Abstract
In recent years, extracellular vesicles (EVs)1 have attracted attention as a new therapeutic tool. In Europe, the United States, and Asia, there is an accelerating trend of moving beyond basic research on clinical trials. However, treatment using EVs is still in the research and development stage, and the general public has insufficient awareness and understanding of the risks involved in ensuring safety and efficacy, the status of laws and regulations, and global research and development trends regarding their use. The Japanese Society for Regenerative Medicine, which has promoted the research and development of regenerative medicine, an innovative medical technology based on the principle of delivering it safely, effectively, and promptly, including the establishment of laws and regulations, would like to express two positions in light of the rapid development of therapies using EVs: 1) concern about treatments that are based solely on the discretion of medical practitioners, and 2) active promotion of treatments based on sound scientific evidence.
Because EVs are released from cells, there are many similarities between EVs and processed cells2 in terms of manufacturing processes and safety hazards. As for efficacy, the mechanism of action of EVs is still unclear, as is the case with specified processed cellsb; in such cases, it is difficult to measure potency, identify efficacy-related quality attributes, and evaluate the comparability of quality before and after a change in the manufacturing process. In other words, the number of quality attributes that can be obtained for EVs is limited because of their complex characteristics, and it is difficult to grasp their quality through specifications and characterization. Therefore, while designing a quality control strategy for EVs, it is important to ensure the quality of the final product (EVs) by controlling the raw materials and manufacturing process.
On the contrary, since EVs do not contain living cell components and are not classified into specified processed cells, non-commercial clinical research on treatments using EVs and individual medical treatments with EVs at the discretion of medical practitioners are out of the scope of the Act on the Safety of Regenerative Medicine of Japan3. At present, there are no relevant laws or regulations for the use of EVs other than the Medical Practitioners’ Act and the Medical Care Act in Japan. Therefore, there is a concern that treatment will be performed without sufficient objective evaluation of the scientific basis for safety and efficacy.
Despite these concerns, the development of therapies using EVs is underway worldwide. This could potentially lead to a wide variety of new therapeutic areas if the methods needed to stably secure and mass cultivate cells as raw materials and the technologies needed for the mass production of EVs can be developed, in addition to understanding the risks involved and developing relevant laws and regulations. As part of the Japanese Society for Regenerative Medicine, we will continue to work on the development of these methods and technologies and hope that such a promising field will be promoted with a high level of safety before reaching the public.
Keywords: Extracellular vesicle, Exosomes, Regenerative medicine, Regulation, Act on the safety of regenerative medicine
1. Introduction
Recently, it has become clear that extracellular vesicles (EVs), which are released not only by cultured human cells but also all kinds of cells such as those of plants, fruit, bacteria, and yeast, have various effects on our biological activities as transmitters of information between cells [1]. Since EVs are unique to the cell from which they are released, a wide range of research on EVs is being conducted in the field of cancer, from diagnosis to therapeutics [[2], [3], [4]]. In particular, small EVs (or exosomes, as they are more frequently called in Japan) with a particle size of approximately 100 nm are attracting attention as new therapeutic tools [[5], [6], [7], [8], [9], [10], [11]]. In addition to basic research, there is a rapidly growing trend in Europe, the US, and Asia to move toward clinical trials, while the global market for exosome diagnosis and treatment is expected to grow rapidly. However, therapies using EVs are still in the research and developmental stages; in addition, there is insufficient awareness and understanding of the risks involved in ensuring safety and efficacy regarding their use, insufficient development of relevant laws and regulations, and insufficient awareness of global research and development trends [12]. More specifically, in Japan, clinical applications of unlicensed EVs (or cell culture supernatants containing EVs) solely at the discretion of medical practitioners are not regulated by any relevant laws other than few very fundamental laws on medical practices, i.e., the Medical Practitioners’ Act and the Medical Care Act4. This contrasts with the fact that the clinical use of processed cells without manufacturing/marketing authorization is strictly regulated by the Act on the Safety of Regenerative Medicine. This further raises concerns that current treatments with EVs are being conducted in environments in which the scientific basis for their safety and efficacy has not been objectively or sufficiently evaluated. The Japanese Society for Regenerative Medicine, which promotes the development of laws for regenerative medicine and cell therapy, held a year-long discussion in its Working Group on EV-based Therapies and Manufacturing of EVs. In this report, it has compiled a consensus on the concerns about the safety and efficacy of EV-based therapies, which can be performed solely at the discretion of medical practitioners in Japan5, and on the need to promote therapies whose safety and efficacy are ensured.
