Abstract
Soft tissue integration is one major difficulty in the wide applications of metal materials in soft tissue-related areas. The inevitable inflammatory response and subsequent fibrous reaction toward the metal implant is one key response for metal implant-soft tissue integration. It is of great importance to modulate this inflammatory-fibrous response, which is mainly mediated by the multidirectional interaction between fibroblasts and macrophages. In this study, macrophages are induced to generate M1 and M2 macrophage immune microenvironments. Their cytokine profiles have been proven to have potentially multi-regulatory effects on fibroblasts. The multi-reparative effects of soft tissue cells (human gingival fibroblasts) cultured on metal material (titanium alloy disks) in M1 and M2 immune microenvironments are then dissected. Fibroblasts in the M1 immune microenvironment tend to aggravate the inflammatory response in a pro-inflammatory positive feedback loop, while M2 immune microenvironment enhances multiple functions of fibroblasts in soft tissue integration, including soft tissue regeneration, cell adhesion on materials, and contraction to immobilize soft tissue. Enlighted by the close interaction between macrophages and fibroblasts, we propose the concept of an “inflammatory-fibrous complex” to disclose possible methods of precisely and effectively modulating inflammatory and fibrous responses, thus advancing the development of metal soft tissue materials.
Keywords: Inflammation, Soft tissue, Macrophage, Fibroblast, Metal material
Graphical abstract
Highlights
-
•
Fibroblasts and macrophages function as an “inflammatory-fibrous complex” on metal materials.
-
•
M1 immune microenvironment tends to promote the proinflammatory effect of fibroblasts in a positive feedback loop.
-
•
M2 immune microenvironment exerts pleiotropic effects on fibroblasts for soft tissue integration.
-
•
Metal implant-soft tissue integration can be improved via modulating the “inflammatory-fibrous complex” response.
1. Introduction
Metal materials, such as hip replacements, dental implants, and cardiovascular stents, have been widely applied in hard tissue replacements or restorations with promising results due to their superior mechanical properties [1,2]. However, metal materials applied in soft tissue-related fields are relatively limited, partially related to the difficulties in establishing effective integration between metal and soft tissue [3,4]. Metal soft tissue materials such as perforating external fracture fixators require an effective soft tissue barrier between internal and external environments to prevent pathogen invasion, tissue inflammation, and implant motions [[4], [5], [6], [7]]. For perforating metal soft tissue materials such as dental implants, a deficiency of soft tissue integration leads to peri-implant mucositis and peri-implantitis, which has become one of the main causes of metal implant failures [8]. Subcutaneous/submucosal metal soft tissue materials such as titanium meshes are prone to complications of soft tissue perforation and mesh exposure, with an incidence rate of 25%∼66% [9,10].
After soft tissue interventions, metal materials inevitably trigger inflammatory and subsequent fibrotic reactions [11,12]. Uncontrolled inflammation can cause function defects, loosening, and failure of the implants [[13], [14], [15]]. The fibrous encapsulation damaged the proper functioning of the metal material, such as hindering the osseointegration and reducing the release efficiency of the drug delivery system [16,17]. Their hard metal surfaces were prone to form scar tissue via activating cells into fibrillar subtypes in the absence of exogenous signals, unlike soft materials such as hydrogels [[18], [19], [20]]. Therefore, numerous studies have attempted to inhibit or even eliminate this unsatisfactory inflammatory-fibrous response to guarantee the proper functioning of the metal material [[21], [22], [23]].
However, the situation becomes more complicated regarding metal-soft tissue integration. Active immune responses are essential to endow material with immune defense ability and regeneration potentials [24]. Fibroblasts are critical functional cells for metal-soft tissue integration. Fibroblasts synthesize, secrete, and remodel the soft tissues around implants. If immune responses and fibroblasts are excessively inhibited or removed, metal soft tissue integration would be significantly suppressed. For metal materials and soft tissue integration, the collaboration of immune cells and fibroblasts under proper inflammatory-fibrous response is required. Therefore, instead of simple inhibition or elimination, we can put a more positive attitude toward this inflammatory-fibrous response and properly regulate this inflammatory-fibrous response, thus obtaining favorable metal-soft tissue integration.
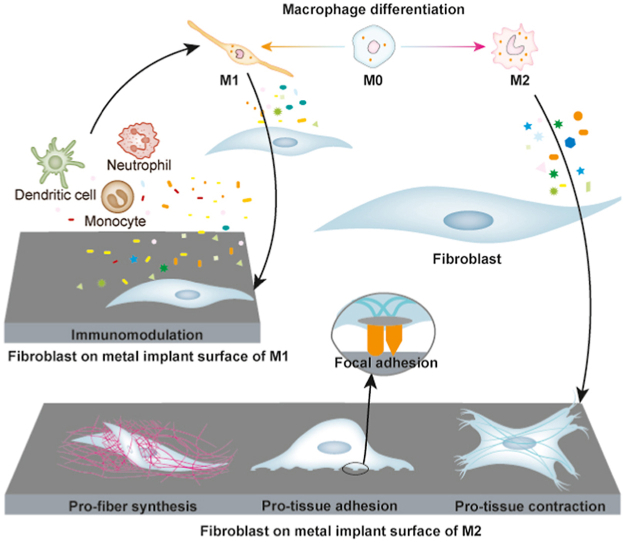
The inflammatory-fibrous response is a complicated interaction between two major cell types: macrophages and fibroblasts. Macrophages have different phenotypes, arousing multifaceted regulatory effects. Their phenotypes are roughly divided into pro-inflammatory M1 and anti-inflammatory M2 phenotypes. M1 macrophages can secrete multiple cytokines to regulate chemotaxis, proliferation, and differentiation of immune cells as well as some progenitor cells, while M2 macrophages promote extracellular matrix (ECM) deposition, angiogenesis, and osteogenesis [[25], [26], [27], [28], [29]]. Fibroblasts have demonstrated multifaceted reparative effects. They can firmly attach to the surface of the metal implant as a layer of metal soft tissue integration. They can also secrete ECM and generate contraction that presses the soft tissue against the implant, forming another layer of metal soft tissue integration. Fibroblasts can also act as immunomodulators, turning metal soft tissue integration into the immune barrier against external stimuli [[30], [31], [32], [33], [34]]. Therefore, we speculate that macrophages and fibroblasts interact closely as one complex. It is of great importance to unveil the multidirectional interactions between fibroblasts and macrophages so that we can precisely and effectively modulate the inflammatory-fibrous complex.
For this purpose, we first utilized different macrophage-derived immune microenvironments to direct human gingival fibroblasts on titanium surfaces. Then, we dissected the multifaceted effects of both macrophages and fibroblasts from the aspect of metal soft tissue integration (Scheme 1). It is of interest to know whether macrophages can activate fibroblasts and whether activated fibroblasts reversely regulate macrophages. The understanding of their multidirectional interactions and the proposal of this “inflammatory-fibrous complex” concept can provide a biological theory for advancing the development of metal soft tissue materials.
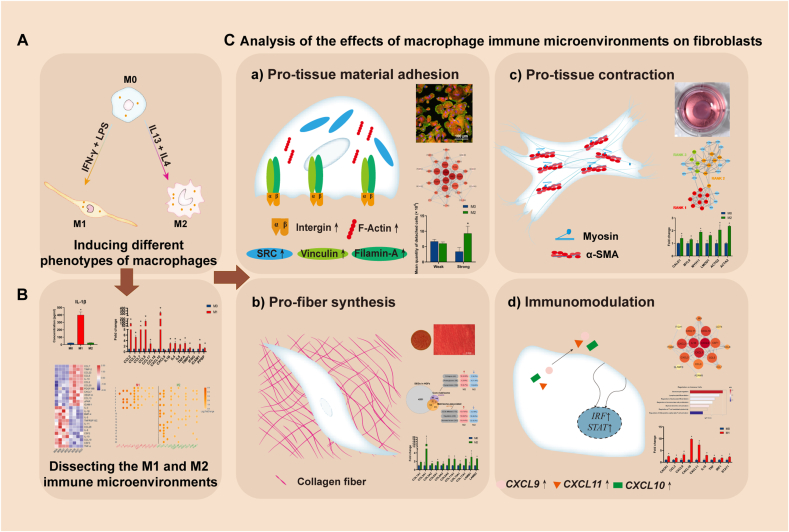
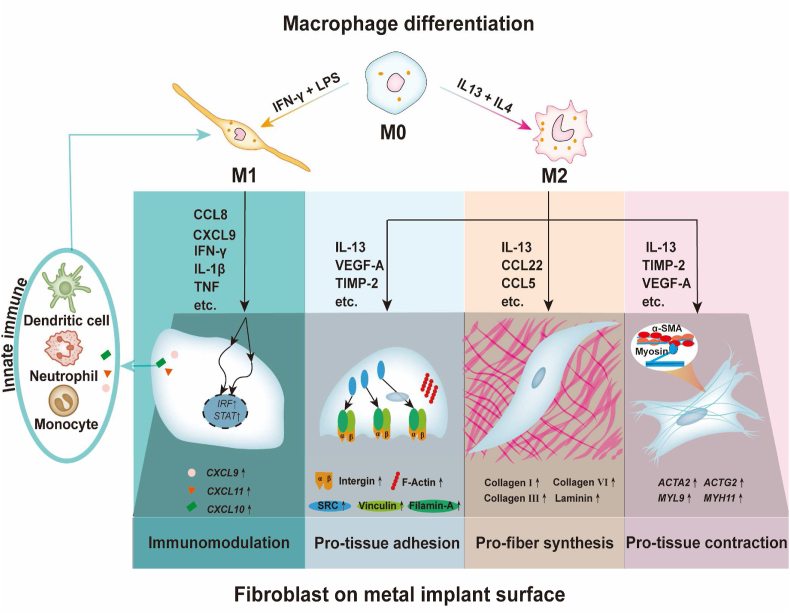
Scheme 1.
