Abstract
Although the clinical approach to the management of musculoskeletal manifestations in systemic lupus erythematosus (SLE) is often similar to that of rheumatoid arthritis (RA), there are distinct differences in immunopathogenesis, structural and imaging phenotypes and therapeutic evidence. Additionally, there are few published comparisons of these diseases. The objective of this narrative literature review is to compare the immunopathogenesis, structural features, magnetic resonance imaging (MRI) and musculoskeletal ultrasound (MSUS) studies and management of joint manifestations in RA and SLE. We highlight the key similarities and differences between the two diseases. Overall, the literature evaluated indicates that synovitis and radiographical progression are the key features in RA, while inflammation without swelling, tendinitis and tenosynovitis are more prominent features in SLE. In addition, the importance of defining patients with RA by the presence or absence of autoantibodies and categorizing patients with SLE by synovitis detected by musculoskeletal ultrasound and by structural phenotype (non-deforming, non-erosive arthritis, Jaccoud’s arthropathy and ‘Rhupus’) with respect to joint manifestations will also be discussed. An increased understanding of the joint manifestations in RA and SLE may inform evidence-based clinical decisions for both diseases.
Keywords: Joint manifestation, Magnetic resonance imaging, Musculoskeletal ultrasound, Rheumatoid arthritis, Systemic lupus erythematosus
Key Summary Points
| The predominant symptom in RA is synovitis, while tendinitis and tenosynovitis are relatively more prominent in SLE. |
| Early inflammatory and structural changes in joints in RA and SLE can be detected by MRI and MSUS. |
| Clinically relevant synovitis and tenosynovitis are underestimated by clinical examination and disease activity instruments in SLE. |
| Joint manifestations of disease can differ due to the presence or absence of autoantibodies in RA and by joint disease phenotype in SLE. |
| Feet are also an important site of joint manifestations in both diseases. |
Introduction
Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are chronic, multifactorial inflammatory autoimmune diseases that significantly impact quality of life. While RA is a progressive disease that targets synovial joints leading to inflammation, erosion and destruction, SLE is more unpredictable and heterogenous, with periods of relapse and remission, and affects many different organs [1, 2]. Genetic and environmental factors predispose to the development of RA or SLE. The prevalence of RA is approximately 0.5–1.0% in the general population, while SLE has a prevalence ranging from 20 to 70 adults per 100,000 [2–4].
Most patients with RA present with symmetrical polyarthritis, but some have monoarthritis or oligoarthritis [5]. RA can affect all joints lined by a synovial membrane (synovial joints) with a predilection for the small joints of the hands and feet [4]. Joint manifestations are also one of the most common and early symptoms of SLE, presenting mostly as non-deforming, non-erosive (NDNE) arthritis [6]. A small proportion of patients with either RA or SLE can develop ‘Rhupus,’ an overlapping disease characterized by the presence of features of both diseases [7].
The aim of this narrative literature review is to compare the structural changes in joints in RA and SLE as visualized by diagnostic imaging techniques, to inform evidence-based clinical decisions.
Methods
A literature search was performed across the PubMed and Embase databases using the following search terms: Rheumatoid arthritis AND systemic lupus erythematosus; rheumatoid arthritis AND joints; rheumatoid arthritis AND imaging; rheumatoid arthritis AND autoantibodies AND joints; systemic lupus erythematosus AND joints; systemic lupus erythematosus AND imaging; systemic lupus erythematosus AND autoantibodies AND joints; rheumatoid arthritis AND autoantibodies AND imaging.
Case reports, congress abstracts, posters, editorials, and articles not published in English or without the full text available were excluded. Additional references were included when they were considered of importance.
Fluorescence optical imaging data were collected within the study with the local ethics number EK1/199/18. Before inclusion in the study, patients gave their written informed consent to participate, including anonymized publication of their imaging data. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments.
Results
Development of Synovitis in RA and SLE
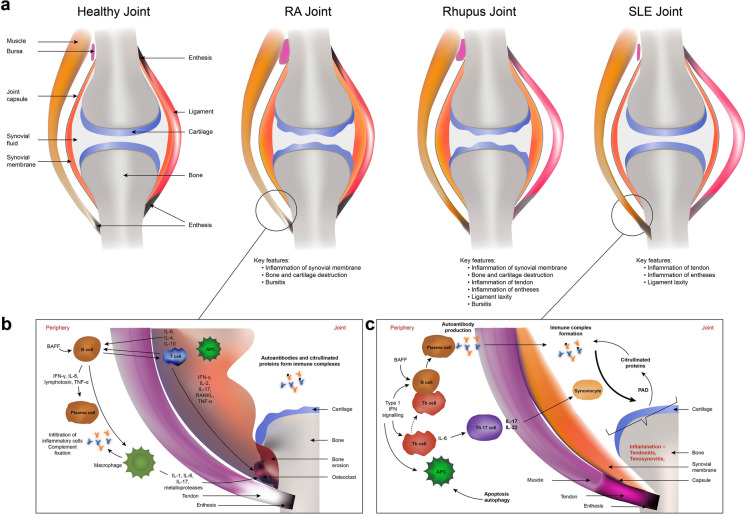
RA develops when genetic susceptibility, environmental factors and/or epigenetic changes result in loss of self-tolerance and autoimmunity. During the preclinical stages of RA, the first evidence of this loss of self-tolerance and autoimmunity is autoantibody production [9, 10]. Approximately 50–70% of patients have anti-citrullinated protein antibodies (ACPAs), including anti-cyclic citrullinated peptides (anti-CCPs), and rheumatoid factors (RF) at diagnosis [2]. Recent evidence indicates that ACPA production can first occur in sites distant from the joints, specifically the periodontium and lungs; however, it is unclear how an autoimmune process to a ubiquitous antigen in distant tissues transforms into a highly specific synovial pathology [11]. Autoantibodies form immune complexes in tissues, including the joints, inducing abundant complement activation and possibly contributing to joint damage [2, 8]. The involvement of tissue-resident macrophages, which normally protect the synovial lining layer, in a process resulting in eventual bone destruction remains to be delineated [12, 13]. However, this overall proinflammatory state drives continuous interaction of macrophages with lymphocytes, release of proinflammatory cytokines, chronic osteoclast activation and consequential bone erosion [2].
Dendritic cell (DC) activation is considered crucial for the initiation and development of synovitis in RA (Fig. 1) [4]. Activated DCs produce cytokines that promote differentiation of T cells into interleukin (IL)-17-producing Th17 cells [4]. IL-17 has many proinflammatory effects, including upregulating RANKL expression on osteoblasts, leading to the formation of osteoclasts and bone destruction, and stimulating leukocytes to produce IL-1, IL-6, tumor necrosis factor alpha (TNF-α) and matrix-degrading enzymes [4]. These cytokines recruit leukocytes to the synovial tissue, amplify proinflammatory signaling cascades and stimulate fibroblasts, which have a crucial role in damage accrual [4, 13]. Unregulated angiogenesis facilitates leukocyte recruitment into the synovial tissue; however, despite the increase in vascularization, the synovial tissue is hypoxic, which further stimulates self-perpetuating inflammatory cycles [13, 14]. Overall, these self-perpetuating processes transform the synovial tissue from a typically oligocellular loose areolar membrane into an invasive ‘pannus,’ resulting in the edematous, hyperplastic synovial tissue that is characteristic of RA [13].
Fig. 1.
Structural and immunological features of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). a Outline of the characteristic anatomical structures of joints in healthy individuals and patients with RA (reflective of seropositive and seronegative RA), Rhupus or SLE (reflective of non-deforming non-erosive SLE and Jaccoud’s arthropathy). b, c Immunological pathways in the joints in RA (b) and SLE (c). APC Antigen presenting cell, BAFF B cell-activating factor, IFN interferon, IL interleukin, PAD peptidylarginine deiminase enzyme, RANKL receptor activator of nuclear factor κβ ligand, TNF tumor necrosis factor. Data from [1, 6, 7, 22, 42, 49, 75, 91, 92]
The pathogenic mechanisms that lead to joint manifestations in SLE are not fully delineated; however, similarly to RA, the immune process appears to start away from the synovium during a long preclinical phase [15]. SLE is proposed to develop due to inadequate clearance of apoptotic material and/or aberrant apoptosis, and subsequent antinuclear antibody (ANA) production (Fig. 1) [15]. In contrast to RA, innate immune responses to nuclear self-antigens leading to type I interferon (IFN) pathway activation in blood, skin and synovium are important in SLE [16, 17]. It remains unclear how this preclinical phase of autoimmunity, which remains stable in most ANA-positive individuals, transforms into a multiorgan inflammatory disease; however, IFN score and family history are predictive factors [15, 18]. Despite differences in autoimmunity initiation, RA and SLE have some similarities in their synovial pathology, which may explain why their clinical phenotypes have some overlapping features.