2. Definition of terms and scope of application
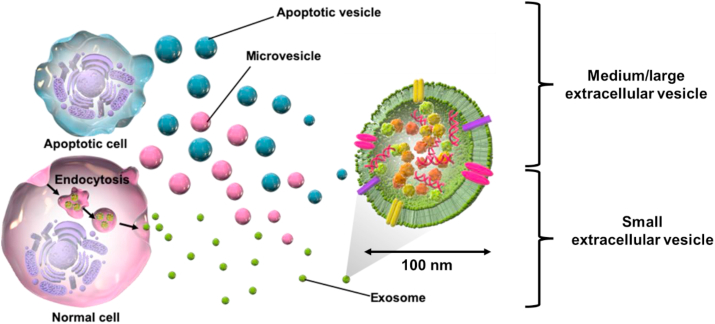
The term “exosome” is often used in Japan, but as exosomes are part of extracellular vesicles, we first clarify the background and definition of the term. Since the 1980s, various cells have been observed to secrete vesicles, which have been termed exosomes, ectosomes, microvesicles, shedding vesicles, apoptotic bodies, oncosomes, and prostasomes, depending on their size and cellular origin. Therefore, the International Society for Extracellular Vesicles (ISEV), an international society for the study of vesicles, recommends the use of extracellular vesicle (EV) as a general term for vesicles secreted by these cells [13,14]. EVs are divided by physical characteristics of EVs in Minimal Information for Studies of Extracellular Vesicles (MISEV) 2018, such as size (“small EVs” (sEVs) and “medium/large EVs” (m/lEVs), with ranges defined, for instance, < 100 nm or < 200 nm [small], or > 200 nm [large and/or medium], respectively) or density (low, middle, high, with each range defined) [14]. Among the EVs, so-called exosomes (very close to sEVs) have received the most attention and are vesicles that form intraluminal membrane vesicles (ILVs) by endocytosis, which engulf receptors on the cell membrane to form endosomes, as shown in Fig. 1. Subsequently, the multivesicular body (MVB), which contains a large amount of IVL, fuses with the cell membrane and is released from the cell. MVBs are known to contain cholesterol, sphingomyelin, ceramide, and lipid raft components in their membranes and many proteins, mRNAs, and miRNAs in their interior. Although these are known to vary from cell to cell as their sources, common markers that characterize most sEVs include membrane transport and fusion proteins (e.g., GTPases, annexins, and flotillin), heat shock proteins (e.g., HSP60, HSP70, and HSP90), tetraspanins (e.g., CD9, CD63, and CD81), MVB formation and transport proteins (e.g., TSG101, ALIX, and Annexins), and cytoskeletal proteins (e.g., actin and tubulin), as per the following Table 3 from the MISEV 2018 [1,14]. sEVs with these properties are not only used for the diagnosis of various diseases, including cancer, but also for novel therapeutic applications as a drug delivery system because of their lipid bilayer coating and the stability of their internal components [11,13,14]. The action mechanism of sEVs is still unknown; however, surface glycans and integrins have been reported to be involved [15].
Fig. 1.
Production pathways and classification of extracellular vesicles (EVs). EVs exist in heterogeneous populations cells with different origins and release processes, with a variety of sizes and properties (exosomes, microvesicles, apoptotic vesicels etc.). The International Society of Extracellular Vesicles (ISEV) states that authors are urged to consider use of operational terms for EV subtypes that refer to a) the physical characteristics of EVs, such as size (“small EVs” (sEVs) and “medium/large EVs” (m/lEVs), with ranges defined, for instance, < 100 nm or < 200 nm [small], or > 200 nm [large and/or medium], respectively) or density (low, middle, high, with each range defined). As for exosome production, endosomes are formed by engulfing receptors in the cell membrane, while intraluminal membrane vesicles are formed inside the endosomes. The multivesicular body is then formed, fusing with the cell membrane, and is further released as an exosome. Exosomes are approximately 100 nm in size and contain cholesterol, sphingomyelin, ceramide, and lipid rafts in their membranes, and many proteins, mRNAs, and miRNAs in the interior.