Experimental flow of this study. A) Inducing macrophages towards M1 and M2 phenotypes; B) Dissecting the multifaceted effects of M1 and M2 macrophages from the aspect of metal soft tissue integration; C) Analyzing different regulatory effects of macrophage-derived immune microenvironments on human gingival fibroblasts on titanium surfaces: a) the cell adhesion behaviors alterations and their potential mechanisms were evaluated using enrichment analysis, cell adhesion assay, RT–qPCR, immunofluorescence, and correlation analysis; b) the fiber synthesis function of fibroblasts and their potential mechanisms were evaluated using enrichment analysis, Sirius red staining, RT–qPCR, immunofluorescence, and correlation analysis; c) the tissue contraction function of fibroblasts and their potential mechanisms were evaluated using enrichment analysis, collagen gel assay, RT–qPCR, immunofluorescence, and correlation analysis; d) the immune regulatory function of fibroblasts and their potential mechanisms were evaluated using enrichment analysis, RT–qPCR, and correlation analysis.
2. Materials and methods
2.1. M0, M1, M2 macrophages induction and conditioned media collection
THP-1 cells were cultured in RPMI media. When stimulated with PMA (100 ng ml−1, Sigma, USA) for 72 h followed by resting for 48 h, THP-1 cells turned into M0 macrophages. M0 macrophages were then polarized into M1 or M2 macrophages under IFN-γ (20 ng ml−1, R&D, USA) plus LPS (100 ng ml−1, InvivoGen, USA) or IL-13 (20 ng ml−1, R&D, USA) plus IL-4 (20 ng ml−1, R&D, USA) stimulation respectively for 48 h. Cell morphologies of THP-1 and the derived macrophage phenotypes were observed with a phase-contrast microscope. Subsequently, RT–qPCR, flow cytometry, and immunofluorescence staining were carried out to identify macrophage differentiation and polarization. For conditioned media collection, M0, M1, and M2 macrophages were cultured in fetal bovine serum (FBS) free media for another 24 h before collection.
2.2. Isolation of primary human gingival fibroblasts and stimulation
Upon written informed consent from patients and ethical approval by the Ethical Review Committee of Hospital of Stomatology, Guanghua School of Stomatology, Sun Yat-sen University, China (No. KQEC-2019-06-02), healthy gingiva samples were obtained from tooth extraction patients. Tissues were digested in Protease Dispase Ⅱ (Sigma, USA) and were cut into small pieces. Gingiva connective tissue pieces were planted on a culture flask in DMEM media with 15% FBS. Fibroblasts crawled out from gingiva tissues were subcultured until reaching 80% confluence. Fibroblasts were cultured on titanium alloy disks with M0, M1, or M2 conditioned media, mimicking the metal implant-soft tissue integration process in vivo. After overnight culture for cell attachment, the media were replaced by M0, M1, or M2 conditioned media for further stimulation.
2.3. Preparation and characterization of titanium alloy disks
The titanium alloy disks were polished with sandpaper to make uniformly smooth titanium surfaces. Subsequently, the titanium alloy disks were ultrasonically cleaned and the surface topography of the titanium alloy disks was assessed using a 3D optical contour instrument. The surface roughness was then determined.
2.4. Flow cytometry
Cell samples were stained with antibodies (CD68 1:100, Human Leukocyte Antigen-DR, HLA-DR 1:1000, CD206 1:1000; Abcam, USA). Cells were stained with Alexa Fluor 488-conjugated (for CD68 and HLA-DR) or PE-conjugated (for CD206) second antibodies (1:500, Beyotime, China). Cytoflex cytometer (BD Biosciences, USA) was applied for detection. All analyses were performed using FlowJo software. The percentage of positive cells concerning macrophages markers expression in this section was calculated.
2.5. Cytokine profiles of polarized macrophage immune microenvironments
Human Cytokine Array C1000 kits (AAH-CYT-1000, Raybiotech, USA) were applied to detect the cytokines in conditioned media following the manufacturer's protocol. ELISA kits (Boster, China) of TNF-α, IL-1β, and IFN-γ were conducted for macrophages to verify the cytokine profiles.
2.6. RT–qPCR
The extracted total RNA was measured by Nanodrop (Thermo Fisher, USA) and then converted to complementary DNA by PrimeScript™ RT Master Mix (Takara, Japan). Aliquots of cDNA samples were loaded for RT–qPCR analysis on an ABI two-step system (Applied Biosystems, USA) using Hieff® qPCR SYBR Green Master Mix (Yeasen, China). Primer sequences were shown in supplementary materials (Table S2 and S3). Samples were calculated based on the 2−ΔΔCt method referring to GAPDH.
2.7. Transcriptome sequencing of fibroblasts
The cDNA library construction and transcriptome sequencing were conducted by the BGI genomics organization (Shenzhen, China) on a BGISEQ-500 platform. Raw data of FASTQ format was generated, further being calculated into read counts and TPM data for downstream analysis. Bioinformatic analysis of transcriptome data was conducted using R studio 4.0 (The R Foundation, USA) and Cytoscape 3.8 (The Cytoscape Consortium, USA) software as previously described [35].
2.8. Immunofluorescence staining
Macrophages were stained by DAPI simultaneously with CD68, HLA-DR, or CD206. Fibroblasts were stained by Actin, DAPI, Collagen Ⅰ antibody (1:100, Abcam, USA), vinculin (VCL) antibody (1:100, Abcam, USA), Integrin β1 antibody (1:100, Abcam, USA), and alpha-smooth muscle actin (α-SMA) antibody (1:100, Abcam, USA) for different purposes.
2.9. Collagen gel contraction assay
Fibroblasts were mixed with type Ⅰ collagen solution (3 mg ml−1) and formed into gel later when 1 M NaOH was added and homogeneously mixed. After 2 days, the collagen gels were carefully separated and began to contract. Observed the contraction of collagen gels 2 days later and photographed with a camera (Canon, Japan).
2.10. Sirius red staining
Fibroblasts in the in vitro model for 4 weeks were fixed with Bouin's fixative (Meilune, China), treated with 0.2% aqueous phosphomolybdic acid (Solarbio, China), and then stained with Sirius red dye (Solarbio, China). After dehydration and sealing, samples were observed and photographed using a confocal laser scanning microscope (Zeiss, Germany). Afterward, fibroblasts on titanium alloy disks were incubated in 0.1 M NaOH on a horizontal shaker (Heidolph, Germany) to dissolve the staining. The optical density of the solution at 550 nm was then measured. The well with 0.1 M NaOH solution was set as a blank control.
2.11. Cell adhesion assay
After culture for 3 h, the model samples were put on a horizontal shaker at 200 rpm for 10 min. Part of the fibroblasts detached from the disk surfaces, which were regarded as weak adhesion. The remaining cells, which were regarded as strong adhesion, were digested with trypsin. The quantities of detached cells and remaining cells were calculated using a Cytometer (Beckman, USA).
2.12. Cell viability and proliferation assay
Fibroblasts were seeded on titanium alloy disks. After overnight attachment, the culture media were replaced with M0, M1, or M2 conditioned media. At 1, 3, 5, and 7 d time points, cell viability and proliferation were detected using Cell Counting Kit-8 (CCK-8, Dojindo, Japan) according to the manufacturer's protocol.
2.13. Wound healing assay
Wound healing assay including lentivirus transduction of GFP labels into fibroblasts and flow-based cell sorting was as previously described [4,36]. To be specific, cells were seeded in each side of the box of the healing insert (Ibidi, Germany). Images were taken at 0, 12, 24, 36, 48, 60 and 72 h.
2.14. Collagen degradation assay
The collagen degradation assay was performed as previously described [37]. Rat tail collagen Ⅰ solution (Corning, USA) was distributed into six-well plates followed by incubation at 37 °C. After dehydration and wash, fibroblasts were seeded and macrophage conditioned media were then added. After 3 days, the cells were removed and the cleavage of collagen was visualized by staining the remaining gels with Coomassie blue (Servicebio, China). The collagen degradation ability was assessed using ImageJ software (NIH, USA).
2.15. Statistical analysis
Experiments were all performed at least in 3 replicates. All the experimental results were shown as mean ± standard deviation (SD). Levels of statistical significance were determined by unpaired two-tailed Student's t-test using SPSS 22.0 software (SPSS Inc., USA) unless otherwise stated. The significance level was set at p < 0.05.
