Highlights
-
•
Among subjects who underwent hysterectomy for endometrial cancer, 19% received adjuvant chemotherapy.
-
•
Platinum and taxane based chemotherapy is the most commonly used adjuvant chemotherapy.
-
•
Platinum and taxane combination therapy was used in 48% of patients at first recurrence.
Abstract
Objective
The objective of this study was to describe patterns of utilization of cytotoxic, hormonal, and immunotherapy in patients with endometrial cancer in the adjuvant setting and at the time of first recurrence.
Methods
We identified patients in the IBM MarketScan database with endometrial cancer who underwent hysterectomy from 2011 to 2019. The use of clinically relevant therapeutic agents and combination regimens was determined in the adjuvant setting and at the time of first recurrence.
Results
A total of 22,632 patients were identified. Of these, 7,147 patients (31.6%) received adjuvant radiation and 4,381 (19.4%) received adjuvant chemotherapy following surgery. Of those who received adjuvant chemotherapy, the most commonly utilized agents were carboplatin (90.3%), paclitaxel (85.8%), cisplatin (9.4%), docetaxel (9.3%), gemcitabine (3.8%), and doxorubicin (2.0%), while bevacizumab was utilized in 1.5% of patients. A combination of platinum and a taxane were utilized as adjuvant therapy in 88.8% of women. Of the cohort, 1,825 patients (8.1%) recurred, of whom 1,017 patients had already received adjuvant chemotherapy. The median time from hysterectomy to initiation of chemotherapy for recurrence was 13.3 months (IQR: 7.6–23.1 months). Overall, platinum and taxane combination therapy was used in 788 (46.8%) of patients with recurrent disease, platinum alone or with other drugs in 194 (11.5%), taxanes alone or with other drugs in 145 (8.6%), and non-platinum and non-taxane based therapy in 31.3%.
Conclusions
Among patients with endometrial cancer who underwent hysterectomy, platinum-taxane combination chemotherapy was used in almost 90% of patients who received adjuvant chemotherapy while nearly 70% of patients who recurred were treated with platinum or taxane based therapy at first recurrence.
1. Introduction
Endometrial cancer is the most common gynecologic malignancy in the United States (Siegel et al., 2021). Most patients with endometrial cancer have stage I disease at presentation, and surgery with hysterectomy is recommended for primary treatment. For patients with high intermediate risk tumors based on pathologic factors, adjuvant therapy (radiation and/or chemotherapy) is often recommended (Keys et al., 2004, Creutzberg et al., 2000, Nout et al., 2010, Randall et al., 2019, de Boer et al., 2018). While a number of cytotoxic agents and combination regimens have been evaluated, carboplatin and paclitaxel is generally considered the preferred chemotherapy regimen for patients receiving adjuvant therapy (Mustea et al., 2013, Jutzi et al., 2013, de Boer et al., 2016).
The recurrence rate for patients with early stage endometrial cancer is approximately 15%, and for most patients, the disease recurs within three years of initial treatment (Fung-Kee-Fung et al., 2006, Tjalma et al., 2004, Morrow et al., 1991). The median survival for patients with recurrent or advanced disease is poor, approximately 12 months (Obel et al., 2006). Recurrence may be treated with radiation, systemic therapy, or both, depending on the site and extent of recurrence and previous treatments. Carboplatin plus paclitaxel is often used first in the recurrent setting, given a response rate of 40–67% and the lower toxicity compared to other regimens (Fleming et al., 2004, Cella et al., 2010, Miller et al., 2020, Sovak et al., 2007, Pectasides et al., 2008, Sorbe et al., 2008). A number of other chemotherapy agents, including immunotherapeutic and targeted therapeutic options, have been studied in the advanced and recurrent setting; in general there are limited data to recommend use of these alternative regimens, with the exception of recent data supporting the approval of lenvatinib in combination with pembroluzimab for advanced endometrial cancer (Makker et al., 2022).
To date, there is little data examining patterns of chemotherapy use in either the adjuvant or recurrent setting for patients with endometrial cancer. This is of particular importance as treatment paradigms are rapidly shifting. The objective of our study was to describe the patterns of utilization of cytotoxic, hormonal, and immunotherapy in patients with endometrial cancer in the adjuvant setting and at the time of first recurrence.
2. Materials and methods
2.1. Data source
The IBM MarketScan Research Databases were used for analysis. The MarketScan commercial insurance database includes health data from approximately 350 payers in all 50 states, and captures claims from around 50 million patients prior to 2015, and around 30 million patients annually since 2015 (Hansen, 2017) For commercially insured patients with Medicare supplemental plans, claims partially covered by Medicare are included in the MarketScan datasets, while claims covered entirely by Medicare may not be included. The Medicaid database contains healthcare service utilization of Medicaid beneficiaries covered under fee-for-service and managed care plans in 12 geographically dispersed states.
The MarketScan database captures information on patient-level clinical services across inpatient and outpatient settings and pharmaceutical claims. It provides longitudinal data to track patients’ medical services and prescriptions of medications over time. The database contains de-identified data and was determined to be non-human subjects research by the Columbia University Institutional Review Board.
