Abstract
Dengue fever is a mosquito-borne-disease of growing public health importance in Africa. The continuous increase of number and frequency of outbreaks of dengue fever, especially in urban area in Africa underline the need to review the current data available on vectors involved in dengue virus transmission in Africa. Here, we summarized the available data on vectors involved in the transmission of dengue virus in the sylvatic and urban environments, vertical transmission, vector competence studies, and vector control strategies used in Africa. The virus was isolated mainly from Aedes furcifer, Ae. luteocephalus, and Ae. taylori in the sylvatic environment and from Ae. aegypti and Ae. albopictus in the urban areas. Prospective and urgently needed studies on vectors biology, behavior, and alternative control strategies are suggested.
Keywords: Dengue, Mosquito vectors, Sylvatic, Urban, Africa
Graphical abstract
Dengue; Mosquito vectors; Sylvatic; Urban; Africa.
1. Introduction
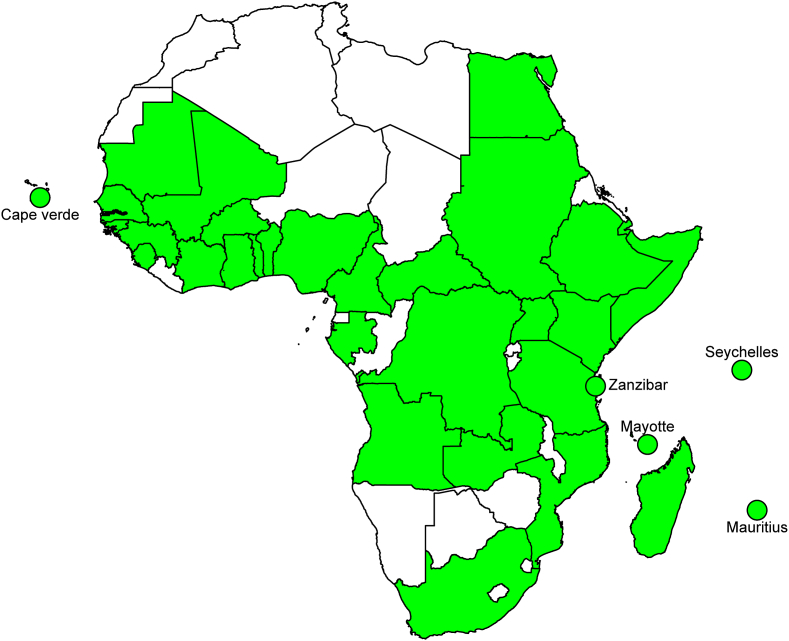
Dengue virus (DENV), the causative agent of Dengue fever, transmitted by several Aedes spp., is one of the most important arboviral diseases in the world. Indeed, this disease caused by four genetically related but antigenically distinct viruses (DENVs 1–4; genus Flavivirus, family Flaviviridae), is endemic in more than 128 countries with around 390 million people infected each year [1, 2]. With 16% of these infections, Africa is one of the most affected regions [3]. Evidence of dengue circulation has been detected in local populations or travelers returning from more than 30 African countries (Figure 1).
Figure 1.
Map showing African countries where dengue circulation has been detected in local populations or travelers.
In Africa, DENV-2 was the most frequently reported serotype before 2000s and was responsible of several epidemics in East Africa (Somalia, Djibouti, Kenya, and Tanzania) and sylvatic emergence with sporadic human cases in West Africa (Senegal, Burkina and Côte d’Ivoire) [4, 5, 6, 7, 8]. In West Africa, DENV-2 epidemics were only detected in Nigeria and Burkina Faso before 2000 [9,10]. The DENV-3 emerged only in Mozambique in 1985 and Somalia in 1993 [8,11], while DENV-1 outbreaks were observed in Sudan in 1984, Comoros in 1993, Nigeria in 1960s and Senegal in 1979 [6,9,12]. In recent decades, the number and frequency of dengue epidemics have increased dramatically in Africa [13, 14, 15]. DENV-2 and 3 are the main serotypes involved in the epidemics on the African continent, although the circulation of the other two serotypes have been documented [16]. In East Africa, DENV-2 has remained very active in countries that were affected during the last century, but has emerged also in several other countries, including Ethiopia in 2013, Tanzania in 2014 and Mozambique in 2014–2015 [17,18,19,20]. Sylvatic amplification and urban epidemics of DENV-2 have occurred in several West African countries (Mali in 2008, Senegal in 2015–2018, Mauritania in 2014–2020, and Burkina Faso in 2013, 2016 and 2017), as well as in Central African countries (Gabon in 2007 and Angola in 2013 and 2018) [13,21,22,23,24]. Outbreaks of DENV-3 have been reported in Tanzania, Zanzibar, Comoros, Benin, Cape Verde, Gabon and Senegal in 2009–2018 [25,26,27,28]. Epidemics of DENV-1 were detected in Angola, Kenya, Senegal and Somalia in 2011–2018 [17,29,30,31].
Because dengue is becoming a major threat to public health in Africa, it is essential to better understand some poorly characterized aspects of the mosquito vectors involved in the epidemiology and transmission of this disease. Since there is no specific treatment or available vaccine for dengue, a better understanding of these factors will be a key driver for designing effective and sustainable vector control methods and strategies.
The goal of this review is to compile the available data on the dengue virus vectors in Africa based mainly on virus isolation from field collected mosquitoes and vector competence studies.
2. Mosquito species associated to dengue virus in the field
Several mosquito species have been associated with DENV in field collected mosquitoes from both urban and sylvatic environments (Table 1).
Table 1.
Mosquito species found naturally infected with dengue virus in Africa.
| Environment | Countries | Species | Years | Serotypes | References |
|---|---|---|---|---|---|
| Urban | Senegal | Ae. (Stegomyia) aegypti | 2009 | 3 | [27] |
| Burkina Faso | 1982, 1986 | 2 | [59] | ||
| Nigeria | 1969 | 2 | [41, 42, 57] | ||
| Côte d’Ivoire | 2017 | 3 | [46] | ||
| Tanzania | 2014 | 2 | [19] | ||
| Kenya | 2014 | 2 | [47] | ||
| Sudan | 2016–17 | ?? | [48] | ||
| Cabo Verde | 2009, 2014-15 | 2, 3, 4 | [45] | ||
| Seychelles | Ae. (Stegomyia) albopictus | 1976–77 | 2 | [51] | |
| Gabon | 2007, 2010 | 2 | [23, 50] | ||
| Rural/Sylvatic | Senegal | Ae. (Stegomyia) aegypti, Ae. (Diceromyia) furcifer, Ae. (Diceromyia) taylori, Ae. (Stegomyia) luteocephalus, Ae. (Aedimorphus) dalzieli, Ae. (Aedimorphus) vittatus, Ma. (Mansonoides) africana | 1974, 1981–82, 1989, 1999–2000 | 2 | [4,53,84] |
| Burkina Faso | Ae. (Stegomyia) luteocephalus, Ae. (Stegomyia) africanus, Ae. (Aedimorphus) cumminsii | 1980, 1983, 1986 | 2 | [44, 59] | |
| Côte d’Ivoire | Ae. (Stegomyia) aegypti, Ae. (Diceromyia) furcifer, Ae. (Diceromyia) taylori, Ae. (Stegomyia) luteocephalus, Ae. (Stegomyia) africanus, Ae. (Stegomyia) opok | 1980, 1985-87 | 2 | [4, 59] | |
| Nigeria |
Ae. (Stegomyia) luteocephalus, Ae. (Stegomyia) africanus Ma. (Mansonioides) africana |
1969, 1977 | 1, 2, 3 | [60, 61] | |
| Guinea Conakry | Ae. (Stegomyia) luteocephalus, Ae. (Stegomyia) africanus | 1981 | 2 | [4] |
2.1. Vectors in the urban environment
In Africa, DENV is transmitted in the urban environment, between humans and the mosquito species Ae. aegypti and Ae. albopictus during epidemics. In urban environments, Aedes vectors were found breeding indoors and outdoors in human associated water storage containers (clay jars, drums, jerrycans, cement tanks, etc), discarded containers, used tires, flower pots, miscellaneous, etc. [32, 33, 34, 35]. These Aedes are mainly anthropophilic, but were also found to have fed on other animals [36, 37, 38]. Aedes aegypti is considered as mainly endophilic, endophagic, and daytime feeder, but it was collected feeding and resting outdoors within used tires, bricks and scrap metals indicating that it can also transmit viruses outdoors [39]. Alternatively, Ae. albopictus is considered as being a more opportunistic and outdoor feeder [37, 38]. Climatically suitable areas for Ae. aegypti related to dengue incidence was predicted to increase in the future [40]. These authors found that temperature and precipitation (by providing breeding sites and stimulates egg hatching) are important climatic factors that will influence Ae. aegypti development and distribution.
