Abstract
SARS-CoV-2 infection is known to result in a range of symptoms with varying degrees of acute-phase severity. In a subset of individuals, an equally diverse collection of long-term sequelae has been reported after convalescence. As survivorship and therefore the number of individuals with ‘long-COVID’ continues to grow, an understanding of the prevalence, origins, and mechanisms of post-acute sequelae manifestation is critically needed. Here, we will explore proposed roles of the anti-SARS-CoV-2 immune response in the onset, severity, and persistence of SARS-CoV-2 post-acute sequelae. We discuss the potential roles of persistent virus and autoantigens in this syndrome, as well as the contributions of unresolved inflammation and tissue injury. Furthermore, we highlight recent evidence demonstrating the potential benefits of vaccination and immunity in the resolution of post-acute symptoms.
Current Opinion in Immunology 2022, 77:102228
This review comes from a themed issue on Special Section on Immune responses to chronic infections
Edited by Rosemary Rochford
For complete overview of the section, please refer to the article collection, “Special Section on Immune responses to chronic infections (August 2022)”
Available online 24th May 2022
https://doi.org/10.1016/j.coi.2022.102228
0952-7915/© 2022 Elsevier Ltd. All rights reserved.
Introduction: the phenomenon of ‘long COVID’
Post-acute sequelae of SARS-CoV-2 (PASC), colloquially referred to as ‘long COVID’, is a poorly understood condition characterized by prolonged Coronavirus Disease 2019 (COVID-19) symptoms and/or the development of new symptoms following the resolution of acute SARS-CoV-2 infection. While there is still no formal clinical definition of PASC, findings reported in individuals experiencing PASC include persistent shortness of breath, sleep disorders, hyperlipidemia, fatigue, gastroesophageal reflux disease (GERD), cough, muscle weakness, joint pain, thromboembolism, kidney disorders, neurological impairments, and cardiopulmonary abnormalities 1••, 2, 3. PASC is differentiated from acute COVID-19 primarily by the timing of symptoms relative to the onset of illness, with acute COVID-19 defined as symptoms persisting for up to four weeks post onset, and PASC restricted to symptoms persisting or developing more than two months after symptom onset. The spectrum of symptoms associated with PASC is not fully unique to SARS-CoV-2 convalescence and includes features associated with recovery from other viral illnesses or sepsis. Nevertheless, an estimated 7–10% of convalescent COVID-19 patients — or 23–33 million people as of January 2022 — are thought to have experienced PASC 4••, 5, with roughly 40% of these individuals stating that PASC significantly impacts their ability to perform basic daily tasks. Both the frequency and overall number of convalescent COVID-19 patients that experience post-acute sequelae of infection make it a significant public health concern.
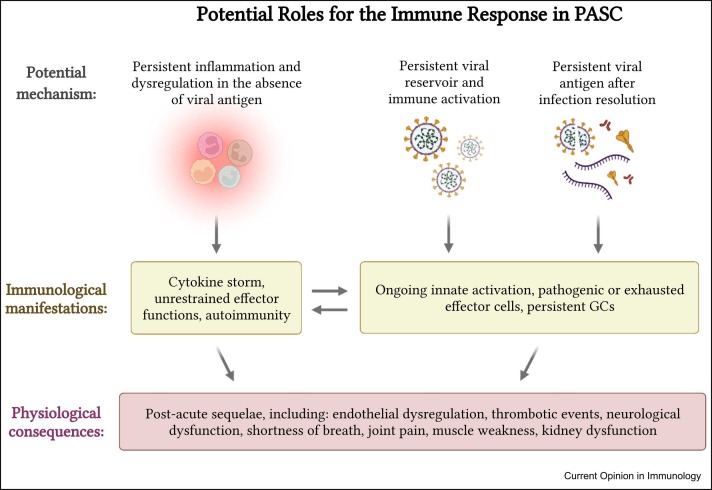
Despite the prevalence and growing awareness of PASC, the physiological mechanisms underpinning the phenomena remain poorly defined. This is in part due to the diverse array of symptoms associated with PASC, the fundamental complexities of COVID-19 pathogenesis, and the lack of a concrete and consistently applied clinical definition of PASC. Only recently have large-scale studies started to shed light on the full clinical burden of PASC and on the risk factors associated with PASC severity and persistence. Both premorbid risk factors — such as hypertension, obesity, and immunosuppression — and the duration and severity of acute COVID-19 illness appear to correlate with the severity and persistence of PASC 3, 4••, 6, 7, 8, 9. However, a common theme is the presence of persistent inflammation and immune activation beyond the acute infectious insult. Here, we will explore the association between persistent inflammation and PASC, as well as several potential mechanisms for the induction and maintenance of dysregulated antigen-specific immune responses following the resolution of acute SARS-CoV-2 infection ( Figure 1).