In addition, it is often difficult to strictly collect sEVs because vesicles of similar size can be mixed during the ultracentrifugation and ultrafiltration processes used to collect them [13,14]. Furthermore, some papers use the term ‘secretome’ because current collection methods include proteins and other components that are not contained in the EVs secreted by cells [16]. In this study, since it is difficult to extract only sEVs from cell culture supernatants, we used the term EVs to include EVs of various size and protein components that may be mixed during preparation (Fig. 1).
3. Risks in ensuring safety and efficacy (manufacturing process, quality)
The potential risks of the clinical use of EVs include: 1) transmission of infectious diseases due to contamination with adventitious agents such as viruses, bacteria, or fungi; 2) undesirable immune reactions such as allergy and rejection; 3) adverse effects of components other than EVs administered simultaneously; 4) variation in efficacy and quality; and 5) undesirable distribution in the body. Because EVs are released by cells, the manufacturing process has many similarities with that of cell therapy products, and they share many of the risks involved regarding safety and efficacy. For example, a common safety challenge is that it is difficult to thoroughly purify the product and to inactivate/remove viruses during the manufacturing process, as is the case with cell therapy products6. Therefore, it is necessary to take consistent measures during all manufacturing processes, including the development of starting materials to prevent contamination with infectious agents. In addition, the phenotypic traits of the cell substrates used for manufacturing may be easily changed, which significantly affects the quality, efficacy, and safety of the EVs released from the cells during the manufacturing process; thus, process control is critical. Furthermore, the mechanism of action of EVs is often unclear, as is the case with cell therapy productsf, where it is difficult to measure the potency of the products, identify efficacy-related quality characteristics, or evaluate quality before and after changing the manufacturing process. In other words, owing to their complex characteristics, only limited quality attributes can be observed in EVs, as well as in cell therapy productsf, which makes their specification and characterization difficult. For this reason, the quality of EVs must be ensured via the quality control of the raw materials and the control of the manufacturing processes, in addition to controlling the final product (e.g., EVs) via their specification and characterization. Therefore, to ensure the quality, efficacy, and safety of EVs, quality and manufacturing controls should be established. In Japan, these should be defined in accordance with the Good Practices for the Provision of Regenerative Medicine under the Act on the Safety of Regenerative Medicine (Section 1, Chapter II of the Ordinance of the Ministry of Health, Labour and Welfare No. 110 of the 2014 Ministerial Ordinance for Enforcement of the Act on the Safety of Regenerative Medicine [last revised in 2020]) or the Good Practices for the Quality Control and Manufacturing Control of Regenerative Medical Products (GCTP: Good Gene, Cellular, and Tissue-based Products Manufacturing Practices) under the Pharmaceuticals and Medical Devices Act.
4. Relevant laws and regulations in Japan
In Japan, under the Pharmaceuticals and Medical Devices Act, whose scope is the manufacture and distribution of therapeutic products intended for marketing, cell therapy and gene therapy products are classified into an independent product category called “regenerative medical products”, and an unique conditional and time-limited approval system may apply to those products [17]. In contrast, EVs containing no living cell component are not classified into regenerative medical products or medical devices, but most likely into drugs, which act principally by pharmacological, immunological, or metabolic action. Furthermore, non-commercial clinical research on EVs and medical treatments with EVs conducted solely at the discretion of medical practitioners are out of the scope of the Act on the Safety of Regenerative Medicine because they are not regarded as specified processed cells as long as they do not contain any viable cell components. Therefore, in Japan, clinical applications of EVs without manufacturing/marketing authorization are conducted solely at the discretion of medical practitioners, leading to the concern that treatments are provided without adequate regulatory oversight other than the Medical Practitioners’ Act and the Medical Care Act and without sufficient scientific safety and efficacy evaluation. Since EVs, like cell therapy products, require risk management againt infectious agent transmission and have complex characteristics, it is necessary to take relevant measures (e.g., enactment of laws, issuance of administrative notices, etc.), which achieve the safety level of EVs, similar to that of specified processed cells under the Regenerative Medicine Safety Acts, in order to protect patients from inappropriate manufacturing and clinical use of EVs. In addition, it is also important to disseminate good practices for the quality and manufacturing controls of EVs based on scientific and clinical expertise to medical practitioners through relevant academic societies.
