Abstract
Objective
This systematic literature review aimed to identify and summarise real-world observational studies reporting the type, prevalence and/or severity of residual symptoms and disease in adults with psoriatic arthritis (PsA) who have received treatment and been assessed against remission or low disease activity targets.
Methods
Patients had received treatment and been assessed with treat-to-target metrics, including minimal disease activity (MDA), Disease Activity Index in PsA (DAPSA) and others. MEDLINE, Embase® and the Cochrane Database of Systematic Reviews (CDSR) were searched using search terms for PsA, treatment targets and observational studies. Screening of search results was completed by two independent reviewers; studies were included if they reported relevant residual disease outcomes in adults with PsA who had received one or more pharmacological treatments for PsA in a real-world setting. Non-observational studies were excluded. Information from included studies was extracted into a prespecified grid by a single reviewer and checked by a second reviewer.
Results
Database searching yielded 2328 articles, of which 42 publications (27 unique studies) were included in this systematic literature review. Twenty-three studies reported outcomes for MDA-assessed patients, and 14 studies reported outcomes for DAPSA-assessed patients. Physician- and patient-reported residual disease was less frequent and/or severe in patients reaching targets, but often not absent, including when patients achieved very low disease activity (VLDA) or remission. For example, studies reported that 0–8% patients in remission according to DAPSA (or clinical DAPSA) had > 1 tender joint, 25–39% had Psoriasis Area and Severity Index (PASI) score > 1 and 0–10% had patient-reported pain > 15. Residual disease was usually less frequent and/or severe among patients achieving MDA-assessed targets versus DAPSA-assessed targets, especially for skin outcomes.
Conclusion
The findings demonstrate a need for further optimisation of care for patients with PsA.
Keywords: Disease burden, Low disease activity, Minimal disease activity, Observational studies, Psoriasis, Psoriatic arthritis, Real world evidence, Remission, Residual disease, Treatment targets
Key Summary Points
| To our knowledge, this is the first systematic review of real-world evidence to describe residual disease burden among patients with psoriatic arthritis who have received treatment and been assessed against treatment targets. |
| This study demonstrates the breadth of different symptoms that can persist despite treatment, even among those patients who have achieved stringent treatment targets. |
| Residual musculoskeletal and skin disease were frequently observed, as well as residual patient-reported pain, fatigue, disability and disease impact on patients’ lives. |
| Our findings demonstrate the variability of residual disease seen with different targets; they also indicate a possible discordance between patients’ and physicians’ perspectives of disease control and treatment success. |
| This review highlights the need for further optimisation of care for patients with psoriatic arthritis. |
Digital Features
This article is published with digital features, including a slide deck to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.19355069.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease affecting 20–30% of patients with psoriasis [1]. Symptoms and signs vary, but typically include peripheral and axial joint pain and swelling, enthesitis, nail disease and skin psoriasis [2–4]. Ongoing inflammation is associated with further comorbidities, including cardiovascular disease, uveitis and subclinical bowel inflammation [1]. PsA disease and comorbidities can result in reduced function and quality of life, and increased mortality [5, 6]. While not currently curable, available treatments for PsA can slow progression and relieve symptoms [3]. However, patients may still experience residual symptoms and disease burden, including persistent joint pain and swelling, anxiety, depression, fatigue and functional disability [7–10].
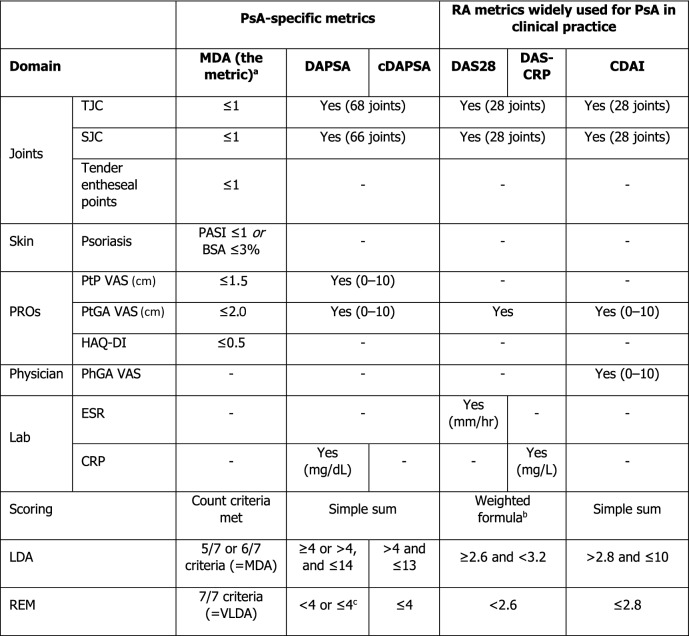
Based on success in rheumatoid arthritis (RA) and documented benefits in the TIght COntrol of Psoriatic Arthritis (TICOPA) study, international groups, including the European Alliance of Associations for Rheumatology (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), support a treat-to-target (T2T) approach for PsA [11–17], recommending remission (REM) as the primary treatment target and low disease activity (LDA) as an alternative [13, 16]. Despite this recommendation, there is a lack of consensus on how REM and LDA should be assessed [12]. Applicable measures include the minimal disease activity (MDA) metric, Disease Activity Index in PsA (DAPSA) and clinical DAPSA (cDAPSA), all of which have been specifically designed to assess PsA disease (Table 1). GRAPPA recommends using the MDA metric over DAPSA, since the MDA metric incorporates psoriasis and enthesitis assessments in addition to peripheral joint disease [13]. However, there is a need to further understand the validity and relative benefits of these metrics, as only the MDA metric has been tested in a T2T strategy trial including patients with PsA [11, 12].
Table 1.
Summary of selected disease activity metrics and low disease activity/remission thresholds
BSA Body surface area, CDAI Clinical Disease Activity Index, cDAPSA clinical DAPSA, CRP C-reactive protein, DAPSA Disease Activity Index in PsA, DAS28 Disease Activity Score 28, DAS-CRP Disease Activity Score-C-reactive protein, ESR erythrocyte sedimentation rate, HAQ-DI Health Assessment Questionnaire-Disability Index, LDA low disease activity, MDA minimal disease activity, PASI Psoriasis Area and Severity Index, PhGA physician’s global assessment, PROs patient-reported outcomes, PsA psoriatic arthritis, PtGA patient’s global assessment, PtP patient pain, RA rheumatoid arthritis, REM remission, SJC swollen joint count, TJC tender joint count, VAS visual analogue scale, VLDA very low disease activity
aModified versions of the MDA metric (e.g. substitution of PASI ≤ 1 with PtGA “Clear”, or requirement for ≥ 6/7 criteria to be met) were also considered relevant in this review
bCut-off varies depending on publication; either threshold was considered relevant in this review. VAS scores use a 0–100 VAS unless stated otherwise
cDAS28 formula: DAS-CRP formula:
This lack of consensus on the optimal measure to define PsA treatment targets is reflected in the modest uptake of T2T among healthcare professionals [12]. Moreover, where T2T is used, there is notable variation in the disease activity metrics employed to define REM or LDA for PsA [18]. The MDA metric, DAPSA and cDAPSA are widely used, as well as metrics designed for RA/other non-PsA arthritides (Table 1) [12, 19, 20] which are less appropriate since they do not account for some PsA-specific aspects of disease [7, 21–23].
Although the achievement of treatment targets has been associated with improved quality of life and slower disease progression, patients may still experience symptoms and a burden of disease. This applies even for the most stringently defined REM by the MDA metric (very low disease activity [VLDA]), and residual disease is likely greater among patients achieving less stringent forms of REM by other disease activity metrics [7, 24, 25]. Our current understanding of unmet need in treated patients is limited, yet this knowledge is essential to refine care to address residual disease. This includes residual disease among patients meeting treatment targets, as well as persistent symptoms in patients not meeting treatment targets.