In SLE, nuclear self-antigens from apoptotic material are presented to T cells, resulting in T cell activation, cytokine production (e.g. IL-6, IL-17) and loss of self-tolerance [19, 20]. Activated T cells interact with and activate B cells, inducing B cell differentiation into autoantibody-producing plasma cells [1]. Th17 cells are activated by IL-6, resulting in IL-17 and IL-22 production, and subsequent activation and inflammation of the synoviocytes [1]. Synoviocytes and fibroblasts produce IFN-β, which downregulates TNF-α and upregulates transforming growth factor-β, IL-10 and IL-1 receptor agonist [21–24]. Unlike RA, synovial biopsy and microarray studies suggest that 50–75% of patients with SLE have a type I IFN signature, which is important in maintaining inflammation in established disease [17, 25, 26]. While IFN activation in blood correlates with overall disease activity and some disease manifestations, such as mucocutaneous manifestations, it is not clearly related to arthritis [27–29]. Nonetheless, anifrolumab, an antibody that blocks IFN-α receptor subunit 1, is effective in patients with SLE experiencing arthritis [30].
Joint Changes in RA and SLE
In RA, joint swelling is a direct consequence of inflammation. Cycles of inflammation, pannus formation and release of matrix-degrading enzymes lead to synovitis, articular cartilage and capsule destruction and, ultimately, joint space narrowing and bone erosions (outlined in Fig. 1), which are detectable by radiographic imaging and define radiographical progression [4].
Seropositive and seronegative RA may have distinct genetic susceptibilities and disease phenotypes [8]. Some studies found that ACPA-positive RA was associated with more severe symptoms, bone erosions, radiographical progression, disability and increased mortality than ACPA-negative RA, while RF-positive RA was associated with increased bone erosions, extra-articular manifestations and disease activity [2, 13, 32–34]. However, as other studies demonstrated greater inflammatory activity at diagnosis in disease-modifying antirheumatic drug (DMARD)-naïve patients with seronegative RA versus those with seropositive RA, comparable radiographical progression between the two groups and slower treatment responses in patients with seronegative RA, more imaging studies of patients with seronegative and seropositive RA are needed to differentiate the phenotypes and natural history of these two type of RA [35, 36].
Joint involvement is the first presenting symptom in up to 50% of patients with SLE, affects approximately 95% of patients during the clinical course and can meet six of the ten points required for classifying SLE based on the European Alliance of Associations for Rheumatology (EULAR)/American College of Rheumatology (ACR) 2019 criteria [37, 38]. In contrast to RA, SLE exhibits heterogenous joint manifestations, ranging from minor arthralgia to severe erosive arthritis [1]. Joint manifestations in SLE are frequently divided into three phenotypes: NDNE arthritis, Jaccoud’s arthropathy (JA) and ‘Rhupus’ syndrome; however, as outlined in this review, modern imaging results suggest this classification may be over-simplified. NDNE arthritis is the most frequent type of arthritis in SLE and generally presents as a transient or migratory persistent joint pain with or without accompanying swelling [1]. NDNE arthritis has a predominantly symmetrical distribution affecting the small joints, particularly in the hands. Tenosynovitis and tendinitis are common symptomatic features but are often undetected unless musculoskeletal ultrasound (MSUS) or magnetic resonance imaging (MRI) studies are performed [39, 40]. Unlike RA, NDNE arthritis rarely results in radiographic bone erosions and deformities [1]. Non-synovial structures, such as entheses and tendons without synovial sheaths, can also be inflamed [6]. Recent advances in joint imaging indicate that erosions may occur in NDNE arthritis [39, 40].
Approximately 3–13% of patients with SLE develop JA, a more severe arthritis type that can lead to deformities like those observed in RA, such as ulnar deviation, swan neck, boutonniere and Z distortion of the thumbs [1]. These deformities occur without the extensive radiographic bone erosion usually seen in RA as deformities in JA occur due to chronic inflammation of periarticular structures (e.g. periarticular swelling, capsular involvement, fibrosis, ligament laxity and muscle imbalance) and not destructive synovitis [1, 42].
A proportion of patients can develop ‘Rhupus.’ While no precise definition of ‘Rhupus’ exists, fulfilment of both the Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) 2012 criteria for SLE and the ACR/EULAR 2010 criteria for RA is the most frequently used classification [7]. The prevalence of ‘Rhupus’ among patients with SLE ranges from 0.1% to 9.7%, although most studies report a prevalence towards the lower end of this scale [7]. The absence of ACPA measurements in historic literature has affected earlier reports of ‘Rhupus’ prevalence. However, as the inclusion criteria and diagnostic approaches in many studies are quite heterogeneous, it is likely that ‘Rhupus’ is underestimated in populations with RA or SLE. ‘Rhupus’ is characterized by the presence of RA-like arthritis and at least one organ with SLE-like involvement [7]. While patients with ‘Rhupus’ generally have less frequent and less severe systemic symptoms than patients with NDNE arthritis, the frequencies of morning stiffness, joint pain, tenderness, symmetrical polyarthritis and swollen and tender joint counts are similar to those in patients with RA [7]. Like RA, joint manifestations in ‘Rhupus’ progress to bone erosions, deformities and disability [7]. Patients with ‘Rhupus’ may have similar titers of ACPA and RF to patients with RA, and of anti-double-stranded DNA (dsDNA), anti-Sm and ANA to patients with SLE [7]. Currently, insufficient data exists on whether these patients have distinct treatment responses.
Management of Musculoskeletal Disease
Treatment outcomes for patients with RA have improved dramatically in the last two decades due to the development of new synthetic and biologic DMARDs, changes in treatment strategies/targets and earlier initiation of DMARD treatments [43]. Similarly, optimized use of immunosuppressive drugs and hydroxychloroquine, attempts to implement treat-to-target strategies, reduced use of glucocorticoids and the availability of rituximab and belimumab have improved treatment options for patients with SLE, resulting in increased life expectancy and delayed accrual of organ damage [31, 44]. However, limited evidence exists on drug efficacy specifically for joint manifestations in SLE. Table 1 summarizes the current treatment options for joint manifestations in RA and SLE.
Table 1.
Summary of the treatment landscape for rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE)
| Treatment landscape for RA and SLE | Rheumatoid arthritis | Systemic lupus erythematosus |
|---|---|---|
| Treatment goals | Remission (primarily for patients with early RA) and low disease activity (mainly for patients with chronic RA) | Remission (primary target) and low disease activity (alternative target) |
| Treatment options for joint manifestations |
Methotrexate is generally the first treatment choice A wide range of bDMARDs and tsDMARDs are available for patients with inadequate responses, intolerance or contraindications to csDMARDs |
No treatment options with proven efficacy in selectively treating musculoskeletal manifestations but musculoskeletal manifestations were prominent in positive trials of belimumab and anifrolumab Treatment options under investigation Baricitinib Dapirolizumab pegol Rituximab |
| Autoantibody effect on treatment outcomes |
Seropositivity for ACPA is associated with increased clinical response to rituximab and abatacept Seropositivity for RF is associated with increased clinical response to rituximab and tocilizumab Seropositivity for both ACPA and RF is associated with more rapid achievement of remission and higher rates of sustained remission compared to other groups |
Seropositivity for anti-dsDNA antibodies, treatment with ≥ 7.5 mg/day prednisolone and SLEDAI-2 K ≥ 10 are positive predictors of response to belimumab treatment |
| Effect of treatment on autoantibodies titers | Treatment may reduce ACPA and RF titers or result in seroconversion to RF negativity | Belimumab, rituximab, anifrolumab, mycophenolate and prednisolone treatment are associated with a reduction in anti-dsDNA antibody titers |
The main treatment targets and summary of treatments for RA and joint manifestation for SLE are outlined. The effect of seropositivity for autoantibodies on treatment response and the effect of treatment on autoantibody titers are summarized. Data from [2, 8, 30, 90–101]
ACPA Anti-citrullinated protein antibodies, bDMARD biologic disease-modifying antirheumatic drugs, csDMARD conventional systemic disease-modifying antirheumatic drugs, dsDNA double-stranded DNA, RF rheumatoid factor, SLEDAI-2 K SLE Disease Activity Index 2000, tsDMARD targeted synthetic disease-modifying antirheumatic drugs
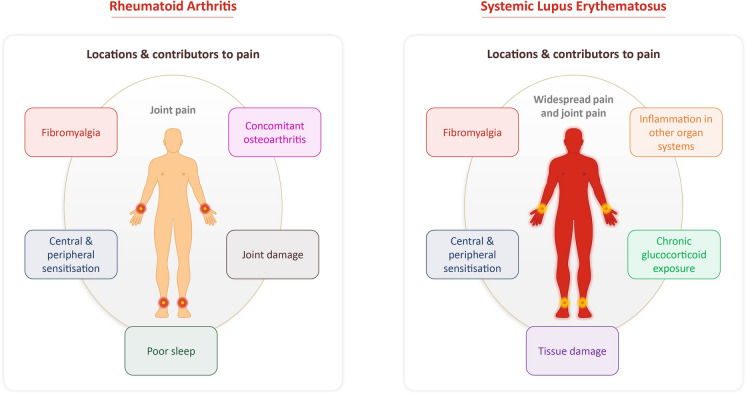
Joint pain can be challenging to treat due to its complex, multifactorial nature [5, 45]. Pain can be potentially exacerbated by comorbidities, disease sequelae and its presence in locations other than the joints (as summarized in Fig. 2), which further complicates its treatment [46, 47]. Despite the availability of effective treatments for inflammation, many patients continue to experience debilitating joint pain, highlighting this unmet medical need [46, 48].
Fig. 2.
