Abstract
Background.
Impaired MFR in the absence of flow-limiting CAD is associated with adverse events. Cardiovascular disease is an important cause of morbidity and mortality in patients with breast cancer. We sought to test the utility of MFR to predict outcomes in a cohort of patients with breast cancer.
Methods.
We retrospectively studied consecutive patients with breast cancer or breast cancer survivors who underwent cardiac stress PET imaging from 2006 to 2017 at Brigham and Women’s Hospital. Patients with a history of clinically overt CAD, LVEF < 45%, or abnormal myocardial perfusion were excluded. Subjects were followed from time of PET to the occurrence of a first major adverse cardiovascular event (MACE) and all-cause death.
Results.
The final cohort included 87 patients (median age 69.0 years, 98.9% female, mean MFR 2.05). Over a median follow-up of 7.6 years after PET, the lowest MFR tertile was associated with higher cumulative incidence of MACE (adjusted subdistribution hazard ratio 4.91; 95% CI 1.68–14.38; p = 0.004) when compared with the highest MFR tertile.
Conclusions.
In patients with breast cancer, coronary vasomotor dysfunction was associated with incident cardiovascular events. MFR may have potential as a risk stratification biomarker among patients with/survivors of breast cancer.
Keywords: Cancer survivorship, Cardio-oncology, Coronary artery calcium, Myocardial flow reserve, Coronary microvascular disease, Positron emission tomography
INTRODUCTION
Coronary vasomotor dysfunction is a manifestation of atherosclerosis affecting the large and small coronary vasculature, which can be present even in the absence of flow-limiting, obstructive epicardial coronary artery disease (CAD).1 Patients with coronary vasomotor dysfunction often present with chest pain, exertional dyspnea, and/or reduced exercise tolerance.2–8 Cardiac stress positron emission tomography (PET) can be used to measure myocardial flow reserve (MFR), defined as the ratio of global stress over rest myocardial blood flow (MBF). In the absence of obstructive epicardial CAD, MFR is a measure of the hemodynamic abnormalities resulting from diffuse nonobstructive atherosclerosis and microcirculatory dysfunction and can therefore be used to identify patients with subclinical coronary vasomotor dysfunction. Independent of other risk factors, coronary vasomotor dysfunction has been shown to be associated with adverse cardiovascular outcomes.3,8–14 However, these studies excluded patients with malignancy.
Patients with breast cancer can have concomitant risk factors for cardiovascular disease and may have been exposed to cardiotoxic therapies including anthracyclines, trastuzumab, and thoracic irradiation, which increases their risk of cardiovascular events.15–18 Both macrovascular and microvascular injury to the endothelium are implicated in cardiotoxicity of cancer therapies, particularly radiation therapy to the chest. Radiation therapy is associated with accelerated atherosclerosis,19 results in vascular endothelial cell damage, and has been linked to reduction in capillary density.20–22 Cardiopulmonary symptoms are common in patients with breast cancer, and survivors are at increased risk of cardiovascular morbidity and mortality.23–26 Therefore, many patients with breast cancer are referred for cardiac stress testing to help guide management decisions.16,27
In this study, we aimed to study if coronary vasomotor dysfunction was a marker of risk even in the absence of clinically overt CAD or left ventricular systolic dysfunction in patients with active or prior breast cancer referred for cardiac PET. We hypothesized that MFR is a biomarker of general vascular health in this population and abnormal MFR would be associated with adverse cardiovascular outcomes.
METHODS
Study Population
The study population included consecutive patients with a diagnosis of breast cancer (prior or currently active at the time of PET) who underwent cardiac PET, including MFR assessment, for evaluation of symptoms (chest pain/dyspnea/syncope/palpitations) or pre-operative assessment between 2006 and 2017 at our center. The cohort was identified using our cardiac PET database and by using ICD-9 and ICD-10 codes to identify patients with breast cancer prior to the date of PET. Patients with a history of clinically overt CAD (defined as a history of myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) surgery), history of end-stage renal disease on dialysis, left ventricular ejection fraction (LVEF) < 45%, or abnormal myocardial perfusion on PET (summed stress score > 2) were excluded. After detailed review of each patient’s longitudinal electronic health record (EHR) (blinded to PET results) to confirm a diagnosis of breast cancer, the final cohort consisted of 87 patients.
Patient demographics, clinical history, and indications for testing were collected prospectively at the time of PET. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL·min−1·1.73 m−2 and anemia was defined as a hematocrit of less than 36%. History of valvular heart disease was defined as at least moderate valvular stenosis or regurgitation, or a history of valve repair or replacement. Detailed review of each patient’s longitudinal EHR was performed retrospectively to obtain breast cancer-related characteristics blinded to PET results. The Mass General Brigham Institutional Review Board approved this study.
