Introduction
Early-stage Lyme-associated erythema migrans most commonly manifests as a targetoid patch. However, several erythema migrans variants have been reported, which may result in misidentification as well as delayed diagnosis and treatment. This case series demonstrates a rare blistering form of erythema migrans known as bullous erythema migrans.1
Case series
Case 1
A 54-year-old woman presented to the emergency department of a rural, academic hospital in New Hampshire, with a worsening rash on the lateral aspect of her left ankle. She reported a sudden onset of localized pain and stinging while walking through a corn maze 11 days previously. An enlarging red lesion appeared soon after and became progressively darker (Fig 1). She developed an intermittent, low-grade fever on the fourth day, and the lesion became progressively more painful, swollen, and purpuric.
Fig 1.
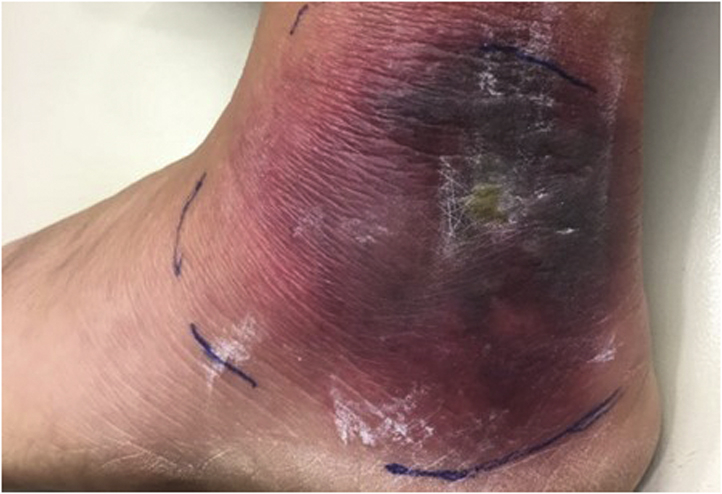
Bright red, annular, edematous, vesicular plaque on the lateral aspect of the left malleolus.
On examination, she was noted to have a 10-cm edematous purpuric plaque with vesiculobullous changes centrally. Diagnoses of bullous Lyme disease, Sweet syndrome, and herpes zoster were considered. A biopsy of a flat portion of the lesion close to the periphery showed hyperkeratosis and acanthosis with prominent overlying papillary edema along with a histiocyte-rich inflammatory infiltrate. Myeloperoxidase staining was negative. Grocott-Gomori methenamine silver stain and Gram stains were negative for microorganisms (Fig 2). Her symptoms improved rapidly with twice-daily treatment with doxycycline 100 mg for 21 days. Positive immunoglobulin M (IgM) serologies via 2-tiered serologic testing at 3 weeks confirmed a Lyme disease diagnosis. Lyme disease antibody serology 1 year previously was negative.
Fig 2.
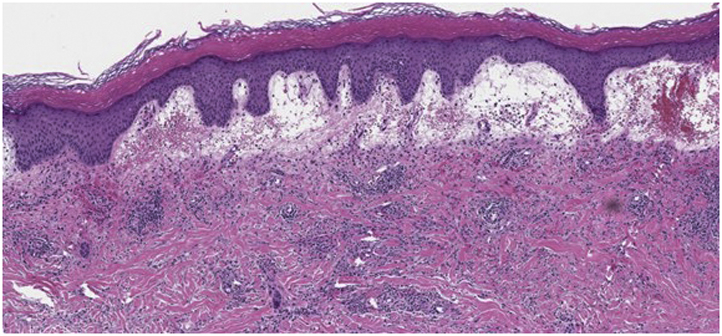
Histopathology demonstrated a dense interstitial and perivascular lymphohistiocytic inflammatory infiltrate within the dermis, extending around adnexal structures and into the deeper aspect of the dermis (hematoxylin-eosin stain; original magnification: ×10).
Case 2
A 49-year-old woman with no known history of tick bite presented to the emergency department 4 days after the appearance of an enlarging, darkening lesion on the posterior aspect of her ankle. On examination, she was noted to have a 9-cm vesiculobullous plaque with erythema (Fig 3). She reported no fever, but slight fatigue. A biopsy of the lesion was not taken. She was treated with a 21-day course of doxycycline and rapidly improved.
Fig 3.