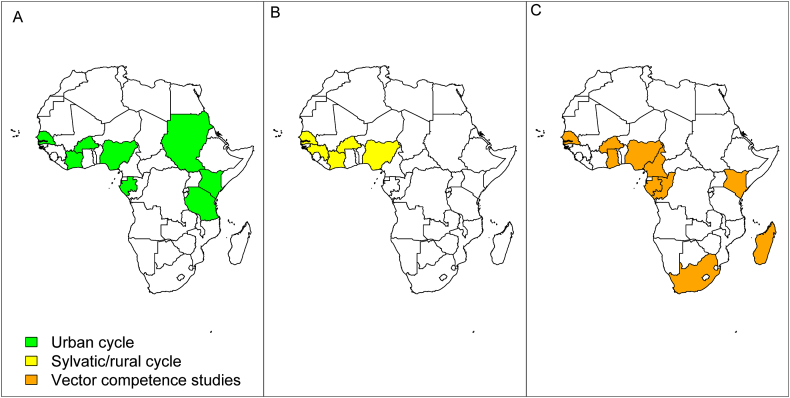
The DENV was associated with Ae. aegypti in an urban cycle, in Senegal, Nigeria, Burkina Faso, Cabo Verde, Tanzania, Kenya and Sudan during outbreak investigations and/or routine entomological studies (Figure 2). Despite its presence in several African countries, Gabon is actually the only country where DENV is associated with Ae. albopictus in continental Africa.
Figure 2.
Map showing African countries where dengue virus was detected from mosquitoes in the urban (A) and sylvatic/Rural cycle (B), and vector competence studies (C) were done.
In Nigeria, Ae. aegypti and Ae. albopictus have been collected during entomological studies but only Ae. aegypti was found infected with all DENV serotypes in the field [41, 42]. A pool of Ae. aegypti collected in August 1969 in Ibadan was found positive for DENV-2. Viral RNAs of the four DENV serotypes were detected in Ae. aegypti collected in different localities of Nigeria in 2001–2002. Three pools of Ae. aegypti were found infected in 2 neighborhoods of Dakar (2 pools in Plateau and 1 pool in Parcelles Assainies), during the first urban epidemic of DENV-3 in Senegal [27]. The Stegomyia indexes in Dakar were above the epidemic risk threshold (between 6.6-195.2 for the Breteau index and 15–63.2 for the container index). Aedes aegypti was also the only potential vector present in high abundance in all the following urban epidemics and sporadic cases of dengue in 2015 (DENV-2; Mbour), 2017 (DENV-3; Louga) and 2018 (DENV-1 and 2 in Fatick; DENV-1 and 3 in Touba; DENV-1 in St Louis) that occurred in Senegal [28, 31, 43]. In Burkina Faso, DENV was isolated from Ae. aegypti in 1982 (1 pool) and 1986 (1 pool) during DENV-2 epidemics in the urban area of Bobo-Dioulasso [44]. During the first dengue epidemic that occurred in Cabo Verde in 2009, DENV-3 was isolated from a pool of Ae. aegypti collected at Praia, the capital city of the country. Two other serotypes (3 strains of DENV-2 and 5 strains of DENV-4) were later detected from the same country by RT-PCR from Ae. aegypti mosquitoes collected in 2014 and 2015, in Palmarejo (7 strains) and Fonton (1 strain), 2 neighborhoods of Praia city [45]. DENV-3 was detected by RT-PCR from 3 Ae. aegypti pools (2 from host-seeking females and 1 from emerging adults collected as larvae) collected at Abidjan, the economic capital city of Côte d’Ivoire, during the 2017 outbreak [46]. The Breteau (213 and 297), House (69 and 82%) and containers (35 and 41%) indexes were high in the 2 localities investigated during this outbreak.
The first association of DENV with Ae. aegypti in Tanzania was detected by an entomological investigation during the 2014 dengue outbreak [19]. DENV-2 was detected in 8.2% of 330 Aedes mosquito pools tested by RT-PCR. Only 2 out of the 27 positives Ae. aegypti pools were collected as adults. The Aedes indices (Breteau index: 20.8–30.6 and container index: 65.2–80.2%) were very high, suggesting high risks of infection in all the districts investigated within Dar es Salaam. DENV-2 was isolated from a pool of 2 males Ae. aegypti collected during an outbreak in Mombasa in 2013–2014, suggesting that this species was the vector of this urban epidemic in eastern Kenya [47]. The Aedes indices were all high in the study area (Breteau index: 123.1–358.6 and container index: 23.3–44.1%). During a routine entomological investigation in Kassala States in eastern Sudan, DENV RNA was detected in one out of 329 Ae. aegypti adults tested by RT-PCR. This species was the only vector identified during this study. The house and Breteau index were 32.8 % and 35.96, respectively [48]. The serotype was not identified during this outbreak but all dengue serotypes have been previously detected in Sudan. DENV-1 and DENV-2 were the most commonly found in this country [48, 49].
While both Ae. aegypti and Ae. albopictus were collected during a DENV-2 outbreak in Gabon in 2007 [23], the virus was only detected in Ae. albopictus (3 pools) in the suburbs of the capital city, Libreville, suggesting Ae. albopictus was the primary vector during this epidemic. Importantly, this was the first detection of DENV in Ae. albopictus in continental Africa and Ae. albopictus was more abundant than Ae. aegypti in this suburban environment. Aedes albopictus was also the vector of a second DENV-2 outbreak in Franceville, in Gabon in 2010 when the virus was detected in 18 out of 46 pools of this species tested [50]. One female was found coinfected with dengue and chikungunya viruses during this outbreak. Prior to the Gabon outbreaks, DENV-2 was isolated from 8 pools of Ae. albopictus collected during a dengue outbreak in the Seychelles in 1976–1977 [51]. Although multiple other dengue fever outbreaks have occurred in several other African countries, data on which mosquito species were involved is lacking because no entomological investigation was carried out during these outbreaks [4, 13, 52].
2.2. Vectors in the sylvatic and rural environments
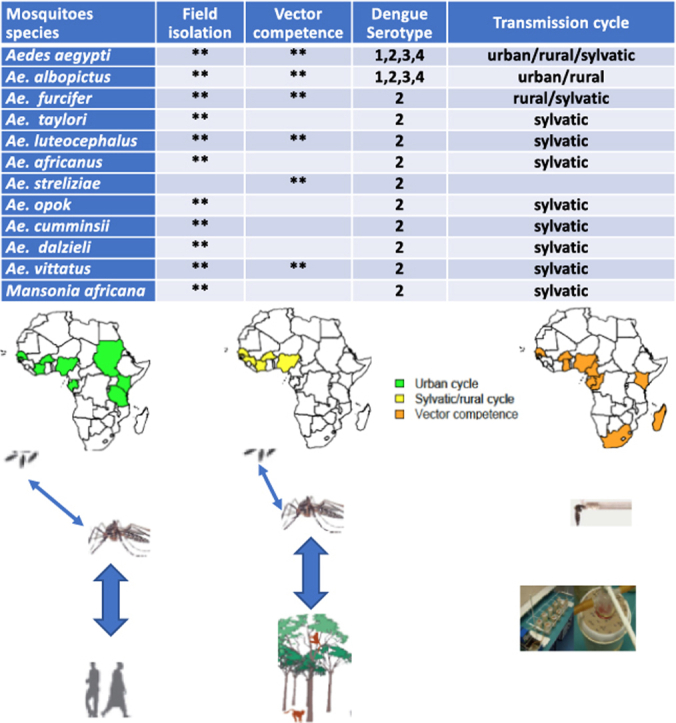
Detection of DENV from several mosquito species (Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. vittatus, Ae. africanus, Ae. opok, Ae. cumminsii, Ae. dalzieli, and Ae. aegypti) in sylvatic and rural areas follows a seasonal pattern. Dengue virus has mainly been detected between August and November in forest galleries and villages [53]. Some of these species (Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. africanus and Ae. opok) are known to be crepuscular feeders [54]. Moreover, depending on the species considered, several of them (Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. africanus, and Ae. opok) seem to feed almost exclusively on human and or non-human primates. Although they are found readily feeding on men, Ae. vittatus, Ae. cumminsii, Ae. dalzieli, sylvatic Ae. aegypti, and Ma. africana are considered as mainly zoophilic [55]. Some species like Ae. luteocephalus and Ae. taylori feed mainly in the forest-canopy, while others could be found feeding in almost all landcover classes found in the sylvatic environment, including villages (outdoors and indoors) and agricultural areas where feeding on humans is possible [56]. Aedes furcifer females infected with DENV were collected within villages investigated in southeastern Senegal from 2009 to 2020, suggesting that they are involved in DENV-2 transmission to humans in this area [53]. The main breeding places are tree holes and fruit husks for most sylvatic vectors (Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. africanus, and Ae. opok), rock pools and puddles for Ae. vittatus [32], ground pools and puddles for Ae. dalzieli and Ae. cumminsii, and grassy ponds for Ma. africana.