3. Results and discussion
3.1. Dissection of M1 and M2 immune microenvironments and prediction of their potential pleiotropic effects on metal implant-soft tissue integration
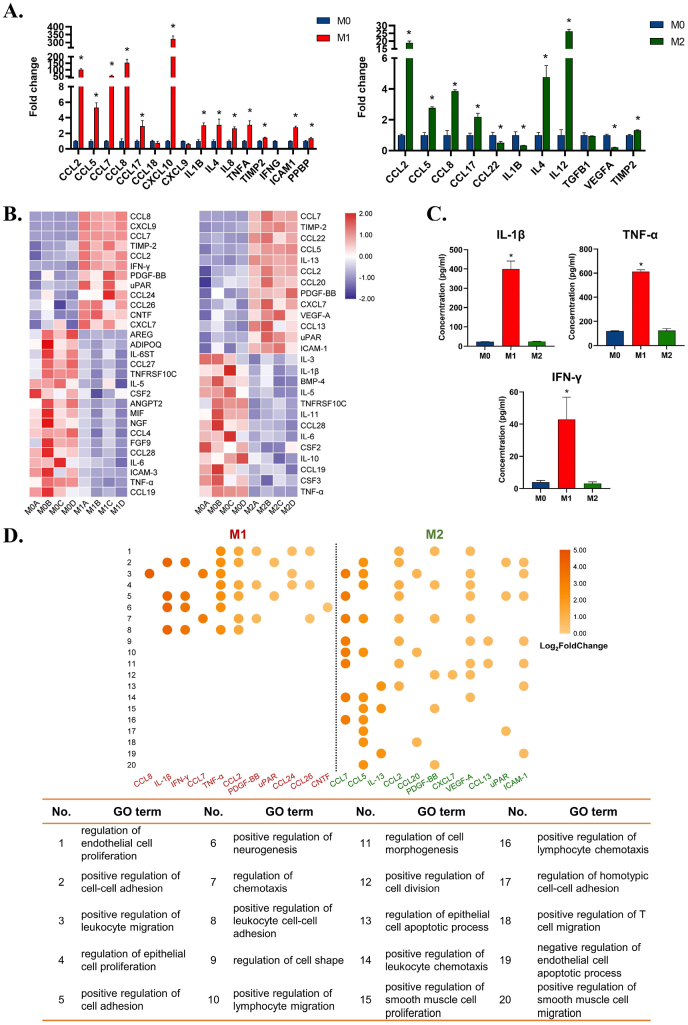
To dissect different macrophage immune microenvironments, we first induced M0, M1, and M2 macrophages. Successful macrophage phenotype switches were confirmed (Figure S1). Different morphologies and surface markers of M0, M1, and M2 macrophages imply their discrepancy in functional status. M1 macrophages tended to express multiple inflammatory cytokine-related genes, showing that the cells were in an active inflammatory regulatory phenotype (Fig. 1A). M2 polarized macrophages not only activated the expression of chemokine-related genes but also upregulated the expression of various tissue repair-related genes, such as interleukin 4 (IL4), transforming growth factor-beta 1 (TGFB1), and IL12, showing that M2 macrophages were in a pro-tissue repair state (Fig. 1B).
Fig. 1.
Dissection of M1 and M2 immune microenvironments and prediction of their potential pleiotropic effects on metal implant-soft tissue integration. A) Gene expression of cytokines in M1 and M2 macrophages by RT–qPCR. B) Differentially expressed cytokines of M1 and M2 immune microenvironments by cytokine array. C) Elisa results of IL-1β, TNF-α, and IFN-γ in M1 and M2 immune microenvironments. D) GO enrichment analysis concerning the biological process of M1 and M2 upregulated cytokines. Data are presented as means ± SD; *p < 0.05.
In addition, the differentially secreted cytokines after macrophage phenotype conversions showed four main regulatory functions: (1) Regulation of tissue matrix formation; (2) Regulation of cell-substrate adhesion; (3) Regulation of cell contraction; and (4) Regulation of the inflammatory response (Fig. 1D, Table S1). These functional items are all closely related to the biological functions of fibroblasts, suggesting that modulating macrophage phenotypic conversion may possess good potential in regulating multiple functions of fibroblasts on metal implants.
M1 macrophages could significantly upregulate inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-1β, and interferon-γ (IFN-γ), which play an important role in promoting angiogenesis, lymphocyte infiltration, macrophage polarization, etc. (Fig. 1A, C and D). M2 macrophages secrete IL-13, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and other tissue regenerative factors, which not only have chemotactic and proangiogenic functions but also possess a range of regulatory effects on soft tissue integration, such as cell adhesion, migration, and proliferation, suggesting their diverse regulatory potential (Fig. 1A and D).
Interestingly, although M1 and M2 immune microenvironments shared some same upregulated cytokines such as CCL2, CCL7, and TIMP-2, functional analysis displayed their different regulatory effects on cell behaviors and functions. This indicates that the immune cytokines work together as a complex rather than independently. The regulation of inflammation should have a more entire view on the secreted immune cytokine profile, rather than targeting each individual cytokine.
3.2. The pleiotropic effects of macrophage immune microenvironments on regulating multi-reparative fibroblasts
To achieve ideal integration between hard metal materials and soft tissue, sufficient soft tissue matrix deposition, connective tissue cell adhesion, and soft tissue contraction are important regulatory targets. Therefore, to verify the possible pleiotropic effects of polarized macrophage-mediated immune microenvironments on fibroblasts, we investigated the tissue-material adhesion, fiber synthesis, tissue contraction, and immune functional changes of fibroblasts after incubation in the M0, M1, or M2 immune microenvironment on smooth titanium surface (Figure S2).
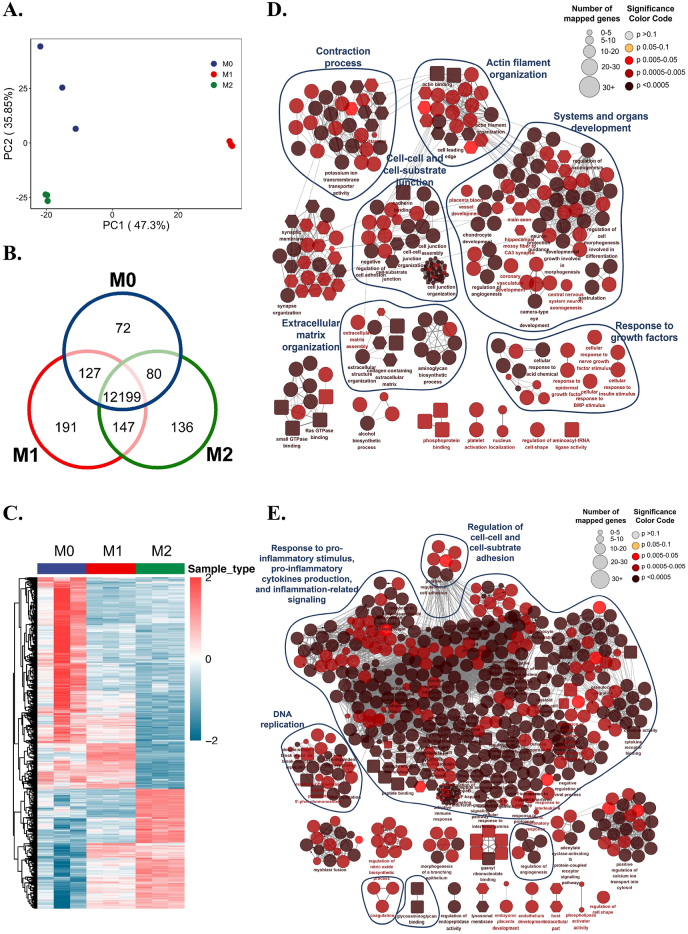
Fibroblasts grew well in different immune environments (Figure S3). Principal component analysis (PCA) and differential analysis showed good quality control of RNA-seq experiments, identifying a variety of differentially expressed genes among fibroblasts of the M0, M1, and M2 groups (Fig. 2A, B, and C). Comprehensive classification of Gene Set Enrichment Analysis (GSEA) showed that the M1 immune microenvironment tremendously elicited the inflammatory response of fibroblasts, given the considerable proportion of enriched pathways related to “response to pro-inflammatory stimulus, pro-inflammatory cytokines production, and inflammation-related signaling” (Fig. 2E), which may lead to an immunoregulatory feedback loop between the fibroblasts and immune cells such as macrophages.
Fig. 2.
The pleiotropic effects of macrophage immune microenvironments on regulating the multi-reparative fibroblasts. A) Principal Component Analysis (PCA) of transcriptome data of fibroblasts in different immune microenvironments. B) Venn diagram shows common and differential gene expression of fibroblasts in different immune microenvironments. C) Heatmap shows the differential gene expression pattern of fibroblasts in different immune microenvironments. D) Overview of fibroblast functions in M2 immune microenvironment by clustering enriched terms of Gene Set Enrichment Analysis (GSEA). E) Overview of fibroblast functions in M1 immune microenvironment by clustering enriched terms of GSEA.
Unlike the M1 microenvironment, which extremely promoted inflammation, the M2 immune microenvironment induced a pleiotropic effect on fibroblasts mainly involving system and organ development, extracellular matrix organization, cell-cell and cell-substrate junctions, cell junctions, and contraction (Fig. 2D). Similar to the functional analysis results of macrophage cytokines, fibroblasts in the M2 immune microenvironment were found to show valuable potential in improving metal soft tissue integration due to their potential in regulating the fiber synthesis, adhesion, and contraction function on titanium alloy in parallel. These functions of fibroblasts are parts of soft tissue regeneration, which shared some pathways with the cluster of systems and organs development. Human gingival fibroblasts have inherent stemness, which is the potential to differentiate into different lineages [38,39]. In combination with these findings, it can be speculated that the fibroblasts may undergo re-development of gingival tissue and reconstruct functional gingival tissue under M2 environment. These results collectively indicate that the multifunctional changes in fibroblasts validated the pleiotropic regulatory effects of M1 and M2 immune microenvironments on metal implant-soft tissue integration. We are interested in how fibroblasts behave under these immune microenvironments in the way the immune microenvironments work.
