Abstract
Introduction:
The inclusion of premanifest Huntington’s Disease (Pre-HD) subjects in clinical trials necessitates selecting those who are near transition to manifest Huntington’s disease (Man-HD). We previously determined that plasma neurofilament light (NfL) levels are significantly correlated with predicted years to Man-HD onset, using established formulae. Recently, a new normalized prognostic index (PIN) score for predicting Pre-HD disease progression has been validated. Our objective was to determine whether plasma NfL levels are similarly associated with PIN score and PIN score-derived years to Man-HD onset (PIN-YTO).
Method:
112 individuals (46 Pre-HD, 66 Man-HD) underwent blood sample collection and clinical assessment, inclusive of the Symbol Digit Modalities Test and Unified Huntington’s Disease Rating Scale Total Motor Score. Plasma NfL levels were measured using a Meso Scale Discovery assay.
Results:
Pre-HD and Man-HD cohorts differed by age (p < .0001), and CAG repeat number (p = .004), but not education level or gender. Plasma NfL levels were significantly correlated with PIN scores (r = 0.69, p < .0001) and PIN-YTO (r = −0.69, p < .0001). Plasma NfL levels were similarly correlated with predicted years to onset scores determined using Langbehn and colleague’s formula (r = −0.68, p < .0001). All significant correlations endured corrections for age and CAG repeat number. A plasma NfL cut-point of <45.0 pg/ml distinguished Pre-HD participants >10 predicted years from Man-HD onset, compared to those ≤10 predicted years.
Conclusions:
We have extensively shown that plasma NfL levels are associated with predicted years to manifest HD onset in Pre-HD participants, and present a plasma NfL cut-point that may help exclude far-from-onset Pre-HD patients from clinical trials.
Keywords: Huntington’s disease, Neurofilament light, Plasma, Blood, Biomarker
1. Introduction
Huntington’s Disease (HD) is a genetic neurodegenerative disorder caused by an unstable CAG trinucleotide repeat expansion in exon 1 of the huntingtin (HTT) gene. While this autosomal mutation with sufficient CAG expansion (CAG >39) guarantees symptom onset during an individual’s lifetime, progression is gradual, and a clinical diagnosis of manifest HD (Man-HD) is not given until the emergence of unequivocal involuntary motor symptoms unattributed to other causes. Motor symptom onset most often occurs between the 4th and 5th decades of life, however other non-specific symptoms, including psychiatric and cognitive decline, can precede the clinical diagnosis [1]. Such symptoms develop gradually during a premanifest phase (Pre-HD), and at some point there is a shift from Pre-HD to Man-HD. The potential to predict years until Man-HD onset is an area of particular interest, given that neurodegenerative changes are known to precede a clinical diagnosis, and it is likely that disease-modifying treatments would be most effective if given prior to diagnosis. Current HD therapeutic trials have included predominantly early-stage Man-HD patients, and results have been disappointing. Not surprisingly, in the wake of these non-efficacious trial outcomes, some of the focus has now shifted to administering candidate therapies earlier in the course of disease and especially prior to prominent symptom onset. Expanding clinical trial design to include Pre-HD patients could therefore help improve the success of future trials [2].
Recently, we showed that plasma neurofilament light (NfL) is associated with predicted years until manifest symptom onset in Pre-HD participants [3], using formulae published by Langbehn and colleagues [4]. NfL has emerged as a promising biomarker for HD, due to its exclusively neuronal expression, and association with neurodegeneration [5]. Our data suggests that plasma NfL may be indicative of neurodegeneration in Pre-HD individuals, which may not be reflected by concurrent clinical indicators or symptom presentation.
