Graphical abstract
Keywords: Bioactive lipids, Omega-3 fatty acids, Anthocyanins, Phenolic compounds, Carotenoids, SARS-CoV-2
Abstract
Since the outbreak of COVID-19 disease, medical and scientific communities are facing a challenge to contain its spread, develop effective treatments, and reduce its sequelae. Together with the therapeutical treatments, the use of dietary bioactive compounds represents a promising and cost-effective strategy to modulate immunological responses. Amazonian oilseeds are great sources of bioactive compounds, thus representing not only a dietary source of nutrients but also of substances with great interest for human health. This narrative review compiled the available evidence regarding the biochemical properties of some Amazonian oilseeds, especially Brazil nut, Açaí berry, Bacaba, Peach palm, Sapucaya and Tucuma fruits, on human health and its immune system. These effects were discussed from an etiological and pathophysiological perspective, emphasizing their potential role as a co-adjuvant strategy against COVID-19. Besides this, the cost associated with these strategies hinders their applicability in many nations, especially low-income countries and communities living in social insecurity.
1. Introduction
Two years have been gone since the outbreak of coronavirus disease (COVID-19) was declared by the World Health Organization as a global pandemic (WHO, 2020). First reported in Wuhan, China, in late 2019, COVID-19 is caused by a respiratory virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and displayed globally high transmission rates as well as varying morbidity and mortality indices through this period. Since them, the world has registered more than 500 million cases and over six million deaths due to COVID-19, according to the WHO Coronavirus (COVID-19) Dashboard (WHO, 2022). Through these couple of years, the WHO, scientists, and many countries, have put forth a series of preventive measures to contain the spread of the SARS-CoV-2 (WHO, 2020). These measures include increasing hygiene practices, using hand sanitizer and facial coverings when in public, and applying strict sanitary control procedures. Furthermore, quarantine and isolation measures have been applied worldwide to curtail mass transmission and give time for the scientific and medical communities to develop effective therapeutic strategies. The scientific community work hardly to develop different antiviral therapies against COVID-19, such as immunotherapy, antiviral therapy, passive immunotherapy, vaccines (inactivated and attenuated), and medications (e.g., dexamethasone) to alleviate symptoms. So far, vaccines (some authorized as emergency use only) and some antiviral therapies for SARS-CoV-2 have been effective in decreasing deaths and severe hospitalizations, while reducing the burden on healthcare services. New variants of the SARS-CoV-2 still defies the available therapies, health systems, and sanitary practices. Even though many vaccines have been developed and approved for use in different age groups of the world population, the process of vaccinating entire populations has been quite slow and costly, especially for developing countries. It has been reported by the Humans Right Council of the WHO that more than 10.5 billion vaccine doses were administrated globally, though only around 13%, were vaccinated in low-income countries, contrarily close to 70% in high-income countries (United Nations, 2022). Therefore, preventing the spread of the virus and/or reducing the number of severe COVID-19 cases remain among the most effective strategies during this pandemic.
Meanwhile, nutritional statuses that negatively affect the immune system—malnutrition, obesity, and other chronic conditions associated with them—can influence COVID-19 severity, representing important risk factors. In addition, those individuals with altered nutritional conditions may have an increased vulnerability to other sources of infection (e.g., parasitic, bacterial and fungal infections) increasing the body’s demand for macro and micronutrients, prolonging and/or worsening clinical manifestations (Barrea et al., 2021; Marcos, Nova, & Montero, 2003). It is known that a well balanced intake of macronutrients and bioactive compounds from dietary sources impart on immunity. Amino acids from protein-rich sources are key nutrients for human metabolism and health, since they participate in regulation of the redox status, gene expression, and production of antibodies, cytokines, and other cytotoxic substances (BourBour et al., 2020). A fiber-rich diet has been correlated with the modulation of intestinal microbiota, which influences the metabolism of other nutrients as well as the inflammatory pathways and immune response (Barrea et al., 2021). Essential fatty acids, especially from omega-3 series, are important substances involved in the synthesis of signaling molecules, such as eicosanoids and docosanoids, which are crucial factors to the immune response (Venter, Eyerich, Sarin, & Klatt, 2020). Moreover, these dietary PUFAs are known by their anti-inflammatory and immune-modulating activities. Despite those macronutrients, many minerals, vitamins and bioactive substances from fruits, plants and herbs and other natural products, e.g., leaves, fruits, seeds, oilseeds and others, have been recognized due to its anti-inflammatory and antiviral effects supporting the immune system and providing a pharmacological basis for the development of novel medical therapies (Antonio et al., 2020, Galanakis et al., 2020).
The Mediterranean diet is an example of a high-quality food intake, rich in whole grains, healthy lipids (rich in PUFAs and poor in SFA), natural antioxidants, and fibers, that impact in the immune system (Barrea et al., 2021). This lifestyle is known by high intake of cereals, fruits, vegetables, legumes, nuts, seeds, especially from olives, as well as a modest dairy products, eggs and fish consumptions, and a very small intake of sweets and red meat (Barrea et al., 2021). Considering that tropical food diets, such as the Amazonian, are generally diverse in fruit and vegetables species as well as fishes as the main animal protein sources (London and Beezhold, 2015, Dufour et al., 2016), is possible to impart a comparison between this and the Mediterranean, although the first one is not well defined so far. If, by one side the biodiversity in foods commonly found in Tropical countries is important from a nutritional and biological-health related point-of-view, on the other hand, it limit a well-defined guide of dietary intake, especially because many of these foods are indigenous, wild, neglected, or underutilized species (Lachat et al., 2018). Many Amazonian fruits and oilseeds are known for their rich content in bioactive compounds, which displays antioxidant, anti-inflammatory, anticarcinogenic, antitumor, cardioprotective, gastroprotective effects and others (Berni et al., 2020, Cruz et al., 2020, Gomes et al., 2019, Nascimento et al., 2019, Yamaguchi et al., 2015, Cândido et al., 2015).
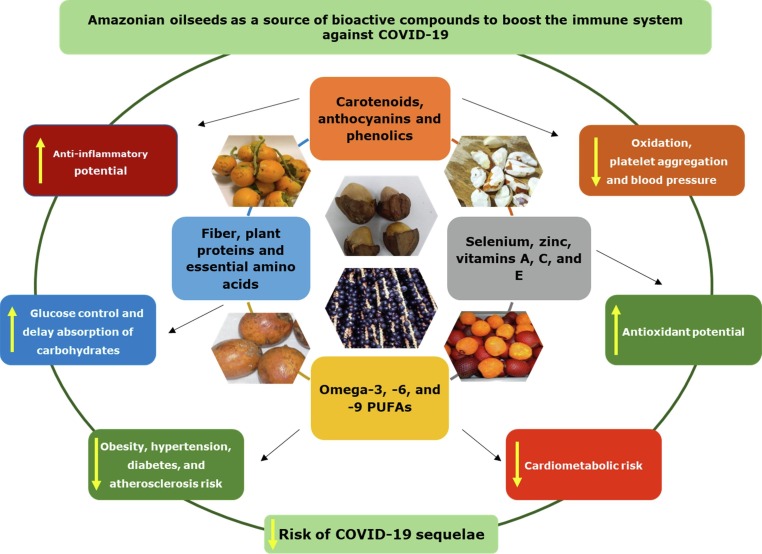
Considering the biodiversity of Amazonian fruits and oilseeds, this narrative review was focused on species with demonstrably high potential for human health benefits: Açaí (Euterpe oleracea), Bacaba (Oenocarpus bacaba), Buriti (Mauritia flexuosa), Brazil nut (Bertholletia excelsa), Sapucaia (Lecythis pisonis), Tucumã (Astrocaryum vulgare), and Peach palm (Bactris gasipaes). These oilseeds are recognized sources of omega−3 and −6 fatty acids, as well as containing relevant bioactive compounds and other micro- and macronutrients. In this narrative review, we aimed to compile and present evidence regarding the biochemical properties of some bioactive compounds found in Amazonian oilseeds, which could support the immune system as well as the human nutritional and health status on the prevention, alleviation, and recovery of COVID-19 symptoms
2. Methods
In this narrative review, the searching for published articles was focused on the following topics: nutrition, immune system, viruses and COVID-19, as well as the following Amazonian oilseeds, Açaí (Euterpe oleracea), Bacaba (Oenocarpus bacaba), Buriti (Mauritia flexuosa), Brazil nut (Bertholletia excelsa), Sapucaia (Lecythis pisonis), Tucumã (Astrocaryum vulgare), and Peach palm (Bactris gasipaes). These data were collected from different online platforms, including Web of Science, PubMed, Scopus, Google Scholar, Science Direct from Elsevier, and ResearchGate from 2002 to 2022.
3. Nutrition and immune system
The relationship between nutrition and immune system is a complex area and under constant research. Dietary compounds, as well as vitamins, essential fatty acids, minerals, and other antioxidant substances plays important role in healthy, and can impair on immunity. Therefore, it is known that the immune system is significantly affected by inappropriate nutrition conditions (Barrea et al., 2021; Marcos, Nova, & Montero, 2003). A reduced risk to develop an allergic and chronic inflammatory disease have been correlated with the intake of specific nutrients and diets (Venter, Eyerich, Sarin, & Klatt, 2020). Consequently, an adequate nutrition can reduce the occurrence of immune-mediated chronic diseases (Barrea et al., 2021).
Amino acids are vital nutrients for supporting and strengthening the immune system. They play a role in the proliferation and activation of T and B lymphocytes, natural killer (NK) cells, and macrophages, as well as in the regulation of the redox status, gene expression, and production of antibodies, cytokines, and other cytotoxic substances (BourBour et al., 2020). Deficient protein intake affects significantly lymphoid organs, causing its atrophy, as well as T-lymphocyte deficiency, and the susceptibility to pathogens, including virus (Barrea et al., 2021). Despite this, loss of peripheral lymphoid tissue, hormonal disparity, loss of leptin, and higher levels of circulating cortisol can be caused by thymic atrophy due to deficient protein malnutrition (Barrea et al., 2021; Marcos, Nova, & Montero, 2003). Arginine can augment the T-cells activity, and it was associated with the regulation of airway function and inflammation and lung diseases, by the production of nitric oxide (BourBour et al., 2020). A protein intake around 0.8 gr/kg/day in healthy adults is recommended (BourBour et al., 2020).
It is known that a fiber-rich diets, such as the Mediterranean diet, can impart on the intestinal microbiota, modulating its activity and the production of metabolites, which influences inflammatory pathways and immune response (Barrea et al., 2021). The consumption of dietary fiber stimulates the growth of specific microbial communities and serve as a substrate to generate fermentation products, such as short-chain fatty acids (e.g., acetate, butyrate, and propionate). In turn, the gut microbiota produces antigens that interact with tissue-resident and systemic immune cells and promotes the maintenance of a healthy mucus barrier, which is a protective layer against pathogen invasion. They can also secrete tryptophan metabolites or secondary bile acids, which regulate intestinal immunity and strengthen the mucus barrier, increasing epithelial integrity, influencing macrophages response, and preventing pathogen adhesion to and growth in epithelial cells (Beukema et al., 2020, Childs et al., 2019, Barrea et al., 2021).
Dietary PUFAs, especially omega-6 and omega-3 series, plays important roles in synthesis of signaling molecules, such as eicosanoids and docosanoids, which are key factors to the immune response (Venter, Eyerich, Sarin, & Klatt, 2020). Generally, fatty acids display relevant function on macrophages, such as phagocytosis capacity, production and secretion of cytokines and chemokines and others (Barrea et al., 2021). The omega-3 fatty acids are linked to anti-inflammatory and immune-modulating pathways, such as leucocyte chemotaxis and endothelial adhesive interactions, as well as, adhesion molecule expression, production of prostaglandins and leukotrienes (from the arachidonic acid), and inflammatory cytokines like TNF- a and IL-1b (Barrea et al., 2021). Mostly of the anti-inflammatory properties of omega-3 PUFAs are linked to their incorporation in membrane cells’ phospholipids, such as phosphatidylcholine and phosphatidylethanolamine (Husson et al., 2016). This incorporation promotes significant changes in the fluidity of the cell membrane, which results in the reduction of microorganisms’ phagocytosis by macrophages and antigen-presenting cells (APCs), formation of phagolysosome, and destroying the macrophages activity (Husson et al., 2016). Despite this, the omega-3 PUFAs also affects the activation B-cells, proliferation of T-cells and activation of Th1 and Th17 (Husson et al., 2016). B-cells are key elements in the immune system because their absence results in recurrent infections, especially in the gastrointestinal and respiratory tracts (Gurzell, Teague, Harris, Clinthorne, Shaikh, & Fenton, 2013). It has been reported that the dietary DHA-enriched fish oil can enhance B-cell activation, which can assist the immune responses associated with pathogen infections (Gurzell et al., 2013).