Figure 1.
Graphical abstract of potential roles of the immune system in PASC.
Immunological misfiring, inflammatory storms, and persistent inflammation in post-acute sequelae of SARS-CoV-2
There is mounting evidence that PASC is accompanied by dysregulated and persistent multiorgan inflammation 10•, 11, 12, 13••. Elevated serum levels of C-reactive protein, TNFα, IFNγ, and IL-6 have been observed in convalescent COVID-19 patients who progressed to develop PASC, with the quantity of these inflammatory markers correlating with the number of patient-supplied PASC symptoms 9, 10•. Notably, Phetsouphanh et al. have described persistent immunological dysfunction in individuals at 8 months after nonsevere SARS-CoV-2 infection characterized by highly activated innate immune signatures and higher expression of both type-I and -III IFN, as well as CXCL9, CXCL10, IL-8, and sTIM-3 [14•]. In individuals with PASC, this unresolved infection-attendant inflammation is postulated to drive tissue damage, endothelial dysregulation, hypoxia-induced injury, and activation of pathogenic effector lymphocyte subsets 15, 16•, 17.
Unchecked inflammation and dysregulated adaptive immune responses have been associated with long-term sequelae following the resolution of many other viral pathogens, including Ebola, Lassa, Chikungunya, and influenza viruses 18, 19, 20. For SARS-CoV-2, persistent immune activation has been shown to result in endothelial dysfunction in convalescent COVID-19 patients [16•]. This study additionally identified circulating activated endothelial cells following the resolution of acute infection, postulating that this indicator of vascular injury was a consequence of proinflammatory cytokine production by activated cytotoxic CD8+ T cells. Notably, they also observed higher frequencies of effector T cells in subjects with pre-existing cardiovascular conditions and other comorbidities associated with increased COVID-19 severity and, consequently, increased probability of developing PASC. In support of this lymphocyte-activation model of PASC, Patterson and colleagues [21] utilized a machine-learning approach to predict the time to resolution of SARS-CoV-2-infection symptoms. The resultant predictive signature of PASC chronicity included elevated levels of plasma IFN-γ and IL-2, suggesting that untempered immune activation may contribute to PASC symptoms after viral clearance.
In addition to triggering unchecked and dysregulated inflammation, it has also been suggested that both severe COVID-19 and PASC are accompanied by the development of self-reactive immune responses. Multiple studies have now demonstrated that the production of autoantibodies can be triggered by SARS-CoV-2 infection and track with the development of anti-SARS-CoV-2 humoral immunity 22, 23. This appears to be especially evident following severe COVID-19 and extends beyond the anti-IFN antibodies that have been identified as playing a role in driving acute COVID-19 severity [24]. Additionally, it has been suggested that SARS-CoV-2 infection can trigger an inflammatory response against other, non-SARS-CoV-2 organisms, thereby resulting in the reactivation of latent viral infections and increasing the risk of opportunistic infections 13••, 25, 26. These observations suggest that SARS-CoV-2-driven inflammation can cause a persistent disruption of immunological homeostasis that results in the propagation of both tissue-level and systemic sequelae.