Pain in RA and SLE. Patients with RA primarily experience pain localized to their joints, while patients with SLE commonly experience both pain in their joints and throughout their body. The main factors that potentially contribute to pain in these diseases are shown. Data from [31, 45–47]
Imaging of Joint Manifestations
Imaging techniques in RA and SLE can support diagnosis, monitor disease progression and improve understanding of the pathological basis of symptoms [1, 39, 49]. Radiographic imaging is reproducible, widely available, validated for erosive changes in RA and the standard outcome assessment in clinical trials, but it is not a very useful diagnostic tool in early RA when inflammatory changes are more important than erosions. Similarly, radiographic imaging has a limited role in SLE and is used primarily for excluding other musculoskeletal diseases.
MRI and MSUS are well-accepted modalities for detecting early changes and monitoring disease activity in patients with RA. In SLE, evidence on these techniques is increasing, but they remain infrequently used in routine practice. The different joint manifestations detected by radiographic imaging, MRI and MSUS are summarized in Table 2. In this section, we compare current knowledge of the structural changes of joints and periarticular structures in RA and SLE as visualized by MRI and MSUS.
Table 2.
Comparison of radiographic imaging, magnetic resonance imaging and musculoskeletal ultrasound techniques in imaging the joint manifestations of rheumatoid arthritis and systemic lupus erythematosus
| Joint manifestations of RA and SLE | Radiographic imaging | Magnetic resonance imaging | Musculoskeletal ultrasound |
|---|---|---|---|
| Synovitis visualization | No | Yes | Yes |
| Tenosynovitis visualization | No | Yes | Yes |
| Bursitis visualization | No | Yes | Yes |
| Joint effusion visualization | Yes | Yes | Yes |
| Bone erosion visualization | Yes, but not in early disease | Yes | Yes |
| Bone marrow edema (BME) | No | Yes | No |
| Joint space narrowing | Yes | Yes | Yes |
| Advantages |
- Gold standard for the detection of radiographical progression in RA - Reproducible - Detects bone erosion and joint space narrowing in advanced disease |
- More sensitive than radiographic imaging - Does not use ionizing radiation - Useful for detecting changes in early disease - MRI measures are sensitive to changes in synovitis, tenosynovitis and BME scores - Can detect bone marrow abnormalities |
- Low cost - Non-invasive - Does not use ionizing radiation - More sensitive than radiographic imaging for detecting erosions (especially in early RA and SLE) - Allows detection of joint inflammation in pre- or early RA and SLE - Can assess multiple locations in one examination |
| Disadvantages |
- Low sensitivity for early inflammatory changes - Cannot detect soft tissue inflammation - Use of ionizing radiation |
- Low-grade abnormalities seen by MRI can be observed in healthy individuals - Generally, MRI assessment is restricted to one location - High cost |
- Low-grade synovitis can be detected in healthy individuals - Cannot visualize bone erosions as well as MRI due to restrictions of the position of the probe and access to all cartilage surfaces - Cannot detect BME - Reproducibility is operator dependent |
The joint manifestations that can be detected by radiographic imaging, MRI and MSUS and the advantages and disadvantages of each imaging technique are summarized. Data from [6, 49, 50, 66, 102–104]
BME Bone marrow edema, MRI magnetic resonance imaging, MSUS musculoskeletal ultrasound, RA rheumatoid arthritis, SLE systemic lupus erythematosus
MRI of Joint Manifestations
MRI provides excellent soft tissue visualization and can detect and evaluate early bone involvement, synovitis, subcutaneous edema/swelling and tenosynovitis, none of which can be detected by clinical examination or radiographic imaging. However, MRI has a high cost, long examination times and restricted anatomical coverage per examination and its use requires further validation as variation in specificity and sensitivity is reported [50, 51].
Proliferative synovitis presents on MRI as a bilateral thickening of the synovial membrane, and as increased vascularization with RA progression [49]. Joint effusion and tenosynovitis are also commonly seen by MRI in early RA [49]. In one study, among patients with early RA, approximately 75% had tenosynovitis, with the flexor tendon at the fifth metacarpophalangeal (MCP) joint, extensor tendons at the second and fourth MCP joints and wrists being the most frequently affected locations [52]. Bursitis is also observed by MRI in RA [49]. Synovitis has been frequently reported in MRI studies of SLE, particularly in the wrists and MCPs, but at varying rates and severities, with one study reporting that 60% of patients had synovitis but of a low grade [1, 53]. In a small SLE study, a JA cohort had no MRI-detected active synovitis compared to 70% of a non-JA SLE cohort; however, the JA cohort had higher rates of edematous tenosynovitis [54]. Tenosynovitis has been reported in many different MRI studies in SLE [1, 53]. Ostendorf et al. reported that 71.4% of patients with SLE had MRI-detected tenosynovitis in their hands [54]. Extensor and flexor tenosynovitis of the wrist in patients with SLE was reported, while another study demonstrated a predominance of tenosynovitis between the MCP and proximal interphalangeal (PIP) joints and only minimal tenosynovitis in the wrists [1, 53].
A small study comparing hand MRIs of patients with RA versus patients with SLE or primary Sjögren’s syndrome demonstrated that tenosynovitis of the right fourth MCP extensor tendon was more frequent in SLE or primary Sjögren’s syndrome compared with RA [55]. Overall, the advanced sensitivity of MRI compared with radiographic imaging for tendinitis and tenosynovitis has resulted in recent MRI studies detecting more periarticular soft tissue inflammation in SLE than was previously reported.
Bone marrow edema (BME) is common in the subchondral bone in early RA and is predictive of future bone erosions and radiographic progression [49, 51]. In one study, patients with RA for longer durations had more BME compared with patients with shorter disease duration (68% vs. 17%) [56]. In another study involving patients with early RA, 64.8% of anti-CCP antibody-seropositive patients had BME compared with 38.5% of seronegative patients (p = 0.03) [57]. However, a study of Brazilian patients with RA reported no difference in Rheumatoid Arthritis Magnetic Resonance Imaging Score (RAMRIS) for BME, synovitis or erosions between patients who were anti-CCP antibody seropositive and those who were seronegative [58].
BME was also detected in the hands and wrists of patients with SLE using MRI [53, 59]. In one study, over 50% of patients had detectable BME in the hands or wrists, although only 10% had a high signal [53]. In an MRI study comparing the hands of patients with RA and SLE, BME was detected in the MCP joints of more patients with RA than SLE (71% vs. 7%) [55]. However, another study showed the prevalence of BME to be similar in RA versus SLE [59]. As BME can also be caused by trauma or mechanical stress in healthy individuals, these findings should be interpreted with caution.
We found few MRI studies of the feet in RA and none in SLE; however, the RA studies demonstrated that feet have similar pathologies to the hands. Specifically, flexor and extensor tenosynovitis at metatarsophalangeal (MTP) joints often occurred with synovitis and BME [60].
Bone erosions have been observed by MRI in high proportions of patient with RA [55, 61]. An MRI study comparing the distribution of bone erosions in patients with RA or SLE showed that the erosive burden in the wrists was comparable between the two groups (10.2 vs. 8.0), while the erosive burden in the hands was significantly higher in patients with RA versus SLE (3.4 vs. 1.3; p = 0.004) [59]. For both RA and SLE, erosions were predominantly in the wrists and distal side of the MCP joints [59]. Erosions were most prevalent in the third, fifth and second phalangeal bases (54, 41 and 41%, respectively) for RA and in the second and third phalangeal basis (both 20%) for SLE [59]. The overall prevalence of erosions in the hands was comparable for patients with RA versus those with SLE (68% vs. 48%) and higher compared to healthy controls (HC) (18%), while the prevalence of erosions in the wrists was similar for patients with RA, SLE and HC (100% vs. 82% vs. 97%) [59]. These observations were consistent with the findings of Mosca et al., who reported at least one erosion in the hand for 48.3% of patients with non-Rhupus SLE (defined as patients not meeting RA classification criteria) and 19.6% of HC, and at least one erosion in the wrist in 98.8% of patients with non-Rhupus SLE and 97.8% of HC [63]. As rates of MRI-detected bone erosions in the hands and wrists of patients with SLE were higher than expected from radiographic studies, the ‘NDNE’ label for most SLE arthritis might need to be reconsidered [39, 62]. However, as high rates of bone erosions were also reported in the wrists of HCs, care must be taken when interpreting the clinical relevance of these data.
Sacroiliitis, a feature not usually found in RA, was detected by MRI in 7.8% of patients with SLE in one study [64]. As another MRI study reported significantly more sacroiliitis in patients with SLE versus HC (73.0% vs. 32.3%; p = 0.001), this feature warrants further investigation [65].
In summary, MRI studies indicate that synovitis is the predominant feature in RA and that tendinitis and tenosynovitis are predominant features in SLE. Synovitis may be present at varying levels in SLE, but at a lower prevalence and reduced severity than observed in RA. Overall, BME and erosions have been found to be present at higher-than-expected levels in patients with SLE; however, as these features can also be observed in HC, the meaning of these results is not clear yet.
MSUS of Joint Manifestations
The use of MSUS in imaging joint manifestations has many benefits as it is low cost and non-invasive and multiple joints can be assessed in the same examination. Similarly to MRI, MSUS can distinguish synovitis from tenosynovitis; however, in contrast to MRI, MSUS cannot penetrate bone or visualize inflammation within the bone marrow, and therefore has a more restricted anatomical coverage [49]. MSUS is particularly useful in imaging joints in early RA disease [66].