While the formulae for predicted years to onset, for a defined degree of probability, published by Langbehn and colleagues is established and commonly used within the HD research field, recently, a new normalized prognostic index (PIN) score for predicting Pre-HD disease progression [4,6], which incorporates patient CAG repeat length, age, Symbol Digit Modalities Test (SDMT) score, and the Unified Huntington’s Disease Rating Scale (UHDRS) Total Motor Score (TMS) [4,6], has been validated. In addition, it has been suggested that an individual’s PIN score may be used to calculate their predicted years to manifest symptom onset, for a defined degree of probability [6] (herein referred to as PIN years to onset (PIN-YTO) values). Given our previous data showing correlations between plasma NfL and both the predicted years to onset using the Langbehn formula, as well as the SDMT [3], we hypothesized that plasma NfL levels would be associated with PIN and PIN-YTO values. Such an association would legitimize the incorporation of plasma NfL levels into future prediction modelling to further improve output accuracy.
2. Methods
2.1. Human Subjects
This study was approved by the University of California, San Diego (UCSD) Institutional Review Board, in accordance with the requirements of the Code of Federal Regulations on the Protection of Human Subjects. Patients were recruited from the UCSD HDSA Center of Excellence and carried a diagnosis of HD with family history of the disorder. Pre-HD individuals had ≥38 CAG repeats, and a UHDRS diagnostic confidence level (DCL) below 4. A CAG repeat minimum of 38 was not part of the eligibility criteria, but rather the minimum CAG repeat length included in this study. All Pre-HD individuals who had consented, and attended a clinical assessment, to provide clinical data and blood samples at UCSD HDSA Center of Excellence were included. Man-HD participants had a DCL of 4, indicating that a clinician had ≥99% certainty that the patient presented with “unequivocal presence of an otherwise unexplained extrapyramidal movement disorder” [7]. The UHDRS was developed by the Huntington Study Group, and is used as a major outcome measure in controlled clinical trials [7,8]. All participants gave written informed consent prior to sample collection. Demographic and disease data were collected at the time of sample collection, including gender, age, CAG repeat length, years of education, age of onset and family history.
2.2. Clinical assessment
Study participants underwent a single clinical assessment, which included the SDMT [9] and UHDRS TMS [7]. PIN scores were calculated using a previously published formula [6]. PIN-YTO values, at probabilities ranging from 50% to 70%, were calculated from the PIN score [6].
2.3. Plasma collection and analysis
Blood was drawn by venipuncture into 2 ml lavender/EDTA tubes. EDTA/whole blood was mixed by inversion and centrifuged (900g, 15 min). The supernatant was isolated, aliquoted into 1 ml aliquots, snap frozen and stored at −80 °C. Plasma levels of NfL were measured using a Meso Scale Discovery (MSD; Rockville, MD) R-Plex Assay (Cat# F217X; researcher determined lower limit of detection: 2.3 pg/ml) and normalized for inter-assay variation as previously described [3].
2.4. Statistical Analysis
Analyses were conducted with GraphPad Prism version 8.4.2 for Windows (GraphPad Software, La Jolla, CA, USA). Analyses which corrected for age and CAG repeat number were conducted using IBM® SPSS® Statistics version 25 for Windows (IBM Corp., NY, USA). Due to a non-normal distribution of data, cohort characteristics were assessed using Mann-Whitney U test. One visual outlier was deemed to be invalid and removed from all correlation analyses (Suppl. Figure 1), which were assessed by Spearman’s nonparametric rho test due to a non-normal distribution of data. Receiver Operating Characteristic Curve (ROC) analyses were used to determine whether a plasma NfL level cut-point could distinguish Pre-HD participants who are far from manifest symptom onset, from those who are near manifest symptom onset. Participant assignment into near and far from manifest symptom onset cohorts was determined on the basis of previously published PIN cut-off scores [2], as well as by grouping into >10 versus ≤10 predicted years until manifest symptom onset. Optimal ROC cut-points were determined using the Youden index [10].
