Appl Microbiol Biotechnol. 1988;29:400–407. [Google Scholar]
- 61.Wariishi H, Valli K, Gold M H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991;176:269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- 62.Wariishi H, Valli K, Gold M H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem. 1992;267:23688–23695. [PubMed] [Google Scholar]