Highlights
-
•
Low-frequency ultrasound (LFUS) effect on cereals and pseudocereals was reviewed.
-
•
LFUS enhances hydration rate, time lag phase and germination of seeds.
-
•
LFUS boosts the extraction of phenolics, polysaccharides and other compounds.
-
•
LFUS modifies starch structure and partially denatures proteins.
-
•
LFUS improves interfacial properties and peptides availability of proteins.
Keywords: By-products, Extraction, Functional properties, Hydration, Meta-analysis
Abstract
Cereals (CE) and pseudocereals (PSCE) play a pivotal role in nourishing the human population. Low-frequency ultrasound (LFUS) modifies the structure of CE and PSCE macromolecules such as starch and proteins, often improving their technological, functional and bioactive properties. Hence, it is employed for enhancing the traditional processes utilized for the preparation of CE- and PSCE-based foods as well as for the upcycling of their by-products. We report recent advances in LFUS treatments for hydration, germination, extraction of bioactive compounds from by-products, and fortification of CEs and PSCE, including kinetic modelling and underlying action mechanisms. Meta-analyses of LFUS influence on compounds extraction and starch gelatinization are also presented. LFUS enhances hydration rate and time lag phase of CE and PSCE, essential for germination, extraction, fermentation and cooking. The germination is improved by increasing hydration, releasing promoters and eliminating inhibitors. Furthermore, LFUS boosts the extraction of phenolic compounds, polysaccharides and other food components; modifies starch structure, affecting pasting properties; causes partial denaturation of proteins, improving their interfacial properties and their peptides availability. Overall, LFUS has an outstanding potential to improve transformation processes and functionalities of CE and PSCE.
1. Introduction
Ultrasound (US) is a mechanical wave with frequency higher than 20 kHz, i.e., beyond the audible frequency range of humans. It can be differentiated in three frequency ranges: 1) 20 to 100 kHz, conventional high-power or low-frequency ultrasound (LFUS); 2) 100 kHz to 2 MHz, sonochemistry range and 3) 5 to 10 MHz, medical or analytical range [1].
Over the past 20 years, the application of LFUS has gained significant potential interest in many industrial sectors because of the versatility in modifying as well as generating microstructures. Thanks to its adaptability, relative simplicity, low energy requirements and limited impact on the environment, LFUS has a very promising future in food technology and has already been applied to solve technical issues such as increasing yield, reducing treatment time [2], [3], [4], extracting with non-toxic solvents [5] and limiting energy needs [6].
LFUS can be employed to make changes in liquids, dispersions, solid and gaseous media, but they are particularly efficient when applied through a liquid [7]. LFUS effects in liquid systems are mainly related to the cavitation phenomenon: during the negative pressure half-wave (rarefaction) the medium is stressed by tensile forces that increase the distance among molecules; once the cavitation threshold is reached, the liquid breaks down and empty bubbles form. When a cavity cannot tolerate the surrounding liquid pressure, it implodes violently, and energy is immediately released. Cavities collapse is far more rapid than heat diffusion, creating a localized hot spot while the liquid bulk remains cold [8]. Inside the cavities, temperatures of 1700–4700 °C and pressures of 180–300 MPa are reached [1]. As temperature rises, cavitation threshold goes down due to drops in surface tension and viscosity, thus a liquid will start to cavitate at lower sound pressure. However, water vapor pressure will be higher and vapor-filled bubbles will form, reducing pressure difference and cushioning bubbles implosions [9]. Very high tensile forces are needed for cavitation. Energy and intensity, along with medium viscosity, surface tension, vapor pressure, nature and concentration of dissolved gas, presence of solid particles, temperature, and treatment pressure, determine the extent of cavitation [10]. Gas-filled bubbles or particulate produce weak spots in the liquid that allow the process to begin; alternatively, pre-generated cavitation bubbles can be starting points.
The most common frequency range applied is 20–40 KHz, because cavitation is hardly achieved at high frequencies unless very great intensities are adopted [8]. The true mechanical power in the liquid is usually far lower than the nominal one; in addition, devices with different efficiencies can be used and the volume may change as well. Therefore, to compare results it is necessary to experimentally evaluate the specific amount of energy per volume unit, an often-overlooked aspect [11]. The volumetric power can be estimated by the following formula [11], [12]:
where m is the water mass (kg), Cp its specific heat (4.186 kJ/(kg °C)) and dT/dt (°C/s) the temperature increase during the first 90 s of sonication.
Besides the mechanical effect, LFUS generates free radicals through water homolytic cleavage: H2O → H• + OH • [1], [8]. However, free radicals are produced at the fastest rate from water at around 200 kHz (sonochemistry range) [1], while at 20 kHz their development is minimal [1], [13].
Food processing procedures using LFUS have been applied to improve product quality, including bioactivity, and process efficiency in cereal and pseudocereals. However, to the best of our knowledge, a review focused on the effect of LFUS on key cereal and pseudocereal processes like hydration, germination and fortification is not available. Additionally, we summarize as forest plots the effect of LFUS on the extraction of bioactive compounds from cereal and pseudocereal by-products. Finally, we report and discuss the influence of LFUS on structural changes of starch, protein, and dietary fibre.
2. Methods
2.1. Research and selection criteria
Literature research from 1994 to 2022 was carried out on Scopus and Web of Science databases. The keywords used were Ultrasound OR Ultrasonic followed by common or Latin name of each cereal and pseudocereal (i.e., Wheat, Rice, Corn or Maize, Barley, Rye, Oat, Sorghum, Teff, Triticale, Millet, Quinoa, Amaranth, Buckwheat, and Chia). All articles concerning the application of low-frequency ultrasound to cereals and pseudocereals, or to derived ingredients, aimed at improving processing, increasing yield, or determining a modification in their chemical and technological behaviour were considered. Sources which presented LFUS as an ameliorating technique but without providing control trials were considered biased and were excluded.
2.2. Statistical elaboration
On two occasions the results were summarized through meta-analysis by creating forest plots with the software RevMan v5.4.1 (https://www.cochrane.org).
For Fig. 1, the effect size was standardized by computing log response ratios and standard errors as illustrated by Borenstein et al. [14]:
When the studies provided multiple non-independent outcomes the effect size was computed as their mean log response ratio, while the variance was determined as follows [14]:
where m is the number of correlated variables and r is the correlation coefficient; when not calculated, r was cautiously assumed to be 1, resulting in a lower weight of the study. The data were plotted with the generic inverse variance method by RevMan, which provides automatic antilogarithm.
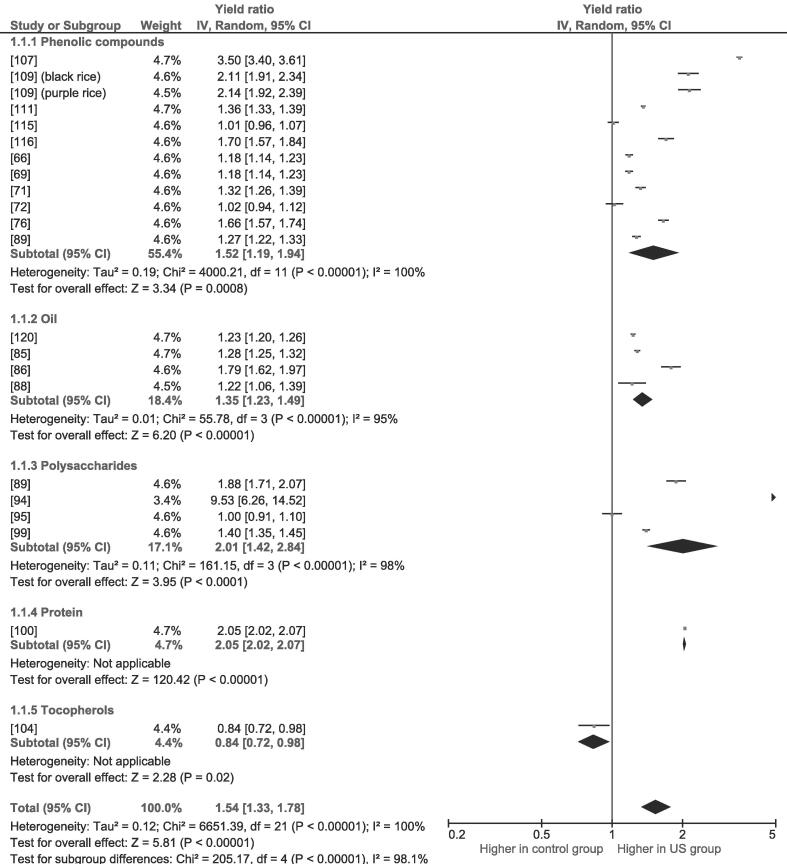
Fig. 1.
Forest plot for random-effects meta-analysis of extraction yield: LFUS-assisted extraction vs. a similar control group of non-US-assisted extraction or vs. traditional extraction. The projection of each point on the abscissa axis represents, if higher than 1, the n of an n-fold increase in the extraction yield.
For Fig. 2, the effect size (i.e., the raw mean difference) and standard error were computed as described by Borenstein et al. [14]:
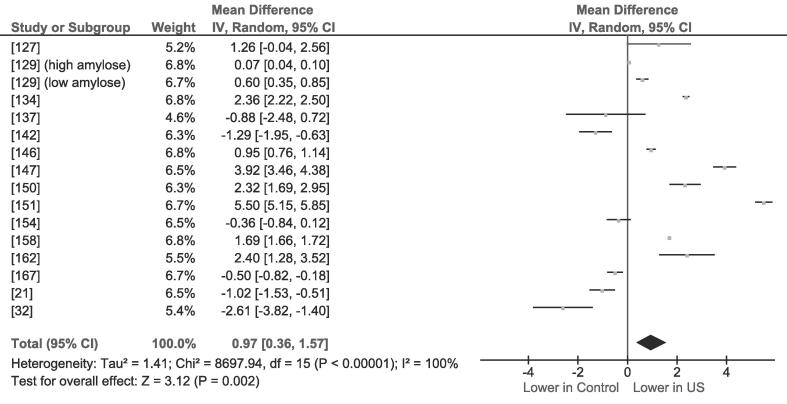
Fig. 2.
Forest plot for random-effects meta-analysis of starch gelatinization enthalpy (ΔH; J/g): control group vs. the LFUS-treated group (under the most severe conditions). The raw mean difference between the control and the LFUS-treated group was used as the effect size.
3. Low-frequency ultrasound application in cereals and pseudocereals processing
Because of ease of utilization, energy-saving, and relatively low costs, high-power LFUS has been proposed for processes as different as analysis, extraction, chemical and technological properties modification, emulsification, drying, freezing [15] and even microbial decontamination [16]. In the cereals area, the research is focused mainly on the effects of LFUS on physical and chemical changes of proteins [17], [18], [19], [20] and starch [21], [22], enzymatic activity [23], antioxidant compounds retrieval [24] and biofortification [25], [26], [27], hydration and germination [11], [28], [29], [30] as well as food technological and sensorial quality improvement [17], [31], [32], [33], [34].
3.1. Hydration
Hydration is essential for many processes, such as cooking, extraction, fermentation, germination and malting [35]. During kernels hydration, the water uptake follows preferential paths, according to grain shape and structure. For instance, in barley caryopses the water penetrates through the hilar fissure and the micropyle, while in maize kernels enters by the tip cap, filling the empty space between germ and endosperm [29], [36].
Several studies suggest that LFUS treatments enhance hydration. Miano et al. [37] proposed two different mechanisms: a) direct, related to inertial flow and sponge effect, where the ultrasound-induced cavitation creates a compression-rarefaction turnover that squeezes and releases the matrix, pumping water through pre-existing pores; and b) indirect, leading to the formation of new micro-channels because of physical damage to the tissues. However, low water activity does not allow cavitation, hence dry caryopses (aw ∼ 0.65) undergo a slow hydration [37]. In fact, new cavities begin to form in the matrix only when an adequate aw is reached, accelerating the hydration process. For the above-mentioned reason, LFUS treatments are more effective when applied on pre-soaked kernels [38]. The relative extent of direct and indirect effects on the kernels varies according to the species [29]. Generally, cereals hydration kinetics exhibit a downward concave curve with one or two steps [35] that can be modelled by the Peleg model [39]:
where Mt is the moisture (% dry weight) at t time, M0 the initial moisture, k1 and k2 are Peleg’s constants. The k1 is equal to the inverse of the initial moisture uptake rate and the k2 is inversely related with equilibrium moisture.
As an alternative the Weibull model, an exponential empirical model useful for its simplicity and good accuracy in describing complex processes with high variability, may be used. [11]:
where Meq is the equilibrium moisture, β the scale parameter and α the shape parameter; α describes the initial water uptake behavior and β represents the time needed to achieve 63% moisture; both parameters are inversely related to the process rate [40]. The parameter α is related to moisture migration during hydration: when α > 1 the process is governed by internal moisture diffusion as well as external mass transfer, while when α < 1 the external mass transfer is negligible [11].
Both Peleg and Weibull models have been used to model the hydration kinetic of wheat treatment with LFUS [11]. The LFUS technology accelerates the hydration process by increasing the hydration rate and reducing the lag phase, contributing to reach a higher final moisture. Li et al. [41] examined the initial 120 min of rice water uptake and observed that ultrasound hastened the maximum absorption rate in a power-dependent manner and that the moisture absorption rate increased according to a Peleg-like curve. Miano et al. [29] reported a 300 min drop in hydration time for flint maize kernels, albeit no new cavities were formed because of their extremely vitreous endosperm, while Patero and Augusto [42] observed a decrease from 320 to 190 min in sorghum kernels. The combination of LFUS and temperature may further improve these results. Kalita et al. [28] compared paddy rice hydration by soaking and LFUS-assisted soaking at different temperatures and noticed that the process was abridged from ≥ 24 h (traditional) to 3 h (LFUS). Similarly, Guimarães et al. [11] observed that LFUS per se shortened wheat hydration time by 28–42%, while the combination of LFUS and temperature (25 °C) cut it by 72%. Patero and Augusto [42] determined that the temperature (53 °C) had a greater effect than LFUS on sorghum hydration, and a similar result was obtained by Shafaei et al. [43] in wheat at different temperatures (from 30 °C to 70 °C). Nevertheless, high temperatures may have relevant drawbacks, including protein denaturation, starch gelatinization or industrial plant insulation costs. Moreover, the increase of processing temperature above 60 °C reduces cavitation, because the amount of water vapor inside the bubble increases and a collapse occurs [8], [44]. Hence, a 35–40 °C range is generally appropriate to accelerate the process without damaging seed properties [36]. In barley malt production, the combination of LFUS and mild temperatures (20–25 °C) accelerates the steeping phase; the LFUS intensity affects water absorption mainly in the early phase (Peleg’s k1), while the temperature has a stronger effect on higher final moisture. Overall, their combination reduces the hydration time by half [45] or even more [36]. Temperatures above 40 °C worsen malt quality, therefore LFUS represents a promising approach to reduce steeping time; additionally, the LFUS mechanical power is dissipated as heat, reducing the energetic costs.