The aim of this systematic literature review (SLR) was to identify and summarise real-world observational studies reporting type, prevalence and/or severity of residual symptoms and disease in adults with PsA who have received treatment and been assessed against REM or LDA targets. Within this aim, the primary objective was to examine residual symptoms of disease (musculoskeletal, skin and patient-reported outcomes [PROs]) in patients whose disease activity was measured using the MDA metric, DAPSA or cDAPSA, these measures being PsA-specific and in common use in PsA care. The secondary objective was to examine residual disease in patients whose disease activity was measured by three widely used non-PsA-specific measures, namely Disease Activity Score 28 (DAS28), DAS with C-reactive protein (DAS-CRP) and Clinical Disease Activity Index (CDAI), and to qualitatively compare these to the PsA-specific measures.
Methods
Search Strategy and Selection Criteria
This SLR was conducted in accordance with a pre-specified protocol (see Electronic Supplementary Material [ESM]) and adheres to the PRISMA guidelines [26]. MEDLINE, Embase® and the Cochrane Database of Systematic Reviews (CDSR) were searched on 8 October 2020, using search terms for PsA, treatment targets and (in the MEDLINE and Embase databases) observational studies (ESM Tables S1–S3). Searches were limited to literature published since 1 January 2015 because of the considerable recent advances in PsA treatment. Supplementary searches included: (1) the bibliographies of relevant SLRs identified through the database searches, and (2) congress abstracts from nine selected congresses (ESM Table S4).
After removing duplicates, records were screened according to the process recommended by the Cochrane Collaboration [27]. Titles and abstracts of the search results were screened against eligibility criteria by two reviewers working independently; discrepancies were resolved by consensus, or by an arbitrator if necessary. Full-text versions of potentially relevant articles were screened using the same process.
To be eligible, studies had to report relevant residual disease outcomes (prevalence or severity of any musculoskeletal, skin or PROs), in adults with PsA who had received one or more pharmacological treatments for their condition in a real-world setting (i.e. reported in an observational study). Interventional, modelling or economic studies, case studies/reports and narrative reviews, editorials or commentaries were excluded. Indicators that are less directly linked to patients’ experience of PsA, such as laboratory, radiology, economic and proxy outcomes, were excluded. The Psoriatic Arthritis Disease Activity Score (PASDAS) was not included in this review given that it is designed for use in clinical trials, and real-world use can be practically challenging [28]. Relevant outcomes had to be reported separately for patients who met relevant thresholds of disease control (MDA/VLDA as measured by the MDA metric criteria, or LDA/REM as measured by DAPSA, cDAPSA, DAS28, DAS-CRP or CDAI; Table 1) and/or those who did not meet these thresholds. No language restrictions were imposed within the eligibility criteria; full eligibility criteria are shown in ESM Table S5.
This SLR is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Unless stated otherwise, the term MDA is hereafter used to refer to the disease activity state of MDA (with 5–6/7 of the criteria met). ‘MDA metric’ is used to describe the seven-criteria disease activity index in general. For ease of comparison, MDA and LDA are presented as equivalent tiers of disease control, as are VLDA and REM (ESM Fig. S1). The terms ‘at least MDA’ or ‘at least LDA’ are used to describe a group of patients who achieved at least MDA (i.e. patients could have been in either MDA or VLDA; also referred to as VLDA + MDA) or at least LDA (i.e. patients could have been in either LDA or REM; also referred to as REM + LDA), respectively (ESM Fig. S1).
Data Analysis
Publications on the same cohort of patients were treated as a single unit. Data extraction and quality assessment followed guidelines from the University of York Centre for Reviews and Dissemination (CRD) [29]. Information from included studies, including group-level outcomes, were extracted into a prespecified grid (ESM Table S6) by a single reviewer and verified and checked for completeness by a second reviewer. Discrepancies were resolved by consensus, or if necessary, by arbitration. The quality of included studies was assessed using an adaptation of a checklist recommended by the National Institute for Health and Care Excellence (NICE) for evaluating the quality of prognosis studies in SLRs (ESM Table S7) [30].
Results
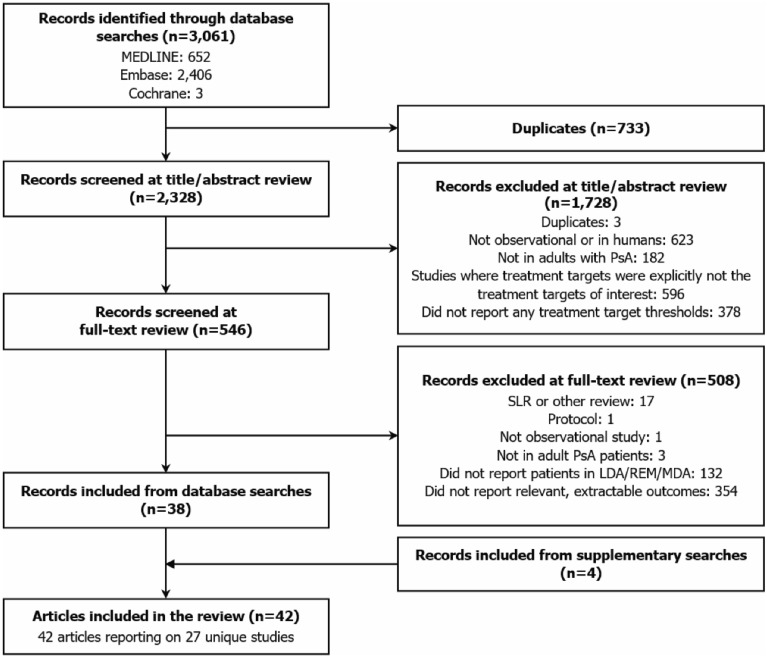
The electronic database search identified 2328 unique records; 546 were included in the full-text review, of which 38 publications were included in the SLR, alongside four articles identified through the supplementary searches (Fig. 1). The 42 included articles comprised 27 unique studies (i.e. some articles referred to the same study) of which 21 were cross-sectional and six were longitudinal. Overall, 23 studies reported relevant outcomes for patients assessed by the MDA metric, while fewer reported outcomes for patients assessed by DAPSA (number of studies [NS] = 14), DAS28 (NS = 3) and CDAI (NS = 2); 13 studies included multiple disease activity metrics. There was substantial heterogeneity in the reporting of outcomes and the grouping of patients (ESM Table S8). ESM Fig. S1 presents an overview of the different ways in which patients were grouped in studies, according to disease activity states.
Fig. 1.
PRISMA flow diagram of study selection. LDA low disease activity, MDA minimal disease activity, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PsA psoriatic arthritis, REM remission, SLR systematic literature review
All 27 studies reported relevant residual disease outcomes, and 20 of the studies included residual disease in their study objectives; one study explicitly used a T2T strategy. Studies were distributed across Europe, Asia and North and South America, including 5138 patients with PsA in total; sample sizes ranged from 20 to 624 patients (NS = 27). Where reported, mean age ranged from 41.4 to 59 years (NS = 21). Mean prior disease duration ranged from 3.8 to 15.3 years (NS = 14). Five studies reported patients’ treatment history prior to enrolment (ESM Table S9). Ten studies reported treatments initiated at enrolment: seven studies included conventional synthetic DMARDs and seven included biologic DMARDs (ESM Table S10). Study characteristics are reported in ESM Tables S11 and S12–S17. The quality assessment identified that potential sources of bias were addressed with varying levels of adequacy across studies, although many studies did not report enough detail to fully assess risk of bias (ESM Table S11). A more detailed summary of the quality assessment is provided in ESM Table S18.
Among patients assessed by PsA-specific metrics, 35–85% of each study population (NS = 5) did not achieve MDA or LDA [7, 31–35], 14–100% (NS = 11) achieved at least MDA or LDA (the most commonly reported group) [7, 31, 33–45] and 12–43% (NS = 6) achieved VLDA or REM (ESM Table S8) [37, 40, 41, 45–49].