PUFAs are important to maintain the structural integrity and fluidity of the cell phospholipid bilayer. Moreover, their metabolic conversions influence the formation of immune system constituents, representing a substrate for the synthesis of lipid mediators (Innes & Calder, 2020). These metabolic conversions comprise a series of steps involving mainly linoleic (n-6) and linolenic (n-3) acids that are catalyzed by different enzymes, inducing elongation (elongase) and desaturation (desaturases) processes. Linoleic acid is the precursor to ARA; after successive enzymatic processes, ARA is converted to docosatetraenoic (22:4n-6) and docosapentaenoic (22:5n-6) acids. These eicosanoids are then catalyzed by cyclooxygenases to form prostanoids (prostaglandins and thromboxanes), or by lipooxygenases to form leukotrienes. These compounds derived from PUFAs and essential fatty acids influence numerous cellular functions, directly affecting metabolic, physiological, pathological, and inflammatory processes in the human body (Innes and Calder, 2020, Rogero et al., 2020). Prostaglandin E2 (PGE2) is an immunoregulator synthesized via cyclooxygenase enzymes that modulate the activity of immune cells such as macrophages, dendritic cells, endothelial cells, and fibroblasts. PGE2 and its specific receptors (EP1-EP4) coupled to protein G are related to pain, vasodilation, and increased endothelial permeability with pro- and anti-inflammatory effects (Kalinski, 2012). Thus, identifying and applying dietary sources of fatty acids in the development of alternative therapeutic strategies may aid immune responses considering that the production of many of its constituents depends on precursors derived from fatty acids. Linolenic acid (n-3) is the precursor to docosahexaenoic acid (22:6n-3). Long-chain fatty acids (e.g., omega-3 PUFAs) can influence different stages of an infectious disease, notably viral entry, and replication. Therefore, healthy levels of PUFAs in the organism are important to control tissue inflammation and promote adequate immune responses (Calder et al., 2020, Messina et al., 2020).
Micronutrients help to maintain structural and functional integrity of mucosal cells (innate barrier), regulate the production of specific proteins that destroy pathogens, thereby aiding in reducing airway infections (Calder et al., 2020, Malaguarnera, 2020, Barrea et al., 2021). Among micronutrients and trace elements, selenium has important antioxidant effects, iron plays an important role as a cofactor of proteins involved in immunological responses, and zinc is essential for T-cell differentiation and activation. Water-soluble vitamins, such as thiamine (vitamin B1) displays anti-inflammatory effects via modulation of pro-apoptotic proteins, mitochondrial membrane integrity, cytochrome C release, and protein kinase activity. Pyridoxine (vitamin B6) plays an important role in T-cell and interleukin production. Moreover, vitamin B12 (cobalamin) assists in white blood cell production and can also act as an immunomodulatory factor, promoting the release of cytotoxic T-cells against viral infections (BourBour et al., 2020, Coelho-Ravagnani et al., 2021). Finally, ascorbic acid (vitamin C) is arguably the most prominent dietary antioxidant. It promotes the deployment of phagocytic cells (e.g., neutrophils) to combat bacteria and viruses and supports epithelial barrier function, as well as modulates the growth and function of innate and adaptive cells, migration of blood cells to infection sites, antibody production, phagocytosis, and microbial death (BourBour et al., 2020, Coelho-Ravagnani et al., 2021).
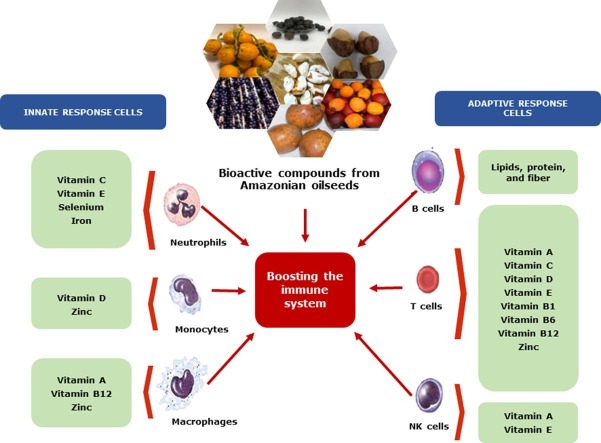
The relationship between nutrition and immune defenses is complex and depends on several intrinsic and extrinsic factors, such as dietary nutrition intake, individual health and nutrition status, intestinal microbiota, metabolic diseases, ageing, food allergies, body weight, lifestyle, and many others. Vitamins, essential fatty acids, amino acids, minerals and other micronutrients can affect the immune system responses, as an innate or adaptive level. Thus, it is crucial in any pathogenic infection that the nutrition status of the patient be kept according to the recommended dietary requirements. As illustrated in Fig. 1 , macro- and micronutrients have several effects on human health that can be applied in managing COVID-19 risk factors or mitigating its post-acute syndrome, through the support of its nutritional status.
Fig. 1.
Bioactive compounds of Amazonian oilseeds and their major potential effects on risk factors associated with COVID-19 and other viruses.
4. Pathophysiology and nutrition requirements of COVID-19
Coronaviruses belong to the Coronaviridae family comprising approximately 30 types of viruses that can infect humans. Their name refers to the “crown-like” appearance of their surface membrane, which is interspersed with glycoproteins and encapsulates their genomic RNA. Human coronaviruses are large (80–120 nm in diameter) and are commonly referred according to the disease that they cause, such as the SARS-CoV and MERS-CoV (Middle East Respiratory Syndrome). Coronaviruses have four structural proteins; among these, the spike protein mediates the attachment of the virus to a host cell, thereby inducing immunological responses (Liu et al., 2020, Thirumdas et al., 2021, Zhu et al., 2020).
These viruses primarily affect the respiratory system, causing pneumonia, although can also affect the cardiac, hepatic, renal, and central nervous systems. Moreover, when the virus affects multiple organs simultaneously, it can cause multiple organs failure, a highly fatal condition. When symptomatic, the effects of SARS-CoV-2 infection occur over a relatively long period of time, encompassing its incubation period (usually 2 weeks) and exponential replication phase in the upper and lower respiratory tract, ultimately leading to pulmonary lesions, and reduced functional lung capacity (Ceribelli et al., 2020, Chan et al., 2020, Heymann and Shindo, 2020, Huang et al., 2020, Liu et al., 2020). As viral replication progresses, symptoms such as fever, cough, dyspnea, myalgia, and intense fatigue may initially occur.
In cases of moderate-to-severe COVID-19, patients may develop severe bilateral interstitial pneumonia and acute respiratory distress syndrome, requiring invasive medical interventions such as mechanical ventilation or intubation (Ceribelli et al., 2020, Heymann and Shindo, 2020, Huang et al., 2020). Patients who develop severe COVID-19 still require intensive care and invasive interventions such as mechanical ventilation support (WHO, 2020). These interventions are costly and not always accessible to those low-income economies, or in situations of socioeconomic vulnerability—which represent most of the world population. Furthermore, recovered individuals often experience long-term effects, a phenomenon recently recognized as “post-acute COVID-19 syndrome.” These include reduced lung capacity owing to lung injuries and fibrosis, cardiovascular symptoms, issues associated with prolonged intubation, muscle depletion, anosmia, hypogeusia, neurological disorders, among others (Bose and McCarthy, 2020, Cothran et al., 2020, Nalbandian et al., 2021, Pan-American Health Organization, 2020, Vindegaard and Benros, 2020).
Immunological responses are innate or adaptive. The former represents the initial response to an invading pathogen and involves cells such as macrophages, neutrophils, and eosinophils. Despite acting quickly, these cells are not specialized and are less effective than those involved in adaptive response, which represents the response to specific pathogens and ultimately builds the immunity acquired over the course of an individual’s life (Childs et al., 2019, Barrea et al., 2021). The cells involved in adaptive response include lymphocytes such as T- and B-cells. They have a high capacity to memorize, recognize, and quickly respond to a pathogen upon reinfection. T-cells comprise several subtypes that are involved in different immune responses: cytotoxic cells promote the destruction of virus-infected and tumor cells, auxiliary cells coordinate immunological responses with other lymphocytes, and regulatory cells maintain immunological tolerance to other elements present in the organism. Meanwhile, B-cells produce immunoglobulins, which assist the immune system in recognizing and destroying pathogens (Childs et al., 2019, Barrea et al., 2021).
Upon infecting the host, SARS-CoV-2 spreads to the respiratory tract, where it faces a complex innate immune response. This precipitates the clinical manifestations that constitute the disease associated with the viral infection, altering the immune system and leading to serious inflammatory responses in critically ill patients (Chowdhury et al., 2020). Studies analyzing demographic and clinical data from patients with COVID-19 in Wuhan, China, reported a close relationship between disease severity and immunological parameters such as low lymphocyte count, high leukocyte count, and low levels of monocytes, eosinophils, and basophils, as well as low levels of helper and suppressor T-cells (Qin et al., 2020, Yang et al., 2020). These findings corroborate those reported by Ni et al. (2020), who indicated an association between SARS-CoV-2 infection and decreased levels of T lymphocytes, especially cytotoxic CD8 + T cells.
When the immune system is functioning properly, the innate and adaptive responses can eliminate pathogens and promote organism recovery. However, SARS-CoV-2 infection may suppress the activation of interferon (IFN)-α and IFN-β—proteins involved in immune response against viral infections—leading to uncontrolled viral replication (Ye et al., 2020). Consequently, inflammatory neutrophils and macrophages increase, generating a marked increase in the release of pro-inflammatory cytokines such as interleukin (IL)-6 and -B1, chemokines, growth factors, and tumor necrosis factor (TNF)-α. This phenomenon, called “cytokine storm or cytokine release syndrome,” is more frequently observed in critically ill patients than in those moderately ill (Huang et al., 2020). This cytokine storm was associated with an inadequately induction of an immune response to the infection. Although other observational studies indicated models in which the SARS-CoV-2 concomitantly induces a proinflammatory state with high levels of some cytokine, as IL-1, IL-6, CXCL8, and TNF (Schultze & Aschenbrenner, 2021). These higher levels of proinflammatory mediators also influences the accumulation of pathogenic inflammatory neutrophils and macrophages leading to a hyperinflammatory state, especially in the lungs, during the second week after infection, and this was more prominent in severe patients (Lucas et al. 2020). It is well-documented that proinflammatory cytokines and mediators are often in higher levels in ICU patients than non-hospitalized with COVID-19 (Li et al., 2021).
Immune system modulation in response to viral infections can mitigate the worsening of clinical symptoms; conversely, modulation impairment leads to increased susceptibility to viral infections and their associated diseases, such as COVID-19. Among the many factors that influence immune system functioning, nutritional status represents one of great importance. Clinical consequences of this disease can affect the food intake and the organism’s capacity to absorb exogenous nutrients, leading to disorders of energy intake as well (Patel et al., 2020, Li et al., 2021). Besides this, replication and amplification in vivo of higher amount of the virus also increases metabolism (Li et al., 2021). Thus, an adequate nutritional status promotes faster and more effective responses against pathogens. In turn, attaining and maintaining this status requires a dietary intake of nutrient-rich food, especially those rich in bioactive compounds with specific and varied effects on immunomodulation, such as amino acids, polyunsaturated fatty acids (PUFAs), fiber, vitamins, and minerals (BourBour et al., 2020, Childs et al., 2019). Some amino acids have been related to protective effects against viral infections and COVID-19 (Alkhatib, 2020). Luo et al. (2020) analyzed the potential of in silico digested proteins to inhibit SARS-CoV-2 binding to host cells. They reported that plant proteins—such as wheat-derived α- and β-gliadin and oat-derived avenin—could bind with the virus’s spike protein, representing a potential therapeutic strategy as a nutritional supplement for patients with COVID-19. Dietary essential PUFAs and their metabolites (known as bioactive lipids) play an important role in controlling acute and chronic inflammation. Specifically, PUFAs are metabolic precursors to specialized pro-resolution lipid mediators that contribute to inflammatory condition improvement, decreasing the level and duration of inflammation (Das, 2020, Weill et al., 2020). Park and Harris (2002) study involving EPA supplementation, showed that changes in platelet aggregation occurred via thromboxanes release. In contrast, DHA supplementation did not lead to platelet aggregation changes, possibly because of a reduction in affinity between thromboxane A2 and prostaglandin H2 receptor. Later, Adili, Hawley, & Holinstat (2018) demonstrated the influence of dietary consumption of EPA, DHA, and ARA on the composition and function of platelet phospholipid membranes. They indicated that the reduction in platelet aggregation was mediated by the release of thromboxanes that interact with cyclooxygenases (COX-1 and 12-LOX) involved in the metabolism of oxyprolines inside the platelets, consequently affecting the progression of thrombotic complications in cardiovascular diseases. Thirumdas et al. (2021) showed the role of food and nutrient supplementation in combating viral infections and their impairing effects on the immune system, emphasizing the effects of omega-3 PUFAs on protecting against hepatitis C virus, SARS-CoV-2, SARS-CoV, and MERS-CoV infections.