3.2. Germination
Germination is the sum of events that break the seed quiescence status and allow the embryonic axis to appear. As a first step, the seed hydrates so the pre-existent enzymes are activated and initiate the Krebs cycle or, in rice, even anaerobic pathways, the endosperm reserves are mobilized, and the protein synthesis becomes intensive. The germination ends with cells elongation, that leads to root and shoot emission [46].
From the point of view of food sciences, the activation of dormant enzymes during germination leads to significant changes in biochemical and nutritional characteristics. The α- and β-amylases rapidly degrade the carbohydrates, with a consequent surge in reducing sugars, while other enzymes decompose cell walls and enhance the accessibility of internal nutrients [47]. The proteins are hydrolyzed, increasing peptides and amino acids availability [47]. Different bioactive compounds, such as tocols, thiamine, riboflavin, folic acid, vitamin C and carotenoids, are biosynthesized to generate the nutrients necessary for seedling growth [47], [48]. The metabolic activities fuel phenolic compounds biosynthesis [49], [50], while the degradation of cell walls leads to an increase of readily available free phenolic compounds [51]. The abundant presence of bioactive molecules boosts the antioxidant capacity [52]. Furthermore, sprouting increases phytase activity, leading to phytic acid degradation and better micronutrients absorption in the gastrointestinal tract [47], [53]. Overall, controlled germination improves the nutritional composition of the kernels, but the enzymatic activity has a negative impact on some technological properties of the flours (e.g., leavening), stressing the importance of a carefully controlled process to avoid excessive hydrolysis [47].
US, together with high pressure, cold plasma, and pulsed electric fields, is an emerging technology used to regulate seeds germination [30]. The LFUS treatment enhances germination primarily by its positive effect on hydration. In fact, cavitation appears to cause fissures in the pericarp, augmenting the availability of water and oxygen [54], [55]. The absorbed water stimulates the embryo to release gibberellic acid, a chemical promoter of germination [55]; at the same time, the hydration promotes the leakage of germination inhibitors, such as the abscisic acid [46]. With regards to other phytohormones, Wei et al. [56] observed that LFUS decreased the indole-3-acetic acid (IAA)/cytokinin ratio in Dendrobium officinale protocorms. IAA is a root growth promoter while cytokinin stimulates shoot growth [46], therefore a higher IAA presence may lead to earlier germination. Moreover, LFUS probably acts facilitating the mobilization of endosperm nutrients by disrupting cell membranes [55], as corroborated by the sonication-induced erosion at the joint between cells observed by Ananthakrishnan et al. [57], which would stimulate a faster flow of water and nutrients. Finally, Chen et al. [58] documented that LFUS-treated wheat seeds exhibited greater antioxidant activities of catalase, superoxide dismutase and glutathione reductase. Apparently, reactive oxygen species (ROS) generated by cavitation elicit an antioxidant response that enhances plant vigor. Other improvements were found in number of germinated seeds, protein and total chlorophyll content, shoot length, fresh and dry biomass, and levels of cell damage indicators [58].
Although the mechanism that induces faster germination is not fully understood, numerous observations have been collected (Table 1). Ding et al. [54] found that LFUS improves rice sprout growth speed by 22.3–26.9%, Xia et al. [30] reported that an additional 10–15% of rice seeds sprouted in the initial 32 h, while Goussous et al. [59] described an enhanced wheat germination rate of about 60% compared to plain water soaking; in addition, a positive interaction between LFUS and temperature was found. Similarly, Yaldagard et al. [55] observed that LFUS reduced barley steeping time from the usual 46 h to 25–30 h, depending on the applied intensity, and that the percentage of germinated seeds increased from 93.3% (control) up to 99.4% in the most intensely treated group. Furthermore, the hair roots were luxuriant and, at the highest intensity, the germination period was shortened from 7 to 4 days. A slight increase in barley germinated seeds was observed also by Miano et al. [60] and was attributed to porosity increment, since plastic bags insulated the seeds from the water during the sonication. Similarly, Hassan et al. [61] observed that 5 min LFUS treatment greatly improved the number of germinated sorghum grains from 78% (control) to 94%; a longer process decreased the germinated seeds, therefore they inferred that at high amplitude the treatment damaged cells and/or enzymes. Kratovalieva et al. [62] studied LFUS application to different cereals and noticed significant effects on coleoptile and mesocotyl elongation. Oat and rye caryopses were very sensitive to LFUS stimulation (+119% and +65% growth, respectively), while triticale and wheat showed smaller increases. In a comparison among different pre-treatment, LFUS proved to be better than microwaves or heat in enhancing buckwheat germination by 4–4.5% [63].
Table 1.
Conditions tested in ultrasound processing for improving seed hydration and germination.
| Sample | Device | Frequency |
Nominal power |
Amplitude |
Volume |
Specific power a |
Time |
T |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| (kHz) | (W) | (%) | (L) | (W/L) | (min) | (°C) | |||
| Barley | Bath | 37 | 154 | 5.7 | 30–480 | 35, 40 | [36] | ||
| Barley | Bath | 20 | 28 | 240 | [60] | ||||
| Buckwheat | Bath | 300 | 30 | 29 | [63] | ||||
| Corn | Bath | 25 | 4 | 41 | 180–300 | [29] | |||
| oat, rye, triticale, wheat | Bath | 30–40 | 300 | 15 | 25 | [62] | |||
| paddy rice | Bath | 35 | <225 | 2 | 15 | 30 | [38] | ||
| paddy rice | Bath | 40 | 50 | 10–120 | 25–40 | [28] | |||
| rice | Bath | 40 | 150–600 | 10–120 | 4 | [41] | |||
| sorghum | Bath | 40 | 28 | 120 | [37] | ||||
| sorghum | Bath | 40 | 2 | 26 | 30 | 25; 53 | [42] | ||
| wheat | Bath | 45 | 160 | 8 | 5–20 | [58] | |||
| wheat | Bath | 40 | 100 | 5–60 | 25 | [59] | |||
| wheat | Bath | 25; 40 | 360–480 | 10 | 30–70 | [43] | |||
| barley | Probe | 20 | 750–1500 | 1 | 51.1; 84.7 | 30–270 | 10–25 | [45] | |
| barley | Probe | 20 | 460 | 20–60 | 0.08 | 5–15 | 30 | [55] | |
| rice | Probe | 25 | 2000 | 125 | 5 | 23; 24 | [54] | ||
| sorghum | Probe | 20 | 750 | 40; 60 | 0.5 | 5; 10 | 35 | [61] | |
| wheat | Probe | 20 | 500–1500 | 1 | 61.5; 83.6; 102.4 | 15–25 | [11] | ||
| wheat | Probe | 22 | 227 W/L | 3 | [65] | ||||
| rice | transducer box x2 | 25 | 16 W/L | 152 | 5 | 23–24 | [64] | ||
| rice | 28 | 400 | 5–30 | [30] |
Determined by the calorimetric method.
Beyond the described effect, sonication before or during germination in rice and wheat appeared to increase the synthesis of γ-aminobutyric acid, a health-enhancing compound, by 1.7 and 3-fold respectively [64], [65].
3.3. Extraction
The most popular LFUS application is improving the extraction of different compounds. Table 2 summarizes a variety of LFUS-assisted extraction trials with their respective optimized experimental conditions. Fig. 1 reports a forest plot for random-effects performed considering twenty studies comparing LFUS-assisted vs. traditional or non-US extractions of different fractions (phenolics compounds, oil, polysaccharides, protein and tocopherols) from diverse substrates; the extraction yields of LFUS-assisted processes were compared with a control group of non-LFUS or traditional processes. The projection of each point on the abscissa axis represents, if higher than 1, the n of an n-fold increase in the extraction yield.
Table 2.
Optimized experimental conditions and yields (mean ± SD) of LFUS-assisted extraction. Experiments marked with asterisk employed LFUS along with another major technique or lasted longer than the net LFUS treatment time.
| Raw material | To be extracted | Device | Power |
Amp. |
Time |
T |
Extraction medium | Ratio |
Yield | Unit | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (W) | (%) | (min) | (°C) | (ml/g) | |||||||
| barley flour | total phenolics | bath | 200 | 20 | 50 | 97 % ethanol | 37 | 24.76 ± 0.15 | mg/g | [107] | |
| barley flour | phenolic acids | bath | 200 | 18 | 50 | 100% ethanol | 60 | 19.65 ± 0.09 | mg/g | [107] | |
| hull-less barley seeds | total phenolics | bath | 2x10 | 20 | ethanol/acetone/water 7:7:6 v/v/v | 300.4 | mg/100 g | [108] | |||
| buckwheat sprouts | flavonoids | bath | 700 | 40 | 56 | 80% [choline chloride/triethyene glycol 1:4] in water | 50 | 21.1 ± 1.2 | mg/g | [77] | |
| oat bran | total phenolics | bath | 600 | 15 | 70 | 80% ethanol | 10 | 173 | mg/100 g | [73] | |
| hull-less oat seeds | total phenolics | bath | 2x10 | 20 | ethanol/acetone/water 7:7:6 v/v/v | 70.3 | mg/100 g | [108] | |||
| black quinoa | total phenolics | bath | 10 | 40 | 80% ethanol | 20 | 236.37 ± 5.26 | mg/100 g dm | [74] | ||
| black rice | free phenolics | bath | 2x15 | 20 | methanol/water 85:15 | 40 | 443 ± 31 | mg/100 g | [72] | ||
| black rice | bound phenolics | bath | 90 | 40 | NaOH 2 M | 20 | 116.1 ± 2.2 | mg/100 g | [72] | ||
| black rice bran | total phenolics | bath | 230 | 23 | 36 | 23.8% ethanol (pH 2.5) | 1978 ± 101 | mg/100 g | [109] | ||
| purple rice bran | total phenolics | bath | 230 | 16 | 31.7 | 31.2% ethanol (pH 2.4) | 2232 ± 115 | mg/100 g | [109] | ||
| black rice husk | total phenolics | bath | 10 | 49.5 | 67.3% ethanol | 40.8 | 1.72 | mg/g | [110]* | ||
| black rice husk | flavonoids | bath | total | 49.5 | 67.3% ethanol | 40.8 | 3.01 | mg/100 g | [110]* | ||
| black rice husk | anthocyanins | bath | 10 | 49.5 | 67.3% ethanol | 40.8 | 3.36 | mg/100 g | [110]* | ||
| wild rice flour | flavonoids | bath | 200 | 51.2 | 76.6% [choline chloride/1,4-butanediol 1:6] in water | 27 | 9.3 | mg/g | [75] | ||
| rye seeds | total phenolics | bath | 2x10 | 20 | ethanol/acetone/water 7:7:6 v/v/v | 189.2 | mg/100 g | [108] | |||
| red sorghum bran | total phenolics | bath | 200 | 21 | 53% ethanol | 52 | 49.74 ± 0.88 | mg/g dm | [111] | ||
| triticale seeds | total phenolics | bath | 2x10 | 20 | ethanol/acetone/water 7:7:6 v/v/v | 49.4 | mg/100 g | [108] | |||
| wheat bran | phenolic acids | bath | 120 | 23 | 45 | ethanol | 30 | [112] | |||
| soft wheat bran | total phenolics | bath | 120 | 40 | 50 | 80% [glycerol/citric acid/glycine 4:1:1 mol/mol/mol] in water (pH 2.9) | 30 | 8.7 ± 0.9 | mg/g | [76]* | |
| soft wheat bran | total phenolics | bath | 120 | 40 | 50 | 80% [glycerol/citric acid/glycine 4:1:1 mol/mol/mol] in water (pH 2.9) | 30 | 94.6 ± 3.0 | mg/g | [76] (+heat)* | |
| wheat chaff | total phenolics | bath | 500 | 10 | 25 | 22.5% ethanol (w/w) in water | 32 | 2.572 ± 0.074 | mg/g | [89] | |
| wheat chaff | total phenolics | bath | 500 | 10 | 25 | ethanol/ammonium sulfate 24.3:23.8 (w/w) in water | 34 | 2.67 ± 0.07 | mg/g | [89] | |
| wheat seeds | total phenolics | bath | 2x10 | 20 | ethanol/acetone/water 7:7:6 v/v/v | 166.8 | mg/100 g | [108] | |||
| wheat germ | total phenolics | bath | 30 | 50 | ethanol | 20 | 646.94 | mg/100 g | [70] | ||
| wheat bran | total phenolics | bath | 250 | 25 | 60 | 64% ethanol | 20 | 3.12 ± 0.03 | mg/g | [68] | |
| chia flour | total phenolics | probe | 400 | 100 | 15 | methanol | 10 | 194 ± 11 | mg/100 g | [113] | |
| purple corn bran | anthocyanins | probe | 400 | 35 (4/8 s on/off) | 40 | 95% ethanol/0.1 M citric acid/water 4:1:3 | 8 | 3.63 ± 0.11 | g/kg | [71] | |
| purple corn | anthocyanins | probe | 105 | 90 | 70 | 74% ethanol | 26 | 0.45 ± 0.01 | g/kg | [80] | |
| purple corn cob | anthocyanins | probe | 500 | 50 | 30 (5/10 s on/off) | 60 | 50% ethanol | 20 | 240.2 | μg/g | [79] |
| purple corn cob | total phenolics | probe | 500 | 50 | 30 (5/10 s on/off) | 60 | 50% ethanol | 20 | 27.7 | mg/g | [79] |
| finger millet seed coat | total phenolics | probe | 550 | 30 | 25 (20/20 s on/off) | 55 | 50% ethanol | 30 | 21.05 ± 0.47 | mg/100 g | [66] |
| red quinoa flour | total phenolics | probe | 250 | 60 | 15 | 75 | 50% ethanol | 10 | 2.62 ± 0.17 | g/100 g extract | [114] |
| rice bran | free phenolics | probe | 315 | 30 | 55 | 80% ethanol | 60 | 7.1 ± 0.3 | mg/g | [115] | |
| wheat seeds from distiller | total phenolics | probe | 400 | 100 | 0.5 | 0 | water | 20 | [67]* | ||
| black wheat bran | total phenolics | 300 | 40 | 50 | 80% ethanol | 2592 ± 109 | μg/g | [116] | |||
| teff seeds | total phenolics | 120 | 2x5 (30/10 s on/off) | 30 | water/ethanol/methanol 49:26:25 v/v/v | 20 | 213.1 ± 6.9 | mg/100 g | [69] | ||
| teff seeds | flavonoids | 120 | 2x5 (30/10 s on/off) | 30 | water/ethanol/methanol 49:26:25 v/v/v | 20 | 106 ± 14 | mg/100 g | [69] | ||
| tartary buckwheat seeds | flavonoids | 200 | 21 | 60 | 72% methanol | 250 | 3.94 ± 0.62 | g/100 g | [117] | ||
| red rice bran | anthocyanins | 400 | 55.2 | acidified ethanol 78.37 % (pH 2.3) | 17.5 | 5.51 | mg/g | [118] | |||
| corn silk | flavonoids | 500 | 21.5 | 33.75% ethanol | 20 | 1.13 | g/100 g | [119] | |||
| chia seeds | oil | bath | 176 | 40 | 50 | ethyl acetate | 12 | 27.19 ± 0.08 | g/100 g | [120] | |
| corn germ | oil | bath | 20 | 40 | cellulase and α-amylase in water | 66.25 ± 0.77 | % recovery | [85]* | |||
| rice | oil | bath | 123 | 37 | 42 | hexane | 10 | 76.93 ± 0.52 | % recovery | [87] | |
| rice bran | oil | bath | 100 | 60 | n-hexane | 5 | 20.35 | g/100 g | [121] | ||
| rice bran | oil | bath | 70 | 25 | water (pH 12) | 20 | g/100 g | [84]* | |||
| rice bran and wax | oil | probe | 300 | 30 | 45 | 0.03 M NaOH | 12 | 17.2 | g/100 g | [82] | |
| rice bran | oil | probe | 93 | 26 (5/5 s on/off) | 35 | ethanol | 6 | 10.8 ± 5.5 | g/100 g | [86] | |