Residual Musculoskeletal Disease
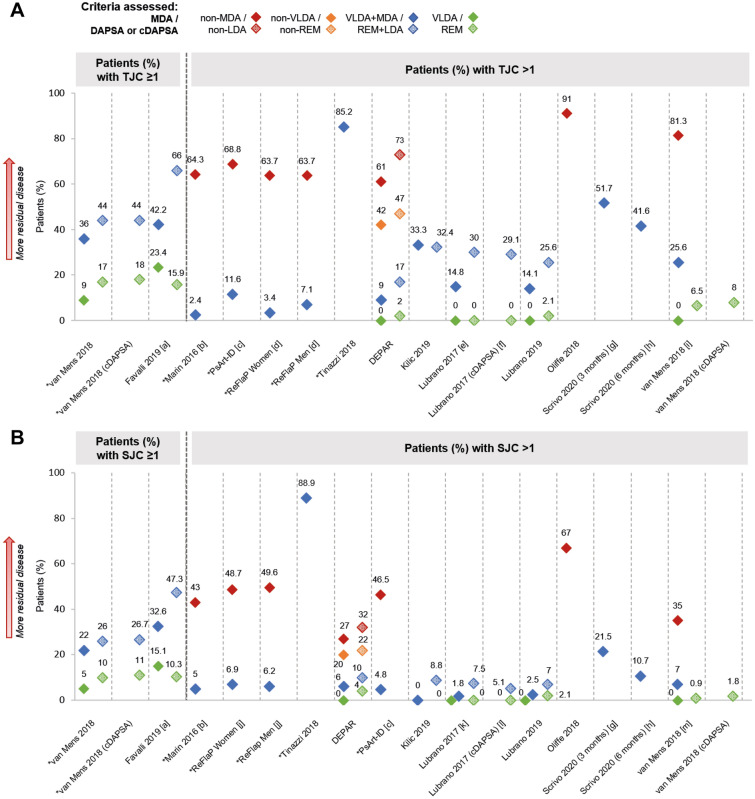
Musculoskeletal outcomes were reported in 18 studies (ESM Tables S8, S19–41). Among groups that did not achieve MDA or DAPSA/cDAPSA LDA, 61–91% patients (NS = 6) had a tender joint count (TJC) > 1 (Fig. 2a) [31, 32, 35, 45, 50, 51], and mean TJC ranged from 1.8 to 10.3 (NS = 2) [50, 51]. Across groups that achieved at least MDA or DAPSA/cDAPSA LDA, 2–85% patients (NS = 10) had TJC > 1 (Fig. 2a) [7, 31, 40, 41, 45, 50–54], and mean TJC ranged from 0.3 to 0.8 (NS = 4) [50, 51, 55, 56]. Residual musculoskeletal disease was least evident among groups that reached VLDA, in which 0% patients had TJC > 1 (NS = 4; 9% patients who reached VLDA had TJC = 1 in van Mens et al. [35]), or DAPSA/cDAPSA REM, in which 0–8% patients had TJC > 1 across the same four studies (Fig. 2a) [40, 41, 44, 45, 54]. Similar patterns of residual disease were reported for swollen joint count (SJC; Fig. 2b). A smaller number of studies reported enthesitis (primarily using the Leeds Enthesitis Index [LEI]), tenosynovitis, oligoarthritis and dactylitis, for which residual disease was also observed (ESM Tables S29–S41).
Fig. 2.
Prevalence of tender joints (a) and swollen joints (b), where reported. Note: Unless stated otherwise, shapes with hatching refer to patients assessed by DAPSA, not cDAPSA. Asterisk indicates that data were inversed for ease of comparison (e.g. percentage of patients with TJC ≤ 1 has been transformed into percentage of patients with TJC > 1). [a] Digitised from a graph in the abstract. [b] p < 0.0001 VLDA + MDA vs. non-MDA. [c] p < 0.001 VLDA + MDA vs. non-MDA. [d] p = 0.341 VLDA + MDA women vs. men; p = 0.174 non-MDA women vs. men (likely a misprint in the data). [e] VLDA group: in a separate subgroup analysis of n = 15, 9 (60%) patients had TJC = 0, while 6 (40%) patients had TJC = 1; DAPSA REM group: in a separate subgroup analysis of n = 18, 13 (72.2%) patients had TJC = 0, while 5 (27.6%) patients had TJC = 1. [f] cDAPSA REM group: in a separate subgroup analysis of n = 22, 16 (72.7%) patients had TJC = 0, while 6 (27.3%) patients had TJC = 1. [g] Data unknown for 11 (7.4%) of participants. [h] Data unknown for 24 (16.1%) of participants. [i] Data for VLDA + MDA group and non-MDA group digitised from a graph in the manuscript; p < 0.001 VLDA + MDA vs non-MDA. [j] p = 0.863 VLDA + MDA women vs. men; p = 0.248 non-MDA women vs. men. [k] VLDA group: in a separate subgroup analysis of n = 15, 14 (93.3%) patients had SJC = 0, while 1 (6.7%) patient had SJC = 1; DAPSA REM group: in a separate subgroup analysis of n = 18, 17 (94.5%) patients had SJC = 0, while 1 (5.5%) patient had SJC = 1. [l] cDAPSA REM group: in a separate subgroup analysis of n = 22, 21 (95.4%) patients had SJC = 0, while 1 (4.5%) patient had SJC = 1. [m] p = 0.000 for VLDA + MDA vs. non-MDA. (c)DAPSA (clinical) Disease Activity Index in PsA, SJC swollen joint count, TJC tender joint count, VLDA very low disease activity
One study reported musculoskeletal outcomes for DAS28-assessed patients, in which 36% patients who had achieved at least LDA (REM + LDA) had TJC > 1; residual SJC, enthesitis, tenosynovitis and dactylitis were also reported for this patient group [7]. One study reported musculoskeletal outcomes for CDAI-assessed patients, in which median TJC28 was 5 (interquartile range: 3–8) among patients in CDAI REM; substantial residual SJC, enthesitis and dactylitis were also reported [57]. For four studies in which patients were assessed using more than one disease activity metric, there were mostly fewer patients with TJC > 1 and SJC > 1 in groups who achieved at least MDA than in groups who achieved at least DAPSA/cDAPSA LDA (Fig. 2) [7, 40, 41, 45]. van Mens et al. reported that 64% patients in VLDA + MDA achieved TJC = 0, compared with 56% in DAPSA REM + LDA (Fig. 2a) [44].
Residual Skin Disease
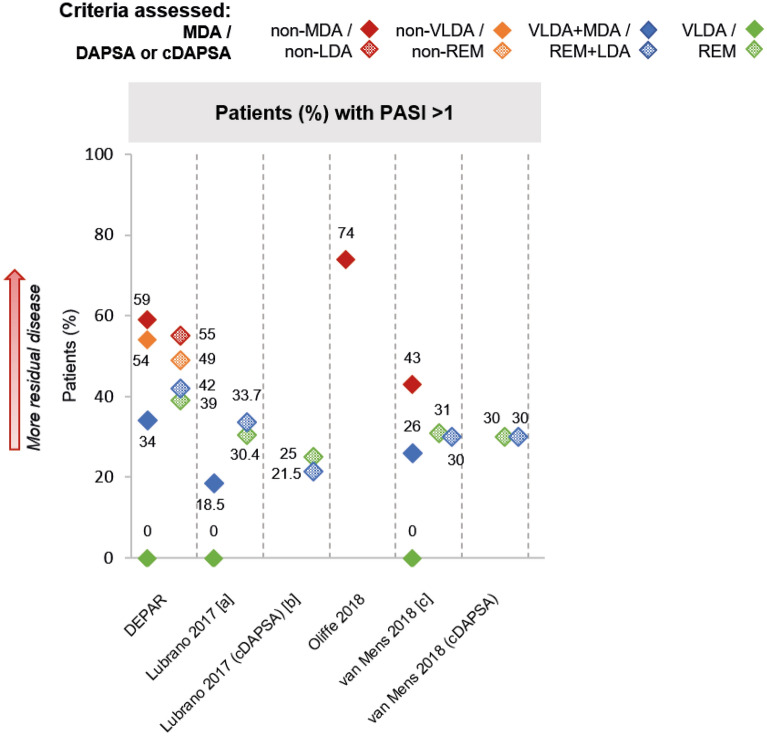
Skin outcomes were reported in 14 studies (ESM Tables S8, S42–S46). Among groups that did not achieve MDA, mean Psoriasis Area and Severity Index (PASI) score ranged from 2.8 to 4 (NS = 3) [33, 42, 51] and mean body surface area was 12% (NS = 1) [51]; 43–74% patients (NS = 3) had PASI > 1 (Fig. 3) [32, 34, 35, 45]. In the one study that reported PASI for groups that did not achieve DAPSA LDA, 55% patients had PASI > 1 [45]. Among groups that achieved at least MDA, 19–34% patients had PASI > 1 (NS = 3) [34, 35, 40, 45], while 22–42% (NS = 3) of those who achieved at least DAPSA/cDAPSA LDA had PASI > 1 [40, 44, 45]. Among VLDA groups, 0% patients had PASI > 1 across the three studies concerned, whereas among DAPSA/cDAPSA REM groups, 25–39% patients had PASI > 1 across the same three studies (Fig. 3) [40, 44, 45]. Only one study reported skin outcomes (PASI scores) for patients assessed by DAS28 (ESM Table S44) [58]; no skin outcomes were reported for CDAI-assessed patients.