Assessment of Coronary Vasomotor Function
Coronary vasomotor function was assessed with quantitative PET imaging, which was performed on a standard hybrid whole-body PET-computed tomography (CT) scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, Wisconsin) with 13N-ammonia or 82Rubidium as flow tracers. Myocardial perfusion images were obtained at rest and in response to vasodilator-stress, as previously described.28 Summed rest, stress, and difference scores were computed.29 Rest LVEF was calculated from gated myocardial perfusion images with commercially available software (Corridor4DM, INVIA Medical Imaging Solutions, Ann Arbor, Michigan). Absolute global MBF (in mL·min−1·g−1 of tissue) was quantified at rest and peak hyperemia using commercial software, as previously described.28 Global MFR was calculated as the ratio of stress to rest MBF. Corrected rest MBF was calculated by normalizing rest MBF by the rate pressure product [(rest MBF/(rest heart rate × rest systolic blood pressure)) × 10,000]. Corrected MFR was calculated as stress MBF/corrected rest MBF.
Coronary Artery Calcium Assessment
The presence and extent of coronary artery calcium (CAC) was assessed using semi-quantitative visual analysis of the low-dose, non-contrast, non-electrocardiogram-gated CT scan obtained for attenuation correction of the PET images.30 Semi-quantitative assessment of CAC was performed by a cardiologist with advanced cardiovascular imaging training for each of the 87 PET/CT scans in a blinded fashion (SD). The degree of CAC was determined to be none, mild, moderate, or severe as previously described by the National Lung Screening Trial investigators.31 This approach was previously deemed comparable to Agatston scoring and strongly associated with cardiovascular death.31
Outcomes
Patients were followed from the time of PET to the occurrence of a first major adverse cardiovascular event (MACE), defined as a composite of cardiovascular death or hospitalization for heart failure, nonfatal MI, or coronary revascularization. Ascertainment of nonfatal MI or heart failure required a discharge note with a primary hospitalization diagnosis of MI and/or heart failure. In addition, only events meeting the 2018 Fourth Universal Definition of MI or defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure, were classified as such.32 Ascertainment of clinical endpoints was determined by blinded adjudication of the EHR, Mass General Brigham Research Patient Data Registry, and the National Death Index. Patient were also followed from the time of PET to all-cause death.
Statistical Analysis
Categorical variables are reported as frequencies with percentage (%). Continuous variables are expressed as mean (± standard deviation) or median (interquartile range (IQR)). We used chi-square and one-way analysis of variance or Kruskal-Wallis to evaluate for differences in categorical and continuous baseline characteristics, respectively, across MFR tertiles. To study the effect of baseline MFR and MFR tertile on incident MACE and account for competing risk of death, univariable Fine and Gray competing risks regression modeling was performed using available covariates. To avoid overfitting the model, demographic and medical history variables (age, sex, symptoms, hypertension, diabetes, hyperlipidemia, smoking history, family history of premature CAD, body mass index (BMI), and estrogen status) were incorporated into the validated Morise clinical risk score for estimating the pre-test probability of CAD (with scores of 0–8, 9–15, and 16–24 indicating low, intermediate, and high pre-test probability of CAD).33 Multivariable adjustment was performed using the Morise score and any covariates not included in the Morise score that had significant univariable association with the outcome. We constructed cumulative incidence curves by MFR tertiles to illustrate time-to-MACE. Differences were tested with the Wald test. Fine and Gray competing risk-adjusted subdistribution hazard functions, with multivariable adjustment using the previously identified covariates, were used to examine the association between cardiovascular events and MFR tertiles.
To study the effect of baseline MFR on all-cause mortality, univariable Cox proportional hazards modeling was performed for adverse event-free survival using available covariates. Multivariable adjustment was not performed as MFR did not have significant univariable association with the outcome. We constructed Kaplan-Meier curves by MFR tertiles to illustrate all-cause survival. Differences were tested with the log-rank test. Graphical methods and Schoenfeld residuals were used to verify that proportional hazards assumptions were met. All tests were 2-sided, and a value of p <0.05 was considered statistically significant. Statistical analysis was performed with the use of Stata version 15.0 (Statacorp, College Station, Texas).
RESULTS
Characteristics of the Study Cohort
Among the 87 patients in the cohort (median age 69.0 years (IQR 59.0–75.8), 98.9% female), 82.8% (n = 72) had cardiovascular symptoms at the time of PET, 63.2% (n = 55) had hypertension, 56.3% (n = 49) had dyslipidemia, 16.1% (n = 14) had diabetes, and 14.9% (n = 13) had chronic kidney disease (Table 1). Additionally, 14.7% (n = 11) of patients had metastatic disease at the time of their breast cancer diagnosis, 21.7% (n = 18) had recurrence of their breast cancer at some point during their course, 94.1% (n = 80) underwent surgery, 65.5% (n = 57) received chest irradiation, 31.0% (n = 27) received chemotherapy, and 46.0% (n = 40) received hormonal therapy. Further baseline and PET characteristics are listed in Table 1.
Table 1.