3. Results
3.1. Participant characteristics
Plasma was collected from 112 individuals: 46 Pre-HD and 66 Man-HD. 26 Pre-HD (57%) and 38 Man-HD (58%) participants from this study were also included in our previous study cohort [3]. Man-HD were significantly older (median, range: 56.0, 36.0–86.0) than Pre-HD (median, range: 38,0, 19.0–66.0; p < .0001). The Man-HD cohort also had significantly higher CAG repeat numbers (median, range: 43.0, 40.0–49.0) than the Pre-HD cohort (median, range: 42.0, 38.0–51.0; p = .004), and significantly higher CAP scores (median, range: 494.3, 333.3–839.3) than the Pre-HD cohort (median, range: 325.7, 176.2–517.1; p < .0001). All Man-HD participants had CAG repeat lengths ≥40; three Pre-HD participants had CAG repeat lengths below 40, considered reduced penetrance mutations (2x 38 CAG, 1x 39 CAG). The Man-HD cohort did not differ from the Pre-HD cohort in years of education (median, range: Man-HD: 16.0, 5.0–21.0; Pre-HD: 16.0, 12.0–22.0; p = .36) or gender balance (men/women: Man-HD: 32/34; Pre-HD: 22/24; p = .95).
3.2. Prognostic potential of plasma NfL
Plasma NfL levels were significantly associated with participant age (r = 0.49, p < .0001) and CAG repeat number (r = 0.26, p = .005), but not gender, irrespective of diagnostic cohort. Plasma NfL levels were significantly correlated with PIN scores in Pre-HD participants (r = 0.69, p < .001), but not Man-HD participants (r = 0.11, p = .36) (Fig. 1A). We next considered PIN cut-off scores previously determined to separate Pre-HD individuals into cohorts closer or further from disease onsets [2]. A plasma NfL cut-point of <45.01 pg/ml accurately distinguished far-from-onset participants with PIN scores of ≤0.0 (AUC = 0.85, p < .00001; Fig. 1B), or ≤0.4 (AUC = 0.75, p = .03; Fig. 1C) from those closer to onset (>0.0 or >0.4 for Fig. 1B and C, respectively). Conversely, the PIN cut-off score with the highest ROC area under the curve value was ≤−0.8 (AUC = 0.91, p < .0001; Fig. 1D).