The sylvatic cycle of DENV has been described in five West African countries and mainly for DENV-2 (Table 1 and Figure 2) [53]. The first isolations of DENV-2 from naturally infected mosquitoes in Africa date to 1969 when one strain was isolated most likely from Ae. (Stegomyia) luteocephalus in Jos in Nigeria [57]. Later, DENV-2 was isolated from mosquitoes collected in wooded areas in Senegal [53, 58], Côte d’Ivoire [59], Guinea [4] and Burkina Faso [44]. The virus was isolated mainly from Ae. luteocephalus, Ae. furcifer and Ae. taylori in Senegal and Côte d’Ivoire, and Ae. luteocephalus in Burkina Faso. Several other species were sporadically found associated with DENV in the sylvatic environment in Senegal (Ae. aegypti, Ae. vittatus, Ae. dalzieli, Ma. africana), Burkina Faso (Ae. africanus, Ae. cumminsii) and Côte d’Ivoire (Ae. africanus, Ae. opok, Ae. cumminsii). DENV-2 was isolated from 4 pools of mosquitoes (3 Ae. africanus and 1 Ae. luteocephalus) collected in November 1981 in Guinea [4].
DENV-1 was isolated from two pools of Ae. africanus collected in the Mamu River Forest reserve in Eastern Nigeria in 1997 [60]. DENV-3 viral RNA was also detected in 30 pools of Ma. africana collected in the rural locality of Ikarama, state of Bayelsa in Nigeria in 2015 [61]. The real importance of Ae. africanus and Ma. africana in the transmission of dengue in the rural human dwelling area need further studies.
3. Vertical transmission studies
In Africa, DENV is likely maintained in nature during unfavorable periods, by vertical transmission as suggested by detection of the virus from male mosquitoes, adults collected as immatures during field studies and progeny of infected females. Indeed, DENV was detected from male mosquitoes belonging to several species including Ae. aegypti in Nigeria [41] and Kenya [47], Ae. furcifer-taylori in Côte d’Ivoire in 1980 [59], Ae. taylori [62] and Ae. furcifer in a forest gallery in Kedougou [53] and unidentified Aedes species in Nigeria [41]. Most of the DENV-2 positive pools of Ae. aegypti (25 out of 27), detected by RT-PCR during the 2014 dengue outbreak in Dar es Salaam, the capital city of Tanzania were collected as immatures [19]. More recently, DENV-3 was detected by RT-PCR from a pool of emerging adults collected as larvae during the 2017 outbreak in Côte d’Ivoire [46]. Dengue virus was detected from one pool of F1 progeny of a population of Ae. aegypti from Nigeria (Lagos), infected with the New Guinea c DENV-2 strain, indicating vertical transmission [63].
4. Vector competence studies
The vector competence of field populations of mosquitoes associated with DENV in Africa is poorly characterized (Table 2) in few countries (Figure 2). Except for DENV-2 [64,65,66], few studies have been performed with DENV-1, 3 and 4 [67,68,69]. Vector competence studies were done mainly with sylvatic and domestic Ae. aegypti populations from Senegal, Cabo Verde, Kenya, Gabon, Cameroon, Ghana, Nigeria, Republic of Congo and Burkina Faso.
Table 2.
Mosquito species originated from Africa competent for dengue virus.
| Species | Mosquito tested |
Titer of the blood meals | Virus serotype (strains; Origin) | Origin | Inf (%) | Diss (%) | Trans (%) | DPE (days) | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Country | localities | |||||||||
| Aedes (Stegomyia) aegypti | Senegal | Kedougou, Koung Koung, Ndougoubene, Ngoye, Dakar and Barkdji |
106.5 and 1.6 × 107 TCID50/mL | DENV-2 (ArD 140875; Kedougou) | Sylvatic | 0–26 | 0–75 | 14 | [64] | |
| 106.5 TCID50/mL | DENV-2 (ArA 6894; Bobo-Dioulasso) | Epidemic | 0–1.85 | 50–100 | 14 | |||||
| Koung Koung, Kedougou | 6.2–8.8 log10 TCID50/mL | DENV-2 (NGC; New Guinea and 1349; Burkina Faso) | Epidemic | 0–25 | 67–100 | 14 | [70] | |||
| 6.2–8.8 log10 TCID50/mL | DENV-2 (PM33974; Guinea and DakAr2022; Burkina Faso) | Sylvatic | 0–3 | 0–100 | 14 | |||||
| Dakar, St-Louis, Kedougou | 4.9 × 106–4.7 × 107 PFU/mL | DENV-1 (SH 29177; Bandia) | Epidemic | 71.4–92.5 | 50–93.8 | 2.9–5.9 | 7,15 | [67] | ||
| 3.5 × 106–2.4 × 107 PFU/mL | DENV-3 (S-162 TvP-3622; Somalia) | Epidemic | 62.5–100 | 50–88.2 | 3.3–7.5 | |||||
| 1.2 × 106–2.6 × 107 PFU/mL | DENV-4 (SH 38549; Dakar) | Epidemic | 71.4–93.8 | 56.7–93.8 | 2.9–20 | |||||
| Saint-Louis, Digale, Dakar, Ngoye, Tambacounda, Goudiry, Niemenike, Ngari, PK10, Deux rivieres, Fongolimbi |
107.5–8.5 pfu/mL | DENV-2 (JAM1409; Jamaica) | Epidemic | up to 90 | 14 | [65] | ||||
| Kedougou, Fatick, Bignona, Richard-Toll, Goudiry, PK10, Mont Rolland, Rufisque |
6.02 log10 PFU/mL | DENV-2 (75505; Kedougou) | Sylvatic | 50–91 | 29–93 | 14 | [71] | |||
| Dakar, Kedougou | 5.104.2 and 5.104.4 MID50 ⁄ mL | DENV3 (H87; Hawaii) | Epidemic | 2.4–15.2 | 0–8.3 | xx | 7, 15,20 | [72] | ||
| Dakar, Kedougou | 5.103.3 and 5.104.3 MID50 ⁄ mL | DENV1_IbH28328; Ibadan) | up to 50 | up to 50 | xx | |||||
| South Africa | Palm Beach, Durban, Richard Bay, Ndumu, Skukuza |
6.1–8.4 Log10 MID50/mL | DENV-1 (Cassim, Durban) | Epidemic | NA | 6–68 | 50–100 | 13–20 | [80] | |
| 7.0–7.9 Log10 MID50/mL | DENV-2 (BC 5007, Taipei) | Epidemic | NA | 11–64 | 33–100 | 14–20 | ||||
| Cape Verde | Santiago (seven counties) | 2 × 105–5 × 104 FFU/mL | DENV-1 (42735; BR PE) | Epidemic | 0–27 | 0–100 | 0 | 7,14,21 | [68] | |
| 1.4 × 105–2 × 105 FFU/mL | DENV-2 (3808; BR-PE) | Epidemic | 50–80 | 20–93.3 | 5.0–65.0 | 7, 14,21 | ||||
| 1.0 × 106–2 × 106 FFU/mL | DENV-3 (85469; BR-PE) | Epidemic | 10–80 | 50–87.5 | 0.0–75.0 | 7,14,21 | ||||
| 1.0 × 106–2 × 106 FFU/mL | DENV-4 (1385; Brazil) | Epidemic | 0–9 | 0 | 0 | 7,14,21 | ||||
| 107 FFU/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 41.6 | 8.3 | 14 | [73] | ||||
| 107 FFU/mL | DENV-3 (Praia; Cabo Verde) | Epidemic | 27.3–80 | 0–20 | 7,10,14 | |||||
| Madagascar | Mahaleja, Joffreville | 108.2 MID50/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 25.0–40.0 | xx | x | 14 | [79] | |
| Cameroon | 19 localities including Douala, Maroua and Yaoundé |
108.1 MID50 ⁄ mL | DENV-2 (D2S32; Bangkok) | Epidemic | 17.2–59.7 | [74] | ||||
| Yaoundé, Douala, Tibati and Bénoué National Park | 107 FFU/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 70.8–100 | 58.8–100 | 0–50 | 14,21 | [75] | ||