Baseline and cardiac stress positron emission tomography/computed tomography characteristics of the study cohort
| Total cohort (n = 87) | |
|---|---|
|
| |
| Age at PET, median (IQR) (years) | 69.0 (59.0–75.8) |
| Cardiovascular symptoms present at time of PET | 72 (82.8%) |
| Female | 86 (98.9%) |
| BMI, mean (SD) (kg·m−2) | 29.8 (8.3) |
| Hypertension | 55 (63.2%) |
| Dyslipidemia | 49 (56.3%) |
| Diabetes | 14 (16.1%) |
| Family history of CAD | 15 (17.2%) |
| Chronic kidney disease | 13 (14.9%) |
| Anemia | 20 (23.3%) |
| History of valvular heart disease | 2 (2.3%) |
| History of prior heart failure admission | 2 (2.3%) |
| Current tobacco use | 5 (5.8%) |
| Any tobacco use | 26 (29.9%) |
| Morise score, mean (SD) | 14.3 (3.4) |
| Pre-test probability of CAD by Morise score | |
| Low (0–8 points) | 5 (5.8%) |
| Intermediate (9–15 points) | 46 (52.9%) |
| High (16–24 points) | 36 (41.4%) |
| Breast cancer treatment | |
| Surgery | 80 (94.1%) |
| Thoracic irradiation | 57 (65.5%) |
| Left chest irradiation | 34 (39.1%) |
| Chemotherapy | 27 (31.0%) |
| Anthracyclines | 16 (18.4%) |
| Trastuzumab | 5 (5.8%) |
| Hormonal therapy | 40 (46.0%) |
| Metastatic disease at breast cancer diagnosis | 11 (14.7%) |
| Breast cancer recurrence after initial therapy | 18 (21.7%) |
| Years between breast cancer diagnosis and PET, median (IQR) | 7.9 (3.8–14.9) |
| Coronary artery calcium present | 44 (50.6%) |
| Mild coronary artery calcium present | 24 (27.6%) |
| Moderate or severe coronary artery calcium present | 20 (23.0%) |
| PET tracer | |
| 82Rubidium | 66 (75.9%) |
| 13N-ammonia | 21 (24.1%) |
| Pharmacologic stress agent | |
| Regadenoson | 51 (58.6%) |
| Dipyridamole | 22 (25.3%) |
| Adenosine | 10 (11.5%) |
| Dobutamine | 4 (4.6%) |
| Rest LVEF, mean (SD) (%) | 64.1 (7.5) |
| Stress MBF, mean (SD) (mL·min−1·g−1) | 2.56 (0.96) |
| Rest MBF, mean (SD) (mL·min−1·g−1) | 1.29 (0.48) |
| MFR, mean (SD) | 2.05 (0.57) |
| Corrected rest MBF, mean (SD) (mL·min−1·g−1) | 1.21 (0.45) |
| Corrected MFR, mean (SD) | 2.18 (0.65) |
BMI, body mass index; CAD, coronary artery disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; MBF, myocardial blood flow; MFR, myocardial flow reserve; PET, positron emission tomography; SD, standard deviation.
Coronary Vasomotor Dysfunction and Coronary Artery Calcification
The median time interval between breast cancer diagnosis and PET was 7.9 years (IQR 3.8–14.9). The characteristics of patients by MFR tertile are listed in Table 2. Patients in the lowest MFR tertile had the highest mean BMI (33.0 kg·m−2 ± 9.9), and a greater proportion of patients with hypertension (86%), diabetes (34%), and anemia (41%).
Table 2.
Characteristics of patients by myocardial flow reserve tertile
| MFR < 1.71 (n = 29) | 1.71 ≤ MFR ≤ 2.24 (n = 29) | MFR > 2.24 (n = 29) | p value | |
|---|---|---|---|---|
|
| ||||
| Age at PET, median (IQR) (years) | 70.5 (63.1–75.0) | 71.0 (64.0–76.7) | 62.0 (58.2–71.5) | 0.14 |
| Cardiovascular symptoms present at time of PET | 24 (83%) | 24 (93%) | 24 (83%) | 1.00 |
| Female | 28 (97%) | 29 (100%) | 29 (100%) | 1.00 |
| BMI, mean (SD) (kg·m−2) | 33.0 (9.9) | 27.8 (8.2) | 28.5 (5.4) | 0.034 |
| Hypertension | 25 (86%) | 17 (59%) | 13 (45%) | 0.003 |
| Dyslipidemia | 20 (69%) | 13 (45%) | 16 (55%) | 0.20 |
| Diabetes | 10 (34%) | 2 (7%) | 2 (7%) | 0.01 |
| Current tobacco use | 0 (0%) | 1 (3%) | 4 (14%) | 0.12 |
| Any tobacco use | 10 (34%) | 4 (14%) | 12 (41%) | 0.058 |
| Family History of CAD | 3 (10%) | 3 (10%) | 9 (31%) | 0.082 |