Persistent antigen production by noninfectious viral RNA
Dysregulated and persistent inflammation does not require the continuous presence of viral antigen after an acute viral illness. However, a growing number of studies posit the existence of a persistent noninfectious SARS-CoV-2 antigen reservoir. While infectious SARS-CoV-2 can generally only be observed in the airway for the first week after symptom onset, viral RNA and protein antigens have been detected in sites such as the central nervous system, intestine, and secondary lymphoid organs for weeks to months after the resolution of acute symptoms 27•, 28, 29••. Autopsies of individuals historically positive for SARS-CoV-2 have detected SARS-CoV-2 RNA up to 230 days post infection in the lung and a wide range of extrapulmonary tissues 27•, 28. However, SARS-CoV-2 RNA found outside of the lung late after acute infection was not accompanied by cytopathic tissue damage or marked inflammation in these individuals [28]. Therefore, while SARS-CoV-2 genetic material may persist long after the initial acute infection, it is unlikely that this represents a truly persistent or latent viral infection. A provocative hypothesis proposed by recent reports is the possibility that SARS-CoV-2 is capable of partial genomic integration, resulting in a somatic source of persistent antigen and subgenomic RNA [30]. This speculation has been intensely disputed 31, 32, 33, and will demand further examination as a potential source of persistent viral antigen that may be capable of driving protracted inflammation.
Some of the strongest evidence supporting a role for SARS-CoV-2-specific immunity in PASC lies in the observed impact of vaccination on PASC prevalence and durability. Recent work by Antonelli and colleagues [34•] demonstrated a 49% reduction in the risk of long-term SARS-CoV-2-infection symptoms in individuals who were vaccinated before infection compared with unvaccinated controls. Moreover, preliminary studies suggest that in some individuals, SARS-CoV-2 vaccination may help to resolve, or abate the worsening of, PASC symptoms [35]. Indeed, some evidence suggests that the duration of acute SARS-CoV-2 infection symptoms is associated with the persistence, but not magnitude, of antigen-specific adaptive immune responses 36, 37. It is therefore plausible that clearance of persistent antigen or a return to immunological baseline as a consequence of vaccination contributes to the resolution of PASC.
Antigen persistence in the absence of viral persistence
While there is evidence suggesting the persistence of SARS-CoV-2 genomic material beyond the acute infection phase, persistent immune activation directed toward SARS-CoV-2 is not dependent on prolonged viral replication or de novo antigen production. Multiple immunological mechanisms exist to retain antigen for the purpose of maturing immunological memory after the resolution of an acute infectious insult 38, 39. Most notably, follicular dendritic cells can retain protein antigens in germinal centers (GCs) within lymph nodes or spleen for months after initial antigen production. Indeed, the long-term retention of SARS-CoV-2 antigen within GCs has been suggested to drive clonal and mutational maturation of memory B cells isolated from convalescent COVID-19 patients 29••, 40•. A separate study identified reduced total IgM or IgG3 levels as a predictor of PASC risk [41], suggesting that not just the magnitude and affinity, but also the isotype of SARS-CoV-2 Ig may play a role in PASC.