The predominant pathological feature detected by MSUS in joints of patients with RA is synovitis, specifically synovial hypertrophy, as well as increased vascularization, which is commonly associated with joint effusion [61, 67, 68]. In one study, synovial hypertrophy (≥ Grade 1) in the wrists and MCP and PIP joints was detected by MSUS in 75.0, 86.7 and 70.0% of patients with RA, respectively, and a power Doppler (PD) signal (≥ Grade 1) was detected in the wrists and MCP and PIP joints of 59.2, 50.8 and 20.8% of patients with RA, respectively [69]. In another study, tenosynovitis and bursitis were also observed by MSUS but at a lower prevalence than synovitis [49]. When patients with RA were grouped by the presence or absence of autoantibodies, MSUS demonstrated that intermetatarsal bursitis was associated with the presence of anti-CCP and RF [70].
In contrast to RA, MSUS-detected synovitis and joint effusion in SLE have been reported with high variability [39]. In a systematic literature review of MSUS assessments of SLE, synovitis and tenosynovitis were reported in 25–94% and 28–65% of patients, respectively, while PD was reported in 10–82% of patients [71]. This marked variation could be attributed to differing patient selection criteria, including controlling for ‘Rhupus’ and glucocorticoid treatment, as well as lack of reporting of Outcome Measures in Rheumatology (OMERACT) grades of abnormality [71]. These methodological issues were addressed in a large cross-sectional study that excluded patients with suspected ‘Rhupus,’ recent changes in glucocorticoid treatment or improving disease, summarizing detailed clinical phenotypes for patients with SLE and reported OMERACT scores [72]. Overall, 68% (60/88) of symptomatic patients had MSUS-detected inflammation (gray-scale ≥ 2 and/or PD ≥ 1 or tenosynovitis) compared with 17% (4/23) of asymptomatic patients [72]. Among the symptomatic patients, clinical inflammation defined by British Isles Lupus Assessment Group (BILAG) A or B was observed in 38% (34/88) of patients while clinical inflammation defined by the SLE Disease Activity Index-Musculoskeletal (SLEDAI-MSK) criterion were observed in 32% (28/88) [72]. Sensitivity (95% confidence interval [CI]) for BILAG A/B and SLEDAI-MSK criterion was 56% (95% CI 41, 69) and 44% (95% CI 31, 59), respectively, while both had specificity of 89% ( 95% CI 72, 96) [72]. Among patients with inflammatory symptoms, 27% (24/88) had no joint swelling but had MSUS-detected inflammation, while 35% (31/88) had no clinical or MSUS-detected inflammation [72]. In a small study of patients with JA in which MSUS-detected abnormalities were assessed using OMERACT definitions, 76.5% of patients had synovial hypertrophy in hands/wrists, but only 29.4% had positive PD signal [73].
Patients with SLE experiencing musculoskeletal pain can have MSUS-detected synovitis without swelling [74]. In a study of 133 patients with SLE and joint pain, patients were assessed at baseline using clinical tools, blinded MSUS results and patient-reported outcomes, then treated with glucocorticoids and re-assessed after 2 and 6 weeks [74]. At baseline, MSUS-detected synovitis was predicted by the presence of joint swelling, symmetrical small joint distribution of arthralgia, presence of anti-ribonucleoprotein antibodies and a high IgG titer [74]. The results showed that patients with MSUS-detected synovitis responded better to glucocorticoid treatment than patients without MSUS-detected synovitis, when patients with fibromyalgia were excluded, indicating that MSUS-detected inflammation is clinically relevant [74].
In contrast to the predominance of synovitis in RA, MSUS studies demonstrated that tenosynovitis and peri-extensor tendon inflammation (tendinitis) were the most common features (collectively affecting 93% of patients) in SLE [40]. Tendon involvement was predominantly localized to the fourth compartment of the wrist extensor, the wrist common flexor and the third finger flexor tendon [40]. MSUS imaging of patients with JA demonstrated that the third flexor tendon was the predominantly affected tendon [73].
A comparison of the hands of patients with RA or SLE by MSUS demonstrated that the synovitis score per affected joint (assessed by gray-scale and PD MSUS) was higher in the RA versus SLE group (2.6 vs. 2.0; p = 0.019) [40]. Likewise, a MSUS study comparing patients with RA and non-Rhupus SLE (defined as patients not meeting diagnostic criteria for RA) reported an increased presence of synovial hypertrophy in the wrists (75.0% vs. 46.8%; p < 0.001) and of PD signals (≥ Grade 1) in the wrists (59.2% vs. 30.6%; p < 0.001) and MCP joints (50.8% vs. 28.2%, p < 0.001) for the RA group [69]. Similarly, there were significantly more synovial proliferation (28/50 vs. 12/52; p < 0.002) and positive PD signals (17/50 vs. 5/52; p < 0.01) in the knees of the patients with RA versus those with SLE [75]. However, tenosynovitis/tendinitis were more frequent in the SLE versus RA group, particularly in the wrists (73% vs. 40%; p = 0.037) [40]. A small study comparing patients with SLE, ‘Rhupus’ or RA demonstrated that patients with ‘Rhupus’ had significantly higher scores for synovial hypertrophy and joint effusion in both the wrist and hands compared with patients with SLE, but scores were similar between patients with ‘Rhupus’ and patients with RA [76].
While MSUS is more limited than MRI in terms of visualizing bone erosions, it can still demonstrate erosions in accessible locations [49]. Erosions increase quickly in the first 2 years of RA disease [50]. A MSUS study comparing erosive disease in long-term versus early (< 12 months disease duration) RA demonstrated that patients with long-term RA had significantly more erosions than those with early RA (89% vs. 67%; p < 0.05) [77]. Another study demonstrated that 60.8, 55.0 and 17.5% of patients with RA had ≥ Grade 2 erosions in the wrist and MCP and PIP joints, respectively, while 8.3, 16.7 and 4.2% of patients had Grade 3 erosions in these respective regions [69]. In MSUS studies of patients with RA grouped by autoantibody status, high titers of anti-CCP antibodies, but not RF antibodies, were associated with the presence of bone erosions [78, 79]. In agreement with this, another study demonstrated that patients with RA with anti-CCP antibodies had significantly more MSUS-detected bone erosions than patients without anti-CCP antibodies (41.4% vs. 12.5%; p < 0.05) [80].
Similar to MRI studies, MSUS studies have shown a higher-than-expected presence of bone erosions in patients with SLE; however, many earlier studies did not adequately control for the presence of ‘Rhupus’ in enrolled patients [71]. A large, more recent, case series that excluded patients with ‘Rhupus’ and JA gave a clearer picture of MSUS erosions in so-called ‘NDNE’ SLE. In this study, overall, 9% of patients had erosions, but this was strongly correlated with BILAG score; in patients with MSK-BILAG A, 29% had erosions, compared to only 4% of those with BILAG C [72].
Only a few MSUS studies of the feet in patients with RA or SLE have been reported; however, foot involvement is a common symptom in SLE and impacts on patients’ quality of life [81–84]. In a MSUS study of asymptomatic feet in patients with RA versus HC, patients with RA had significantly more synovitis and a higher frequency of PD signals in the talocalcaneal, talonavicular, and first-to-fourth MTP (all dorsal face) joints compared with the HC [85]. Intermetatarsal bursitis was detected in the feet of 20.6% of patients with RA by MSUS, with the second-third MTP and third-fourth MTP spaces being the most frequently affected sites [70]. An MSUS study of patients with SLE demonstrated that 27% had synovitis in the feet, with 9% and 2% having mild and moderate-to-severe PD synovitis scores, respectively [72]. In another study, MSUS-detected synovial PD signals were significantly higher in the first to fourth MTP joints of patients with SLE versus HC [86]. MSUS evaluation of the feet demonstrated significantly more erosions in asymptomatic patients with RA compared with HC (34.5% vs. 2.9%; p < 0.001), while the presence of erosions in the joints of the feet were similar in patients with SLE and HC (the highest prevalence was 3.7% in the first MTP joint) [72, 85, 86].
Enthesitis, a feature usually not observed in RA, has been clearly demonstrated in patients with SLE by MSUS and PD signal, mainly at the distal insertion of the patellar tendon, calcaneal insertion of the Achilles tendon and the proximal enthesis of the patellar tendon [6].