2.2. Study population
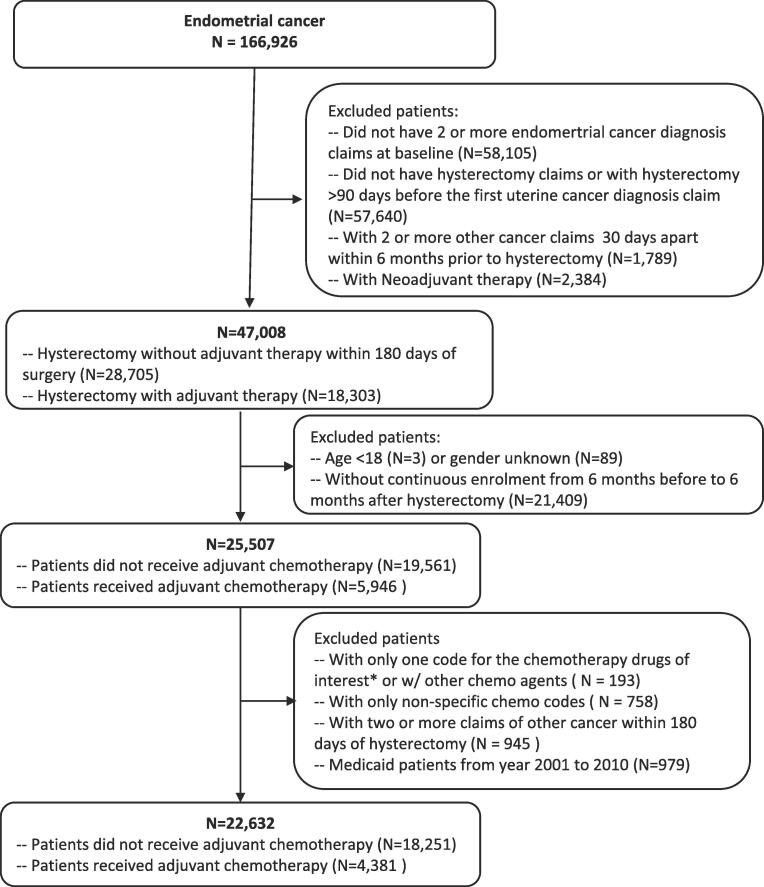
Patients aged 18 years or older with endometrial cancer, diagnosed from January 1, 2011 to December 31, 2019, were included in the analysis. To capture incident cases of endometrial cancer, the analysis was limited to patients who underwent hysterectomy and had at least two billing claims for endometrial cancer (Supplemental Table 1). Patients who did not have continuous health insurance and drug coverage from 6 months before to 6 months after hysterectomy were excluded. Neoadjuvant therapy was defined as the presence of chemotherapy or radiation claims within 180 days before hysterectomy, and patients with neoadjuvant therapy were excluded. Patients with two or more claims for other cancer diagnoses during the period from 6 months before to 6 months after hysterectomy were excluded (Fig. 1). Each patient was followed longitudinally from the time of hysterectomy until the maximum of insurance enrollment.
Fig. 1.
Cohort selection flow chart for patients with commercial insurance and Medicaid insurance.
2.3. Adjuvant chemotherapy and radiation
Chemotherapeutic agents were identified by matching generic drug names and National Drug Codes (NDCs) to the MarketScan RED BOOK, which provides specific drug dosing concentration (mg/unit) and route of administration. HCPCS codes were used to capture chemotherapy agents administered intravenously. Non-specific chemotherapy administration was captured by ICD-9-CM and ICD-10-CM and Current Procedural Terminology (CPT) codes.
Adjuvant chemotherapy was defined as the occurrence of at least two claims for chemotherapy on differing days within 6 months after hysterectomy. The chemotherapy agents of interest included drugs that were utilized by more than 2% of patients (including carboplatin, paclitaxel, cisplatin, docetaxel, gemcitabine) and bevacizumab. The combination of chemotherapy agents was classified into four sub-groups: platinum-taxanes for patients receiving both carboplatin or cisplatin and paclitaxel or docetaxel including with other agents; platinum with or without non-taxane drugs including carboplatin or cisplatin alone or with any other non-taxane drug; taxanes with or without non-platinum drugs including paclitaxel or docetaxel alone or with any other non-platinum drug; and non-platinum and non-taxane drugs. The duration of adjuvant chemotherapy was defined as the period from the start of chemotherapy to the end of the same chemotherapy agent combination. Patients were censored at the last date of continuous insurance enrollment.
Adjuvant radiation was defined as the identification of specific or non-specific radiation claims identified by ICD and/or CPT codes, within 180 days of hysterectomy. The combination of adjuvant chemotherapy and radiation was classified as receipt of both, receipt of chemotherapy alone, receipt of radiation alone, and none.
2.4. First-line chemotherapy for recurrence
Recurrence was defined based on the introduction of a new therapeutic agent or a gap of greater than 3 months from adjuvant therapy for patients who received adjuvant chemotherapy. For those who did not receive adjuvant chemotherapy, recurrence was defined as initiation of chemotherapy more than 180 days after hysterectomy. First line recurrence chemotherapy agents with utilization rates of >2% or clinically relevant drugs including carboplatin, paclitaxel, docetaxel, bevacizumab, gemcitabine, doxorubicin, cisplatin, topotecan, pembrolizumab, tamoxifen, megestrol acetate, ifosfamide, letrozole, cyclophosphamide, methotrexate, anastrozole, and lenvatinib were analyzed. The median number of months from hysterectomy to initiation of chemotherapy for recurrence was noted for each patient.
Since platinum therapy and taxanes are often used concomitantly in early settings, treatment combinations for first line recurrence were classified into the same four sub-groups as adjuvant chemotherapy, including platinum-taxanes, platinum with or without non-taxane drugs, taxanes with or without non-platinum drugs, and non-platinum and non-taxane drugs. The non-platinum and non-taxane drugs group was further classified as hormonal therapy if a patient received tamoxifen, megestrol acetate, letrozole, anastrozole, or exemestane; cytotoxic therapy if a patient received doxorubicin, gemcitabine, methotrexate, 5-fluorouracil, cyclophosphamide, ifosfamide, or topotecan; immunotherapy if a patient received pembrolizumab; and targeted therapy if a patient received bevacizumab or lenvatinib. To be consistent with the algorithm used in defining adjuvant chemotherapy, the treatment combinations for first line recurrence were limited to the patients who had claims on at least two separate days within three months of initiation to treatment of the recurrence.
2.5. Covariates and statistical analysis
Patients were stratified into two groups based on health insurance coverage: commercial insurance or Medicaid. Patient demographics at the time of surgery, including year of hysterectomy, age at surgery (<40, 40–49, 50–59, 60–69, ≥70 years), and Elixhauser comorbidity score (0, 1, ≥2) were recorded (Moore et al., 2017). Information on race and ethnicity (White, Black, Hispanic, other/unknown) was only available for Medicaid patients, and geographic information including metropolitan statistical area (yes, no, unknown) and region (northeast, north central, south, west, unknown) were only available for commercially insured patients. Patients’ procedure year, age at surgery, and comorbidity score were compared by health insurance status using chi-square tests.