3.2.1. The effects of the macrophage immune microenvironment on the pro-tissue material adhesion function of fibroblasts
For metal material surfaces, it is difficult for connective tissue to attain mechanical retention, such as osteointegration, so cell adhesion in metal materials is vital [40,41]. To achieve stronger adhesion on metal material, cells are supposed to develop focal adhesion, which connects actin cytoskeleton and ECM components attached to the material surface via integrins [42,43]. As demonstrated above, the M2 immune microenvironment improved fibroblast adhesion ability. We further explored how M2 immune microenvironments regulate fibroblasts to form focal adhesions on metal surfaces.
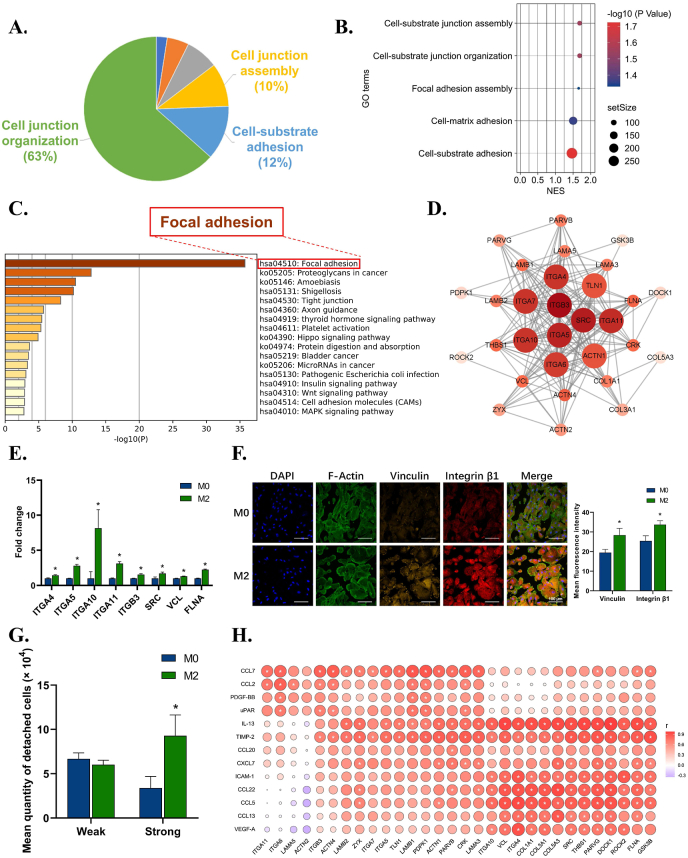
The dissection of adhesion clusters revealed enrichment of the cell-substrate adhesion pathway in the second rank (Fig. 3A). Further analysis of these subterms in the pathway showed increasing assembly and organization of cell-material junctions (Fig. 3B). To clarify the specific structure at the cell-substrate interface, we profiled the core genes in the subterms and found that focal adhesion was significantly upregulated (Fig. 3C). Hub gene analysis indicated that SRC, integrins, and actinin (ACTN) could be critical to enhance fibroblast anchorage (Fig. 3D). PCR further confirmed the upregulation of both membrane integrins (integrin α4, α5, α10, α11, and β3) and cytoskeleton-related genes, such as SRC, VCL, and filamin A (FLNA) (Fig. 3E). Immunofluorescence results showed rising expression of focal adhesion markers VCL and integrin β1 (Fig. 3F). The cell adhesion assay also proved that fibroblasts in the M2 group elevated the level of strong adhesion (Fig. 3G), while the wound healing assay showed reduced migration ability at 72 h (Figure S4). These results collectively indicated the pro-adhesion effects and potentially increased formation of focal adhesion of fibroblasts under the stimulation of M2 immune microenvironments.
Fig. 3.
The effects of macrophage immune microenvironment on the pro-tissue material adhesion function of fibroblasts. A) Leading terms analysis reveals components of the adhesion clusters, with cell-substrate adhesion at top 2. B) GO terms reveals subterms in cell-substrate adhesion components. C) KEGG analysis of cell-substrate adhesion components reveals focal adhesion is the most significant pathway in cell-substrate adhesion. D) Hub gene analysis of focal adhesion pathway in M2 group. E) RT–qPCR results confirm the up-regulation of genes related to focal adhesion. F) Representative immunofluorescence images and semi-quantitative statistical analysis of VCL and integrin β1 expression of fibroblasts in M2 group. G) Cell adhesion assay reveals an increasing percentage of strong adhesion cells in M2 group. H) Correlation analysis between up-regulated cytokines of M2 immune microenvironment and up-regulated focal adhesion-related genes of fibroblast. Data are presented as means ± SD; *p < 0.05.
To further investigate how macrophage environments induce pro-adhesion effects of fibroblasts, a correlation analysis between upregulated cytokines of M2 and core genes of focal adhesion in fibroblasts was conducted (Fig. 3H). Cytokines IL-13, VEGF-A, and tissue metalloproteinase inhibitor 2 (TIMP-2) in the M2 immune microenvironment were positively related to the core genes in focal adhesion. IL-13 can signal through its receptor IL-13Rα2 to enhance focal adhesion protein activation [44]. VEGF-A is reported to induce the recruitment of focal adhesion proteins, thus favoring the assembly of focal adhesion [45,46]. Lack of TIMP-2 leads to selective loss of integrin and compromises focal adhesion [47]. It can be inferred that various cytokines in the M2 immune microenvironment, such as IL-13, VEGF-A, and TIMP-2, can enhance fibroblast expression of integrin receptors as well as SRC and VCL in the cytoskeleton, thus possibly inducing assembly of focal adhesion. Via focal adhesion, fibroblasts can better attach to the titanium surface, indicating a stable and dynamic fibroblast layer as part of metal soft tissue integration.
3.2.2. The effects of the macrophage immune microenvironment on the pro-fiber synthesis function of fibroblasts
To achieve ideal integration between hard metal materials and soft tissue, the soft tissue matrix around the metal implant is the most essential regulatory target. The mature tissue matrix has stronger tensile strength, and a more stable scaffold structure can improve metal-soft tissue integration. In this process, fibroblasts play a crucial role in synthesizing, secreting, and organizing various collagen fibers, which are the major constituents of the tissue matrix. As mentioned above, the macrophage immune microenvironment elicits significant effects in regulating soft tissue regeneration. It is of great interest to unveil how the immune microenvironment regulates the pro-fiber synthesis function of fibroblasts and eventually achieves metal material-soft tissue integration.
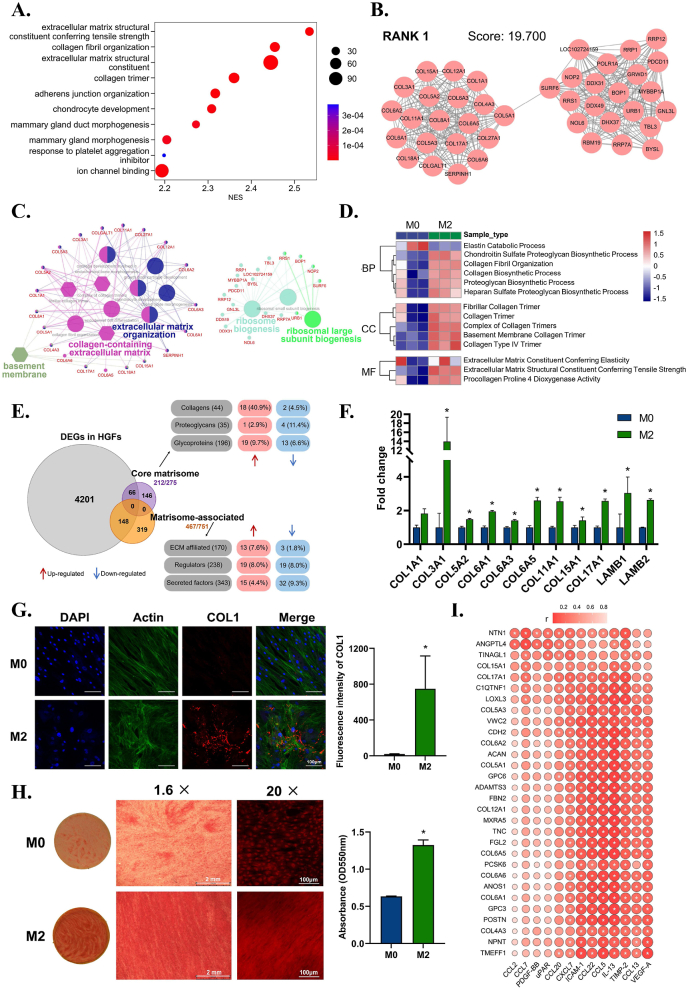
Among the multiple functional effects of fibroblasts in M2 immune microenvironments, we found that the activation of ECM organization was the most significant (Fig. 4A). The most highly interconnected (RANK1) module in the upregulated gene network of the M2 group further showed enrichment of ECM organization and ribosome biogenesis (Fig. 4B and C). These results indicated that the biosynthetic function of fibroblasts, especially ECM protein synthesis, was highly active in the M2 immune microenvironment.
Fig. 4.