| rice bran | oil | probe | 160 | 40 | 40 | supercritical CO2 14.82 g/min | 12.65 | g/100 g | [88]* | ||
| wheat chaff | xylo-oligosaccharides | bath | 500 | 10 | 25 | water | 32 | 15.2 ± 0.1 | mg/g | [89] | |
| wheat chaff | xylo-oligosaccharides | bath | 500 | 10 | 25 | ethanol/ammonium sulfate 24.3:23.8 (w/w) in water | 34 | 16.02 ± 0.74 | mg/g | [89] | |
| wheat bran | arabinoxylan | bath | 180 | 70 | 50 | 4.5 g/l endoxylanase | 20 | 142.6 ± 1.7 | mg/g | [99]* | |
| hull less barley flour | β-glucans | probe | 500 | 99 | 4.8 | 50 | water (pH 5) | 10 | 3.87 | g/100 g | [96] |
| enzyme free barley flour | β-glucans | probe | 400 | 16 (0.9 cycle) | 55 | water | 10 | 65.6 ± 1.2 | % recovery | [92] | |
| brewer’s spent grain | arabinoxylan | probe | 750 | 100 | 10 (5/5 s on/off) | 2 M KOH | 25 | 20.3 ± 0.4 | g/100 g | [95]* | |
| corn cob | xylan | probe | 200 | 10 | 70 | 5% NaOH | 25 | 36.8 | g/100 | [90]* | |
| corn bran | arabinoxylan | probe | 500 | 25 | 70 | 0.3% NaOH | 30 | 27.78 ± 0.17 | g/100 g | [97] | |
| dried corn silk | polysaccharides | 250 | 17 | 56 | water | 20 | 6.02 ± 0.02 | g/100 g | [98] | ||
| wheat bran | heteroxylans | probe | 100 | 5 | 40 | 5% NaOH | 15 | 45.6 | g/100 | [91]* | |
| dewaxed wheat straw | hemicellulose | probe | 100 | 35 | 35 | 0.5 KOH | 30 | 25.5 | g/100 | [94] | |
| chia seeds | hetero-polysaccharides | probe | 400 | 40 | 60 (1/1 s on/off) | 50 | water (pH 9) | 30 | 10.39 ± 0.57 | g/100 | [93]* |
| quinoa | protein | bath | 320 | 20 | 25 | water buffered pH 9 | 5 | 4.10 ± 0.18 | g/100 g | [101] | |
| rice dreg | protein | probe | 448 | 20 (10/6 s on/off) | 50 | 0.08 M NaOH | 20 | 88.44 ± 0.40 | % recovery | [100] | |
| defatted wheat germ | protein | probe | 363 | 24 (2.4/2 s on/off) | Na-docusate/isooctane/KCl reverse micelles in water | 100 | 57 | % recovery | [102] | ||
| corn meal | carotenoids | probe | 900 | >60 | 38 | ethanol | 6 | [106] | |||
| corn gluten meal | zeaxanthin | bath | 250 | 45 | 56 | 95% ethanol | 7.9 | 212 | μg/g | [122] | |
| corn gluten meal | lutein | bath | 250 | 45 | 56 | 95% ethanol | 7.9 | 185 | μg/g | [122] | |
| malted barley flour | α-amylase | probe | 200 | 25 | 20 (5/5 s on/off) | 30 | 50 mM Na phosphate pH 8 | 5.3 | 213.46 | μmol/(min g) | [123] |
| quinoa seed hulls | betacyanins | probe | 70 | 9.2 s (0.6 cycle) | water | 100 | 96.5 | mg/100 g wb | [103] | ||
| quinoa seed hulls | betaxanthins | probe | 90 | 40 s (0.7 cycle) | water | 100 | 201 | mg/100 g wb | [103] | ||
| rice bran | γ-oryzanol | probe | 500 | 40 | 40 | 45 | soybean oil | 5 | 493 ± 44 | μg/g oil | [104] |
| rice bran | α-tocopherol | probe | 500 | 40 | 40 | 45 | soybean oil | 5 | 139 ± 18 | μg/g oil | [104] |
| rice bran | γ-tocopherol | probe | 500 | 40 | 40 | 45 | soybean oil | 5 | 212 ± 31 | μg/g oil | [104] |
| rice bran | δ-tocopherol | probe | 500 | 40 | 40 | 45 | soybean oil | 5 | 241 ± 23 | μg/g oil | [104] |
| red rice seeds | melatonin | probe | 200 | 30 | 10 (0.2 cycle) | 40 | 50% methanol in water pH 3.5 | 2.5 | 72.67 | % recovery | [124] |
| red rice seeds | tryptophan and derivatives | probe | 200 | 30 | 5 (0.7 cycle) | 30 | 8% methanol in water pH 3 | 5 | 100 | % recovery | [125] |
| sorghum husk | biocolorants | probe | 360 | 20 (2/2 s on/off) | 55 | acidified ethanol/water 70/30 | 30 | 16.7 ± 0.6 | g/100 g | [105] |
An overall pooled increase of 54% in yield is achieved by implementing LFUS. Extraction of phenolic compounds, especially from by-products and waste materials, was the most studied process. Although in some cases yield improvements are negligible, other advantages must be considered. In fact, LFUS-assisted extraction generally allows to shorten the extraction period, to employ food-grade non-hazardous solvents and to use lower temperatures. For instance, in Balasubramaniam et al. [66] experiments, although yields were similar, the phenolic compounds extraction from millet was performed in half the time (30 vs. 60 min) and at lower temperature (50 vs. 60 °C). Izadifar [67] found that a 30 s sonication increased the extracted phenolics content from wheat by 14.3%. Further reductions in time were reported by Wang et al. [68], from 15 h of Soxhlet extraction to 25 min; Giopato Viell et al. [69], 5 min instead of 10; Teslić et al. [70], 30 min instead of 24 h, with 11% higher yield; Chen et al. [71], 32.5% higher yields in half the time; Melini and Acquistucci [72], 15 min instead of 1 h. As reported by Chen et al. [73], even though the highest yields were achieved at high temperature (70 °C), at 20 °C LFUS extracted in 5 min 54% more phenolics than a 65 min control trial. Therefore, LFUS represents a strategy to perform shorter low-temperature extractions and deserves to be introduced in analytical protocols to ensure correct quantification of phenolics [74].
LFUS-assisted extraction in some cases can efficiently use ethanol in place of chlorinated methanol [66], [71]. On the other hand, a certain amount of methanol contributes to lower the average viscosity of solvent mixture, thus facilitating cavitation [69]. In addition, with LFUS, green new developed deep eutectic solvents (DESs) may substitute or outperform the traditional ones. DES was 13% more effective than 30% ethanol in extracting ferulic and sinapic acid from corn silk [75] and extracted phenolic compounds from wheat bran better than 60% EtOH (+33%) and alkaline hydrolysis (+71%) [76]. Besides, properly formulated DESs can be compatible with HPLC as dilution solvent [77].
The LFUS ability at improving extraction is probably due to cavitation-driven cellular damage (especially cell walls), particle size reduction, strong mixing, increase in porosity and in specific surface area. These power- and time-dependent events allow the solvent to easily access tissues and dissolve phenolic compounds [67], [72], [76], [78]. In enzyme-assisted extraction, cavitation leads to higher available surface for enzyme digestion [78]. Giopato Viell et al. [69] suggested that part of extracted flavonoids from teff could come from release of bound fraction because of cavitation, while Chen et al. [73] inferred that at 70 °C heat-led cleavage of glycosidic bonds could have freed phenolics from bound forms.
Nevertheless, a LFUS overtreatment may worsen the extraction because of phenolics oxidation or destruction [67], [79] due to temperature increase [67] and radicals generation [78], [80]. According to Dzah et al. [81], although above 65 °C the phenolic molecules are degraded by hydrogen peroxide and their antioxidant activity decreases, the sonication time has not a direct impact on them.
Improving oil extraction is another LFUS application. In a study by Cravotto et al. [82], alkaline aqueous extraction with LFUS support outperformed hexane, resulting in 22% higher yield. The extraction time was also cut by 8-fold (4 h to 30 min). Additionally, in the presence of LFUS rice wax was hydrolyzed to policosanols mixture under milder temperature and alkali concentration [82], [83]. Khoei and Chekin [84] tested LFUS as pre-treatment in aqueous extraction carried out at 45 °C and pH 12 for 15 min, and achieved an 20% yield, similar to a Soxhlet extraction (23.4%) which, nevertheless, used hexane. In addition, the small Soxhlet extraction surplus in yield appeared linked to free fatty acids content, 3.2% in hexane-extracted oil vs. almost zero in aqueous-extracted oil: this could suggest that the aqueous extraction is selective against free fatty acids. Han et al. [85] confirmed that LFUS, being more efficient than steam and heat, could be a viable pre-treatment also in enzyme-led aqueous extraction. In Krishnan et al. [86] experiments, LFUS-assisted extraction of oil with ethanol achieved a yield 29% higher compared to the traditional process with hexane, and 78.5% higher compared to non-US ethanol extraction. Additionally, no changes in oil composition or modification in peroxide value were found [86], [87]. Therefore, a green solvent like ethanol could be conveniently used in oil extraction. By implementing a LFUS phase in their supercritical CO2 extraction, Soares et al. [88] improved the yield by 27% and cut by 60% the time, while extracting four oryzanol precursors, campesterol, β-sitosterol, stigmasterol and 4-methylenecycloartanol.
As above mentioned, sometimes LFUS over-treatment may lead to poor performances. According to Xu et al. [87], a power excess lowers yields since cavitation bubbles act as hindrance for wave propagation, while too long a treatment increases the quantity of suspended impurities and worsens solvent penetration. An over-treatment could also result in lipids emulsification, hence lower extraction yield [84]. Lastly, Khoei and Chekin [84], Han et al. [85] and Xu et al. [87] pointed out the cushioning effect as one of the major factors preventing yield enhancement by raising temperature.
Polysaccharides are a third class of compounds whose LFUS-assisted extraction has been studied. Ðordević and Antov [89] developed a protocol to extract at the same time xylo-oligosaccharides and phenolic compounds from wheat chaff, and the LFUS treatment improved by 2 and 1.3-fold the respective yields. In xylan extraction from corn cob, a 10 min LFUS pre-treatment at 70 °C could substitute the traditional one at 95 °C for 1 h [90]. In a more recent work, Hromádková et al. [91] confirmed that, thanks to LFUS implementation, heteroxylans extraction from wheat bran could be hastened by 55 min and with minor yield loss. Successively, Benito-Román et al. [92] found that LFUS-assisted extraction of β-glucans from barley outperformed the stirred tank technique by improving yield up to 58% and hastening the processing time from 3 h to 16 min. Conversely, Sun and Tomkinson [93] found a negligible increase in hemicellulose extraction yield from wheat straw. Wang et al. [94] optimized the extraction of mucilage (i.e., heteropolysaccharides) from chia seeds and observed that the adoption of LFUS increased 10-fold the extraction yield.
Besides yields, LFUS proved to be energy-efficient and timesaving. Reis et al. [95] designed a 25 min-long LFUS-assisted extraction from brewer’s spent grain that yielded the same amount of arabinoxylan they got with a 7 h optimized alkaline extraction. In another case the LFUS-assisted extraction of β-glucan from hull-less barley gave a 6% lower yield, but was still convenient because lasted 5 min instead of the standard 90 min [96]. Shorter times often allow to save energy because heating is needed for a briefer period. According to Benito-Román et al. [92], LFUS could cut energy demand by at least 52%.
The yield loss, in case of over-treatment, seems to be the main LFUS drawback [97], [98], [99]. Although the most drastic ultrasound conditions resulted in lower polysaccharides molecular weight, due to cavitation-driven chain-breaking, experimental parameters could be adjusted to minimize unwanted depolymerization [90], [92]. In addition, at the highest amplitude, unspecific disruption of cell compartments results in different polysaccharides release, thus threatening the extract purity [96].
As detailed in the last rows of Table 2, LFUS-assisted extraction was tested for a variety of other compounds as well. Protein can be extracted efficiently with LFUS: Li et al. [100] reported a 2-fold increase in yield, thanks to better solvent penetration. Similarly, Quintero-Quiroz et al. [101] saw a 2.4-fold higher yield, while Zhu et al. [102] stated that LFUS improved forward mass transfer in reverse micelles extraction. Laqui-Vilca et al. [103] extracted betalains from quinoa seed hulls in 10–40 s instead of 10 min. Loypimai et al. [104] developed a LFUS-assisted soybean oil enriching process: the oil was directly employed as green solvent to simultaneously extract tocopherols and γ-oryzanol from rice bran; the yields were slightly lower than with conventional extraction, but this technique simplified the process and avoided the use of hazardous solvents. Wizi et al. [105] recovered 3.6-fold higher red colorants, mainly apigeninidin and luteolinidin, from sorghum husk by coupling ultrasound and microwave technologies. Ye et al. [106] extracted carotenoids from corn meal and observed that LFUS were better than magnetic stirring, inasmuch that after 60 min they achieved 0.08 mg/ml vs. 0.004 mg/ml. However, the experiment did not last enough to find out the optimum conditions.
3.4. Fortification
Because of its capacity to produce cracks and pores, LFUS has also been tested as a method to fortify grains. Bonto et al. [25] treated polished white rice in a 53 Hz ultrasonic bath: a time-dependent fragmentation of the cells external layer was observed, and the starchy endosperm arrangement was disrupted, but no starch granules were damaged. Although at first sonication led to complete B-vitamins loss, after soaking in pantothenic acid the LFUS-treated grains absorbed and retained 140% more vitamin B5 than non-sonicated rice. Similarly, Tiozon et al. [27] fortified rice by soaking it in 800 ppm folic acid solution after 5 min-long sonication. Brown rice absorbed about 1982-fold its natural folic acid content, while a 4054-fold increase was seen in white rice; in addition, after washing and cooking, they retained 93.5% and 86.5% folic acid, respectively. Yanova et al. [126] attempted mineral fortification of barley and oat groats by LFUS treatment with 65 mg/L solutions of Fe2+ and Zn2+. A positive linear relationship between amount of absorbed minerals, temperature and treatment duration was found; furthermore, at higher ultrasound frequency an additional amount of iron and zinc was soaked up, possibly because of the higher number of impingement cycles. In must be stressed that higher frequencies are less likely to produces grains fractures because cavitation collapses are less violent [1].