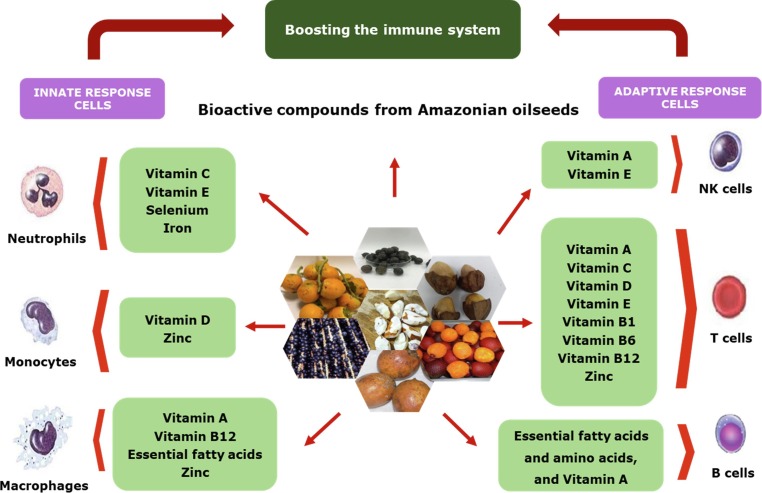
Concerning other viral infections, fatty acids can induce leakage or even lysis of invading cells (including rupture of virus protein envelopes) as well as oxidative phosphorylation decoupling, cellular respiration inhibition, amino acid transportation disruption, among other effects. Leukocytes, alveolar macrophages, and NK, B-, and T-cells release fatty acids in the cellular environment in which they are inserted when confronting invasive pathogens, including viruses such as MERS-CoV, SARS-CoV, and SARS-CoV-2, thus inactivating them and protecting the lungs (Das, 2020). Conversely, this relationship between fatty acids and the immune system implies that fatty acids deficiency may increase an individual’s susceptibility to viral infections, including COVID-19. Importantly, according to Conte & Toraldo (2020), gut microbiota dysbiosis can contribute to COVID-19 development. Furthermore, vitamins A, C, D, and E are regulatory compounds that also exhibit anti-inflammatory, antimicrobial, and antioxidant effects, helping curtail acute respiratory infections, viral infections, and airway inflammation, all prominent symptoms of COVID-19 (BourBour et al., 2020, Teymoori-Rad et al., 2019). Notably, vitamin D induces the production of cathelicidin and defensins, which protect the respiratory tract through viral encapsulation, impairing SARS-CoV-2′s binding abilities (Muscogiuri et al., 2020). Fig. 2 illustrates the relationships between these compounds and the innate and adaptive immune responses.
Fig. 2.
Macro- and micronutrients involved in modulating the immune system response.
Clinical manifestations and post-acute infection syndrome differ among patients with mild, moderate, and severe COVID-19. In a longitudinal study, Lucas et al. (2020) monitored patients hospitalized with COVID-19 and compared their clinical characteristics with those of a control group. They observed that patients with moderate COVID-19 had low levels of inflammatory markers and high levels of protein requirement owing to organic reconstitution and tissue recovery needs. Conversely, patients with severe COVID-19 exhibited high levels of IFN-α, IL-1 receptor antagonist, and proteins associated with T helper cells (Th1 and Th2) even after the viral replication phase had ended. In this way, the relationship between the immune system and anti-inflammatory effects as well as the role of protein-mediated immune responses are evident. Considering the nutritional conditions of hospitalized patients with COVID-19, a recent work from Li et al. (2021) evaluated its nutritional risk and therapy in 523 patients with severe and critical COVID-19 from four hospitals in Wuhan, China. In this work, the authors found that patients admitted to the Intensive Care Unit (ICU) or died in it had a higher risk of malnutrition, especially liked to a low body mass index (BMI) and proteins levels, such as albumin. They noted that patients with a BMI lower than 20.5 had lower hospitalization survival than those with higher BMI (>20.5). Despite this, these authors also noted that when the patients were admitted to the ICU and received the nutrition support in less than 48 h, they were less susceptible to death. In another work, up to 45% (from 50 cases) of patients with COVID-19 in a Rehabilitation Unit at the San Raffaele Scientific Institute (Milan, Italy) had malnutrition status, based upon the Malnutrition Universal Screen Tool (Brugliera et al., 2020). In their research, Brugliera et al. (2020), also noted that more than 90% of COVID-19 patients showed some degree of dysphagia, needing for an adaptive diet such as by nasogastric feeding. Moreover, it is known that malnutrition can increase hospitalization periods and burden the patient’s recovery. Martindale, Patel, Taylor, Arabi, Warren, & McClave (2020) recommended the administration of Enteral Nutrition (EN) within 24–36 h from ICU admission, since this intervention can reduce mortality and infections. Some nutritional recommendation to COVID-19 patients hospitalized in ICUs are listed in Table 1 . Thus, preventive as well as alternative supplementary therapeutic and nutritional strategies are needed to aid in reducing the severity of COVID-19 manifestations and post-acute syndrome. It is worth mentioning that every therapeutic and/or nutritional strategy should always be guided by specialized professionals, such as medical doctors, nutritionists, pharmacists and others, to avoid health risks.
Table 1.
Some nutritional recommendation to COVID patients hospitalized in ICUs.
| Nutrients | Nutritional recommendation | |||
|---|---|---|---|---|
| Brugliera et al., 2020 | Azzolino, Passarelli, D’Addona, & Cesari (2021) | Pironi et al. (2021) | Martindale et al. (2020) | |
| Energy | 27–30 kcal\kg\day | 27–30 Kcal/Kg of BW/day | 660 Kcal/day | − 15–20 kcal/kg BW/day − 11–14 kcal/kg BW/day (patients with BMI of 30–50) − 22–25 kcal/kg BW/day (patients with BMI > 50) |
| Protein | up to 1.5 g\kg\day | − 1.0 g/Kg of BW/day − 1.2–1.5 g/Kg of BW/day (in the case of acute or chronic diseases) - Up to 2.0 g/Kg of BW/day (highly catabolic conditions) |
− 20.0 g/day (oral nutrition) − 23–61 g/day (enteral nutrition) − 49–75 g/day (parenteral nutrition) |
− 1.2–2 g/kg ABW/day (initial low dose) − 2–2.5 g/kg (ideal body weight) − 2–2.5 g/kg BW/day (critically ill patients undergoing renal replacement therapy) |
| Essential aminoacids | 10–15 g with at least 3 g of leucine | – | – | |
| Lipid:Carbohydrate ratio | 30:70 to 50:50 | – | – | – |
| Vitamins and minerals | – | According to daily allowances | – | – |
| Water | Adequate hydration | – | – | – |
ICU: Intensive Care Unit; BW: Body weight.
5. Potential effects of Amazonian fruits and oilseeds to strengthen the immune system against COVID-19
Some studies have focused on exploring the potential of bioactive compounds derived from dietary sources in the development of therapeutic strategies that harness and strengthen the immune system and its natural responses, thereby lessening the signs and symptoms of viral diseases such as COVID-19 and its post-acute syndrome (BourBour et al., 2020, Galanakis et al., 2020). Table 2 highlights the oilseed species explored in this review, their bioactive compounds, and their potential health effects according to recent studies. In this context, Amazonian oilseed species represent important dietary sources of immunologically active compounds such as PUFAs (e.g., arachidonic [ARA], eicosapentaenoic [EPA], and docosahexaenoic [DHA] acids), which can modulate and potentiate anti-inflammatory mechanisms, and amino acids that play a role in cytokine and antibody production. Moreover, these oilseeds also can have high protein content, which can aid in tissue muscle reconstitution in recovered COVID-19 patients.
Table 2.
Bioactive compounds, and potential biological effects of Amazonian oilseeds on health.
| Food | Used part | Bioactive compound | Assay/ study type | Potential health benefits | References |
|---|---|---|---|---|---|
| Açaí (Euterpe oleracea) | Lyophilized fruit pulp, tablet and jam. | Anthocyanins and phenolic compounds | In vitro | Antioxidant activity | Aliaño-González et al. (2020); |
| Fruit pulp juice | Anthocyanins, phenolic compounds, unsaturated fatty acids. | Randomized cross-over study | ↑ HDL-c concentration; ↑ total antioxidant capacity; ↓ oxidative stress index |
Liz et al. (2020) | |
| Freeze-dried hydroalcoholic extract | Different phenolic compounds (orientin, p-coumaric acid, apigenin, cyanidyn, luteolin, epicatechin, and others). | In vitro | ↓ pro-inflammatory; ↑ anti-inflammatory cytokines; modulation of NLRP3 inflammasome protein expression | Machado et al. (2019) | |
| Fruit pulp | Anthocyanins and phenolic compounds | Clinical study with forty healthy volunteer women | ↑ concentration of apolipoprotein A-I; ↓ radical oxidative stress, ox-LDL and malondialdehyde; ↑ total antioxidant capacity. | Pala et al. (2018) | |
| Fruit pulp | Anthocyanins and phenolic compounds | Cross-sectional, retrospective, and analytical study | Suggests a reduction of occurrence of diabetes and hypertension in women | Silva et al. (2020); | |
| Bacaba (Oenocarpus ssp.) | Fruit powder | Total phenolics and anthocyanins | In vitro | Antioxidant activity | Nascimento et al. (2019) |
| Fruit pulp oil | Total phenolics and PUFAs | In vitro | Antioxidant activity | Pinto et al. (2018) | |
| Fruit pulp | Phenolics and flavonoids | In vitro | Antioxidant properties | Carvalho et al. (2016) | |
| Fruit pulp extract | Phenolic compounds | Cell culture of MCF-7 cells and cell proliferation | Antiproliferative capacities; induced apoptosis in MCF-7 breast cancer cells through the mitochondrial pathway; highest activation caspase-9. | Finco et al. (2016) | |
| Aqueous, methanolic and acetonic pulp extracts | Phenolic compounds | In vitro | Antioxidant properties; protective against DNA damage. | Leba et al. (2014) | |
| Pulp phenolic extract | Phenolic compounds | In vitro cell culture (3T3-L1 preadipocytes) | Reduced accumulation of intracellular lipids and protein expression of adipogenic markers including PPARγ, C/EBPα, FABP4, IR-β, and adiponectin; decreased lipid accumulation; adipogenesis inhibition. | Lauvai et al. (2017) | |
| Buriti (Mauritia flexuosa) | Freeze–dried pulp | Carotenoids | In vitro | Antioxidant properties. | Berni et al. (2020) |
| Leaves extracts | Flavonoids: tricin-7-O-rutinoside, apigenin-6-C-arabinoside, 8-Cglucoside, kaempferol-3-O-rutinoside, quercetin-3-O-rutinoside, luteolin-8-C-glucoside and luteolin-6-C-glucoside. | In vitro | Not applied | Oliveira, Siqueira, Nunes, & Cota (2013) | |
| Freeze–dried pulp | Carotenoids | In vitro | Antioxidant properties. | Cândido, Silva, & Agostini-Costa (2015) | |
| Pulp oil | Carotenoids and unsaturated fatty acids | In vitro | Non-toxic to human blood mononuclear phagocytes; increased rate of cellular phagocytosis in enteropathogenic Escherichia coli | Cruz et al. (2020) | |
| Fruit pulp and sweet dessert | Phenolic compounds and carotenoids | In vitro | Antioxidant properties. | Nascimento-Silva, Silva, & Silva, (2020) | |
| Pulp, peel and endocarp extracts | Phenolic compounds | In vitro bioaccessibility against rat blood cells; in vitro antioxidant assays | The buriti extracts protected rat blood cells against lysis induced by peroxyl radicals, and antioxidant properties. | Pereira-Freire et al., 2018 | |
| Methanolic pulp extract | Phenolic compounds and carotenoids | In vitro evaluation of lipid oxidative damage of red blood cell (RBC) membranes | Antioxidant properties, and the IC50 related to the lipid peroxidation suggests that the extract could be useful in counteracting pathologies associated with reactive oxygen species. | Abreu-Naranjo, Paredes-Moreta, Granda-Albuja, Iturralde, González-Paramás, & Alvarez-Suarez (2020) | |
| Fruit pulp extract | Phenolic compounds and carotenoids | In vitro assay in breast tumor cells line (MDA – MB − 231) | High antioxidant capacity; no changes of MDA – MB – 231 cells viability at 20 a 320 μg /mL extract concentration after 24/48 h. | Pelosi et al. (2020). | |