Fluorescence Optical Imaging of Joint Manifestations
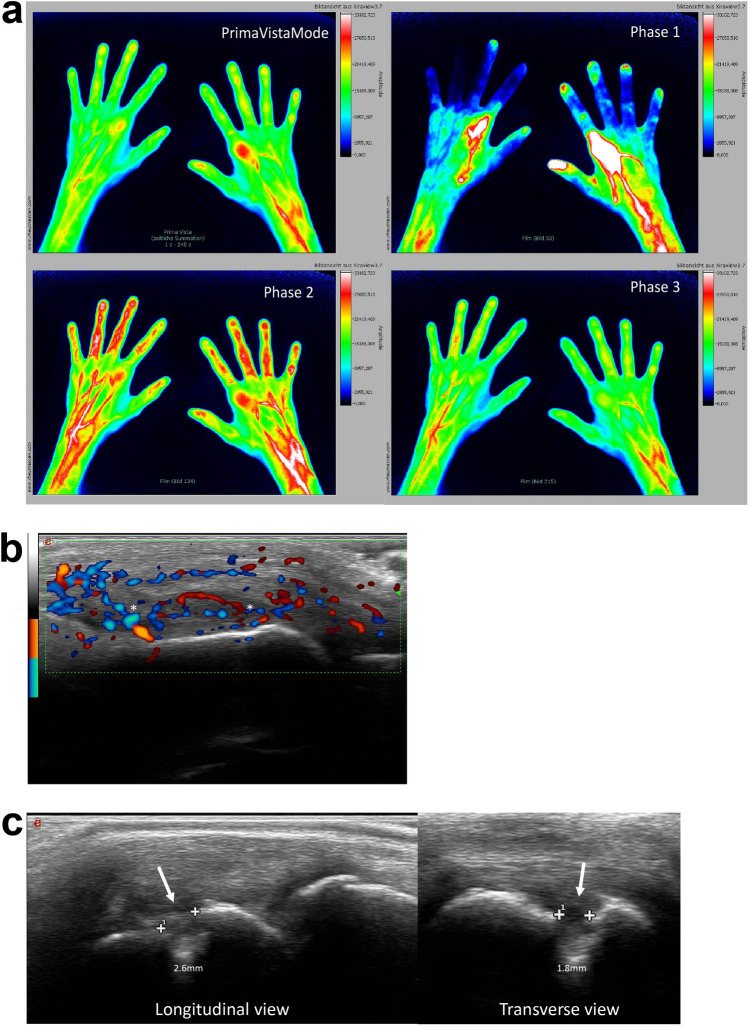
In recent years, indocyanine green enhanced fluorescence optical imaging (FOI) (Xiralite®; Xiralite GmbH, Berlin, Germany), a novel imaging technique, has been used to study RA and other rheumatic diseases; this diagnostic tool has been approved for clinical use in the European Union and USA. FOI in rheumatology is based on the visualization of impaired microcirculation due to inflammation in the joints of hands. The examination of both hands lasts 6 min and one image per second is recorded, resulting in a total of 360 images. A recently published comprehensive review summarized the role of FOI in research and clinical practice and outlined how FOI can complement our current imaging repertoire in rheumatology [87]. While some studies have compared FOI to MRI and MSUS in patients with RA and demonstrated that it is a useful technique and can be used to distinguish RA from osteoarthritis, as of yet, there are no published studies on the use of FOI in SLE [87]. However, initial data comparing FOI and MSUS images of the hands of a patient with ‘Rhupus’ suggest that FOI may be a useful technique for detecting joint manifestations in patients with ‘Rhupus’ and possibly SLE (Fig. 3) (S. Ohrndorf and G. Schmittat, unpublished data;—see Electronic Supplementary Material for more details).
Fig. 3.
a Fluorescence optical imaging (FOI) in Prima Vista mode (PVM) and Phase 1 to Phase 3 in a patient with ‘Rhupus’ showing strong enhancements in bilateral metacarpophalangeal (MCP)2 joints in Phase 1 and moderate enhancements in bilateral MCP2 joints in PVM and Phase 2. Musculoskeletal ultrasound images in power Doppler (b) and gray-scale (c) in the same patient with ‘Rhupus’. b Power Doppler active synovitis (denoted by asterisks) is visible in the right MCP2. c An erosion on the radial side (denoted by arrows) visible on longitudinal and transverse gray-scale view. (Original images from Sarah Ohrndorf and Gabriela Schmittat)
In summary, while MRI and MSUS imaging techniques were used less frequently for SLE than for RA, the published evidence demonstrates that radiographical progression was not identified in most patients with SLE. Overall, the higher sensitivity of MRI and MSUS compared with radiographic imaging demonstrated that tendinitis and tenosynovitis are the key features of joint manifestations in SLE and that certain sheaths, specifically the fourth extensor tendon sheath, may be preferentially affected. However, not all features of RA and SLE joint manifestations were detected in both MRI and MSUS studies, which may reflect anatomical restrictions of imaging techniques, differences in imaging methods, measurements and the definition of pathologies in studies, as well as heterogeneity between patients [88].
Synovitis was detected at varying rates and severities in patients with SLE and at a lower presence compared with patients with RA. Synovitis in SLE may be a secondary consequence of the mechanical stress induced by capsular–ligament involvement [7]. Moreover, in contrast to RA, joint manifestations in SLE are not limited to structures with synovial sheaths as inflammatory features, such as sacroiliitis and enthesitis, have been observed.
These studies highlight the importance of differentiating patients with RA or SLE based on seropositivity or joint phenotypes, respectively, as these subgroups can be associated with different disease severities and pathologies. Seropositive patients with RA appear to have more severe disease and radiographical progression than seronegative patients. Similarly, the NDNE, JA and ‘Rhupus’ phenotypes in SLE have different disease burdens and different associations with radiographical progression and autoantibodies. In particular, patients with ‘Rhupus’ need to be more carefully evaluated as their rate of structural damage appears more similar to patients with RA than to those with SLE [76]. Many of these studies did not differentiate between SLE phenotypes, suggesting that the presence of patients with ‘Rhupus’ increased the prevalence of synovitis, BME and erosions in SLE groups in these studies. Differentiation between SLE phenotypes in future studies will provide valuable information on joint manifestations in this disease. The presence of erosions in patients with JA and, to a lesser extent, NDNE arthritis raises important questions about synovitis in patients with SLE. Factors predisposing some patients with SLE to progress to bone erosions and structural damage also needs further investigation.
Discussion
Overall, the results presented in this review demonstrate that RA and SLE have both distinct and overlapping features in terms of joint manifestations of the diseases (summarized in Table 3). While most studies reported here did not differentiate patients with RA by the presence or absence of autoantibodies or patients with SLE by joint phenotype, the studies that did include these differentiations indicate that this is key to improving our knowledge on joint manifestations for each disease subgroup.
Table 3.
Comparison of the key features of rheumatoid arthritis and systemic lupus erythematosus
| Key features | Rheumatoid arthritis | Systemic lupus erythematosus | |||
|---|---|---|---|---|---|
| Seropositive | Seronegative | Non-deforming non-erosive (NDNE) arthritis | Jaccoud’s arthropathy (JA) | Rhupus syndrome | |
| Predominant feature(s) | Synovitis | Synovitis | Tendinitis, tenosynovitis | Tendinitis, tenosynovitis | Synovitis |
| Secondary feature(s) | Tenosynovitis, bursitis, joint effusion, BME | Tenosynovitis, bursitis, joint effusion, BME. BME may be lower than in seropositive patients | Synovitis and BME (lower prevalence or grade than JA, Rhupus and RA) | Synovitis, BME (higher prevalence than NDNE arthritis but lower than Rhupus and RA) | Tendinitis, tenosynovitis, bursitis, BME, joint effusion |
| Erosions detected by radiographic imaging | Yes, increases as disease progresses | Yes, increases as disease progresses | No | No | Yes. Similar to RA |
| Erosions detected by MSUS or MRI | Yes | Yes, but at a lower level than seropositive patients | Yes, but at a lower number and lower severity than JA, Rhupus and RA | Yes | Yes. Similar to RA |
| Enthesitis | Usually absent | Usually absent | Present | Present | Present |
| Radiographical progression | Progression to erosion and deformity | Progression to erosion and deformity | Usually minimal progression to erosions and no deformity | Progression to deformity only | Progression to erosion and deformity |
| Deformities present in untreated or inadequately treated disease | Yes—irreversible | Yes—irreversible | No | Yes—reversible | Yes—irreversible |
| Autoantibody formation | Yes | No | Yes | Yes | Yes |
The most common features affecting hands and wrists in patients with RA or SLE detected by MRI and MSUS imaging are summarized in this table. RA is categorized by seropositivity or seronegativity and SLE is categorized by NDNE arthritis, Jaccoud’s arthropathy (JA) or ‘Rhupus’ phenotype. A well-defined disease progression, the development of deformities in untreated or inadequately treated disease and the presence of autoantibodies are also noted. Data from [1, 2, 4, 6, 7, 31, 40–42, 49, 50, 54, 57, 59, 61, 67, 68, 71, 73, 76, 78, 80, 105–107]
BME Bone marrow edema, JA Jaccoud’s arthropathy, MRI Magnetic resonance imaging, MSUS Musculoskeletal ultrasound, NDNE Non-deforming non-erosive, RA Rheumatoid arthritis, SLE Systemic lupus erythematosus
While most imaging studies in RA and SLE focus on the hands, the feet may also be an important site of joint manifestations in both diseases. In RA, feet are frequently involved early in the disease course and often show the first radiographical evidence of joint pathology [89]. While the number of studies investigating the feet in SLE is limited, the published data suggest that joint manifestations in SLE do occur in the feet and are similar to those observed in the hands and wrists [72, 86].
The data summarized in this review clearly demonstrate that MRI and MSUS imaging are very effective diagnostic tools for detecting joint manifestations in RA and SLE; however, as these imaging techniques have higher sensitivity than clinical examination, some structural changes detected in patients with RA or SLE can also be detected in HC [59, 63]. Scoring systems, such as RAMRIS and OMERACT, and predefined threshold scores could be used to overcome this problem and prevent over-diagnosing of patients [66].
Additionally, as a substantial proportion of patients with SLE with musculoskeletal pain have MSUS-detected synovitis without accompanying swelling, the use of MRI and MSUS may provide clinically relevant information that cannot be garnered from clinical examination [74]. In agreement with this, USEFUL, a multicenter longitudinal study, demonstrated that among patients with SLE experiencing inflammatory joint pain with or without accompanying swelling, patients with MSUS-detected synovitis responded better to glucocorticoid treatment than patients without MSUS-detected synovitis, when patients with fibromyalgia were excluded [74].