Fig. 3.
Prevalence of skin disease by PASI, where reported. Note: Unless stated otherwise, shapes with hatching refer to patients assessed by DAPSA, not cDAPSA. Asterisk indicates that data were inversed for ease of comparison (e.g. percentage of patients with PASI ≤ 1 has been transformed into percentage of patients with PASI > 1). [a] VLDA group: in a separate subgroup analysis of n = 15, 12 (80.0%) patients had PASI 0–0.3, 2 (13.3%) patients had PASI 0.4–0.6 and 1 (6.7%) patient had PASI 0.7–1; DAPSA REM group: in a separate subgroup analysis of n = 18, 11 (61.1%) patients had PASI 0–0.3, 2 (11.1%) patients had PASI 0.4–0.6 and 5 (27.7%) patients had PASI > 1. [b] cDAPSA REM: in a separate subgroup analysis of n = 22, 14 (63.6%) patients had PASI 0–0.3, 2 (9.0%) patients had PASI 0.4–0.6, 1 (4.5%) patient had PASI 0.7–1 and 5 (22.7%) patients had PASI > 1. [c] p = 0.002 VLDA + MDA vs. non-MDA. PASI Psoriasis Area and Severity Index
Residual Patient-Reported Disease
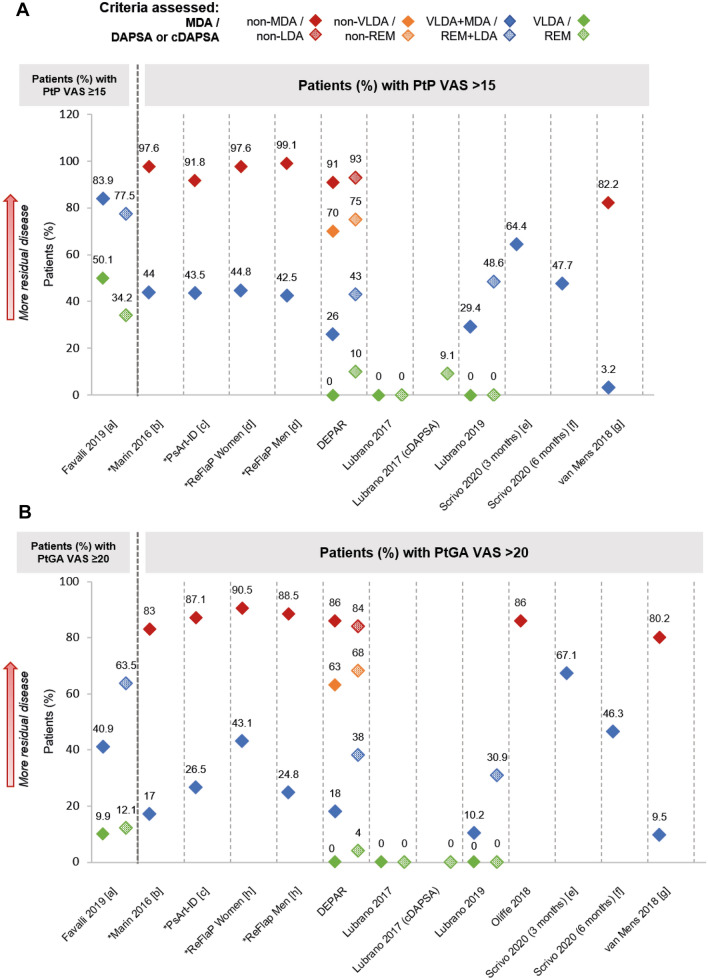
PROs were reported in 26 studies (ESM Tables S8, S47–S70). Among groups that did not achieve MDA or DAPSA/cDAPSA LDA, mean patient pain (PtP) on a 0–100 visual analogue scale (VAS; 1–10 VAS transformed where necessary) ranged from 44 to 63 (NS = 3) [39, 51, 58] and from 47 to 66, respectively (NS = 2) [39, 50]. Among groups that achieved at least MDA or DAPSA/cDAPSA LDA, mean PtP VAS ranged from 9 to 25 (NS = 5) [39, 51, 55, 56, 58] and from 18 to 27 (NS = 3) [39, 50, 55], respectively; it was also common to see PtP VAS > 15 in these groups (Fig. 4a). In three studies that assessed patients using multiple disease activity metrics, prevalence of PtP VAS > 15 among patients who achieved VLDA was 0% in the three studies, whereas for patients who achieved DAPSA REM, prevalence of PtP VAS > 15 was 0% in two studies (one of which also reported PtP VAS > 15 for 9% patients in cDAPSA REM) [46] and 10% in the other (Fig. 4a) [41, 45, 46]. PtP VAS was also reported in two studies that used DAS28 and in one study that assessed patients by CDAI [7, 57, 58]; within these studies, residual PtP VAS was reported across all tiers of disease activity, including DAS28/CDAI REM (Tables ESM S47–S51).
Fig. 4.
Patient pain VAS (a) and patient global assessment VAS (b), where reported. Note: Unless stated otherwise, shapes with hatching refer to patients assessed by DAPSA, not cDAPSA. Asterisk indicates data that were inversed for ease of comparison (e.g. percentage of patients with PtP VAS ≤ 15 has been transformed into percentage of patients with PtP VAS > 15). [a] Digitised from a graph in the abstract. [b] p < 0.0001 VLDA + MDA vs. non-MDA. [c] p < 0.001 VLDA + MDA vs. non-MDA. [d] p = 0.771 VLDA + MDA women vs. men; p = 0.355 non-MDA women vs. men. [e] Data unknown for 11 (7.4%) participants. [f] Data unknown for 24 (16.1%) participants. [g] Digitised from a graph in the manuscript; p < 0.001 VLDA + MDA vs. non-MDA. [h] p = 0.011 VLDA + MDA women vs. men; p = 0.594 non-MDA women vs. men. PtGA patient global assessment, PtP patient pain, VAS visual analogue scale
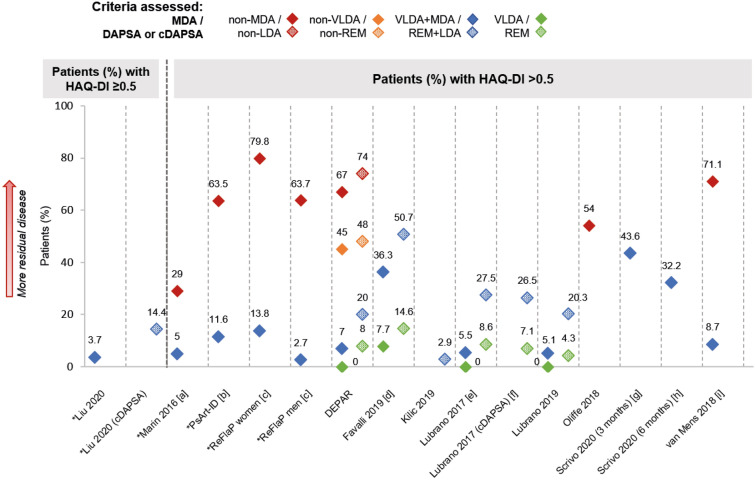
Similar patterns of residual disease were observed in findings from the patient global assessment (PtGA) VAS (Fig. 4b) and Health Assessment Questionnaire Disability Index (HAQ-DI; Fig. 5). Among groups that did not achieve MDA or DAPSA/cDAPSA LDA, 80–91% patients (NS = 6) [31, 32, 35, 45, 50, 51] and 84% patients (NS = 1) [45] had PtGA VAS > 20, respectively; 29–80% patients (NS = 6) [31, 32, 35, 45, 50, 51] and 74% patients (NS = 1) [45] had HAQ-DI > 0.5, respectively. Trends like those observed in musculoskeletal and skin outcomes were also seen for both these PROs, with less residual disease in groups with more stringent disease control, and less residual disease in MDA and VLDA groups compared to DAPSA/cDAPSA LDA and REM groups, respectively (Figs. 4b; 5). For example, among VLDA groups, no patients had HAQ-DI > 0.5 in three studies [40, 41, 45, 46] and 8% patients were above this threshold in a further study [37]; among DAPSA/cDAPSA REM groups, 4–15% patients (NS = 4) [37, 40, 41, 45, 46] had HAQ-DI > 0.5.