The effects of macrophage immune microenvironment on the pro-fiber synthesis function of fibroblasts. A) Gene Set Enrichment Analysis (GSEA) reveals the prominent enrichment of processes related to extracellular matrix in the top 10 up-regulated GO terms in M2 group. B) MCODE analysis reveals the most highly interconnected module in the up-regulated genes network of M2 group. C) ClueGO analysis reveals the GO enrichment of the genes from the RANK 1 module. D) Gene Set Variation Analysis (GSVA) reveals the enriched events related to ECM proteins synthesis in terms of biological process, cellular component, and molecular function. E) Analysis based on matrisome database shows the types and quantities of up-regulated ECM-related proteins of M2 group, in which collagen proteins account for the largest proportion. F) RT–qPCR results verify the up-regulation of collagen and other ECM genes of fibroblasts in M2 group. G) Representative immunofluorescence images and semi-quantitative statistical analysis of collagen Ⅰ expression of fibroblasts in M2 group. H) Sirius red staining and semi-quantitative statistical analysis of collagen fibers of fibroblasts in M2 group. I) Correlation analysis between up-regulated cytokines of M2 immune microenvironment and up-regulated collagen-related genes of fibroblast. Data are presented as means ± SD; *p < 0.05.
To dissect what ECM constituents were actively synthesized, Gene Set Variation Analysis (GSVA) showed collagen fibril organization and biosynthetic process, and proteoglycan biosynthetic process were mainly active synthetic events (Fig. 4D). Moreover, the upregulated genes encoding the ECM of fibroblasts in the M2 group further showed a remarkable percentage of collagen proteins (Fig. 4E). Among the upregulated genes of collagen and related proteins shown in the heatmap, we found collagen Ⅰ, Ⅲ, Ⅳ, Ⅴ, Ⅵ, Ⅺ, and Ⅻ as well as enzymes related to collagen biosynthesis (Figure S5), which were further confirmed by RT–qPCR (Fig. 4F). Collagen Ⅰ, the most important collagen in the soft tissue matrix, was also upregulated (Fig. 4G). Sirius red staining demonstrated the increased formation of collagen fibers by fibroblasts in the M2 immune microenvironment (Fig. 4H). These results collectively indicated the pro-fiber synthesis effect of fibroblasts under stimulation of the M2 immune microenvironment.
To further investigate how the macrophage environment affects the pro-fiber synthesis function of fibroblasts, a correlation analysis between upregulated cytokines from the M2 immune microenvironment and upregulated collagen-related genes of fibroblasts was carried out. Intercellular adhesion molecule (ICAM-1), C–C motif chemokine ligand 22 (CCL22), CCL5, IL-13, TIMP-2, VEGF-A, CCL13, and chemokine (C-X-C motif) ligand 7 (CXCL7) from the M2 immune microenvironment were positively associated with collagen synthesis gene expression in fibroblasts (Fig. 4I). Among these factors, CCL22 produced by macrophages can increase ECM deposition to promote wound healing [48]. IL-13 is reported to play a role via extracellular signal-regulated kinase (ERK) to activate type Ⅰ collagen production [49]. CCL5 can stimulate fibroblasts to produce striated collagen Ⅵ [50]. These results collectively indicated that in the M2 immune microenvironment, especially in the presence of multiple cytokines, such as CCL22, IL-13, and CCL5, fibroblasts tend to synthesize collagens and organize into collagen fibers that improve the tensile strength of the ECM. The newly formed mature ECM built up a more stable scaffold for metal materials and soft tissue integration. This new soft tissue integration with metal materials establishes mechanical and biological barriers against deleterious stimuli, allowing hard metal implants to easily coexist with soft tissue.
In addition, fibroblasts expressed multiple types of collagens with parallel bundles appearance [51]. Similar to fetal wound healing, the high level of collagen Ⅲ endowed the new fiber network with superiority of tensility, flexibility, and softness [52]. These indicate that the fiber synthesis of fibroblasts was closer to normal wound healing rather than fibrosis. Notably, the balance between collagen synthesis and degradation determines the extent of collagen deposition and the resulting rehabilitation of tissue. Therefore, since fibroblasts in M2 immune microenvironment had enhanced collagen synthesis, we took further exploration into the collagen degradation of fibroblasts in M2 group. As a result, in our gene set enrichment analysis of fibroblast transcriptome (Figure S6A), the collagen degradation process was not significantly enriched in M2 group. Gene set variation analysis also showed the enrichment of multiple extracellular matrix biosynthetic processes coupled with only one degradation process, i.e., the elastin catabolic process (Fig. 4D). Collagen degradation assay and RT-qPCR further confirmed the results (Figure S6B and S6C). These implied that collagen synthesis and degradation balance is shifted towards the synthesis extreme in M2 immune microenvironment.
3.2.3. The effects of the macrophage immune microenvironment on the pro-tissue contraction function of fibroblasts
For metal material integration, fibroblasts play an important role in mediating soft tissue contraction and wound closure to ensure the ideal integration of materials and prevent bacterial invasion and excessive inflammatory responses [32,53]. To achieve this aim, fibroblasts should differentiate into myofibroblasts, which are rich in α-smooth muscle actin (actin alpha 2, ACTA2 gene)-like smooth muscle cells. Differentiated myofibroblasts can generate stress fibers and produce contractile forces [54]. As mentioned above, the macrophage immune microenvironment elicits significant effects in regulating tissue contraction. It is of great interest to unveil how the immune microenvironment regulates the tissue contraction function of fibroblasts and myofibroblast differentiation.
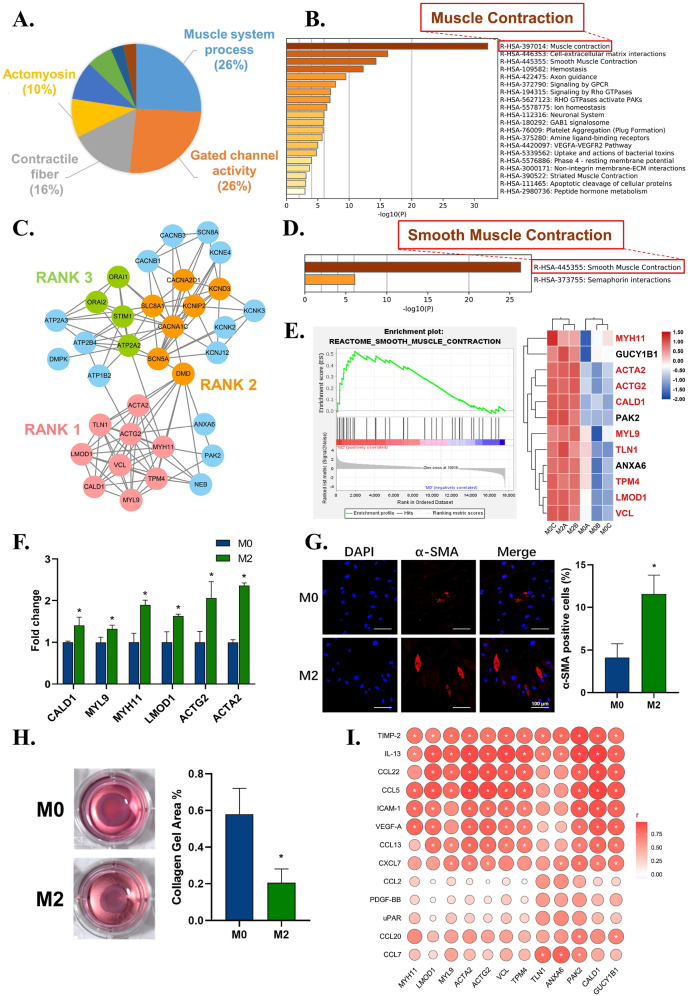
From the enrichment results, we found large numbers of upregulated genes in the M2 immune microenvironment related to muscle system processes, contractile fiber, and actomyosin, which were further enriched in muscle contraction (Fig. 5A and B). To dissect how this immune microenvironment regulates the contraction function of fibroblasts, the most highly interconnected module in muscle contraction-related genes (Fig. 5C) showed that smooth muscle contraction was the most significant event (Fig. 5D). GSEA further showed that smooth muscle contraction was significantly upregulated, with core genes including ACTA2 (Fig. 5E). Smooth muscle contraction-related genes, including ACTA2, actin gamma 2 (ACTG2), caldesmon 1 (CALD1), myosin light chain 9 (MYL9), MYH11, leiomodin 1 (LMOD1), and VCL, were further verified by RT–qPCR, which confirmed that these genes were upregulated in the M2 group and that ACTA2 was the most significantly upregulated gene (Fig. 5F). Immunofluorescent staining of α-SMA further confirmed its upregulation (Fig. 5G). The collagen gel contraction assay demonstrated stronger collagen contractile forces in the M2 group (Fig. 5H). These results collectively indicated the pro-tissue contraction effect and the potential myofibroblast differentiation of fibroblasts under stimulation of the M2 immune microenvironment.
Fig. 5.
The effects of macrophage immune microenvironment on the pro-tissue contraction function of fibroblasts. A) Leading terms analysis reveals the contraction terms clustered. B) Reactome enrichment analysis of muscle system process, contractile fiber, and actomyosin. C) MCODE analysis reveals the top 3 highly interconnected modules in the up-regulated genes network of muscle contraction. D) Reactome enrichment analysis of the genes from the RANK 1 module. E) Gene Set Enrichment Analysis (GSEA) confirms upregulation of smooth muscle contraction event and the heatmap shows the core genes. F) RT–qPCR results show the up-regulation of contraction-related genes of fibroblasts in M2 immune microenvironment. G) Representative immunofluorescence images and semi-quantitative statistical analysis of α-SMA expression of fibroblasts in M2 group. H) Collagen gel assay and semi-quantitative statistical analysis verifies the enhanced contraction ability of fibroblasts in M2 group. I) Correlation analysis between up-regulated cytokines of M2 immune microenvironment and up-regulated contraction-related genes of fibroblast. Data are presented as means ± SD; *p < 0.05.