Fig. 1.
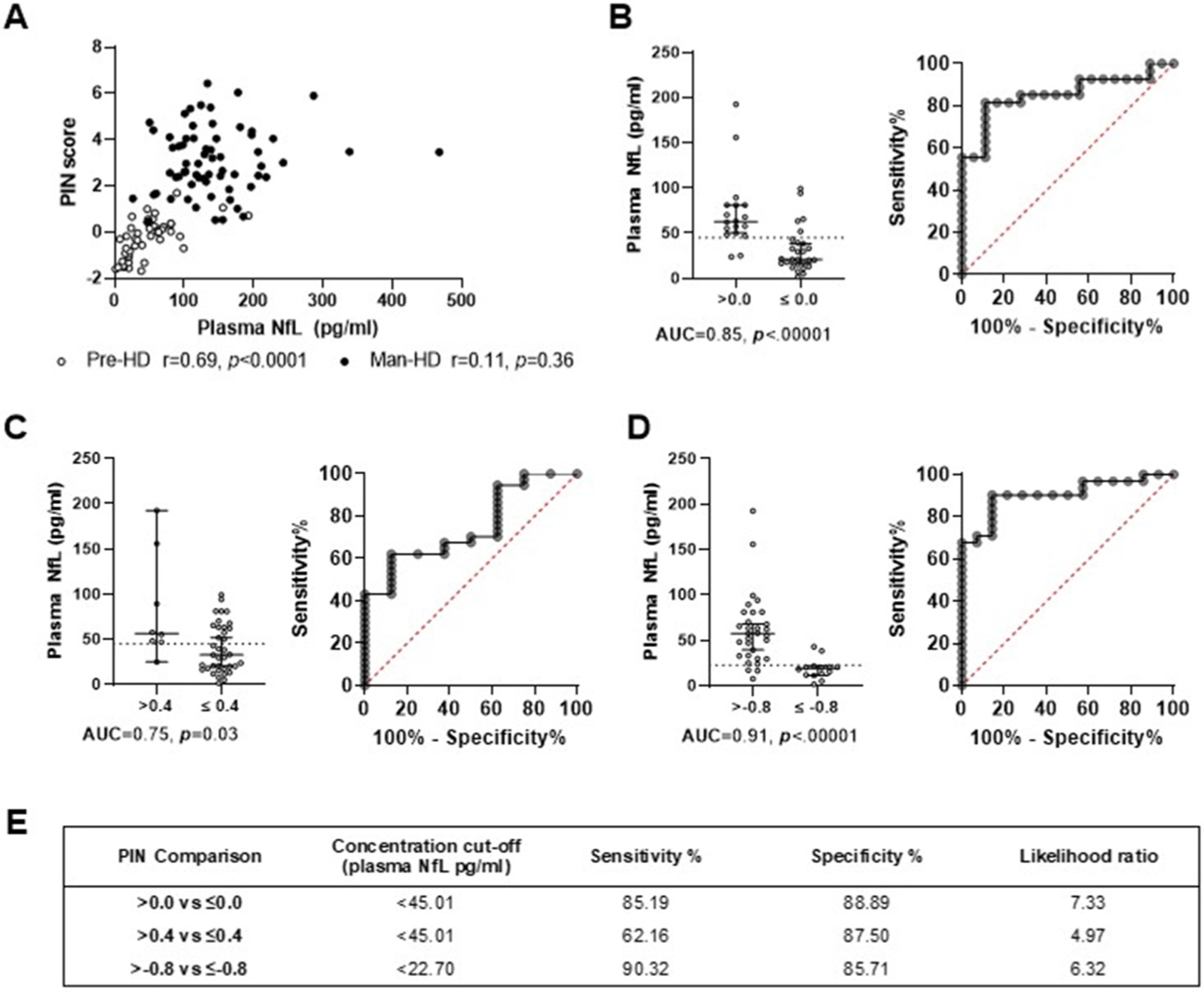
PIN scores, used to determine PIN YTO, were significantly correlated with plasma NfL levels in premanifest HD (Pre-HD; open circles) but not manifest HD (Man-HD; filled circles) participants (A). Using receiver operating characteristic curve (ROC) analyses, a plasma NfL cut-off level of <45.01 pg/ml (dotted line) distinguished premanifest HD participants with PIN scores equal to or less than 0.0 (B) or 0.4 (C), cohorts previously proposed to be far from manifest disease onset [2]. A PIN score cut-off of <=−0.8 provided the highest area under the curve (AUC) value (D; <22.70 pg/ml cut-off value [dotted line]). ROC curve specificity and sensitivity values for B-D are detailed in (E).
Participant PIN scores were used to determine PIN-YTO values at 50%, 60% and 70% probability, which were significantly correlated with plasma NfL levels in Pre-HD participants at all measured probabilities (r = 0.69, p = .0001 for all; open circles, Fig. 2A–C), which endured adjustment for age and CAG (r = −0.43, p = .004). PIN-YTO values were not correlated with plasma NfL levels in Man-HD participants at any probability measured, both before and after correcting for age and CAG (p > .05 for all; filled circles, Fig. 2A–C). ROC analyses determined that a plasma NfL <45.01 pg/ml cut-off score accurately distinguished participants with >10 predicted years until manifest symptom onset, compared to those ≤10 years (AUC = 0.86 p < .00001; AUC = 0.79, p = .002, AUC = 0.75, p = .03) for Fig. 2D–F respectively; see Fig. 2G for a summary).
Fig. 2.