3.5. Other applications
Cui et al. [32] and Dang et al. [127] suggested LFUS as a viable alternative to reduce brown rice cooking time by about 16%, with the additional advantage that less vitamins and other solids are lost; the LFUS pre-treatment resulted also in higher grain expansion volume, water uptake and softness. The rice cooking time reduction was confirmed by Yang et al. [128]. In addition, the glycemic index was subjected to negligible changes with respect to untreated brown rice [127], [129]. LFUS washing at acidic pH improved rice bran quality by reducing lipase and lipoxygenase activities, heavy metals contaminants, phytic acid and coliforms [130]. Habuš et al. [131], [132] confirmed that the shelf life of wheat bran can be extended with an ultrasound treatment thanks to the reduction of the enzymatic activities of lipase, lipoxygenase and polyphenol oxidase. However, the effect is partly due to thermal denaturation from the heat generated, because when the sample was cooled the LFUS treatment appeared less effective than the conventional heating.
According to Yüksel and Elgün [33] submitting wheat to 30 s sonication during tempering should improve its bread-making quality, although the wet gluten amount was slightly diminished, its quality was better as higher gluten index, dough stability, energy, loaf volume and specific volume were observed. In addition, LFUS-assisted mixing may result in better air incorporation and thus higher loaf development [31]. Similarly, sonication during pre-mixing of corn bread dough resulted in better softness, porosity, general sensory acceptability and yellower crust and crumb [133]. Hence, LFUS treatment of wheat and quinoa flour suspension could produce tailored-made flours with defined rheological properties, such as decrease in gelatinization enthalpy, lower viscosities and lower gel hardness and cohesiveness [134], [135].
The use of LFUS during corn nixtamalization cooking, reduced the steeping time needed to reach the correct kernel softness from 20 to 1 h; a slight inferior mass leaching was also observed [136]. A further study confirmed that LFUS reduces steeping time (from 18 to 1.5 h) and does not affect nixtamal quality as well as sensory acceptability of derived products like tortilla chips [17].
4. Effect of low-frequency ultrasound on cereals and pseudocereals components
4.1. Starch
Table 3 reports the broad range of different conditions tested for modifying cereal starches. Ultrasound treatments induce pores, depressions and cracks in starch granules because of the rapid water jets produced by the cavitation bubbles collapse. Yang et al. [137] even reported that rice starch granules were peeled off as power increased. However, LFUS augmented the total pores volume but not their average diameter [19] and affect mainly the largest granules [138], [139]. Additionally, in the range 25–55 °C the temperature effect is inversely correlated to the damage [140] because the collapse of the bubbles favors cavitation but is less violent at higher temperature [1]. Conversely, the application of multiple frequencies is more effective due to the sum of cavitation events [141], [142].
Table 3.
Conditions tested in ultrasound processing for modifying cereal starches.
| Sample |
Suspension concentration |
Frequency |
Device |
Power |
Amplitude |
Time |
T |
Reference |
|---|---|---|---|---|---|---|---|---|
| (g/100 ml) | (kHz) | (W) | (%) | (min) | (°C) | |||
| buckwheat | bath | 450 | 30 (5/3 s on/off) | [150] | ||||
| corn | 5 | 20;25;20 + 25 | bath | 400 | 5–40 | 30 | [158] | |
| corn | 5 | 20;25;20 + 25 | bath | 400 | 40 | 30 | [141] | |
| corn | 30 | 40 | bath | 420–540 | 20–40 | 40–60 | [146] | |
| corn | 30 (w/w) | 40 | bath | 100 | 30 | 30 | [152] | |
| millet | 5 | 40 + 80;80 | bath | 720 | 25;60 | [142] | ||
| millet | 10–30 (w/w) | bath | 400 | 15–60 | [154] | |||
| foxtail millet | 30 | 40 | bath | 20 | [155] | |||
| corn | 30 | probe | 500 | 3–15 (15/5 s on/off) | [148] | |||
| corn | 2 | 20 | probe | 400 | 100 | 15–105 (2/2 s on/off) | 10 | [143] |
| corn | 5;10 | 20 | probe | 750 | 0.5–20 | [144] | ||
| corn | 40 (w/w) | 20 | probe | 800 | 240 (3/3 s on/off) | [145] | ||
| corn | 10–20 | 24 | probe | 150 | 50–100 | 5–15 (80% duty cycle) | 25–65 | [140] |
| corn | 30 | 24 | probe | 400 | 80 | 1–16 | 20 | [21] |
| corn | 10 (w/w) | 24 | probe | 100–400 | 15;30 | [151] | ||
| corn, wheat, rice | 30 | 20 | probe | 170 | 30 | 20 | [19], [139] | |
| waxy corn | 30 (w/w) | 15 | probe | 100;400 | 40 | 25 | [147] | |
| rice | 30 (w/w) | 22 | probe | 150–600 | 20 (5/5 s on/off) | 25 | [137] | |
| rice | 20 | 20 | probe | 750 | 100 | 30 | [167] | |
| non-waxy rice | 5 | 24 | probe | 100–1000 | up to 120 | 20–55 | [156] | |
| waxy rice | 1;7 | 20 | probe | 600 | 60–2880 | [149] | ||
| wheat flour | 10 | 20 | probe | 750 | 50 | 120–1200 (3/1 s on/off) | [134] | |
| wheat | 10 (w/w) | 30 | probe | 100 | 100 | 15;30 (80% duty cycle) | [138] | |
| brown rice | whole grains | 16 | 2000 | 30 | 25–55 | [32] | ||
| waxy rice | 5 (w/w) | 211 | 2.5;4.1 | up to 60 | 25–70 | [161] |
Boufi et al. [143] reported that LFUS ruptured starch particles into smaller ones: a 15 min treatment reduced both 1200 nm and 950 nm granules to 600 nm, while a 90 min process trimmed them down to 40 nm, a size reduction easily visualized by beam light scattering, because the starch suspension becomes clearer over the time. Similar observations were reported by Kang et al. [144] for waxy starches, but the amylose-rich samples underwent a slight particles diameter increase due to amylose aggregation, as observed also by Li et al. [145]. The correlation between granule size and degree of polymerization is not clear. For instance, Yang et al. [137] reported a decrease in particle size but no changes in chain-length. However, many studies describe a depolymerization process. According Kang et al. [144] and Li et al. [146], LFUS first disrupts weak interactions and then cleaves covalent C-O-C α-1,6 glycosidic bonds (apparently less stable than α-1,4 bonds) thus leading to amylopectin debranching [147]. Coherently, Li et al. [145] saw a roughly halved weight average molar mass (Mw) and Huang et al. [148] reported a drastic increase in hydrolysis degree. Furthermore, the number average molecular weight (Mn) and polydispersity (Mw∕Mn) diminished with sonication time and their decreasing rate was related to temperature and power [149]. Due to these events, Zhou et al. [150] indicated LFUS as the best alternative to produce flavonoids-enriched starch complex from Tartary buckwheat. In addition, they saw an increase in resistant starch (+20%) and a slower starch digestion.
Swelling power and solubility were generally increased by LFUS [138], [139], [140], [151], [152], [153] and the temperature-time interaction was positively correlated to these parameters [140]. A slight increase in suspension transparency was noticed by Sujka and Jamroz [139], Amini et al. [140] and Li et al. [154]. Jambrak et al. [151] observed that the sonicated suspension became clear after one-day storage. However, Dey and Sit [155] stated that LFUS turned starch 21% darker.
Fig. 2 reports the forest plot for random effects performed considering fourteen different studies related to LFUS effect on starch gelatinization enthalpy (ΔH); the raw mean difference between the control and the LFUS-treated group was used as the effect size.
The overall pooled LFUS treatment causes a slight decrease (0.97 J/g) in ΔH, a tendency also observed by Amini et al. [140], Boufi et al. [143], Li et al. [145], Huang et al. [148], Zhou et al. [150] and Yu et al. [156]. The decrease in ΔH was related to the loss of amylopectin double helices, essential structures for granule integrity preservation [157]. Controlled-temperature LFUS treatments allow to obtain starches that demand lower energy when residual helical structures unfold [151], [158]. Since amorphous lamellae are disrupted first [148], [152], [159], their disappearance could determine a temporary higher crystallinity, till the inner lamellae are attacked [21], [142]. In fact, the onset temperature (To) increased slightly, hence when weak structures are damaged, the remaining stronger crystals will require higher energy to melt [158], [159]. Similarly, a rise-and-fall pattern due to short-lived increased interactions between crystals and amorphous chains was reported by Li et al. [41]. Ultrasound power is inversely related to ΔH [147], [151], [156], although an opposite behavior has been observed at very high power (600–1000 W) probably because small amounts of amylose leaked out from the granules and gelled, insulating the surface from external water [137], [156]. These behavioral discrepancies may be explained by the different nature of starch granules: A-type granules are characterized by a tight monoclinic lattice, while B-type granules have a hexagonal arrangement enclosing a cavity with freezable water. Consequently, A-type granules are more resistant, while B-types are more susceptible to LFUS-induced disruption [152], [160]. Yang et al. [137], Yang et al. [147] and Hu et al. [158] provided observations that support this crystallinity changes theory, Karwasra et al. [138], Hu et al. [142] and Luo et al. [152] did not observe any significant modification: probably, different experimental conditions (e.g., ultrasound power, time, volume treated, higher efficiency of the probe system with respect to bath) led to higher total energy per volume unit. In fact, conclusive evidence from Boufi et al. [143] showed that LFUS flattened thermogram and x-ray diffraction spectrum, thus supporting the formation of amorphous particles.
Because of LFUS-induced starch modification, the pasting properties of cereal starches and flours are also modified. LFUS treatment decrease starch paste peak viscosity, breakdown, and setback, but did not affect pasting temperature [141], [145], [146], [147], [148], [149], [152], [154], [161]. However, Cui et al. [32], Yang et al. [137] and Park and Han [162] reported lower pasting temperature, higher peak viscosity, and breakdown. According to Li et al. [146] and Zuo et al. [161] the viscosity drop is due to amylose and long linear amylopectin depolymerization, a finding consistent with the results by Luo et al. [152], who saw no modification in waxy maize starch, but a viscosity fall in amylose-rich Amylomaize V. Amylose is mainly responsible for the viscosity increase during setback [163], [164], therefore its hydrolysis will result in a weaker network. The magnitude of changes in pasting behavior varies from almost negligible to complete starch liquefaction, as in thermal assisted (60 °C) sonication [149]. The LFUS and temperature interaction effect on pasting properties was confirmed by Zuo et al. [161], who reported a peak viscosity decrease down to a quarter. These findings encourage the use of LFUS to further improve starch saccharification, as described by Montalbo-Lomboy et al. [165] and Shewale and Pandit [166].
4.2. Proteins
Ultrasound-assisted modification of cereal and pseudocereal protein isolates has been extensively studied. Table 4 reports the broad range of conditions tested for modifying cereals and pseudocereals proteins. The results demonstrate that cavitation disrupts weak interactions (electrostatic bonds, hydrogen bonds, hydrophobic effect), thus leading to conformational changes in the secondary and tertiary structures. The proteins unravel and the hydrophobic cores, rich in phenylalanine, tyrosine, and tryptophan, are exposed, thus augmenting the surface hydrophobicity [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185]. Covalent disulphide bonds are also affected by LFUS, and the amount of free-sulfhydryls and disulphides depends on the power intensity used185, 186]. In fact, far higher energy is required to reduce S-S bonds (226 kJ/mol) than to break weak interactions (13 kJ/mol) [175]. An increase in SH groups, accompanied by a less evident decrease in S-S, was reported [168], [179], [180], [183], [184], [185], [187], leading to conclude that the breaking of disulphide bonds LFUS led to a much looser gluten matrix [20], [180], [182]. With regards to the secondary structure, the relative number of α-helices decreases in favor of β-sheets and random coils [20], [171], [173], [178], [180], [183], [185], while intramolecular β-sheets are converted to intermolecular β-sheets [20]. Ultrasound-induced denaturation may result in better digestibility due to increased accessibility for the digestive enzymes: in fact, Jin et al. [170] observed an improvement from 41.4 (control) to 58.2% (LFUS-treated) with buckwheat protein isolate.
Table 4.
Conditions tested in ultrasound processing for modifying cereal proteins.
| Sample |
Suspension concentration |
Frequency |
Device |
Power |
Amplitude |
Time |
T |
Reference |
|---|---|---|---|---|---|---|---|---|
| (Protein isolate) | (g/100 ml) | (kHz) | (W) | (%) | (min) | (°C) | ||
| corn gluten meal | 3 | 20;28;40 | bath | 100 | 240 | 30 | [189] | |
| amaranth | 10 | 24 | probe | 100 | 30–90 | 15;30 | 3 | [168] |
| buckwheat protein | 4 | 20 | probe | 60 | 10 (10/5 s on/off) | 20 | [170] | |
| corn germ defatted | 5 | 20–52 | 28–80 | 15 | 30 | [193] | ||
| corn gluten meal | 3–7 | 20;28;40 | 150 W/L | 2–10 (10/5 s on/off) | 60 | [190] | ||
| millet | 10 (w/w) | 20 | probe | 100 | 20–100 | 5–20 | 20–30 | [174] |
| oat | 0.5–6 | 20 | probe | 250–1250 | 10–50 (2/2 s on/off) | 27 | [192] | |
| quinoa | 5 | 20 | probe | 200–600 | 20 | 21–40 | [173] | |
| quinoa | 20 | probe | 700 (39 net) | 20 | 5–30 (17–83% duty cycle) | 20 | [188] | |
| quinoa protein | 4 | 20 | probe | 360 | 10 kJ/ml | [181] | ||
| rice | 4 (w/w) | 28 | probe | 58 W/L | 15 (3/2 s on/off) | 50 | [171], [172] | |
| rice | 4.6 | 20–50 | 50 W/L | 10 | 53 | [179] | ||
| rice | 6 | 20–60 | 300x6 | 30 | [178] | |||
| wheat | 0.1–3 (w/w) | 20 | probe | 750 | 95 | up to 2 | >45 | [175] |
| wheat germ | 10 | 20 | probe | 200–1800 | 5–60 (2/2 s on/off) | 25 | [184] | |
| wheat germ defatted | 1 | 20 | probe | 900–1800 | 20 (2/2 s on/off) | [169] | ||
| wheat gluten | 6 | 20 | probe | 540–900 | 60–100 | 10 | 25 | [34] |
| wheat gluten | 3 | 20 + 35 | probe x2 | 80–160 W/L | 30 (5/5 s on/off) | 30 | [186] | |
| wheat gluten | 1–5 | 20 | probe x5 | 494 | 20 (4/3 s on/off) | 30 | [20] | |
| wheat gluten | 3 | 20–50 | probe x5 | 100–300 W/L | 5–25 (5/5 s on/off) | 30 | [183] | |
| wheat gluten | 1–5 | 20–50 | probe x5 | 100–300 W/L | 15 | 30 | [182] | |
| wheat gluten | 8 | 150;300 | 0.16–1 | [187] | ||||
| wheat gluten | 3 | 20–80 | 67 W/L | 10 (10/5 s on/off) | 30 | [180] | ||
| wheat gliadin | 0.2 in 65% ethanol | 20–25 | probe | 200–600 | 10 (5/1 s on/off) | 30 | [185] | |
| zein | 1 | 22–68 | 600 | 30 | 25 | [191] | ||
| zein | 1 | 33;68 | 600 | 40 (10/3 s on/off) | [177] | |||
| zein | 1 | 40 | 600 | 40 (10/3 s on/off) | 25 | [176] |
These changes do not affect protein molecular weight but disrupt aggregates [185]. Nevertheless, overtreatment may result in new intermolecular disulphide and hydrophobic bonds that increase the dimensions of the aggregates [101], [168], [175], [184], [185], [188]. Constantino and Garcia-Rojas [168] suggested that hydrogen peroxide generated by hydroxy radical addition could act as an oxidizing agent, thus converting free sulfhydryl into disulphide bridge.