To further investigate how the macrophage environment affects the pro-tissue contraction function of fibroblasts, a correlation analysis between upregulated cytokines from the M2 immune microenvironment and upregulated contraction-related genes of fibroblasts was carried out. IL-13, TIMP-2, CCL22, CCL5, ICAM-1, VEGF-A, CCL13, and CXCL7 from the M2 immune microenvironment were positively associated with the contraction gene expression of fibroblasts (Fig. 5I). Among these factors, IL-13 is reported to induce the expression of both α-SMA and desmin, two known markers for myofibroblasts, and further promote collagen contraction [55,56]. TIMP-2 can also activate fibroblasts into contractile myofibroblasts to remodel the ECM at lower concentrations [57]. A lack of VEGF-A is crucial for decreased neoangiogenesis and myofibroblast accumulation [58]. These results collectively indicate that multiple cytokines from the M2 immune microenvironment, such as IL-13, TIMP-2, and VEGF-A, can promote ACTA2, ACTG2, MYH11, and MYL9 expression in fibroblasts to assemble the contractile apparatus of smooth muscle. This results in the differentiation of fibroblasts to myofibroblasts and further induces contractile forces. The contractile force can resist soft tissue mobility, fix soft tissue on metal materials and reinforce soft tissue seals, thus improving metal material-soft tissue integration.
3.2.4. The effects of the macrophage immune microenvironment on the immune functions of fibroblasts
In addition to its multiple functions in tissue repair, fibroblasts are also reported to act as both immunoregulatory cells regulating inflammation and inflammatory cells that actively participate in inflammation [59,60]. As mentioned above, upon stimulation of the M1 immune microenvironment, fibroblasts demonstrate significant immune-related features. Therefore, we further analyzed fibroblast immune functions under M1 microenvironment activation.
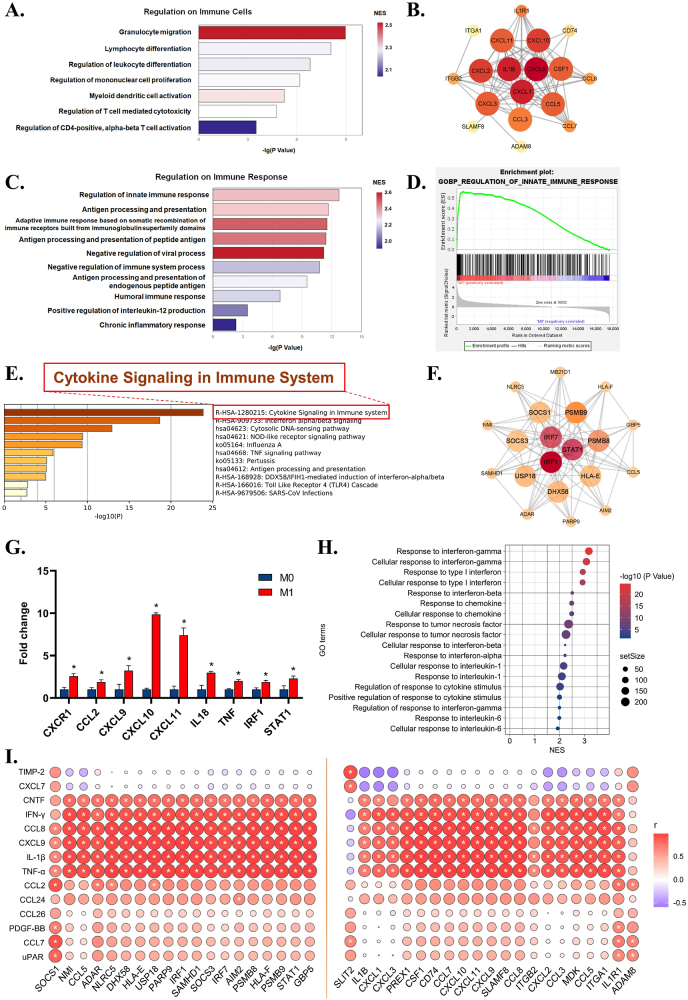
Leading terms of the immune-related cluster indicated that activated fibroblasts demonstrated multiple immunoregulatory effects, including chemotaxis, differentiation, proliferation, activation, and immune effects of various immune cells (Fig. 6A). Granulocyte migration appears to be the most significant pathway. Granulocytes are key effectors of the innate response that identify, ingest and destroy pathogens during early and acute inflammation [61,62]. Core gene analysis of the pathway further revealed upregulation of various CXC motif chemokine ligands and CC chemokines (Fig. 6B). CXC and CC chemokines are potent chemoattractants that recruit not only granulocytes but also macrophages, NK cells, and dendritic cells [63]. These results collectively indicate that fibroblasts might take an active part in the innate response via secreting chemokines to recruit immune cells.
Fig. 6.
The effects of macrophage immune microenvironment on the immune function of fibroblasts. A) Leading terms analysis reveals the upregulation of granulocyte migration. B) Hub gene analysis reveals enrichment of various chemokines for innate immune. C) Leading terms analysis shows the most significant enrichment of regulation of innate immune response. D) Gene Set Enrichment Analysis (GSEA) analysis confirms the upregulated event of regulation of innate immune response. E) KEGG analysis reveals cytokine-related signaling pathway in M1 group. F) Hub gene analysis of the term of innate immune response. G) RT–qPCR confirms the upregulation of chemokines, inflammatory factors, and innate immune genes in M1 group. H) Leading term analysis reveals IFN, chemokines, TNF and IL-1 possibly contributed to fibroblasts' immune function in M1 group. I) Correlation analysis between up-regulated cytokines of M1 immune microenvironment and up-regulated immune-related genes of fibroblast. Data are presented as means ± SD; *p < 0.05.
We then reviewed the leading terms related to the immune response and found that the pathway named “regulation of innate immune response” was also enriched (Fig. 6C), which further confirms our hypothesis. GSEA also confirmed the upregulation of the regulation of the innate immune response (Fig. 6D). Functional analysis of its core genes revealed that fibroblasts might actively participate in cytokine signaling in the immune system (Fig. 6E). Core gene analysis demonstrated fibroblast upregulation of multiple innate immune-related genes, such as interferon regulatory factor (IRF) and signal transducer and activator of transcription (STAT) (Fig. 6F). Interestingly, active IRF and STAT are hallmarks of IFN-γ/LPS-activated macrophages, and M1 macrophages react to pathogens [64], which suggests that fibroblasts in M1 immune microenvironments tend to enhance the M1-like innate immune response, amplifying the origin of M1 proinflammation. RT–qPCR further confirmed the fibroblast upregulation of CXC chemokines for granulocyte migration and IL18, TNF and IRF1, STAT1 for innate immunity (Fig. 6G).
To determine the cytokines that change fibroblasts, leading terms were overviewed so that we found that pathways related to the response to IFN, chemokines, TNF, and IL-1 were enriched (Fig. 6H). Correlation analysis between M1 upregulated cytokines and fibroblast core genes further confirmed that IFN-γ, IL-1β, TNF-α, CXCL9, and CCL8 in the M1 immune microenvironment were positively related to the core genes in both events (Fig. 6I). We can infer that the M1 immune microenvironment turned fibroblasts not only into immunoregulatory-like cells that secrete CXCL9, CXCL10, and CXCL11 to recruit innate immune cells but also into immune-like cells that express IRF and STAT to amplify M1 dominant inflammation. In addition, this possible inflammation-amplification effect of fibroblasts implies the existence of an inflammatory-fibrous complex.
3.3. Implications for the modulation of inflammatory-fibrous complexes to advance metal soft tissue material development
Metal material implantation could inevitably trigger inflammatory-fibrous responses, of which macrophages and fibroblasts play a key role, after implantation. In this study, we dissected the multidirectional functional response of fibroblasts in macrophage-mediated immune microenvironments and showed their interactive relationship as a complex rather than separate behavior. M1 macrophages can regulate the expression of a variety of immune-related transcription factors of fibroblasts and endow them with an inflammatory regulatory phenotype, and fibroblasts could, in turn, affect the function of a variety of innate immune cells, including macrophages, by releasing chemokines. M2 macrophage-fibroblast responses manifest as relatively weak immune feedback and strong terminal effects, which significantly regulate the multifaceted functions of fibroblasts, including collagen secretion, adhesion, contraction, etc., thus affecting the fibrous response of materials.
As M1 and M2 macrophages are intermingled and dynamically balanced in the macrophage response mediated after material implantation, the interactions between macrophages and fibroblasts and their mixing behaviors could be more intricate and complex and may be beyond current comprehension. Inflammatory-fibrous responses after material implantation are more like a complex containing various cells, such as fibroblasts and M1 and M2 macrophages, as well as their delicate interactions. Immune microenvironment changes usually occur with the changes of multiple regulatory cytokines. Upon the stimulation of multi-signal, fibroblasts would demonstrate multi-functional changes, unlike a simple function change elicited by one single cytokine. Such interactions and the balance of multiple modes in this intertwining complex determine the reaction characteristics of the complex and mediate the outcome of the material reaction. How to comprehensively understand the characteristics and function of the inflammatory-fibrous complex and further regulate its behavior should be a crucial scientific question in metal soft tissue material biology.