| Chronic kidney disease | 4 (14%) | 4 (14%) | 5 (17%) | 1.00 |
| Anemia | 12 (41%) | 4 (14%) | 4 (14%) | 0.026 |
| History of valvular heart disease | 1 (3%) | 0 (0%) | 1 (3%) | 1.00 |
| History of prior heart failure admission | 2 (7%) | 0 (0%) | 0 (0%) | 0.33 |
| Morise score, mean (SD) | 15.2 (2.9) | 14.2 (3.4) | 13.4 (3.7) | 0.12 |
| Pre-test probability of CAD by Morise score | 0.50 | |||
| Low (0–8 points) | 1 (3%) | 1 (3%) | 3 (10%) | |
| Intermediate (9–15 points) | 13 (45%) | 16 (55%) | 17 (59%) | |
| High (16–24 points) | 15 (52%) | 12 (41%) | 9 (31%) | |
| Years between cancer diagnosis and PET, median (IQR) | 8.7 (3.1–19.8) | 6.7 (2.8–13.2) | 8.5 (3.8–16.8) | 0.60 |
| Metastatic disease at breast cancer diagnosis | 4 (16%) | 2 (8%) | 5 (19%) | 0.60 |
| Breast cancer recurrence | 9 (32%) | 5 (18%) | 4 (15%) | 0.33 |
| Treatment with chemotherapy | 9 (31%) | 7 (24%) | 11 (38%) | 0.57 |
| Treatment with anthracyclines | 3 (10%) | 5 (17%) | 8 (28%) | 0.27 |
| Treatment with trastuzumab | 1 (3%) | 1 (3%) | 3 (10%) | 0.61 |
| Treatment with hormonal therapy | 13 (45%) | 17 (59%) | 10 (34%) | 0.20 |
| Treatment with chest irradiation | 18 (62%) | 20 (69%) | 19 (66%) | 0.96 |
| Treatment with left chest irradiation | 14 (78%) | 10 (50%) | 10 (53%) | 0.17 |
| Treatment with surgery | 28 (97%) | 25 (93%) | 27 (93%) | 0.86 |
| Rest LVEF, mean (SD) (%) | 64.8 (7.4) | 62.9 (7.3) | 64.6 (8.1) | 0.57 |
| Stress MBF, mean (SD) (mL·min−1·g−1) | 2.14 (0.86) | 2.40 (0.73) | 3.15 (1.00) | <0.001 |
| Rest MBF, mean (SD) (mL·min−1·g−1) | 1.45 (0.57) | 1.24 (0.42) | 1.17 (0.41) | 0.079 |
| MFR, mean (SD) | 1.48 (0.18) | 1.96 (0.14) | 2.72 (0.36) | <0.001 |
| Corrected rest MBF, Mean (SD) (mL·min−1·g−1) | 1.26 (0.55) | 1.17 (0.43) | 1.21 (0.36) | 0.75 |
| Corrected MFR, mean (SD) | 1.72 (0.34) | 2.16 (0.58) | 2.67 (0.60) | <0.001 |
BMI, body mass index; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MBF, myocardial blood flow; MFR, myocardial flow reserve; PET, positron emission tomography; SD, standard deviation.
To account for the effect of diffuse atherosclerosis on measurements of coronary vasomotor dysfunction, we reviewed the attenuation correction CT images for CAC. The presence of CAC did not differ significantly between treatment groups (Supplemental Table 1). The severity of CAC also did not differ significantly between MFR tertiles (Table 3). Of note, 45%, 48%, and 55% of patients in the lowest, middle, and highest MFR tertiles, respectively, had no evidence of CAC. Conversely, severe CAC was present in only 3%, 10%, and 0% of patients in the lowest, middle, and highest MFR tertiles, respectively (Table 3). There was no relationship between tracer or pharmacologic stress agent used and MFR tertile (Supplemental Table 2).
Table 3.
Degree of coronary artery calcification by myocardial flow reserve tertile in patients with normal myocardial perfusion imaging
| MFR < 1.71 (n = 29) | 1.71 ≤ MFR ≤ 2.24 (n = 29) | MFR > 2.24 (n = 29) | p value | |
|---|---|---|---|---|
|
| ||||
| No CAC | 13 (45%) | 14 (48%) | 16 (55%) | 0.80 |
| Mild CAC | 8 (28%) | 8 (28%) | 8 (28%) | 1.00 |
| Moderate CAC | 7 (24%) | 4 (14%) | 5 (17%) | 0.69 |
| Severe CAC | 1 (3%) | 3 (10%) | 0 (0%) | 0.32 |
There were no statistically significant differences in either the overall presence or severity of coronary artery calcification seen on transmission computed tomography scans between the myocardial flow reserve tertiles.
CAC, coronary artery calcium; MFR, myocardial flow reserve.