| Brazil nuts (Bertholletia excelsa) | Intake of Brazilian nuts | Macronutrients and selenium content | Population study and biochemical assays | The intake of Brazil nuts improved the Se deficient; increased the blood concentration of high-density lipoprotein cholesterol, thus reducing cardiovascular risks. | Cominetti, de Bortoli, Garrido, & Cozzolino (2012) |
| Brazil nuts in daily meals | Macronutrients and selenium content | Population study and biochemical assays | The addition of Brazil nuts in children meals elevated Se levels in their blood, without selenosis symptoms. | Martens et al. (2015). | |
| Microencapsulated cake extract powder | Phenolic compounds | In vitro | High selenium content; phenolics stability up to 120 days; potential ingredient for functional foods. | Gomes et al. (2019) | |
| Daily nut intake (1 nut/day for 60 days) | Macronutrients, phenolics and selenium content (1 Brazil nut (approximately 1261 μg/Se) | Randomized controlled trial and in vitro assays | Increased expression levels of 2 miRNAs (miR-454-3p and miR-584-5p) after Brazil nut intake; the study suggest a linkage between Se intake, vitamin D metabolism, and calcium homeostasis. | Reis et al. (2019) | |
| Brazil nut aqueous extract | Phenolics and selenium content | In vitro modulation of cell growth and pro-oxidative and antioxidant markers | The extract at 75 ng Se/mL increased cell growth and decreased oxidative metabolism indicators; minimized negative effects in both directions of the superoxide and hydrogen peroxide imbalance. | Schott et al. (2018) | |
| Daily nuts intake | Macronutrients, phenolics and selenium content | Systematic review and meta-analysis of randomized controlled trials | The intake of Brazil nuts does not change body weight, reduces triglyceride and cholesterol levels, Low Density Lipoprotein but not C-reactive protein | Hou et al. (2020) | |
| Daily nuts intake | Macronutrients, phenolics and selenium content | Systematic review and meta-analysis of randomized controlled trials | The intake of Brazil nuts increased the effect on plasma selenium levels and glutathione peroxidase but had no significant effect on T3 a thyroid hormone. | Li et al., (2020) | |
| Peach palm fruit or pupunha (Bactris gasipaes) | Stem portion of peach palm by-product | Phenolic compounds and sugars | In vitro | High values of antioxidant activity, which could be related to phenolics, gallic, hydroxy benzoic and chlorogenic acids. | Giombelli et al. (2020); |
| Fruit peels | Carotenoids | In vitro | High carotenoid sources, superior to those found in the Pulp; β-carotene was the major carotenoid; high potential to be used as bioactive ingredient. | Matos et al. (2019); | |
| Fruit pulp oil | Phenolic compounds, carotenoids and unsaturated fatty acids | In vitro | The oil displayed good antiatherogenic, antithrombogenic and hypocholesterolemic indices, which could decrease the risk of cardiovascular diseases. | Santos et al. (2020). | |
| Fruit pulp extracts | Phenolic compounds | In vitro | The fruit exhibited good antioxidant properties; IC50 < 65 μg/mL of the vitro inhibitory activities for pancreatic lipase (obesity) and α-glucosidase and α-amylase (type 2 diabetes). | Faria et al. (2021) | |
| Extracts from peach palm biomass | Phenolic compounds and carotenoids | In vitro and in vivo studies | The peach palm carotenoids displayed antioxidant activity on the kidney; anti-inflammatory effect, and the Wistar rats supplemented with carotenoids had lower weight. | Santamarina et al. (2022). | |
| Sapucaia (Lecythis pisonis) | Ethanolic leaves extract | Phenolic compounds | In vitro studies | High antioxidant activity associated to the high level of phenols and flavonoids. | Ferreira et al. (2014) |
| Sapucaia nut flours | Macronutrients, fibers and phenolics | In vitro studies | The sapucaia flours is is a source of proteins (31–49%) and carbohydrates (17–31%). | Teixeira et al. (2018). | |
| Sapucaia nut cake milk | Macronutrients and phenolics | In vitro studies | Good antioxidant activity due to phenolic compounds (gallic, vanillic, ferulic, sinapic and salicylic acids, catechin, taxifolin and sinapaldehyde); promising bioactive food ingredient. | Demoliner et al. (2018) | |
| Sapucaia nut oil | Macronutrients, Omega-3 and omega-6 fatty acids and phenolics | In vitro studies | The oil displayed good antiatherogenic, antithrombogenic and hypocholesterolemic indices, which could decrease the risk of cardiovascular diseases. | Santos, Carvalho, Costa, & Lannes (2019). | |
| Tucumã (Astrocaryum vulgare) | Tucumã oil | Macronutrients, Omega-3 and omega-6 fatty acids and phenolics | In vitro and in vivo studies | Tucumã oil is able to modulate cholinergic neurotransmission of neurons by modulating enzymatic antioxidant defenses, improving or avoiding memory deficits. | Baldissera et al. (2017) |
| Tucumã extracts | Phenolic compounds | In vitro studies | The extract inhibited macrophage proliferation, increased antioxidant defenses, reduced oxidative stress, and modulated genes from inflammatory responses. | Cabral et al. (2020); | |
| Ethanolic tucumã extracts | Phenolic compounds | In vitro studies | Tucumã extract improved cell viability, stimulating cell proliferation, and did not cause oxidative damage. | Ongaratto et al. (2020); | |
| Nano-structured lipid carrier containing tucuma butter/oil | Macronutrients, Omega-3 and omega-6 fatty acids and phenolics | In vitro studies | The nano-structured lipid carrier showed anti- and pro-inflammatory and healing activity. | Rossato et al. (2020); |
In this section, we presented and discussed biochemical properties of some bioactive compounds from Amazonian oilseeds, which can be used to improve human nutritional and health status, and consequently support the immune system on the prevention, alleviation, and recovery of COVID-19 symptoms.
5.1. Fruits from Euterpe oleraceae and Oenocarpus bacaba
Among the various oilseed species, Açaí (E. oleraceae)—one of the most widely recognized Amazonian oily fruit standing out as a “super-fruit”— is consumed in many forms, in natura by the native populations of the Amazon Rainforest region, as well as different food products and industrial by-products by markets all over the world (Yamaguchi et al., 2015). A quick look using the keywords açaí powder, açai oil and international shipping, on online searching platforms can reveal the world-wide commercial accessibility of this fruit in powder, as a puree or its oil to be purchased at different locations, making this super fruit available for everyone's consumption. Moreover, among the oilseeds presented in Table 2, açaí represents the most studied species to date, with many of its components and functions being elucidated.
Assaí, acaí or açaí, is a berry-like fruit rich in natural antioxidants, phenolic compounds and vitamins (Aliaño-González et al., 2020, Machado et al., 2019, Silva et al., 2019). Responsible for its light- to purple-color variations, anthocyanins are the major polyphenols found in açaí. Close to 90 bioactive substances were found present in it, majorly consisting of flavonoids (31%), phenolic compounds (23%), lignoids (11%) and anthocyanins (9%) (Yamaguchi et al., 2015). The predominant anthocyanins in açaí are cyanidin-3-glucoside and cyanidin-3-rutinoside, while the most frequent phenolics are ferulic acid, p-hydroxybenzoic, as well as the gallic, protocatechuic, ellagic, vanillic, p-coumaric acids, and ellagic acid glycoside (Yamaguchi et al., 2015). Some of these phenolic compounds, gallic acid, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, were also found in the fixed oils extracted from their pulp (Pacheco-Palencia, Mertens-Talcott, & Talcott, 2008), confirming its powerful antioxidant activity (Teixeira-Costa, Silva Pereira, Lopes, & Tristão Andrade, 2020). These secondary metabolites assume various functions, including plant defense against pathogens, auto-oxidation prevention, and photoprotection. In the human body, these compounds can modulate inflammatory and oxidative processes, sequestering free radicals and chelating transition metal ions (Aliaño-González et al., 2020, Machado et al., 2019).
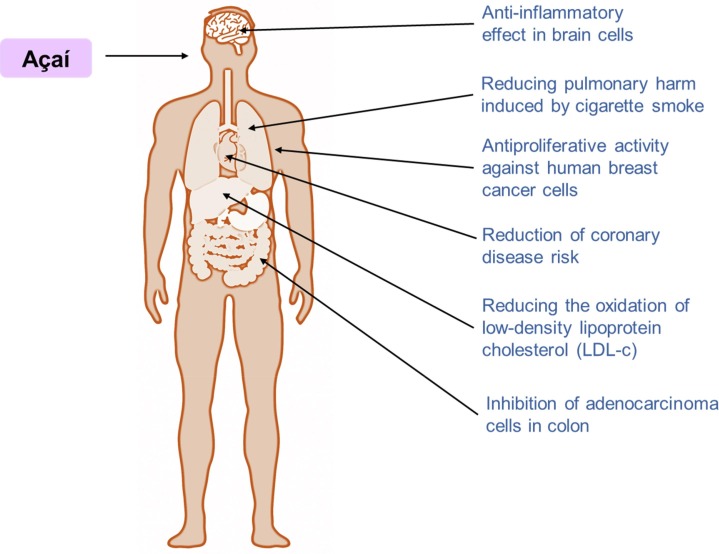
The fruit pulp has displayed an important anti-inflammatory effect in brain cells, reducing signals related to neurodegenerative disorders, such a nitric oxide release, and levels of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), mitogen-activated protein kinase (MAPK), tumor necrosis factor α (TNF α), and nuclear factor κB (NF-κB) (Poulose et al., 2012). In an in vitro study, Ford et al. (2016) demonstrated the role of anthocyanins in the release of pro-inflammatory cytokines by CD4 + T lymphocytes. They incubated cells with anthocyanins and stimulated them with lipopolysaccharides. Their findings showed that these açaí-derived polyphenols decreased the release of pro-inflammatory cytokines, especially of IL-2 and INF-γ. Pala et al. (2018) evaluated the lipid profile, apolipoproteins, and oxidative stress biomarkers of study participants before and after daily intake of açaí. They observed that the concentrations of apolipoprotein A1 and cholesteryl ester transfer proteins increased. These findings demonstrate the role of bioactive compounds of açaí affects the metabolism of high-density lipoprotein cholesterol (HDL-c), reducing the oxidation status of low-density lipoprotein cholesterol (LDL-c) and increasing the endogenous production of reactive oxygen species (ROS), thereby regulating innate immune responses, especially those involving neutrophils.
In another study, Kim et al. (2018) investigated the effects of long-term açaí intake in drink form. They observed that the bioactive compounds present in this fruit can reduce the levels of plasma IFN and urinary 8-isoprostane in patients with metabolic syndrome, demonstrating its health applications against dysfunction of inflammatory processes and oxidative stress. Furthermore, clinical studies (phase 2) conducted by the University of Toronto, Canada, are investigating the anti-inflammatory effect of the intake of 1,560 mg of açaí extracts per day in humans over 40 years old with COVID-19 (Farkouh & Andreazza, 2020). So far, the researchers have found that 1 µg mL-1of açaí extract reduce pro-inflammatory and increase anti-inflammatory cytokines, suggesting that it can be a powerful alternative anti-inflammatory therapy (Machado et al., 2019).
Other clinical trials have been conducted to study the effect açai consumption in health and to prevent metabolic diseases. When using the keyword açai to search for clinical studies at the platform Clinical Trials (available at https://clinicaltrials.gov), it was found a total of 8 studies with açai. Some of these studies have been using the fruit in different forms, as nutritional supplement or as drug active ingredients, such as smoothie (code NCT04748575), puree frozen blended it with banana (code NCT02292329), juice product (code NCT01521949), and drink (code NCT00975728). This highlights the potential use of natural products in reducing or alleviating COVID-19 complications caused by generalized inflammation.