| Kenya | Kilifi, Nairobi | 105.08 PFU/ml | DENV-2 (008/01/2012; Mandera) | Epidemic | 6.8–21 | 7.02–42.9 | 7,14,21 | [66] | ||
| Gabon | Franceville | 108.2 MID50/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 52.0, 69.6 | 14 | [76] | |||
| Nigeria | Lagos | 0 .001 PFU/mL | DENV-2 (NGC; New Guinea) | Epidemic | 37–56 | [63] | ||||
| Burkina Faso | Kari | 0 .001 PFU/mL | DENV-2 (NGC; New Guinea) | Epidemic | 0 | [63] | ||||
| Ghana | Hohoe, Accra, Larabanga, Jirapa | 1×106 FFU/mL | DENV-1 (/NIID100/2014; Saitama) | 15.4–75.9 | 0–90.9 | 0 | 7,14 | [69] | ||
| 1×106 FFU/mL | DENV-2 (F299/2017; Ghana) | 0–30.2 | 0–12.5 | |||||||
| Republic of Congo | Brazzaville | 107 ffu/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 77.3–95.8 | 88.2–95.6 | 36.4 | 14,21 | [75] | |
| Aedes (Stegomyia) albopictus | Madagascar, | Anamakia, Antsiranana, Joffreville | 108.2 MID50/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 33.3–93.0 | xx | xx | 14 | [79] |
| Gabon | Libreville | 108 MID50 ⁄ mL | DENV-2 (D2S32; Bangkok) | Epidemic | 13, 21.4 | 14 | [77] | |||
| Cameroon | 12 localities including Yaoundé and Douala | 108.1 MID50/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 13.3–47.5 | 14 | [74] | |||
| Yaoundé, Douala, Tibati | 14,21 | [75] | ||||||||
| Republic of Congo | Brazzaville | 107 ffu/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 14,21 | [75] | ||||
| La Reunion Island | 10 localities including La Marine, Sainte Marie, Saint Pierre |
108.2 MID50/mL | DENV-2 (D2S32; Bangkok) | Epidemic | 18–52 | 14 | [78] | |||
| Ae. (Diceromyia) furcifer | Senegal | Kedougou | 6.5–9.2 TCID50/mL | DENV-2 (NGC; New Guinea and 1349; Burkina Faso) | Epidemic | 58–94 | 22–75 | 14 | [70] | |
| Kedougou | 7.0–8.0 TCID50/mL | DENV-2 (PM33974; Guinea and DakAr2022; Burkina Faso) | Sylvatic | 26–97 | 0–48 | |||||
| Kedougou | 1.6 × 106 | DENV-4 (Haiti73; Haiti) | Epidemic | 88.5 | 84.6 | 0 | [67] | |||
| 3.1 × 106 | DENV-3 (Carec 01–11828; Barbados) | Epidemic | 86.6 | 77.8 | 0 | |||||
| South Africa | Mica | 6.8–7.1 Log10 MID50/mL | DENV-1 (Cassim; Durban) | 0–3 | 0 | 15,17 | [80] | |||
| Mica, Ndumu | 8.2–8.4 Log10 MID50/mL | DENV-2 (BC 5007; Taipei) | 9, 17 | 50 | 15–18 | |||||
| Ae. (Stegomyia) luteocephalus | Senegal | Kedougou | 5.5–8.2 TCID50/mL | DENV-2 (NGC; New Guinea and 1349; Burkina Faso) | Epidemic | 0–89 | 50 | [70] | ||
| 5.5–9.2 TCID50/mL | DENV-2 (PM33974; Guinea and DakAr2022; Burkina Faso) | Sylvatic | 58–79 | 13–27 | ||||||
| 1.4 × 106 PFU/mL | DENV-4 (SH 38549; Dakar) | Epidemic | 77.3 | 72.7 | 4.5 | 7,15 | [67] | |||
| Ae. (Diceromyia) taylori | Kedougou | 4.9 × 106 PFU/mL | DENV-1 (SH 29177; Bandia) | Epidemic | 68 | 64 | 0 | [67] | ||
| 3.5 × 106 PFU/mL | DENV-3 (S-162 TvP-3622; Somalia) | Epidemic | 76.7 | 76.7 | 6.7 | 7,15 | ||||
| 2.6 × 107 PFU/mL | DENV-4 (SH 38549; Dakar) | Epidemic | 83.9 | 77.4 | 0 | |||||
| Ae. (Stegomyia) streliziae | South Africa | Palm Beach | 7.1 | DENV-1 (Cassim; Durban) | Epidemic | 33 | 0 | 12,14 | [80] | |
| Palm Beach, Armadale | 7.1–7.8 | DENV-2 (BC 5007; Taipei) | Epidemic | 54, 60 | 29 | 14–16 | ||||
| Ae. (Aedimorphus) vittatus | Senegal | Kedougou | 5.8 TCID50/mL | DENV-2 (NGC; New Guinea) | Epidemic | 0 | 14 | [70] | ||
| 6.5–8.8 TCID50/mL | DENV-2 (PM33974; Guinea and DakAr2022; Burkina Faso) | Sylvatic | 6 and 19 | 60, 100 | ||||||
TCID50/mL: 50% tissue culture infectious doses; MID50/mL: 50% of mosquito infectious dose for Ae. aegypti; PFU/ml: Plaque formed unit; ffu/ml: Foci-Formed unit; Inf: infection rate; Diss: Dissemination rate; Trans: transmission rate; DPE: days post exposure when mosquitoes were tested.
Populations of Ae. aegypti from Nigeria (Lagos) and Burkina Faso (Kari) infected with a DENV-2 (New Guinea c strain) by intrathoracic inoculation were able to transmit the virus to suckling mice [63]. The first study by Diallo et al. [70] showed a low susceptibility of two populations of Ae. aegypti from Senegal to an epidemic and sylvatic DENV-2 strains. In this study, the infection and dissemination rates ranged between 0-25% and 67–100%, respectively. The low susceptibility of six populations of Ae. aegypti from Senegal, from different bioclimatic zones, to DENV-2 (a sylvatic and an epidemic strain) was confirmed later by Diallo et al. [64]. After 14 days post exposure, they found that these populations of Ae. aegypti had low infection rates (0–26%) and dissemination rates ranging between 10 and 100%. The authors did not find geographic variations of the vector competence of Ae. aegypti for DENV-2 in this study. The low infection rates obtained during these studies have been explained, among others, by the virus titer used and/or the genetic variability of populations used [64]. Later, 11 populations of Senegalese Ae. aegypti were challenged with a DENV-2 strain from Jamaica, and showed a northwest-southeast cline of susceptibility [65]. The main finding of these authors was that the northwestern Ae. aegypti aegypti populations had higher disseminated infection rates than the southeastern Ae. aegypti formosus populations. These results were discordant with those of a following study using a DENV-2 strain isolated from Ae. luteocephalus from Senegal to test 8 populations of Ae. aegypti collected across Senegal. Indeed, all these populations, including those from the southeastern part of the country, showed high infection and dissemination rates [71] highlighting the role that the viral isolate plays in vector competence. After the first DENV-3 outbreak that occurred in Dakar, Senegal, in 2009, the ability of Ae. aegypti populations from Dakar and Kedougou to transmit DENV-1 and 3 was evaluated [72]. The results showed low susceptibility to DENV-3 but high infection and dissemination rates with DENV-1. However, the oral DENV doses used were low and transmission potential was not tested. This study was followed by a larger vector competence study using several mosquito species including one sylvatic and 2 urbans Ae. aegypti populations from Senegal for DENV-1, DENV-3 and DENV-4 using experimental oral infection [67]. The virus was detected in the saliva of all three populations. These results indicated that these Ae. aegypti populations are more susceptible to DENV-3 than the other serotypes.