Unexpectedly, LFUS denaturation increases protein solubility in water [168], [173], [174], [176], [180], [188]. Nazari et al. [174] suggested that sonication leads to the emergence of hidden residues with a negatively-charged side chain, thus explaining the more negative zeta potential (25% and 14%) observed by Vera et al. [188] and Zhang et al. [181], respectively. Alterations in spatial arrangement improve the interfacial properties of the protein because expose residues in accordance with polarity, thus acting like surfactants [168], [181]. LFUS-treated wheat gluten increased foam capacity (+138%), foam stability (+42–118%), emulsion activity index (2-fold) and emulsion stability (>3-fold) [182]. Similarly, LFUS improved millet protein foaming capacity (2.75-fold), foam stability (22-fold), emulsion activity index (almost 2-fold) and emulsion stability (4.4-fold) [174]. By emulsifying finer oil droplets in water, LFUS enhanced wheat proteins foaming even better than Tween 80 [175]. Recently, Zhang et al. [181] produced a high internal phase emulsion, which mimics a solid fat, using quinoa protein nanoparticles as an emulsifier whose interfacial properties were adjusted by varying the ultrasonic density.
The low-power high-frequency ultrasound has often been utilized as pre-treatment to produce bioactive peptides from cereals. The LFUS-assisted enzymolysis was very effective at enhancing the ACE inhibitory activity of peptides by 8–99% [20], [169], [176], [177], [178], [182], [183], [189], [190], [191]. Protein denaturation, as mentioned above, leads to enhanced exposure of the enzymatic cleavage sites, improving the quality of the digested products, because hydrophobic-ending peptides have higher ACE inhibitory activity [169], [178], [189]. Furthermore, cavitation disaggregates protein-starch complexes, thus exposing larger attack surfaces [190]. Enzymatic affinity is increased, because of the reduction (13–42%) in Michaelis-Menten constant [20], [169], [190], [192] causes higher initial hydrolysis rates [20], [169], [192], [193]. The affinity towards the substrate may also be raised by conformational changes in the enzymes [190].
4.3. Dietary fibre
Ultrasound modification of dietary fibre was experimented by Hassan et al. [194] on the insoluble fraction pre-extracted from chia seeds, and by Wei et al. [195] on purified soluble fraction from millet bran: the LFUS treatments produced about two-fold increases of both water and oil holding capacity. Those effects were attributed to the shear stress leading to disruption of structure and fragmentation of particles, with the consequent increase of hydrophilic functional groups, but also to a greater access to hydrophobic cavities where oil can be retained. LFUS capability to shatter dietary fibre was evidenced by Vaitkeviciene et al. [196]: they ultrasonicated rice bran and observed an average 10% decrease of insoluble fibre, and an increase of the soluble fraction; formation of resistant starch, likely due to chains rearrangement, was also detected. Similarly, Zadeike et al. [197], documented a coarse surface with multiple fractures in LFUS-treated rice bran and an increase in its absorption of water and oil.
Li et al. [198] observed a reduction in the number-average molecular weight of wheat bran arabinoxylan (AX) treated with LFUS (120–160 W for 15–45 min) and hypothesized a power and time-dependent chain-breaking effect because of cavitation, while Fan et al. [199] confirmed the LFUS role in the reduction of AX molecular weight; both group of authors suggested that this could represent an alternative way to regulate the rheological properties of AX fraction. In addition, AX covalently binds phenolic acids [198], thus its heightened depolymerization could imply a transition of phenolic compounds from insoluble to soluble forms. In fact, Fan et al. [199] observed an increase in ferulic acid after sonication, but being AX a major component of wheat bran [200] this phenomenon deserves further, more detailed examination.
5. Conclusions
Low-frequency ultrasound (LFUS) is a promising green technology for improving key processing steps in cereals and pseudocereals as well as for modifying structural, physical, chemical, technological, functional, and biological properties of macromolecules such as carbohydrates and proteins. Moreover, in addition to saving energy and reducing processing time in agreement with its environment-friendly nature, LFUS allows to improve many extraction processes and to obtain value-added ingredients from cereal and pseudocereal by-products in the frame of a circular economy.
LFUS enhances the hydration rate and the time lag phase during pre-treatments essential for cooking, extraction, fermentation, and germination of cereals and pseudocereals. Additionally, it improves and accelerates sprouting by increasing hydration, which in turn releases promoters and eliminates inhibitors of germination. Therefore, LFUS could be easily employed to speed-up the synthesis of bioactive compound (e.g., phenolics) in cereals and pseudocereals during the germination under stress-inducing condition that promote the production of antioxidants. Additionally, LFUS boosts the extraction rate of bioactive compounds, such as phenolic compounds and polysaccharides, is compatible with the use of some green solvents and improves the fortification with vitamin and minerals due to its ability to produce cracks and pores. However, the mechanism underlying such improvements still need be investigated in detail to increase scientific knowledge and optimize LFUS treatments.
An important property of LFUS is that can improve and regulate the technological properties of fundamental food macromolecules like carbohydrates (starch and dietary fibre) and proteins. Peptides produced from the enzymatic hydrolysis of proteins denatured by LFUS present enhanced bioavailability and bioactivity. Therefore, LFUS may represent an efficient and viable alternative to produce nanoparticles of proteins or carbohydrates and bioactive compounds with improved bioavailability and bioactivity. Nevertheless, to scale-up similar processes to future industrial applications, LFUS conditions should be carefully assessed and measured by calorimetric and chemical dosimetry, rarely studied in cereals and pseudocereals.
Although LFUS has demonstrated numerous possible applications in cereals and pseudocereals, its use in manufacturing has been scarcely developed. The challenge currently is to go beyond basic research and to transfer the promising lab results to pilot and industrial scale.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
The authors acknowledge the support of the APC central fund of the University of Milan.
References
- 1.Mason T.J., Peters D. Woodhead Publishing; 2002. Practical Sonochemistry: Power ultrasound uses and applications. [Google Scholar]
- 2.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: A review. Ultrason Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason T.J., Cintas P. Handbook of Green Chemistry and Technology. Blackwell Science Ltd; Oxford, UK: 2002. pp. 372–396. [Google Scholar]
- 4.Mohammadi Ziarani G., Kheilkordi Z., Gholamzadeh P. Ultrasound-assisted synthesis of heterocyclic compounds. Mol. Divers. 2020;24:771–820. doi: 10.1007/s11030-019-09964-1. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari B.K. Ultrasound: A clean, green extraction technology. Trends Analyt Chem. 2015;71:100–109. doi: 10.1016/j.trac.2015.04.013. [DOI] [Google Scholar]
- 6.T. J. Mason, L. Paniwnyk, J. P. Lorimer. The uses of ultrasound in food technology. Ultrason Sonochem. 3. (1996). S253–260. 10.1016/S1350-4177(96)00034-X.
- 7.H. Feng, W. Yang. Ultrasound processing. In: H. Q. Zhang, G. V. Barbosa-Cánovas, V. M. Balasubramaniam, C. P. Dunne, D. F. Farkas, J. T. C. Yuan (Eds.). Nonthermal processing technologies for food, UK: John Wiley & Sons, Wiley-Blackwell and IFT Press. 2011. 135–154. 10.1002/9780470958360.
- 8.Suslick K.S. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 9.Mason T.J., Lorimer J.P. Applied sonochemistry: The uses of power ultrasound in chemistry and processing. Wiley-VCH. 2002 doi: 10.1002/352760054X. [DOI] [Google Scholar]
- 10.Soria A.C., Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- 11.Guimarães B., Polachini T.C., Augusto P.E.D., Telis-Romero J. Ultrasound-assisted hydration of wheat grains at different temperatures and power applied: Effect on acoustic field, water absorption and germination. Chem Eng Process. 2020;155 doi: 10.1016/j.cep.2020.108045. [DOI] [Google Scholar]
- 12.Margulis M.A., Margulis I.M. Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrason Sonochem. 2003;10:343–345. doi: 10.1016/S1350-4177(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 13.Ashokkumar M., Sunartio D., Kentish S., Mawson R., Simons L., Vilkhu K., Versteeg C.K. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov Food Sci Emerg Technol. 2008;9:155–160. doi: 10.1016/j.ifset.2007.05.005. [DOI] [Google Scholar]
- 14.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to meta-analysis. Wiley. 2009 doi: 10.1002/9780470743386. [DOI] [Google Scholar]
- 15.Awad T.S., Moharram H.A., Shaltout O.E., Asker D., Youssef M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012;48:410–427. doi: 10.1016/j.foodres.2012.05.004. [DOI] [Google Scholar]
- 16.Schmidt M., Zannini E., Arendt E.K. Screening of post-harvest decontamination methods for cereal grains and their impact on grain quality and technological performance. Eur. Food Res. Technol. 2019;245:1061–1074. doi: 10.1007/s00217-018-3210-5. [DOI] [Google Scholar]
- 17.B. Janve, W. Yang, C. Sims, Sensory and quality evaluation of traditional compared with power ultrasound processed corn (Zea mays) tortilla chips, J. Food Sci. 80 (2015) S1368–S1376. 10.1111/1750-3841.12892. [DOI] [PubMed]
- 18.Liang Q., Ren X., Ma H., Li S., Xu K., Oladejo A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J Food Qual. 2017;2017 doi: 10.1155/2017/2784146. [DOI] [Google Scholar]
- 19.Sujka M. Ultrasonic modification of starch – Impact on granules porosity. Ultrason Sonochem. 2017;37:424–429. doi: 10.1016/j.ultsonch.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Ma H., Wang B., Qu W., Li Y., He R., Wali A. Effects of ultrasound pretreatment on the enzymolysis and structural characterization of wheat gluten. Food Biophys. 2015;10:385–395. doi: 10.1007/s11483-015-9393-4. [DOI] [Google Scholar]
- 21.Flores-Silva P.C., Roldan-Cruz C.A., Chavez-Esquivel G., Vernon-Carter E.J., Bello-Perez L.A., Alvarez-Ramirez J. In vitro digestibility of ultrasound-treated corn starch. Starke. 2017;69:1700040. doi: 10.1002/star.201700040. [DOI] [Google Scholar]
- 22.Kaur H., Gill B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019;126:367–375. doi: 10.1016/j.ijbiomac.2018.12.149. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira H.M., Correia V.S., Segundo M.A., Fonseca A.J., Cabrita A.R. Does ultrasound improve the activity of alpha amylase? A comparative study towards a tailor-made enzymatic hydrolysis of starch, LWT. 2017;84:674–685. doi: 10.1016/j.lwt.2017.06.035. [DOI] [Google Scholar]
- 24.Sharma S., Saxena D.C., Riar C.S. Changes in the GABA and polyphenols contents of foxtail millet on germination and their relationship with in vitro antioxidant activity. Food Chem. 2018;245:863–870. doi: 10.1016/j.foodchem.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 25.Bonto A.P., Camacho K.S.I., Camacho D.H. Increased vitamin B5 uptake capacity of ultrasonic treated milled rice: A new method for rice fortification. LWT. 2018;95:32–39. doi: 10.1016/j.lwt.2018.04.062. [DOI] [Google Scholar]
- 26.Miano A.C., Augusto P.E.D. The ultrasound assisted hydration as an opportunity to incorporate nutrients into grains. Food Res. Int. 2018;106:928–935. doi: 10.1016/j.foodres.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Tiozon R.N., Camacho D.H., Bonto A.P., Oyong G.G., Sreenivasulu N. Efficient fortification of folic acid in rice through ultrasonic treatment and absorption. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127629. [DOI] [PubMed] [Google Scholar]
- 28.Kalita D., Jain S., Srivastava B., Goud V.V. Sono-hydro priming process (ultrasound modulated hydration): Modelling hydration kinetic during paddy germination. Ultrason Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miano A.C., Ibarz A., Augusto P.E.D. Ultrasound technology enhances the hydration of corn kernels without affecting their starch properties. J Food Eng. 2017;197:34–43. doi: 10.1016/j.jfoodeng.2016.10.024. [DOI] [Google Scholar]
- 30.Xia Q., Tao H., Li Y., Pan D., Cao J., Liu L., Zhou X., Barba F.J. Characterizing physicochemical, nutritional and quality attributes of wholegrain Oryza sativa L. subjected to high intensity ultrasound-stimulated pre-germination. Food Control 108. 2020 doi: 10.1016/j.foodcont.2019.106827. [DOI] [Google Scholar]
- 31.Che Pa N.F., Chin L.N., Yusof Y.A., Abd Aziz N. Agriculture and Agricultural Science Procedia 2. 2014. Power ultrasound assisted mixing effects on bread physical properties; pp. 60–66. [DOI] [Google Scholar]
- 32.Cui L., Pan Z., Yue T., Atungulu G.G., Berrios J. Effect of ultrasonic treatment of brown rice at different temperatures on cooking properties and quality. Cereal Chem. 2010;87:403–408. doi: 10.1094/CCHEM-02-10-0034. [DOI] [Google Scholar]
- 33.Yüksel Y., Elgün A. Determination of the effect of high energy ultrasound application in tempering on flour quality of wheat. Ultrason Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105129. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H., Claver I.P., Zhu K.-X., Zhou H. The effect of ultrasound on the functional properties of wheat gluten. Molecules. 2011;16:4231–4240. doi: 10.3390/molecules16054231. [DOI] [Google Scholar]
- 35.Miano A.C., Augusto P.E.D. The hydration of grains: A critical review from description of phenomena to process improvements. Compr. Rev. Food Sci. Food Saf. 2018;17:352–370. doi: 10.1111/1541-4337.12328. [DOI] [PubMed] [Google Scholar]
- 36.Borsato V.M., Jorge L.M.M., Mathias A.L., Jorge R.M.M. Ultrasound assisted hydration improves the quality of the malt barley. J Food Process Eng. 2019;42:e13208. [Google Scholar]
- 37.Miano A.C., Ibarz A., Augusto P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason Sonochem. 2016;29:413–419. doi: 10.1016/j.ultsonch.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Chatchavanthatri N., Junyusen T., Moolkaew P., Arjharn W., Junyusen P. Effect of soaking and sprouting treatment on germination rate of paddy. E3S Web of Conferences 187. 2020:04016. doi: 10.1051/e3sconf/202018704016. [DOI] [Google Scholar]
- 39.Peleg M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988;53(4) doi: 10.1111/j.1365-2621.1988.tb13565.x. [DOI] [Google Scholar]