Adjuvant chemotherapy and chemotherapy for first recurrence are presented descriptively by health insurance. Patterns of first line chemotherapy use were further stratified by receipt of adjuvant chemotherapy. Chi-square tests were utilized for two group comparisons. A time to event analysis was used to calculate the cumulative recurrence rate at different follow-up time points by adjuvant chemotherapy setting. Separate analyses of first line chemotherapy use stratified by insurance status are also presented. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). All hypothesis tests were two-sided. A p-value of <0.05 was considered statistically significant.
3. Results
A total of 22,632 patients were identified, including 94.2% (21,327) with commercial insurance and 5.8% (1,305) with Medicaid coverage. The median age at the time of surgery was 59.0 years (IQR 54.0–64.0 years). Patients with Medicaid coverage were slightly younger. Within the Medicaid cohort, 56.6% of patients were White, 23.3% Black, and 1.8% Hispanic (Supplemental Table 2). Compared to commercially insured patients, Medicaid beneficiaries were more likely to have a comorbidity score ≥ 2 (62.2% vs. 27.4%, P < 0.0001) and were less likely to receive adjuvant radiation (24.4% vs. 32.0%, P < 0.0001) and adjuvant chemotherapy (14.7% vs. 19.6%, P < 0.0001) (Table 1).
Table 1.
Demographics and clinical factors of endometrial cancer patients who underwent hysterectomy stratified by health insurance coverage.
| Total | Commercially insured patients | Medicaid patients | P-values* | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Total No. of patients | 22,632 | 21,327 | 1305 | |
| Hysterectomy year | <0.0001 | |||
| 2011 | 1689 (7.5) | 1625 (7.6) | 64 (4.9) | |
| 2012 | 3182 (14.1) | 3109 (14.6) | 73 (5.6) | |
| 2013 | 2942 (13.0) | 2869 (13.5) | 73 (5.6) | |
| 2014 | 2949 (13.0) | 2782 (13.0) | 167 (12.8) | |
| 2015 | 2745 (12.1) | 2552 (12.0) | 193 (14.8) | |
| 2016 | 2691 (11.9) | 2460 (11.5) | 231 (17.7) | |
| 2017 | 2256 (10.0) | 2047 (9.6) | 209 (16) | |
| 2018 | 2180 (9.6) | 1974 (9.3) | 206 (15.8) | |
| 2019 | 1998 (8.8) | 1909 (9.0) | 89 (6.8) | |
| Age at surgery (years) | <0.0001 | |||
| Median [IQR] years | 59.0 [54.0–64.0] | 60.0 [54.0–64.0] | 55.0 [47.0–61.0] | |
| <40 | 784 (3.5) | 647 (3.0) | 137 (10.5) | |
| 40–49 | 2533 (11.2) | 2268 (10.6) | 265 (20.3) | |
| 50–59 | 8106 (35.8) | 7603 (35.7) | 503 (38.5) | |
| 60–69 | 8158 (36.1) | 7792 (36.5) | 366 (28.0) | |
| 60–64 | 6193 (27.4) | 5868 (27.5) | 325 (24.9) | |
| 65–69 | 1965 (8.7) | 1924 (9.0) | 41 (3.1) | |
| ≥70 | 3051 (13.5) | 3017 (14.2) | 34 (2.6) | |
| Race | NA | |||
| White | NA | NA | 738 (56.6) | |
| Black | NA | NA | 304 (23.3) | |
| Hispanic | NA | NA | 24 (1.8) | |
| Other/Unknown | NA | NA | 239 (18.3) | |
| Metropolitan statistics area | NA | |||
| Non-MSA | 2908 (12.8) | 2908 (13.6) | NA | |
| MSA | 17,064 (75.4) | 17,064 (80.0) | NA | |
| Unknown | 2660 (11.8) | 1355 (6.4) | NA | |
| Region | NA | |||
| Northeast | 4850 (21.4) | 4850 (22.7) | NA | |
| North central | 5620 (24.8) | 5620 (26.4) | NA | |
| South | 7489 (33.1) | 7489 (35.1) | NA | |
| West | 3213 (14.2) | 3213 (15.1) | NA | |
| Unknown | 1460 (6.5) | 155 (0.7) | NA | |
| Elixhauser comorbidity score | <0.0001 | |||
| 0 | 10,502 (46.4) | 10,241 (48.0) | 261 (20) | |
| 1 | 5481 (24.2) | 5249 (24.6) | 232 (17.8) | |
| ≥2 | 6649 (29.4) | 5837 (27.4) | 812 (62.2) | |
| Adjuvant radiation | <0.0001 | |||
| No | 15,485 (68.4) | 14,499 (68.0) | 986 (75.6) | |
| Yes | 7147 (31.6) | 6,828 (32.0) | 319 (24.4) | |
| Adjuvant chemotherapy | <0.0001 | |||
| No | 18,251 (80.6) | 17,138 (80.4) | 1113 (85.3) | |
| Yes | 4381 (19.4) | 4,189 (19.6) | 192 (14.7) | |
| Adjuvant chemotherapy and radiation | <0.0001 | |||
| Both | 3723 (16.5) | 3,276 (15.4) | 148 (11.3) | |
| Chemotherapy alone | 957 (4.2) | 913 (4.3) | 44 (3.4) | |
| Radiation alone | 3723 (16.5) | 3,552 (16.7) | 171 (13.1) | |
| None | 14,528 (64.2) | 13,586 (63.7) | 942 (72.2) |
Commercially insured patients included patients with or without Medicare supplemental plan. For patients with Medicare supplemental plan, claims partially covered by Medicare were included in the analysis, while claims covered 100% by Medicare were not included in the analysis.
Definition of adjuvant radiation: Patients who had any radiation codes, specific or non-specific, within 180 days after hysterectomy.
Definition of adjuvant chemotherapy: Patients with at least two claims of chemotherapy agents within 6 months of surgery.
P-values calculated from Chi-Square test.
A total of 7,147 patients (31.6%) received adjuvant radiation, 4,381 (19.4%) received adjuvant chemotherapy, and 3,723 (16.5%) received both (Table 1). Of those who received adjuvant chemotherapy, the most commonly utilized agents were carboplatin (90.3%) and paclitaxel (85.8%) (Table 2). Other agents utilized included cisplatin (9.4%), docetaxel (9.3%), gemcitabine (3.8%), and doxorubicin (2.0%), while bevacizumab was utilized in 1.5% of patients. A combination of a platinum and a taxane was utilized as adjuvant therapy in 88.8% of patients who received adjuvant chemotherapy while non-platinum/non-taxane therapy was utilized in 0.6% of these patients. Platinum-based therapy alone or with a non-taxane agent was utilized in 6.6% of the cohort, while 4.0% of the cohort received taxane-based therapy alone or with non-platinum agents. The median duration of adjuvant chemotherapy use was 3.6 months (IQR 2.6–4.7 months). Overall, the patterns of adjuvant chemotherapy use were similar among patients with commercial insurance and those with Medicaid.