A study of Ae. aegypti populations from Nairobi and Kilifi, in Kenya, showed that 12.6 % of the 1117 mosquitoes tested with DENV-2 were infected. The population from Nairobi had a significantly higher infection rate with 16.8% of the mosquitoes with a detectable midgut infection. Mosquito infection rates were higher in high temperatures for both populations, indicating an impact of temperature in the Ae. aegypti susceptibility to DENV-2 [66]. In Cabo Verde, the susceptibility of Ae. aegypti population from Santiago Island showed moderate susceptibility for DENV-3 and low susceptibility for DENV-2 [73]. This Santiago population was later challenged with the four serotypes of DENV and tested for infection, dissemination and transmission after 7, 14, and 21 days post-infection. They showed high vector competence for DENV-2 and 3 and a low susceptibility to the other serotypes [68]. The vector competence of four populations of Ae. aegypti (one urban, one semi-urban and two rural) from Ghana were tested for DENV-1 and 2 isolated from human in Japan for DENV-1 and Ghana for DENV-2 [69]. The urban and semi-urban populations were more susceptible to DENV-1 (45 and 41%, respectively) compared to DENV-2 (4 and 3%, respectively) while the rural populations were refractory to both DENV serotypes tested. The highest dissemination rate (92% at 14 dpi) was observed in the urban population for DENV-1. The other dissemination rates for DENV-1 were 29% in the semi-urban, 27 and 0% in the rural populations. None of the populations disseminated the DENV-2 strain. Around 35% of the urban population and 20% of the semi-urban population that disseminated the DENV-1 had infectious saliva at 14 dpi.
The oral susceptibility of 19 populations of Ae. aegypti from Cameroon to a DENV-2 virus strain isolated from a human sample in Bangkok, Thailand, showed statistically different disseminated infection rates ranging from 17.2 to 59.7% [74]. Later, 3 populations of Ae. aegypti from rural and urban localities of Cameroon and one population of the Republic of Congo (Brazzaville) orally infected with the same DENV-2 strain, showed a competence to transmit the virus [75]. A population of Ae. aegypti from Franceville, Gabon, orally tested using the same DENV-2 strain, showed after 14 days post exposure, infection rates of 52 and 69.6% for the F2 and F3 generations, respectively [76].
Several populations of Ae. albopictus from Gabon, La Reunion Island, Madagascar, Republic of Congo, and Cameroon were infected with a DENV-2 virus strain isolated from a human sample in Bangkok, Thailand. Two populations from the city Libreville, Gabon, showed low dissemination rates of 13 and 21.4% [77]. Ten populations from La Reunion Island showed variables infection rates ranging between 18 and 59% [78]. These infection rates varied geographically and were higher in the east coast of the island, while the disseminated infection rates of the 12 populations from Cameroon that ranged between 13.3 and 47.5% and were comparable [74]. Populations of Ae. albopictus collected from Cameroon (3 populations) and the Republic of Congo (1 population from Brazzaville) in 2017–2018, orally infected with a DENV-2 strain from Bangkok, were all able to transmit the virus [75]. Three populations from Madagascar showed susceptibility to the virus ranging between 33.3 and 93% [79].
The first vector competence study on sylvatic DENV vectors from Senegal showed high susceptibility of Ae. furcifer (infection rates: 26–97%; dissemination rates: 0–75%) and Ae. luteocephalus (infection rates: 0–89%; dissemination rates: 13–50%) to infection by the sylvatic and epidemic DENV-2 strains [70]. Ae. vittatus also showed low infection (0–19%) but high dissemination rates (60–100%) to DENV-2 during this study. Following the first DENV-3 outbreak in Senegal in 2009, another study tested the vector competence of Ae. furcifer (for DENV-3 and 4), Ae. taylori (for DENV-1, 3 and 4) and Ae. luteocephalus (for DENV-4) populations from Senegal [67]. The infection and dissemination rates of Ae. furcifer varied, between 86.6 to 88.5% and 77.8–84.6%, respectively. Like Ae. furcifer, the infection and dissemination rates of the other sylvatic vectors were high for all serotypes tested. Only Ae. luteocephalus (for DENV-4; 4.5%) and Ae. taylori (for ENV-3; 6.7%) transmitted the virus among sylvatic species tested. Populations Ae. furcifer and Ae. streliziae from South Africa were susceptible to DENV-1 and 2 with head squash infection rates varying between 0 and 17% for Ae. furcifer and 33–60% for Ae. streliziae [80]. The transmission rates were 0–50% for Ae. furcifer and 0–29% for Ae. streliziae.
Thus, vector competence data indicate great variability in the susceptibility of mosquito vectors to DENVs. This variability has been observed between populations of different geographic origins, and for the same population with different viral strains. The influence of the temperature on Ae. aegypti vector competence to DENVs was also showed [66].
5. Vector control
Very little data is available on vector control efforts during outbreaks in Africa. Vector control during dengue outbreaks in Africa relay on several interventions including insecticides spraying, sources reduction and larviciding [81]. Insecticide spraying in and around houses of dengue positive cases has been widely used during dengue outbreaks in Senegal, Cabo Verde and Côte d’Ivoire. Massive space spraying of insecticides outdoors in the neighborhood or entire city of positive cases has been the most widely used control method used in Senegal, Mauritania, Cabo Verde and Côte d’Ivoire. The susceptibility status of the targeted vector populations to the used insecticides were generally not studied [81]. Insecticides susceptibly tests should be always done before insecticide spraying operations [82]. To the best of our knowledge, larviciding using abate and larvivorous fishes to control dengue vectors were only used in Cabo Verde. Domiciliary visits with social sensitization associated with the removal of breeding sites were implemented during the 2018 dengue outbreak in Fatick and Touba, Senegal. The effectiveness of these interventions has never been evaluated.
6. Knowledge gaps and prospects for the future
Nationwide distribution, vector competence and insecticides susceptibilities status of Ae. aegypti and other dengue vectors remain largely unknown for most African countries. These questions deserve urgent attention of the medical entomology community while urban dengue epidemics number and frequency are increasing in Africa.
It is also essential to study the bio-ecology of mosquito populations, in particular Aedes populations in urban areas in Africa. Most of the data currently available has been obtained in the framework of epidemic investigations [27, 46]. However, these investigations are punctual and usually take place at times when the dynamics and diversity of the vectors do not necessarily reflect the situation that caused the epidemic. A simplified dichotomous morphological key of dengue vectors is needed for the rapid identification of these species in the field. This key will allow scientists involved in dengue studies and mosquito control personal to more rapidly characterize mosquito populations, assess the epidemic risk, and respond efficiently to dengue outbreaks.
It would also be very useful to investigate the spatial distribution of vectors and the environmental and socio-economic risk factors associated with epidemic dynamics in order to propose targeted, efficient and sustainable control strategies [83]. The control strategies usually implemented during epidemics have never been monitored and evaluated. New innovative control strategies need to be developed and evaluated. These strategies should ideally use a package of low-cost technologies developed in Africa. These technologies must be produced and maintained at local and national levels. Strategies must be easy to understand, culturally acceptable and not labor intensive to implement by local communities. Finally, the efficacy and efficiency of these strategies must be regularly monitored and evaluated by public health authorities.
There is very little data available on other Aedes species present in urban areas in Africa that could play an important role in dengue transmission. With the progressive urbanization and gradual destruction of the forests, which are the natural habitats of the sylvatic vectors, it is essential to examine the evolution of these sylvatic Aedes to understand how they will adapt to the urban environment. Until now, DENV-2 is the main serotype detected in the forest environment in Africa [53]. The possibility of DENV-1, 3, and 4 to invade the forest environment and establish sylvatic cycles is unknown and should be investigated. Adaptation of sylvatic Aedes into a more urban environment and DENV-1,3 and 4 to the sylvatic environment could complicate the epidemiology of dengue fever in Africa and result in more frequent outbreaks. Finally, it is essential to study the vector competence of the Ae. aegypti, Ae. albopictus and all Aedes species populations in all African cities where they are abundantly present for all dengue serotypes from Africa, Asia and the Americas.