- 40.Machado M.F., Oliveira F.A.R., Cunha L.M. Effect of milk fat and total solids concentration on the kinetics of moisture uptake by ready-to-eat breakfast cereal. Int. J. Food Sci. Technol. 1999;34:47–57. doi: 10.1046/j.1365-2621.1999.00238.x. [DOI] [Google Scholar]
- 41.Li S., Luo Z., Guan X., Huang K., Li Q., Zhu F., Liu J. Effect of ultrasonic treatment on the hydration and physicochemical properties of brewing rice. J. Cereal Sci. 2019;87:78–84. doi: 10.1016/j.jcs.2019.03.002. [DOI] [Google Scholar]
- 42.Patero T., Augusto P.E.D. Ultrasound (US) enhances the hydration of sorghum (Sorghum bicolor) grains. Ultrason Sonochem. 2015;23:11–15. doi: 10.1016/j.ultsonch.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Shafaei S.M., Nourmohamadi-Moghadami A., Rahmanian-Koushkaki H., Kamgar S. Neural computing efforts for integrated simulation of ultrasound-assisted hydration kinetics of wheat. Inf. Process. Agric. 2019;6:357–374. doi: 10.1016/j.inpa.2019.01.001. [DOI] [Google Scholar]
- 44.Kentish S.E. In: Ultrasound: Advances for Food Processing and Preservation. Bermudez-Aguirre D., editor. Academic Press; 2017. Engineering principles of ultrasound technology; pp. 1–13. [DOI] [Google Scholar]
- 45.de Carvalho G.R., Polachini T.C., Darros-Barbosa R., Bon J., Telis-Romero J. Effect of intermittent high-intensity sonication and temperature on barley steeping for malt production. J. Cereal Sci. 2018;82:138–145. doi: 10.1016/j.jcs.2018.06.005. [DOI] [Google Scholar]
- 46.Srivastava L.M. Plant Growth and Development. Hormones and Environment. Academic Press; 2002. Seed germination, mobilization of food reserves, and seed dormancy; pp. 447–471. [DOI] [Google Scholar]
- 47.Olaerts H., Courtin C.M. Impact of preharvest sprouting on endogenous hydrolases and technological quality of wheat and bread: a review. Compr. Rev. Food Sci. Food Saf. 2018;17:698–713. doi: 10.1111/1541-4337.12347. [DOI] [PubMed] [Google Scholar]
- 48.Aborus N.E., Tumbas Šaponjac V., Čanadanović-Brunet J., Ćetković G., Hidalgo A., Vulić J., Šeregelj V. Sprouted and freeze-dried wheat and oat seeds – phytochemical profile and in vitro biological activities. Chem. Biodivers. 2018;15:e1800119. doi: 10.1002/cbdv.201800119. [DOI] [PubMed] [Google Scholar]
- 49.Benincasa P., Galieni A., Manetta A.C., Pace R., Guiducci M., Pisante M., Stagnari F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015;95:1795–1803. doi: 10.1002/jsfa.6877. [DOI] [PubMed] [Google Scholar]
- 50.Hidalgo A., Tumbas-Šaponjac V., Ćetković G., Šeregelj V., Čanadanović-Brunet J., Chiosa D., Brandolini A. Antioxidant properties and heat damage of water biscuits enriched with sprouted wheat and barley. LWT. 2019;114 doi: 10.1016/j.lwt.2019.108423. [DOI] [Google Scholar]
- 51.Van Hung P., Hatcher D.W., Barker W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011;126:1896–1901. doi: 10.1016/j.foodchem.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- 53.Gupta R.K., Gangoliya S.S., Singh N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015;52:676–684. doi: 10.1007/s13197-013-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding J., Hou G.G., Dong M., Xiong S., Zhao S., Feng H. Physicochemical properties of germinated dehulled rice flour and energy requirement in germination as affected by ultrasound treatment. Ultrason Sonochem. 2018;41:484–491. doi: 10.1016/j.ultsonch.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Yaldagard M., Mortazavi S.A., Tabatabaie F. Application of ultrasonic waves as a priming technique for accelerating and enhancing the germination of barley seed: Optimization of method by the Taguchi approach. J. Inst. Brew. 2008;114:14–21. doi: 10.1002/j.2050-0416.2008.tb00300.x. [DOI] [Google Scholar]
- 56.Wei M., Yang C.-Y., Wei S.-H. Enhancement of the differentiation of protocorm-like bodies of Dendrobium officinale to shoots by ultrasound treatment. J. Plant Physiol. 2012;169:770–774. doi: 10.1016/j.jplph.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Ananthakrishnan G., Xia X., Amutha S., Singer S., Muruganantham M., Yablonsky S., Fischer E., Gaba V. Ultrasonic treatment stimulates multiple shoot regeneration and explant enlargement in recalcitrant squash cotyledon explants in vitro. Plant Cell Rep. 2007;26:267–276. doi: 10.1007/s00299-006-0235-1. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y.-P., Liu Q., Yue X.-Z., Meng Z.-W., Liang J. Jing Liang, Ultrasonic vibration seeds showed improved resistance to cadmium and lead in wheat seedling. Environ. Sci. Pollut. Res. 2013;20(7):4807–4816. doi: 10.1007/s11356-012-1411-1. [DOI] [PubMed] [Google Scholar]
- 59.Goussous S.J., Samarah N.H., Alqudah A.M., Othman M.O. Enhancing seed germination of four crop species using an ultrasonic technique. Exp. Agric. 2010;46:231–242. doi: 10.1017/S0014479709991062. [DOI] [Google Scholar]
- 60.Miano A.C., Forti V.A., Abud H.F., Gomes-Junior F.G., Cicero S.M., Augusto P.E.D. Effect of ultrasound technology on barley seed germination and vigourEffect of ultrasound technology on barley seed germination and vigour. Seed Sci. Technol. 2015;43(2):297–302. [Google Scholar]
- 61.Hassan S., Imran M., Ahmad M.H., Khan M.I., Xu C., Khan M.K., Muhammad N. Phytochemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int J Food Prop. 2020;23(1):853–863. [Google Scholar]
- 62.Kratovalieva S., Srbinoska M., Popsimonova G., Selamovska A., Meglic V., Andjelkovic V. Ultrasound influence on coleoptile length at Poaceae seedlings as valuable criteria in prebreeding and breeding processes. Genetika. 2012;44:561–570. doi: 10.2298/GENSR1203561K. [DOI] [Google Scholar]
- 63.Wang J., Bian Z., Wang S., Zhang L. Effects of ultrasonic waves, microwaves, and thermal stress treatment on the germination of Tartary buckwheat seeds. J Food Process Eng. 2020;43:e13494. [Google Scholar]
- 64.Ding J., Ulanov A.V., Dong M., Yang T., Nemzer B.V., Xiong S., Zhao S., Feng H. Enhancement of gamma-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason Sonochem. 2018;40:791–797. doi: 10.1016/j.ultsonch.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 65.Naumenko N., Potoroko I., Kalinina I. Stimulation of antioxidant activity and -aminobutyric acid synthesis in germinated wheat grain Triticum aestivum L. by ultrasound: increasing the nutritional value of the product. Ultrason Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balasubramaniam V.G., Ayyappan P., Sathvika S., Antony U. Effect of enzyme pretreatment in the ultrasound assisted extraction of finger millet polyphenols. J. Food Sci. Technol. 2019;56:1583–1594. doi: 10.1007/s13197-019-03672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izadifar Z. Ultrasound pretreatment of wheat dried distiller’s grain (DDG) for extraction of phenolic compounds. Ultrason Sonochem. 2013;20:1359–1369. doi: 10.1016/j.ultsonch.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 69.Giopato Viell F.L., Madeira T.B., Nixdorf S.L., Terezinha S., Gomes M., Bona E., Matsushita M. Comparison between ultra-homogenisation and ultrasound for extraction of phenolic compounds from teff (Eragrostis tef (Zucc.)) Int. J. Food Sci. Technol. 2020;55:2700–2709. doi: 10.1111/ijfs.14523. [DOI] [Google Scholar]
- 70.Teslić N., Bojanić N., Rakić D., Takači A., Zeković Z., Fišteš A., Bodroža-Solarov M., Pavlić B. Defatted wheat germ as source of polyphenols—Optimization of microwave-assisted extraction by RSM and ANN approach. Chem Eng Process. 2019;143 doi: 10.1016/j.cep.2019.107634. [DOI] [Google Scholar]
- 71.Chen L., Yang M., Mou H., Kong Q. Ultrasound-assisted extraction and characterization of anthocyanins from purple corn bran. J. Food Process. Preserv. 2018;42:e13377. [Google Scholar]
- 72.Melini V., Acquistucci R. Extraction of free and insoluble-bound phenolic compounds from pigmented rice by commonly used procedures: a comparative study. J. Food Meas. Charact. 2017;11:2151–2159. doi: 10.1007/s11694-017-9600-8. [DOI] [Google Scholar]
- 73.Chen C., Wang L., Wang R., Luo X., Li Y., Li J., Li Y., Chen Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and β-glucan contents: compositional and kinetic studies. J Food Eng 222. 2018:1–10. doi: 10.1016/j.jfoodeng.2017.11.002. [DOI] [Google Scholar]
- 74.Melini V., Melini F. Modelling and optimization of ultrasound-assisted extraction of phenolic compounds from black quinoa by response surface methodology. Molecules. 2021;26:3616. doi: 10.3390/molecules26123616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng J., Dou Y., Yan N., Li N., Zhang H., Tan J.-N. Optimizing ultrasound-assisted deep eutectic solvent extraction of bioactive compounds from Chinese wild rice. Molecules. 2019;24:2718. doi: 10.3390/molecules24152718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherif M.M., Grigorakis S., Halahlah A., Loupassaki S., Makris D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Process. 2020;7:845–859. doi: 10.1007/s40710-020-00449-0. [DOI] [Google Scholar]
- 77.Mansur A.R., Song N.-E., Jang H.W., Lim T.-G., Yoo M., Nam T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019;293:438–445. doi: 10.1016/j.foodchem.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Gadalkar S.M., Rathod V.K. Pre-treatment of ferulic acid esterases immobilized on MNPs to enhance the extraction of ferulic acid from defatted rice bran in presence of ultrasound, Biocatal. Agric. Biotechnol. 2017;10:342–351. doi: 10.1016/j.bcab.2017.03.016. [DOI] [Google Scholar]
- 79.Muangrat R., Pongsirikul I., Blanco P.H. Ultrasound assisted extraction of anthocyanins and total phenolic compounds from dried cob of purple waxy corn using response surface methodology. J. Food Process. Preserv. 2018;42:e13447. [Google Scholar]
- 80.Jiang T., Zhan S., Li S., Zhu Z., He J., Lorenzo J.M., Barbac F.J. From ‘green’ technologies to ‘red’ antioxidant compounds extraction of purple corn: a combined ultrasound-ultrafiltration-purification approach. J. Sci. Food Agric. 2018;98:4919–4927. doi: 10.1002/jsfa.9024. [DOI] [PubMed] [Google Scholar]
- 81.Dzah C.S., Duan Y., Zhang H., Boateng N.A.S., Ma H. Ultrasound-induced lipid peroxidation: Effects on phenol content and extraction kinetics and antioxidant activity of tartary buckwheat (Fagopyrum tataricum) water extract. Food Biosci. 2020;37 doi: 10.1016/j.fbio.2020.100719. [DOI] [Google Scholar]
- 82.Cravotto G., Binello A., Merizzi G., Avogadro M. Improving solvent-free extraction of policosanol from rice bran by high-intensity ultrasound treatment. Eur J Lipid Sci Technol. 2004;106:147–151. doi: 10.1002/ejlt.200300914. [DOI] [Google Scholar]
- 83.Liu Y., Yu J., Wang X. Extraction of policosanols from hydrolysed rice bran wax by high-intensity ultrasound. Int. J. Food Sci. Technol. 2008;43:763–769. doi: 10.1111/j.1365-2621.2006.01232.x. [DOI] [Google Scholar]
- 84.Khoei M., Chekin F. The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 2016;194:503–507. doi: 10.1016/j.foodchem.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 85.Han C., Liu Q., Jing Y., Wang D., Zhao Y., Zhang H., Jiang L. Ultrasound-assisted aqueous enzymatic extraction of corn germ oil: analysis of quality and antioxidant activity. J Oleo Sci. 2018;67:745–754. doi: 10.5650/jos.ess17241. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan V.C.A., Kuriakose S., Rawson A. Ultrasound assisted extraction of oil from rice bran: a response surface methodology approach. J Food Process Technol. 2015;6:1000454. doi: 10.4172/2157-7110.1000454. [DOI] [Google Scholar]
- 87.Xu G., Liang C., Huang P., Liu Q., Xu Y., Ding C., Li T. Optimization of rice lipid production from ultrasound-assisted extraction by response surface methodology. J. Cereal Sci. 2016;70:23–28. doi: 10.1016/j.jcs.2016.05.007. [DOI] [Google Scholar]
- 88.Soares J.F., Dal Prá V., Barrales F.M., dos Santos P., Kuhn R.C., Rezende C.A., Martínez J., Mazutti M.A. Extraction of rice bran oil using supercritical CO2 combined with ultrasound, Brazilian. J Chem. Eng. 2018;35:785–794. doi: 10.1590/0104-6632.20180352s20160447. [DOI] [Google Scholar]
- 89.Ðordević T., Antov M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidants from wheat chaff. Sep. Purif. Technol. 2017;182:52–58. doi: 10.1016/j.seppur.2017.03.025. [DOI] [Google Scholar]
- 90.Hromádková Z., Kováčiková J., Ebringerová A. Study of the classical and ultrasound-assisted extraction of the corn cob xylan. Ind Crops Prod. 1999;9:101–109. doi: 10.1016/S0926-6690(98)00020-X. [DOI] [Google Scholar]
- 91.Hromádková Z., Kováčiková J., Ebringerová A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran. Ultrason Sonochem. 2008;15:1062–1068. doi: 10.1016/j.ultsonch.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 92.Benito-Román Ó., Alonso E., Cocero M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT. 2013;50:57–63. doi: 10.1016/j.lwt.2012.07.006. [DOI] [Google Scholar]
- 93.Sun R.C., Tomkinson J. Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw. Carbohydr. Polym. 2002;50:263–271. doi: 10.1016/S0144-8617(02)00037-1. [DOI] [Google Scholar]
- 94.Wang W.-H., Lu C.-P., Kuo M.-I. Combination of ultrasound and heat in the extraction of chia seed (Salvia hispanica L.) mucilage: impact on yield and technological properties. Processes. 2022;10:519. doi: 10.3390/pr10030519. [DOI] [Google Scholar]
- 95.Reis S.F., Coelho E., Coimbra M.A., Abu-Ghannam N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason Sonochem. 2015;24:155–164. doi: 10.1016/j.ultsonch.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Sourki A.H., Koocheki A., Elahi M. Ultrasound-assisted extraction of β-D-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017:462–475. doi: 10.1016/j.ijbiomac.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 97.Jiang Y., Bai X., Lang S., Zhao Y., Liu C., Yu L. Optimization of ultrasonic-microwave assisted alkali extraction of arabinoxylan from the corn bran using response surface methodology. Int. J. Biol. Macromol. 2019;128:452–458. doi: 10.1016/j.ijbiomac.2019.01.138. [DOI] [PubMed] [Google Scholar]
- 98.Maran J.P., Manikandan S., Thirugnanasambandham K., Vigna Nivetha C., Dinesh R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013:604–611. doi: 10.1016/j.carbpol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Sun B., Liu Y., Zhang H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014;150:482–488. doi: 10.1016/j.foodchem.2013.10.121. [DOI] [PubMed] [Google Scholar]
- 100.Li K., Ma H., Li S., Zhang C., Dai C. Effect of ultrasound on alkali extraction protein from rice dreg flour. J Food Process Eng. 2017;40:e12377. [Google Scholar]
- 101.Quintero-Quiroz J., Celis-Torres A., Ciro-Gómez G., Torres J., Corrales-García L., Rojas J. Physicochemical properties and functional characteristics of ultrasound-assisted legume-protein isolates: a comparative study. J. Food Sci. Technol. 2021 doi: 10.1007/s13197-021-05126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu K.-X., Sun X.-H., Zhou H.-M. Optimization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles. J. Cereal Sci. 2009;50:266–271. doi: 10.1016/j.jcs.2009.06.006. [DOI] [Google Scholar]