Article 37 of the Declaration of Helsinki (Ethical Principles for Medical Research Involving Human Subjects) states the following regarding unproven treatments in clinical practice: “In the treatment of an individual patient, where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorized representative, may use an unproven intervention if in the physician's judgement it offers hope of saving life, re-establishing health or alleviating suffering. This intervention should subsequently be the object of research designed to evaluate safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.” It is a matter of course that any therapy using EVs with unproven efficacy and safety, even if it needs to be conducted, must follow the Declaration of Helsinki and be based on the high ethical standards of medical professionals.
5. EV-based therapies
Mesenchymal stem cell (MSC)-derived EVs have recently been implicated in the mechanism underlying the therapeutic effects of MSCs [6,9,11,18,19], with both merits and demerits. The advantage of MSC therapy is that the risks to safety and efficacy are clear, regulations are in place, and the path to clinical practice is well-defined. The disadvantages include the effects caused by the size of the cells, especially when administered intravascularly, and the need to prevent complications of embolization. In addition, alterations to the local environment at the site of administration, such as inflammation, may affect the production of EVs, which, in turn, may affect the predicted therapeutic effect. In addition, because most of the cells are primary cultured cells, there are differences among organs of origin and lots, and difficulties in quality control during the manufacturing process due to cell aging. On the contrary, among the merits, EVs themselves are isolated and purified cell-secreted components, there is no risk of the therapeutic effect being affected by local conditions, and the possibility of embolization is theoretically low due to their small size compared with that of cells. In addition, EVs are easily transferred to the target tissue, multiple administrations are possible (unlike in cell therapy), and they can be easily quantified as the active ingredient that exerts a therapeutic effect. The disadvantages include the need for a larger number of cells than that required for cell administration and the unclear risks in terms of safety and efficacy; and immaturity in terms of quality control, manufacturing control, and legislation. Altogether, predicting the path to a clinical trial is difficult [1,6,9,11,[18], [19], [20], [21], [22]].
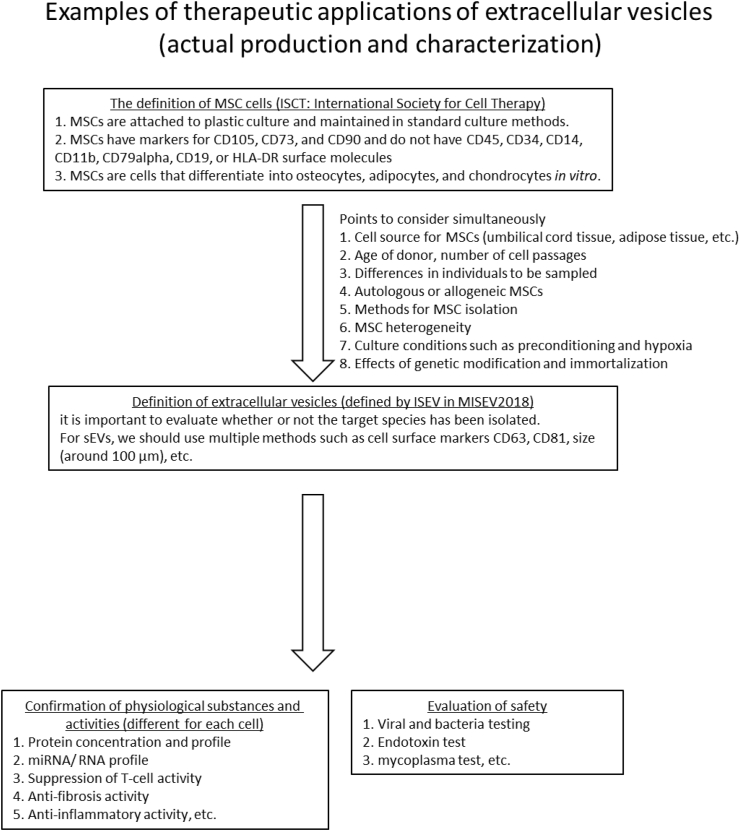
The Journal of Extracellular Vesicles, the official journal of the ISEV, defined the treatment of MSC-derived EVs in 2019 [13,14,23]. Although the cells used as the sources of EVs may vary in characteristics from cell to cell, examples of the current standards and characterization of EVs used in therapy can be found (Fig. 2).