7. Concluding remarks
The objective of this paper was to review available data on dengue vectors in Africa. Evidences incriminated Ae. aegypti as a dengue vector in the urban setting in several countries in Africa (Senegal, Nigeria, Burkina Faso, Cabo Verde, Tanzania, and Kenya), while Ae. albopictus was only incriminated in Gabon. The sylvatic cycle of DENV, involving mainly arboreal mosquitoes and non-human primates, was described for DENV-2 in Nigeria, Senegal, Côte d’Ivoire and Burkina Faso. Several Aedes species (Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. vittatus, Ae. africanus, Ae. opok, Ae. cumminsii, Ae. dalzieli, and Ae. aegypti) were associated with DENV in the sylvatic environment. Detection of the virus from male mosquitoes and adults collected as immatures during field studies in some countries suggested that DENV is probably maintained in nature by vertical transmission.
Vector competence studies done with sylvatic and domestic Ae. aegypti populations (from Senegal, Cabo Verde, Kenya, Gabon and Cameroon) showed great variability in the susceptibility of these populations. This variability was geographic origin, viral serotypes, and temperature dependent. Several populations of Ae. albopictus from Gabon, La Reunion Island, and Cameroon infected with a DENV-2 virus showed variables susceptibilities. Vector competence studies on sylvatic DENV vectors from Senegal, showed high susceptibility of Ae. furcifer and Ae. luteocephalus to infection. Ae. vittatus also showed low infection but high dissemination rates to DENV-2. The infection and dissemination rates of Ae. taylori were also high for all serotypes tested.
Data on distribution, vector competence and insecticide susceptibilities status of Ae. aegypti and other dengue vectors are lacking for most African countries. These questions need to be investigated. Specifically, studies on the bio-ecology of Aedes species in urban areas are needed. A simplified dichotomous key of dengue vectors will be helpful for that purpose. The other questions that need to be investigated include environmental and socio-economic risk factors associated with dengue vectors dynamics, control strategies and adaptation of vectors and viral serotypes to new environments.
Declarations
Author contribution statement
Diawo Diallo, Babacar Diouf, Alioune Gaye, El hadji NDiaye, Ndeye Marie Sene, Ibrahima Dia and Mawlouth Diallo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo C., Zhou Z., Wen Z., Liu Y., Zeng C., Xiao D., et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 2017;7:317. doi: 10.3389/fcimb.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SHARP Tyler M. Unveiling the burden of dengue in Africa [Internet] https://blogs.cdc.gov/publichealthmatters/2015/07/unveiling-the-burden-of-dengue-in-africa/ blogs.cdc.gov/publichealthmatters. [cited 2021 May 5]. Available from:
- 4.Cornet M. 39–47. World Health Organization; Geneva: 1993. (Dengue in Africa. Epidemiology of Dengue and Dengue Hemorrhagic Fever Monograph on Dengue/dengue Hemorrhagic Fever). [Google Scholar]
- 5.Saleh A.S., Hassan A., Scott R.M., Mellick P.W., Oldfield E.C., Podgore J.K. Dengue in north-East Africa. Lancet. 1985;2:211–212. doi: 10.1016/s0140-6736(85)91521-1. [DOI] [PubMed] [Google Scholar]
- 6.Hyams K.C., Oldfield E.C., Scott R.M., Bourgeois A.L., Gardiner H., Pazzaglia G., et al. Evaluation of febrile patients in Port Sudan, Sudan: isolation of dengue virus. Am. J. Trop. Med. Hyg. 1986;35:860–865. doi: 10.4269/ajtmh.1986.35.860. [DOI] [PubMed] [Google Scholar]
- 7.Rodier G.R., Gubler D.J., Cope S.E., Cropp C.B., Soliman A.K., Polycarpe D., et al. Epidemic dengue 2 in the city of Djibouti 1991-1992. Trans. R. Soc. Trop. Med. Hyg. 1996;90:237–240. doi: 10.1016/s0035-9203(96)90228-x. [DOI] [PubMed] [Google Scholar]
- 8.Kanesa-thasan N., Iacono-Connors L., Magill A., Smoak B., Vaughn D., Dubois D., et al. Dengue serotypes 2 and 3 in US forces in Somalia. Lancet. 1994;343:678. doi: 10.1016/s0140-6736(94)92678-6. [DOI] [PubMed] [Google Scholar]
- 9.Carey D.E., Causey O.R., Reddy S., Cooke A.R. Dengue viruses from febrile patients in Nigeria, 1964-68. Lancet. 1971;1:105–106. doi: 10.1016/s0140-6736(71)90840-3. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez J.P., Du Saussay C., Gautun J.C., McCormick J.B., Mouchet J. Dengue in Burkina Faso (ex-Upper Volta): seasonal epidemics in the urban area of Ouagadougou. Bull. Soc. Pathol. Exot. Filiales. 1985;78:7–14. [PubMed] [Google Scholar]
- 11.Gubler D.J., Sather G.E., Kuno G., Cabral J.R. Dengue 3 virus transmission in Africa. Am. J. Trop. Med. Hyg. 1986;35:1280–1284. doi: 10.4269/ajtmh.1986.35.1280. [DOI] [PubMed] [Google Scholar]
- 12.Boisier P., Morvan J.M., Laventure S., Charrier N., Martin E., Ouledi A., et al. [Dengue 1 epidemic in the grand comoro island (federal islamic republic of the comores). March-may 1993] Ann. Soc. Belg. Med. Trop. 1994;74:217–229. [PubMed] [Google Scholar]
- 13.Mwanyika G.O., Mboera L.E., Rugarabamu S., Ngingo B., Sindato C., Lutwama J.J., et al. Dengue virus infection and associated risk factors in Africa: a systematic review and meta-analysis. Viruses. 2021;13:536. doi: 10.3390/v13040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . World Health Organization. Regional Office for Africa; 2021. Weekly Bulletin on Outbreak and Other Emergencies: Week 19: 03-09 May 2021. [Google Scholar]
- 15.Otu A., Ebenso B., Etokidem A., Chukwuekezie O. Dengue fever–an update review and implications for Nigeria, and similar countries. Afr. Health Sci. 2019;19:2000–2007. doi: 10.4314/ahs.v19i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaenisch T., Junghanss T., Wills B., Brady O.J., Eckerle I., Farlow A., et al. Dengue expansion in Africa—not recognized or not happening? Emerg. Infect. Dis. 2014;20 doi: 10.3201/eid2010.140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis E.M., Neatherlin J.C., Delorey M., Ochieng M., Mohamed A.H., Mogeni D.O., et al. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woyessa A.B., Mengesha M., Kassa W., Kifle E., Wondabeku M., Girmay A., et al. The first acute febrile illness investigation associated with dengue fever in Ethiopia, 2013: a descriptive analysis. Ethiop. J. Health Dev. 2014;28:155–161. [Google Scholar]
- 19.Mboera L.E.G., Mweya C.N., Rumisha S.F., Tungu P.K., Stanley G., Makange M.R., et al. The risk of dengue virus transmission in dar es Salaam, Tanzania during an epidemic period of 2014. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massangaie M., Pinto G., Padama F., Chambe G., da Silva M., Mate I., et al. Clinical and epidemiological characterization of the first recognized outbreak of dengue virus-type 2 in Mozambique, 2014. Am. J. Trop. Med. Hyg. 2016;94:413–416. doi: 10.4269/ajtmh.15-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridde V., Agier I., Bonnet E., Carabali M., Dabiré K.R., Fournet F., et al. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): research and public health implications. Infect. Dis. Povert. 2016;5:23. doi: 10.1186/s40249-016-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarnagda Z., Cissé A., Bicaba B.W., Diagbouga S., Sagna T., Ilboudo A.K., et al. Dengue fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018;24:170–172. doi: 10.3201/eid2401.170973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leroy E.M., Nkoghe D., Ollomo B., Nze-Nkogue C., Becquart P., Grard G., et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill S.C., Neto de Vasconcelos J., Granja B.G., Thézé J., Jandondo D., Neto Z., et al. Early genomic detection of cosmopolitan genotype of dengue virus serotype 2, Angola, 2018. Emerg. Infect. Dis. 2019;25:784–787. doi: 10.3201/eid2504.180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautret P., Simon F., Hervius Askling H., Bouchaud O., Leparc-Goffart I., Ninove L., et al. Dengue type 3 virus infections in European travellers returning from the Comoros and Zanzibar. Euro Surveill. 2010;15:19541. February-April 2010. [PubMed] [Google Scholar]