Table 2.
Adjuvant chemotherapy in women with newly diagnosed endometrial cancer.
| Total | Commercially insured patients | Medicaid patients | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total Patients | 4381 | 4189 | 192 |
| Drugs of interest | |||
| Carboplatin | 3958 (90.3) | 3,792 (90.5) | 166 (86.5) |
| Paclitaxel | 3758 (85.8) | 3,592 (85.8) | 166 (86.5) |
| Cisplatin | 411 (9.4) | 393 (9.4) | 18 (9.4) |
| Docetaxel | 409 (9.3) | 391 (9.3) | 18 (9.4) |
| Gemcitabine | 165 (3.8) | 159 (3.8) | 6 (3.1) |
| Doxorubicin | 89 (2.0) | 88 (2.1) | 1 (0.5) |
| Bevacizumab | 66 (1.5) | 62 (1.5) | 4 (2.1) |
| Combination patterns | |||
| Platinum-taxanes | 3892 (88.8) | 3,726 (88.9) | 166 (86.5) |
| Platinum with or without non-taxanes drugs | 287 (6.6) | 270 (6.4) | 17 (8.6) |
| Taxanes with or without non-platinum drugs | 174 (4.0) | 166 (4.0) | 8 (4.2) |
| Non-platinum and non-taxane drugs | 28 (0.6) | 27 (0.6) | 1 (0.5) |
| Duration of adjuvant chemotherapy (month) | |||
| Median (IQR) | 3.6 (2.6 – 4.7) | 3.6 (2.8 – 4.7) | 3.5 (2.2 – 4.7) |
| ≤1 month | 149 (3.4) | 141 (3.4) | 8 (4.2) |
| 1.1–3 months | 1053 (24.0) | 1,002 (23.9) | 51 (26.6) |
| 3.1–6 months | 2599 (59.3) | 2,495 (59.6) | 104 (54.2) |
| 6.1–9 months | 251 (5.7) | 243 (5.8) | 8 (4.2) |
| 9.1–12 months | 126 (2.9) | 118 (2.8) | 8 (4.2) |
| >12 months | 203 (4.6) | 190 (4.5) | 13 (5.8) |
Combination patterns:
1. Platinum-taxanes for patients receiving both carboplatin/cisplatin and paclitaxel/docetaxel.
2. Platinum with or without non-taxane drugs include carboplatin/cisplatin alone or carboplatin/cisplatin and any other non-taxane drug.
3. Taxanes with or without non-platinum drugs include paclitaxel/docetaxel alone or paclitaxel/docetaxel with any other non-platinum and non-taxane drug.
4. Non-platinum and non-taxane drugs.
Duration of adjuvant chemotherapy was defined as the start of chemotherapy of interest or non-specific chemotherapy codes to the end of targeted chemo with a 3-months gap or the start of new agent which ever came first. Patients were censored at the last date of maximum continuous enrollment.
Therapy for recurrent endometrial cancer was initiated in 1,825 patients (8.1% of the entire cohort) (Table 3). Chemotherapy for recurrent disease was initiated in 23.2% of those who received adjuvant chemotherapy after initial surgery and in 4.4% of those who did not. The most commonly utilized agents in the recurrent setting were carboplatin (52.4%), paclitaxel (47.8%), doxorubicin (22.0%), bevacizumab (18.3%), gemcitabine (10.6%), docetaxel (9.6%), cisplatin (8.4%), tamoxifen (7.7%), cyclophosphamide (3.7%), megestrol acetate (3.4%), topotecan (3.2%), letrozole (3.1%), methotrexate (3.0%), pembrolizumab (2.7%), and anastrazole (2.7%). Lenvatinib and ifosfamide were utilized in 1.8% and 1.4% of patients, respectively. Overall, platinum and taxane combination therapy was used in 788 (46.8%) patients, platinum-based therapy alone or with a non-taxane drug was used in 194 patients (11.5%), while taxanes alone or with a non-platinum agent were used in 145 (8.6%). Non-platinum/non-taxane cytotoxic therapy was initiated in 281 (16.7%) patients, while 167 (9.9%) were treated with hormonal therapy, and 28 (1.7%) with immunotherapy. The overall platinum re-challenge rate was 14.5% (605/4179) in the overall population, 14.4% (574/3996) in commercially insured patients, and 16.9% (31/183) in Medicaid beneficiaries.
Table 3.
Therapy for first line recurrence in women with endometrial cancer stratified by receipt of adjuvant chemotherapy.