This “inflammatory-fibrous complex” concept is of great significance for advancing the development of metal soft tissue materials (Scheme 2). Our study also shows that the regulation of the inflammatory-fibrous complex has an important positive potential in shaping soft tissue integration. An inflammatory-fibrous complex modulation-based strategy may significantly improve metal and soft tissue integration and promote the research and development of metal soft tissue materials. We should bear in mind that the immune cells and fibroblast closely interact, and the regulation should be targeted in the complex rather than one single cell type. We can tune the surface physical-chemical properties of metal soft tissue materials to manipulate the immune environment, thus regulating fibrous response. We can also regulate the immune environment to improve soft tissue integration on the same metal surface as we did in this study. Take dental implant abutment as an example. The surface properties of the abutment can be modified to induce a favorable immune microenvironment to improve gingival fibroblast-mediated soft tissue integration. The optimal aim is to enhance the immune response against the external stimulus and facilitate better soft tissue integration, contributing to wide and flexible applications prospects.
Scheme 2.
Schematic diagram shows that fibroblasts in the M1 immune microenvironment tend to aggravate the inflammatory response in a proinflammatory positive feedback loop, while M2 immune microenvironment enhances multiple functions of fibroblasts in soft tissue integration, including soft tissue regeneration, cell adhesion on materials, and contraction for soft tissue immobilization.
Due to the important roles of macrophages and fibroblasts in the response to metal materials, we have limited research subjects on macrophages and fibroblasts. However, this does not mean that macrophage is the only cell involved. The results should be more complicated. Other immune cells, such as lymphocytes, neutrophils, mast cells, etc. should participate in this inflammatory-fibrous response, which needs to be investigated in the future. In addition, the cytokine secretion protein confirmation assay is hard to be carried out due to the disturbance of original M1 cytokines in the conditioned medium. Applying indirect Transwell co-culture to investigate their interaction could be a solution to thoroughly dissect the cytokine secretion from each cell.
4. Conclusion
By tuning macrophage polarization, the multi-reparative aspects of fibroblasts on titanium metal surfaces can be modulated. In M1 immune microenvironments, fibroblasts appeared to aggravate inflammation in a positive loop, whereas in M2 immune microenvironments, fibroblasts develop better soft tissue regeneration, adhesion to metal materials, and contraction, which can contribute to favorable metal material-soft tissue integration. Immune cells and fibroblasts work together as a complex. Educating macrophages to modulate fibroblasts is an effective approach to regulate the inflammatory-fibrous complex, thus improving soft tissue integration.
CRediT authorship contribution statement
Peina Huang: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Jieyun Xu: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Lv Xie: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Guangqi Gao: Validation, Investigation, Visualization. Shoucheng Chen: Writing – original draft, Funding acquisition, Visualization. Zhuohong Gong: Investigation. Xiaomei Lao: Resources. Zhengjie Shan: Investigation. Jiamin Shi: Investigation. Zhaocai Zhou: Investigation, Validation. Zhuofan Chen: Supervision. Yang Cao: Supervision, Project administration. Yan Wang: Supervision, Project administration. Zetao Chen: Term, Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Conflict of interest
All authors declare no conflicts of interest.
Acknowledgments
P.H., J.X., and L.X. contributed equally to this work. This work was financially supported by the Natural Science Foundation of Guangdong Province [grant numbers 2018B030306030], National Natural Science Foundation of China [grant numbers 82071167], International Team for Implantology (ITI) Research Grant [grant numbers 1536_2020], Guangdong Financial Fund for High-Caliber Hospital Construction, Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds) [grant numbers pdjh2021a0005], China Postdoctoral Science Foundation [grant numbers 2021TQ0379].
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.05.013.
Contributor Information
Peina Huang, Email: huangpn@mail2.sysu.edu.cn.
Jieyun Xu, Email: xujy33@mail2.sysu.edu.cn.
Lv Xie, Email: xielv@mail2.sysu.edu.cn.
Guangqi Gao, Email: gaogq5@mail2.sysu.edu.cn.
Shoucheng Chen, Email: chenshch8@mail.sysu.edu.cn.
Zhuohong Gong, Email: gongzhh5@mail2.sysu.edu.cn.
Xiaomei Lao, Email: laoxm3@mail.sysu.edu.cn.
Zhengjie Shan, Email: shanzhj@mail2.sysu.edu.cn.
Jiamin Shi, Email: shijm8@mail.sysu.edu.cn.
Zhaocai Zhou, Email: zhouzhc@mail2.sysu.edu.cn.
Zhuofan Chen, Email: chzhuof@mail.sysu.edu.cn.
Yang Cao, Email: caoyang@mail.sysu.edu.cn.
Yan Wang, Email: wangyan9@mail.sysu.edu.cn.
Zetao Chen, Email: chenzet3@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim T., See C.W., Li X., Zhu D. Orthopedic implants and devices for bone fractures and defects: past, present and perspective. Eng. Regen. 2020;1:6–18. [Google Scholar]
- 2.Chua K., Khan I., Malhotra R., Zhu D. Additive manufacturing and 3D printing of metallic biomaterials. Eng. Regen. 2021;2:288–299. [Google Scholar]
- 3.Hanawa T. Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019;7:170. doi: 10.3389/fbioe.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu R., Chen S., Huang P., Liu G., Luo P., Li Z., Xiao Y., Chen Z., Chen Z. Immunomodulation‐based strategy for improving soft tissue and metal implant integration and its implications in the development of metal soft tissue materials. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 5.Wang L., Luo Q., Zhang X., Qiu J., Qian S., Liu X. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 2021;6:64–74. doi: 10.1016/j.bioactmat.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarino N., Carbone G., Müser M., Ciavarella M. Modeling and simulation in tribology across scales: an overview. Tribol. Int. 2022;125:169–199. [Google Scholar]
- 7.Yin W., Chen M., Bai J., Xu Y., Wang M., Geng D., Pan G. Recent advances in orthopedic polyetheretherketone biomaterials: material fabrication and biofunction establishment. Smart Mater. Med. 2022;3:20–36. [Google Scholar]
- 8.Schwarz F., Derks J., Monje A., Wang H.L. Peri-implantitis. J. Periodontol. 2018;89(Suppl 1):S267–S290. doi: 10.1002/JPER.16-0350. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y., Li S., Zhang T., Wang C., Cai X. Titanium mesh for bone augmentation in oral implantology: current application and progress. Int. J. Oral Sci. 2020;12:37. doi: 10.1038/s41368-020-00107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann A., Seiler M. Minimizing risk of customized titanium mesh exposures - a retrospective analysis. BMC Oral Health. 2020;20:36. doi: 10.1186/s12903-020-1023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y., Li L., Zhao W., Qian Y., Dong L., Fang Y., Yang L., Fan Y. Surface modification of electrospun fibers with mechano-growth factor for mitigating the foreign-body reaction. Bioact. Mater. 2021;6:2983–2998. doi: 10.1016/j.bioactmat.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Agrawal D.K., Thankam F.G. Biomaterials-driven sterile inflammation. Tissue Eng. B Rev. 2022;28:22–34. doi: 10.1089/ten.teb.2020.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson S., Trobos M., Hoffman M., Norlindh B., Petronis S., Lausmaa J., Suska F., Thomsen P. A novel soft tissue model for biomaterial-associated infection and inflammation–bacteriological, morphological and molecular observations. Biomaterials. 2015;41:106–121. doi: 10.1016/j.biomaterials.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Zhang S., Huo S., Tang H., Qu X., Yue B. Effects of staphylococcal infection and aseptic inflammation on bone mass and biomechanical properties in a rabbit model. J. Orthop. Transl. 2020;21:66–72. doi: 10.1016/j.jot.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bank R.A. Limiting biomaterial fibrosis. Nat. Mater. 2019;18 doi: 10.1038/s41563-019-0428-y. 781-781. [DOI] [PubMed] [Google Scholar]
- 17.Farah S., Doloff J.C., Müller P., Sadraei A., Han H.J., Olafson K., Vyas K., Tam H.H., Hollister-Lock J., Kowalski P.S. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat. Mater. 2019;18:892–904. doi: 10.1038/s41563-019-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson M.D., Burdick J.A., Wells R.G. Engineered biomaterial platforms to study fibrosis. Adv. Healthc Mater. 2020;9 doi: 10.1002/adhm.201901682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noskovicova N., Hinz B., Pakshir P. Implant fibrosis and the underappreciated role of myofibroblasts in the foreign body reaction. Cells. 2021;10:1794. doi: 10.3390/cells10071794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y., Liu R., He L., Feng H., Li Y., Li Z. Recent development of implantable and flexible nerve electrodes. Smart Mater Med. 2020;1:131–147. [Google Scholar]