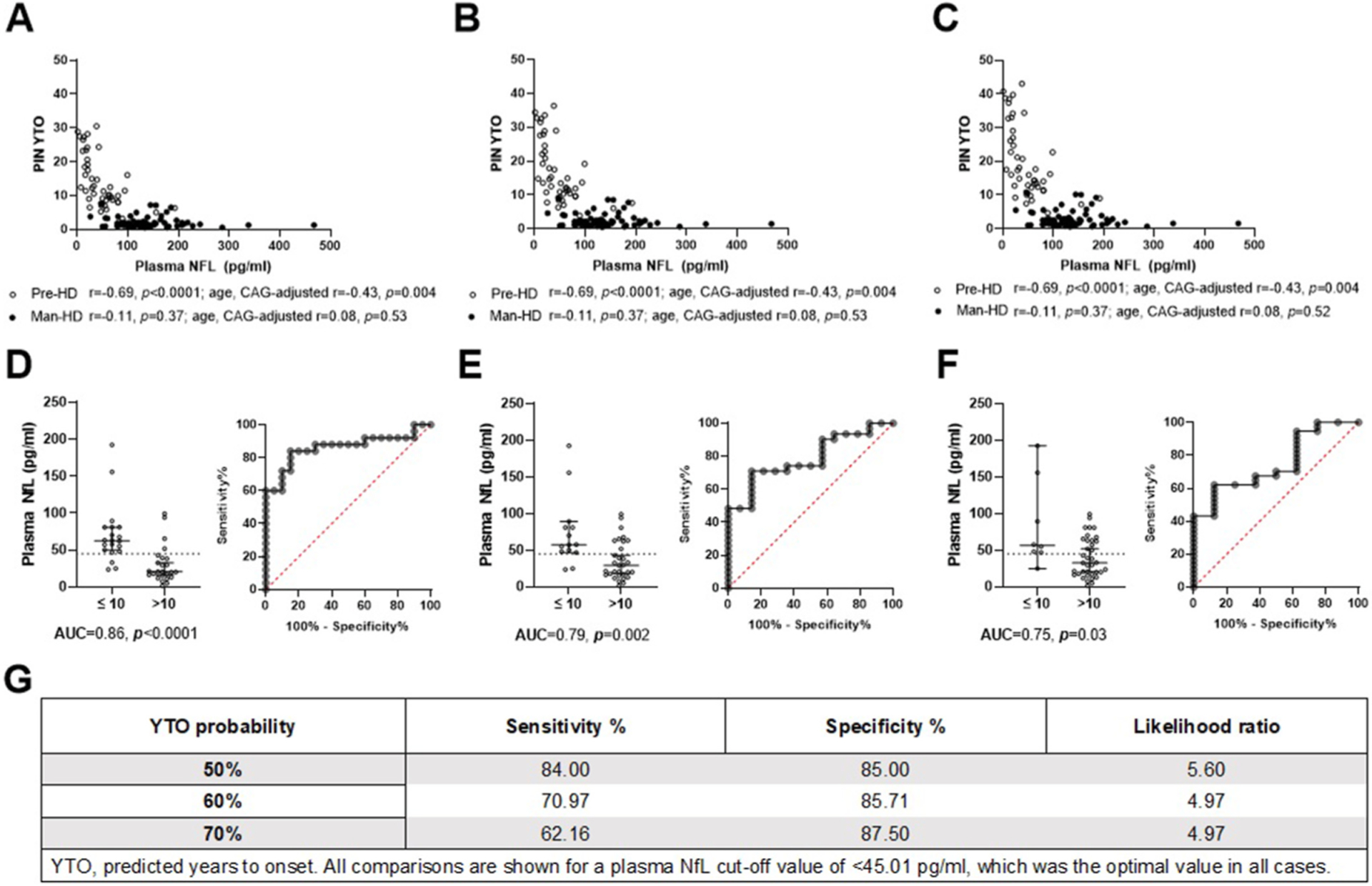
Plasma NfL levels are significantly correlated with predicted years to manifest disease onset (YTO) at 50% (A), 60% (B) and 70% (C) probability in premanifest Huntington’s Disease patients (Pre-HD, open circles), but not manifest HD patients (Man-HD, filled circles). Using receiver operating characteristic curve analyses, a plasma NfL cut-off value of <45.01 pg/ml (dotted line) accurately distinguished Pre-HD participants with more than 10 years until manifest symptom onset, from those with less than 10 years, at 50% (D), 60% (E) and 70% (F) probability of onset. Graph D-E cut-off accuracy is specified in (G).