|
Total |
Patients who received adjuvant chemotherapy |
Patients who did not receive adjuvant chemotherapy |
|
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Recurrence rate | 1825 (8.1) | 1017 (23.2) | 808 (4.4) |
| Median months from hysterectomy to 1L recurrence among patients with recurrence [IQR] | 13.3 [7.6–23.1] | 10.2 [6.6–18.0] | 17.7 [10.6–31.6] |
| 1st line recurrence chemotherapy agents# | |||
| Carboplatin | 956 (52.4) | 574 (56.4) | 382 (47.3) |
| Paclitaxel | 873 (47.8) | 487 (47.9) | 386 (47.8) |
| Docetaxel | 176 (9.6) | 93 (9.1) | 83 (10.3) |
| Bevacizumab | 335 (18.4) | 213 (20.9) | 122 (15.1) |
| Gemcitabine | 194 (10.6) | 117 (11.5) | 77 (9.5) |
| Doxorubicin | 401 (22.0) | 288 (28.3) | 113 (14) |
| Cisplatin | 154 (8.4) | 91 (8.9) | 63 (7.8) |
| Topotecan | 59 (3.2) | 52 (5.1) | 7 (0.9) |
| Pembrolizumab | 50 (2.7) | 47 (4.6) | 3 (0.4) |
| Tamoxifen | 141 (7.7) | 56 (5.5) | 85 (10.5) |
| Megestrol acetate | 62 (3.4) | 24 (2.4) | 38 (4.7) |
| Letrozole | 56 (3.1) | 21 (2.1) | 35 (4.3) |
| Cyclophosphamide | 68 (3.7) | 22 (2.2) | 46 (5.7) |
| Methotrexate | 55 (3.0) | 12 (1.2) | 43 (5.3) |
| Anastrozole | 49 (2.7) | 14 (1.4) | 35 (4.3) |
| Combination patterns within 90 days of first line recurrence start* | N = 1,684 | N = 939 | N = 745 |
| Platinum-taxanes | 788 (46.8) | 457 (48.7) | 331 (44.4) |
| Platinum with or without non-taxane drugs | 194 (11.5) | 146 (15.5) | 57 (7.7) |
| Taxanes with or without non-platinum drugs | 145 (8.6) | 73 (7.8) | 79 (10.6) |
| Non-platinum and non-taxane drugs | 527 (31.3) | 263 (28.0) | 278 (37.3) |
| Hormonal therapy | 167 (9.9) | 44 (4.7) | 123 (16.5) |
| Cytotoxic therapy | 281 (16.7) | 164 (17.5) | 117 (15.7) |
| Immunotherapy | 28 (1.7) | 28 (3.0) | 0 (0.0) |
| Targeted therapy | 124 (7.4) | 69 (7.3) | 55 (7.4) |
Notes:
1. Platinum-taxanes include both carboplatin/cisplatin and paclitaxel/docetaxel with or without other non-platinum and non-taxane drugs.
2. Platinums with or without other drugs include carboplatin/cisplatin alone OR carboplatin/cisplatin and any other non-taxane drug.
3. Taxanes with or without other drugs include paclitaxel/docetaxel alone OR paclitaxel/docetaxel w/ any other non-platin drug.
4. Non-platinum and non-taxane drugs (not-mutually exclusive).
Hormonal therapy if tamoxifen OR megestrol OR letrozole OR anastrozole OR exemestane.
Cytotoxic therapy if doxorubicin OR gemcitabine OR methotrexate OR fluorouracil OR cyclophosphamide OR ifosfamide OR topotecan.
Immunotherapy if pembrolizumab.
Targeted therapy if bevacizumab or lenvatinib.
Drugs need to have at least two codes within 90 days of recurrence start dates to be included.
Drugs with a utilization rate ≥ 2% are reported.
For first line recurrence, platinum and taxane therapy were utilized in 48.7% of those who received adjuvant chemotherapy compared to 44.4% of patients who had not received adjuvant chemotherapy (Table 3). Non-platinum, non-taxane based therapy was more common in those who had not received prior adjuvant chemotherapy than those who received adjuvant chemotherapy (37.3% vs. 28.0%), as was hormonal therapy (16.5% vs. 4.7%). Compared to patients with commercial insurance, Medicaid beneficiaries were less likely to receive platinum and taxane based therapy and more likely to receive hormonal therapy (Supplemental Tables 3 and 4). Platinum and taxane based therapy was utilized in 49.6% of patients with commercial insurance who had received adjuvant chemotherapy and in 45.2% of commercially insured patients who had not received adjuvant therapy. The corresponding rates of platinum-taxane use were 34.0% and 33.3%, respectively, among Medicaid recipients.
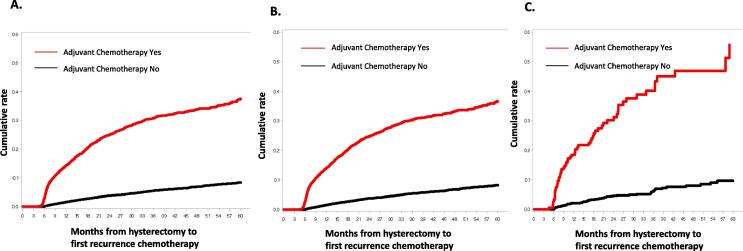
The median time from hysterectomy to initiation of chemotherapy for recurrence was 13.3 months (IQR 7.6 to 23.1 months). Patients who received adjuvant chemotherapy initiated chemotherapy for recurrent disease at a median of 10.2 months after hysterectomy compared to 17.7 months for those who had not received adjuvant chemotherapy (Table 3). A time to event analysis for patients based on receipt of adjuvant chemotherapy demonstrated that 14.2% of patients treated with adjuvant chemotherapy compared to 1.5% of those who had not received adjuvant chemotherapy initiated chemotherapy for recurrence by 12 months after hysterectomy (Fig. 2A). At 24 months, the corresponding rates of treatment for first line recurrence were 25.0% vs. 3.8%, respectively, while the rates by 36 months were 30.7% vs. 5.4% at 36 months, respectively.
Fig. 2.
Cumulative rate of recurrence by adjuvant chemotherapy setting in overall cohort (A), commercially insured = cohort (B), and Medicaid cohort (C).
In an analysis of the Medicaid cohort that included race, the patterns of adjuvant chemotherapy use were similar among Black and White patients (Supplemental Table 5). Among patients treated for recurrence, use of platinum-taxane therapy was most common, with similar rates of use among Black and White patients. However, White patients were more likely to receive hormonal therapy, and Black patients were more likely to receive taxanes alone or with other non-platinum drugs (Supplemental Table 6).
4. Discussion
Among patients with endometrial cancer who underwent hysterectomy between 2011 and 2019, 19% of patients received adjuvant chemotherapy and the majority (89%) were treated with a combination of a platinum and a taxane. At the time of first recurrence, platinum and taxane combination therapy was used in 48% of patients, while non-platinum/non-taxane therapy was initiated in 32% of patients, with cytotoxic therapy being more commonly used compared to hormonal and immunotherapy options.
Treatment for advanced stage and recurrent endometrial cancer has gradually evolved over time. Historically, doxorubicin was considered the most active agent for endometrial cancer and early trials utilizing single agent doxocubicin demonstrated response rates of approximately 20–25% (Thigpen et al., 1994, Aapro et al., 2003). Subsequent trials added cisplatin and paclitaxel to doxorubicin and noted improved response rates (as high as 57%), as well as higher progression-free and overall survival rates (Fleming et al., 2004, Aapro et al., 2003, Thigpen et al., 2004). A recent cooperative group trial (GOG 209) established the non-inferiority of carboplatin plus paclitaxel (TC) compared to doxorubicin, carboplatin, and paclitaxel (TAP) with lower toxicity, establishing TC as the standard first-line chemotherapy in advanced and recurrent endometrial cancer (Miller et al., 2020). It should be noted, however, that GOG 209 excluded patients who had previously received chemotherapy.