In RA (particularly seropositive RA), the well-defined progression of synovitis to bone damage and deformities can be interrupted by targeted DMARD treatment, resulting in low disease activity or remission [2]. As little evidence is available on assessing and treating musculoskeletal disease in SLE, and not all symptomatic inflammation will be considered under the commonly used definitions of low disease activity or remission, the use of imaging techniques in clinical trials can potentially identify patients who will benefit from innovative and existing treatments and improve assessment of treatment responses.
Conclusions
Differences exist in the type, severity and progression of joint manifestations between RA and SLE, and these can be further differentiated by categorizing patients by the presence or absence of autoantibodies in RA or by joint phenotype in SLE. Our knowledge of joint manifestations in SLE is limited in comparison to RA, and further research is required to improve understanding and treatment of joint manifestations in SLE.
Acknowledgements
Funding
Medical writing, editorial support and the journal’s Rapid Service Fee were provided/funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Thomas Dörner, Rieke Alten, Natalia Bello, Ewa Haladyj, Gerd Burmester. Writing—original draft: Ewa Haladyj. Writing—reviewing and editing: Thomas Dörner, Edward M Vital, Sarah Ohrndorf, Rieke Alten, Natalia Bello, Ewa Haladyj, Gerd Burmester. Visualization: Gerd Burmester. Supervision: Gerd Burmester. Validation: Sarah Ohrndorf, Ewa Haladyj. Investigation: Sarah Ohrndorf. Formal analysis: Ewa Haladyj. Resources: Sarah Ohrndorf, Ewa Haladyj.
Medical Writing and/or Editorial Assistance
The authors would like to thank Dr. Gabrielle Stack, a medical writer and employee of Eli Lilly and Company, for writing and editorial support, and Praveen Shetty and Jeevan R, employees of Eli Lilly, for development of graphical images for the manuscript.
Disclosures
Thomas Dörner reports conflicts of interest from Eli Lilly and Company, AbbVie, Novartis, Janssen, Roche, Genentech and EULAR. Edward M. Vital reports conflicts of interest from Eli Lilly and Company, AstraZeneca, Sandoz, Roche, Aurinia, UCB, and GSK. Sarah Ohrndorf reports no conflicts of interest. Rieke Alten reports conflicts of interest from Eli Lilly, Abbvie, BMS, Celltrion, Galapagos, Novartis, Pfizer, Roche and UCB. Gerd Burmester reports conflicts of interest from Eli Lilly and Company. Ewa Haladyj and Natalia Bello are employees and minor shareholders of Eli Lilly and Company.
Compliance with Ethics Guidelines
The fluorescence optical imaging data were collected within the study with the local ethics number EK1/199/18. Before inclusion in the study, patients gave their written informed consent to participate including anonymized publication of their imaging data. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Thomas Dörner, Edward M. Vital and Sarah Ohrndorf contributed equally to this work.
References
- 1.Ceccarelli F, Perricone C, Cipriano E, et al. Joint involvement in systemic lupus erythematosus: from pathogenesis to clinical assessment. Semin Arthritis Rheum. 2017;47(1):53–64. doi: 10.1016/j.semarthrit.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharif K, Sharif A, Jumah F, Oskouian R, Tubbs RS. Rheumatoid arthritis in review: clinical, anatomical, cellular and molecular points of view. Clin Anat. 2018;31(2):216–223. doi: 10.1002/ca.22980. [DOI] [PubMed] [Google Scholar]
- 5.Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):ITC1–ITC16. doi: 10.7326/AITC201901010. [DOI] [PubMed] [Google Scholar]
- 6.Di Matteo A, Isidori M, Corradini D, et al. Ultrasound in the assessment of musculoskeletal involvement in systemic lupus erythematosus: state of the art and perspectives. Lupus. 2019;28(5):583–590. doi: 10.1177/0961203319834671. [DOI] [PubMed] [Google Scholar]
- 7.Antonini L, Le Mauff B, Marcelli C, Aouba A, de Boysson H. Rhupus: a systematic literature review. Autoimmun Rev. 2020;19(9):102612. doi: 10.1016/j.autrev.2020.102612. [DOI] [PubMed] [Google Scholar]
- 8.Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. 2020;110:102400. doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- 9.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 10.Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 11.Lucchino B, Spinelli FR, Iannuccelli C, Guzzo MP, Conti F, Di Franco M. Mucosa-environment interactions in the pathogenesis of rheumatoid arthritis. Cells. 2019;8(7):700. doi: 10.3390/cells8070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385(7):628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- 13.Veale DJ, Orr C, Fearon U. Cellular and molecular perspectives in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):343–354. doi: 10.1007/s00281-017-0633-1. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy A, Ng CT, Biniecka M, et al. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum. 2010;62(3):711–721. doi: 10.1002/art.27287. [DOI] [PubMed] [Google Scholar]
- 15.Pentony P, Duquenne L, Dutton K, et al. The initiation of autoimmunity at epithelial surfaces: a focus on rheumatoid arthritis and systemic lupus erythematosus. Discov Med. 2017;24(133):191–200. [PubMed] [Google Scholar]
- 16.Psarras A, Alase A, Antanaviciute A, et al. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat Commun. 2020;11(1):6149. doi: 10.1038/s41467-020-19918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nzeusseu Toukap A, Galant C, Theate I, et al. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56(5):1579–1588. doi: 10.1002/art.22578. [DOI] [PubMed] [Google Scholar]
- 18.Md Yusof MY, Psarras A, El-Sherbiny YM, et al. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann Rheum Dis. 2018;77(10):1432–1439. doi: 10.1136/annrheumdis-2018-213386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382(9894):819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 20.Sippl N, Faustini F, Rönnelid J, et al. Arthritis in systemic lupus erythematosus is characterized by local IL-17A and IL-6 expression in synovial fluid. Clin Exp Immunol. 2021;205:44–52. doi: 10.1111/cei.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis. 2005;64(12):1780–1782. doi: 10.1136/ard.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Holten J, Reedquist K, Sattonet-Roche P, et al. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2004;6(3):R239–R249. doi: 10.1186/ar1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adriaansen J, Kuhlman RR, van Holten J, Kaynor C, Vervoordeldonk MJ, Tak PP. Intraarticular interferon-beta gene therapy ameliorates adjuvant arthritis in rats. Hum Gene Ther. 2006;17(10):985–996. doi: 10.1089/hum.2006.17.985. [DOI] [PubMed] [Google Scholar]
- 24.Treschow AP, Teige I, Nandakumar KS, Holmdahl R, Issazadeh-Navikas S. Stromal cells and osteoclasts are responsible for exacerbated collagen-induced arthritis in interferon-beta-deficient mice. Arthritis Rheum. 2005;52(12):3739–3748. doi: 10.1002/art.21496. [DOI] [PubMed] [Google Scholar]
- 25.Merrill JT, Furie R, Werth VP, et al. Anifrolumab effects on rash and arthritis: impact of the type I interferon gene signature in the phase IIb MUSE study in patients with systemic lupus erythematosus. Lupus Sci Med. 2018;5(1):e000284. doi: 10.1136/lupus-2018-000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman RW, Merrill JT, Alarcón-Riquelme MM, et al. Gene expression and pharmacodynamic changes in 1,760 systemic lupus erythematosus patients from two phase III trials of BAFF blockade with tabalumab. Arthritis Rheumatol. 2017;69(3):643–654. doi: 10.1002/art.39950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiche L, Jourde-Chiche N, Whalen E, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014;66(6):1583–1595. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Sherbiny YM, Md Yusof MY, Psarras A, et al. B cell tetherin: a flow cytometric cell-specific assay for response to type I interferon predicts clinical features and flares in systemic lupus erythematosus. Arthritis Rheumatol. 2020;72(5):769–779. doi: 10.1002/art.41187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Sherbiny YM, Psarras A, Md Yusof MY, et al. A novel two-score system for interferon status segregates autoimmune diseases and correlates with clinical features. Sci Rep. 2018;8(1):5793. doi: 10.1038/s41598-018-24198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382(3):211–221. doi: 10.1056/NEJMoa1912196. [DOI] [PubMed] [Google Scholar]