Apart from anthocyanins, açaí also contains other macro- and micronutrients that influence its effects on human health, such as minerals. Iron is a transition metal present in various foods of plant and animal origin that is essential for the differentiation and growth of epithelial tissues as well as for the functioning of proteins that are central components of the immune system. Moreover, iron is involved in the production of ROS by neutrophils during responses to pathogen invasion. Santos et al., 2021 found that the fat from açaí contains approximately 0.5 µg g−1 of Fe, a much lower value when compared to its pulp, which presents about 44 µg g−1 of Fe, while the pulp from the white variety of açai presented the highest value, around 64 µg g−1 of Fe. Zinc is another essential mineral element present in açaí (∼31 – 42 µg g−1) (Santos et al., 2021). Zn is also vital for the development, differentiation, and activation of T lymphocytes. In fact, zinc deficiency is associated with immune system impairment and a reduction in macrophages and monocytes, leading to a pathological increase in oxidative stress (BourBour et al., 2020, Coelho-Ravagnani et al., 2021, Muscogiuri et al., 2020).
Many in vitro studies have indicated that açaí consumption increases catalase activity, decreasing the production of ROS in polymorphonuclear cells. They suggested that regular açaí intake can positively influence endogenous antioxidant processes, thereby protecting cells from oxidative damage. Moreover, açaí consumption can affect lipid metabolism, displaying beneficial effects on LDL-c and HDL-c control (Aliaño-González et al., 2020, Liz et al., 2020, Machado et al., 2019, Magalhães et al., 2020, Silva et al., 2019, Silva et al., 2020, Souza et al., 2020). Furthermore, when studying the biological properties of the açaí oil, several researchers found a powerful in vitro antioxidant activity from it as well as a great content of phenolic compounds non-anthocyanins, which could be potentialized when encapsulated into micro- or nanoparticles to a controlled release in specific targets (Pacheco-Palencia et al., 2008, Rufino et al., 2011, Teixeira-Costa et al., 2020). In Fig. 3 is shown a summary of main biological activities of açaí on human health.
Fig. 3.
Summary of main biological activities of açaí (Euterpe oleracea) on human health.
The Oenocarpus bacaba, popularly known in Brazil as Bacaba, is another oily fruit from the Amazon Rainforest region. This is widely consumed in a similar way to açaí. Recent studies revealed that this oilseed is rich in omega-3 and −6 PUFAs as well as flavonoids, especially anthocyanins, with its effects concentrating on inflammatory processes control, cardiovascular protection, and oxidative stress control (Nascimento et al., 2019, Pinto et al., 2018). Besides anthocyanins, bacaba also contains carotenoids (around 1.4 mg 100 g−1 of oil), vitamin C (30 mg 100-1 of pulp) and other flavonoids (close to 36 mg 100-1 of pulp), such as quercetin (Agostini-Costa, 2018). A derivate from this compound, quercetin glycosylated derivate was found to have angiotensin-converting enzyme type 1 and 2 inhibitor activity (ACEIn and ACE2In), which are the main action searched in the development of anti-COVID-19 therapies (Antonio, Wiedemann, & Veiga-Junior, 2020). Thus, quercetins and its derivates could interfere in various stages of the pathogen's virulence, such as virus entry and replication, and protease inhibition, by their closely related active sites to both ACE and ACE2. Furthermore, the therapeutic effects of flavonoids are potentiated when administered concomitantly with ascorbic acid (vitamin C) (Carvalho et al., 2016, Biancatelli et al., 2020).
The studies from Finco, Kloss, & Graeve (2016) have demonstrated that a rich-phenolic extract from Oenocarpus bacaba induces apoptosis in the MCF-7 breast cancer cell line through a mitochondria-dependent pathway. In another study, a O. bacaba phenolic extract was able to attenuates adipogenesis through the down regulation of the initial and intermediate stages of PPARγ2 and C/EBPα differentiation, demonstrating the potential of this oilseed as a source of antioxidants with biological relevance in adipogenesis (Lauvai et al., 2017).
5.2. Seeds from Bertholetia excelsa and Lecythis pisonis
The Brazil nut, Bertholetia excelsa, is another popular and world widely recognized Amazonian oilseed. The Brazil nut is one of the main sources of selenium, an essential microelement, as well as omega-3 and −6 PUFAs, and phenolic compounds, such as tocopherols (vitamin E) (Gomes et al., 2019, Cardoso et al., 2017). Sapucaia, Lecythis pisonis, belongs to the same botanic family, Leticidaceae, as the Brazil nuts and is an oilseed rich of unsaturated fatty acids, i.e., oleic, and linoleic acids, γ-tocopherol and β-sitosterol, as well as a source of proteins, dietary fiber, and selenium (Demoliner et al., 2018). These authors have been studied this seed and its by-products as a rich source of phenolic compounds, such as myricetin and, vanillic, ferulic, and ellagic acids. These bioactive compounds may display anti-inflammatory, antimicrobial, and antioxidant effects, which have contributed to the potential use of L. pisonis for pharmaceutical, cosmetic and food applications, such as a plant-based milk enhancer, confectionery flour and others (Barreto et al., 2020, Demoliner et al., 2020, Lopes et al., 2021, Teixeira et al., 2018). Moreover, polyphenols present in Brazil nuts and Sapucaia nuts could bind to the angiotensin-converting enzyme 2 (ACE-2), thus preventing that virus entry in host cells, as they may hold the same binding site that S protein from SARS-CoV-2 (Paraiso, Revel, & Stevens, 2020). Likewise, polyphenols can also influence lung injury severity through the regulation of ACE-2 expression. Although, polyphenols are promising inhibitors for viral proteases implied on its replication, more studies are necessary to elucidate safety and effectiveness of those effects in humans (Paraiso, Revel, & Stevens, 2020).
Selenium is an important mineral associated with immunological functions owing to its role complexation to proteins called selenoproteins, which contain selenomethionine or selenocysteine residues, represented by selenoprotein K and enzymes such as glutathione peroxidase and thioredoxin reductases. Moreover, this mineral is an immunostimulant for T and NK cells, which are part of the adaptive immune response, thus influencing vaccination response, pathogen immunity, and tissue inflammation (Qian et al., 2019, Muscogiuri et al., 2020). Studies have provided evidence regarding the relationships between zinc, selenium, and vitamins and the improvement of immune system against COVID-19. Zhang et al. (2020) observed a direct association between reported COVID-19 recovery rates and the selenium status of a population living outside the city of Hubei, China, which was estimated using the selenium concentration in hair samples. Another study suggests selenium supplementation to prevent viral infections, including COVID-19 (Kieliszek and Lipinski, 2020, Moghaddam et al., 2020). In this context, as Brazil nuts are a great natural source of selenium (∼31 to 60 µg g−1), their intake could be a benefic alternative supplementation of it (Santos, Silva Júnior, & Muccillo-Baisch, 2017).
Other micronutrients present in Amazonian oilseeds, such as vitamins A, B6, B12, C, D and E, as well as folate and trace elements, zinc, iron, magnesium, and copper, are important in preventing viral infections owing to their immune system supportive effects (Alkhatib, 2020), indicating that the consumption of Brazil nuts and Sapucaia nuts can boost the immune system and health against viral infections such as COVID-19. Reis et al. (2019) reported that the daily intake of one unit of Brazil nut increases the expression of miR-454-3p and miR-584-5p in obese women. They also conducted a network analysis that indicated a link between selenium intake, vitamin D metabolism, and calcium homeostasis, positively affecting the risk of developing chronic diseases in women with obesity. Moreover, the consumption of Brazil nuts can improve the oxidative metabolism of chemically unbalanced human fibroblasts in a nutrigenomic way (Hou et al., 2020, Li et al., 2020, Schott et al., 2018).
Tocopherols with vitamin E activity, specially α-tocopheryl acetate, represents another class of potent antioxidants commonly present in Amazonian oilseeds. They stimulate the immune system and prevent pathogen-induced diseases while maintaining the integrity of T cell membranes. Tocopherols inhibit the activity of cyclooxygenase-2 (COX-2), improving the formation of immune synapses in T-cells and modulating the Th1/Th2 balance (Barrea et al., 2021, BourBour et al., 2020). Moreover, Alasalvar, Salvadó, & Ros (2020) have critically reviewed the health outcomes and the correlated diseases prevention by nuts’ consumption. In their work, they suggested that the many evidence have shown the reduction of cardiovascular and coronary diseases, as well as the blood lipids profile by the daily intake of nuts, improving health.
5.3. Fruits fom Mauritia flexuosa, Bactris gasipaes and Astrocaryum vulgare
Buriti, M. flexuosa, is a tropical palm tree found in the Amazon Rainforest and Cerrado regions, located in Brazil. This palm tree produces a yellow- to dark-reddish brown fruit color. Buriti fruits presents a dark yellow oily pulp with a unique flavor and scent, and for this is commonly used to prepare confectionary, ice creams, and other food products. It has been recognized as a high source of provitamin A carotenoids, around 53 mg 100 g−1, mainly as β- and α-carotene, as well as lutein and gallic acid (Bailão et al., 2015, Cândido et al., 2015). Besides, its isolated by-products have been gaining attention because of its high content of omega-3 and −6 PUFAs, flavonoids, carotenoids, and other antioxidants, demonstrating potential anti-inflammatory, cardioprotective, antitumor, and gastroprotective effects (Berni et al., 2020, Cruz et al., 2020, Nascimento-Silva et al., 2020, Pelosi et al., 2020).
The lipid-soluble vitamins present in buriti and other oilseeds act as regulatory compounds of high functional relevance. Vitamin A, for example, displays anti-inflammatory effects that improve immune system functioning and maintain the integrity of the mucosa that protects the body against infections. These effects occur through different mechanisms, including TNF-α production, NK cell regulation, and IL-2 production (BourBour et al., 2020, Gombart et al., 2020). Besides, the intake of buriti fruits or its high vitamin A by-products may be used to reverse xerophtalmia and restore liver reserves of vitamin A combating deficiency of this nutrient in needing populations (Bailão et al., 2015).
Studies have reported the in vitro antimicrobial activity of buriti oil when combined with aminoglycoside antibiotics, using the microdilution transfer plate technique to determine the minimum inhibitory concentration of buriti oil against Staphylococcus aureus, Escherichia coli, and other multidrug-resistant bacterial strains (Nobre et al., 2018, Pereira et al., 2018). Another study conducted in human blood indicated that buriti pulp oil can act as an immunomodulator against enteropathogenic Escherichia coli (Cruz et al., 2020), corroborating the body of evidence regarding the potential of buriti in human health applications, especially those involving anti-inflammatory and immunomodulatory effects.
Peach palm, also known as pupunha, B. gasipaes, and tucumã, A. vulgare, fruits have generated great scientific interest owing to their high content of carotenoids, tocopherols, and other antioxidant compounds that have anti-inflammatory, cardioprotective, gastroprotective, and pro-vitamin A effects (Felisberto et al., 2020, Giombelli et al., 2020, Matos et al., 2019, Santos et al., 2020). The oil extracted from the pupunha mesocarp or seed has high levels of monounsaturated fatty acids (52%–70% oleic acid) and sterols (sitosterol), which are commonly associated with a decrease in LDL-c levels and cardiovascular disease risk (Bezerra & Da Silva, 2016).
Baldissera et al. (2017) reported that tucumã oil has potentially protective effects against diabetes. These authors also suggested that its side effects, such as changes in the purinergic system, could improve immune response, demonstrating the potential of tucumã’ intake to the prevention and treatment of chronic diseases. Furthermore, studies with tucumã oil have described its antimicrobial and anti-inflammatory effects as well as its role in the prevention of pathologies, including viral ones (Cabral et al., 2020, Matos et al., 2019, Ongaratto et al., 2020, Rossato et al., 2020). In fact, pupunha and tucumã fruits contain zinc, a transition metal element that influences endogenous antioxidant defense and oxidative response to pathogen invasion (Cure and Cumhur Cure, 2020, Santos et al., 2018), thus contributing to their immunomodulatory effects that could influence the response to respiratory infections, including COVID-19 (Jayawardena et al., 2020).
Regarding the carotenoids, which are present in tucumã, pupunha, and buriti fruits and are responsible for their characteristic colors, Rowles & Erdman (2020) compiled the effects of the most common dietary carotenoids and correlated them to the 10 most commonly diagnosed cancers. They reported that the consumption of dietary carotenoids was related to a reduction in carcinogenesis. Bohn (2019) reviewed the levels of carotenoids and other oxidative stress biomarkers in observational studies and evaluated the relationship between these and chronic diseases, such as cardiovascular diseases and diabetes. Their findings demonstrated the main biological functions of carotenoids in addition to its antioxidant action on endogenous ROS through the activation of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, and enzymes that interact with nuclear factor erythroid 2–related factor 2. Moreover, the only adverse effects observed were in smokers supplemented with β-carotene.