Fig. 2.
Standards and characterization of mesenchymal stem cell (MSC)-derived EVs. Evaluation items during the clinical application of MSC-derived EVs assumed when developing treatments.
6. Conclusions
Although EV-based therapy is still in its infancy, it is expected to become a new treatment for intractable diseases, and the competition for its development will be global. Because this is a new concept, there is currently insufficient regulation of clinical research and free medical treatment using EVs, which raises the concern that treatments may be carried out without sufficient evaluation of the scientific basis for their safety and efficacy. However, if a stable supply of cells is used as the raw material along with the development of mass culture and an appropriate extraction technology for EVs, a clarification of the risks in terms of safety and efficacy, and an improvement in relevant laws and regulations, EVs may be used as a new treatment method for many diseases. In addition, further progress and development is expected in this field by modifying EVs with sugar chains and introducing target proteins or nucleic acid drugs, which will be necessary to respond to the progress in technological development [24,25]. In summary, the Japanese Society for Regenerative Medicine is concerned about the implementation of free medical treatment using EVs that lacks scientific evidence in terms of ensuring efficacy and safety. It is our hope that new and promising therapies will be developed and promoted in an environment in which laws and manufacturing processes are in place to ensure safety and reach to the public.
Statement of originality
This material has not been published and is not currently under consideration by another journal.
Author contributions
Conceptualization, S.T. and M.K.; writing original manuscript draft and investigation, A.T.; methodology, I.H., Y.H., A.S., N.N., Y.S., and T.O.; review & editing, S.T., Y.S., T.O., and M.K.
Funding
For this research, we did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analyzed during the current study.
Declaration of competing interest
Shuji Terai receives funding from ROHTO Pharmaceutical Co., Ltd., MOCHIDA PHARMACEUTICAL CO., LTD, and FUJIFILM Wako Pure Chemical Corporation for basic research of exosomes/extracellular vesicles.
Acknowledgements
The need for this article was established by the Working Group of Attitudes for Preparation and Treatment of Exosomes of the Japanese Society for Regenerative Medicine.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Extracellular vesicles; the positioning paper of the International Society of Extracellular Vesicles (ISEV) (Minimal Information for Studies of Extracellular Vesicles [MISEV] 2018, Journal of Exarcellular Vesicles [JEV]; Updating MISEV, 2021, JEV) states that authors are urged to consider use of operational terms for EV subtypes that refer to a) the physical characteristics of EVs, such as size (“small EVs” (sEVs) and “medium/large EVs” (m/lEVs), with defined ranges, for instance, <100 nm or <200 nm [small], or >200 nm [large and/or medium], respectively) or density (low, middle, high, with each range defined). Therefore, although we use the term EVs in this report, we are particularly conscious of sEVs and so-called exosomes. In addition, we recognize that EVs include extracellular vesicles of various sizes and production processes other than EVs that may be included at the time of preparation, as well as protein components included in cell culture supernatants.
The term “processed cells” is defined in the Act on the Safety of Regenerative Medicine (RM Safety Act), which regulates individual regenerative medicine and cell therapy (RM/CT) at the discretion and responsibility of medical practitioners, as well as non-commercial clinical research on RM/CT. In the RM Safety Act, a cell-based therapeutic product that is manufactured by substantial or more-than-minimal manipulations of somatic/stem cells is called “processed cells.” “Processed cells” other than “cell-processed products,” as defined in the Pharmaceuticals and Medical Devices Act (PMD Act), are called “specified processed cells” in the RM Safety Act. In other words, the term “cell-processed products” in the PMD Act refers to “processed cells” that are intended for marketing.
The scope of the RM Safety Act is non-commercial clinical research on treatments using specified processed cells and individual medical treatments with specified processed cells at the discretion of medical practitioners.