In the current analysis, platinum and taxane therapy was the most commonly utilized regimen at the time of first recurrence. The next most commonly used agents in this setting were doxorubicin (22.0%) and bevacizumab (18.3%). Phase II studies have shown that bevacizumab is active and well tolerated both as single agent therapy and when used in combination with carboplatin and paclitaxel in the recurrent setting, (Aghajanian et al., 2011, Simpkins et al., 2015, Aghajanian et al., 2018, Lorusso et al., 2019). and the addition of bevacizumab to carboplatin/paclitaxel has shown improved response rate (72% vs 54%) and numerically greater but not statistically significantly improved progression-free survival and overall survival in a recent phase II randomized study (MITO-END) (Lorusso et al., 2019).
Many trials evaluating chemotherapy for recurrent endometrial cancer did not include patients who had previously received chemotherapy, and there remains limited data on standard first-line chemotherapy agent use for recurrence after previous adjuvant chemotherapy. The concept of “platinum sensitivity,” which is popular in ovarian cancer, has been evaluated in a retrospective study in endometrial cancer (Nagao et al., 2013). This multicenter retrospective cohort study demonstrated higher progression-free and overall survival for patients with platinum-free intervals of greater than 12 months. While retrospective analyses have been prognostic, there are no randomized clinical trials to support this concept for endometrial cancer, and the clinical utility has not been defined, mostly because of lack of agents with significant activity in endometrial cancer (Moore et al., 2010, Matoda et al., 2014). Our data suggest that platinum-based chemotherapy is the most commonly used treatment in the recurrent setting, especially when there has been a longer interval since initial adjuvant treatment. In our cohort, platinum and taxane combination therapy was the most frequently used regimen for patients with recurrent endometrial cancer, regardless of whether they had been treated with adjuvant chemotherapy. However, there was substantial variability in patterns of care in this setting and a number of patients received other cytotoxic agents or hormonal therapy.
We identified notable disparities in patterns of care for recurrent endometrial cancer based on insurance status. Compared to patients with commercial insurance, Medicaid beneficiaries were less likely to receive platinum and taxane based therapy and more likely to receive hormonal therapy. Similarly, among Medicaid beneficiaries there were differences between Black and White patients in chemotherapy selection. Racial and insurance related disparities in endometrial cancer have previously been well documented and also appear to extend to selection of adjuvant and recurrent therapy (Olson et al., 2012, Wright et al., 2009, Bregar et al., 2017, Long et al., 2013). Similarly, we noted differences in patterns of care for patients with endometrial cancer based on race. Further elucidating the mechanisms driving this disparity is clearly needed.
Molecular characterization of endometrial cancer is advancing rapidly with the recent emergence of a number of new immunotherapeutic and targeted therapeutic options for patients with advanced and recurrent endometrial cancer (Arend et al., 2018). Recent data have demonstrated a role for immune checkpoint inhibitor therapy in this setting. In the current analysis, 25 patients (1.5%) with recurrent disease were treated with immunotherapy. A phase II study evaluating the role of pembrolizumab, an anti-programmed death 1 (PD-1) monoclonal antibody, included patients with previously treated, advanced microsatellite instability-high or mismatch repair-deficient (MSI-H/dMMR) tumors, including endometrial cancer; the results of this study demonstrated durable antitumor activity (objective response rate of 48%) and manageable toxicity (O'Malley et al., 2022). A more recent phase III trial looking at the combination of pembroluzimab and lenvatinib, a multiple kinase inhibitor, in advanced endometrial cancer demonstrated improved progression-free and overall survival with lenvatinib in combination with pembroluzimab compared with physician’s choice of treatment (doxorubicin or paclitaxel) (Makker et al., 2022). This combination regimen is now approved by the FDA for patients with advanced endometrial carcinoma who have disease progression following prior systemic therapy, and should be considered for patients in this setting. As these agents have only recently gained regulatory approval, the majority of patients in our analysis were treated with cytotoxic chemotherapy. Future studies with more contemporary data will be important in evaluating the uptake and outcomes of these new therapeutic regimens.
Our study has a number of limitations. As with any claims-based data, there may have been under coding of some of the chemotherapeutics of interest. Similarly, use of non-specific chemotherapy administration codes limited our ability to discern the specific agents utilized in a small number of women. Our algorithm to identify incident endometrial cancer may have not captured a small number of patients with newly diagnosed tumors. Second, MarketScan does not contain a number of important oncologic variables (histology, grade, stage), patient characteristics, and molecular markers that are now used to guide treatment. We acknowledge that these factors undoubtedly influenced treatment decision making and outcomes, and additional studies that can further adjust for these covariates are clearly needed. Third, based on the structure of Marketscan, the majority of patients included were commercially insured. As such, the data presented may not be generalizable to the overall population of patients with endometrial cancer which is older and more heavily represented with Medicare.
In conclusion, this study demonstrates that among patients with endometrial cancer who underwent hysterectomy, platinum-taxane combination chemotherapy was used in nearly 90% of patients as adjuvant therapy while nearly 70% of patients were treated with platinum or taxane based therapy at first recurrence. There is limited data to guide chemotherapy agent selection for recurrent endometrial cancer, and further research is warranted to determine the best regimen based on specific patient and tumor characteristics.
Author contributions
Knisely: analysis of data, manuscript drafting, review and editing, final approval of manuscript.
Huang: study design, analysis of data, manuscript drafting, review and editing, final approval of manuscript.
Li: study design, analysis of data, manuscript review and editing, final approval of manuscript.
Prabhu: study design, analysis of data, manuscript review and editing, final approval of manuscript.
Wright: study design, analysis of data, manuscript drafting, review and editing, final approval of manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Dr. Wright has received royalties from UpToDate.