- 26.Franco L., Di Caro A., Carletti F., Vapalahti O., Renaudat C., Zeller H., et al. Recent expansion of dengue virus serotype 3 in West Africa. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 27.Faye O., Ba Y., Faye O., Talla C., Diallo D., Chen R., et al. Urban epidemic of dengue virus serotype 3 infection, Senegal, 2009. Emerg. Infect. Dis. 2014;20:456–459. doi: 10.3201/eid2003.121885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Regional Office for Africa . 2018. Weekly Bulletin on Outbreaks and Other Emergences.http://apps.who.int/iris/bitstream/handle/10665/275620/OEW43-2026102018.pdf [Internet]. [cited 2021 Apr 1]. Available from: [Google Scholar]
- 29.Caron M., Grard G., Paupy C., Mombo I.M., Bikie Bi Nso B., Kassa Kassa F.R., et al. First evidence of simultaneous circulation of three different dengue virus serotypes in Africa. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz E., Meltzer E., Mendelson M., Tooke A., Steiner F., Gautret P., et al. Detection on four continents of dengue fever cases related to an ongoing outbreak in Luanda, Angola, March to May 2013. Euro Surveill. 2013;18 [PubMed] [Google Scholar]
- 31.Dieng I., Cunha M dos P., Diagne M.M., Sembène P.M., Zanotto PM. de A., Faye O., et al. Origin and spread of the dengue virus type 1, genotype V in Senegal, 2015–2019. Viruses. 2021;13:57. doi: 10.3390/v13010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diallo D., Diagne C.T., Hanley K.A., Sall A.A., Buenemann M., Ba Y., et al. Larval ecology of mosquitoes in sylvatic arbovirus foci in southeastern Senegal. Parasites Vectors. 2012;5:286. doi: 10.1186/1756-3305-5-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diallo D., Dia I., Diagne C.T., Gaye A., Diallo M. In: Chikungunya and Zika Viruses. Higgs S., Vanlandingham D.L., Powers A.M., editors. Elsevier; Amsterdam, The Netherlands: 2018. Emergences of chikungunya and Zika in Africa; pp. 87–133. [Google Scholar]
- 34.Simard F., Nchoutpouen E., Toto J.C., Fontenille D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005;42:726–731. doi: 10.1093/jmedent/42.5.726. [DOI] [PubMed] [Google Scholar]
- 35.Kamgang B., Happi J.Y., Boisier P., Njiokou F., Herve J.P., Simard F., et al. Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med. Vet. Entomol. 2010;24:132–141. doi: 10.1111/j.1365-2915.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 36.Diouf B., Sene N.M., Ndiaye E.H., Gaye A., Ngom E.H.M., Gueye A., et al. Resting behavior of blood-fed females and host feeding preferences of Aedes aegypti (Diptera: Culicidae) morphological forms in Senegal. J. Med. Entomol. 2021 doi: 10.1093/jme/tjab111. [DOI] [PubMed] [Google Scholar]
- 37.Kamgang B., Nchoutpouen E., Simard F., Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasites Vectors. 2012;5:1–4. doi: 10.1186/1756-3305-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawley W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- 39.Diallo D., Diallo M. Resting behavior of Aedes aegypti in southeastern Senegal. Parasites Vectors. 2020;13:356. doi: 10.1186/s13071-020-04223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sintayehu D.W., Tassie N., De Boer W.F. Present and future climatic suitability for dengue fever in Africa. Infect. Ecol. Epidemiol. 2020;10:1782042. doi: 10.1080/20008686.2020.1782042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baba M., Saron M.-F., Vorndam A., Adeniji J., Diop O., Olaleye D. Dengue virus infections in patients suspected of malaria/typhoid in Nigeria. J. Am. Sci. 2009;5:129–134. [Google Scholar]
- 42.Fagbami A.H., Fabiyi A. Epidemiology of dengue infections in Nigeria: virus isolations and clinical observations, 1972-1975. J. Trop. Med. Hyg. 1976;79:226–228. [PubMed] [Google Scholar]
- 43.Dieng I., Hedible B.G., Diagne M.M., El Wahed A.A., Diagne C.T., Fall C., et al. Mobile laboratory reveals the circulation of dengue virus serotype I of Asian origin in medina gounass (guediawaye), Senegal. Diagnostics. 2020;10:408. doi: 10.3390/diagnostics10060408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert V., Lhuillier M., Meunier D., Sarthou J.L., Monteny N., Digoutte J.P., et al. [Yellow fever virus, dengue 2 and other arboviruses isolated from mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and epidemiological considerations] Bull. Soc. Pathol. Exot. 1993;86:90–100. [PubMed] [Google Scholar]
- 45.Guedes D.R.D., Gomes E.T.B., Paiva M.H.S., Melo-Santos MAV de, Alves J., Gómez L.F., et al. Circulation of DENV2 and DENV4 in Aedes aegypti (Diptera: Culicidae) mosquitoes from Praia, Santiago island, Cabo Verde. J. Insect Sci. 2017;17 doi: 10.1093/jisesa/iex057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kone B.A., Konan L.K., Fofana D., Koffi A.F., Coulibaly D., Benie J.B.V. Entomological evaluation of the risk of spread of the dengue 3 epidemic in the health district of Cocody (Abidjan, Côte d’Ivoire) Int. J. Mosq. Res. 2018;5:19–24. [Google Scholar]
- 47.Lutomiah J., Barrera R., Makio A., Mutisya J., Koka H., Owaka S., et al. Dengue outbreak in Mombasa city, Kenya, 2013-2014: entomologic investigations. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elaagip A., Alsedig K., Altahir O., Ageep T., Ahmed A., Siam H.A., et al. Seroprevalence and associated risk factors of Dengue fever in Kassala state, eastern Sudan. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphrey J.M., Cleton N.B., Reusken C.B.E.M., Glesby M.J., Koopmans M.P.G., Abu-Raddad L.J. Dengue in the Middle East and north Africa: a systematic review. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caron M., Paupy C., Grard G., Becquart P., Mombo I., Nso B.B.B., et al. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin. Infect. Dis. 2012;55:e45–53. doi: 10.1093/cid/cis530. [DOI] [PubMed] [Google Scholar]
- 51.Metselaar D., Grainger C., Oei K., Reynolds D., Pudney M., Leake C., et al. An outbreak of type 2 dengue fever in the Seychelles, probably transmitted by Aedes albopictus (Skuse) Bull. World Health Organ. 1980;58:937. [PMC free article] [PubMed] [Google Scholar]
- 52.Teles F.R. Update on dengue in Africa. Dengue Bull. 2011;35:35–51. [Google Scholar]
- 53.Diallo M., Ba Y., Sall A.A., Diop O.M., Ndione J.A., Mondo M., et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999-2000: entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 2003;9:362–367. doi: 10.3201/eid0903.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornet M., Chateau R., Valade M., Dieng P., Raymond H., Lorand A. Données bio-écologiques sur les vecteurs potentiels du virus amaril au Sénégal oriental. Rôle des différentes espéces dans la transmission du virus. Cah ORSTOM, Entomol. Med. Parasitol. 1978;16:315–541. [Google Scholar]
- 55.Cornet M., Robin Y., Chateau R., Hème G., Adam C., Valade M., et al. Isolements d’arbovirus au Sénégal oriental a partir de moustiques (1972–1977) et notes sur l’épidémiologie des virus transmis par les Aedes, en particulier du virus amaril. Cah ORSTOM. Entomol. Med. Parasitol. 1979;17:149–163. [Google Scholar]