Fig. 5.
HAQ-DI, where reported. Note: Unless stated otherwise, shapes with hatching refer to patients assessed by DAPSA, not cDAPSA. Asterisk indicates data that were inversed for ease of comparison (e.g. percentage of patients with HAQ-DI ≤ 0.5 has been transformed into percentage of patients with HAQ-DI > 0.5). [a] p = 0.004 VLDA + MDA vs. non-MDA. [b] p < 0.001 VLDA + MDA vs. non-MDA. [c] p = 0.005 VLDA + MDA women vs. men; p = 0.003 non-MDA women vs. men. [d] Digitised from a graph in the abstract. [e] VLDA group: in a separate subgroup analysis of n = 15, all patients had HAQ-DI ≤ 0.5; DAPSA REM group: in a separate subgroup analysis of n = 18, 17 (94.5%) patients achieved HAQ-DI ≤ 0.5, while 1 (5.5%) patient had HAQ-DI > 0.5. [f] cDAPSA REM group: in a separate subgroup analysis of n = 22, 20 (90.9%) patients achieved HAQ-DI ≤ 0.5, while 2 (9.1%) patients had HAQ-DI > 0.5. [g] Data unknown for 11 (7.4%) participants. [h] Data unknown for 24 (16.1%) participants. [i] Digitised from a graph in the manuscript; p < 0.001 VLDA + MDA vs non-MDA. HAQ-DI Health Assessment Questionnaire-Disability Index
Five studies that reported PtGA VAS also reported physician global assessment (PhGA) VAS. In different studies and patient groups, there were differing degrees of alignment between PtGA and PhGA; for example, in van Mens et al., median PtGA and PhGA VAS were 6 and 7, respectively, among patients who achieved at least MDA, while medians were 37 and 23, respectively, in patients who did not achieve MDA [35]. Generally, there was a trend for PtGA to be higher (i.e. worse) than PhGA, as demonstrated in the PsArt-ID study in which median PtGA and PhGA VAS among patients who achieved at least MDA were 20 and 10, respectively, while in patients who did not achieve MDA, median PtGA and PhGA were 50 and 35, respectively [31].
Residual disease in treated patients, including those who had met treatment targets, was also evident across PROs that are not components of the MDA metric, DAPSA or the other included metrics (Table 1). These PROs encompassed patient-reported fatigue, disability and quality of life measures, as detailed in ESM Tables S8 and S65–70. The Psoriatic Arthritis Impact of Disease 12-item questionnaire (PsAID-12; range: 0–10, where 10 represents the worst score) [59] was among the more commonly reported of these PROs. Among groups that did not achieve MDA or DAPSA/cDAPSA LDA, mean PsAID-12 ranged from 3.8 to 7.1 (NS = 5) [33, 36, 39, 42, 60] and from 3.9 to 5.3 (NS = 3) [38, 39, 50], respectively. Mean PsAID-12 ranged from 1.1 to 3.5 (NS = 5) among groups that achieved at least MDA [33, 36, 39, 42, 60], and from 1.7 to 2.7 (NS = 3) [38, 39, 50] for those that achieved at least DAPSA/cDAPSA LDA. Mean PsAID-12 in the one study that reported this outcome for a VLDA group was 1.1 [48, 49]; among DAPSA/cDAPSA REM groups, mean PsAID-12 scores of 1.3 and 1.7 were reported in one study [48]. Yedimenko et al. reported median PsAID-12 for patients meeting targets assessed by the MDA metric, DAPSA, and CDAI; for those in MDA or DAPSA/CDAI LDA, median PsAID-12 was 1.5, 2.4 and 1.7, respectively, and in the VLDA or DAPSA/CDAI REM groups, median PsAID-12 was 0.4, 0.7 and 0.5, respectively [61]. None of the included studies reported PsAID-12 for DAS28-assessed patients.
Discussion
This systematic review is, to our knowledge, the first to characterise the real-world evidence of residual disease among treated PsA patients grouped by thresholds of disease control. The evidence demonstrated that, despite treatment, a large proportion of patients may not reach treatment targets and consequently face substantial residual disease. As expected, this review showed that patients with disease control usually considered optimal (VLDA/REM or MDA/LDA) tended to have less residual disease than patients who did not achieve such control. However, even patients who achieved the most stringent targets (VLDA or REM) still experienced a range of residual disease, including (but not limited to) tender and swollen joints, enthesitis, skin disease and patient-reported pain, disability and disease impact on patients’ lives.
Persistence of patient-reported symptoms, even among patients in VLDA or REM, indicates that achieving stringent targets may still not result in satisfactory resolution of disease and acceptable disease impact from the patient’s perspective. While some of the PROs did demonstrate residual disease impact among patients achieving targets, patients’ wider perceptions of their residual disease were beyond the scope of this review. Published evidence on the views of patients with PsA is limited, although it is recognised that there can be a discord between patient and physician perspectives, consistent with findings in this review (PtGA vs. PhGA VAS). Patient-reported assessments have been found to show worse disease than physicians’ assessments, especially for patients in remission, and patient–physician discordance has also been observed when considering which symptoms are most important or burdensome [62, 63]. These findings suggest that treatment strategies might be improved by more explicitly considering patient perspectives, a strategy which has also been proposed for the treatment of patients with RA [64]. For instance, the target metric could be complemented by a patient-reported measure of disease impact (e.g. PsAID-12, an outcome reported in some included studies in this SLR), such that care can be personalised to address issues important to the individual patient.
Many studies included combined groups of patients with at least MDA (VLDA + MDA), or at least LDA (REM + LDA), depending on the metric. There was considerable between-study variation in the extent of residual disease among these groups; low residual disease was reported in some studies [40, 41, 50, 51, 65], while others reported a relatively high prevalence and/or severity of residual disease [37, 52, 53]. In the studies where patients achieving VLDA or REM were assessed separately from groups achieving MDA or LDA, the prevalence of residual disease was lower, but often not absent. These findings are consistent with the T2T guidelines, where VLDA or REM are recommended as primary treatment targets [13, 16]. However, targeting the most stringent threshold of disease control may not necessarily lead to the optimal outcome for an individual patient. The stringency of a target is likely to be balanced against how attainable it is for the patient, as there may be side effects, comorbidities or other factors that become relevant in the context of a change to the patient’s drug treatment. Attainability may in turn affect treatment adherence and overall response to therapy. The secondary targets of MDA or LDA may therefore be suitable alternatives in some cases [13, 16]. This emphasises the importance of patient education and shared decision-making when considering how to address patients’ residual disease burden, for both pharmacological interventions such as treatment escalation and the overall management of disease activity and disease impact [66].
Among patients in the same ‘tier’ of disease control (as assessed using the same metric; e.g. all in DAPSA LDA), there was heterogeneity in residual disease between studies. Studies differed in terms of inclusion and exclusion criteria, treatment and the assessment timepoints. However, the between-study variation in residual disease may also reflect the breadth of disease activity that can occur within each tier (e.g. DAPSA LDA encompasses scores of 4–14).