Diseases that affect the respiratory system, such as COVID-19, chronic obstructive pulmonary disease, and lung cancer—often compounded by smoking—are significative causes of deaths worldwide. Rakic & Wang (2020) investigated the relationship between high cholesterol levels and chronic obstructive pulmonary disease. They also highlighted the importance of identifying and consuming dietary sources of bioactive compounds to prevent and decrease the incidence of these diseases. Specifically, they recommend a high intake of carotenoids and lycopene owing to their associations with a reduced risk of chronic lung injuries and as an effective strategy to target the main proteins involved in reverse cholesterol transport. Therefore, these bioactive compounds and their dietary sources should be considered when developing alternative therapeutic strategies to prevent or alleviate respiratory system injuries caused by COVID-19.
Finally, the COVID-19 pandemic accelerated the development of vaccines, which represent the most promising and advanced therapeutical strategy, with several already in use worldwide (WHO, 2020). In vaccines, their main active pharmaceutical ingredients are encapsulated and protected by lipid nanomaterials. In this context, studies may turn to natural sources of lipids to develop bioactive encapsulated materials for use in drug delivery systems, including those used in vaccines. Thus, considering the evidence presented herein, they have potential for application in therapeutic strategies for patients with COVID-19, as well as in the development of antiviral, antibacterial, and anti-inflammatory drugs that could be used to treat these patients.
6. Conclusion
In this review, we compiled the potential benefits and effects of the dietary consumption of Amazonian oilseed species based on their bioactive compounds—especially PUFAs and phenolic substances—including possible applications as alternative or preventive therapeutic strategies for patients with COVID-19. The bioactive compounds of the species highlighted in this study display important effects for the prevention and treatment of viral infections, including COVID-19. These include anti-inflammatory, anticoagulant, antiviral, immunomodulatory, and antioxidant effects that directly affect platelet aggregation, cytokine storm conditions, oxidative stress, and other adverse phenomena. Furthermore, the regular consumption of Amazonian oilseeds may contribute to the prevention of comorbidities associated with COVID-19 severity, such as obesity, diabetes, hypertension, and atherosclerosis. The biolipids from Amazonian oilseeds are widely used in drug delivery systems, including in the protection and encapsulation of the active ingredients in vaccines, and other diverse pharmaceutical applications. In a near future, clinical studies could be conducted to clear and confirm those health benefits in cell and animal models. Moreover, works should consider these sources of bioactive compounds as economically advantageous and widely available materials with relevant applications in human health, nutrition, and immune responses. Especially for underdeveloped or developing countries, the use of dietary therapeutic alternatives may aid in reducing the cost of hospital interventions and health burden incurred during this pandemic, representing a research topic of great interest for various health and research agencies worldwide.
CRediT authorship contribution statement
Orquídea Vasconcelos dos Santos: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Supervision. Ana Clara da C. Pinaffi Langley: Writing – review & editing. Ana Júlia Mota de Lima: Writing – original draft. Vinícius Sidonio Vale Moraes: Writing – original draft. Stephanie Dias Soares: Writing – original draft. Barbara Elisabeth Teixeira-Costa: Resources, Writing – original draft, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments and funding
The authors acknowledge to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil, as this study was partly financed in part by CAPES Finance Code 001, and to the Pró-Reitoria de Pesquisa e Pós-Graduação from the Universidade Federal do Pará - PROPESP/UFPA (PAPQ) for publishing funding support (Edital 02/2022 - PAPQ). This research did not receive any specific grant from funding agencies in the commercial or not-for-profit sectors.
References
- Abreu-Naranjo R., Paredes-Moreta J.G., Granda-Albuja G., Iturralde G., González-Paramás A.M., Alvarez-Suarez J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adili R., Hawley M., Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins & Other Lipid Mediators. 2018;139:10–18. doi: 10.1016/j.prostaglandins.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini-Costa T. da S. Bioactive compounds and health benefits of some palm species traditionally used in Africa and the Americas – A review. Journal of Ethnopharmacology. 2018;224:202–229. doi: 10.1016/j.jep.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Alasalvar C., Salvadó J.S., Ros E. Bioactives and health benefits of nuts and dried fruits. Food chemistry. 2020;314 doi: 10.1016/j.foodchem.2020.126192. [DOI] [PubMed] [Google Scholar]
- Aliaño-González M.J., Espada-Bellido E., Ferreiro-González M., Carrera C., Palma M., Ayuso J., Álvarez J.Á., Barbero G.F. Extraction of anthocyanins and total phenolic compounds from açai (Euterpe oleracea Mart.) using an experimental design methodology. Part 2: Ultrasound-assisted extraction. Agronomy. 2020;10(3):326. doi: 10.3390/agronomy10030326. [DOI] [Google Scholar]
- Alkhatib A. Antiviral functional foods and exercise lifestyle prevention of Coronavirus. Nutrients. 2020;12(9):2633. doi: 10.3390/nu12092633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio A.D.S., Wiedemann L.S.M., Veiga-Junior V.F. Natural products’ role against COVID-19. RSC Advances. 2020;10(39):23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolino D., Passarelli P.C., D’Addona A., Cesari M. Nutritional strategies for the rehabilitation of COVID-19 patients. European Journal of Clinical Nutrition. 2021;75(4):728–730. doi: 10.1038/s41430-020-00795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailão E.F.L.C., Devilla I.A., da Conceição E.C., Borges L.L. Bioactive compounds found in Brazilian cerrado fruits. International Journal of Molecular Sciences. 2015;16:23760–23783. doi: 10.3390/ijms161023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera M.D., Souza C.F., Grando T.H., Sagrillo M.R., da Silva A.S., Stefani L.M., Monteiro S.G. The use of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice: Effects on behavior, oxidant/antioxidant status, and enzymes involved in brain neurotransmission. Molecular and Cellular Biochemistry. 2017;436(1–2):159–166. doi: 10.1007/s11010-017-3087-9. [DOI] [PubMed] [Google Scholar]
- Barrea, L., Muscogiuri, G., Frias-Toral, E., Laudisio, D., Pugliese, G., Castellucci, B., Garcia-Velasquez, E., Savastano, S., & Colao, A. (2021). Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Critical reviews in food science and nutrition, 1–25. Advance online publication. 10.1080/10408398.2020.1792826. [DOI] [PubMed]
- Barreto, K. G., Moreira, L. L. P. F., Gomes, J. S. X., Matos, C. R. R., & Mathias, L. (2020). Phytochemical profile and antioxidant activity of a Lecythis pisonis Cambess. specimen (Lecythidaceae). Revista Virtual de Química, 12, 1511–1528. http://doi.org/10.21577/1984-6835.20200118 [Brazilian Portuguese].
- Berni P., Pinheiro A.C., Bourbon A.I., Guimarães M., Canniatti-Brazaca S.G., Vicente A.A. Characterization of the behavior of carotenoids from pitanga (Eugenia uniflora) and buriti (Mauritia flexuosa) during microemulsion production and in a dynamic gastrointestinal system. Journal of Food Science and Technology. 2020;57(2):650–662. doi: 10.1007/s13197-019-04097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukema M., Faas M.M., de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Experimental & Molecular medicine. 2020;52(9):1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra C.V., Da Silva L.H.M. In: Traditional Foods. Integrating Food Science and Engineering Knowledge Into the Food Chain. Kristbergsson K., Oliveira J., editors. Springer; 2016. Pupunha (Bactris gasipaes): General and Consumption Aspects; pp. 399–405. [DOI] [Google Scholar]
- Biancatelli R.C., Berrill M., Catravas J.D., Marik P.E. Quercetin and Vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Frontiers In Immunology. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants (Basel, Switzerland) 2019;8(6):179. doi: 10.3390/antiox8060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R., McCarthy J.R. Direct SARS-CoV-2 infection of the heart potentiates the cardiovascular sequelae of COVID-19. Drug discovery today. 2020;25(9):1559–1560. doi: 10.1016/j.drudis.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BourBour, F., Mirzaei Dahka, S., Gholamalizadeh, M., Akbari, M. E., Shadnoush, M., Haghighi, M., Taghvaye-Masoumi, H., Ashoori, N., & Doaei, S. (2020). Nutrients in prevention, treatment, and management of viral infections; special focus on Coronavirus. Archives of physiology and biochemistry, 1–10. Advance online publication. https://doi.org/10.1080/13813455.2020.1791188. [DOI] [PubMed]
- Brugliera L., Spina A., Castellazzi P., Cimino P., Arcuri P., Negro A., Houdayer E., Alemanno F., Giordani A., Mortini P., Iannaccone S. Nutritional management of COVID-19 patients in a rehabilitation unit. European Journal of Clinical Nutrition. 2020;74(6):860–863. doi: 10.1038/s41430-020-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F.L., Bernardes V.M., Passos D.F., de Oliveira J.S., Doleski P.H., Silveira K.L., Horvarth M.C., Bremm J.M., Barbisan F., Azzolin V.F., Teixeira C.F., de Andrade C.M., da Cruz I., Ribeiro E.E., Leal D. Astrocaryum aculeatum fruit improves inflammation and redox balance in phytohemagglutinin-stimulated macrophages. Journal of Ethnopharmacology. 2020;247 doi: 10.1016/j.jep.2019.112274. [DOI] [PubMed] [Google Scholar]
- Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12(4):1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cândido T.L.N., Silva M.R., Agostini-Costa T.S. Bioactive compounds and antioxidant capacity of buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon biomes. Food Chemistry. 2015;177:313–319. doi: 10.1016/j.foodchem.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Cardoso B.R., Duarte G.B.S., Reis B.Z., Cozzolino S.M.F. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Research International. 2017;100:9–18. doi: 10.1016/j.foodres.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Carvalho A.V., Da Silveira T.F., De Sousa S.H.B., De Moraes M.R., Godoy H.T. Phenolic composition and antioxidant capacity of bacaba-de-leque (Oenocarpus distichus Mart.) genotypes. Journal of Food Composition and Analysis. 2016;54:1–9. doi: 10.1016/j.jfca.2016.09.013. [DOI] [Google Scholar]
- Ceribelli A., Motta F., De Santis M., Ansari A.A., Ridgway W.M., Gershwin M.E., Selmi C. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. Journal of autoimmunity. 2020;109 doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.-F.-W., Yuan S., Kok K.-H., To K.-K.-W., Chu H., Yang J.…Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C.E., Calder P.C., Miles E.A. Diet and Immune Function. Nutrients. 2019;11(8):1933. doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M.A., Hossain N., Kashem M.A., Shahid M.A., Alam A. Immune response in COVID-19: A review. Journal of infection and public health. 2020;13(11):1619–1629. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Ravagnani, C. de F., Corgosinho, F. C., Sanches, F. L. F. Z., Prado, C. M. M., Laviano, A., & Mota, J. F. (2021). Dietary recommendations during the COVID-19 pandemic. Nutrition Reviews, 79(4), 382–393. https://doi.org/10.1093/nutrit/nuaa067. [DOI] [PMC free article] [PubMed]
- Cominetti C., de Bortoli M.C., Garrido A.B., Cozzolino S.M.F. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutrition Research. 2012;32(6):403–407. doi: 10.1016/j.nutres.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Conte L., Toraldo D.M. Targeting the gut–lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Therapeutic Advances in Respiratory Disease. 2020;14 doi: 10.1177/1753466620937170. 175346662093717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothran T.P., Kellman S., Singh S., Beck J.S., Powell K.J., Bolton C.J., Tam J.W. A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID-19. Brain, behavior, and immunity. 2020;88:957–958. doi: 10.1016/j.bbi.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M.B., Oliveira W.D.S., Araújo R.L., Honório França A.C., Pertuzatti P.B. Buriti (Mauritia Flexuosa L.) pulp oil as an immunomodulator against enteropathogenic Escherichia coli. Industrial Crops and Products. 2020;149(112330) doi: 10.1016/j.indcrop.2020.112330. [DOI] [Google Scholar]
- Cure E., Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes & Metabolic Syndrome. 2020;14(4):349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U.N. Can bioactive lipids inactivate coronavirus (COVID-19)? Archives of Medical Research. 2020;51(3):282–286. doi: 10.1016/j.arcmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoliner F., de Britto Policarpi P., Vasconcelos L.F.L., Vitali L., Micke G.A., Block J.M. Sapucaia nut (Lecythis pisonis Cambess) and its by-products: A promising and underutilized source of bioactive compounds. Part II: Phenolic compounds profile. Food Research International. 2018;112:434–442. doi: 10.1016/j.foodres.2018.06.050. [DOI] [PubMed] [Google Scholar]
- Demoliner F., Carvalho L.T., Liz G.R., Prudêncio E.S., Ramos J.C., Bascuñan V.L.A.F., Vitali L., Block J.M. Improving the nutritional and phytochemical compounds of a plant-based milk of Sapucaia nut cake using block freeze concentration. International Journal of Food Science & Technology. 2020;55:3031–3042. doi: 10.1111/ijfs.14568. [DOI] [Google Scholar]
- Dufour D.L., Piperata B.A., Murrieta R.S.S., Wilson W.M., Williams D.D. Amazonian foods and implications for human biology. Annals of Human Biology. 2016;43(4):330–348. doi: 10.1080/03014460.2016.1196245. [DOI] [PubMed] [Google Scholar]
- Faria J.V., Valido I.H., Paz W.H.P., da Silva F.M.A., de Souza A.D.L., Acho L.R.D., Lima E.S., Boleti A.P.A., Marinho J.V.N., Salvador M.J., dos Santos E.L., Soares P.K., López-Mesas M., Maia J.M.F., Koolen H.H.F., Bataglion G.A. Comparative evaluation of chemical composition and biological activities of tropical fruits consumed in Manaus, central Amazonia, Brazil. Food Research International. 2021;139 doi: 10.1016/j.foodres.2020.109836. [DOI] [PubMed] [Google Scholar]
- Farkouh, M. & Andreazza, A. (2020). The açaí berry COVID-19 anti-inflammation trial (ACAI). ClinicalTrials.gov. Available at <https://clinicaltrials.gov/ct2/show/NCT04404218> accessed on June 15, 2021.