Coronary Vasomotor Dysfunction, Major Adverse Cardiovascular Events, and All-Cause Mortality
Over a median follow-up of 7.6 years (IQR 3.14–9.41) after PET, there were 15 major adverse cardiovascular events: 12 cardiovascular hospitalizations (8 heart failure, 3 non-fatal MI, and 1 coronary revascularization) and 3 cardiovascular deaths (which were not preceded by a cardiovascular hospitalization). MFR was significantly associated with incident MACE after accounting for competing risk of death (subdistribution hazard ratio (SHR) 0.18, 95% CI 0.05–0.67; p = 0.01), and this association persisted after multivariable adjustment (which adjusted for Morise score and chronic kidney disease) (SHR 0.28; 95% CI 0.10–0.76; p = 0.013). MFR tertile was also significantly associated with incident MACE (SHR 2.06; 95% CI 1.10–3.84; p = 0.023), and this association also persisted after multivariable adjustment (SHR 1.14; 95% CI 1.14–3.15; p = 0.013) (Supplemental Table 3). Rest MBF (SHR 0.62; 95% CI 0.19–1.97; p = 0.416) was not significantly associated with incident MACE. Stress MBF was associated with incident MACE (SHR 0.37; 95% CI 0.17–0.81; p= 0.013), but this association did not persist after multivariable adjustment (SHR 0.45; 95% CI 0.20–1.02; p = 0.057).
Compared with patients in the highest MFR tertile, those in the lowest MFR tertile had a higher incidence of MACE during the follow-up period on a univariable basis (SHR 4.85; 95% CI 1.12–21.14; p = 0.035) and after multivariable adjustment (adjusted SHR 4.91; 95% CI 1.68–14.38; p = 0.004) (Figure 1). Compared with patients in the highest MFR tertile, those in the middle tertile did not have a statistically significant higher incidence of MACE during the follow-up period on a univariable basis (SHR 2.96; 95% CI 0.60–14.51; p = 0.181). There were 23 deaths during the follow-up period: 5 cardiovascular and 18 non-cardiovascular (11 cancer-related deaths, 3 non-cancer related deaths, 4 unknown cause of death). Neither MFR (HR 0.49, 95% CI 0.22–1.09; p = 0.079) nor MFR tertile (p = 0.081) were significantly associated with all-cause mortality (Figure 2).
Figure 1.
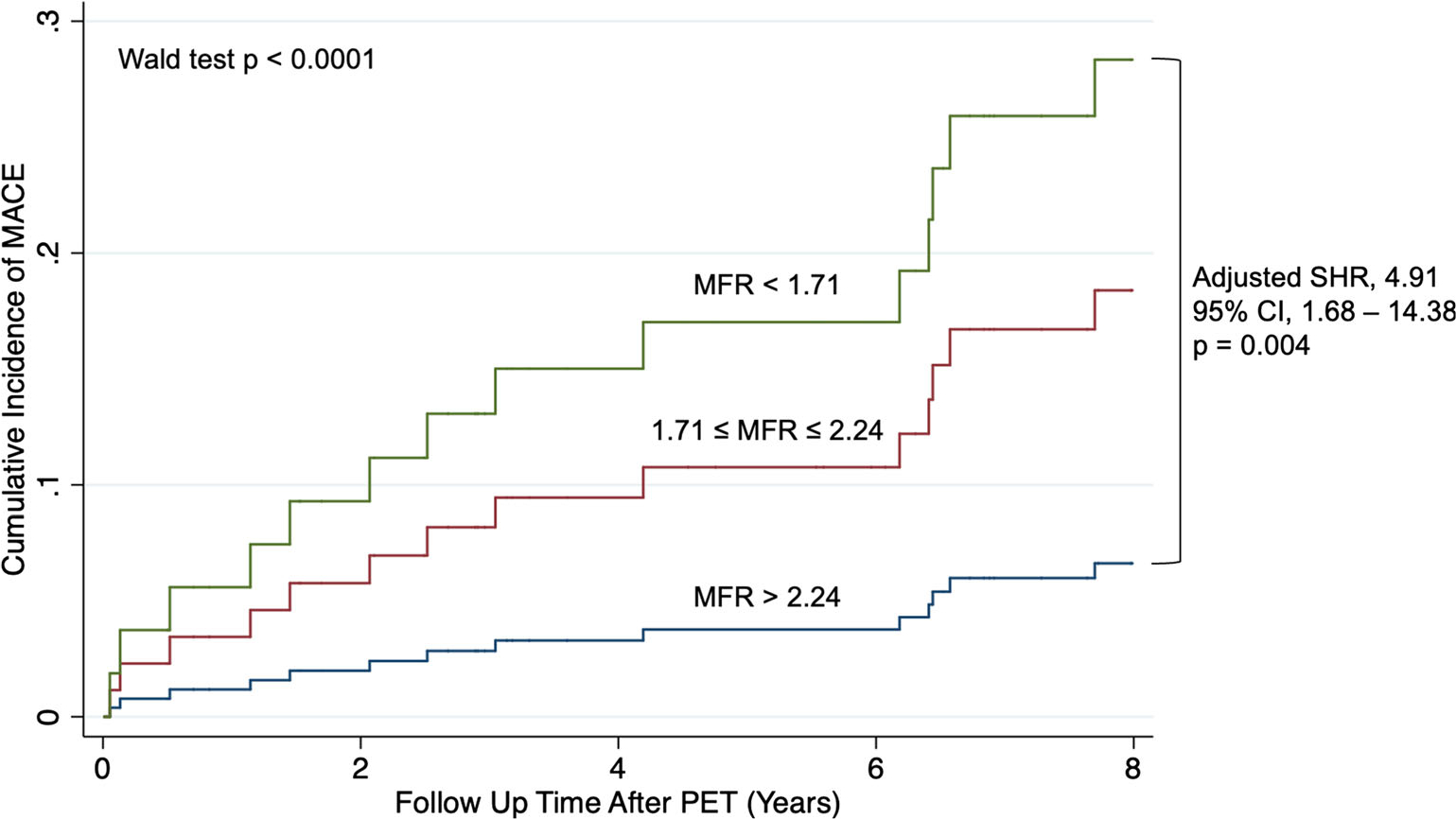
Time to incident major adverse cardiovascular event by MFR tertile. Cumulative incidence of MACE for the cohort is presented stratified by MFR tertile. Multivariable analysis (considering competing risk of death) adjusted for Morise score and chronic kidney disease. CI, confidence interval, MACE, major adverse cardiovascular event; MFR, myocardial flow reserve; PET, positron emission tomography; SHR, subdistribution hazard ratio.