How the persistence of SARS-CoV-2 antigen may mechanistically lead to individual post-acute symptoms remains unclear, but a number of investigations into the immunological basis for PASC and isolated case studies have identified dysregulation of CD8+ T-cell responses as a correlate of risk 42, 43•, 44•. Immune signatures of bronchoalveolar lavage (BAL) fluid in older individuals with PASC at 60–90 days post infection revealed a persistent increase in the frequency of CD69+ CD103- CD8+ TRM cells, as well as inflammatory signatures among myeloid cells [44•]. Following peptide stimulation, the CD69+ CD103- CD8+ T cells in convalescent subjects were polyfunctional for cytotoxic cytokine production, and expressed granzyme K, which can promote fibroblast activation. In contrast, Peluso et al. [43•] observed PASC patients 4 months out from infection to have a reduced frequency of degranulation-prone CD107a+ CD8+ T cells in the peripheral blood. Moreover, the frequency of nucleocapsid-specific IFN-γ-secreting CD8+ T cells declined more quickly in individuals with PASC in this study. It is possible that the preferential antigen-specific activation of pathogenic CD103- CD8+ T cells leads to cytotoxic lung-tissue damage and pathology, or that cytotoxic CD8+ T-cell exhaustion limits the capacity for complete viral or viral antigen clearance. Importantly, these possibilities are not mutually exclusive.
Stimulation of B cells via antigen–receptor binding is known to lead to the expansion of atypical memory B cells (atMBCs) during chronic or repeated intracellular infection or vaccination [45]. atMBCs are also associated with the production of autoantibodies in several autoimmune diseases, and were observed to be expanded in the peripheral blood of individuals with PASC before the onset of symptoms [13]. Cheon and colleagues [44•] also observed elevated frequencies of activated resident memory-like CD27+ CD69+ B cells in the BAL fluid of aged convalescent subjects, and several studies have shown or suggested a potential relationship between peripheral autoantibody levels and PASC 46, 47•. Further investigation will be required to determine whether atMBCs and circulating autoantibodies detected in PASC patients contribute to symptoms, or are a consequence of PASC itself.
Conclusions and significant open questions for the field
Despite the abundance of epidemiological information about PASC, numerous critical questions remain about the role of the immune system in PASC risk, resolution, and severity. It has become clear that considerable heterogeneity exists in the presentation — and likely also the origins — of specific PASC. As has been suggested for acute SARS-CoV-2 disease, the kinetics of the immune response and the interaction of polarized subsets of immune cells are likely to be the link between identified PASC risk factors and clinical outcomes. As observed by Su et al. [13], it is possible that early or inappropriately skewed acute T- and B-cell response could cause local tissue-damage-related PASC. Alternatively, delayed activation or exhaustion of the adaptive immune response to SARS-CoV-2 infection could result in untempered inflammation and secondary viral/antigen dissemination and inflammation at distal sites. Indeed, respiratory and GI PASC were proposed to be driven by divergent early transcriptional programs [13], and although these programs may have pre-existing pathological origins, differences in the kinetics of recruitment could also account for these disparities.
Another outstanding question is the nature and mechanistic underpinnings of severe post-acute symptoms observed in some younger individuals. One subgroup of PASC symptoms, Multisystem Inflammatory Syndrome in Children (MIS-C), is a rare acute shock-like syndrome affecting pediatric populations during SARS-CoV-2 convalescence. Recent analyses have identified marked activation of vascular patrolling CX3CR1+ CD8+ T cells [48] and dysregulated IFN-γ responses [49] during MIS-C. It will be important to determine whether some cases of PASC in adults follow a parallel mechanism of development, considering the similarities between the infectious context and potential immunological basis for these conditions. Additionally, it will be intriguing to explore whether pre-existing cross-reactive adaptive immune cells from endemic hCoVs or heterologous SARS-CoV-2 exposures influence the development or resolution of symptoms in the post-acute phase.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
K.L.N. and A.T.W. wrote and edited the paper.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgements
We gratefully acknowledge J. Wilmore and A. Wegman for their helpful comments.
Graphics
Graphical abstract created with BioRender.com.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest.
- 1••.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]; This article provided extensive characterization of symptoms and comorbidities associated with SARS-CoV-2 post-acute illness.
- 2.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Xie Y., Bowe B., Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the results of a comprehensive data analysis using medical records from the Veterans Affairs healthcare system. A correlation between disease severity and the risk of PASC was identified after adjusting for confounders.
- 5.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University: COVID-19 Dashboard; 2022.