- Felisberto, M. H. F., Souza Costa, M., Villas Boas, F., Lopes Leivas, C., Maria Landi Franco, C., Michielon de Souza, S., Pedrosa Silva Clerici, M. T., & Mach Côrtes Cordeiro, L. (2020). Characterization and technological properties of peach palm (Bactris gasipaes var. gasipaes) fruit starch. Food Research International, 136, 109569. https://doi.org/10.1016/j.foodres.2020.109569. [DOI] [PubMed]
- Ferreira, É. L. de F., Mascarenhas, T. S., Oliveira, J. P. de C., Chaves, M. H., Araújo, B. Q., & Alberto José Cavalheiro. (2014). Phytochemical investigation and antioxidant activity of extracts of Lecythis pisonis Camb. Journal of Medicinal Plants Research, 8(8), 353–360. https://doi.org/10.5897/JMPR2013.5153.
- Finco F.D.A.B., Kloss L., Graeve L. Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS Journal. 2016;5:5–15. doi: 10.1016/j.nfs.2016.11.001. [DOI] [Google Scholar]
- Ford C.T., Richardson S., McArdle F., Lotito S.B., Crozier A., McArdle A., Jackson M.J. Identification of (poly)phenol treatments that modulate the release of pro-inflammatory cytokines by human lymphocytes. The British journal of nutrition. 2016;115(10):1699–1710. doi: 10.1017/S0007114516000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C.M., Aldawoud T.M.S., Rizou M., Rowan N.J., Ibrahim S.A. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods. 2020;9(11):1701. doi: 10.3390/foods9111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giombelli C., Iwassa I.J., da Silva C., Barros B.C.B. Valorization of peach palm by-product through subcritical water extraction of soluble sugars and phenolic compounds. The Journal of Supercritical Fluids. 2020;165 doi: 10.1016/j.supflu.2020.104985. [DOI] [Google Scholar]
- Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S., Finotelli P.V., Sardela V.F., Pereira H.M.G., Santelli R.E., Freire A.S., Torres A.G. Microencapsulated Brazil nut (Bertholletia excelsa) cake extract powder as an added-value functional food ingredient. LWT - Food science and Technology. 2019;116 doi: 10.1016/j.lwt.2019.108495. [DOI] [Google Scholar]
- Gurzell E.A., Teague H., Harris M., Clinthorne J., Shaikh S.R., Fenton J.I. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. Journal of Leukocyte Biology. 2013;93(4):463–470. doi: 10.1189/jlb.0812394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Shindo N. COVID-19: What is next for public health? The Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, L., Rashid, M., Chhabra, M., Chandrasekhar, B., Amirthalingam, P., Ray, S., & Li, Z. (2020). The effect of Bertholletia excelsa on body weight, cholestrol, and c-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Complementary therapies in medicine, 57, 102636. Advance online publication. https://doi.org/10.1016/j.ctim.2020.102636. [DOI] [PubMed]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.…Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson M.O., Ley D., Portal C., Gottrand M., Hueso T., Desseyn J.L., Gottrand F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. Journal of Infection. 2016;73(6):523–535. doi: 10.1016/j.jinf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Innes J.K., Calder P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An update for 2020. International Journal of Molecular Sciences. 2020;21(4):1362. doi: 10.3390/ijms21041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes & Metabolic Syndrome. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P. (2012). Regulation of immune responses by prostaglandin E2. Journal of Immunology (Baltimore, Md.: 1950), 188(1), 21–28. https://doi.org/10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed]
- Kim H., Simbo S.Y., Fang C., McAlister L., Roque A., Banerjee N., Talcott S.T., Zhao H., Kreider R.B., Mertens-Talcott S.U. Açaí (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food & function. 2018;9(6):3097–3103. doi: 10.1039/c8fo00595h. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Medical Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachat C., Raneri J.E., Smith K.W., Kolsteren P., Van Damme P., Verzelen K., Penafiel D., Vanhove W., Kennedy G., Hunter D., Odhiambo F.O., Ntandou-Bouzitou G., De Baets B., Ratnasekera D., Ky H.T., Remans R., Termote C. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proceedings of the National Academy of Sciences. 2018;115(1):127–132. doi: 10.1073/pnas.1709194115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvai J., Schumacher M., Abadio Finco F.D.B., Graeve L. Bacaba phenolic extract attenuates adipogenesis by down-regulating PPARγ and C/EBPα in 3T3-L1 cells. NFS Journal. 2017;9:8–14. doi: 10.1016/j.nfs.2017.09.001. [DOI] [Google Scholar]
- Leba L.-J., Brunschwig C., Saout M., Martial K., Vulcain E., Bereau D., Robinson J.-C. Optimization of a DNA Nicking Assay to Evaluate Oenocarpus bataua and Camellia sinensis Antioxidant Capacity. International Journal of Molecular Sciences. 2014;15(10):18023–18039. doi: 10.3390/ijms151018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhou C., Ba Y., Wang Y., Song B., Cheng X., Dong Q., Wang L., You S. Nutritional risk and therapy for severe and critical COVID-19 patients: A multicenter retrospective observational study. Clinical Nutrition. 2021;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Clark C., Abdulazeeme H.M., Salehisahlabadi A., Rahmani J., Zhang Y. The effect of Brazil nuts on selenium levels, glutathione peroxidase, and thyroid hormones: A systematic review and meta-analysis of randomized controlled trials. Journal of King Saud University - Science. 2020;32(3):1845–1852. doi: 10.1016/j.jksus.2020.01.019. [DOI] [Google Scholar]
- Liz S., Cardoso A.L., Copetti C., Hinnig P.F., Vieira F., da Silva E.L., Schulz M., Fett R., Micke G.A., Di Pietro P.F. Açaí (Euterpe oleracea Mart.) and juçara (Euterpe edulis Mart.) juices improved HDL-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clinical nutrition (Edinburgh, Scotland) 2020;39(12):3629–3636. doi: 10.1016/j.clnu.2020.04.007. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS central science. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London D.S., Beezhold B. A phytochemical-rich diet may explain the absence of age-related decline in visual acuity of Amazonian hunter-gatherers in Ecuador. Nutrition Research. 2015;35(2):107–117. doi: 10.1016/j.nutres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Lopes B.O., Coelho C.C.S., Souza A.G.C., Freitas-Silva O. Non-timber Amazonian Forest products and their valuable edible nuts: Cutia nut, Egg nut, Sapucaia nut and Brazil nut. Journal of Agricultural Studies. 2021;9:286–302. doi: 10.5296/jas.v9i1.18050. [DOI] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T., Silva J., Sundaram M.…Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Su K., Zhang X. Potential of plant proteins digested In Silico by gastrointestinal enzymes as nutritional supplement for COVID-19 Patients. Plant foods for human nutrition (Dordrecht, Netherlands) 2020;75(4):583–591. doi: 10.1007/s11130-020-00850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A.K., Cadoná F.C., Assmann C.E., Andreazza A.C., Duarte M.M.M.F., Branco C.S., Zhou X., Souza D.V., Ribeiro E.E., Cruz I.B.M. Açaí (Euterpe oleracea Mart.) has anti-inflammatory potential through NLRP3-inflamma some modulation. Journal of Functional Foods. 2019;56:364–371. doi: 10.1016/j.jff.2019.03.034. [DOI] [Google Scholar]
- Magalhães T.A., de Oliveira Macedo P.C., Converti A., Neves de Lima Á.A. The use of Euterpe oleracea Mart. as a new perspective for disease treatment and prevention. Biomolecules. 2020;10(6):813. doi: 10.3390/biom10060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera L. Vitamin D3 as potential treatment adjuncts for COVID-19. Nutrients. 2020;12(11):3512. doi: 10.3390/nu12113512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos A., Nova E., Montero A. Changes in the immune system are conditioned by nutrition. European Journal of Clinical Nutrition. 2003;57(S1):S66–S69. doi: 10.1038/sj.ejcn.1601819. [DOI] [PubMed] [Google Scholar]
- Martens I.B.G., Cardoso B.R., Hare D.J., Niedzwiecki M.M., Lajolo F.M., Martens A., Cozzolino S.M.F. Selenium status in preschool children receiving a Brazil nut–enriched diet. Nutrition. 2015;31(11–12):1339–1343. doi: 10.1016/j.nut.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition Therapy in Critically Ill Patients With Coronavirus Disease 2019. Journal of Parenteral and Enteral Nutrition. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos K.A.N., Praia Lima D., Pereira Barbosa A.P., Zerlotti Mercadante A., Campos Chisté R. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chemistry. 2019;272:216–221. doi: 10.1016/j.foodchem.2018.08.053. [DOI] [PubMed] [Google Scholar]
- Messina G., Polito R., Monda V., Cipolloni L., Di Nunno N., Di Mizio G., Murabito P., Carotenuto M., Messina A., Pisanelli D., Valenzano A., Cibelli G., Scarinci A., Monda M., Sessa F. Functional role of dietary intervention to improve the outcome of COVID-19: A hypothesis of work. International Journal of Molecular Sciences. 2020;21(9):3104. doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscogiuri G., Barrea L., Savastano S., Colao A. Nutritional recommendations for CoVID-19 quarantine. European Journal of Clinical Nutrition. 2020;74(6):850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S.…Wan E.Y. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento R.A., Andrade E.B., Ribeiro N.F.P., Costa C.M.L., Faria L.J.G. Bacaba powder produced in spouted bed: An alternative source of bioactive compounds and energy food product. Brazilian Journal of Food Technology. 2019;22:1–15. doi: 10.1590/1981-6723.22918. [DOI] [Google Scholar]
- Nascimento-Silva N.R.R., Silva F.A., Silva M.R. Physicochemical composition and antioxidants of buriti (Mauritia flexuosa Linn. F.) – pulp and sweet. Journal of Bioenergy and Food. Science. 2020;7(1):1–12. doi: 10.18067/jbfs.v7i1.279. [DOI] [Google Scholar]
- Ni M., Tian F.B., Xiang D.D., Yu B. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. Journal of medical virology. 2020;92(11):2600–2606. doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre C.B., De Sousa E.O., De Lima Silva J.M.F., Melo Coutinho H.D., Da Costa J.G.M. Chemical composition and antibacterial activity of fixed oils of Mauritia flexuosa and Orbignya speciosa associated with aminoglycosides. European Journal of Integrative Medicine. 2018;23:84–89. doi: 10.1016/j.eujim.2018.09.009. [DOI] [Google Scholar]
- Oliveira D.M., Siqueira E.P., Nunes Y.R.F., Cota B.B. Flavonoids from leaves of Mauritia flexuosa. Revista Brasileira de Farmacognosia. 2013;23(4):614–620. doi: 10.1590/S0102-695X2013005000061. [DOI] [Google Scholar]
- Ongaratto, F., Bonadiman, B. S. R., Marafon, F., Kosvoski, G. C., Chaves, C. C., Chaves, C. M., Cruz, I. B. M., & Bagatini, M. D. (2020). Effect on the glass of Tucuman extract (Astrocaryum aculeatum) on peripheral blood mononuclear cells. Brazilian Journal of Health Review, 3(3), 5055–5062. https://doi.org/10.34119/bjhrv3n3-087 [Brazilian Portuguese].