- 21.Witherel C.E., Abebayehu D., Barker T.H., Spiller K.L. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv. Healthc Mater. 2019;8 doi: 10.1002/adhm.201801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch N.G., Winkler D.A., Thissen H. Antifibrotic strategies for medical devices. Adv. Drug Deliv. Rev. 2020;167:109–120. doi: 10.1016/j.addr.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Wu S., Xia B., Mai S., Feng Z., Wang X., Liu Y., Liu R., Li Z., Xiao Y., Chen Z., Chen Z. Sodium fluoride under dose range of 2.4–24 μM, a promising osteoimmunomodulatory agent for vascularized bone formation. ACS Biomater. Sci. Eng. 2018;5:817–830. doi: 10.1021/acsbiomaterials.8b00570. [DOI] [PubMed] [Google Scholar]
- 24.Cooke J.P. Inflammation and its role in regeneration and repair: a caution for novel anti-inflammatory therapies. Circ. Res. 2019;124:1166–1168. doi: 10.1161/CIRCRESAHA.118.314669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye J., Xie C., Wang C., Huang J., Yin Z., Heng B.C., Chen X., Shen W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact. Mater. 2021;6:4096–4109. doi: 10.1016/j.bioactmat.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandorkar Y., K R., Basu B. The foreign body response demystified. ACS Biomater. Sci. Eng. 2019;5:19–44. doi: 10.1021/acsbiomaterials.8b00252. [DOI] [PubMed] [Google Scholar]
- 27.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015;2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H.T., Lee C.W., Li M.Y., Wang Y.F., Yung P.S.H., Lee O.K.S. The shift in macrophages polarisation after tendon injury: a systematic review. J. Orthop. Transl. 2020;21:24–34. doi: 10.1016/j.jot.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abaricia J.O., Farzad N., Heath T.J., Simmons J., Morandini L., Olivares-Navarrete R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021;133:58–73. doi: 10.1016/j.actbio.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoudi S., Mancini E., Xu L., Moore A., Jahanbani F., Hebestreit K., Srinivasan R., Li X., Devarajan K., Prélot L., Ang C.E., Shibuya Y., Benayoun B.A., Chang A.L.S., Wernig M., Wysocka J., Longaker M.T., Snyder M.P., Brunet A. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature. 2019;574:553–558. doi: 10.1038/s41586-019-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anjum R., Nishida K., Matsumoto H., Murakami D., Kobayashi S., Anada T., Tanaka M. Attachment and growth of fibroblast cells on poly (2-methoxyethyl acrylate) analog polymers as coating materials. Coatings. 2021;11:461. [Google Scholar]
- 32.Hannan R.T., Peirce S.M., Barker T.H. Fibroblasts: diverse cells critical to biomaterials integration. ACS Biomater. Sci. Eng. 2018;4:1223–1232. doi: 10.1021/acsbiomaterials.7b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson S., Coles M., Thomas T., Kollias G., Ludewig B., Turley S., Brenner M., Buckley C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y., Li J., Guan S., Witte F. The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Eng. Regen. 2021;2:182–194. [Google Scholar]
- 35.Wu S., Shan Z., Xie L., Su M., Zeng P., Huang P., Zeng L., Sheng X., Li Z., Zeng G. Mesopore controls the responses of blood clot‐immune complex via modulating fibrin network. Adv. Sci. 2022;9 doi: 10.1002/advs.202103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y., Mi J., Ye C., Ao Y., Shi M., Shan Z., Li B., Chen Z., Chen Z., Vasilev K. A practical guide to promote informatics-driven efficient biotopographic material development. Bioact. Mater. 2022;8:515–528. doi: 10.1016/j.bioactmat.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W., Fang M., Song F., Windsor L.J. Effects of cigarette smoke condensate and nicotine on human gingival fibroblast‐mediated collagen degradation. J. Periodontol. 2011;82:1071–1079. doi: 10.1902/jop.2010.100540. [DOI] [PubMed] [Google Scholar]
- 38.Hsu S.H., Huang G.S., Feng F. Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials. 2012;33:2642–2655. doi: 10.1016/j.biomaterials.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Egusa H., Sonoyama W., Nishimura M., Atsuta I., Akiyama K. Stem cells in dentistry–part I: stem cell sources. J. Prosthodont. Restor. 2012;56:151–165. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Bacakova L., Filova E., Parizek M., Ruml T., Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011;29:739–767. doi: 10.1016/j.biotechadv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Chen L., Yan C., Zheng Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today. 2018;21:38–59. [Google Scholar]
- 42.Kolodziej C.M., Kim S.H., Broyer R.M., Saxer S.S., Decker C.G., Maynard H.D. Combination of integrin-binding peptide and growth factor promotes cell adhesion on electron-beam-fabricated patterns. J. Am. Chem. Soc. 2012;134:247–255. doi: 10.1021/ja205524x. [DOI] [PubMed] [Google Scholar]
- 43.Geiger B., Bershadsky A., Pankov R., Yamada K.M. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 44.Bartolome R.A., Garcia-Palmero I., Torres S., Lopez-Lucendo M., Balyasnikova I.V., Casal J.I. IL13 receptor alpha2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 2015;75:2434–2444. doi: 10.1158/0008-5472.CAN-14-3650. [DOI] [PubMed] [Google Scholar]
- 45.Cezar-de-Mello P.F., Nascimento-Silva V., Villela C.G., Fierro I.M. Aspirin-triggered lipoxin A4 inhibition of VEGF-induced endothelial cell migration involves actin polymerization and focal adhesion assembly. Oncogene. 2006;25:122–129. doi: 10.1038/sj.onc.1209002. [DOI] [PubMed] [Google Scholar]
- 46.Lyu J., Hu Y., Xu X., Zhang H. Dynamics of focal adhesions and reorganization of F-actin in VEGF-stimulated NSCs under varying differentiation states. J. Cell. Biochem. 2013;114:1744–1759. doi: 10.1002/jcb.24517. [DOI] [PubMed] [Google Scholar]
- 47.Kandalam V., Basu R., Moore L., Fan D., Wang X., Jaworski D.M., Oudit G.Y., Kassiri Z. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124:2094–2105. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson H.N., Roberts E.R., Stafford A.R., Banyard K.L., Matteucci P., Mace K.A., Hardman M.J. Tissue iron promotes wound repair via M2 macrophage polarization and the chemokine (C-C motif) ligands 17 and 22. Am. J. Pathol. 2019;189:2196–2208. doi: 10.1016/j.ajpath.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Bhogal R.K., Bona C.A. Regulatory effect of extracellular signal-regulated kinases (ERK) on type I collagen synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13. Int. Rev. Immunol. 2008;27:472–496. doi: 10.1080/08830180802430974. [DOI] [PubMed] [Google Scholar]
- 50.Brett E., Sauter M., Timmins E., Azimzadeh O., Rosemann M., Merl-Pham J., Hauck S.M., Nelson P.J., Becker K.F., Schunn I., Lowery A., Kerin M.J., Atkinson M., Kruger A., Machens H.G., Duscher D. Oncogenic linear collagen VI of invasive breast cancer is induced by CCL5. J. Clin. Med. 2020;9:991. doi: 10.3390/jcm9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez A.C.d.O., Costa T.F., Andrade Z.d.A., Medrado A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016;91:614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomes R.N., Manuel F., Nascimento D.S. The bright side of fibroblasts: molecular signature and regenerative cues in major organs. NPJ Regen. Med. 2021;6:1–12. doi: 10.1038/s41536-021-00153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bainbridge P. Wound healing and the role of fibroblasts. J. Wound Care. 2013;22(407–408):410–412. doi: 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- 54.Darby I.A., Laverdet B., Bonte F., Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Invest. Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Firszt R., Francisco D., Church T.D., Thomas J.M., Ingram J.L., Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-beta1 in airway fibroblasts in asthma. Eur. Respir. J. 2014;43:464–473. doi: 10.1183/09031936.00068712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X., Kohyama T., Wang H., Zhu Y.K., Wen F.Q., Kim H.J., Romberger D.J., Rennard S.I. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L1049–L1056. doi: 10.1152/ajplung.00321.2001. [DOI] [PubMed] [Google Scholar]
- 57.Ngu J.M., Teng G., Meijndert H.C., Mewhort H.E., Turnbull J.D., Stetler-Stevenson W.G., Fedak P.W. Human cardiac fibroblast extracellular matrix remodeling: dual effects of tissue inhibitor of metalloproteinase-2, Cardiovasc. For. Pathol. 2014;23:335–343. doi: 10.1016/j.carpath.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alizadeh N., Pepper M.S., Modarressi A., Alfo K., Schlaudraff K., Montandon D., Gabbiani G., Bochaton-Piallat M.L., Pittet B. Persistent ischemia impairs myofibroblast development in wound granulation tissue: a new model of delayed wound healing. Wound Repair Regen. 2007;15:809–816. doi: 10.1111/j.1524-475X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 59.Correa-Gallegos D., Jiang D., Rinkevich Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 2021;302:147–162. doi: 10.1111/imr.12972. [DOI] [PubMed] [Google Scholar]
- 60.Bautista-Hernandez L.A., Gomez-Olivares J.L., Buentello-Volante B., Bautista-de Lucio V.M. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017;7:151–157. doi: 10.1556/1886.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin A., Lore K. Granulocytes: new members of the antigen-presenting cell family. Front. Immunol. 2017;8:1781. doi: 10.3389/fimmu.2017.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kobayashi S.D., DeLeo F.R. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009;1:309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esche C., Stellato C., Beck L.A. Chemokines: key players in innate and adaptive immunity. J. Invest. Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 64.Platanitis E., Decker T. Regulatory networks involving STATs, IRFs, and NFkappaB in inflammation. Front. Immunol. 2018;9:2542. doi: 10.3389/fimmu.2018.02542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.