Funding for the study was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Drs. Li and Prabhu are employees of Merck.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.101002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aapro M.S., van Wijk F.H., Bolis G., Chevallier B., van der Burg M.E.L., Poveda A., de Oliveira C.F., Tumolo S., Scotto di Palumbo V., Piccart M., Franchi M., Zanaboni F., Lacave A.J., Fontanelli R., Favalli G., Zola P., Guastalla J.P., Rosso R., Marth C., Nooij M., Presti M., Scarabelli C., Splinter T.A.W., Ploch E., Beex L.V.A., ten Bokkel Huinink W., Forni M., Melpignano M., Blake P., Kerbrat P., Mendiola C., Cervantes A., Goupil A., Harper P.G., Madronal C., Namer M., Scarfone G., Stoot J.E.G.M., Teodorovic I., Coens C., Vergote I., Vermorken J.B. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol. 2003;14(3):441–448. doi: 10.1093/annonc/mdg112. [DOI] [PubMed] [Google Scholar]
- Aghajanian C., Sill M.W., Darcy K.M., Greer B., McMeekin D.S., Rose P.G., Rotmensch J., Barnes M.N., Hanjani P., Leslie K.K. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2011;29(16):2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian C., Filiaci V., Dizon D.S., Carlson J.W., Powell M.A., Secord A.A., Tewari K.S., Bender D.P., O'Malley D.M., Stuckey A., Gao JianJiong, Dao F., Soslow R.A., Lankes H.A., Moore K., Levine D.A. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018;150(2):274–281. doi: 10.1016/j.ygyno.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend R.C., Jones B.A., Martinez A., Goodfellow P. Endometrial cancer: Molecular markers and management of advanced stage disease. Gynecol. Oncol. 2018;150(3):569–580. doi: 10.1016/j.ygyno.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Bregar A.J., Alejandro Rauh-Hain J., Spencer R., Clemmer J.T., Schorge J.O., Rice L.W., del Carmen M.G. Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis. Gynecol. Oncol. 2017;145(1):114–121. doi: 10.1016/j.ygyno.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Cella D., Huang H., Homesley H.D., Montag A., Salani R., De Geest K., Lee R., Spirtos N.M. Patient-reported peripheral neuropathy of doxorubicin and cisplatin with and without paclitaxel in the treatment of advanced endometrial cancer: Results from GOG 184. Gynecol. Oncol. 2010;119(3):538–542. doi: 10.1016/j.ygyno.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Creutzberg C.L., van Putten W.LJ., Koper P.CM., Lybeert M.LM., Jobsen J.J., Wárlám-Rodenhuis C.C., De Winter K.AJ., Lutgens L.C., van den Bergh A.CM., van de Steen-Banasik E., Beerman H., van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. The Lancet. 2000;355(9213):1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C., Ottevanger P.B., Ledermann J.A., Khaw P., Colombo A., Fyles A., Baron M.-H., Kitchener H.C., Nijman H.W., Kruitwagen R.F., Nout R.A., Verhoeven-Adema K.W., Smit V.T., Putter H., Creutzberg C.L. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(8):1114–1126. doi: 10.1016/S1470-2045(16)30120-6. [DOI] [PubMed] [Google Scholar]
- de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C., Ottevanger P.B., Ledermann J.A., Khaw P., Colombo A., Fyles A., Baron M.-H., Jürgenliemk-Schulz I.M., Kitchener H.C., Nijman H.W., Wilson G., Brooks S., Carinelli S., Provencher D., Hanzen C., Lutgens L.C.H.W., Smit V.T.H.B.M., Singh N., Do V., D'Amico R., Nout R.A., Feeney A., Verhoeven-Adema K.W., Putter H., Creutzberg C.L., McCormack M., Whitmarsh K., Allerton R., Gregory D., Symonds P., Hoskin P.J., Adusumalli M., Anand A., Wade R., Stewart A., Taylor W., Kruitwagen R.F.P.M., Hollema H., Pras E., Snyers A.n., Stalpers L., Jobsen J.J., Slot A., Mens J.-W., Stam T.C., Van Triest B., Van der Steen - Banasik E.M., De Winter K.A.J., Quinn M.A., Kolodziej I., Pyman J., Johnson C., Capp A., Fossati R., Gribaudo S., Lissoni A.A., Ferrero A., Artioli G., Davidson C., McLachlin C.M., Ghatage P., Rittenberg P.V.C., Souhami L., Thomas G., Duvillard P., Berton-Rigaud D., Tubiana-Mathieu N. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol.. 2018;19(3):295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming G.F., Brunetto V.L., Cella D., Look K.Y., Reid G.C., Munkarah A.R., Kline R., Burger R.A., Goodman A., Burks R.T. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2004;22(11):2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- Fung-Kee-Fung M., Dodge J., Elit L., Lukka H., Chambers A., Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol. Oncol. 2006;101(3):520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- L. Hansen. The Truven Health MarketScan Databases for life sciences researchers. 2017.