- Pacheco-Palencia L.A., Mertens-Talcott S., Talcott S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açai (Euterpe oleracea Mart.) Journal of Agricultural and Food Chemistry. 2008;56(12):4631–4636. doi: 10.1021/jf800161u. [DOI] [PubMed] [Google Scholar]
- Pala D., Barbosa P.O., Silva C.T., de Souza M.O., Freitas F.R., Volp A.C.P., Maranhão R.C., de Freitas R.N. Açai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clinical Nutrition. 2018;37(2):618–623. doi: 10.1016/j.clnu.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Pan-American Health Organization, World Health Organization. (2020). Alerta Epidemiológico. Complicações e sequelas da COVID-19. Retrieved from https://www.paho.org/bra/index.php?option=com_docman&view=download&alias=2046-alerta-epidemiologico-complicacoes-e-sequelas-da-covid-19&category_slug=covid-19-materiais-de-comunicacao-1&Itemid=965. Accessed March 30, 2021 [Brazilian Portuguese].
- Park Y., Harris W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids. 2002;37(10):941–946. doi: 10.1007/s11745-006-0984-1. [DOI] [PubMed] [Google Scholar]
- Pereira, Y. F., do Socorro Costa, M., Relison Tintino, S., Esmeraldo Rocha, J., Fernandes Galvão Rodrigues, F., de Sá Barreto Feitosa, M. K., de Menezes, I., Douglas Melo Coutinho, H., da Costa, J., & de Sousa, E. O. (2018). Modulation of the Antibiotic Activity by the Mauritia flexuosa (Buriti) fixed oil against methicillin-resistant Staphylococcus aureus (MRSA) and other multidrug-resistant (MDR) bacterial strains. Pathogens (Basel, Switzerland), 7(4), 98. https://doi.org/10.3390/pathogens7040098. [DOI] [PMC free article] [PubMed]
- Paraiso I.L., Revel J.S., Stevens J.F. Potential use of polyphenols in the battle against COVID-19. Current Opinion in Food Science. 2020;32:149–155. doi: 10.1016/j.cofs.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.J., Martindale R.G., McClave S.A. Relevant nutrition therapy in COVID-19 and the constraints on its delivery by a unique disease process. Nutrition in Clinical Practice. 2020;35(5):792–799. doi: 10.1002/ncp.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi, M. S., Leite, I., Passos, L., Ferreira, C., Sabaa-sur, A.U.O., & Fialho, E. (2020). Physico-chemical evaluation and antioxidant capacity of buriti extract and antitumor activity in breast cancer cell line MDA-MB-231. Brazilian Journal of Development, 6(4), 17493–17515. http://doi:10.34117/bjdv6n4-064 [Brazilian Portuguese].
- Pereira-Freire, J. A., Oliveira, G. L. da S., Lima, L. K. F., Ramos, C. L. S., Arcanjo-Medeiros, S. R., Lima, A. C. S. De, Teixeira, S. A., Oliveira, G. A. L. De, Nunes, N. M. F., Amorim, V. R., Lopes, L. D. S., Rolim, L. A., Costa-Júnior, J. S. Da, & Ferreira, P. M. P. (2018). In Vitro and Ex Vivo Chemopreventive Action of Mauritia flexuosa Products. Evidence-Based Complementary and Alternative Medicine, 2018, 1–12. https://doi.org/10.1155/2018/2051279. [DOI] [PMC free article] [PubMed]
- Pinto R.H.H., Sena C., Santos O.V., Costa W.A., Rodrigues A.M.C., Carvalho-Junior R.N. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas y aceites. 2018;69(2):246. doi: 10.3989/gya.0883171. [DOI] [Google Scholar]
- Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., Brodosi L., Leoni L., Mari G.A., Musio A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clinical Nutrition. 2021;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose S.M., Fisher D.R., Larson J., Bielinski D.F., Rimando A.M., Carey A.N., Schauss A.G., Shukitt-Hale B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. Journal of Agricultural and Food Chemistry. 2012;60(4):1084–1093. doi: 10.1021/jf203989k. [DOI] [PubMed] [Google Scholar]
- Qian F., Misra S., Prabhu K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Critical Reviews in Biochemistry and Molecular Biology. 2019;54(6):484–516. doi: 10.1080/10409238.2020.1717430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases an official publication of the Infectious Diseases Society of America. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, B. Z., Duarte, G., Vargas-Mendez, E., Ferreira, L., Barbosa, F., Jr, Cercato, C., Rogero, M. M., & Cozzolino, S. (2019). Brazil nut intake increases circulating miR-454-3p and miR-584-5p in obese women. Nutrition research (New York, N.Y.), 67, 40–52. https://doi.org/10.1016/j.nutres.2019.05.004. [DOI] [PubMed]
- Rakic J.M., Wang X.D. Role of lycopene in smoke-promoted chronic obstructive pulmonary disease and lung carcinogenesis. Archives of Biochemistry and Biophysics. 2020;689 doi: 10.1016/j.abb.2020.108439. [DOI] [PubMed] [Google Scholar]
- Rogero M.M., Leão M.C., Santana T.M., Pimentel M., Carlini G., da Silveira T., Gonçalves R.C., Castro I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radical Biology & Medicine. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato A., Silveira L.S., Oliveira P.S., Filho W.P.S., Wagner R., Klein B., Souza D., Baldissera M.D., Sagrillo M.R. Evaluation of anti-inflammatory and healing activity of a nano-structured lipid carrier containing tucuman butter oil and butter. Disciplinarum Scientia. 2020;21(3):99–108. doi: 10.37779/nt.v21i3.3551. [DOI] [Google Scholar]
- Rowles J.L., Erdman J.W., Jr Carotenoids and their role in cancer prevention. Biochimica et biophysica acta. Molecular and Cell Biology of. Lipids. 2020;1865(11) doi: 10.1016/j.bbalip.2020.158613. [DOI] [PubMed] [Google Scholar]
- Rufino, M. do S. M., Pérez-Jiménez, J., Arranz, S., Alves, R. E., de Brito, E. S., Oliveira, M. S. P., & Saura-Calixto, F. (2011). Açaí (Euterpe oleraceae) ‘BRS Pará’: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Research International, 44(7), 2100–2106. https://doi.org/10.1016/j.foodres.2010.09.011.
- Santamarina A.B., de Souza Mesquita L.M., Casagrande B.P., Sertorio M.N., Vitor de Souza D., Mennitti L.V., Ribeiro D.A., Estadella D., Ventura S.P.M., de Rosso V.V., Pisani L.P. Supplementation of carotenoids from peach palm waste (Bactris gasipaes) obtained with an ionic liquid mediated process displays kidney anti-inflammatory and antioxidant outcomes. Food Chemistry: X. 2022;13(February) doi: 10.1016/j.fochx.2022.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., da Silva Júnior F.M.R., Muccillo-Baisch A.L. Selenium content of Brazilian foods: A review of the literature values. Journal of Food Composition and Analysis. 2017;58:10–15. doi: 10.1016/j.jfca.2017.01.001. [DOI] [Google Scholar]
- Santos, M. M. R., Fernandes, D. S., Cândido, C. J., Cavalheiro, L. F., Silva, A. F. da, Nascimento, V. A. do, Ramos Filho, M. M., Santos, E. F. dos, & Hiane, P. A. (2018). Physical-chemical, nutritional and antioxidant properties of tucumã (Astrocaryum huaimi Mart.) fruits. Semina: Ciências Agrárias, 39(4), 1517. https://doi.org/10.5433/1679-0359.2018v39n4p1517.
- dos Santos O.V., Carvalho R.N., da Costa C.E.F., Lannes S.C.da S. Chemical, chromatographic-functional, thermogravimetric-differential and spectroscopic parameters of the sapucaia oil obtained by different extraction methods. Industrial Crops and Products. 2019;132:487–496. doi: 10.1016/j.indcrop.2019.02.043. [DOI] [Google Scholar]
- Santos O.V., Soares S.D., Dias P.C., Duarte S.P.A., Santos M.P.L., Nascimento F.C.A. Chromatographic profile and bioactive compounds found in the composition of pupunha oil (Bactris gasipaes Kunth): Implications for human health. Revista de Nutrição. 2020;33:1–12. doi: 10.1590/1678-9805202033e190146. [DOI] [Google Scholar]
- Santos, G. A., Carvalho, A. A. C., Oliveira, A. P., Naozuka, J., Matta, F. V., Felipe-Sotelo, M., Ward, N. I., Corrêa, N. C. F., & Nomura, C. S. (2021). Bioaccessibility of Essential Elements in Açaí (Euterpe oleracea Mart.) Pulp. ACS Food Science & Technology, acsfoodscitech.1c00070. https://doi.org/10.1021/acsfoodscitech.1c00070.
- Schott K.L., Assmann C.E., Teixeira C.F., Boligon A.A., Waechter S.R., Duarte F.A., Ribeiro E.E., da Cruz I. Brazil nut improves the oxidative metabolism of superoxide-hydrogen peroxide chemically-imbalanced human fibroblasts in a nutrigenomic manner. Food and Chemical Toxicology. 2018;121:519–526. doi: 10.1016/j.fct.2018.09.038. [DOI] [PubMed] [Google Scholar]
- Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D.F., Neto C., da Silva M.A., Rodrigues G.M., Vidal F., Barbosa M., Oliveira Brito L.M., Bezerra G., Muniz Filho W.E., Borges K., Rosa I.G., de Carvalho J., Soares Branda O., Nascimento M. Açaí (Euterpe oleracea Mart) consumption and prevention of chronic diseases: Is there an association? A preliminary study. The Scientific World Journal. 2020;2020:5782485. doi: 10.1155/2020/5782485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.P., Cunha A.V.M.B., Sousa S.H.B., Menezes E.G.O., Bezerra P.N., Neto J.T.F.N., Filho G.N.R., Araújo M.E., Carvalho R.N., Jr Supercritical CO2 extraction of lyophilized Açaí (Euterpe oleracea Mart.) pulp oil from three municipalities in the state of Pará, Brazil. Journal of CO2 utilization. 2019;31:226–234. doi: 10.1016/j.jcou.2019.03.019. [DOI] [Google Scholar]
- Souza T.C.M., Souza G.V.M., Volp A.C.P. Inflammatory pathways involved in adipose tissue hypertrophy and the effect of Acai (Euterpe oleracea Martius) on the modulation of this process: A review. Research, society and development. 2020;9(9) doi: 10.33448/rsd-v9i9.6813. [DOI] [Google Scholar]
- Teixeira G.L., Ávila S., Hornung P.S., Barbi R.C.T., Ribani R.H. Sapucaia nut (Lecythis pisonis Cambess.) flour as a new industrial ingredient: Physicochemical, thermal, and functional properties. Food Research International. 2018;109:572–582. doi: 10.1016/j.foodres.2018.04.071. [DOI] [PubMed] [Google Scholar]
- Teixeira-Costa B.E., Silva Pereira B.C., Lopes G.K., Tristão Andrade C. Encapsulation and antioxidant activity of assai pulp oil (Euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocolloids. 2020;109 doi: 10.1016/j.foodhyd.2020.106097. [DOI] [Google Scholar]
- Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Reviews in medical virology. 2019;29(2) doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- Thirumdas R., Kothakota A., Pandiselvam R., Bahrami A., Barba F.J. Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: A review. Trends in Food Science & Technology. 2021;110:66–77. doi: 10.1016/j.tifs.2021.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. (2022). UN rights chief leads call for global COVID-19 vaccine equity. UN News. Retrieved from https://news.un.org/en/story/2022/03/1113672, accessed on March 29, 2022.
- Venter C., Eyerich S., Sarin T., Klatt K.C. Nutrition and the immune system: a complicated Tango. Nutrients. 2020;12(3):818. doi: 10.3390/nu12030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain, behavior, and immunity. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in COVID-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO - World Health Organization (2020). Rolling updates on coronavirus disease (COVID-19). WHO characterizes COVID-19 as a pandemic. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed February 20, 2020.
- WHO - World Health Organization (2022). WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed on April 22, 2022.
- Yamaguchi, K. K. de L., Pereira, L. F. R., Lamarão, C. V., Lima, E. S., & da Veiga-Junior, V. F. (2015). Amazon acai: Chemistry and biological activities: A review. Food Chemistry, 179, 137–151. https://doi.org/10.1016/j.foodchem.2015.01.055. [DOI] [PubMed]
- Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: Immunopathogenesis and immunotherapeutics. Signal transduction and targeted therapy. 2020;5(1)(128):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm' in COVID-19. The Journal of infection. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. The American Journal of Clinical Nutrition. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]