- 56.Diallo D., Sall A.A., Buenemann M., Chen R., Faye O., Diagne C.T., et al. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in southeastern Senegal. PLoS Neglected Trop. Dis. 2012;6:e1649. doi: 10.1371/journal.pntd.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore D.L., Causey O.R., Carey D.E., Reddy S., Cooke A.R., Akinkugbe F.M., et al. Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann. Trop. Med. Parasitol. 1975;69:49–64. doi: 10.1080/00034983.1975.11686983. [DOI] [PubMed] [Google Scholar]
- 58.Robin Y., Cornet M., Heme G., Le Gonidec G. Isolement du virus de la dengue au Sénégal. Ann. Virol. (Paris) 1980;131:149–154. [Google Scholar]
- 59.Roche J., Cordellier R., Hervy J., Digoutte J., Monteny N. Isolement de 96 souches de virus dengue 2 à partir de moustiques capturés en Cote-d’Ivoire et Haute-Volta. Ann. Virol. (Paris) 1983;143:233–244. [Google Scholar]
- 60.Fabiyi A. Arboviruses in the Mediterranean Zbl Bakt Suppl. Vol. 9. 1980. Epidemiology of arbovirus zoonoses in west, east, central and South Africa; pp. 215–218. [Google Scholar]
- 61.Agwu J.E., Clement I., Igbinosa I.B. Detection of yellow fever and dengue viruses in mosquitoes between 2014 and 2015 in bayelsa and benue states of Nigeria. Acta Entomol. Serbica. 2019;24:59–78. [Google Scholar]
- 62.Cornet M., Saluzzo J., Hervy J.-P., Digoutte J., Germain M., Chauvancy M., et al. Dengue 2 au Sénégal oriental: une poussée épizootique en milieu selvatique: isolements du virus à partir de moustiques et d’un singe et considérations épidémiologiques. Cah ORSTOM, Entomol. Med. Parasitol. 1984;22:313–323. [Google Scholar]
- 63.Jousset F.-X. Geographic Aedes aegypti strains and dengue-2 virus: susceptibility, ability to transmit to vertebrate and transovarial transmission. Ann. Virol. (Paris) 1981;132(3):357–370. [Google Scholar]
- 64.Diallo M., Ba Y., Faye O., Soumare M.L., Dia I., Sall A.A. Vector competence of Aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans. R. Soc. Trop. Med. Hyg. 2008;102:493–498. doi: 10.1016/j.trstmh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Sylla M., Bosio C., Urdaneta-Marquez L., Ndiaye M., Black WC th. Gene flow, subspecies composition, and dengue virus-2 susceptibility among Aedes aegypti collections in Senegal. PLoS Neglected Trop. Dis. 2009;3:e408. doi: 10.1371/journal.pntd.0000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chepkorir E., Lutomiah J., Mutisya J., Mulwa F., Limbaso K., Orindi B., et al. Vector competence of Aedes aegypti populations from Kilifi and Nairobi for dengue 2 virus and the influence of temperature. Parasites Vectors. 2014;7:435. doi: 10.1186/1756-3305-7-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaye A., Wang E., Vasilakis N., Guzman H., Diallo D., Talla C., et al. Potential for sylvatic and urban Aedes mosquitoes from Senegal to transmit the new emerging dengue serotypes 1, 3 and 4 in West Africa. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.da Moura A.J.F., de Melo Santos M.A.V., Oliveira C.M.F., Guedes D.R.D., de Carvalho-Leandro D., da Cruz Brito M.L., et al. Vector competence of the Aedes aegypti population from Santiago Island, Cape Verde, to different serotypes of dengue virus. Parasites Vectors. 2015;8:114. doi: 10.1186/s13071-015-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amoa-Bosompem M., Kobayashi D., Itokawa K., Murota K., Faizah A.N., Azerigyik F.A., et al. Determining vector competence of Aedes aegypti from Ghana in transmitting dengue virus serotypes 1 and 2. Parasites Vectors. 2021;14:228. doi: 10.1186/s13071-021-04728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diallo M., Sall A.A., Moncayo A.C., Ba Y., Fernandez Z., Ortiz D., et al. Potential role of sylvatic and domestic African mosquito species in dengue emergence. Am. J. Trop. Med. Hyg. 2005;73:445–449. [PubMed] [Google Scholar]
- 71.Dickson L.B., Sanchez-Vargas I., Sylla M., Fleming K., Black W.C. Vector competence in West African Aedes aegypti is Flavivirus species and genotype dependent. PLoS Neglected Trop. Dis. 2014;8:e3153. doi: 10.1371/journal.pntd.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaye A., Faye O., Diagne C.T., Faye O., Diallo D., Weaver S.C., et al. Oral susceptibility of Aedes aegypti (Diptera: Culicidae) from Senegal for dengue serotypes 1 and 3 viruses. Trop. Med. Int. Health. 2014;19:1355–1359. doi: 10.1111/tmi.12373. [DOI] [PubMed] [Google Scholar]
- 73.Vazeille M., Yébakima A., Lourenço-de-Oliveira R., Andriamahefazafy B., Correira A., Rodrigues J.M., et al. Oral receptivity of Aedes aegypti from Cape Verde for yellow fever, dengue, and chikungunya viruses. Vector Borne Zoonotic Dis. 2013;13:37–40. doi: 10.1089/vbz.2012.0982. [DOI] [PubMed] [Google Scholar]
- 74.Paupy C., Ollomo B., Kamgang B., Moutailler S., Rousset D., Demanou M., et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in central Africa. Vector Borne Zoonotic Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 75.Kamgang B., Vazeille M., Tedjou A.N., Wilson-Bahun T.A., Yougang A.P., Mousson L., et al. Risk of dengue in Central Africa: vector competence studies with Aedes aegypti and Aedes albopictus (Diptera: Culicidae) populations and dengue 2 virus. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vazeille-Falcoz M., Failloux A.B., Mousson L., Elissa N., Rodhain F. [Oral receptivity of Aedes aegypti formosus from Franceville (Gabon, central Africa) for type 2 dengue virus] Bull. Soc. Pathol. Exot. 1999;92:341–342. [PubMed] [Google Scholar]
- 77.Vazeille M., Moutailler S., Pages F., Jarjaval F., Failloux A.-B. Introduction of Aedes albopictus in Gabon: what consequences for dengue and chikungunya transmission? Trop. Med. Int. Health. 2008;13:1176–1179. doi: 10.1111/j.1365-3156.2008.02123.x. [DOI] [PubMed] [Google Scholar]
- 78.Paupy C., Girod R., Salvan M., Rodhain F., Failloux A.B. Population structure of Aedes albopictus from La Réunion Island (Indian Ocean) with respect to susceptibility to a dengue virus. Heredity. 2001;87:273–283. doi: 10.1046/j.1365-2540.2001.00866.x. [DOI] [PubMed] [Google Scholar]
- 79.Vazeille M., Mousson L., Rakatoarivony I., Villeret R., Rodhain F., Duchemin J.B., et al. Population genetic structure and competence as a vector for dengue type 2 virus of Aedes aegypti and Aedes albopictus from Madagascar. Am. J. Trop. Med. Hyg. 2001;65:491–497. doi: 10.4269/ajtmh.2001.65.491. [DOI] [PubMed] [Google Scholar]
- 80.Jupp P.G., Kemp A. The potential for dengue in South Africa: vector competence tests with dengue 1 and 2 viruses and 6 mosquito species. Trans. R. Soc. Trop. Med. Hyg. 1993;87:639–643. doi: 10.1016/0035-9203(93)90271-q. [DOI] [PubMed] [Google Scholar]
- 81.Dia I., Diagne C.T., Ba Y., Diallo D., Konate L., Diallo M. Insecticide susceptibility of Aedes aegypti populations from Senegal and Cape Verde Archipelago. Parasites Vectors. 2012;5:238. doi: 10.1186/1756-3305-5-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sene N.M., Mavridis K., Ndiaye E.H., Diagne C.T., Gaye A., Ngom E.H.M., et al. Insecticide resistance status and mechanisms in Aedes aegypti populations from Senegal. PLoS Neglected Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chadee D.D. Resting behaviour of Aedes aegypti in Trinidad: with evidence for the re-introduction of indoor residual spraying (IRS) for dengue control. Parasites Vectors. 2013;6:255. doi: 10.1186/1756-3305-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Traore-Lamizana M., Zeller H., Monlun E., Mondo M., Hervy J.P., Adam F., et al. Dengue 2 outbreak in southeastern Senegal during 1990: virus isolations from mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1994;31:623–627. doi: 10.1093/jmedent/31.4.623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.