- 6.Tenforde M.W., Billig Rose E., Lindsell C.J., Shapiro N.I., Files D.C., Gibbs K.W., Prekker M.E., Steingrub J.S., Smithline H.A., Gong M.N., et al. Characteristics of adult outpatients and inpatients with COVID-19 — 11 academic medical centers, United States, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helleberg M., Niemann C.U., Moestrup K.S., Kirk O., Lebech A.-M., Lane C., Lundgren J. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103–1107. doi: 10.1093/infdis/jiaa446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., Doucet L., Berkani S., Oliosi E., Mallart E., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans R.A., McAuley H., Harrison E.M., Shikotra A., Singapuri A., Sereno M., Elneima O., Docherty A.B., Lone N.I., Leavy O.C., et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work identified elevated serum C-reactive protein in individuals with PASC.
- 10•.Peluso M.J., Lu S., Tang A.F., Durstenfeld M.S., Ho H.-E., Goldberg S.A., Forman C.A., Munter S.E., Hoh R., Tai V., et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that individuals with PASC have evidence of persistent immune activation and inflammation early after SARS-CoV-2 recovery.
- 11.Sun B., Tang N., Peluso M.J., Iyer N.S., Torres L., Donatelli J.L., Munter S.E., Nixon C.C., Rutishauser R.L., Rodriguez-Barraquer I., et al. Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021;10 doi: 10.3390/cells10020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., Garcia-Valtanen P., Al-Delfi Z., Gummow J., Ferguson C., et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20 doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used plasma and single-cell multiomic approaches to provide the most comprehensive analysis to date of predictors of PASC risk from medical records and biological specimens. The authors identified pre-existing co-morbidities, SARS-CoV-2 RNAemia, EBV viremia, and autoantibodies as risk factors, and found that immunological polarization was divergent early post-infection and associated with specific PASC symptoms.
- 14•.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]; This study examined the peripheral blood immune profile of SARS-CoV-2 convalescents and age and sex-matched controls eight months following infection, finding persistent activation of innate immune cells, and elevated inflammatory and antiviral markers.
- 15.Chun H.J., Coutavas E., Pine A.B., Lee A.I., Yu V.L., Shallow M.K., Giovacchini C.X., Mathews A.M., Stephenson B., Que L.G., et al. Immunofibrotic drivers of impaired lung function in postacute sequelae of SARS-CoV-2 infection. JCI Insight. 2021;6 doi: 10.1172/jci.insight.148476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Chioh F.W., Fong S.-W., Young B.E., Wu K.-X., Siau A., Krishnan S., Chan Y.-H., Carissimo G., Teo L.L., Gao F., et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife. 2021;10 doi: 10.7554/eLife.64909. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study suggests that persistent immune activation in the post-acute phase causes endothelial dysfunction.
- 17.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paquin-Proulx D., Gunn B.M., Alrubayyi A., Clark D.V., Creegan M., Kim D., Kibuuka H., Millard M., Wakabi S., Eller L.A., et al. Associations between antibody Fc-mediated effector functions and long-term sequelae in Ebola virus survivors. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.682120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiedemann A., Foucat E., Hocini H., Lefebvre C., Hejblum B.P., Durand M., Krüger M., Keita A.K., Ayouba A., Mély S., et al. Long-lasting severe immune dysfunction in Ebola virus disease survivors. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschenberger M., Hunszinger V., Sparrer K.M.J. Implications of innate immunity in post-acute sequelae of non-persistent viral infections. Cells. 2021;10 doi: 10.3390/cells10082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson B.K., Guevara-Coto J., Yogendra R., Francisco E.B., Long E., Pise A., Rodrigues H., Parikh P., Mora J., Mora-Rodríguez R.A. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.700782. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used a machine learning approach to generate predictive parameters for the development of PASC and suggests individuals with PASC generate peripheral effector T cells lacking appropriate homing signals.