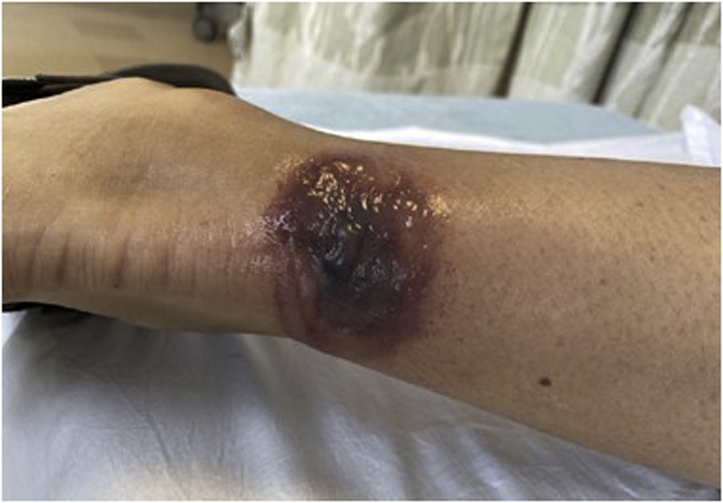
Vesiculobullous plaque on the posterior aspect of the right leg with surrounding erythema.
Three weeks later, a positive IgM Lyme result via 2-tiered serologic testing confirmed an atypical Lyme disease diagnosis. She did not have previous Lyme disease serology data for comparison.
Case 3
A 65-year-old woman presented to the emergency department with a 3-day history of a red, swollen, painful plaque on the left side of the flank (Fig 4).
Fig 4.
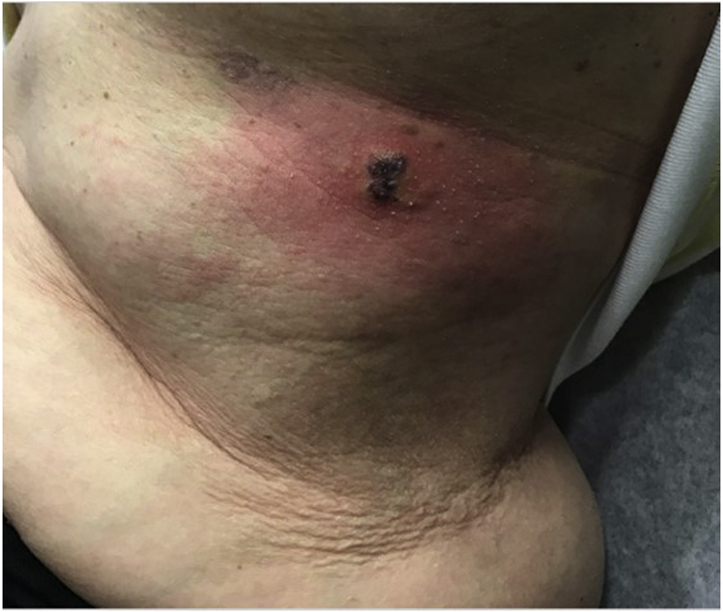
Rash displaying a bright red patch with central dusky papulovesicles.
Central dusky papulovesicles developed within a day after the appearance of the plaque. She reported a low-grade fever and malaise and denied a known history of tick bite. Treatment for herpes zoster with valacyclovir and for Lyme disease with doxycycline was initiated. Direct fluorescent antibody staining and viral culture were negative for varicella-zoster virus and herpes simplex virus. Polymerase chain reaction was negative for herpes simplex virus. Lyme serology after 21 days was positive for IgM via 2-tiered serologic testing, confirming a Lyme disease diagnosis. A Lyme disease antibody test 7 years previously had been negative.
Discussion
We report 3 cases of otherwise healthy women with no known history of tick bites who presented with vesiculobullous lesions caused by Lyme disease. All 3 cases were considered likely infectious processes and resolved with empiric doxycycline. Follow-up serologies were positive for IgM at 3 weeks, supporting a Lyme disease diagnosis.