- 103.Laqui-Vilca C., Aguilar-Tuesta S., Mamani-Navarro W., Montaño-Bustamante J., Condezo-Hoyos L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind Crops Prod. 2018;111:606–614. doi: 10.1016/j.indcrop.2017.11.034. [DOI] [Google Scholar]
- 104.Loypimai P., Moongngarm A., Sittisuanjik A., Khamanan S. Optimization of tocols and γ-oryzanol extraction from rice bran using ultrasound and soybean oil as a green solvent. Food Research 4. 2020;4(6):2322–2332. [Google Scholar]
- 105.Wizi J., Wang L., Hou X., Tao Y., Ma B., Yang Y. Ultrasound-microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind Crops Prod. 2018;120:203–213. doi: 10.1016/j.indcrop.2018.04.068. [DOI] [Google Scholar]
- 106.Ye J., Feng L., Xiong J., Xiong Y. Ultrasound-assisted extraction of corn carotenoids in ethanol. Int. J. Food Sci. Technol. 2011;46:2131–2136. doi: 10.1111/j.1365-2621.2011.02727.x. [DOI] [Google Scholar]
- 107.Wang X.J., Qi J.C., Wang X., Cao L.P. Extraction of polyphenols from barley (Hordeum vulgare L.) grain using ultrasound-assisted extraction technology. Asian J. Chem. 2013;25:1324–1330. [Google Scholar]
- 108.Kruma Z., Tomsone L., Kince T., Galoburda R., Senhofa S., Sabovics M., Straumite E., Sturite I. Effects of germination on total phenolic compounds and radical scavenging activity in hull-less spring cereals and triticale. Agron. Res. 2016;14:1372–1383. [Google Scholar]
- 109.Das A.B., Goud V.V., Das C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling, Ind Crops. Prod. 2017;95:332–341. doi: 10.1016/j.indcrop.2016.10.041. [DOI] [Google Scholar]
- 110.Jha P., Das A.J., Deka S.C. Optimization of ultrasound and microwave assisted extractions of polyphenols from black rice (Oryza sativa cv. Poireton) husk. J. Food Sci. Technol. 2017;54:3847–3858. doi: 10.1007/s13197-017-2832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo X., Cui J., Zhang H., Duan Y., Zhang D., Cai M., Chen G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind Crops Prod 112. 2018:296–304. doi: 10.1016/j.indcrop.2017.12.019. [DOI] [Google Scholar]
- 112.Cengiz M.F., Babacan U., Akinci E., Kesci S.T., Kaba A. Extraction of phenolic acids from ancient wheat bran samples by ultrasound application. J. Chem. Technol. Biotechnol. 2021;96:134–141. doi: 10.1002/jctb.6519. [DOI] [Google Scholar]
- 113.Corona-Jiménez E., Martínez-Navarrete N., Ruiz-Espinosa H., Carranza-Concha J. Ultrasound-assisted extraction of phenolics compounds from chia (Salvia hispanica L.) seeds and their antioxidant activity. Agrociencia. 2016;50:403–412. [Google Scholar]
- 114.Navarro del Hierro J., Herrera T., García-Risco M.R., Fornari T., Reglero G., Martin D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018;109:440–447. doi: 10.1016/j.foodres.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 115.de Souza M.M., Da Silva B., Costa C.S.B., Badiale-Furlong E. Free phenolic compounds extraction from Brazilian halophytes, soybean and rice bran by ultrasound-assisted and orbital shaker methods. An. Acad. Bras. Cienc. 2018;90:3363–3372. doi: 10.1590/0001-3765201820170745. [DOI] [PubMed] [Google Scholar]
- 116.Chen X., Li X., Zhu X., Wang G., Zhuang K., Wang Y., Ding W. Optimization of extrusion and ultrasound-assisted extraction of phenolic compounds from Jizi439 black wheat bran. Processes. 2020;8:1153. doi: 10.3390/pr8091153. [DOI] [Google Scholar]
- 117.Peng L.-X., Zou L., Zhao J.-L., Xiang D.-B., Zhu P., Zhao G. Response surface modeling and optimization of ultrasound-assisted extraction of three flavonoids from tartary buckwheat (Fagopyrum tataricum) Pharmacogn Mag. 2013;9:210–215. doi: 10.4103/0973-1296.113266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y., Zhao L., Zhang R., Yang X., Sun Y., Shi L., Xue P. Optimization of ultrasound-assisted extraction by response surface methodology, antioxidant capacity, and tyrosinase inhibitory activity of anthocyanins from red rice bran. Food Sci. Nutr. 2020;8:921–932. doi: 10.1002/fsn3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng L.-L., Wen G., Yuan M.-Y., Gao F. Ultrasound-assisted extraction of total flavonoids from corn silk and their antioxidant activity. J Chem. 2016 doi: 10.1155/2016/8768130. [DOI] [Google Scholar]
- 120.de Mello B.T., dos Santos Garcia V.A., da Silva C. Ultrasound-assisted extraction of oil from chia (Salvia hispanica L.) seeds: optimization extraction and fatty acid profile. J Food Process Eng. 2017;40:e12298. [Google Scholar]
- 121.Djaeni M., Listyadevi Y.L. The ultrasound-assisted extraction of rice bran oil with n-hexane as a solvent. J Phys Conf Ser. 2019;1295 doi: 10.1088/1742-6596/1295/1/012027. [DOI] [Google Scholar]
- 122.Wang L., Lu W., Li J., Hu J., Ding R., Lv M., Wang Q. Optimization of ultrasonic-assisted extraction and purification of zeaxanthin and lutein in corn gluten meal. Molecules. 2019;24:2994. doi: 10.3390/molecules24162994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yaldagard M., Mortazavi S.A., Mousavi S.M., Tabatabaie F. Investigation of the effects of ultrasound on extraction of α-amylase from the flour of malted barley. J Am Soc Brew Chem. 2009;67:141–145. [Google Scholar]
- 124.Setyaningsih W., Duros E., Palma M., Barroso C.G. Optimization of the ultrasound-assisted extraction of melatonin from red rice (Oryza sativa) grains through a response surface methodology. Appl Acoust. 2016;103:129–135. doi: 10.1016/j.apacoust.2015.04.001. [DOI] [Google Scholar]
- 125.Setyaningsih W., Saputro I.E., Palma M., Barroso C.G. Optimization of the ultrasound-assisted extraction of tryptophan and its derivatives from rice (Oryza sativa) grains through a response surface methodology. J. Cereal Sci. 2017;75:192–197. doi: 10.1016/j.jcs.2017.04.006. [DOI] [Google Scholar]
- 126.Yanova M.A., Khizhnyak S.V., Khaliullin R. Fortification of cereal groats with iron and zinc using ultrasound. IOP Conf. Ser.: Earth Environ. Sci. 2020;421(8):082019. [Google Scholar]
- 127.Dang L.T.K., Therdthai N., Ratphitagsanti W. Effects of ultrasonic and enzymatic treatment on physical and chemical properties of brown rice. J Food Process Eng. 2019;42:e13016. [Google Scholar]
- 128.Yang Z., Lin X., Wang L., Li C., Liu S. Effects of ultrasonic treatment on the cooking and fermentation properties of Shanlan rice. J. Cereal Sci. 2020;95 doi: 10.1016/j.jcs.2020.103003. [DOI] [Google Scholar]
- 129.Kunyanee K., Luangsakul N. The effects of ultrasound – assisted recrystallization followed by chilling to produce the lower glycemic index of rice with different amylose content. Food Chem. 2020;323 doi: 10.1016/j.foodchem.2020.126843. [DOI] [PubMed] [Google Scholar]
- 130.Mohammadi F., Marti A., Nayebzadeh K., Hosseini S.M., Tajdar-oranj B., Jazaeri S. Effect of washing, soaking and pH in combination with ultrasound on enzymatic rancidity, phytic acid, heavy metals and coliforms of rice bran. Food Chem. 2021;334 doi: 10.1016/j.foodchem.2020.127583. [DOI] [PubMed] [Google Scholar]
- 131.Habuš M., Novotni D., Gregov M., Čukelj Mustač N., Voučko B., Ćurić D. High-intensity ultrasound treatment for prolongation of wheat bran oxidative stability. LWT 151. 2021 doi: 10.1016/j.lwt.2021.112110. [DOI] [Google Scholar]
- 132.Habuš M., Novotni D., Gregov M., Štifter S., Čukelj Mustač N., Voučko B., Ćurić D. Influence of particle size reduction and high-intensity ultrasound on polyphenol oxidase, phenolics, and technological properties of wheat bran. J Food Process Preserv. 2021;45:e15204. [Google Scholar]
- 133.Jalali M., Sheikholeslami Z., Elhamirad A.H., Khodaparast M.H.H., Karimi M. The effect of the ultrasound process and pre-gelatinization of the corn flour on the textural, visual, and sensory properties in gluten-free pan bread. J. Food Sci. Technol. 2020;57:993–1002. doi: 10.1007/s13197-019-04132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cui R., Zhu F. Effect of ultrasound on structural and physicochemical properties of sweetpotato and wheat flours. Ultrason Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2020.105118. [DOI] [PubMed] [Google Scholar]
- 135.Zhu F., Li H. Modification of quinoa flour functionality using ultrasound. Ultrason Sonochem. 2019;52:305–310. doi: 10.1016/j.ultsonch.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 136.Janve B., Yang W., Kozman A., Sims C., Teixeira A., Gunderson M.A., Rababah T.M. Enhancement of corn nixtamalization by power ultrasound. Food Bioproc Tech 6. 2013;6(5):1269–1280. [Google Scholar]
- 137.Yang W., Kong X., Zheng Y., Sun W., Chen S., Liu D., Zhang H., Fang H., Tian J., Ye X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason Sonochem. 2019;59 doi: 10.1016/j.ultsonch.2019.104709. [DOI] [PubMed] [Google Scholar]
- 138.Karwasra B.L., Kaur M., Gill B.S. Impact of ultrasonication on functional and structural properties of Indian wheat (Triticum aestivum L.) cultivar starches. Int. J. Biol. Macromol. 2020;164:1858–1866. doi: 10.1016/j.ijbiomac.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 139.Sujka M., Jamroz J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013;31:413–419. doi: 10.1016/j.foodhyd.2012.11.027. [DOI] [Google Scholar]
- 140.Amini A.M., Razavi S.M.A., Mortazavi S.A. Morphological, physicochemical, and viscoelastic properties of sonicated corn starch. Carbohydr. Polym. 2015;122:282–292. doi: 10.1016/j.carbpol.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 141.Hu A., Jiao S., Zheng J., Li L., Fan Y., Chen L., Zhang Z. Ultrasonic frequency effect on corn starch and its cavitation. LWT. 2015;60:941–947. doi: 10.1016/j.lwt.2014.10.048. [DOI] [Google Scholar]
- 142.Hu A., Li Y., Zheng J. Dual-frequency ultrasonic effect on the structure and properties of starch with different size. LWT. 2019;106:254–262. doi: 10.1016/j.lwt.2019.02.040. [DOI] [Google Scholar]
- 143.Boufi S., Haaj S.B., Magnin A., Pignon F., Impéror-Clerc M., Mortha G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason Sonochem. 2018;41:327–336. doi: 10.1016/j.ultsonch.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 144.Kang N., Zuo Y.J., Hilliou L., Ashokkumar M., Hemar Y. Viscosity and hydrodynamic radius relationship of high-power ultrasound depolymerised starch pastes with different amylose content. Food Hydrocoll. 2016;52:183–191. doi: 10.1016/j.foodhyd.2015.06.017. [DOI] [Google Scholar]
- 145.Li C., Liu W., Gu Z., Fang D., Hong Y., Cheng L., Li Z. Ultrasonic pretreatment improves the high-temperature liquefaction of corn starch at high concentrations. Starke. 2017;69:1600002. doi: 10.1002/star.201600002. [DOI] [Google Scholar]
- 146.Li M., Li J., Zhu C. Effect of ultrasound pretreatment on enzymolysis and physicochemical properties of corn starch. Int. J. Biol. Macromol. 2018;111:848–856. doi: 10.1016/j.ijbiomac.2017.12.156. [DOI] [PubMed] [Google Scholar]
- 147.Yang Q.-Y., Lu X.-X., Chen Y.-Z., Luo Z.-G., Xiao Z.-G. Fine structure, crystalline and physicochemical properties of waxy corn starch treated by ultrasound irradiation. Ultrason Sonochem. 2019;51:350–358. doi: 10.1016/j.ultsonch.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Huang Q., Li L., Fu X. Ultrasound effects on the structure and chemical reactivity of cornstarch granules. Starke. 2007;59:371–378. doi: 10.1002/star.200700614. [DOI] [Google Scholar]
- 149.Isono Y., Kumagai T., Watanabe T. Ultrasonic Degradation of Waxy Rice Starch. Biosci. Biotechnol. Biochem. 1994;58:1799–1802. doi: 10.1271/bbb.58.1799. [DOI] [Google Scholar]
- 150.Zhou X., Wang S., Zhou Y. Study on the structure and digestibility of high amylose Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch-flavonoid prepared by different methods. J. Food Sci. 2021;86:1463–1474. doi: 10.1111/1750-3841.15657. [DOI] [PubMed] [Google Scholar]
- 151.Jambrak A.R., Herceg Z., Šubarić D., Babić J., Brnčić M., Brnčić S.R., Bosiljkov T., Čvek D., Tripalo B., Gelo J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010;79:91–100. doi: 10.1016/j.carbpol.2009.07.051. [DOI] [Google Scholar]
- 152.Luo Z., Fu X., He X., Luo F., Gao Q., Yu S. Effect of ultrasonic treatment on the physicochemical properties of maize starches differing in amylose content. Starke. 2008;60:646–653. doi: 10.1002/star.200800014. [DOI] [Google Scholar]
- 153.Montalbo-Lomboy M., Johnson L., Khanal S.K., van Leeuwen J.H., Grewell D. Sonication of sugary-2 corn: A potential pretreatment to enhance sugar release. Bioresour. Technol. 2010;101:351–358. doi: 10.1016/j.biortech.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 154.Li Y., Hu A., Zheng J., Wang X. Comparative studies on structure and physiochemical changes of millet starch under microwave and ultrasound at the same power. Int. J. Biol. Macromol. 2019;141:76–84. doi: 10.1016/j.ijbiomac.2019.08.218. [DOI] [PubMed] [Google Scholar]
- 155.Dey A., Sit N. Modification of foxtail millet starch by combining physical, chemical and enzymatic methods. Int. J. Biol. Macromol. 2017;95:314–320. doi: 10.1016/j.ijbiomac.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 156.Yu S., Zhang Y., Ge Y., Zhang Y., Sun T., Jiao Y., Zheng X.-Q. Effects of ultrasound processing on the thermal and retrogradation properties of nonwaxy rice starch. J Food Process Eng. 2013;36:793–802. doi: 10.1111/jfpe.12048. [DOI] [Google Scholar]
- 157.Cooke D., Gidley M.J. Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohydr. Res. 1992;227:103–112. doi: 10.1016/0008-6215(92)85063-6. [DOI] [Google Scholar]
- 158.Hu A., Li L., Zheng J., Lu J., Meng X., Liu Y., Rehman R. Different-frequency ultrasonic effects on properties and structure of corn starch. J. Sci. Food Agric. 2014;94:2929–2934. doi: 10.1002/jsfa.6636. [DOI] [PubMed] [Google Scholar]
- 159.Amini A.M., Razavi S.M.A. Ultrasound-assisted acid-thinning of corn starch: Morphological, physicochemical, and rheological properties. Starke. 2015;67:640–653. doi: 10.1002/star.201400228. [DOI] [Google Scholar]
- 160.Pérez S., Baldwin P.M., Gallant D.J. Structural features of starch granules I. Chemistry and technology, Elsevier; Starch: 2009. [DOI] [Google Scholar]
- 161.Zuo J.Y., Knoerzer K., Mawson R., Kentish S., Ashokkumar M. The pasting properties of sonicated waxy rice starch suspensions. Ultrason Sonochem. 2009;16:462–468. doi: 10.1016/j.ultsonch.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 162.Park D.-J., Han J.-A. Quality controlling of brown rice by ultrasound treatment and its effect on isolated starch. Carbohydr. Polym. 2016;137:30–38. doi: 10.1016/j.carbpol.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 163.W. R. Mason, Starch use in foods, in: J. BeMiller, R. Whistler (Eds.), Starch: Chemistry and technology, Elsevier, 2009. 10.1016/B978-0-12-746275-2.00020-3.