- 22.Chang S.E., Feng A., Meng W., Apostolidis S.A., Mack E., Artandi M., Barman L., Bennett K., Chakraborty S., Chang I., et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun. 2021;12 doi: 10.1038/s41467-021-25509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 24.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregorova M., Morse D., Brignoli T., Steventon J., Hamilton F., Albur M., Arnold D., Thomas M., Halliday A., Baum H., et al. Post-acute COVID-19 associated with evidence of bystander T-cell activation and a recurring antibiotic-resistant bacterial pneumonia. eLife. 2020;9 doi: 10.7554/eLife.63430. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work provides support for SARS-CoV-2 as a catalyst for nonspecific immune activation and increased susceptibility to opportunistic infection.
- 26.Drago F., Ciccarese G., Rebora A., Parodi A. Human herpesvirus-6, -7, and Epstein–Barr virus reactivation in pityriasis rosea during COVID-19. J Med Virol. 2021;93:1850–1851. doi: 10.1002/jmv.26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Van Cleemput J., van Snippenberg W., Lambrechts L., Dendooven A., D’Onofrio V., Couck L., Trypsteen W., Vanrusselt J., Theuns S., Vereecke N., et al. Organ-specific genome diversity of replication-competent SARS-CoV-2. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes evidence of disseminated SARS-CoV-2 infection and persistence.
- 28.Chertow D., Stein S., Ramelli S., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., Winkler C., Dickey J., Ylaya K., et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Res Sq. 2021 doi: 10.21203/rs.3.rs-1139035/v1. pre-print (In Review) [DOI] [Google Scholar]; This pre-print describes the results of autopsies of individuals who died following infection with SARS-CoV-2. The authors describe the location and quantity of SARS-CoV-2 culturable virus, RNA, and sub-genomic RNA in multiple organs and tissues and from subjects at varying time points post-infection.
- 29••.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence of SARS-CoV-2 antigen persistence based on the apparent evolution of memory B cell profiles.
- 30.Zhang L., Richards A., Barrasa M.I., Hughes S.H., Young R.A., Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2105968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smits N., Rasmussen J., Bodea G.O., Amarilla A.A., Gerdes P., Sanchez-Luque F.J., Ajjikuttira P., Modhiran N., Liang B., Faivre J., et al. No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parry R., Gifford R.J., Lytras S., Ray S.C., Coin L.J.M. No evidence of SARS-CoV-2 reverse transcription and integration as the origin of chimeric transcripts in patient tissues. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2109066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazachenka A., Kassiotis G. SARS-CoV-2-host chimeric RNA-sequencing reads do not necessarily arise from virus integration into the host DNA. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.676693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., Canas L.S., Graham M.S., Klaser K., Modat M., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work found that risk of long-term sequelae was reduced by 49% in vaccinated individuals, compared with those unvaccinated.
- 35.Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., Hamilton F.W. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: a case series. Ann Intern Med. 2021;174:1334–1336. doi: 10.7326/M21-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]; This small observational study is one of the first to provide evidence of the improvement of PASC symptoms with subsequent vaccination.
- 36.Files J.K., Sarkar S., Fram T.R., Boppana S., Sterrett S., Qin K., Bansal A., Long D.M., Sabbaj S., Kobie J.J., et al. Duration of post-COVID-19 symptoms are associated with sustained SARS-CoV-2 specific immune responses. JCI Insight. 2021;6 doi: 10.1172/jci.insight.151544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang H., Wegman A.D., Ripich K., Friberg H., Currier J.R., Thomas S.J., Endy T.P., Waickman A.T. Persistent COVID-19 symptoms minimally impact the development of SARS-CoV-2-specific T cell immunity. Viruses. 2021;13 doi: 10.3390/v13050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cirelli K.M., Crotty S. Germinal center enhancement by extended antigen availability. Curr Opin Immunol. 2017;47:64–69. doi: 10.1016/j.coi.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heesters B.A., van Megesen K., Tomris I., de Vries R.P., Magri G., Spits H. Characterization of human FDCs reveals regulation of T cells and antigen presentation to B cells. J Exp Med. 2021;218 doi: 10.1084/jem.20210790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Poon M.M.L., Rybkina K., Kato Y., Kubota M., Matsumoto R., Bloom N.I., Zhang Z., Hastie K.M., Grifoni A., Weiskopf D., et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl9105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work assessed numerous tissues from organ donations after SARS-CoV-2 infection, demonstrating that antigen-specific memory B and T cells are present in the bone marrow, spleen, lung, and lymph nodes for up to 6 months after infection. It provides strong evidence for local coordination of long-term SARS-CoV-2 immune responses.