Figure 2.
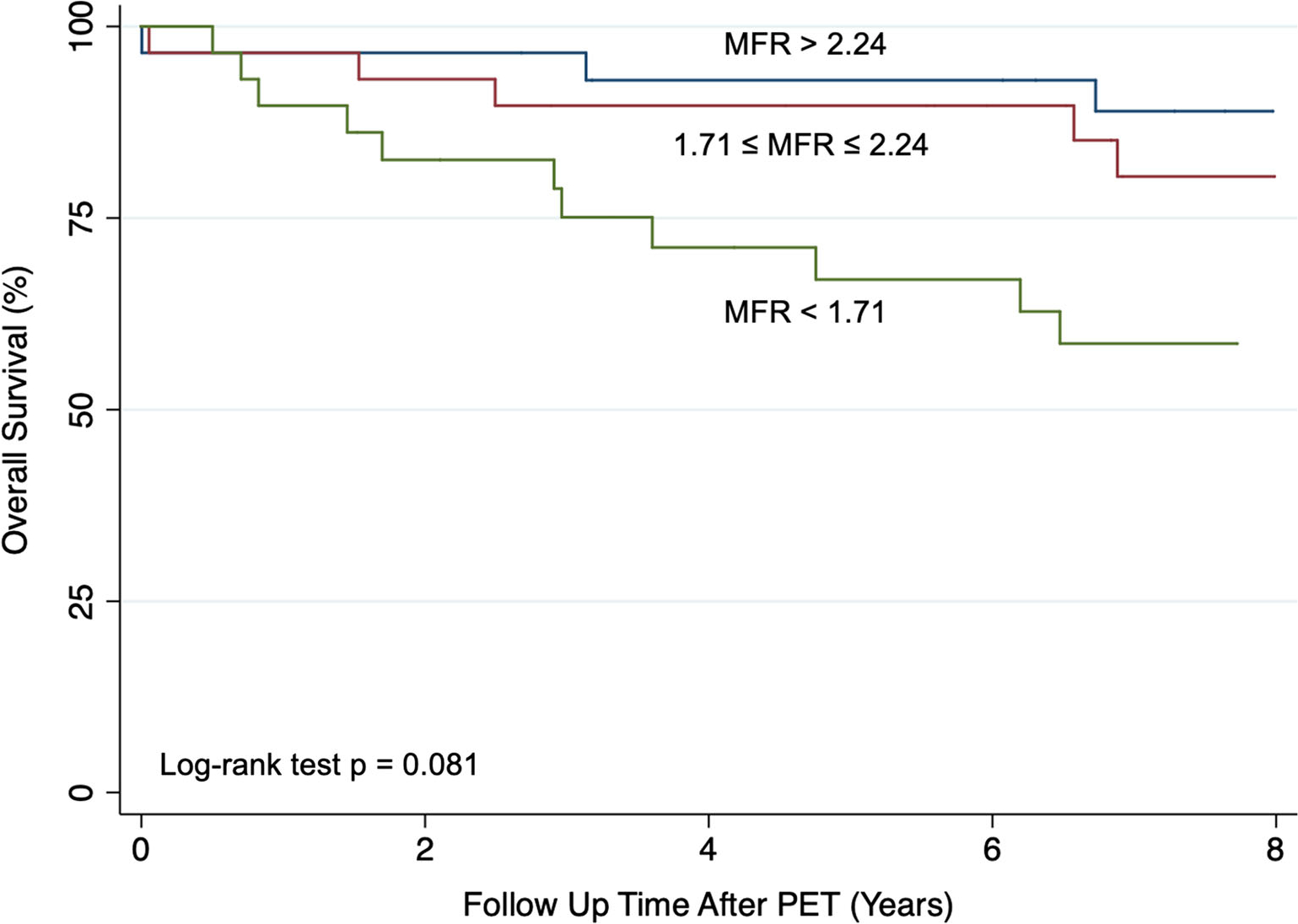
Kaplan-Meier estimate of overall survival by MFR tertile. Overall survival for the cohort is presented stratified by MFR tertile. MFR, myocardial flow reserve; PET, positron emission tomography.