- 164.Varavinit S., Shobsngob S., Varanyanond W., Chinachoti P., Naivikul O. Effect of amylose content on gelatinization, retrogradation and pasting properties of flours from different cultivars of Thai rice. Starke. 2003;55:410–415. doi: 10.1002/star.200300185. [DOI] [Google Scholar]
- 165.Montalbo-Lomboy M., Khanal S.K., van Leeuwen J.H., Raman D.R., Dunn L., Jr., Grewell D. Ultrasonic pretreatment of corn slurry for saccharification: A comparison of batch and continuous systems. Ultrason Sonochem. 2010;17:939–946. doi: 10.1016/j.ultsonch.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 166.Shewale S.D., Pandit A.B. Enzymatic production of glucose from different qualities of grain sorghum and application of ultrasound to enhance the yield. Carbohydr. Res. 2009;344:52–60. doi: 10.1016/j.carres.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 167.Bassetto Bisinella R.Z., Beninca C., Delinski Bet C., de Oliveira C.S., Mottin Demiate I., Schnitzler E. Thermal, structural and morphological characterization of organic rice starch after physical treatment. J. Therm. Anal. Calorim. 2022;147:3615–3623. doi: 10.1007/s10973-021-10712-7. [DOI] [Google Scholar]
- 168.Constantino A.B.T., Garcia-Rojas E.E. Modifications of physicochemical and functional properties of amaranth protein isolate (Amaranthus cruentus BRS Alegria) treated with high-intensity ultrasound. J. Cereal Sci. 2020;95 doi: 10.1016/j.jcs.2020.103076. [DOI] [Google Scholar]
- 169.Jia J., Ma H., Zhao W., Wang Z., Tian W., Luo L., He R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010;119:336–342. doi: 10.1016/j.foodchem.2009.06.036. [DOI] [Google Scholar]
- 170.Jin J., Okagu O.D., Yagoub A.E.A., Udenigwe C.C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li S., Yang X., Zhang Y., Ma H., Liang Q., Qu W., He R., Cunshan Zhou C., Mahunu G.K. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason Sonochem. 2016;31:20–28. doi: 10.1016/j.ultsonch.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 172.Li S., Yang X., Zhang Y., Ma H., Qu W., Ye X., Muatasim R., Oladejo A.O. Enzymolysis kinetics and structural characteristics of rice protein with energy-gathered ultrasound and ultrasound assisted alkali pretreatments. Ultrason Sonochem. 2016;31:85–92. doi: 10.1016/j.ultsonch.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 173.Li X., Da S., Li C., Xue F., Zang T. Effects of high-intensity ultrasound pretreatment with different levels of power output on the antioxidant properties of alcalase hydrolyzates from quinoa (Chenopodium quinoa Willd.) protein isolate. Cereal Chem. 2018;95:518–526. doi: 10.1002/cche.10055. [DOI] [Google Scholar]
- 174.Nazari B., Mohammadifar M.A., Shojaee-Aliabadi S., Feizollahi E., Mirmoghtadaie L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason Sonochem. 2018;41:382–388. doi: 10.1016/j.ultsonch.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 175.O’Sullivan J., Park M., Beevers J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016;69:77–84. doi: 10.1016/j.jcs.2016.02.013. [DOI] [Google Scholar]
- 176.Ren X., Liang Q., Ma H. Effects of sweeping frequency ultrasound pretreatment on the hydrolysis of zein: angiotensin-converting enzyme inhibitory activity and thermodynamics analysis. J. Food Sci. Technol. 2018;55:4020–4027. doi: 10.1007/s13197-018-3328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ren X., Zhang X., Liang Q., Hou T., Zhou H. Effects of different working modes of ultrasound on structural characteristics of zein and ACE inhibitory activity of hydrolysates. J Food Qual. 2017 doi: 10.1155/2017/7896037. [DOI] [Google Scholar]
- 178.Yang X., Li Y., Li S., Oladejo A.O., Ruan S., Wang Y., Huang S., Ma H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason Sonochem. 2017;38:19–28. doi: 10.1016/j.ultsonch.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 179.Yang X., Wang L., Zhang F., Ma H. Effects of multi-mode S-type ultrasound pretreatment on the preparation of ACE inhibitory peptide from rice protein. Food Chem. 2020;331 doi: 10.1016/j.foodchem.2020.127216. [DOI] [PubMed] [Google Scholar]
- 180.Zhang H., Chen G., Liu M., Mei X., Yu Q., Kan J. Effects of multi-frequency ultrasound on physicochemical properties, structural characteristics of gluten protein and the quality of noodle. Ultrason Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105135. [DOI] [PubMed] [Google Scholar]
- 181.Zhang X., Zuo Z., Ma W., Yu P., Li T., Wang L. Assemble behavior of ultrasound-induced quinoa protein nanoparticles and their roles on rheological properties and stability of high internal phase emulsions. Food Hydrocoll. 2021;117 doi: 10.1016/j.foodhyd.2021.106748. [DOI] [Google Scholar]
- 182.Zhang Y., Li J., Li S., Ma H., Zhang H. Mechanism study of multimode ultrasound pretreatment on the enzymolysis of wheat gluten. J. Sci. Food Agric. 2018;98(4):1530–1538. doi: 10.1002/jsfa.8624. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Y., Ma H., Wang B., Qu W., Wali A., Zhou C. Relationships between the structure of wheat gluten and ACE inhibitory activity of hydrolysate: stepwise multiple linear regression analysis. J. Sci. Food Agric. 2015;96:3313–3320. doi: 10.1002/jsfa.7509. [DOI] [PubMed] [Google Scholar]
- 184.Zhou C., Ma H., Yu X., Liu B., Abu E.-G., Yagoub Z.P. Pretreatment of defatted wheat germ proteins (by-products of flour mill industry) using ultrasonic horn and bath reactors: Effect on structure and preparation of ACE-inhibitory peptides. Ultrason Sonochem. 2013;20:1390–1400. doi: 10.1016/j.ultsonch.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 185.Zhang K., Wen Q., Li T., Wang Y., Zhang Y., Luo D. Comparative study of the effects of ultrasonic power on the structure and functional properties of gliadin in wheat and green wheat. J. Food Sci. 2022;87:1020–1034. doi: 10.1111/1750-3841.16050. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Y., Li Y., Li S., Zhang H., Ma H. In situ monitoring of the effect of ultrasound on the sulfhydryl groups and disulfide bonds of wheat gluten. Molecules. 2018;23:1376. doi: 10.3390/molecules23061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jin H.-L., Wang J.-S., Bian K. Characteristics of enzymatic hydrolysis of the wheat gluten proteins treated by ultrasound wave. Adv Mat Res. 2012;343–344:1015–1022. doi: 10.4028/www.scientific.net/AMR.343-344.1015. [DOI] [Google Scholar]
- 188.Vera A., Valenzuela M.A., Yazdani-Pedram M., Tapia C., Abugoch L. Conformational and physicochemical properties of quinoa proteins affected by different conditions of high-intensity ultrasound treatments. Ultrason Sonochem. 2019;51:186–196. doi: 10.1016/j.ultsonch.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 189.Luo L., Zhang Y., Wang K., Ma H., Dong M. In situ and real-time monitoring of an ultrasonic-assisted enzymatic hydrolysis process of corn gluten meal by a miniature near infrared spectrometer. Anal. Methods. 2017;9:3795–3803. doi: 10.1039/C7AY00887B. [DOI] [Google Scholar]
- 190.Qu W., Sehemu R.M., Zhang T., Song B., Yang L., Ren X., Ma H. Immobilized enzymolysis of corn gluten meal under triple-frequency ultrasound. Int. J. Food Eng. 2018 doi: 10.1515/ijfe-2017-0347. [DOI] [Google Scholar]
- 191.Ren X., Ma H., Mao S., Zhou H. Effects of sweeping frequency ultrasound treatment on enzymatic preparations of ACE-inhibitory peptides from zein. Eur. Food Res. Technol. 2014;238:435–442. doi: 10.1007/s00217-013-2118-3. [DOI] [Google Scholar]
- 192.Wang B., Atungulu G.G., Khir R., Geng J., Ma H., Li Y., Wu B. Ultrasonic treatment effect on enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophys. 2015;10:244–252. doi: 10.1007/s11483-014-9375-y. [DOI] [Google Scholar]
- 193.Musa A., Ma H., Gasmalla M.A.A., Sarpong F., Awad F.N., Duan Y. Effect of multi-frequency counter-current S type ultrasound pretreatment on the enzymatic hydrolysis of defatted corn germ protein: Kinetics and thermodynamics. Process Biochem. 2019;87:112–118. doi: 10.1016/j.procbio.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 194.Hassan Z., Imran M., Ahmad M.H., Khan M.K. Ultrasound-assisted modification of insoluble dietary fiber from chia (Salvia hispanica L.) seeds. J Food Qual. 2021:5035299. doi: 10.1155/2021/5035299. [DOI] [Google Scholar]
- 195.Wei C., Ge Y., Liu D., Zhao S., Wei M., Jiliu J., Hu X., Quan Z., Wu Y., Su Y., Wang Y., Cao L. Effects of high-temperature, high-pressure, and ultrasonic treatment on the physicochemical properties and structure of soluble dietary fibers of millet bran. Front. Nutr. 2022;8 doi: 10.3389/fnut.2021.820715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Vaitkeviciene R., Zadeike D., Gaizauskaite Z., Valentaviciute K., Marksa M., Mazdzieriene R., Bartkiene E., Lele V., Juodeikiene G., Jakstas V. Functionalisation of rice bran assisted by ultrasonication and fermentation for the production of rice bran-lingonberry pulp-based probiotic nutraceutical. Int. J. Food Sci. Technol. 2021;57:1462–1472. doi: 10.1111/ijfs.15053. [DOI] [Google Scholar]
- 197.Zadeike D., Vaitkeviciene R., Degutyte R., Bendoraitiene J., Rukuiziene Z., Cernauskas D., Svazas M., Juodeikiene G. A comparative study on the structural and functional properties of water-soluble and alkali-soluble dietary fibres from rice bran after hot-water, ultrasound, hydrolysis by cellulase, and combined pre-treatments. Int. J. Food Sci. Technol. 2022;57:1137–1149. doi: 10.1111/ijfs.15480. [DOI] [Google Scholar]
- 198.Li L., Ma S., Fan L., Zhang C., Pu X., Zheng X., Wang X. The influence of ultrasonic modification on arabinoxylans properties obtained from wheat bran. Int. J. Food Sci. Technol. 2016;51:2338–2344. doi: 10.1111/ijfs.13239. [DOI] [Google Scholar]
- 199.Fan L., Ma S., Wang X., Zheng X. Improvement of Chinese noodle quality by supplementation with arabinoxylans from wheat bran. Int. J. Food Sci. Technol. 2016;51:602–608. doi: 10.1111/ijfs.13042. [DOI] [Google Scholar]
- 200.Andersson A.A., Andersson R., Piironen V., Lampi A.-M., Nyström L., Boros D., Fras A., Gebruers K., Courtin C.M., Delcour J.A., Rakszegi M., Bedo Z., Ward J.L., Shewry P.R., Åmana P. Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem. 2013;136:1243–1248. doi: 10.1016/j.foodchem.2012.09.074. [DOI] [PubMed] [Google Scholar]