When comparing participant PIN-YTO values to those determined using Langbehn and colleague’s formula (herein referred to as Langbehn-YTO) [4], PIN-YTO and Langbehn-YTO values were significantly correlated at all probabilities (50–70%), with PIN-YTO values comparatively lower than Langbehn-YTO values for the same participant (Suppl. Figure 2).
Plasma NfL values were significantly correlated with Langbehn-YTO values in Pre-HD participants at 50% (r = 0.69, p < .0001), 60% (r = 0.68, p < .0001), and 70% (r = 0.68, p < .0001) probability, which survived adjustment for age and CAG (r = −0.46, p = .002 for all probabilities) (Suppl. Figure 3). ROC analyses determined that a plasma NfL <45.01 pg/ml cut-off score accurately distinguished participants with >10 predicted years until manifest symptom onset, compared to those ≤10 years, at 50% (AUC = 0.83 p = .0001), 60% (AUC = 0.77, p = .004) and 70% (AUC = 0.74, p = .04) probability (Suppl. Figure 3).
4. Discussion
In this study, we found that plasma NfL levels were significantly correlated with PIN scores, and PIN score-derived predicted years to manifest symptom onset [6] in premanifest HD participants. While these findings are in line with those we have published previously using Langbehn’s formula and an overlapping cohort [3], they contrast findings reported by Scahill and colleagues, who found no association between plasma NfL and Pre-HD predicted years to onset [11]. The Pre-HD cohort analyzed by Scahill and colleagues was an average of 23.6 predicted years from onset, whereas the cohort in the present study was an average of 17.7 predicted years from onset (median of 15.2 years). It is possible that plasma NfL may be less useful when predicting years to onset for those who are far from onset. We did not observe a correlation between PIN or PIN-YTO scores and plasma NfL levels in manifest HD participants, which is in line with the absence of correlation between plasma NfL levels and disease, clinical or cognitive measures we have previously reported [3]. The plasma NfL value that distinguished Pre-HD participants who were far from manifest onset (as determined by previously published PIN cut-off scores [2]), and participants with more than 10 years until manifest symptom onset (using PIN-YTO value) was the same (<45.0 pg/ml). A PIN cut-off of 0.0 was previously associated with greater than 80% 10-year probability of onset, and our data further validates these cut-off scores and the use of plasma NfL values, using an independent cohort. The plasma cut-off value of <45.0 pg/ml was similar to the value we had previously determined using the Langbehn-YTO formula in a smaller cohort (<45.66 pg/ml) [3], and matched that determined using the Langbehn-YTO formula in the current cohort (Suppl. Figure 3). These findings suggest that a plasma NfL value cut-off value of <45.0 pg/ml may be a useful complement to clinical trial prognostic indicators such as the PIN score, although this value should be validated in a separate cohort of patients. In addition, while we observed similar associations between plasma NfL and the PIN-YTO score, and plasma NfL and the Langbehn-YTO score, use of the PIN-YTO formula reduced the specificity of the optimal plasma NfL cut-off score (see Suppl. Figure 3G). However, from our cross-sectional data, we cannot conclusively say that plasma NfL cut-point specificity is an indication of years to onset predictive formula accuracy, rather than vice versa. Indeed, it is possible that the PIN and PIN-YTO score may captures predictive indicators not related to plasma NfL levels. Therefore, while we suggest the incorporation of plasma NfL values into predictive models, we do not suggest that it should replace current clinical trial prognostic indicators such as the PIN score. Such incorporation would allow a plasma NfL cut-point to complement, and be complemented by, other indicators of neurodegeneration and disease progression, such as age, cognitive and motor function, as well as the emergence of other biological markers. Overall, validation of plasma NfL levels as indicators of predicted years to onset may assist clinicians and patients in predicting disease progression and planning clinical treatment.