Across musculoskeletal, skin and PROs, patients in VLDA (by the MDA metric) tended to have less residual disease than patients in DAPSA REM, as did patients in MDA compared with DAPSA LDA. These findings were supported by studies that classified the same patients using both metrics, allowing residual disease to be more accurately compared between ‘equivalent’ groups. In the DEPAR study for example, prevalence of > 1 residual tender or swollen joints, > 1 tender entheseal points, PASI > 1, PtP VAS > 15, PtGA VAS > 20 or HAQ-DI > 0.5 was lower among MDA-achievers than among DAPSA LDA-achievers [22, 45, 67–70]. Across the MDA metric and DAPSA, we noted that these listed thresholds were most often exceeded for PASI, PtP and PtGA. This was least likely for the musculoskeletal outcomes. The differences in residual disease between the PsA-specific metrics MDA and DAPSA are likely related to their composition. DAPSA is an additive sum of several components, whereas the MDA metric is a count of stringent thresholds met for seven disease domains. Importantly, the MDA metric domains better reflect the multifaceted nature of PsA disease through the inclusion of skin and entheseal assessments, which are not included in DAPSA. This is likely a key reason for the very low levels of residual skin disease among patients in VLDA, versus the substantial residual skin disease among patients in DAPSA REM, as well as the relatively similar skin outcomes for DAPSA LDA and DAPSA REM.
Notably, despite T2T being recommended by international groups, only one of the 27 studies explicitly reported that patients were treated using a T2T approach. However, within the context of T2T for PsA, this review supports GRAPPA’s recommendation for the use of the MDA metric over DAPSA [13, 71], insofar as the achievement of MDA or VLDA is associated with less residual disease compared with DAPSA LDA or REM. Very few studies classified patients using DAS28 [7, 58, 72] or CDAI [57, 61], limiting the qualitative comparisons with PsA-specific metrics. The available data indicated that patients meeting DAS28 and CDAI targets have substantial residual disease.
Strengths of this review included adherence to best-practice systematic review methods [26], as well as relevance to clinical practice by including only observational studies that covered a range of different regions and populations. However, the focus on observational studies also introduced limitations. It is important to consider the substantial heterogeneity between studies, including differences in study design, treatment and baseline patient characteristics, all of which complicated interpretation and comparison of study results. Furthermore, most included studies were cross-sectional and did not assess patients at a consistent timepoint within their disease or treatment pathway, hence some patients may have spent less time on treatment with less opportunity for a disease response. Additionally, the prevalence and/or severity of residual disease were reported in numerous ways across different studies, further complicating comparisons of their results. Congress abstracts were included in this SLR in accordance with Cochrane Collaboration recommendations [27], although we note the increased risk of selection bias associated with such publications.
Conclusions
Overall, the findings of this study highlight the need for further effective treatments and for continued refinement of strategies to reduce residual disease, both in patients aiming to achieve treatment targets and in those who have achieved them. Although this review has characterised residual disease among treated patients with PsA, including that reported by patients themselves, patients’ views about their residual disease and care were beyond the scope of this review; this topic warrants further research. Future studies should also aim to evaluate whether patients’ perceptions of their symptoms and disease, and their knowledge and expectations of available treatments and outcomes, may affect the burden of disease they experience. This may ultimately help advance patient education and engagement, and tailoring of treatment strategies to address the aspects of PsA that are of greatest importance to patients.
Acknowledgements
The authors acknowledge Heather Edens, PhD, UCB Pharma for publication coordination. The authors also acknowledge Jill Crich, MSc, and Aaditya Rawal, MSc, from Costello Medical, for support with the SLR, and James Evry, MSc, and Lucy Berry, MBBS, from Costello Medical, for medical writing and editorial assistance based on the authors’ input and direction. This study was funded by UCB Pharma.
Funding
This study and its publication, including the Rapid Service Fee, was sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by James Evry, MSc, and Lucy Berry, MBBS, Costello Medical, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Substantial contributions to study conception and design: LCC, MdW, ABH, MS, AS, ARO; substantial contributions to analysis and interpretation of the data: LCC, MdW, ABH, MS, AS, ARO; drafting the article or revising it critically for important intellectual content: LCC, MdW, ABH, MS, AS, ARO; final approval of the version of the article to be published: LCC, MdW, ABH, MS, AS, ARO.
Disclosures
Laura C. Coates reports speaker's bureau for AbbVie, Amgen, Biogen, Celgene, Gilead, GSK, Janssen, Eli Lilly, Medac, Novartis, Pfizer and UCB Pharma; consulting fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Domain, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, Serac and UCB Pharma; and grant/research support from AbbVie, Amgen, Celgene, Gilead, Janssen, Eli Lilly, Novartis, Pfizer and UCB Pharma. Maarten de Wit: over the last 5 years Stichting Tools has received fees for lectures or consultancy provided by Maarten de Wit, from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Pfizer, Roche and UCB Pharma. Amy Buchanan-Hughes is an employee of Costello Medical. Maartje Smulders is an employee of Astellas Pharma Europe B.V. and was formerly contracted by UCB Pharma during the conduct of the SLR. Anna Sheahan is a shareholder and was formerly an employee of UCB Pharma during the conduct of the SLR. Alexis R. Ogdie received grant/research support from Pfizer, Novartis and Amgen; and is a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Janssen, Eli Lilly, Novartis and Pfizer.
Compliance with Ethics Guidelines
This SLR is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
This study is an SLR and no novel data were generated. All data relevant to the study are either included in the article or uploaded as supplementary information.
Contributor Information
Laura C. Coates, Email: laura.coates@ndorms.ox.ac.uk
Maarten de Wit, Email: martinusdewit@hotmail.com.
Amy Buchanan-Hughes, Email: amy.buchanan-hughes@costellomedical.com.
Maartje Smulders, Email: msmulders@kubadili.com.
Anna Sheahan, Email: anna.e.dow@gmail.com.
Alexis R. Ogdie, Email: ogdiea@pennmedicine.upenn.edu
References
- 1.Ocampo DV, Gladman D. Psoriatic arthritis [version 1; peer review: 2 approved]. F1000Research 2019;8(F1000 Faculty Rev):1665. 10.12688/f1000research.19144.1.