- 31.Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393(10188):2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 32.Mouterde G, Rincheval N, Lukas C, et al. Outcome of patients with early arthritis without rheumatoid factor and ACPA and predictors of rheumatoid arthritis in the ESPOIR cohort. Arthritis Res Ther. 2019;21(1):140. doi: 10.1186/s13075-019-1909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hetland ML, Østergaard M, Stengaard-Pedersen K, et al. Anti-cyclic citrullinated peptide antibodies, 28-joint Disease Activity Score, and magnetic resonance imaging bone oedema at baseline predict 11 years' functional and radiographic outcome in early rheumatoid arthritis. Scand J Rheumatol. 2019;48(1):1–8. doi: 10.1080/03009742.2018.1466362. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi T, Soen S, Ishiguro N, et al. Predictors of new bone erosion in rheumatoid arthritis patients receiving conventional synthetic disease-modifying antirheumatic drugs: analysis of data from the DRIVE and DESIRABLE studies. Mod Rheumatol. 2021;31(1):34–41. doi: 10.1080/14397595.2019.1703484. [DOI] [PubMed] [Google Scholar]
- 35.Nordberg LB, Lillegraven S, Aga AB, et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open. 2018;4(2):e000752. doi: 10.1136/rmdopen-2018-000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordberg LB, Lillegraven S, Lie E, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naïve patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis. 2017;76(2):341–345. doi: 10.1136/annrheumdis-2015-208873. [DOI] [PubMed] [Google Scholar]
- 37.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 38.Zoma A. Musculoskeletal involvement in systemic lupus erythematosus. Lupus. 2004;13(11):851–853. doi: 10.1191/0961203303lu2021oa. [DOI] [PubMed] [Google Scholar]
- 39.Tani C, Carli L, Stagnaro C, et al. Imaging of joints in systemic lupus erythematosus. Clin Exp Rheumatol. 2018;36 Suppl 114(5):68–73. [PubMed] [Google Scholar]
- 40.Ogura T, Hirata A, Hayashi N, et al. Comparison of ultrasonographic joint and tendon findings in hands between early, treatment-naïve patients with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2017;26(7):707–714. doi: 10.1177/0961203316676375. [DOI] [PubMed] [Google Scholar]
- 41.van Vugt RM, Derksen RH, Kater L, Bijlsma JW. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis. 1998;57(9):540–544. doi: 10.1136/ard.57.9.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabba A, Piga M, Vacca A, et al. Joint and tendon involvement in systemic lupus erythematosus: an ultrasound study of hands and wrists in 108 patients. Rheumatology (Oxford) 2012;51(12):2278–2285. doi: 10.1093/rheumatology/kes226. [DOI] [PubMed] [Google Scholar]
- 43.Burgers LE, Raza K, van der Helm-van Mil AH. Window of opportunity in rheumatoid arthritis—definitions and supporting evidence: from old to new perspectives. RMD Open. 2019;5(1):e000870. doi: 10.1136/rmdopen-2018-000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urowitz MB, Ohsfeldt RL, Wielage RC, Kelton KA, Asukai Y, Ramachandran S. Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Ann Rheum Dis. 2019;78(3):372–379. doi: 10.1136/annrheumdis-2018-214043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Franco M, Guzzo MP, Spinelli FR, et al. Pain and systemic lupus erythematosus. Reumatismo. 2014;66(1):33–38. doi: 10.4081/reumatismo.2014.762. [DOI] [PubMed] [Google Scholar]
- 46.Nichilatti LP, Fernandes JM, Marques CP. Physiopathology of pain in systemic erythematosus lupus. Lupus. 2020;29(7):721–726. doi: 10.1177/0961203320919872. [DOI] [PubMed] [Google Scholar]
- 47.McWilliams DF, Walsh DA. Pain mechanisms in rheumatoid arthritis. Clin Exp Rheumatol. 2017;35 Suppl 107(5):94–101. [PubMed] [Google Scholar]
- 48.Harrington R, Al Nokhatha SA, Conway R. JAK inhibitors in rheumatoid arthritis: an evidence-based review on the emerging clinical data. J Inflamm Res. 2020;13:519–531. doi: 10.2147/JIR.S219586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sudoł-Szopińska I, Jans L, Teh J. Rheumatoid arthritis: what do MRI and ultrasound show. J Ultrason. 2017;17(68):5–16. doi: 10.15557/JoU.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubin DA. MRI and ultrasound of the hands and wrists in rheumatoid arthritis. I. Imaging findings. Skeletal Radiol. 2019;48(5):677–695. doi: 10.1007/s00256-019-03179-z. [DOI] [PubMed] [Google Scholar]
- 51.McQueen FM, Chan E. Insights into rheumatoid arthritis from use of MRI. Curr Rheumatol Rep. 2014;16(1):388. doi: 10.1007/s11926-013-0388-1. [DOI] [PubMed] [Google Scholar]
- 52.Mathew AJ, Danda D, Conaghan PG. MRI and ultrasound in rheumatoid arthritis. Curr Opin Rheumatol. 2016;28(3):323–329. doi: 10.1097/BOR.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 53.Zollars ES, Hyer M, Wolf B, Chapin R. Measuring lupus arthritis activity using contrasted high-field MRI. Associations with clinical measures of disease activity and novel patterns of disease. Lupus Sci Med. 2018;5(1):e000264. doi: 10.1136/lupus-2018-000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostendorf B, Scherer A, Specker C, Mödder U, Schneider M. Jaccoud's arthropathy in systemic lupus erythematosus: differentiation of deforming and erosive patterns by magnetic resonance imaging. Arthritis Rheum. 2003;48(1):157–165. doi: 10.1002/art.10753. [DOI] [PubMed] [Google Scholar]
- 55.Boutry N, Hachulla E, Flipo RM, Cortet B, Cotten A. MR imaging findings in hands in early rheumatoid arthritis: comparison with those in systemic lupus erythematosus and primary Sjögren syndrome. Radiology. 2005;236(2):593–600. doi: 10.1148/radiol.2361040844. [DOI] [PubMed] [Google Scholar]
- 56.Savnik A, Malmskov H, Thomsen HS, et al. Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol. 2001;28(10):2193–2200. [PubMed] [Google Scholar]
- 57.Tamai M, Kawakami A, Uetani M, et al. The presence of anti-cyclic citrullinated peptide antibody is associated with magnetic resonance imaging detection of bone marrow oedema in early stage rheumatoid arthritis. Ann Rheum Dis. 2006;65(1):133–134. doi: 10.1136/ard.2005.04138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porto LS, Tavares WCJ, Costa DA, Lanna CC, Kakehasi AM. Anti-CCP antibodies are not a marker of severity in established rheumatoid arthritis: a magnetic resonance imaging study. Rev Bras Reumatol Engl Ed. 2017;57(1):15–22. doi: 10.1016/j.rbr.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 59.Tani C, D'Aniello D, Possemato N, et al. MRI pattern of arthritis in systemic lupus erythematosus: a comparative study with rheumatoid arthritis and healthy subjects. Skeletal Radiol. 2015;44(2):261–266. doi: 10.1007/s00256-014-2054-8. [DOI] [PubMed] [Google Scholar]
- 60.Dakkak YJ, Jansen FP, DeRuiter MC, Reijnierse M, van der Helm-van Mil AHM. Rheumatoid arthritis and tenosynovitis at the metatarsophalangeal joints: an anatomic and MRI study of the forefoot tendon sheaths. Radiology. 2020;295(1):146–154. doi: 10.1148/radiol.2020191725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sudoł-Szopińska I, Kontny E, Maśliński W, et al. The pathogenesis of rheumatoid arthritis in radiological studies. Part I: formation of inflammatory infiltrates within the synovial membrane. J Ultrason. 2012;12(49):202–213. doi: 10.15557/JoU.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ball EM, Tan AL, Fukuba E, et al. A study of erosive phenotypes in lupus arthritis using magnetic resonance imaging and anti-citrullinated protein antibody, anti-RA33 and RF autoantibody status. Rheumatology (Oxford) 2014;53(10):1835–1843. doi: 10.1093/rheumatology/keu215. [DOI] [PubMed] [Google Scholar]
- 63.Mosca M, Tani C, Carli L, et al. The role of imaging in the evaluation of joint involvement in 102 consecutive patients with systemic lupus erythematosus. Autoimmun Rev. 2015;14(1):10–15. doi: 10.1016/j.autrev.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Yilmaz N, Yazici A, Özulu TÜrkmen B, Karalok I, Yavuz Ş. Sacroiliitis in systemic lupus erythematosus revisited. Arch Rheumatol. 2020;35(2):254–258. doi: 10.46497/ArchRheumatol.2020.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kayacan Erdogan E, Gokcen N, Badak SO, Bicakci YK, Arslan D. Sacroiliitis in systemic lupus erythematosus: the rates of involvement of the forgotten joint. Z Rheumatol. 2021;80(5):447–455. doi: 10.1007/s00393-020-00879-z. [DOI] [PubMed] [Google Scholar]
- 66.Baker JF, Conaghan PG, Gandjbakhch F. Update on magnetic resonance imaging and ultrasound in rheumatoid arthritis. Clin Exp Rheumatol. 2018;36 Suppl 114(5):16–23. [PubMed] [Google Scholar]
- 67.Teh J, Østergaard M. What the rheumatologist is looking for and what the radiologist should know in imaging for rheumatoid arthritis. Radiol Clin North Am. 2017;55(5):905–916. doi: 10.1016/j.rcl.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Filippucci E, Cipolletta E, Mashadi Mirza R, et al. Ultrasound imaging in rheumatoid arthritis. Radiol Med. 2019;124(11):1087–1100. doi: 10.1007/s11547-019-01002-2. [DOI] [PubMed] [Google Scholar]
- 69.Buosi AL, Natour J, Machado FS, Takahashi RD, Furtado RN. Hand ultrasound: comparative study between “no rhupus” lupus erythematosus and rheumatoid arthritis. Mod Rheumatol. 2014;24(4):599–605. doi: 10.3109/14397595.2013.857583. [DOI] [PubMed] [Google Scholar]