The Medical Practitioners' Act regulates the examination, licensing, and professional rights and duties of physicians. The Medical Care Act is the core of Japan's medical care system. It governs the establishment and management of hospitals, clinics, and midwifery centers; the maintenance and management systems of these medical facilities; medical plans established by prefectures; regulations concerning medical corporations; provision of information on medical care; etc.
In Japan, a private medical practice can perform an EV-based therapy using an unlicensed medicinal product at the discretion of a medical practitioner under the Medical Practitioners' Act and the Medical Care Act. This is similar to that based on the “specials” scheme in Article 5 (1) of the Directive 2001/83/EC in the EU and the UK (https://www.legislation.gov.uk/eudr/2001/83/contents).
The term “cell therapy products” is used here to refer to “processed cells” as defined in the Act on the Safety of Regenerative Medicine.
Contributor Information
Shuji Terai, Email: terais@med.niigata-u.ac.jp.
Masahiro Kino-oka, Email: kino-oka@bio.eng.osaka-u.ac.jp.
References
- 1.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Othman N., Jamal R., Abu N. Cancer-derived exosomes as effectors of key inflammation-related players. Front Immunol. 2019;10:2103. doi: 10.3389/fimmu.2019.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An T., Qin S., Xu Y., Tang Y., Huang Y., Situ B., et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J Extracell Vesicles. 2015;4:27522. doi: 10.3402/jev.v4.27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thind A., Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi: 10.3402/jev.v5.31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T., Tsuchiya A., Takeuchi S., Nojiri S., Yoshida T., Ogawa M., et al. Development of a non-alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regenerative Therapy. 2020;14:252–261. doi: 10.1016/j.reth.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan D., Tieu A., Lalu M., Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9:39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiner A.T., Witwer K.W., van Balkom B.W.M., de Beer J., Brodie C., Corteling R.L., et al. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl Med. 2017;6:1730–1739. doi: 10.1002/sctm.17-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde E., Pachler K., Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21:581–592. doi: 10.1016/j.jcyt.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Russell A.E., Sneider A., Witwer K.W., Bergese P., Bhattacharyya S.N., Cocks A., et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. J Extracell Vesicles. 2019;8:1684862. doi: 10.1080/20013078.2019.1684862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witwer K.W., Van Balkom B.W.M., Bruno S., Choo A., Dominici M., Gimona M., et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couch Y., Buzas E.I., Vizio D.D., Gho Y.S., Harrison P., Hill A.F., et al. A brief history of nearly EV-erything - the rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10:e12144. doi: 10.1002/jev2.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lener T., Gimona M., Aigner L., Borger V., Buzas E., Camussi G., et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida-Aoki N., Tominaga N., Kosaka N., Ochiya T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J Extracell Vesicles. 2020;9:1713527. doi: 10.1080/20013078.2020.1713527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S., Liu C., Ji H.L. Concise review: therapeutic potential of the mesenchymal stem cell derived secretome and extracellular vesicles for radiation-induced lung injury: progress and hypotheses. Stem Cells Transl Med. 2019;8:344–354. doi: 10.1002/sctm.18-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara A., Sato D., Sahara Y. New governmental regulatory system for stem cell-based therapies in Japan. Ther Innov Regul Sci. 2014;48:681–688. doi: 10.1177/2168479014526877. [DOI] [PubMed] [Google Scholar]
- 18.Elahi F.M., Farwell D.G., Nolta J.A., Anderson J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cell. 2020;38:15–21. doi: 10.1002/stem.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuchiya M., Terai H., Mizutani K., Funai Y., Tanaka K., Yamada T., et al. General anesthesia management for adult mucopolysaccharidosis patients undergoing major spine surgery. Med Princ Pract. 2019;28:581–585. doi: 10.1159/000503051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulcahy L.A., Pink R.C., Carter D.R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terai S., Tsuchiya A. Status of and candidates for cell therapy in liver cirrhosis: overcoming the "point of no return" in advanced liver cirrhosis. J Gastroenterol. 2017;52:129–140. doi: 10.1007/s00535-016-1258-1. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe Y., Tsuchiya A., Seino S., Kawata Y., Kojima Y., Ikarashi S., et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl Med. 2019;8:271–284. doi: 10.1002/sctm.18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Mendt M., Kamerkar S., Sugimoto H., McAndrews K.M., Wu C.C., Gagea M., et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.