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 3.Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17:65–70. doi: 10.7861/clinmedicine.17-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:569–579. doi: 10.1016/j.rdc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Gladman DD. Recent advances in understanding and managing psoriatic arthritis [version 1; peer review: 2 approved]. F1000Research. 2016;5:2670. 10.12688/f1000research.9592.1. [DOI] [PMC free article] [PubMed]
- 6.Helliwell PS, Ruderman EM. Natural history, prognosis, and socioeconomic aspects of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41:581–591. doi: 10.1016/j.rdc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Kilic G, Kilic E, Nas K, Kamanlı A, Tekeoglu İ. Residual symptoms and disease burden among patients with psoriatic arthritis: is a new disease activity index required? Rheumatol Int. 2019;39:73–81. doi: 10.1007/s00296-018-4201-3. [DOI] [PubMed] [Google Scholar]
- 8.Husted JA, Tom BD, Schentag CT, Farewell VT, Gladman DD. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553–1558. doi: 10.1136/ard.2008.098202. [DOI] [PubMed] [Google Scholar]
- 9.Husni ME, Merola JF, Davin S. The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. 2017;47:351–360. doi: 10.1016/j.semarthrit.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Freire M, Rodríguez J, Möller I, et al. Prevalence of symptoms of anxiety and depression in patients with psoriatic arthritis attending rheumatology clinics. Reumatol Clin. 2011;7:20–26. doi: 10.1016/j.reuma.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet. 2015;386:2489–2498. doi: 10.1016/s0140-6736(15)00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dures E, Shepperd S, Mukherjee S, et al. Treat-to-target in PsA: methods and necessity. RMD Open. 2020;6. 10.1136/rmdopen-2019-001083. [DOI] [PMC free article] [PubMed]
- 13.Coates LC, FitzGerald O, Merola JF, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/outcome measures in rheumatology consensus-based recommendations and research agenda for use of composite measures and treatment targets in psoriatic arthritis. Arthritis Rheumatol. 2018;70:345–355. doi: 10.1002/art.40391. [DOI] [PubMed] [Google Scholar]
- 14.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77:3–17. doi: 10.1136/annrheumdis-2017-211734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 18.Hagege B, Tan E, Gayraud M, Fautrel B, Gossec L, Mitrovic S. Remission and low disease activity in psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology (Oxford) 2020;59:1818–1825. doi: 10.1093/rheumatology/keaa030. [DOI] [PubMed] [Google Scholar]
- 19.Tucker LJ, Ye W, Coates LC. Novel concepts in psoriatic arthritis management: Can we treat to target? Curr Rheumatol Rep. 2018;20:71. 10.1007/s11926-018-0781-x. [DOI] [PMC free article] [PubMed]
- 20.Mease PJ, Etzel CJ, Huster WJ, et al. Understanding the association between skin involvement and joint activity in patients with psoriatic arthritis: experience from the Corrona Registry. RMD Open. 2019;5:e000867. doi: 10.1136/rmdopen-2018-000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helliwell PS, Kavanaugh A. Comparison of composite measures of disease activity in psoriatic arthritis using data from an interventional study with golimumab. Arthritis Care Res (Hoboken). 2014;66:749–756. doi: 10.1002/acr.22204. [DOI] [PubMed] [Google Scholar]
- 22.Wervers K, Luime JJ, Tchetverikov I, et al. Comparison of disease activity measures in early psoriatic arthritis in usual care. Rheumatology (Oxford) 2019;58:2251–2259. doi: 10.1093/rheumatology/kez215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coates LC, Merola JF, Mease PJ, et al. Performance of composite measures used in a trial of etanercept and methotrexate as monotherapy or in combination in psoriatic arthritis. Rheumatology (Oxford). 2021;60(3):1137–47. 10.1093/rheumatology/keaa271. [DOI] [PMC free article] [PubMed]
- 24.Aletaha D, Alasti F, Smolen JS. Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann Rheum Dis. 2017;76:418–421. doi: 10.1136/annrheumdis-2016-209511. [DOI] [PubMed] [Google Scholar]
- 25.Kavanaugh A, van der Heijde D, Beutler A, et al. Radiographic progression of patients with psoriatic arthritis who achieve minimal disease activity in response to golimumab therapy: results through 5 years of a randomized, placebo-controlled study. Arthritis Care Res (Hoboken). 2016;68:267–274. doi: 10.1002/acr.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1. 2020. https://training.cochrane.org/handbook/archive/v6.1. Accessed Aug 2020.
- 28.Tillett W, McHugh N, Orbai AM, et al. Outcomes of the 2019 GRAPPA Workshop on Continuous Composite Indices for the Assessment of Psoriatic Arthritis and Membership-recommended Next Steps. J Rheumatol Suppl. 2020;96:11–18. doi: 10.3899/jrheum.200121. [DOI] [PubMed] [Google Scholar]
- 29.Centre for Reviews and Disseminatio (CRD). Systematic reviews: CRD's guidance for undertaking reviews in health care. York: Centre for reviews and dissemination, University of York; 2008.
- 30.National Institute for Health and Care Excellence (NICE). The guidelines manual: appendices B-I. 2012. https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-pdf-3304416006853. Accessed Aug 2020. [PubMed]
- 31.Bakirci S, Solmaz D, Al Osaimi N, et al. What are the main barriers to achieve minimal disease activity in psoriatic arthritis in real life? Clin Exp Rheumatol. 2019;37:808–812. [PubMed] [Google Scholar]
- 32.Oliffe M, Sinnathura P, Briggs F, et al. Application of the omeract definition of minimal disease activity in a real-world cohort of patients with psoriatic arthritis. Internal Med. 2018;48.
- 33.Queiro R, Brandy A, Rosado MC, et al. Minimal disease activity and patient-acceptable symptom state in psoriatic arthritis: a real-world evidence study with ustekinumab. JCR. 2018;24:381–384. doi: 10.1097/rhu.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 34.van Mens LJJ, van Kuijk AW, Baeten DL. THU0443 Unmet needs in psoriatic arthritis: one third of the patients with quiescent disease according to the rheumatologist's opinion do not achieve minimal disease activity. Ann Rheumatic Dis. 2016;75:351. doi: 10.1136/annrheumdis-2016-eular.2124. [DOI] [Google Scholar]
- 35.van Mens LJJ, Turina MC, van de Sande MGH, Nurmohamed MT, van Kuijk AWR, Baeten DLP. Residual disease activity in psoriatic arthritis: discordance between the rheumatologist's opinion and minimal disease activity measurement. Rheumatology (Oxford). 2018;57:283–90. 10.1093/rheumatology/kex183. [DOI] [PubMed]
- 36.Mosquera Martínez JA, García-Porrúa C, Fernández-Dominguez L, Guerra-Vazquez JL, Pinto-Tasende J. AB0823 Minimal disease activity in psoriatic arthritis is associated with low impact of disease on PsAID12 questionaire. Ann Rheumatic Dis. 2020;79:1715. 10.1136/annrheumdis-2020-eular.4722.
- 37.Favalli EG, Idolazzi L, Bugatti S, et al. SAT0656 DAPSA or MDA/VLDA criteria for defining the treatment target in psoriatic arthritis? Cross-sectional analysis from a multicenter Italian cohort. Ann Rheum Dis. 2019;78:1426–1427. doi: 10.1136/annrheumdis-2019-eular.4217. [DOI] [Google Scholar]
- 38.García-Porrúa C, Fernández-Dominguez L, Guerra-Vazquez JL, Mosquera Martínez JA, Pinto-Tasende J. AB0771 Dapsa remission/low disease activity is associated with low psaid12 in psoriatic arthritis. Ann Rheumatic Dis. 2020;79:1683–4. 10.1136/annrheumdis-2020-eular.4775.
- 39.Gossec L, Bergmans P, Vlam Kd, et al. FRI0436 Achieving the treatment targets of remission or low disease activity (LDA) in psoriatic arthritis (PSA) is associated with significantly improved quality of life, function and pain. Ann Rheumatic Dis. 2019;78:909. 10.1136/annrheumdis-2019-eular.2379.
- 40.Lubrano E, De Socio A, Perrotta FM. Comparison of composite indices tailored for psoriatic arthritis treated with csDMARD and bDMARD: a cross-sectional analysis of a longitudinal cohort. J Rheumatol. 2017;44:1159–1164. doi: 10.3899/jrheum.170112. [DOI] [PubMed] [Google Scholar]
- 41.Lubrano E, Scriffignano S, Perrotta FM. Residual disease activity and associated factors in psoriatic arthritis. J Rheumatol. 2020;47:1490–1495. doi: 10.3899/jrheum.190679. [DOI] [PubMed] [Google Scholar]
- 42.Queiro R, Cañete JD. Impact of cardiovascular risk factors on the achievement of therapeutic goals in psoriatic arthritis: is there any association? Clin Rheumatol. 2018;37:661–666. doi: 10.1007/s10067-018-4004-7. [DOI] [PubMed] [Google Scholar]
- 43.Queiro R, Cañete JD, Torre JC, et al. THU0427 Study of prevalence and predictors of minimal disease activity (MDA) state in a spanish population with psoriatic arthritis. maaps study. Ann Rheumatic Dis. 2016;75:344. 10.1136/annrheumdis-2016-eular.3250.