- 41.Cervia C., Zurbuchen Y., Taeschler P., Ballouz T., Menges D., Hasler S., Adamo S., Raeber M.E., Bächli E., Rudiger A., et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13 doi: 10.1038/s41467-021-27797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turner J.S., Day A., Alsoussi W.B., Liu Z., O’Halloran J.A., Presti R.M., Patterson B.K., Whelan S.P.J., Ellebedy A.H., Mudd P.A. SARS-CoV-2 viral RNA shedding for more than 87 days in an individual with an impaired CD8+ T cell response. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.618402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Peluso M.J., Deitchman A.N., Torres L., Iyer N.S., Munter S.E., Nixon C.C., Donatelli J., Thanh C., Takahashi S., Hakim J., et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed that individuals with PASC demonstrate a lower frequency of CD8+T cells expressing CD107a, a marker of degranulation, and a more rapid decline in the frequency of N-specific interferon g-producing CD8+ T cells, but did not have higher levels of persistent inflammation than non-PASC subjects.
- 44•.Cheon I.S., Li C., Son Y.M., Goplen N.P., Wu Y., Cassmann T., Wang Z., Wei X., Tang J., Li Y., et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abk1741. [DOI] [PMC free article] [PubMed] [Google Scholar]; This group performed comprehensive immunoprofiling of the peripheral blood and BAL from nondeceased aged SARS-CoV-2 convalescent subjects. High dimensional flow cytometry and single-cell RNA sequencing provide insights into potential immunological drivers of post-acute lung sequelae.
- 45.Sutton H.J., Aye R., Idris A.H., Vistein R., Nduati E., Kai O., Mwacharo J., Li X., Gao X., Andrews T.D., et al. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodruff M.C., Walker T.A., Truong A.D., Dixit A.N., Han J.E., Ramonell R.P., Runnstrom M.C., Rudolph M.E., Khosroshahi A., Lee F.E.-H., et al. Evidence of persisting autoreactivity in post-acute sequelae of SARS-CoV-2 infection. MedRxiv pre-print. 2021 doi: 10.1101/2021.09.21.21263845. [DOI] [Google Scholar]
- 47•.Seeßle J., Waterboer T., Hippchen T., Simon J., Kirchner M., Lim A., Müller B., Merle U. Persistent symptoms in adult patients 1 year after Coronavirus Disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74(7):1191–1198. doi: 10.1093/cid/ciab611. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective study examined autoantibody levels in individuals with PASC, finding that neurocognitive symptom frequency was significantly higher in the group with elevated ANA titers.
- 48.Vella L.A., Giles J.R., Baxter A.E., Oldridge D.A., Diorio C., Kuri-Cervantes L., Alanio C., Pampena M.B., Wu J.E., Chen Z., et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of subjects with MIS-C shows that vascular patrolling CX3CR1+ CD8+ T cells are distinctly activated during MIS-C.
- 49.Diorio C., Shraim R., Vella L.A., Giles J.R., Baxter A.E., Oldridge D.A., Canna S.W., Henrickson S.E., McNerney K.O., Balamuth F., et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat Commun. 2021;12 doi: 10.1038/s41467-021-27544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]