Lyme disease is an increasingly common diagnosis throughout regions of the United States and Europe, with an estimated 300,000 cases diagnosed annually.2 The initial stage of cutaneous Lyme is most often characterized by the erythema-migrans patch, which appears within a month of the tick bite. A quarter of these lesions display the characteristic bull’s-eye appearance.3 However, recent publications have also reported urticarial, linear, granulomatous, and bullous erythema migrans presentations.1,4,5
This case series demonstrates the rare vesicobullous erythema migrans variant. All 3 cases exhibited rapidly developing bullous lesions in the presence of systemic symptoms, ranging from fatigue and malaise to fever. Histologic findings in the first case displayed relatively nonspecific inflammatory findings; the histologic features of Lyme disease are varied and may be nonspecific.1,4,5 As such, these lesions were originally considered to represent Sweet syndrome, herpes simplex virus infection, varicella-zoster virus infection, or a spider bite, in addition to atypical Lyme disease. One of the challenges in confirming the diagnosis of Lyme disease is the delayed positivity of Lyme-specific antibodies and the lack of specific findings on histopathology. Histopathologic features of Lyme disease often include a superficial and deep perivascular and interstitial infiltrate consisting of lymphocytes and plasma cells, and may include eosinophils and neutrophils; however, a variety of histopathologic patterns have been described and often suggest a broad histopathologic differential diagnosis.6,7 The testing options for early-stage Lyme disease (<30 days since symptom onset) include polymerase chain reaction, enzyme-linked immunosorbent assay (ELISA), and Western blot (Table I).8 The Center for Disease Control and Prevention currently recommends a 2-tier approach, involving an initial serum enzyme immunoassay, followed by IgG and IgM Western blotting or a secondary enzyme immunoassay. All 3 patients underwent 2-tiered serologic testing, using a VlsE1/pepC10 ELISA followed by a whole-cell sonicate ELISA (Zeus Scientific).
Table I.
Ninety-five percent confidence intervals of sensitivity and specificities of common Lyme disease serology tests for stage 1 disease (adapted from Waddell et al).8
| Testing method | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| PCR (blood sample) | 33.8%-62.0% | NA |
| ELISA–pepC10 | 32.7%-44.0% | 97.7%-99.5% |
| ELISA–VlsE1 | 47%-77% | 98%-99% |
| ELISA–WCS | 60.9%-78.8% | 59.5%-83.5% |
| Western blot (Marblot/GenBio) | 42.7%-76.0% | 91.9%-98.7% |
CI, Confidence intervals; ELISA, enzyme-linked immunosorbent assay; NA, not applicable; PCR, polymerase chain reaction; WCS, whole-cell sonicate.
This case series highlights a rare variant of cutaneous Lyme disease. Clinicians in Lyme-endemic areas should be aware that Lyme disease might exhibit a broad range of clinical and histologic findings, including bullous presentations. Thus, a low threshold for considering Lyme disease in the differential diagnosis of bullous lesions is warranted in endemic areas with empiric treatment and follow-up serologies for disease confirmation.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Tiger J.B., Guill M.A., III, Chapman M.S. Bullous Lyme disease. J Am Acad Dermatol. 2014;71(4):e133–e134. doi: 10.1016/j.jaad.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Nelson C.A., Saha S., Kugeler K.J., et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21(9):1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malane M.S., Grant-Kels J.M., Feder H.M., Jr., Luger S.W. Diagnosis of Lyme disease based on dermatologic manifestations. Ann Intern Med. 1991;114(6):490–498. doi: 10.7326/0003-4819-114-6-490. [DOI] [PubMed] [Google Scholar]
- 4.Badin D.J., O'Hern K., Simmons B.J., Mann J.A., Momtahen S. Localized reactive granulomatous dermatitis secondary to erythema migrans. JAAD Case Rep. 2020;6(12):1236–1238. doi: 10.1016/j.jdcr.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feder H.M., Jr., Whitaker D.L. Misdiagnosis of erythema migrans. Am J Med. 1995;99(4):412–419. doi: 10.1016/s0002-9343(99)80190-9. [DOI] [PubMed] [Google Scholar]
- 6.Wilson T.C., Legler A., Madison K.C., Fairley J.A., Swick B.L. Erythema migrans: a spectrum of histopathologic changes. Am J Dermatopathol. 2012;34(8):834–837. doi: 10.1097/DAD.0b013e31825879be. [DOI] [PubMed] [Google Scholar]
- 7.Miraflor A.P., Seidel G.D., Perry A.E., Castanedo-Tardan M.P., Guill M.A., Yan S. The many masks of cutaneous Lyme disease. J Cutan Pathol. 2016;43(1):32–40. doi: 10.1111/cup.12620. [DOI] [PubMed] [Google Scholar]
- 8.Waddell L.A., Greig J., Mascarenhas M., Harding S., Lindsay R., Ogden N. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]