- 44.van Mens LJJ, van de Sande MGH, van Kuijk AWR, Baeten D, Coates LC. Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real-life cohort. Ann Rheumatic Dis. 2018;77:251. 10.1136/annrheumdis-2017-211998. [DOI] [PubMed]
- 45.Wervers K, Vis M, Tchetveriko I, et al. Burden of psoriatic arthritis according to different definitions of disease activity: comparing minimal disease activity and the disease activity index for psoriatic arthritis. Arthritis Care Res (Hoboken) 2018;70:1764–70. 10.1002/acr.23571. [DOI] [PMC free article] [PubMed]
- 46.Coates LC, Lubrano E, Perrotta FM, Emery P, Conaghan PG, Helliwell PS. What should be the primary target of "treat to target" in psoriatic arthritis? J Rheumatol. 2019;46:38–42. 10.3899/jrheum.180267. [DOI] [PubMed]
- 47.Lorenzo A, Pardo E, Charca L, Pino M, Queiro R. Enthesitis and joint erosions are disease traits associated with cardiovascular risk in psoriatic arthritis. Clin Rheumatol. 2020;39:2973–9. 10.1007/s10067-020-05088-2. [DOI] [PubMed]
- 48.Queiro R, Cañete JD, Montilla C, et al. Very low disease activity, DAPSA remission, and impact of disease in a spanish population with psoriatic arthritis. J Rheumatol. 2019;46:710–5. 10.3899/jrheum.180460. [DOI] [PubMed]
- 49.Queiro R, Canete JD, Montilla-Morales CA, Abad MA, Castro SG, Cabez A. Psoriatic arthritis patients who attain a very low disease activity state have a minimal impact of the disease on their lives. In: Arthritis Rheumatol. Conference: American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Scientific Meeting; November 3–8, 2017. San Diego, CA. p. 69 [abstract].
- 50.Orbai AM, Perin J, Gorlier C, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res (Hoboken) 2020;72:1772–9. 10.1002/acr.24090. [DOI] [PMC free article] [PubMed]
- 51.Marin J, Acosta Felquer ML, Ferreyra Garrot L, Ruta S, Rosa J, Soriano ER. Patients with psoriatic arthritis fulfilling the minimal disease activity criteria do not have swollen and tender joints, but have active skin. J Rheumatol. 2016;43:907–910. doi: 10.3899/jrheum.151101. [DOI] [PubMed] [Google Scholar]
- 52.Scrivo R, Giardino AM, Salvarani C, et al. An observational prospective study on predictors of clinical response at 6 months in patients with active psoriatic arthritis treated with golimumab. Clin Exp Rheumatol. 2020;38:107–114. [PubMed] [Google Scholar]
- 53.Tinazzi I, McGonagle D, Aydin SZ, Chessa D, Marchetta A, Macchioni P. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann Rheumatic Dis. 2018;77:922–5. 10.1136/annrheumdis-2017-212681. [DOI] [PubMed]
- 54.van Mens LJJ, Kuijk AWRv, Baeten DL, Coates LC. SAT0464 The ideal target for psoriatic arthritis? comparison of remission and inactive disease states in a real life cohort. Ann Rheumatic Dis. 2017;76:949. doi: 10.1136/annrheumdis-2017-eular.2926. [DOI] [PubMed] [Google Scholar]
- 55.Liu V, Fong W, Kwan YH, Leung YY. AB0699 residual disease burden present in axial spondyloarthritis and psoriatic arthritis patients achieving low disease activity states. Ann Rheumatic Dis. 2020;79:1645–6. 10.1136/annrheumdis-2020-eular.3700.
- 56.Mease PJ, Karki C, Liu M, et al. SAT0471 Trends in clinical characteristics associated with achievement of minimal disease activity in response to biologic therapy in psoriatic arthritis—analyses from the corrona psoriatic arthritis/spondyloarthritis (PSA/SPA) registry. Ann Rheumatic Dis. 2017;76:953. 10.1136/annrheumdis-2017-eular.1479.
- 57.Ogdie A, Palmer JL, Greenberg J, et al. Predictors of achieving remission among patients with psoriatic arthritis initiating a tumor necrosis factor inhibitor. J Rheumatol. 2019;46:475–482. doi: 10.3899/jrheum.171034. [DOI] [PubMed] [Google Scholar]
- 58.Hughes C NN, Garrood T, Kirkham B. Comparison of quality-of-life, function and psoriasis measures in minimal disease activity and DAS28 states in routine care of patients with psoriatic arthritis. In: Arthritis Rheumatol. Conference: American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Scientific Meeting; November 3–8, 2017. San Diego, CA. p. 69 [abstract].
- 59.Holland R, Højgaard P, Tillett W, et al. Evidence for psoriatic arthritis impact of disease (PsAID12) as core instrument to measure health-related quality of life in psoriatic arthritis: a systematic review of psychometric properties. J Psoriasis Psoriatic Arthritis. 2020;5:12–22. doi: 10.1177/2475530319890832. [DOI] [Google Scholar]
- 60.Kasavkar G, Banerjee S, Gullick N. SAT0381 Minimal disease activity in psoriatic arthritis predicts low impact of disease. Ann Rheumatic Dis. 2019;78:1274–5. 10.1136/annrheumdis-2019-eular.7172.
- 61.Yedimenko J, Walsh J, Ogdie A, et al. Patient-Reported Outcomes differentiate between remission and low disease activity in psoriatic arthritis. In: Arthritis Rheumatol. Conference: American College of Rheumatology Convergence; November 5–9, 2020. All virtual. p. 72 [abstract].
- 62.Desthieux C, Granger B, Balanescu AR, et al. Determinants of patient-physician discordance in global assessment in psoriatic arthritis: a multicenter European study. Arthritis Care Res. 2017;69:1606–11. 10.1002/acr.23172. [DOI] [PubMed]
- 63.Husni ME, Fernandez A, Hauber B, et al. Comparison of US patient, rheumatologist, and dermatologist perceptions of psoriatic disease symptoms: results from the DISCONNECT study. Arthritis Res Ther. 2018;20:102. 10.1186/s13075-018-1601-4. [DOI] [PMC free article] [PubMed]
- 64.Ferreira RJO, Ndosi M, de Wit M, et al. Dual target strategy: a proposal to mitigate the risk of overtreatment and enhance patient satisfaction in rheumatoid arthritis. Ann Rheum Dis. 2019;78:e109. 10.1136/annrheumdis-2018-214199. [DOI] [PubMed]
- 65.Gossec L, Wit Md, Gorlier C, et al. SAT0635 Patient-perceived residual burden of psoriatic arthritis in patients in remission/low disease: an analysis of 444 patients. Ann Rheumatic Dis. 2019;78:1415. 10.1136/annrheumdis-2019-eular.1206.
- 66.Schoemaker CG, de Wit MPT. Treat-to-target from the patient perspective is bowling for a perfect strike. Arthritis Rheumatol. 2021;73:9–11. doi: 10.1002/art.41461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.den Braanker H, Wervers K, Mus AMC, et al. Achieving sustained minimal disease activity with methotrexate in early interleukin 23-driven early psoriatic arthritis. RMD Open. 2020;6:e001175. doi: 10.1136/rmdopen-2020-001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wervers K, Braanker Hd, Luime J, et al. SAT0401 Use of and response to methotrexate in early psoriatic arthritis: results from the real world cohort, depar study. Ann Rheumatic Dis. 2019;78:1287. 10.1136/annrheumdis-2019-eular.5371.
- 69.Wervers K, Luime JJ, Tchetveriko I, et al. Impact of time to minimal disease activity and quality of life one year after diagnosis of psoriatic arthritis. In: Arthritis Rheumatol. Conference: American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP) Annual Scientific Meeting; October 19–24, 2018. Chicago, IL. p. 70(suppl 10) [abstract].
- 70.Wervers K, Luime JJ, Tchetverikov I, et al. Time to minimal disease activity in relation to quality of life, productivity, and radiographic damage 1 year after diagnosis in psoriatic arthritis. Arthritis Res Ther, 2019;21:25. 10.1186/s13075-019-1811-4. [DOI] [PMC free article] [PubMed]
- 71.Tillett W, FitzGerald O, Coates LC, et al. Composite measures for clinical trials in psoriatic arthritis: testing pain and fatigue modifications in a UK multicenter study. J Rheumatol. 2021. 10.3899/jrheum.201674. [DOI] [PubMed]
- 72.Arancibia Aguila L, Medeiros AC, Gonçalves CR, Sampaio Barros PD, Goldenstein Schainberg C. AB0816 Altered sleep patterns, fatigue, anxiety and depression levels are important features among patients with psoriatic arthritis. Ann Rheumatic Dis 2015;74:1172. 10.1136/annrheumdis-2015-eular.1619.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is an SLR and no novel data were generated. All data relevant to the study are either included in the article or uploaded as supplementary information.