DISCUSSION
The results from our study support the hypothesis that MFR, a marker of coronary vasomotor dysfunction that is associated with adverse outcomes in patients without cancer, is also associated incident major adverse cardiovascular events in this cohort of patients with breast cancer. These results advance our understanding of the prognostic implications of abnormal MFR and may provide the basis for further evaluation of MFR as a biomarker of general vascular health and clinical risk in this population.
It is well-established that cardiovascular disease and breast cancer have overlapping risk factors, such as obesity and tobacco use.34 The data from our study provide further evidence of this overlap as patients in the lowest MFR tertile had the highest BMI. Additionally, in older women diagnosed with breast cancer, cardiovascular disease is the leading cause of death.35 The hypothesis-generating data from our study point to coronary vasomotor dysfunction as a potential biomarker, and possible therapeutic target, in breast cancer patients/survivors who are at increased risk of adverse cardiovascular events, even in the absence of clinically overt obstructive CAD and/or left ventricular systolic dysfunction, and in some cases even in the absence of coronary artery calcifications/non-obstructive CAD. It is notable that the lowest MFR tertile had significant coronary vasomotor dysfunction as MFR values in this group (<1.71) were much lower than the mean for the entire cohort (2.56).
We hypothesize that MFR may be a surrogate marker of underlying cardiovascular risk in patients with breast cancer, and that cancer therapies may affect MFR in this population. Recent pre-clinical work studying human ex-vivo microvascular responses identified impaired coronary arteriolar function after anthracycline treatment.36 Atherosclerotic disease after radiation therapy has also been shown to be partly due to microvascular injury associated with reduced capillary density, fibrosis, and abnormal vascular reactivity.19,22,37 The absence of CAC in 49% of our total study cohort and 45% of those in the lowest MFR tertile is consistent with a contribution of microvascular dysfunction to increased risk in this population. While it is possible that a portion of these patients may have had noncalcified atherosclerosis, it is unlikely that all did. Additionally, we have previously shown an inverse correlation between mean cardiac radiation dose and coronary vasomotor function in 35 patients referred for clinical stress PET following radiation therapy for a variety of malignancies.38 Finally, though neither MFR nor MFR tertile were significantly associated with all-cause mortality, there was a trend toward significance for both. These data suggest that the inability to significantly augment MBF in response to a vasodilator-stress in patients without clinically overt obstructive CAD or left ventricular systolic dysfunction may be a surrogate marker for overall reserve and/or fitness. Additional study is needed with larger sample sizes to further test this hypothesis.
Study limitations
Our study has important limitations. It is a single-center, observational study in which the population consisted of patients referred clinically for cardiac PET. Given the retrospective nature of the study, we did not have PET data pre- and post-diagnosis nor pre- and posttreatment. This limited our ability to assess if cancer therapy affected MFR, and to what degree cardiovascular risk factors alone were responsible for abnormalities in MFR. One of the aims of the now-enrolling Cardiotoxicity in Locally Advanced Lung Cancer Patients Treated With Chemoradiation Therapy (CLARITY) study (ClinicalTrials.gov Identifier: NCT04305613) is to measure baseline MFR, and the effect of cancer therapy on MFR in patients with a new diagnosis of lung cancer. To focus on the prognostic implications of coronary vasomotor dysfunction, we excluded patients with known clinically overt CAD, left ventricular systolic dysfunction, or abnormal myocardial perfusion. Though it is possible that some patients with multivessel, obstructive CAD without perfusion abnormalities on PET were included, we have previously demonstrated that this is unlikely.39 We did not evaluate the effect of baseline medications on MFR nor on outcomes. Finally, CAC was assessed qualitatively and not via formal calcium scoring. However, this approach is supported by societal guidelines 40 and we followed previously published methods.30,31 Understanding these important limitations, our data still suggest potential clinical value for abnormal MFR as a biomarker of cardiovascular risk in this population.
CONCLUSIONS
In patients with breast cancer or survivors of breast cancer referred for cardiac stress PET, coronary vasomotor dysfunction was associated with higher incidence of cardiovascular events. The data from our study suggest that MFR may have value as a biomarker of cardiovascular risk in patients with breast cancer. Further investigation with larger sample sizes may provide more supportive data for the use of MFR as a general biomarker of vascular health/fitness in this population.
NEW KNOWLEDGE GAINED
In a retrospective analysis of a cohort of 87 consecutive patients with breast cancer or survivors of breast cancer clinically referred for a cardiac stress PET, coronary vasomotor dysfunction (via myocardial flow reserve (MFR)) was associated with incident major adverse cardiovascular events. MFR may have potential in risk stratification among patients with/survivors of breast cancer.