- 70.Hammer HB, Kvien TK, Terslev L. Intermetatarsal bursitis is frequent in patients with established rheumatoid arthritis and is associated with anti-cyclic citrullinated peptide and rheumatoid factor. RMD Open. 2019;5(2):e001076. doi: 10.1136/rmdopen-2019-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zayat AS, Md Yusof MY, Wakefield RJ, Conaghan PG, Emery P, Vital EM. The role of ultrasound in assessing musculoskeletal symptoms of systemic lupus erythematosus: a systematic literature review. Rheumatology (Oxford) 2016;55(3):485–494. doi: 10.1093/rheumatology/kev343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zayat AS, Mahmoud K, Md Yusof MY, et al. Defining inflammatory musculoskeletal manifestations in systemic lupus erythematosus. Rheumatology (Oxford) 2019;58(2):304–312. doi: 10.1093/rheumatology/key277. [DOI] [PubMed] [Google Scholar]
- 73.Ceccarelli F, Massaro L, Perricone C, et al. Jaccoud's arthropathy in systemic lupus erythematosus: clinical, laboratory and ultrasonographic features. Clin Exp Rheumatol. 2017;35(4):674–677. [PubMed] [Google Scholar]
- 74.Mahmoud K, Zayat AS, Yusof MYM, et al. Ultrasound to identify SLE patients with musculoskeletal symptoms who respond best to therapy: the USEFUL longitudinal multicentre study. Rheumatology (Oxford). 2021;60(11):5194-204. 10.1093/rheumatology/keab288. [DOI] [PMC free article] [PubMed]
- 75.Ossandon A, Iagnocco A, Alessandri C, Priori R, Conti F, Valesini G. Ultrasonographic depiction of knee joint alterations in systemic lupus erythematosus. Clin Exp Rheumatol. 2009;27(2):329–332. [PubMed] [Google Scholar]
- 76.Tani C, D'Aniello D, Delle Sedie A, et al. Rhupus syndrome: assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmun Rev. 2013;12(4):537–541. doi: 10.1016/j.autrev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Tămaş MM, Filippucci E, Becciolini A, et al. Bone erosions in rheumatoid arthritis: ultrasound findings in the early stage of the disease. Rheumatology (Oxford) 2014;53(6):1100–1107. doi: 10.1093/rheumatology/ket484. [DOI] [PubMed] [Google Scholar]
- 78.Lu CH, Chen LF, Huang YM, Cheng CF, Hsieh SC, Li KJ. Anti-cyclic citrullinated peptide antibodies correlate to ultrasound synovitis in rheumatoid arthritis better than C-reactive protein. J Clin Rheumatol. 2021;27(8):e412-17. 10.1097/rhu.0000000000001499. [DOI] [PMC free article] [PubMed]
- 79.Tan YK, Li H, Allen JC, Jr, Thumboo J. Anti-cyclic citrullinated peptide but not rheumatoid factor is associated with ultrasound-detected bone erosion among rheumatoid arthritis patients with at least moderate disease activity. Int J Rheum Dis. 2020;23(10):1337–1343. doi: 10.1111/1756-185X.13933. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Shao Q, Wu J. Correlation between high-frequency ultrasonography of patients with early rheumatoid arthritis and anti-CCP antibody. Medicine (Baltimore) 2019;98(6):e14083. doi: 10.1097/MD.0000000000014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens MJ, Walker-Bone K, Culliford DJ, et al. Work participation, mobility and foot symptoms in people with systemic lupus erythematosus: findings of a UK national survey. J Foot Ankle Res. 2019;12:26. doi: 10.1186/s13047-019-0335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams AE, Blake A, Cherry L, et al. Patients' experiences of lupus-related foot problems: a qualitative investigation. Lupus. 2017;26(11):1174–1181. doi: 10.1177/0961203317696590. [DOI] [PubMed] [Google Scholar]
- 83.Cherry L, Alcacer-Pitarch B, Hopkinson N, et al. The prevalence of self-reported lower limb and foot health problems experienced by participants with systemic lupus erythematosus: results of a UK national survey. Lupus. 2017;26(4):410–416. doi: 10.1177/0961203316670730. [DOI] [PubMed] [Google Scholar]
- 84.Williams AE, Cherry L, Blake A, et al. An investigation into the scale and impact of self-reported foot problems associated with systemic lupus erythematosus: a study protocol and survey questionnaire development. Musculoskeletal Care. 2016;14(2):110–115. doi: 10.1002/msc.1119. [DOI] [PubMed] [Google Scholar]
- 85.Sant'Ana Petterle G, Natour J, Rodrigues da Luz K, et al. Usefulness of US to show subclinical joint abnormalities in asymptomatic feet of RA patients compared to healthy controls. Clin Exp Rheumatol. 2013;31(6):904–912. [PubMed] [Google Scholar]
- 86.Morales-Lozano R, Martínez-Barrio J, González-Fernández ML, et al. The feet in systemic lupus erythematosus; are we underestimating their involvement and functional impact? Clin Exp Rheumatol. 2016;34(4):609–617. [PubMed] [Google Scholar]
- 87.Ohrndorf S, Glimm AM, Ammitzbøll-Danielsen M, Ostergaard M, Burmester GR. Fluorescence optical imaging: ready for prime time? RMD Open. 2021;7(2):e001497. doi: 10.1136/rmdopen-2020-001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong PC, Lee G, Sedie AD, et al. Musculoskeletal ultrasound in systemic lupus erythematosus: systematic literature review by the lupus task force of the OMERACT Ultrasound Working Group. J Rheumatol. 2019;46(10):1379–1387. doi: 10.3899/jrheum.181087. [DOI] [PubMed] [Google Scholar]
- 89.Stolt M, Suhonen R, Leino-Kilpi H. Foot health in patients with rheumatoid arthritis—a scoping review. Rheumatol Int. 2017;37(9):1413–1422. doi: 10.1007/s00296-017-3699-0. [DOI] [PubMed] [Google Scholar]
- 90.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 91.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 92.Franklyn K, Lau CS, Navarra SV, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS) Ann Rheum Dis. 2016;75(9):1615–1621. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 93.Gatto M, Zen M, Iaccarino L, Doria A. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol. 2019;15(1):30–48. doi: 10.1038/s41584-018-0133-2. [DOI] [PubMed] [Google Scholar]
- 94.van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS) Ann Rheum Dis. 2017;76(3):554–561. doi: 10.1136/annrheumdis-2016-209519. [DOI] [PubMed] [Google Scholar]
- 95.Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92(7):1129–1143. doi: 10.1016/j.mayocp.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Pope JE, Movahedi M, Rampakakis E, et al. ACPA and RF as predictors of sustained clinical remission in patients with rheumatoid arthritis: data from the Ontario Best practices Research Initiative (OBRI) RMD Open. 2018;4(2):e000738. doi: 10.1136/rmdopen-2018-000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arriens C, Wren JD, Munroe ME, Mohan C. Systemic lupus erythematosus biomarkers: the challenging quest. Rheumatology (Oxford) 2017;56(suppl_1):i32–i45. doi: 10.1093/rheumatology/kew407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ginzler EM, Wofsy D, Isenberg D, Gordon C, Lisk L, Dooley MA. Nonrenal disease activity following mycophenolate mofetil or intravenous cyclophosphamide as induction treatment for lupus nephritis: findings in a multicenter, prospective, randomized, open-label, parallel-group clinical trial. Arthritis Rheum. 2010;62(1):211–221. doi: 10.1002/art.25052. [DOI] [PubMed] [Google Scholar]
- 99.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carreira PL, Isenberg DA. Recent developments in biologic therapies for the treatment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2019;58(3):382–387. doi: 10.1093/rheumatology/key064. [DOI] [PubMed] [Google Scholar]
- 101.Iaccarino L, Andreoli L, Bocci EB, et al. Clinical predictors of response and discontinuation of belimumab in patients with systemic lupus erythematosus in real life setting. Results of a large, multicentric, nationwide study. J Autoimmun. 2018;86:1–8. doi: 10.1016/j.jaut.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Sudoł-Szopińska I, Zaniewicz-Kaniewska K, Warczyńska A, Matuszewska G, Saied F, Kunisz W. The pathogenesis of rheumatoid arthritis in radiological studies. Part II: Imaging studies in rheumatoid arthritis. J Ultrason. 2012;12(50):319–328. doi: 10.15557/JoU.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vyas S, Bhalla AS, Ranjan P, Kumar S, Kumar U, Gupta AK. Rheumatoid arthritis revisited—advanced imaging review. Pol J Radiol. 2016;81:629–635. doi: 10.12659/PJR.899317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Østergaard M, Boesen M. Imaging in rheumatoid arthritis: the role of magnetic resonance imaging and computed tomography. Radiol Med. 2019;124(11):1128–1141. doi: 10.1007/s11547-019-01014-y. [DOI] [PubMed] [Google Scholar]
- 105.Pfeil A, Oelzner P, Bornholdt K, et al. Joint damage in rheumatoid arthritis: assessment of a new scoring method. Arthritis Res Ther. 2013;15(1):R27. doi: 10.1186/ar4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan YK, Li H, Allen JC, Jr, Thumboo J. Extended 36-joint sonographic examination: What it reveals about bone erosions in patients with rheumatoid arthritis. J Clin Ultrasound. 2020;48(1):14–18. doi: 10.1002/jcu.22785. [DOI] [PubMed] [Google Scholar]
- 107.Piga M, Saba L, Gabba A, et al. Ultrasonographic assessment of bone erosions in the different subtypes of systemic lupus erythematosus arthritis: comparison with computed tomography. Arthritis Res Ther. 2016;18(1):222. doi: 10.1186/s13075-016-1125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.