- Jutzi L., Hoskins P., Lim P., Aquino-Parsons C., Tinker A., Kwon J.S. The importance of adjuvant chemotherapy and pelvic radiotherapy in high-risk early stage endometrial carcinoma. Gynecol. Oncol. 2013;131(3):581–585. doi: 10.1016/j.ygyno.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Keys H.M., Roberts J.A., Brunetto V.L., Zaino R.J., Spirtos N.M., Bloss J.D., Pearlman A., Maiman M.A., Bell J.G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2004;92(3):744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Long B., Liu F.W., Bristow R.E. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol. Oncol. 2013;130(3):652–659. doi: 10.1016/j.ygyno.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso D., Ferrandina G., Colombo N., Pignata S., Pietragalla A., Sonetto C., Pisano C., Lapresa M.T., Savarese A., Tagliaferri P., Lombardi D., Cinieri S., Breda E., Sabatucci I., Sabbatini R., Conte C., Cecere S.C., Maltese G., Scambia G. Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2 - A randomized phase II trial. Gynecol. Oncol. 2019;155(3):406–412. doi: 10.1016/j.ygyno.2019.10.013. [DOI] [PubMed] [Google Scholar]
- Makker V., Colombo N., Casado Herráez A., Santin A.D., Colomba E., Miller D.S., Fujiwara K., Pignata S., Baron-Hay S., Ray-Coquard I., Shapira-Frommer R., Ushijima K., Sakata J., Yonemori K., Kim Y.M., Guerra E.M., Sanli U.A., McCormack M.M., Smith A.D., Keefe S., Bird S., Dutta L., Orlowski R.J., Lorusso D. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022;386(5):437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoda M., Omatsu K., Yamamoto A., et al. Importance of platinum-free interval in second-line chemotherapy for advanced or recurrent endometrial cancer. Eur. J. Gynaecol. Oncol. 2014;35:224–229. [PubMed] [Google Scholar]
- Miller D.S., Filiaci V.L., Mannel R.S., Cohn D.E., Matsumoto T., Tewari K.S., DiSilvestro P., Pearl M.L., Argenta P.A., Powell M.A., Zweizig S.L., Warshal D.P., Hanjani P., Carney M.E., Huang H., Cella D., Zaino R., Fleming G.F. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209) J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2020;38(33):3841–3850. doi: 10.1200/JCO.20.01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.N., Tian C., McMeekin D.S., Thigpen J.T., Randall M.E., Gallion H.H. Does the progression-free interval after primary chemotherapy predict survival after salvage chemotherapy in advanced and recurrent endometrial cancer?: a Gynecologic Oncology Group ancillary data analysis. Cancer. 2010;116(23):5407–5414. doi: 10.1002/cncr.25480. [DOI] [PubMed] [Google Scholar]
- Moore B.J., White S., Washington R., Coenen N., Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med. Care. 2017;55:698–705. doi: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- Morrow C.P., Bundy B.N., Kurman R.J., Creasman W.T., Heller P., Homesley H.D., Graham J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol. Oncol. 1991;40(1):55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- Mustea A., Koensgen D., Belau A., Sehouli J., Lichtenegger W., Schneidewind L., Sommer H., Markmann S., Scharf J.P., Ehmke M., Ledwon P., Braicu I., Zygmunt M., Koehler G. Adjuvant sequential chemoradiation therapy in high-risk endometrial cancer: results of a prospective, multicenter phase-II study of the NOGGO (North-Eastern German Society of Gynaecological Oncology) CancerChemotherapy Pharmacol. 2013;72(5):975–983. doi: 10.1007/s00280-013-2276-9. [DOI] [PubMed] [Google Scholar]
- Nagao S., Nishio S., Michimae H., Tanabe H., Okada S., Otsuki T., Tanioka M., Fujiwara K., Suzuki M., Kigawa J. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: the SGSG-012/GOTIC-004/Intergroup study. Gynecol. Oncol. 2013;131(3):567–573. doi: 10.1016/j.ygyno.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Nout R.A., Smit V.T., Putter H., Jürgenliemk-Schulz I.M., Jobsen J.J., Lutgens L.C., van der Steen-Banasik E.M., Mens J., Slot A., Kroese M.C.S., van Bunningen B.N., Ansink A.C., van Putten WLJ, Creutzberg C.L. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet (London, England) 2010;375(9717):816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- Obel J.C., Friberg G., Fleming G.F. Chemotherapy in endometrial cancer. Clin. Adv. Hematol. Oncol.: H&O. 2006;4:459–468. [PubMed] [Google Scholar]
- Olson S.H., Atoria C.L., Cote M.L., Cook L.S., Rastogi R., Soslow R.A., Brown C.L., Elkin E.B. The impact of race and comorbidity on survival in endometrial cancer. Cancer Epidemiol. Biomarkers Prev. 2012;21(5):753–760. doi: 10.1158/1055-9965.EPI-11-0735. [DOI] [PubMed] [Google Scholar]
- O'Malley D.M., Bariani G.M., Cassier P.A., Marabelle A., Hansen A.R., De Jesus Acosta A., Miller W.H., Safra T., Italiano A., Mileshkin L., Xu L., Jin F., Norwood K., Maio M. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2022;40(7):752–761. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pectasides D., Xiros N., Papaxoinis G., Pectasides E., Sykiotis C., Koumarianou A., Psyrri A., Gaglia A., Kassanos D., Gouveris P., Panayiotidis J., Fountzilas G., Economopoulos T. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol. Oncol. 2008;109(2):250–254. doi: 10.1016/j.ygyno.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Randall M.E., Filiaci V., McMeekin D.S., von Gruenigen V., Huang H., Yashar C.M., Mannel R.S., Kim J.-W., Salani R., DiSilvestro P.A., Burke J.J., Rutherford T., Spirtos N.M., Terada K., Anderson P.R., Brewster W.R., Small W., Aghajanian C.A., Miller D.S. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2019;37(21):1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Simpkins F., Drake R., Escobar P.F., Nutter B., Rasool N., Rose P.G. A phase II trial of paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma (EMCA) Gynecol. Oncol. 2015;136(2):240–245. doi: 10.1016/j.ygyno.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Sorbe B., Andersson H., Boman K., Rosenberg P., Kalling M. Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel-long-term follow-up. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2008;18(4):803–808. doi: 10.1111/j.1525-1438.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- Sovak M.A., Dupont J., Hensley M.L., Ishill N., Gerst S., Abu-Rustum N., Anderson S., Barakat R., Konner J., Poyner E., Sabbatini P., Spriggs D.R., Aghajanian C. Paclitaxel and carboplatin in the treatment of advanced or recurrent endometrial cancer: a large retrospective study. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2007;17(1):197–203. doi: 10.1111/j.1525-1438.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- Thigpen J.T., Blessing J.A., DiSaia P.J., Yordan E., Carson L.F., Evers C. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 1994;12(7):1408–1414. doi: 10.1200/JCO.1994.12.7.1408. [DOI] [PubMed] [Google Scholar]
- Thigpen J.T., Brady M.F., Homesley H.D., Malfetano J., DuBeshter B., Burger R.A., Liao S. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2004;22(19):3902–3908. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- Tjalma W.A.A., Van Dam P.A., Makar A.P., Cruickshank D.J. The clinical value and the cost-effectiveness of follow-up in endometrial cancer patients. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2004;14(5):931–937. doi: 10.1111/j.1048-891X.2004.014532.x. [DOI] [PubMed] [Google Scholar]
- Wright J.D., Fiorelli J., Schiff P.B., Burke W.M., Kansler A.L., Cohen C.J., Herzog T.J. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–1285. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.