Supplementary Material
Funding
Dr. Divakaran and Dr. Zhou were supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301). Dr. Divakaran was also supported by a joint KL2/Catalyst Medical Research Investigator Training (CMeRIT) award from Harvard Catalyst and the Boston Claude D. Pepper Older Americans Independence Center (5P30AG031679–10). Mr. Caron was supported in part by the Goodman Master Clinician Scholar Award (awarded to Dr. Groarke) and the Gelb Master Clinician Scholar Award (awarded to Dr. Nohria). Dr. Taqueti was supported by Grant Number K23 HL135438 from the National Heart, Lung, and Blood Institute. Dr. Dorbala was supported by Grant Number R01 HL130563 from the National Heart, Lung, and Blood Institute. Dr. Di Carli was supported by Grant Number R01 HL132021 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr. Dorbala is a member of an advisory board for Proclara, Pfizer, and General Electric Health Care, and receives grant support from Pfizer. Dr. Blankstein receives research support from Amgen Inc. and Astellas Inc. Dr. Groarke receives research support from Amgen, Inc. Dr. Nohria receives research support from Amgen, Inc. and consulting fees from Takeda Oncology, AstraZeneca Pharmaceuticals, and Boehringer Ingelheim. Dr. Di Carli has received investigator-initiated institutional research grant support from Spectrum Dynamics and Gilead Sciences, and consulting fees from Bayer and Janssen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations
- CAC
Coronary artery calcium
- CAD
Coronary artery disease
- LVEF
Left ventricular ejection fraction
- MACE
Major adverse cardiovascular event
- MFR
Myocardial flow reserve
- PET
Positron emission tomography
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12350-021-02825-1.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Ahmed Tawakol, MD.
References
- 1.Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2625–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 3.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J 2016;37:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: The iPOWER study. J Am Heart Assoc 2016;5:e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–53. [DOI] [PubMed] [Google Scholar]
- 7.Shah NR, Cheezum MK, Veeranna V, Horgan SJ, Taqueti VR, Murthy VL, et al. Ranolazine in symptomatic diabetic patients without obstructive coronary artery disease: Impact on microvascular and diastolic function. J Am Heart Assoc 2017;6:e005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj NS, Singh A, Zhou W, Gupta A, Fujikura K, Byrne C, et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in patients with chronic kidney impairment. Circulation 2020;141:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol 2019;74:2350–60. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, et al. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015; 131:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborne MT, Bajaj NS, Taqueti VR, Gupta A, Bravo PE, Hainer J, et al. Coronary microvascular dysfunction identifies patients at high risk of adverse events across cardiometabolic diseases. J Am Coll Cardiol 2017;70:2835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sardar P, Kundu A, Chatterjee S, Nohria A, Nairooz R, Bangalore S, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer: A systematic review and meta-analysis. Clin Cardiol 2017;40:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barac A, Murtagh G, Carver JR, Chen MH, Freeman AM, Herrmann J, et al. Cardiovascular health of patients with cancer and cancer survivors: A roadmap to the next level. J Am Coll Cardiol 2015;65:2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. [DOI] [PubMed] [Google Scholar]
- 19.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: A report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:721–40. [DOI] [PubMed] [Google Scholar]
- 20.Schultz-Hector S Radiation-induced heart disease: Review of experimental data on dose response and pathogenesis. Int J Radiat Biol 1992;61:149–60. [DOI] [PubMed] [Google Scholar]
- 21.Schultz-Hector S, Balz K. Radiation-induced loss of endothelial alkaline phosphatase activity and development of myocardial degeneration. An ultrastructural study. Lab Invest 1994;71:252–60. [PubMed] [Google Scholar]
- 22.Stewart FA, Hoving S, Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res 2010;174:865–9. [DOI] [PubMed] [Google Scholar]
- 23.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 24.Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C, Darby SC, et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol 2017;123:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson KE, Reulen RC, Winter DL, Bright CJ, Fidler MM, Frobisher C, et al. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age: The teenage and young adult cancer survivor study. Circulation 2016;134:1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dess RT, Sun Y, Matuszak MM, Sun G, Soni PD, Bazzi L, et al. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol 2017;35:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 28.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: Comparison with (13)N-ammonia PET. J Nucl Med 2009;50:1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002;18:539–42. [PubMed] [Google Scholar]
- 30.Einstein AJ, Johnson LL, Bokhari S, Son J, Thompson RC, Bateman TM, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol 2010;56:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the national lung screening trial: A comparison of three scoring methods. Radiology 2015;276:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;138:e618–51. [DOI] [PubMed] [Google Scholar]
- 33.Morise AP, Haddad WJ, Beckner D. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med 1997;102:350–6. [DOI] [PubMed] [Google Scholar]
- 34.Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular disease and breast cancer: where these entities intersect: A scientific statement from the American Heart Association. Circulation 2018;137:e30–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hader SN, Zinkevich N, Norwood Toro LE, Kriegel AJ, Kong A, Freed JK, et al. Detrimental effects of chemotherapy on human coronary microvascular function. Am J Physiol Heart Circ Physiol 2019;317:H705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: Current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groarke JD, Divakaran S, Nohria A, Killoran JH, Dorbala S, Dunne RM, et al. Coronary vasomotor dysfunction in cancer survivors treated with thoracic irradiation. J Nucl Cardiol 2020. 10.1007/s12350-020-02255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11:74–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