Whether plasma NfL values are truly an appropriate predictor of years to manifest disease onset would be best confirmed through longitudinal tracking of these participants, to ascertain which individuals experience manifest disease onset within 10 years. We acknowledge that a cross-sectional design is a limitation of the current study. Currently available data of longitudinal Pre-HD plasma NfL levels suggests a gradual increase over time and, significantly, progression to manifest HD within 3 years by those with approximately 45 pg/ml or greater baseline plasma NfL levels [5,12]. Overall, we have shown that a plasma NfL cut-point of <45.01 pg/ml may allow the exclusion of Pre-HD participants who are far from manifest onset from clinical trials, thereby fostering the enrichment of clinical trials with Pre-HD participants who are nearing manifest symptom onset. Longitudinal follow-up of participants included in this short communication is currently underway.
Supplementary Material
Acknowledgement
This work was supported the National Institutes of Health (NS111655 to E.A.T.); the UCSD Huntington’s Disease Society of America Center of Excellence; and the UCSD Shiley-Marcos Alzheimer’s Disease Research Center NIH P50 AG005131. These funding sources had no role in study design, conduct, analysis, interpretation or writing of the manuscript, or in the decision to submit the manuscript.
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parkreldis.2022.02.023.
References
- [1].Paulsen JS, Miller AC, Hayes T, Shaw E, Cognitive and behavioral changes in Huntington disease before diagnosis, Handb. Clin. Neurol 144 (2017) 69–91. [DOI] [PubMed] [Google Scholar]
- [2].Langbehn DR, Hersch S, Clinical outcomes and selection criteria for prodromal Huntington’s disease trials, Mov. Disord 35 (12) (2020) 2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parkin GM, Corey-Bloom J, Snell C, Castleton J, Thomas EA, Plasma neurofilament light in Huntington’s disease: a marker for disease onset, but not symptom progression, Park. Relat. Disord 87 (2021) 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Langbehn DR, Hayden MR, Paulsen JS, PREDICT-HD Investigators, CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches, Am. J. Med. Genet. B Neuropsychiatr. Genet 153 (2) (2010) 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RA, et al. , Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis, Lancet Neurol 16 (8) (2017) 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Long JD, Langbehn DR, Tabrizi SJ, Landwehrmeyer BG, Paulsen JS, Warner J, et al. , Validation of a prognostic index for Huntington’s disease, Mov. Disord 32 (2) (2017) 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kieburtz K, Penney JB, Corno P, Ranen N, Shoulson I, Feigin A, et al. , Unified Huntington’s disease rating scale: reliability and consistency, Neurology 11 (2) (2001) 136–142. [Google Scholar]
- [8].Sun W, Zhou D, Warner JH, Langbehn DR, Hochhaus G, Wang Y, Huntington’s disease progression: a population modeling approach to characterization using clinical rating scales, J. Clin. Pharmacol 60 (8) (2020) 1051–1060. [DOI] [PubMed] [Google Scholar]
- [9].Smith A, Symbol digit modalities test, in: Spreen O, Strauss E (Eds.), A Compendium of Neuropsychological Tests, second ed., Western Psychological Services, Los Angeles, 1982. [Google Scholar]
- [10].Fluss R, Faraggi D, Reiser B, Estimation of the Youden Index and its associated cutoff point, Biom. J.: J. Math. Methods Biosci 47 (4) (2005) 458–472. [DOI] [PubMed] [Google Scholar]
- [11].Scahill RI, Zeun P, Osborne-Crowley K, Johnson EB, Gregory S, Parker C, et al. , Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington’s disease Young Adult Study (HD-YAS): a cross-sectional analysis, Lancet Neurol 19 (6) (2020) 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rodrigues FB, Byrne LM, Tortelli R, Johnson EB, Wijeratne PA, Arridge M, et al. , Mutant huntingtin and neurofilament light have distinct longitudinal dynamics in Huntington’s disease, Sci. Transl. Med 12 (574) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


